Published online Dec 7, 2021. doi: 10.3748/wjg.v27.i45.7813
Peer-review started: April 26, 2021
First decision: June 13, 2021
Revised: June 26, 2021
Accepted: September 2, 2021
Article in press: September 2, 2021
Published online: December 7, 2021
Processing time: 221 Days and 0 Hours
Surgical resection is a treatment of choice for gallbladder cancer (GBC) patients but only 10% of patients have a resectable disease at presentation. Even after surgical resection, overall survival (OS) has been poor due to high rates of recurrence. Combination of surgery and systemic therapy can improve outcomes in this aggressive disease.
To summarize our single-center experience with multimodality management of resectable GBC patients.
Data of all patients undergoing surgery for suspected GBC from January 2012 to December 2018 was retrieved from a prospectively maintained electronic database. Information extracted included demographics, operative and perioperative details, histopathology, neoadjuvant/adjuvant therapy, follow-up, and recurrence. To know the factors associated with recurrence and OS, univariate and multivariate analysis was done using log rank test and cox proportional hazard analysis for categorical and continuous variables, respectively. Multivariate analysis was done using multiple regression analysis.
Of 274 patients with GBC taken up for surgical resection, 172 (62.7%) were female and the median age was 56 years. On exploration, 102 patients were found to have a metastatic or unresectable disease (distant metastasis in 66 and locally unresectable in 34). Of 172 patients who finally underwent surgery, 93 (54%) underwent wedge resection followed by anatomical segment IVb/V resection in 66 (38.4%) and modified extended right hepatectomy in 12 (7%) patients. The postoperative mortality at 90 d was 4.6%. During a median follow-up period of 20 mo, 71 (41.2%) patients developed recurrence. Estimated 1-, 3-, and 5-years OS rates were 86.5%, 56%, and 43.5%, respectively. Estimated 1- and 3-year disease free survival (DFS) rates were 75% and 49.2%, respectively. On multivariate analysis, inferior OS was seen with pT3/T4 tumor (P = 0.0001), perineural invasion (P = 0.0096), and R+ resection (P = 0.0125). However, only pT3/T4 tumors were associated with a poor DFS (P < 0.0001).
Multimodality treatment significantly improves the 5-year survival rate of patients with GBC up to 43%. R+ resection, higher T stage, and perineural invasion adversely affect the outcome and should be considered for systemic therapy in addition to surgery to optimize the outcomes. Multimodality treatment of GBC has potential to improve the survival of GBC patients.
Core Tip: Gallbladder cancer (GBC) is an aggressive malignancy with only 10% of cases amenable to resection at presentation and a dismal overall 5-year survival rate of 5%-13% after curative surgery. Recently, several experts have recommended that multimodality treatment, including neoadjuvant and adjuvant therapies, can improve survival. In this study, we share our experience with multimodality approach in GBC. Five-year overall survival was approaching 50%, and therefore we suggest that such approach can improve survival in this aggressive malignancy.
- Citation: Goel S, Aggarwal A, Iqbal A, Talwar V, Mitra S, Singh S. Multimodality management of gallbladder cancer can lead to a better outcome: Experience from a tertiary care oncology centre in North India. World J Gastroenterol 2021; 27(45): 7813-7830
- URL: https://www.wjgnet.com/1007-9327/full/v27/i45/7813.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i45.7813
Gallbladder cancer (GBC) is the most common and most aggressive malignant disease of the biliary tract. A distinct geographical variability has been observed in the prevalence of GBC. Countries like India, Pakistan, Chile, Korea, and Japan have reported a higher prevalence as compared to the Western world. The highest incidence has been reported in regions like Delhi, India (21.5/100000), La Paz, Bolivia (15.5/100000), South Karachi, Pakistan (13.8/100000), and Quito, Ecuador (12.9/100000)[1,2].
Surgical resection is the treatment of choice but only 10% of patients have a resectable disease at presentation. Even after surgical resection, overall survival (OS) has been poor due to high rates of recurrence[2]. Recently, there has been an increased interest in multimodality treatment including both neoadjuvant and adjuvant therapy to improve outcomes. Although there are no randomized trials on the issue but improved outcomes have recently been reported using multimodality treatment. A recent expert consensus statement on GBC recommended that all patients with clinical T3–4 N+ disease should be considered for neoadjuvant chemotherapy (NACT) trials[3]. After curative resection, patients with T2 or higher and N+ disease should undergo adjuvant systemic chemotherapy or chemoradiotherapy (CRT). Adjuvant CRT should be used in patients with positive margins after resection[3]. With advancements in surgical approach and systemic therapy, multimodality approach has a potential to obtain favorable outcomes in this aggressive disease[4].
We have adapted various aspects of the multimodality approach for GBC in the last decade. In this study, we aimed to analyze our outcomes for multimodality mana
Institutional review board approval was taken for waiver of informed consent (RGCIRC/IRB-BHR/48/2020). All patients undergoing surgery for suspected gall bladder cancer from January 2012 to December 2018 were included. Data containing demographics, operative and perioperative details, histopathology, and neoadju
All patients with suspicion of GBC were evaluated by contrast-enhanced computed tomography (CECT) of the abdomen and pelvis. CEA and CA19-9 were routinely measured in all cases. Since April 2015, all patients who had a resectable disease on CECT underwent an additional 18-FDG positron emission tomography (PET) scan to rule out distant metastasis as a part of the study to evaluate the role of PET scan in GBC[5]. All patients who presented with jaundice underwent magnetic resonance cholangiopancreatography to confirm the level of obstruction and biliary drainage procedure as indicated. Patients who underwent laparoscopic cholecystectomy outside for a benign disease and were found to have to have GBC on histopathology were defined as incidental GBC. They were evaluated similarly except that they were excluded from PET scan study due to possible high false positivity rate in view of ongoing inflammation at the postoperative site. Patients with locally advanced diseases were considered for NACT after discussion in multidisciplinary board. The following criteria were used to select patients for neoadjuvant therapy in primary GBC patients: (1) T4 lesion involving two or more adjacent organs or the hepatic hilum; (2) Extensive hepatic infiltration which required major liver resection (> 2 segments); (3) N2 disease (AJCC 7th); (4) Bulky regional nodes (> 3 cm in short axis); and (5) During waiting period after portal vein embolization.
The main aim for NACT was to select good tumor biology patients and improve R0 resection rate. Incidental cases were referred for NACT if they have a history of bile spillage in index surgery. Neoadjuvant CRT was not done in any patient.
The most commonly used regimen for NACT was gemcitabine (1000 mg/m2 intravenously over 30-60 min) on days 1 and 8, and cisplatin (75 mg/m2 intravenously over 2 h) on day 1, every 21 d. In case of renal compromise, carboplatin was used. After three cycles, patients were reassessed for response using PET-computed tomography (CT) and CECT of the abdomen and pelvis. Due to the retrospective nature of this study, the type and duration of chemotherapy were not controlled and were decided by the team of medical oncologists.
Data collection was done in concordance with ethical guidelines of Declaration of Helsinki. All patients provided informed consent prior to any intervention, chemotherapy, or surgery.
All patients underwent staging laparoscopy to rule out distant metastases. This was followed by exploratory laparotomy and inter-aortocaval (IAC) lymph node sampling for frozen section. Definitive procedure was generally abandoned if IAC nodes were positive for malignancy except for select cases. Resectable primary GBC underwent radical cholecystectomy which included en bloc resection of the gallbladder with a non-anatomical liver wedge (2 cm liver margin) or segment IVB/V resection with regional lymphadenectomy including retropancreatic lymph nodes (station 13) and common hepatic artery nodes (station 8) along with all the soft tissue around and in between hilar structures (station 12). In the initial period, the decision between non-anatomical wedge and segment IVB/V was taken by operative surgeon intraoperatively, but since 2014, all patients were part of a randomized controlled trial (RCT) comparing wedge resection and segment IVB/V resection for GBC (CTRI/2018/05/014324). Selected cases with extensive liver involvement or infiltration into right portal structures underwent modified extended right hepatectomy (en bloc resection of the gallbladder along with segments V, VI, VII, VIII, and IVB) with regional lymphadenectomy. We did not perform hepato-pancreatoduodenectomy or vascular resections for GBC at our centre. Port sites were resected for all patients with incidental GBC before 2016, but it is not done routinely now. Common bile duct resection and adjacent organ (colon/stomach/duodenum) resections were performed only when necessary to achieve R0 status. All intraoperative and perioperative data was recorded. Postoperative complications were recorded and graded according to the Clavien-Dindo classification[6]. Histopathological data for all patients were retrieved and staging was done as per AJCC 8th classification[7].
