Published online Dec 7, 2021. doi: 10.3748/wjg.v27.i45.7771
Peer-review started: April 27, 2021
First decision: June 13, 2021
Revised: June 25, 2021
Accepted: November 20, 2021
Article in press: November 20, 2021
Published online: December 7, 2021
Processing time: 219 Days and 23.5 Hours
Chronic rejection (CR) of liver allografts causes damage to intrahepatic vessels and bile ducts and may lead to graft failure after liver transplantation. Although its prevalence has declined steadily with the introduction of potent immunosuppressive therapy, CR still represents an important cause of graft injury, which might be irreversible, leading to graft loss requiring re-transplantation. To date, we still do not fully appreciate the mechanisms underlying this process. In addition to T cell-mediated CR, which was initially the only recognized type of CR, recently a new form of liver allograft CR, antibody-mediated CR, has been identified. This has indeed opened an era of thriving research and renewed interest in the field. Liver biopsy is needed for a definitive diagnosis of CR, but current research is aiming to identify new non-invasive tools for predicting patients at risk for CR after liver transplantation. Moreover, the minimization or withdrawal of immunosuppressive therapy might influence the establishment of subclinical CR-related injury, which should not be disregarded. Therapies for CR may only be effective in the “early” phases, and a tailored management of the immunosuppression regimen is essential for preventing irreversible liver damage. Herein, we provide an overview of the current knowledge and research on CR, focusing on early detection, identification of non-invasive biomarkers, immuno
Core Tip: Chronic rejection (CR) still represents a cause of graft loss after liver transplantation. Recent advances in understanding the pathways leading to CR, through a T cell-mediated or antibody mediated injury, are opening new strategies for its management. Early detection of CR, tailored immunosuppressive regimen and strict monitoring are essential to prevent graft loss rejection-related requiring re-trans
- Citation: Angelico R, Sensi B, Manzia TM, Tisone G, Grassi G, Signorello A, Milana M, Lenci I, Baiocchi L. Chronic rejection after liver transplantation: Opening the Pandora’s box. World J Gastroenterol 2021; 27(45): 7771-7783
- URL: https://www.wjgnet.com/1007-9327/full/v27/i45/7771.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i45.7771
Chronic rejection (CR) of a liver allograft is an immunologically mediated insult to the parenchyma, resulting in damage to vessels and bile ducts[1]. The prevalence of CR after liver transplantation (LT) has been declining over the last years, mainly due to the introduction of new potent immunosuppressive (IS) regimens. However, when CR occurs, it may be successfully treated only in the early phases, while irreversible injury may lead to graft loss requiring re-transplantation[1]. Despite CR being a post-transplantation complication that has been recognized for a long time, its path
CR is the consequence of the activation of the host’s immune system against the liver allograft and can occur through two general mechanisms: (1) T cell-mediated and (2) Antibody-mediated pathways[1,2]. T cell-mediated chronic rejection (TCMCR) is a long-known complication of LT. Antibody-mediated chronic rejection (AMCR) instead has been observed initially for heart, kidney and pancreas transplants. Yet, it was thought to spare liver allografts, because the liver is a relatively immune-privileged organ and is commonly not targeted by antibodies. Today, it is known that AMCR affects liver allografts as well, albeit rarely, and with not-so-clear clinical conse
IS drugs play a crucial role in influencing the onset of CR. After LT, the “adequate” IS is considered as this which maintains a viable graft and healthy patients, balancing the risk of rejection (maintaining the target drugs’ blood trough levels) and the risk of IS-associated side effects. Yet, different strategies of IS regimens are currently adopted depending of a large number of variables (such as recipient’s renal function, cardiovascular disease, risk of infections and tumours, recurrence of underling liver disease). Nevertheless, there is not a unique definition for the optimal IS regimen since it should be tailored to each recipient.
In this narrative review, we highlight the current knowledge and hint to future perspectives on CR in LT, which today represents a growing field for the long-term outcomes of LT recipients.