All patients were discussed in multidisciplinary meetings for planning adjuvant therapy. Since January 2015, all T2/node positive GBC patients were included in an institutional RCT comparing adjuvant chemotherapy and CRT after radical cholecystectomy (R0 resection) [CTRI/2018/01/011296]. The patients randomized to chemotherapy were given single agent gemcitabine 1 gm/m2 on days 1, 8, and 21 in each cycle for six cycles starting 3 wk after surgery. Chemo-radiation group received external beam radiation therapy (50.4 Gy, 1.8 Gy for 28 fractions). Radiation area included gallbladder fossa, tumor bed, and adjacent liver and regional nodes. Chemotherapy included injection of 5-FU 750 mg/m2 on days 1-5 and on last days of radiotherapy in a concurrent fashion. All patients who received NACT completed a total of six cycles of perioperative chemotherapy. Patients with R1 resection received radiation therapy in addition to chemotherapy.
All patients were kept on regular follow-up, every 3 mo for first 2 years, and every 6 mo for next 3 years. At each visit, physical examination and tumor marker (CA19-9 and CEA) measurement were done. CECT of whole abdomen was done every 6 mo and those with suspicious or equivocal findings underwent PET-CT followed by histological confirmation of recurrence. All patients with recurrence were counselled for palliative therapy.
Demographic and preoperative data was given for all patients, including those who were found to have an unresectable/metastatic disease intraoperatively. But these patients were excluded from final analysis. Categorical variables are described using counts/percentages and the mean/median was used for continuous variables. OS and disease free survival (DFS) were calculated using Kaplan-Meier curves. OS was calculated from the date of diagnosis to death or last follow-up and DFS was calculated from the date of surgery to recurrence of disease. To know the factors associated with recurrence and OS, univariate and multivariate analysis was done using log rank test and cox proportional hazard analysis for categorical and continuous variables, respectively. Multivariate analysis was done using multiple regression analysis. The statistical review of the study was performed by a biomedical statistician.
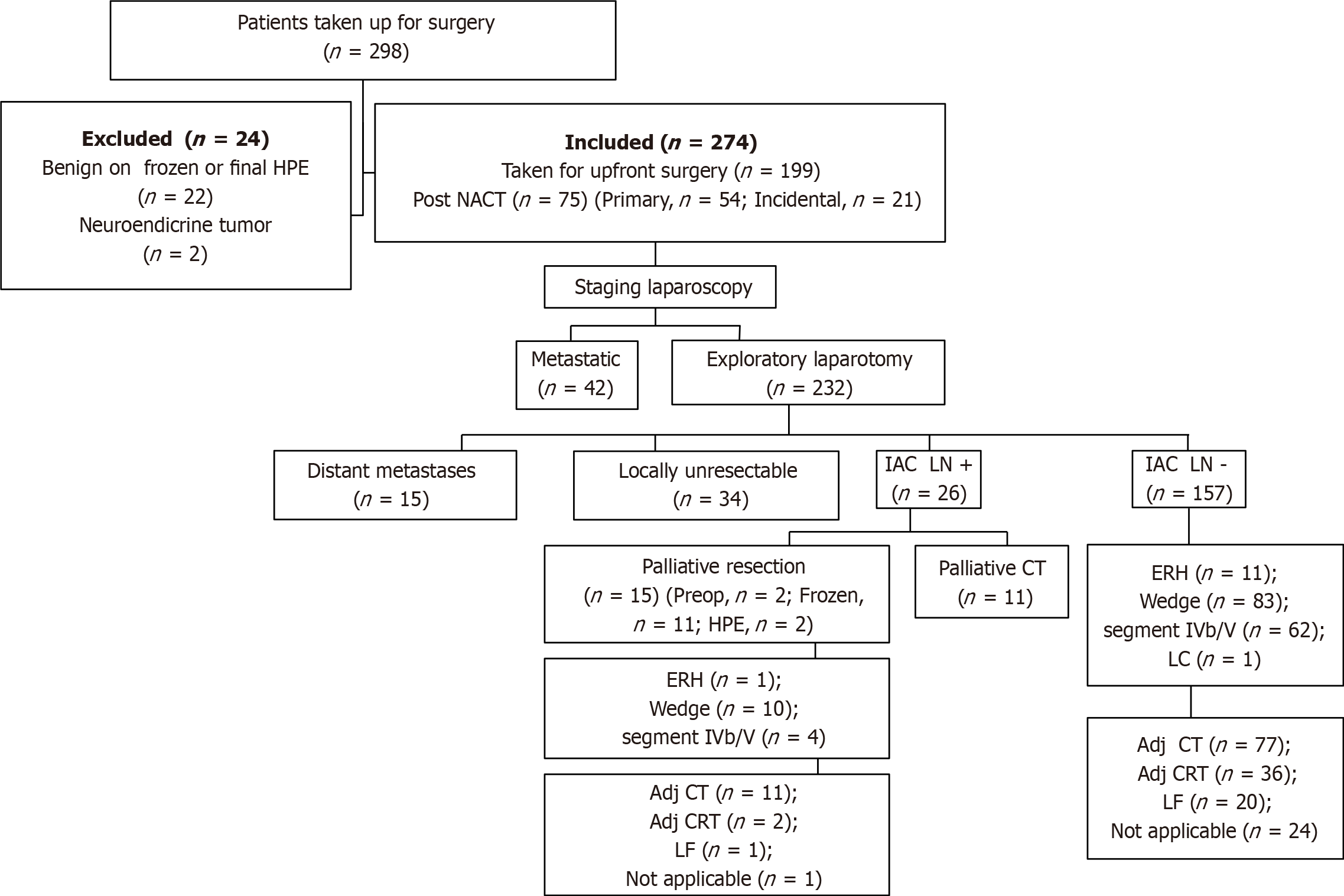
From January 2012 to December 2018, a total of 298 patients were taken up for surgery for a suspected GB malignancy. Out of these, 22 patients were found to have benign disease on final histopathology and 2 had neuroendocrine tumors of the gallbladder, so they were excluded from final analysis (Figure 1). Among 274 patients with a confirmed histopathological diagnosis of GBC, 172 (62.7%) were female and the median age was 56 (range, 28-80) years. The most common presenting symptom was abdominal pain (80.7%), followed by jaundice (8.1%), non-specific symptoms (5.5%), dyspepsia, weight loss, loss of appetite, and fever. Ninety-six (35%) patients had incidental presentation and the median time interval between cholecystectomy and radical surgery was 30 (range, 11-175) d. Cholelithiasis was seen in 173 (63.1%) cases. Although CEA and CA19-9 levels were not available in some patients, CEA was raised in 57/174 (32.8%) and CA19-9 was raised in 94/209 (45%) cases.
Twenty-seven percent (75/274) of all patients received NACT. Out of the 75 patients, 21 had incidental presentation and the rest 54 were non-incidental. Fifty-seven percent (43/75) of patients who received NACT could undergo curative resection and the rest 43% (32/75) were found to have an either metastatic or locally unresectable disease on exploration. Of 43 patients who successfully underwent surgery, 29 received gemcitabine with cisplatin, 12 received gemcitabine with carboplatin, and 2 received gemcitabine only. After NACT, 37 patients underwent radical cholecystectomy (22 had wedge liver resection, and 15 underwent anatomical segment IVb/V resection) and 6 had modified extended right hepatectomy.
On exploration, 102 (staging laparoscopy, 42; laparotomy, 60) patients were found to have a metastatic or unresectable disease. Distant metastasis was seen in 66 patients (peritoneum, 40; liver, 15; IAC nodes, 11) and 34 had a locally unresectable disease on exploration. Two patients who were planned for major hepatectomy were found to have liver cirrhosis and surgery was abandoned. Of 172 patients who finally underwent surgical resection, 93 (54%) underwent wedge resection followed by anatomical segment IVb/V resection in 66 (38.4%) and modified extended right hepatectomy in 12 (7%) patients. One patient underwent laparoscopic cholecystectomy but was found to have T1a disease on final histopathology. Adjacent organ resection was done in 66 patients (CBD, 31; colon, 11; stomach/duodenum, 13; and multiple organs, 11). Median blood loss was 200 (range, 50-2000) mL and median duration of surgery was 270 (range, 120-540) min.
The postoperative mortality at 90 d was 4.6% (8/172), and the most common cause of death was bile leak and subsequent sepsis (n = 3) followed by postoperative liver failure (n = 2), acute myocardial infarction (n = 2), and ARDS (n = 1). Overall morbidity rate was 30.8% (53/172) but clinically significant complications (Clavien-Dindo grade III or more) were seen in only 12.2% (21/172) of cases. Median hospital stay was 9 (range, 3-54) d.
Histopathological details are described in Table 1. The most common histological diagnosis was adenocarcinoma seen in 160/172 (93%) cases. All patients were staged according to the AJCC 8th TNM classification. The majority of patients had a T2/T3 (83%) disease and 55/172 (32%) had a node positive disease. Median number of lymph nodes resected was 9 (range, 1-25). On final staging, the maximum number of patients had a stage III disease (III, 73; II, 45; IV, 33; I, 21).
| Patient characteristic | n (%) |
| Type of surgery | |
| Wedge resection | 93 (54) |
| Anatomical segment IVb/V resection | 66 (38.4) |
| Modified extended right hepatectomy | 12 (7) |
| Lap cholecystectomy | 1 (0.6) |
| Histology | |
| Adenocarcinoma | 160 (93) |
| Adenosquamous | 9 (5.2) |
| Carcinosarcoma | 2 (1.2) |
| Squamous | 1 (0.6) |
| Histological grade | |
| Well differentiated | 33 (19.2) |
| Moderately differentiated | 116 (67.4) |
| Poorly differentiated | 23 (13.4) |
| pT stage | |
| T1 | 25 (14.5) |
| T2 | 70 (40.7) |
| T3 | 73 (42.4) |
| T4 | 4 (2.4) |
| pN stage | |
| N0 | 117 (68) |
| N+ | 55 (32) |
| LVI positive | 54 (31.4) |
| PNI positive | 56 (32.5) |
| IAC positive | 15 (8.7) |
| R0/R1 resection | |
| R0 | 161 (93.6) |
| R1 | 11 (6.4) [liver (n = 4), cystic duct (n = 4), bile duct (n = 3)] |
| Final stage (AJCC 8th) | |
| I | 21 (12.2) |
| II | 45 (26.2) |
| III | 73 (42.4) |
| IV | 33 (19.2) |
| Postoperative morbidity | |
| Overall | 53 (30.8) |
| Clavien-Dindo grade III & above | 21 (12.2) |
| Bile leak | 14 (8.1) |
| Clinically significant | 5 (2.9) |
| 90 d mortality | 8 (4.6) |
| Adjuvant therapy | 117/147 (79.6) |
| Chemotherapy only | 80 |
| Chemoradiotherapy | 37 |
| Advised but not taken/incomplete | 30 |
| Not indicated | 25 |
Excluding the patients who had a stage I (n = 21) disease on final histopathology, 151 patients were eligible for adjuvant therapy. Approximately 86% (126/147) of patients received adjuvant therapy. Out of these, 88 received chemotherapy only and 38 received CRT. Ninety-seven percent of patients in the radiotherapy group (37/38) and 90.9% (80/88) patients in the chemotherapy group completed the intended treatment. Overall, 117 out of 126 (92.8%) patients completed the adjuvant therapy.
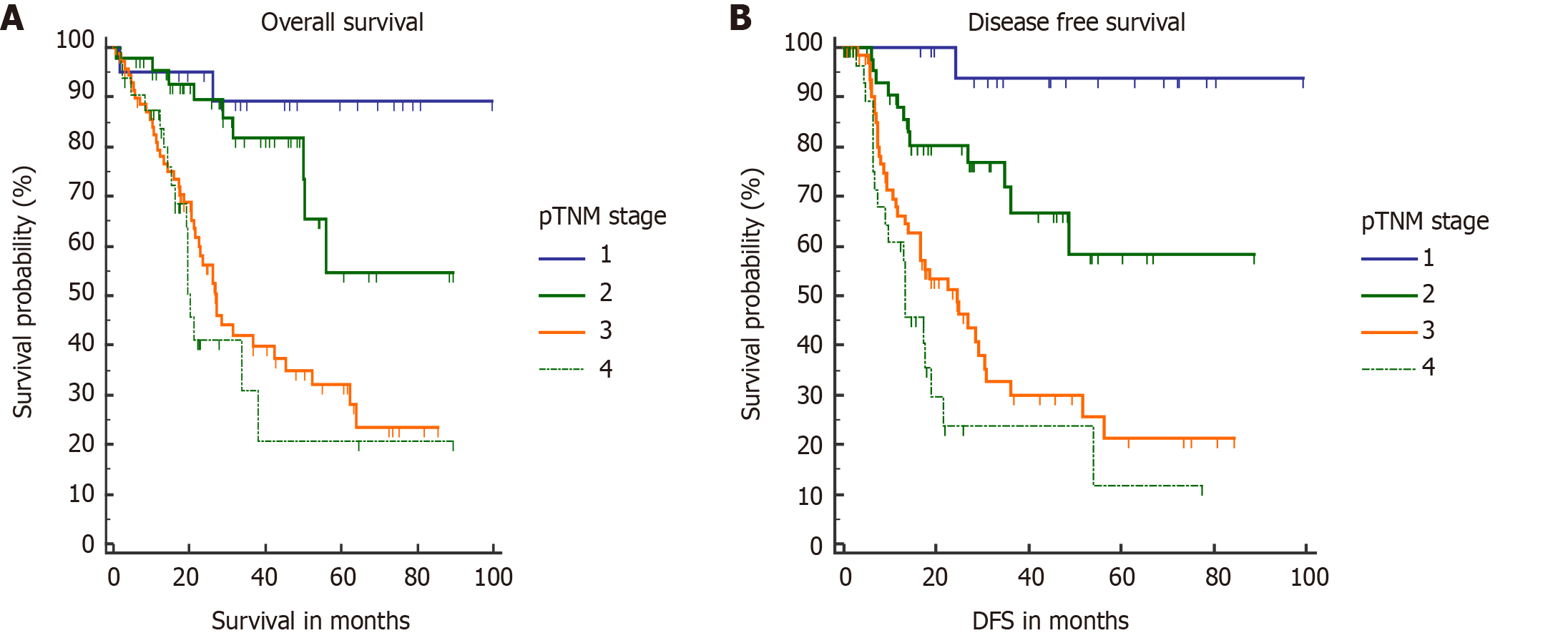
During a median follow-up period of 20 mo, 71 (41.2%) patients developed recurrence. In the majority of them, recurrence was seen at a distant site (47/71, 66.2%) followed by loco-regional failure in 18/71 (25.4%) and at multiple sites in 6 (8.4%). The most common site of distant metastases was the peritoneum (n = 22) followed by the liver (n = 15), distant nodes (n = 9), and lung (n = 1). Median DFS and OS were not reached in our study. However, median OS for stage III and stage IV patients was 27.1 mo and 19.6 mo, respectively. Median DFS for stage III and stage IV patients was 24 mo and 13 mo, respectively. Estimated 1-, 3-, and 5-year OS rates were 86.5%, 56%, and 43.5%, respectively. Estimated 1- and 3-year DFS rates were 75% and 49.2%, respectively. Stagewise OS and DFS are shown in Figure 2. On log rank test, they correlated significantly.
On univariate analysis, inferior OS and DFS were associated with upfront presentation (non-incidental), positive resection margin, lymph node involvement, higher T stage (T3 or T4), and lymphovascular and perineural invasion (PNI) (Table 2). Neoadjuvant therapy was given in advanced cases, hence the cohort was associated with a poor outcome. However, on multivariate analysis, inferior OS was seen with pT3/T4 tumour (P = 0.0001), PNI (P = 0.0096), and R+ resection (P = 0.0125). On multivariate analysis, only pT3/T4 tumors were associated with a poor DFS (P < 0.0001). Also, association of R+ resection with early recurrence was approaching the level of significance (P = 0.0513).
| Overall survival | Disease free survival | |||||
| Factor | HR | 95%CI | P value | HR | 95%CI | P value |
| Sex | ||||||
| Female | 0.92 | [0.56-1.51] | 0.76 | 0.98 | [0.61-1.6] | 0.96 |
| Male | ||||||
| Jaundice | ||||||
| Yes | 0.9 | [0.37-2.16] | 0.81 | 1.19 | [0.48-2.93] | 0.67 |
| No | ||||||
| Incidental | ||||||
| Yes | 0.24 | [0.07-0.81] | 0.001 | 0.54 | [0.34-0.86] | 0.01 |
| No | ||||||
| Neoadjuvant therapy | ||||||
| Yes | 2.2 | [1.27-3.81] | < 0.001 | 2.91 | [1.64-5.16] | < 0.001 |
| No | ||||||
| Resection | ||||||
| R+ | 4.08 | [1.22-13.64] | < 0.001 | 4.13 | [1.22-13.9] | < 0.001 |
| R0 | ||||||
| Lymph node status | ||||||
| Positive | 1.91 | [1.13-3.25] | 0.006 | 2.44 | [1.4-4.17] | 0.001 |
| Negative | ||||||
| T stage | ||||||
| T1a-T2 | ||||||
| T3-T4 | 5.01 | [3.06-8.18] | < 0.001 | 4.21 | [2.55-6.94] | < 0.001 |
| LVI | ||||||
| Yes | 2.08 | [1.22-3.5] | 0.001 | 2.12 | [1.23-3.64] | 0.001 |
| No | ||||||
| PNI | ||||||
| Yes | 3.06 | [1.75-5.37] | < 0.001 | 2.54 | [1.45-4.45] | < 0.001 |
| No | ||||||
| Poorly differentiated | ||||||
| Yes | ||||||
| No | 1.75 | [0.81-3.7] | 0.07 | 1.43 | [0.66-3.08] | 0.28 |
In our study, 147 patients were advised to receive adjuvant therapy, out of which 117 patients completed the adjuvant therapy (adjuvant group) whereas 30 patients did not take/complete adjuvant therapy (non-adjuvant group). These two groups were comparable in baseline characteristics except for a higher incidence of post-cholecystectomy GBC in the adjuvant group (Table 3).