The liver allograft is uniquely immunologically privileged organ and can be transplanted successfully despite positive crossmatch, irrespective of HLA matching. LT requires less amount of induction therapy and maintenance IS compared to other organs, and features of CR rates are low even when IS regimens are minimized or absent.
The mechanisms underlying LT tolerance have not been fully elucidated yet, but appear to be related to particular characteristics of the liver micro-environment. Firstly, the liver contains both innate immune cells expressing undetectable levels of major histocompatibility complex antigens and a large population of migratory immune cells, which have immunoregulatory activity inducing its privileged status[1,2]. Secondly, the liver has evolved to clear high concentrations of bacterial antigens gut-derived coming from the portal circulation, without inducing useless and potentially harmful immune activation. In particular, with high intrahepatic antigen load, T-cells activated in the liver can induce to a dysfunctional response or to a regulatory phenotype. This may be due to high concentrations of interleukin 10 produced by Kupffer cells or due to antigen presentation by sinusoidal endothelial cells or stellate cells, which have poor costimulatory capacity. Therefore, the in
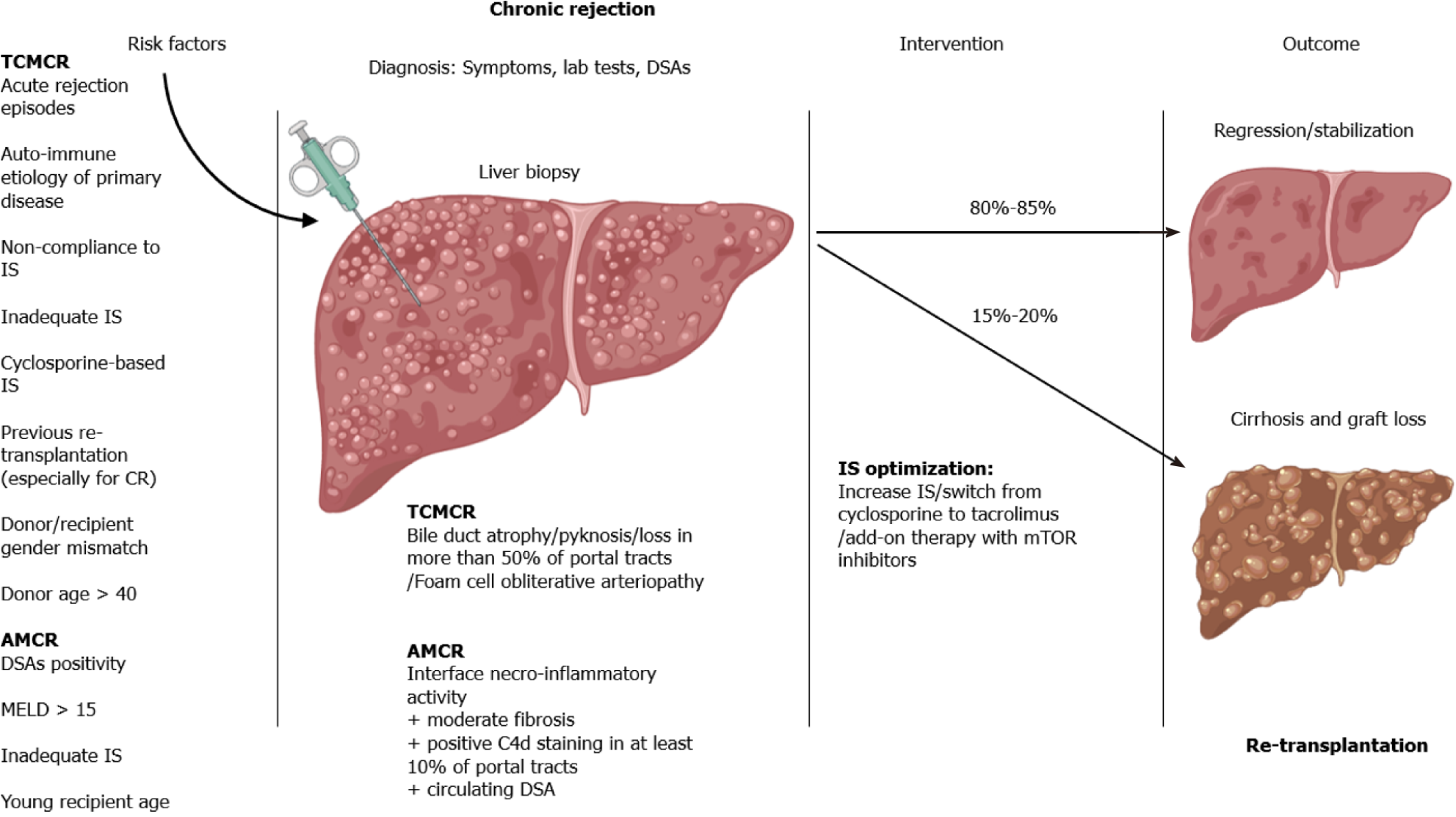
The diagnosis of CR is made on the basis of clinical, laboratory, histologic and radiological criteria (Table 1); among these, the histological recognition of CR is a fundamental step in diagnosis.
| T cell-mediated chronic rejection | Antibody-mediated chronic rejection | |
| Histological definition (according to the 2016 Banff Group[1]) | Presence of bile duct atrophy/pyknosis affecting the majority of bile ducts; OR Bile duct loss in more than 50% of the portal tracts; OR Foam cell obliterative arteriopathy | At least mild mononuclear portal and/or perivenular inflammation with interface and/or perivenular necroinflammatory activity; AND At least moderate portal/periportal, sinusoidal or perivenular fibrosis; AND Positive C4d staining in at least 10% of the portal tracts; AND Circulating DSAs in serum samples collected within 3 months of biopsy; AND Other causes have reasonably been excluded |
| Incidence | 2%-5% | Unknown |
| Risk factors | (1) History of T cell-mediated acute rejection episodes; (2) Autoimmune aetiology of the primary liver disease; (3) Non-compliance with IS therapy; (4) Cyclosporine-based IS regimens as opposed to tacrolimus-based regimens; (5) Previous re-transplantation for rejection; (6) Donor/recipient gender mismatch; and (7) Donor age greater than 40 | (1) Donor-specific antibodies (especially de novo anti-HLA class II antigens); (2) Inadequate IS (cyclosporine regimens or low CNI concentrations); (3) MELD score > 15; (4) Young age at transplantation; and (5) Re-transplantation |
| Clinical implications | 15%-20% graft loss | Increased fibrosis and graft failure in an unknown percentage of patients |
The most widely accepted histologic criteria for the diagnosis of CR are those proposed by the Banff Working Group, an international expert panel, which are periodically refined and updated[1,2].
The criteria for TCMCR include the presence of three features: Bile duct atrophy/ pyknosis affecting the majority of bile ducts, bile duct loss in more than 50% of portal tracts and foam cell obliterative arteriopathy[2]. The latter feature is considered pathognomonic. Yet unfortunately, it is rarely found in needle biopsy specimens, while it has traditionally been observed in lost allografts at re-transplantation or autopsy. Therefore, the diagnosis relies mainly on the detection of bile duct atrophy and bile duct loss. Both of these features, instead, are rather unspecific, often making CR a diagnosis of exclusion, which requires a thorough exclusion of other causes, including arterial stenosis or biliary strictures, drug-mediated injury and cytomegalovirus infection. An important point in the differential diagnosis is the general absence of “ductular reactions” in TCMCR specimens, in contrast to what is common in other biliary diseases. Small arterial branches may also be missing in TCMCR, making the identification of portal tracts difficult, as well as a distinction between bile ducts and ductular reactions. Staining for cytokeratin may be helpful, as well as epithelial membrane antigen, which preferentially stains bile ducts, as opposed to ductules[3].
Pathologists have also developed TCMCR grading criteria, which are particularly useful, as they correlate with the reversibility of the condition and with prognosis[2]. TCMCR is distinguished into early and late stages according to the Banff schema[2], as summarized in Table 2. Histologically, the most important characteristic in the differentiation between early and late CR is the loss of bile ducts, which occurs in less than 50% of portal tracts (with associated degenerative changes in other ducts), early rejection and greater than 50% in late rejection. Other diagnostic criteria are bridging perivenular fibrosis and small arterial loss, which have all been correlated with a high rate of graft failure. The staging distinction of TCMCR is clinically important; early CR is potentially reversible, whereas late-stage CR is generally irreversible.
| Structure | Early CR | Late CR |
| Small bile ducts (< 60 μm) | (1) Degenerative changes involving the majority of ducts: Eosinophilic transformation of the cytoplasm; Increased nucleus: Cytoplasm ratio; nuclear hyperchromasia; uneven nuclear spacing; ducts only partially lined by biliary epithelial cells; and (2) Bile duct loss in < 50% of the portal tracts | (1) Degenerative changes in remaining bile ducts; and (2) Loss in > 50% of the portal tracts |
| Terminal hepatic venules and zone 3 hepatocytes | (1) Intimal/luminal inflammation; (2) Lytic zone 3 necrosis and inflammation; and (3) Mild perivenular fibrosis | (1) Focal obliteration; (2) Variable inflammation; and (3) Severe (bridging) fibrosis |
| Portal tract hepatic arterioles | Occasional loss involving < 25% of the portal tracts | Loss involving > 25% of the portal tracts |
| Other | So-called "transition" hepatitis with spotty necrosis of hepatocytes | Sinusoidal foam cell accumulation and marked cholestasis |
| Large perihilar hepatic artery branches | Intimal inflammation and focal foam cell deposition without luminal compromise | (1) Luminal narrowing by subintimal foam cells; and (2) Fibrointimal proliferation |
| Large perihilar bile ducts | Inflammation damage and focal foam cell deposition | Mural fibrosis |
The existence of AMCR has been elusive and a strict definition has only been attained in recent years (Table 1)[4]. This has been mainly explored through studies on suboptimally immunosuppressed patients, in those undergoing IS weaning and in the paediatric population, as most primary paediatric diseases requiring LT are not immune-mediated and do not recur[5]. The development of AMCR seems to be multifactorial. It has been proposed that a first independent liver insult (i.e., acute rejection and low immunosuppression) may be necessary to precipitate AMCR by inducing up-regulation of class II HLA molecules and consequently rendering the liver vulnerable to donor-specific antibody (DSA)-mediated damage.
The difficulty in identifying this condition is due to the lack of specific histologic findings. According to the 2016 Banff conference report, AMCR is histologically defined as inflammation with an interface of necroinflammatory activity, moderate fibrosis and evidence of C4d-positive staining in at least 10% of the portal tracts in a patient with circulating human leukocyte antigen (HLA) DSAs in serum samples collected within 3 mo of biopsy and when other causes have reasonably been excluded[1].
Finally, a combination of the two types of rejection (AMCR and TCMCR) is also possible. Suspicion of combined AMCR should be raised when late TCMCR presents with severe fibrosis and/or is resistant to therapy. In these cases, C4d staining and DSA dosing should be performed.
For the time being, a definitive diagnosis of CR can only be made with histologic confirmation and therefore requires a liver biopsy, which is a fairly invasive pro
Nowadays, CR is relatively uncommon in liver allografts, and its incidence has declined vertiginously in the last decades due to improved IS, especially after the introduction of tacrolimus (TAC)-based IS regimens. Incidence rates of 15%-20% documented in the first LT series have now been minimized to 2%-5% in the adult population after a median of 5 years, while it has been recorded in up to 16% of the paediatric population[6,7]. These figures primarily refer to TCMCR, as the incidence of AMCR today remains unknown. In fact, AMCR develops in a fraction of patients with persistent or de novo DSAs, which themselves account for around 15% of recipients.
After LT, the incidence of CR is significantly lower compared to other solid organ transplants, such as heart (25%-60%), combined kidney and pancreas (20%-40%), pancreas alone (30%-70%) and lung (28%-45%) transplantation. Notably, in contrast to different organ transplants, the risk of CR is highest during the first year and seems to decrease thereafter[6].
Risk factors for the development of TCMCR have been extensively studied. The most important and consistently reported risk factor is the number and severity of preceding T cell-mediated acute rejection episodes (Figure 1)[1,2]. Other frequently cited risk factors include autoimmune aetiology of the primary liver disease, non-compliance with IS therapy, cyclosporine-based IS therapy (as opposed to TAC-based regimens), previous re-transplantation for rejection, donor/recipient gender mismatch and donor age greater than 40 years[6,7]. The risk factors themselves seem to be influenced by specific IS regimens, with patients on TAC exhibiting loss of association with most risk factors, with the exception of acute rejection episodes[6]. A newly identified risk factor is donor/recipient mismatch in the minor histocompatibility antigen glutathione S-transferase T1.
DSAs are considered a risk factor for the development of AMCR, as they are a necessary, although insufficient, requirement for the diagnosis of AMCR[8]. Other risk factors for AMCR include possibly inadequate IS therapy [cyclosporine regimens or low concentrations of calcineurin inhibitors (CNIs)], recipient’s Mayo End-Stage Liver Disease (MELD) score > 15, young age at transplantation and re-transplantation.
TCMCR can develop at any time after LT and no specific time parameter exists, although most cases occur more than 90 d after LT. It can manifest as a spectrum of disease severity, ranging from mild and inconsequential alterations in blood tests to liver failure and death. The most typical presentation is that of a cholestatic-pattern in liver function tests (LFTs), with the preferential elevation of gamma glutamyl-transpeptidase and alkaline phosphatase, in a patient with previous episodes of acute T cell-mediated rejection. At other times, this scenario may develop as a consequence of untreated or therapy-refractory acute rejection. Rarely, it may evolve insidiously with worsening of LFTs in patients without previous acute rejection.
Early TCMCR can also be found in protocol biopsies without clinical or laboratory alterations, which is a rare event in adults, but found in up to 20% of cases of paediatric LT[9,10]. The clinical implication of this last situation is uncertain. Symp
Progression or regression of TCMCR can be predicted based on the histological grading and timing of occurrence[2,9]. Early TCMCR can be reversible with prompt adoption of an adequate therapy. On the other hand, late TCMCR is associated with a much higher chance of evolution into liver failure. Deterioration may be rapid and allograft failure may typically occur within the first year[6]. TCMCR occurring more than 1 year after LT is usually seen in inadequately immunosuppressed patients, either as a result of non-compliance, intolerable side effects or failed IS weaning attempts. Overall, around 15%-20% of patients with TCMCR will ultimately face graft loss and require re-transplantation.