| Patient characteristic | Adjuvant group (n = 117), n (%) | Non-adjuvant Group (n = 30), n (%) | P value |
| Age, yr (mean) | 54.5 | 60 | 0.01 |
| Sex (M:F) | 47:70 | 10:20 | 0.63 |
| Incidental GBC | 55 (47) | 7 (23.3) | 0.03 |
| Neoadjuvant therapy | 33 (28.2) | 7 (23.3) | 0.76 |
| Type of surgery | 0.71 | ||
| Wedge resection | 62 (53) | 16 (53.3) | |
| Anatomical segment IVb/V resection | 48 (41) | 11 (36.7) | |
| Modified extended right hepatectomy | 7 (6) | 3(10) | |
| Histology | 0.91 | ||
| Adenocarcinoma | 108 (92.3) | 28 (93.3) | |
| Adenosquamous | 7(6) | 2 (6.7) | |
| Carcinosarcoma | 1 | 0 | |
| Squamous | 1 | 0 | |
| Histological grade | 0.12 | ||
| Well differentiated | 21 (18) | 2 (6.7) | |
| Moderately differentiated | 82 (70.1) | 21 (70) | |
| Poorly differentiated | 14 (11.9) | 7 (23.3) | |
| pT stage | 0.56 | ||
| T1 | 6 (5.1) | 2 (6.7) | |
| T2 | 57 (48.7) | 11 (36.7) | |
| T3 | 52 (44.4) | 17 (56.6) | |
| T4 | 2 (1.7) | 0 | |
| pN stage | 0.47 | ||
| N0 | 77 (65.8) | 17 (56.7) | |
| N+ | 40 (34.2) | 13 (43.3) | |
| LVI positive | 37 (31.6) | 13 (43.3) | 0.32 |
| PNI positive | 40 (34.2) | 12 (40) | 0.7 |
| R0/R1resection | 0.84 | ||
| R0 | 109 (93.2) | 27 (90) | |
| R1 | 8 (6.8) | 3 (10) | |
| Final stage (AJCC 8th) | 0.79 | ||
| I | 3 (2.6) | 1 (3.3) | |
| II | 36 (30.8) | 8 (26.7) | |
| III | 56 (47.9) | 13 (43.3) | |
| IV | 22 (18.1) | 8 (26.7) |
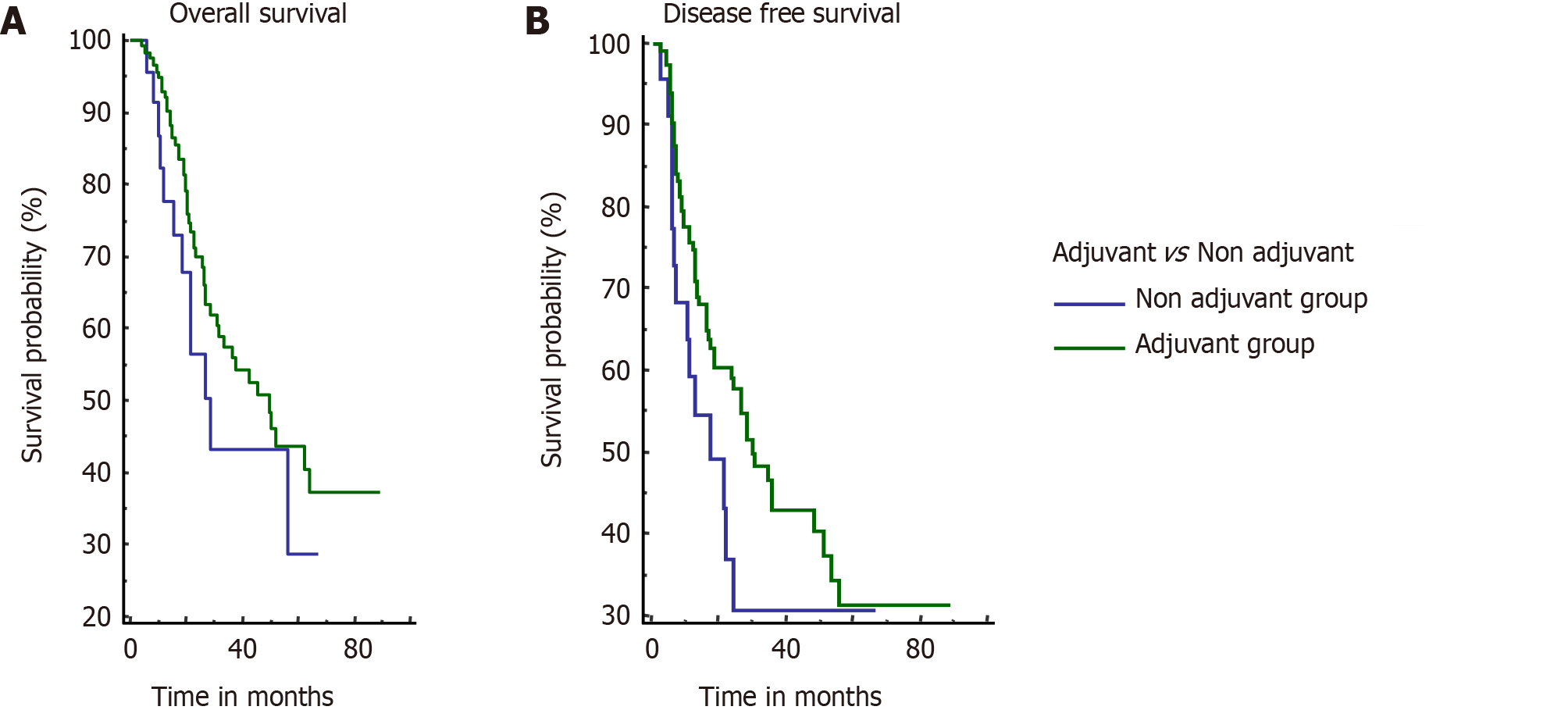
Estimated median OS for the adjuvant group and non-adjuvant group was 49.9 mo and 28.5 mo, respectively; however, the difference was not significant (P = 0.21). Estimated median DFS was 30.6 mo and 17.7 mo for the adjuvant and non-adjuvant group, respectively (P = 0.14) (Figure 3).
According to GLOBOCAN 2018 data, GBC accounts for 1.2% of all cancer diagnoses worldwide with a median survival of less than a year in advanced cases[8]. It is an aggressive malignancy with usually late presentation with an overall estimated 5-year survival rate of 5%-13%[9-11]. Radical surgery is the mainstay of treatment but survival with surgery alone is dismal in locally advanced cases[10].
Presentation is usually a decade late in Western patients as compared to those in our series[12], which can be attributed to endemicity of GBC in Indian subcontinent which has higher composition of younger population. It is diagnosed either incidentally (where cholecystectomy is performed for benign conditions) or mostly in advanced stage where patients present with cachexia with or without jaundice.
Cholelithiasis has been associated with GBC in several studies with a prevalence of stones in approximately 70%-88% of cases of GBC[13,14]. Our study showed the absence of gallstones in approximate one-third of cases, which might be explained by environmental and genetic predisposition of the study population to GBC.