The clinical implication of AMCR is particularly controversial. Its existence has been debated for a long time, yet in the last years, there is mounting evidence that AMCR can portend poor graft outcomes after LT.
DSAs can either be existent before LT (preformed), and may disappear or persist, or they can be generated de novo after LT. Generally, preformed DSAs are more closely correlated to acute rejection, while de novo DSAs are more tied to AMCR[8,11]. De novo DSAs may appear in 0.4%-8% of patients 1 year after LT. O’Leary et al[4] showed that the prevalence of de novo DSAs was 62% in patients with CR and 38% in patients without CR (P = 0.047). In the same study, the difference was even more significant within the first year after LT, where DSAs were found in 44% and 13% of patients with and without CR, respectively (P = 0.004)[4]. The positivity of DSAs have been associated with higher mortality after LT. On the other hand, recently Feng et al[5] published the long-term results of IS withdrawal in 12 paediatric patients and reported the absence of fibrosis or inflammation in protocol biopsies, despite the presence of DSAs in nine recipients.
In this scenario, nowadays, a major goal in the field of transplant immunology is to understand the role of serum DSAs in LT recipients with normal LFTs. Recently, Höfer et al[12] correlated DSA testing with liver allograft histological findings in patients with normal graft function, aiming to screen for subclinical rejection/fibrosis in a prospective biopsy-based program. Their results indicated that DSA positivity was associated with greater graft inflammation [odds ratio (OR): 5.4] and fibrosis (OR: 4.2) and to histological criteria that excluded the possibility of the minimization of im
These data indicate that serum DSA monitoring in post-liver transplant follow-up may be highly helpful, especially in patients in whom IS minimization is attempted, as in those with long-term graft dysfunction.
In 2017, the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group, an expert panel of transplant clinicians who provide practical recommendations for the identification and management of modifiable risk factors to maximize graft and patient survival after transplantation, recommended testing for serum DSAs, when available, in all patients with a well-functioning graft at 1, 5 and 10 years after transplantation. According to the COMMIT Group, DSA positivity should warrant protocol graft biopsies or, at least, the use of non-invasive testing for fibrosis[14]. However, these recommendations are based on a low level of evidence from the currently available literature. Moreover, despite there being growing evidence for the possible benefit of measuring serum DSAs in LT, routine screening for DSAs is not yet available in all transplant centres.
All these data suggest that DSA testing should be implemented both in adult and paediatric LT recipients during long-term follow-up to better understand their role in the post-transplant liver injury process.
Judicious management of immunosuppression is crucial for the long-term outcomes and quality of life of LT recipients. The perfect management of IS regimens rests on a thin line, with rejection-mediated graft damage on one side and drug-related side effects on the other. The adverse effects of immunosuppression should never be underestimated and may include the development of kidney injury, metabolic syndrome, neurological complications, malignancies, cytomegalovirus and other opportunistic infections. Furthermore, the aetiology of primary liver dysfunction should always be kept in mind as immunosuppression may have impact on its recurrence. Recurrence of hepatocellular carcinoma (HCC) or unresolved HCV in
Generally, after solid organ transplantation, the IS regimen is manipulated mainly for drug-related toxicity or for oncologic prevention (de novo or recurrent tumours) and different strategies can be adopted, such as: (1) Switch to another IS regimen; (2) IS minimization; or (3) IS withdrawal. However, all these actions may affect the development of CR, a possibility which should not be disregarded. In the following paragraphs, we will analyse how IS management might influence the establishment of CR-related injury.
TAC-based IS therapy is currently the most commonly used regimen after LT, as there is evidence that TAC decreases the chance of graft failure. Moreover, there is consistent proof in the literature that TAC-based IS regimens also decrease the risk of CR[6].
To prevent graft rejection, initial TAC trough levels should be kept between 6 and 10 ng/mL. A large meta-analysis has demonstrated that levels < 10 ng/mL guarantee no increase in rejection episodes, while halving the incidence of nephrotoxicity, compared to levels > 10 ng/mL. The first month after transplantation, the TAC dosage may be decreased with a target of 4-8 ng/mL. Thus, while initial TAC target levels are largely endorsed in the literature, there is no consensus on the optimal level in the long term. The COMMIT Group states that “lower” TAC levels may be acceptable in combination therapy, when TAC-related toxicity is an issue[14]. Also, the International Liver Transplant Society (ILTS) suggests that, from year 1 after LT onward, TAC trough levels can be dropped to 3 ng/mL; furthermore, after the fifth year, keeping drug levels “above detectable” is fine as long as graft function is optimal[15].
TAC is often initially used with other IS drugs, the most common of which are corticosteroids and mycophenolate mofetil (MMF). During the first 90 d after LT, steroids should be gradually tapered, leading to suspension after the third month. When aiming for TAC monotherapy, higher trough levels should be maintained, while dual therapy permits lower levels to be acceptable. Indeed, in a retrospective study aiming to assess CR in LT recipients receiving low-dose CNI monotherapy, TAC levels < 5 ng/mL were not associated with silent CR on protocol liver biopsies[16].
When TAC needs to be reduced or suspended due to adverse events, the guidelines recommend adjunct or substitution with MMF or other IS agents, such as mammalian target of rapamycin (mTOR) inhibitors (everolimus and sirolimus), to keep a low threshold for suspicion of rejection[15].
Nephrotoxicity is the major side effect of TAC requiring dose adjustments of the drug. When nephrotoxicity is anticipated to be an issue before transplantation, induction with basiliximab plus MMF and delayed TAC introduction may be used to decrease renal impairment in the first weeks, as demonstrated by the DIAMOND study[17]. In this multicentre prospective randomized trial, delayed initiation of high-dose TAC significantly reduced renal function impairment compared with immediate post-transplant administration, while the risk of rejection was similar[17].
For later appearing nephrotoxicity, renal-sparing regimens are often used, with most being common alternative agents to CNIs. Several studies have shown that switching from TAC to mTOR inhibitor-based regimens, such as everolimus monotherapy, significantly improved renal function after LT. Everolimus significantly increases the estimated glomerular filtration rate (amounting to 5.6 mL/min), with a low incidence of histologically proven rejection (5%) and side effects[18]. In contrast, for sirolimus, a randomized trial comparing ab initio standard TAC with low-dose TAC plus sirolimus found an excess of graft loss mortality in the combination therapy arm, which was due to hepatic artery/portal vein thrombosis or sepsis.
TAC-based IS regimens are usually also later replaced with mTOR inhibitor regimens in patients transplanted for HCC. In fact, TAC trough levels above 10 ng/mL during the first 30 d post-LT seem to increase the rate of HCC recurrence; hence, mTOR inhibitors, which have an anti-proliferative effect, may offer protection from tumour recurrence, maintaining the same risk of post-transplant graft rejection.
As rejection is considered a relatively uncommon cause of graft failure after LT, current guidelines suggest to employ IS minimization strategies in an effort to limit the morbidity associated with excessive immunosuppression, which represents the main cause of late mortality after LT[14]. In fact, IS minimization has been recognized to be feasible and is nowadays incorporated in existing recommendations[14,19]. However, IS minimization protocols need to be rigorous in order to avoid the risk of favouring acute and chronic rejection[19].
First, IS minimization strategies should be entertained after the third post-transplantation month, in patients with stable laboratory exams for a minimum of 4 wk and under tight control. Liver allograft biopsy is not required prior to IS mi
However, in LT recipients under an IS minimization regimen, insufficient TAC serum concentrations have been linked with AMCR. In a retrospective cohort of 749 adult LT recipients, Kaneku et al[21] reported that de novo serum DSA formation (incidence rate: 8%) was favoured by low CNI levels (TAC < 3 ng/mL or cyclosporine < 75 ng/mL) and by the use of cyclosporine in place of TAC, while it was inversely related to age > 60 years old and a MELD score > 15[21]. Patients with de novo DSAs were found at significantly higher risk for graft loss and death. Hence, serum DSA monitoring in patients who are candidates for IS minimization may be useful. Accordingly, the ILTS guidelines encourage clinicians to screen for serum DSAs before minimization attempts and to consider biopsy if strong DSA positivity is detected[15].
Additionally, an association between variability in TAC levels and late CR has been implied, especially in paediatric LT recipients. The largest of these studies included a heterogeneous population (n = 144) of children (age: 8-18 years) transplanted with different solid organ grafts (liver, kidney, heart or lung), where high variability in TAC blood levels increased the risk of late CR and graft loss, hampering the generalization of results to adult liver grafts[22]. Therefore, until new evidence emerges, excess variability in TAC levels should probably be best avoided.
Nonetheless, many studies have gone further and investigated the possibility of IS withdrawal, a unique chance offered to LT recipients[23]. The aim of IS withdrawal is to avoid long-term IS-related side effects, which influence patient/graft survival, as well as the quality of life of LT recipients. Overall, among IS withdrawal trials, CR had a very low incidence, accounting for 0%-3%[24].
Initial pioneering studies demonstrated the feasibility and safety of IS discontinuation after LT, but they lacked sample size assessments, clear withdrawal protocols, histopathologic surveillance and long-term follow up[25]. In these studies, acute rejection episodes could generally be managed successfully, and only three cases of CR were reported, two of which culminated in graft loss and re-transplantation.
A prospective trial in 102 patients found that 42 (41.17%) LT recipients could be weaned off completely and did not suffer rejection, as assessed by protocol liver biopsies[22]; moreover, the time elapsed from transplantation was the key variable influencing the success of IS weaning, with high chances 10 years from trans
Recently, Jucaud et al[26] described the prevalence and impact of de novo DSAs during a multicentre IS withdrawal trial in adult LT recipients. Patients who were IS-free had a high (66.7%) prevalence of de novo DSAs, in particular for HLA-I. Yet, these de novo DSAs did not seem to be deleterious to the graft. However, their significance in the long term is still unknown, as well as their possible association with CR in patients who achieved a successful and sustained suspension of IS therapy.
Nowadays, around 20% of LT recipients can ultimately be weaned off IS therapy completely[24]. Yet, IS withdrawal is not a common practice and should only be attempted in the controlled setting of a clinical trial. There is accumulating evidence that under-immunosuppression may lead to de novo DSA formation, and in turn, DSA formation may lead to AMCR or mixed CR and eventually to graft loss[10,24]. Therefore, further IS weaning studies should include protocol biopsies and DSA testing[24].
The treatment of LT recipients who have developed CR is difficult and not entirely defined, as in general, there is still a low level of evidence. Thus, most recommendations are only based on expert opinions[14,15].
Generally speaking, patients with TCMCR may benefit from increased IS blood levels. Nonetheless, the increase in dosage should be gradual. High variability in TAC concentrations should be avoided, especially when graft function is impaired, as this may increase mortality through over-IS-related events[14]. Therefore, TAC dosage modifications should be carried out progressively and with special caution in patients with liver dysfunction[14].
Despite there being limited evidence to support a specific IS regimen for CR, patients taking a cyclosporine-based IS regimen should be switched to a TAC-based one[6]. The conversion from cyclosporine to TAC is associated to a 70% response rate and graft survival; moreover, an “early” conversion to TAC, before bilirubin levels exceed 10 mg/dL, seems to be fundamental to achieve good outcomes[6].
In a recent report, 23 patients with biopsy-proven CR were treated with a “rescue therapy” consisting of addition of an mTOR inhibitor (either everolimus or sirolimus) to the baseline IS regimen[27]. In 12 patients (52%), this strategy was successful and reversed CR, while the other patients had a poor outcome and most of them required re-transplantation. Although evidence is scarce and further data are needed, these preliminary data suggest that mTOR inhibitor add-on therapy may be valuable in treating TCMCR, and its potential benefits should be further explored in this setting.
While acute antibody-mediated rejection has different potential treatment strategies, most of which are transposed from the kidney transplantation experience, none of these is indicated for AMCR. So far, the treatment of AMCR remains an unmet clinical need, with no study to date on the subject, to the best of our knowledge.