Incidental detection of GBC after cholecystectomy usually confers a favorable prognosis as the malignancy is usually detected in early stage[15,16]. Non-incidental cases are more likely to have advanced T stage, high-grade tumors, lymphovascular invasion, positive lymph nodes, and R2 resection[16]. Optimal timing of completion of radical cholecystectomy is still debatable. Early surgery may lead to higher morbidity due to recent inflammation and adhesions and is also associated with a higher rate of unresectability due to breach of tumor and dissemination with seeding of tumor cells in the peritoneal cavity during index surgery[17]. Recently, a multi-institutional study showed a better survival when re-resection was performed between 4-8 wk from the index surgery although the retrospective and observational nature of the study casts apprehension over its universal application[18]. However, it is pertinent to give importance to bile spillage during index surgery, residual disease, and tumor biology rather than relying solely on the time interval[10,19]. In the present study, median time interval between index and redo surgery was 4 wk. Surgery was usually delayed with administration of NACT if there was any evidence of bile spillage. Future outcome also correlates well with the presence of residual disease on final exploration. Risk factors for finding residual disease include T3 tumors, PNI, and lymphovascular invasion[20]. Even half of the patients with incidental T1b/T2 GBC have residual disease on re-exploration and subsequently have a poor outcome[21]. However, higher T stage and poorly differentiated tumors have shown a high probability of residual disease at redo surgery[22]. In our study, 39.7% (29/73) of patients with incidental GBC were found to have residual disease at re-exploration. Incidental cases were found to have a significantly better survival on univariate analysis but not on multivariate analysis. This might be due to a smaller sample size of incidental cases in view of its lesser prevalence as compared to Western studies (Table 4). Also the number of truly incidental GBC (pT1) was much higher in Western studies as compared to our series (pT1 = 16.4%)[23].
| Parameter | Our study | Patkar et al[4], 2018 | Creasy et al[15], 2019 |
| Sample size | 274 | 400 | 437 |
| Patients who underwent complete resection | 172 | 320 | 255 |
| Unresectable | 102 | 80 | 182 |
| Major liver resection, (%) | Yes (7) | No | Yes (24.3) |
| Incidental GBC, (%) | 35 | 40 | 60.7 |
| R1 resection, n (%) | 11/172 (6.4) | 10/320 (3.1) | 15/255 (5.9) |
| Neoadjuvant in resectable group, n (%) | 43/172 (25) | 83/320 (25.9) | 16/255 (6.3) |
| Final stage III/IV, n (%) | 106/172 (61.6) | 232/400 (58) | 306/437 (70) |
| LN positivity, n (%) | 56/172 (32) | 98/320 (30.62) | NA |
| Residual disease in incidental cases, n (%) | 29/73 (39.7) | 68/160 (42.5) | 172/276 (62.3) |
| Adjuvant therapy, n (%) | 117/147 (79.6) | 206/320 (64.4) | 78/255 (30.7) |
| Recurrence, n (%) | 71/172 (41.2) | 98/320 (30.6) | NA |
| Dying of disease, n (%) | 69/172 (40.1) | 45/320 (14) | 149/255 (58.4) |
| Estimated 3 yr OS, (%) | 56 | 64 | NA |
| Estimated 5 yr OS, (%) | 43.5 | NA | 43 (only survivors) |
| Estimated 3 yr DFS, (%) | 49.2 | 49 | 36 |
Curative surgery with R0 resection improves the survival of GBC patients. The tendency of GBC to have early systemic dissemination often rules out radical surgery. A recently published study from our centre showed that routine application of 18-FDG PET changed management in approximately one-fourth of all resectable primary GBC patients and in one-third of locally advanced cases due to detection of unsuspected distant metastasis[5]. Similarly, routine application of staging laparoscopy before surgical exploration prevented non-therapeutic laparotomy in 23% of overall GBC patients with higher yield in locally advanced cases[24]. We universally applied staging laparoscopy in GBC patients before proceeding with curative surgery. It prevented laparotomy in 15.3% (42/274) of cases and helped in not only preventing surgical morbidity but also leading to quick commencement of palliative treatment. Staging laparoscopy is now routinely recommended prior to laparotomy for all suspected or proven GBC cases[3].
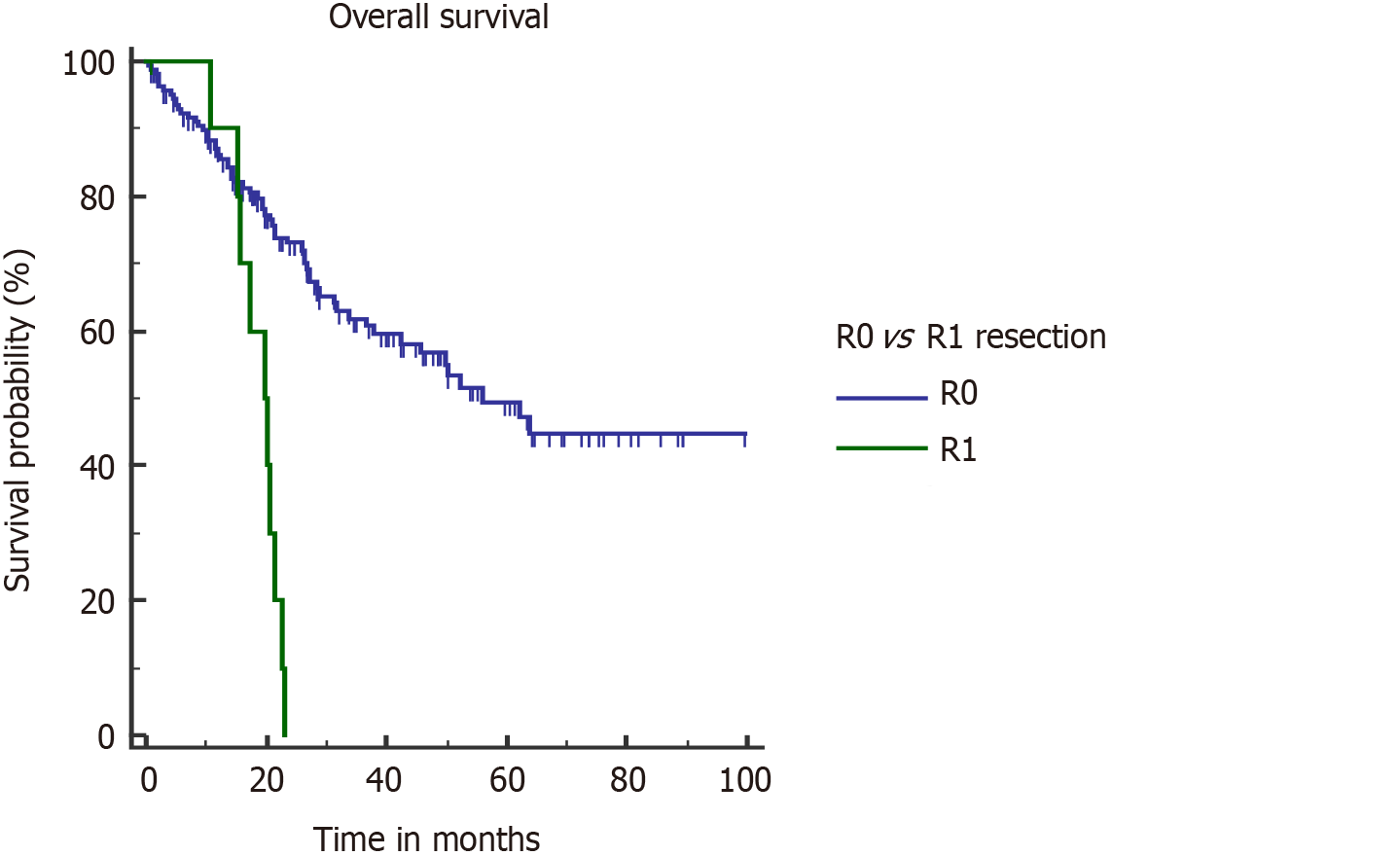
For non–metastatic GBC, standard surgical treatment is radical cholecystectomy which includes non-anatomical wedge or segment IVb/V resection with locoregional lymphadenectomy. Adjacent organ resection or major hepatectomy may be necessary to achieve negative margins. R0 resection was one of the major factors that significantly affected OS survival in our series. R1 resection was associated with a higher risk of death (hazard ratio [HR] = 4.08, 95%CI: 1.22-13.64, P < 0.001) and recurrence (HR = 4.13, 95%CI: 1.22-13.9, P < 0.001). All the patients with positive microscopic margin had a stage III or IV disease. Median OS in patients with R1 resection was significantly poor (19.6 vs 56.1 mo) (Figure 4). Patkar et al[4] also showed an inferior survival after R1 resection (17 vs 71 mo). It seems logical to give neoadjuvant treatment to avoid R1 resection in cases where tumor is close to resection margins on imaging, which is mostly in stages III and IV disease.