In case of a strong positivity for serum DSAs is detected (MFI > 5000), irrespective of the class of anti-HLA antibodies, and the histological pattern is consistent with AMCR, the COMMIT Group suggested to reinforce the baseline IS regimen by increasing the CNI trough level (if tolerated) or by the introduction of MMF or other agents in patients receiving CNI monotherapy[14]. Moreover, if the histological pattern is suggestive of de novo autoimmune hepatitis with positive DSAs, the addition of corticosteroids is recommended. In all cases, strict follow-up and evaluation of therapeutic changes need to be based on repeated liver biopsies and DSA/MFI monitoring. Yet, with the new recent international definition of AMCR and the evidence that its incidence is expanding, a new field of study has been opened, and it is likely that the treatment of AMCR will be the object of many investigations in the near future.
In summary, despite the lack of evidence based on randomized trials, so far literature data suggest that when CR is diagnosed early (before the development of significant ductopenia, perivenular fibrosis and obliterative arteriopathy), CR is potentially reversible by increasing or changing the IS regimen.
Improved histology and resolution of liver function abnormalities can be seen in successfully treated patients. In contrast, late CR is usually unresponsive to increased IS therapy and generally requires re-transplantation.
When CR is severe enough to compromise graft function, the chances of regression are quite low and most patients will ultimately face graft loss. In such cases of advanced CR, the only chance for survival is therefore re-transplantation.
In the early series, CR represented the most frequent indication for re-trans
On the other hand, re-transplantation is a technically challenging procedure with significantly worse outcomes than primary LT. The technical difficulty mainly lies in a more complex dissection, with a large percentage of patients necessitating vascular and biliary reconstructions[30-32]. In analyses of the UNOS database, the 1-year risk of graft failure was 37.8% and patient survival at 1, 3 and 5 years was 67%, 60% and 53%, respectively, which is far below the corresponding rates for primary LT[30]. In fact, to avoid futility of liver re-transplantation, many studies have proposed stringent predictive scores be used to select appropriate candidates[33].
Among patients candidate for liver re-transplantation, most deaths are due to sepsis and are directly related to the MELD score, time from the primary LT (liver re-transplantation within 1 year from the first LT has the highest mortality), and warm ischaemic time. Other studies have found a correlation with donor age above 60, recipient age above 50, bilirubin levels and intraoperative transfusions[34]. Moreover, MELD scores higher than 30 are associated with prohibitive mortality risks (42% at 1 year and 21% at 5 years)[33-34]. However, no increased postoperative mortality has been reported in liver re-transplantation for CR, as opposed to other indications[33]. IS in re-transplantation for CR remains an issue as to date there is no data to guide choice of therapeutic regimens. Induction therapy with antibodies such as basiliximab is reasonable yet controversial. In particular, some studies suggest lower rates of acute rejection episodes while others do not confirm such results[35-37].
The future of CR prevention, diagnosis and management holds promise. The recent recognition of AMCR in liver allografts has opened a whole new area of research. One of the main objectives would be to recognize patients at high risk for rejection. Many studies are currently aiming to identify useful biomarkers for rejection. Yet, translation of the results into clinical practice is still lagging behind. Despite new promising biomarkers, such as intracellular IFN-γ and IL-2, being proposed to select patients at higher risk for acute rejection, no biomarker has yet been recognized to identify CR[38].
Another area of interest is the definition of non-invasive methodologies for the diagnosis of CR. Lutz et al[39] have shown how liver ultrasound, with calculation of the right hepatic vein resistance index, and transient elastography have a high correlation with graft fibrosis. Unfortunately, although fibrosis is indeed a component of CR, it is not specific; however, detection of significant fibrosis may represent a valuable tool to select asymptomatic patients to be biopsied.
Another potential benefit of identifying non-invasive biomarkers for CR would be to drive a tailored IS regimen according to the individual patients risk to develop CR[38]. For example, they could predict a response or adverse effects of diverse drugs in the individual patient. Nowadays, as a similar experience is growing in kidney transplant recipients in this field, we foresee that it will also occur for LT recipients.
Finally, rejection prevention would be the ultimate goal. Schlegel et al[40] have demonstrated in a mouse model that graft preconditioning with hypothermic oxygenated perfusion can induce immune tolerance and prevent graft failure, even in the absence of IS therapy. Their results suggest that one day operational tolerance could be “induced” rather than depend strictly on the so far unpredictable graft-host interaction, reducing or abolishing the risk of CR.