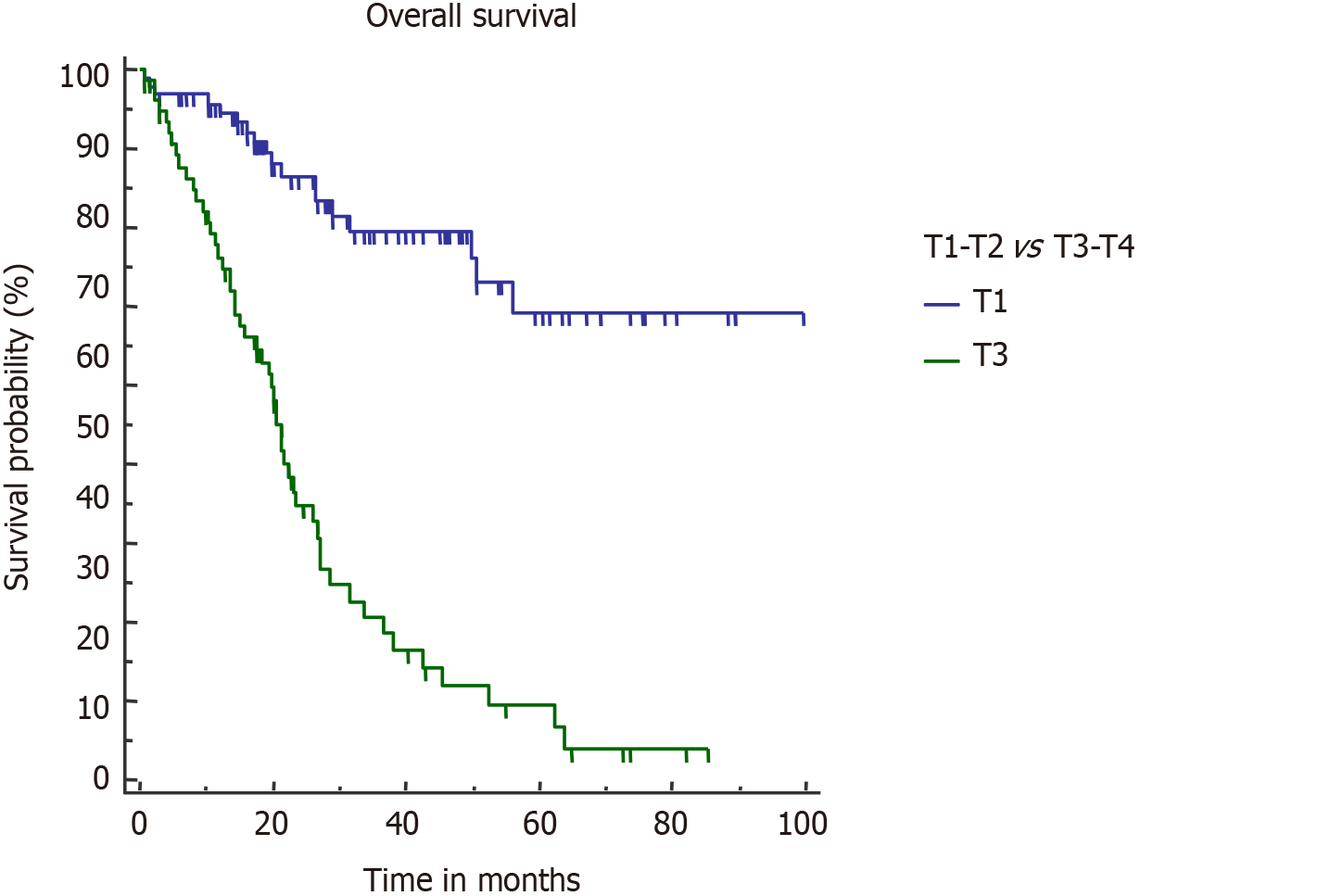
T stage is an important determinant of final outcome of GBC patients[25,26]. Increasing T stage is also associated with a higher probability of lymph nodal involvement and PNI[4,27]. Higher T stage (pT3/T4) was the only factor which negatively impacted both OS and DFS in our study. Median OS in pT3/T4 tumors was 21.5 mo (Figure 5).
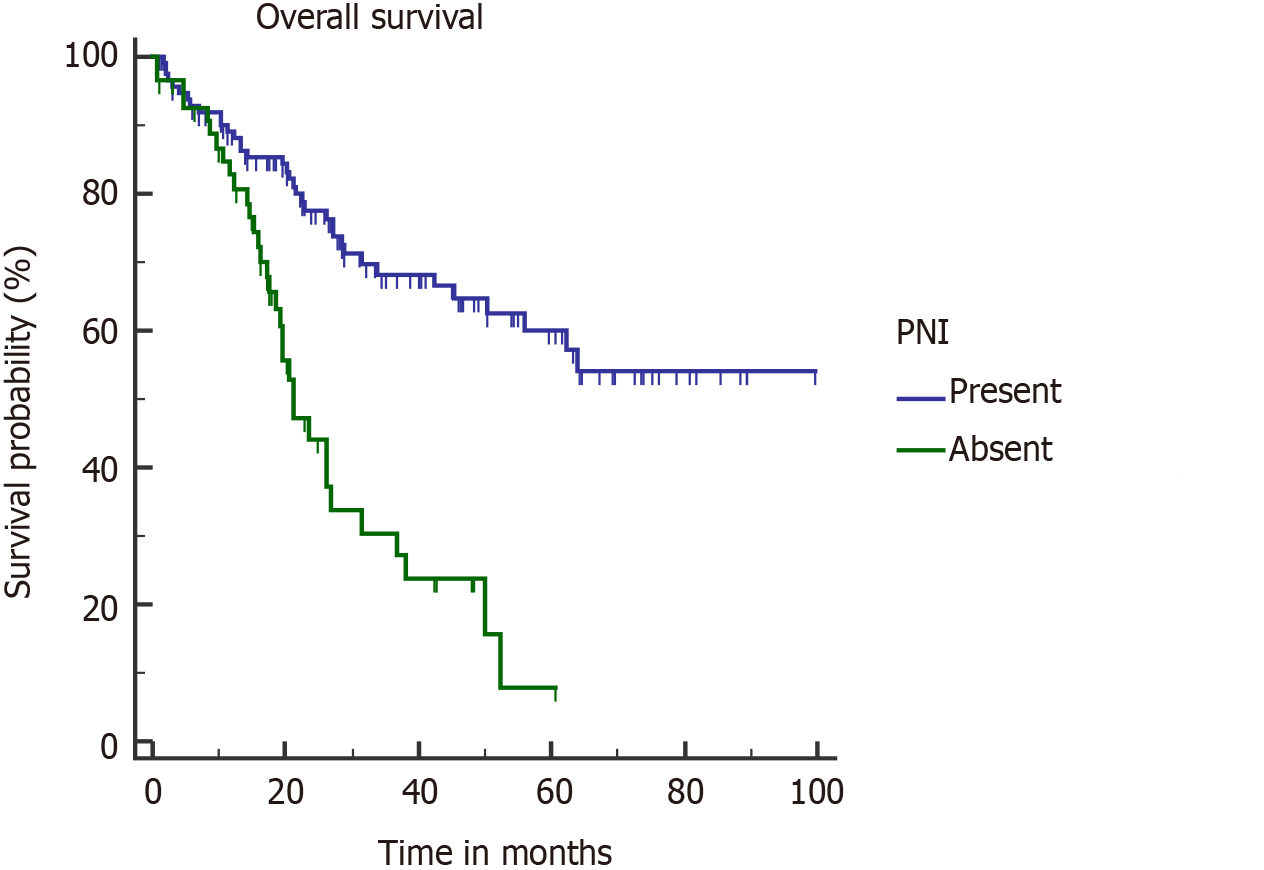
PNI is acknowledged as a poor pathological factor with inferior outcome[26,28]. PNI is more frequently found in proximal tumors (tumors located in GB neck and cystic duct) and with higher T stage[27]. PNI positive patients are shown to have significantly lower OS and DFS[26-28]. In our study, on multivariate analysis, PNI adversely affected OS (median OS, 21.3 mo) in PNI positive patients (Figure 6). Median OS was not reached in the PNI negative cohort. None of the patients with stage I disease was found to have PNI positivity, which correlates with the results of a recent study[27]. However, almost half of combined stage III/IV patients had PNI (48/106).
In past, various studies have reported about the adverse impact of node positivity on survival[4,15,29]. From the AJCC 8th edition, N classification of GBC was modified with more emphasis laid on the number rather than the location of involved nodes. Suspicious or confirmed involvement of lymph nodes is also one of the indications for neoadjuvant therapy[30]. In our study, 32% of operated patients had pathological involvement of lymph nodes but it did not affect survival or recurrence on multivariate analysis. LN sampling was adequate, with a median LN harvest of 9. Seventy-three percent of node positive patients completed intended adjuvant therapy. This might explain partly why lymph node positivity did not affect survival and recurrence in the present study.
Chemotherapy is used as an adjunct to surgery in several settings of GBC: (1) As adjuvant therapy after surgical resection, with or without radiation to minimize recurrence; and (2) As neoadjuvant therapy in locally advanced GBC to downstage disease and select good biology tumors for surgery. Due to the rarity of GBC in the West, the data is often clubbed with other biliary malignancies, which leads to heterogeneity of data and hampers their applicability to GBC. Recently published studies from high volume centres have highlighted the need of multimodality management of GBC patients for further improvement in outcomes (Table 4).
Neoadjuvant therapy for GBC is still not standardized in terms of indications, regimen, and duration. Institutions follow their own protocols based on the local data in the absence of randomized trials. The most suitable cases for implementation of NACT in GBC would be incidental GBC patients with residual mass on imaging or evidence of bile spillage during index surgery or locally advanced GBC where R0 resection is not feasible. Locally advanced GBC usually refers to T3 tumors with extensive liver involvement, T4 tumors, or those with any T stage and nodal involvement on imaging.
No randomized control trial has been conducted till date to test the efficacy of neoadjuvant therapy in GBC. A recent systematic analysis reviewed eight studies, out of which five were from India and only two were prospective studies. This calls attention to the paucity of the literature on neoadjuvant therapy for GBC[31]. The median OS for locally advanced cases that undergo curative resection following neoadjuvant therapy is found to be significantly better than that of patients who did not have surgery following neoadjuvant therapy[30,31]. In one of the largest studies, on a retrospective review of 160 patients, Chaudhari et al[30] reported a response rate of 52% with surgery feasible in 41% of cases. In another study from the same centre, 74% of patients who received neoadjuvant therapy could undergo R0 resection[4]. In a study from the West, Creasy et al[15] showed a median survival of 50 mo in locally advanced GBC patients who underwent surgery after preoperative gemcitabine based chemotherapy. In our study, the neoadjuvant therapy cohort had a poor survival due to the advanced nature of the disease in this subclass. However, 57% of patients with locally advanced disease initially could undergo surgery after NACT. Improvement in chemotherapeutic drug regimen with possible addition of targeted therapy might further improve resectability rate in future.
Even after R0 resection, 30%-70% of patients develop recurrence over the time[4,15,32]. On analysis, 41% (71/172) of our patients developed recurrence after surgery, out of which 2/3 relapsed at distant sites. Higher rate of distant relapse in spite of R0 resection emphasizes on the need of inclusion of novel systemic therapies for further improvement in outcome and survival.
In contrast to neoadjuvant therapy, adjuvant therapy has been tested in the RCT setting with mixed results. In a meta-analysis by Ma et al[33], patients with positive lymph nodes, R1 resection, and non-stage I, benefited most from administration of adjuvant chemotherapy. Recently, several studies have highlighted various chemotherapy drug combinations with promising results after surgery. In the ABC-02 trial, 410 patients with advanced or metastatic biliary malignancy (36% cases were GBC) were randomized to receive gemcitabine + cisplatin or cisplatin alone. The results demonstrated significant improvements in OS (11.7 vs 8.1 mo, P < 0.001) with the combination regimen[34]. Another French study (PRODIGE-12/ACCORD-18) evaluated 196 patients with biliary malignancy after surgical resection, out of which only 20% of patients had GBC. The trial randomized patients to receive gemcitabine + oxaliplatin or observation alone. The study found no survival benefit in the chemotherapy group. The study was criticized for including a lower proportion of high-risk patients (R1 resection and node positive patients) who can derive maximum benefit from adjuvant therapy[35].
More recently, in a study from UK (BILCAP trial), patients with biliary malignancies were randomized to receive either adjuvant capecitabine or observation alone after surgery. A total of 447 patients were included in the study, out of which only 18% were GBC cases. This study clearly demonstrated the benefit of adjuvant therapy in improving the OS and decreasing the recurrence rate during the first 2 years after surgery. However, in this study, there were issues with quality of surgery performed as 54% of cases had positive microscopic margins and also 38% had node positive disease which is a subclass that derives maximum benefit from adjuvant therapy[36].
In view of a 25%-68% rate of recurrence in loco-regional basin, researchers have been advocating administration of adjuvant CRT[4,37]. In a study from the United States (SWOG0809 trial), 79 patients with biliary tract cancer were analyzed after receiving adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine. GBC comprised 32% of the study population. The local recurrence at 2 years was 11% with a median OS of 35 mo. In spite of the lack of a control group, this study provided clinicians with a well-supported regimen[38]. However, Fareed et al[39] found no survival benefit with adjuvant chemoradiation in resected GBC patients. In a recent multi-institutional analysis, resected GBC patients with high-risk features such as T3/T4 tumor, lymph node positivity, and R1 resection were found to derive maximum benefit after adjuvant therapy[40]. In present times, the data is still insufficient to conclusively advocate adjuvant chemotherapy over chemoradiation in node negative R0 resected patients. However, adjuvant chemoradiation is unanimously considered to be the treatment of choice in patients with R1/2 resection margins[3].
In the absence of standard clinical guidelines, in the current study, all patients with T stage ≥ 2 and/or positive lymph node were advised to receive adjuvant therapy. Three–fourth of all our patients received adjuvant therapy. Estimated 5-year OS rate was 43.5%, which is comparable to that observed in the MSKCC study[15] (Table 4). Historically, the 5-year OS rate after aggressive resection for GBC was 16%[41]. Even after all the advancements in surgical technique and perioperative care, the median survival for patients with stage I-III disease was 12.9 mo and 5.8 mo for those presenting with stage IV disease in the absence of multimodality treatment at MSKCC in 2008 with improvement in survival after increase in administration of systemic therapy[15,42]. Our study showed a better median survival for stage III and IV cases with multimodality treatment (27 mo and 20 mo, respectively). When comparing early stage disease (stages I and II) with locally advanced stage GBC (stages III and IV), the former had a significantly better survival (73.1 vs 41.4 mo, respectively, P < 0.0001), which emphasizes on the need for better chemotherapeutic regimen as well as uniform application of systemic therapy in the adjuvant setting.
Our study is one of the largest studies worldwide reporting improved outcomes following multimodality treatment in surgically resected patients. In wake of the scarcity of data on multimodality management of GBC, our study highlights the feasibility of better outcomes with proper utilization of systemic therapy with surgery to obtain optimum results. Correlation between specific chemotherapy regimens and survival is beyond the scope of this study due to its retrospective nature. Despite inherent limitations with potential biases, our study stresses on the urgent need for conducting randomized trials to form consensus on tackling an aggressive disease like GBC. In future, addition of genomic profiling-guided targeted therapy may potentially improve the survival and personalize the therapy of GBC patients.
GBC is an aggressive malignancy which warrants equally aggressive measures to provide patients with a meaningful survival. With addition of systemic therapy to curative surgery, the 5-year survival rate in our study was 43%. R+ resection, higher T stage, and PNI adversely affected the outcome. Patients with higher stage (III/IV), nodal involvement, and high-risk features should be considered for systemic therapy in addition to surgery to optimize the outcomes. Multimodality treatment of GBC has a potential to improve the survival of these patients.
Gallbladder cancer (GBC) is an aggressive biliary tract cancer with only 10% of cases amenable to resection at presentation with a dismal overall 5-year survival of less than 15% after surgery. Even after surgical resection, overall survival (OS) has been poor due to high rates of recurrence. With advancements in surgical approach and systemic therapy, multimodality approach has a potential to obtain favorable outcomes in this aggressive disease; however, there is a paucity of data in the literature for its uniform application.
In the management of patients with GBC, adoption of a multimodality approach should be considered.
The research purpose was to share our experience and give an overview on mul
All the data of patients undergoing surgery for suspected GBC from January 2012 to December 2018 was retrieved from a prospectively maintained electronic database and analyzed.
Multimodality treatment significantly improved the 5-year survival of patient with GBC. Microscopically positive resection margin, higher T stage, and perineural invasion adversely affected the outcome.
Gallbladder cancer has a favorable survival when treated with multimodality approach. Patients with high-risk features may particularly benefit from this approach
Multimodality treatment of GBC has a potential to improve the survival of GBC patients.