CR of liver allografts has become a relatively rare entity due to stronger IS regimens, but it still represents a diagnostic and management challenge to the transplant clinician. In the last decade, the definition of antibody-mediated rejection has opened the way for an entire new research field in the study of CR, effectively unleashing a Pandora’s box. The next few years hold promise to improve CR management by the identification of non-invasive biomarkers predicting patients at risk for CR, which could hopefully be prevented by the use of tailored IS regimens, thus no longer becoming an issue after LT.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gencdal G, Tavabie OD, Xu X S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Demetris AJ, Bellamy C, Hübscher SG, O'Leary J, Randhawa PS, Feng S, Neil D, Colvin RB, McCaughan G, Fung JJ, Del Bello A, Reinholt FP, Haga H, Adeyi O, Czaja AJ, Schiano T, Fiel MI, Smith ML, Sebagh M, Tanigawa RY, Yilmaz F, Alexander G, Baiocchi L, Balasubramanian M, Batal I, Bhan AK, Bucuvalas J, Cerski CTS, Charlotte F, de Vera ME, ElMonayeri M, Fontes P, Furth EE, Gouw ASH, Hafezi-Bakhtiari S, Hart J, Honsova E, Ismail W, Itoh T, Jhala NC, Khettry U, Klintmalm GB, Knechtle S, Koshiba T, Kozlowski T, Lassman CR, Lerut J, Levitsky J, Licini L, Liotta R, Mazariegos G, Minervini MI, Misdraji J, Mohanakumar T, Mölne J, Nasser I, Neuberger J, O'Neil M, Pappo O, Petrovic L, Ruiz P, Sağol Ö, Sanchez Fueyo A, Sasatomi E, Shaked A, Shiller M, Shimizu T, Sis B, Sonzogni A, Stevenson HL, Thung SN, Tisone G, Tsamandas AC, Wernerson A, Wu T, Zeevi A, Zen Y. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am J Transplant. 2016;16:2816-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 428] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 2. | Demetris A, Adams D, Bellamy C, Blakolmer K, Clouston A, Dhillon AP, Fung J, Gouw A, Gustafsson B, Haga H, Harrison D, Hart J, Hubscher S, Jaffe R, Khettry U, Lassman C, Lewin K, Martinez O, Nakazawa Y, Neil D, Pappo O, Parizhskaya M, Randhawa P, Rasoul-Rockenschaub S, Reinholt F, Reynes M, Robert M, Tsamandas A, Wanless I, Wiesner R, Wernerson A, Wrba F, Wyatt J, Yamabe H. Update of the International Banff Schema for Liver Allograft Rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. Hepatology. 2000;31:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 360] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 3. | Herman HK, Abramowsky CR, Caltharp S, Metry D, Cundiff CA, Romero R, Gillespie SE, Shehata BM. Identification of Bile Duct Paucity in Alagille Syndrome: Using CK7 and EMA Immunohistochemistry as a Reliable Panel for Accurate Diagnosis. Pediatr Dev Pathol. 2016;19:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | O'Leary JG, Cai J, Freeman R, Banuelos N, Hart B, Johnson M, Jennings LW, Kaneku H, Terasaki PI, Klintmalm GB, Demetris AJ. Proposed Diagnostic Criteria for Chronic Antibody-Mediated Rejection in Liver Allografts. Am J Transplant. 2016;16:603-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Feng S, Demetris AJ, Spain KM, Kanaparthi S, Burrell BE, Ekong UD, Alonso EM, Rosenthal P, Turka LA, Ikle D, Tchao NK. Five-year histological and serological follow-up of operationally tolerant pediatric liver transplant recipients enrolled in WISP-R. Hepatology. 2017;65:647-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Blakolmer K, Jain A, Ruppert K, Gray E, Duquesnoy R, Murase N, Starzl TE, Fung JJ, Demetris AJ. Chronic liver allograft rejection in a population treated primarily with tacrolimus as baseline immunosuppression: long-term follow-up and evaluation of features for histopathological staging. Transplantation. 2000;69:2330-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Tannuri AC, Lima F, Mello ES, Tanigawa RY, Tannuri U. Prognostic factors for the evolution and reversibility of chronic rejection in pediatric liver transplantation. Clinics (Sao Paulo). 2016;71:216-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Kovandova B, Slavcev A, Honsova E, Erhartova D, Skibova J, Viklicky O, Trunecka P. De novo HLA Class II antibodies are associated with the development of chronic but not acute antibody-mediated rejection after liver transplantation - a retrospective study. Transpl Int. 2020;33:1799-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Blakolmer K, Seaberg EC, Batts K, Ferrell L, Markin R, Wiesner R, Detre K, Demetris A. Analysis of the reversibility of chronic liver allograft rejection implications for a staging schema. Am J Surg Pathol. 1999;23:1328-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Feng S, Bucuvalas JC, Demetris AJ, Burrell BE, Spain KM, Kanaparthi S, Magee JC, Ikle D, Lesniak A, Lozano JJ, Alonso EM, Bray RA, Bridges NE, Doo E, Gebel HM, Gupta NA, Himes RW, Jackson AM, Lobritto SJ, Mazariegos GV, Ng VL, Rand EB, Sherker AH, Sundaram S, Turmelle YP, Sanchez-Fueyo A. Evidence of Chronic Allograft Injury in Liver Biopsies From Long-term Pediatric Recipients of Liver Transplants. Gastroenterology. 2018;155:1838-1851.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 11. | Legaz I, Boix F, López M, Alfaro R, Galián JA, Llorente S, Campillo JA, Botella C, Ramírez P, Sánchez-Bueno F, Pons JA, Moya-Quiles MR, Minguela A, Muro M. Influence of Preformed Antibodies in Liver Transplantation. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Höfer A, Jonigk D, Hartleben B, Verboom M, Hallensleben M, Manns MP, Jaeckel E, Taubert R. Non-invasive screening for subclinical liver graft injury in adults via donor-specific anti-HLA antibodies. Sci Rep. 2020;10:14242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | O'Leary JG, Kaneku H, Jennings L, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific alloantibodies are associated with fibrosis progression after liver transplantation in hepatitis C virus-infected patients. Liver Transpl. 2014;20:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Neuberger JM, Bechstein WO, Kuypers DR, Burra P, Citterio F, De Geest S, Duvoux C, Jardine AG, Kamar N, Krämer BK, Metselaar HJ, Nevens F, Pirenne J, Rodríguez-Perálvarez ML, Samuel D, Schneeberger S, Serón D, Trunečka P, Tisone G, van Gelder T. Practical Recommendations for Long-term Management of Modifiable Risks in Kidney and Liver Transplant Recipients: A Guidance Report and Clinical Checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group. Transplantation. 2017;101:S1-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 15. | Charlton M, Levitsky J, Aqel B, OʼGrady J, Hemibach J, Rinella M, Fung J, Ghabril M, Thomason R, Burra P, Little EC, Berenguer M, Shaked A, Trotter J, Roberts J, Rodriguez-Davalos M, Rela M, Pomfret E, Heyrend C, Gallegos-Orozco J, Saliba F. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation. 2018;102:727-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 16. | Barbier L, Garcia S, Cros J, Borentain P, Botta-Fridlund D, Paradis V, Le Treut YP, Hardwigsen J. Assessment of chronic rejection in liver graft recipients receiving immunosuppression with low-dose calcineurin inhibitors. J Hepatol. 