The authors would like to thank Dr. Jaipuria J, Consultant (Surgical Oncology), for his valuable inputs.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tashkandi E S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Lai CH, Lau WY. Gallbladder cancer--a comprehensive review. Surgeon. 2008;6:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010;15:168-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 3. | Aloia TA, Járufe N, Javle M, Maithel SK, Roa JC, Adsay V, Coimbra FJ, Jarnagin WR. Gallbladder cancer: expert consensus statement. HPB (Oxford). 2015;17:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 317] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 4. | Patkar S, Ostwal V, Ramaswamy A, Engineer R, Chopra S, Shetty N, Dusane R, Shrikhande SV, Goel M. Emerging role of multimodality treatment in gall bladder cancer: Outcomes following 510 consecutive resections in a tertiary referral center. J Surg Oncol. 2018;117:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Goel S, Aggarwal A, Iqbal A, Gupta M, Rao A, Singh S. 18-FDG PET-CT should be included in preoperative staging of gall bladder cancer. Eur J Surg Oncol. 2020;46:1711-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8555] [Article Influence: 534.7] [Reference Citation Analysis (0)] |
| 7. | Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2018;25:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 544] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 8. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55673] [Article Influence: 7953.3] [Reference Citation Analysis (132)] |
| 9. | Rückert JC, Rückert RI, Gellert K, Hecker K, Müller JM. Surgery for carcinoma of the gallbladder. Hepatogastroenterology. 1996;43:527-533. [PubMed] |
| 10. | Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg. 1994;219:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 209] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9954] [Article Influence: 995.4] [Reference Citation Analysis (0)] |
| 12. | Ries LA, Young JL, Keel GE. editors. SEER survival monograph: cancer survival among adults: U.S. SEER Program, 1988-2001, patient and tumorcharacteristics. National Cancer Institute, SEER Program. Bethesda (MD): NIH, 2007. |
| 13. | Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda LC, Wang BS, Shen MC, Zhang BH, Niwa S, Chen J, Fraumeni JF Jr. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97:1577-1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Pilgrim CH, Groeschl RT, Christians KK, Gamblin TC. Modern perspectives on factors predisposing to the development of gallbladder cancer. HPB (Oxford). 2013;15:839-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Creasy JM, Goldman DA, Gonen M, Dudeja V, O'Reilly EM, Abou-Alfa GK, Cercek A, Harding JJ, Balachandran VP, Drebin JA, Allen PJ, Kingham TP, D'Angelica MI, Jarnagin WR. Evolution of surgical management of gallbladder carcinoma and impact on outcome: results from two decades at a single-institution. HPB (Oxford). 2019;21:1541-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Ethun CG, Le N, Lopez-Aguiar AG, Pawlik TM, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Krasnick BA, Weber SM, Salem A, Martin RCG, Scoggins CR, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Russell MC, Maithel SK. Pathologic and Prognostic Implications of Incidental versus Nonincidental Gallbladder Cancer: A 10-Institution Study from the United States Extrahepatic Biliary Malignancy Consortium. Am Surg. 2017;83:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Tsirlis T, Ausania F, White SA, French JJ, Jaques BC, Charnley RM, Manas DM. Implications of the index cholecystectomy and timing of referral for radical resection of advanced incidental gallbladder cancer. Ann R Coll Surg Engl. 2015;97:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Ethun CG, Postlewait LM, Le N, Pawlik TM, Buettner S, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Jin LX, Weber SM, Salem A, Martin RC, Scoggins C, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Kooby DA, Maithel SK. Association of Optimal Time Interval to Re-resection for Incidental Gallbladder Cancer With Overall Survival: A Multi-Institution Analysis From the US Extrahepatic Biliary Malignancy Consortium. JAMA Surg. 2017;152:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Lundgren L, Muszynska C, Ros A, Persson G, Gimm O, Andersson B, Sandström P. Management of incidental gallbladder cancer in a national cohort. Br J Surg. 2019;106:1216-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Hickman L, Contreras C. Gallbladder Cancer: Diagnosis, Surgical Management, and Adjuvant Therapies. Surg Clin North Am. 2019;99:337-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 21. | Watson H, Dasari B, Wyatt J, Hidalgo E, Prasad R, Lodge P, Toogood G. Does a second resection provide a survival benefit in patients diagnosed with incidental T1b/T2 gallbladder cancer following cholecystectomy? HPB (Oxford). 2017;19:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Creasy JM, Goldman DA, Gonen M, Dudeja V, Askan G, Basturk O, Balachandran VP, Allen PJ, DeMatteo RP, D'Angelica MI, Jarnagin WR, Peter Kingham T. Predicting Residual Disease in Incidental Gallbladder Cancer: Risk Stratification for Modified Treatment Strategies. J Gastrointest Surg. 2017;21:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Søreide K, Guest RV, Harrison EM, Kendall TJ, Garden OJ, Wigmore SJ. Systematic review of management of incidental gallbladder cancer after cholecystectomy. Br J Surg. 2019;106:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 24. | Agarwal AK, Kalayarasan R, Javed A, Gupta N, Nag HH. The role of staging laparoscopy in primary gall bladder cancer--an analysis of 409 patients: a prospective study to evaluate the role of staging laparoscopy in the management of gallbladder cancer. Ann Surg. 2013;258:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Foster JM, Hoshi H, Gibbs JF, Iyer R, Javle M, Chu Q, Kuvshinoff B. Gallbladder cancer: Defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol. 2007;14:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Kwon YJ, Lee KG. Is T-stage the only significant factor determining the extent of surgery for gallbladder cancer? Hepatogastroenterology. 2014;61:304-306. [PubMed] |
| 27. | Maruyama S, Kawaida H, Hosomura N, Amemiya H, Saito R, Shimizu H, Furuya S, Akaike H, Kawaguchi Y, Sudo M, Inoue S, Kono H, Ichikawa D. Indications for extrahepatic bile duct resection due to perineural invasion in patients with gallbladder cancer. World J Surg Oncol. 2019;17:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Lee H, Choi DW, Park JY, Youn S, Kwon W, Heo JS, Choi SH, Jang KT. Surgical Strategy for T2 Gallbladder Cancer According to Tumor Location. Ann Surg Oncol. 2015;22:2779-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Shirai Y, Wakai T, Sakata J, Hatakeyama K. Regional lymphadenectomy for gallbladder cancer: rational extent, technical details, and patient outcomes. World J Gastroenterol. 2012;18:2775-2783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Chaudhari VA, Ostwal V, Patkar S, Sahu A, Toshniwal A, Ramaswamy A, Shetty NS, Shrikhande SV, Goel M. Outcome of neoadjuvant chemotherapy in "locally advanced/borderline resectable" gallbladder cancer: the need to define indications. HPB (Oxford). 2018;20:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Hakeem AR, Papoulas M, Menon KV. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer - A systematic review. Eur J Surg Oncol. 2019;45:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, DeMatteo RP, Wagman R, Blumgart LH, Fong Y. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 33. | Ma N, Cheng H, Qin B, Zhong R, Wang B. Adjuvant therapy in the treatment of gallbladder cancer: a meta-analysis. BMC Cancer. 2015;15:615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine vs gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3147] [Article Influence: 209.8] [Reference Citation Analysis (1)] |
| 35. | Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, Boudjema K, Fartoux L, Bouhier-Leporrier K, Jouve JL, Faroux R, Guerin-Meyer V, Kurtz JE, Assénat E, Seitz JF, Baumgaertner I, Tougeron D, de la Fouchardière C, Lombard-Bohas C, Boucher E, Stanbury T, Louvet C, Malka D, Phelip JM. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol. 2019;37:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 362] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 36. | Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J; BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 810] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 37. | Ben-David MA, Griffith KA, Abu-Isa E, Lawrence TS, Knol J, Zalupski M, Ben-Josef E. External-beam radiotherapy for localized extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2006;66:772-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, Thomas CR Jr, Alberts SR, Dawson LA, Micetich KC, Thomas MB, Siegel AB, Blanke CD. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol. 2015;33:2617-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 273] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 39. | Fareed MM, DeMora L, Esnaola NF, Denlinger CS, Karachristos A, Ross EE, Hoffman J, Meyer JE. Concurrent chemoradiation for resected gall bladder cancers and cholangiocarcinomas. J Gastrointest Oncol. 2018;9:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Kim Y, Amini N, Wilson A, Margonis GA, Ethun CG, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Krasnick B, Weber SM, Salem A, Martin RC, Scoggins C, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Cardona K, Maithel SK, Pawlik TM. Impact of Chemotherapy and External-Beam Radiation Therapy on Outcomes among Patients with Resected Gallbladder Cancer: A Multi-institutional Analysis. Ann Surg Oncol. 2016;23:2998-3008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Piehler JM, Crichlow RW. Primary carcinoma of the gallbladder. Surg Gynecol Obstet. 1978;147:929-942. [PubMed] |
| 42. | Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, D'Angelica M, Dematteo RP, Blumgart LH, O'Reilly EM. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol. 2008;98:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 313] [Article Influence: 19.6] [Reference Citation Analysis (0)] |