2013;59:1223-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | TruneČka P, Klempnauer J, Bechstein WO, Pirenne J, Friman S, Zhao A, Isoniemi H, Rostaing L, Settmacher U, Mönch C, Brown M, Undre N, Tisone G; DIAMOND† study group. Renal Function in De Novo Liver Transplant Recipients Receiving Different Prolonged-Release Tacrolimus Regimens-The DIAMOND Study. Am J Transplant. 2015;15:1843-1854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Hüsing A, Schmidt M, Beckebaum S, Cicinnati VR, Koch R, Thölking G, Stella J, Heinzow H, Schmidt HH, Kabar I. Long-Term Renal Function in Liver Transplant Recipients After Conversion From Calcineurin Inhibitors to mTOR Inhibitors. Ann Transplant. 2015;20:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Di Maira T, Little EC, Berenguer M. Immunosuppression in liver transplant. Best Pract Res Clin Gastroenterol. 2020;46-47:101681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Rodríguez-Perálvarez M, García-Caparrós C, Tsochatzis E, Germani G, Hogan B, Poyato-González A, O'Beirne J, Senzolo M, Guerrero-Misas M, Montero-Álvarez JL, Patch D, Barrera P, Briceño J, Dhillon AP, Burra P, Burroughs AK, De la Mata M. Lack of agreement for defining 'clinical suspicion of rejection' in liver transplantation: a model to select candidates for liver biopsy. Transpl Int. 2015;28:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Kaneku H, O'Leary JG, Banuelos N, Jennings LW, Susskind BM, Klintmalm GB, Terasaki PI. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant. 2013;13:1541-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand AI, Hebert D, Ng VL, Solomon M, McCrindle BW, Grant D. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant. 2010;14:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Benítez C, Londoño MC, Miquel R, Manzia TM, Abraldes JG, Lozano JJ, Martínez-Llordella M, López M, Angelico R, Bohne F, Sese P, Daoud F, Larcier P, Roelen DL, Claas F, Whitehouse G, Lerut J, Pirenne J, Rimola A, Tisone G, Sánchez-Fueyo A. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58:1824-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 24. | Clavien PA, Muller X, de Oliveira ML, Dutkowski P, Sanchez-Fueyo A. Can immunosuppression be stopped after liver transplantation? Lancet Gastroenterol Hepatol. 2017;2:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Tran L, Humar A. Tolerance studies in liver transplantation: are we fooling ourselves? Curr Opin Organ Transplant. 2020;25:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Jucaud V, Shaked A, DesMarais M, Sayre P, Feng S, Levitsky J, Everly MJ. Prevalence and Impact of De Novo Donor-Specific Antibodies During a Multicenter Immunosuppression Withdrawal Trial in Adult Liver Transplant Recipients. Hepatology. 2019;69:1273-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Choudhary NS, Saraf N, Saigal S, Gautam D, Rastogi A, Goja S, Bhangui P, Srinivasan T, Yadav SK, Soin A. Revisiting chronic rejection following living donor liver transplantation in the tacrolimus era: A single center experience. Clin Transplant. 2018;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Adam R, Karam V, Cailliez V, O Grady JG, Mirza D, Cherqui D, Klempnauer J, Salizzoni M, Pratschke J, Jamieson N, Hidalgo E, Paul A, Andujar RL, Lerut J, Fisher L, Boudjema K, Fondevila C, Soubrane O, Bachellier P, Pinna AD, Berlakovich G, Bennet W, Pinzani M, Schemmer P, Zieniewicz K, Romero CJ, De Simone P, Ericzon BG, Schneeberger S, Wigmore SJ, Prous JF, Colledan M, Porte RJ, Yilmaz S, Azoulay D, Pirenne J, Line PD, Trunecka P, Navarro F, Lopez AV, De Carlis L, Pena SR, Kochs E, Duvoux C; all the other 126 contributing centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA). 2018 Annual Report of the European Liver Transplant Registry (ELTR) - 50-year evolution of liver transplantation. Transpl Int. 2018;31:1293-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 329] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 29. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 706] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 30. | Kim H, Lee KW, Yi NJ, Lee HW, Choi Y, Suh SW, Jeong J, Suh KS. Outcome and technical aspects of liver retransplantation: analysis of 25-year experience in a single major center. Transplant Proc. 2015;47:727-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Pfitzmann R, Benscheidt B, Langrehr JM, Schumacher G, Neuhaus R, Neuhaus P. Trends and experiences in liver retransplantation over 15 years. Liver Transpl. 2007;13:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 32. | Akpinar E, Selvaggi G, Levi D, Moon J, Nishida S, Island E, DeFaria W, Pretto E, Ruiz P, Tzakis AG. Liver retransplantation of more than two grafts for recurrent failure. Transplantation. 2009;88:884-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Berumen J, Hemming A. Liver Retransplantation: How Much Is Too Much? Clin Liver Dis. 2017;21:435-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Kitchens WH, Yeh H, Markmann JF. Hepatic retransplant: what have we learned? Clin Liver Dis. 2014;18:731-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Zhang GQ, Zhang CS, Sun N, Lv W, Chen BM, Zhang JL. Basiliximab application on liver recipients: a meta-analysis of randomized controlled trials. Hepatobiliary Pancreat Dis Int. 2017;16:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Penninga L, Wettergren A, Ch W, Aw C, Da S, Gluud C. Antibody induction vs corticosteroid induction for liver transplant recipients ( Review ). Cochrane Database Syst Rev. 2014;5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Lmj B, Leung J, Sc F. Induction immunosuppression in adults undergoing liver transplantation: a network meta-analysis (Review). Cochrane Database Syst Rev. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Brunet M, Shipkova M, van Gelder T, Wieland E, Sommerer C, Budde K, Haufroid V, Christians U, López-Hoyos M, Barten MJ, Bergan S, Picard N, Millán López O, Marquet P, Hesselink DA, Noceti O, Pawinski T, Wallemacq P, Oellerich M. Barcelona Consensus on Biomarker-Based Immunosuppressive Drugs Management in Solid Organ Transplantation. Ther Drug Monit. 2016;38:S1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Lutz HH, Schroeter B, Kroy DC, Neumann U, Trautwein C, Tischendorf JJ. Doppler Ultrasound and Transient Elastography in Liver Transplant Patients for Noninvasive Evaluation of Liver Fibrosis in Comparison with Histology: A Prospective Observational Study. Dig Dis Sci. 2015;60:2825-2831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Schlegel A, Kron P, Graf R, Clavien PA, Dutkowski P. Hypothermic Oxygenated Perfusion (HOPE) downregulates the immune response in a rat model of liver transplantation. Ann Surg. 2014;260:931-937; discussion 937-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |