Published online Nov 14, 2021. doi: 10.3748/wjg.v27.i42.7376
Peer-review started: June 13, 2021
First decision: July 14, 2021
Revised: July 27, 2021
Accepted: October 25, 2021
Article in press: October 25, 2021
Published online: November 14, 2021
Processing time: 149 Days and 18.9 Hours
Few studies have fully described endoscopic ultrasound (EUS) features of newly diagnosed autoimmune pancreatitis (AIP) involving both typical findings and chronic pancreatitis (CP) features. The typical EUS findings are prevalent in the diffuse type AIP but may not be as common for the focal type, and the differences between the diffuse and focal AIP need to be specified.
To demonstrate the EUS features of newly diagnosed AIP and the difference between diffuse and focal AIP.
This retrospective single center study included 285 patients of newly diagnosed type 1 AIP following the international consensus diagnostic criteria, with the EUS procedures accomplished before corticosteroid initiation. We explored the EUS features and compared the typical AIP and CP features between the diffuse and focal AIP cases. The Rosemont criteria were employed for CP features definition and CP change level comparison.
For the typical AIP features, there were significantly more patients in the diffuse group with bile duct wall thickening (158 of 214 cases, 73.4% vs 37 of 71 cases, 52.1%, P = 0.001) and peripancreatic hypoechoic margin (76 of 214 cases, 35.5% vs 5 of 71 cases, 7.0%, P < 0.001). For the CP features, there were significantly more patients in the focal group with main pancreatic duct dilation (30 of 214 cases, 14.0% vs 18 of 71 cases, 25.3%, P = 0.03). The cholangitis-like changes were more prevalent in the focal cases with pancreatic head involvement. The CP change level was relatively limited for newly diagnosed AIP cases in both groups.
This study demonstrated the difference in the typical AIP and CP features between diffuse and focal AIP and indicated the limited CP change level in newly diagnosed AIP.
Core Tip: The endoscopic ultrasound (EUS) features of newly diagnosed autoimmune pancreatitis (AIP) involving both typical findings and chronic pancreatitis (CP) features have rarely been described. The EUS typical features of AIP can help to differentiate diffuse AIP from classic CP and differentiate focal AIP from pancreatic cancer. This study demonstrated the EUS features of newly diagnosed AIP and the difference in the typical AIP features and CP features between the diffuse and focal AIP cases on the basis of the largest number of cases and indicated the relatively limited CP change in newly diagnosed AIP cases.
- Citation: Zhang SY, Feng YL, Zou L, Wu X, Guo T, Jiang QW, Wang Q, Lai YM, Tang SJ, Yang AM. Endoscopic ultrasound features of autoimmune pancreatitis: The typical findings and chronic pancreatitis changes. World J Gastroenterol 2021; 27(42): 7376-7386
- URL: https://www.wjgnet.com/1007-9327/full/v27/i42/7376.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i42.7376
In 1995, Yoshida et al[1] first proposed the concept of autoimmune pancreatitis (AIP) as a unique disease entity, which can be divided into two subtypes. Type 1 AIP, which is the most common type in Asia, is the pancreatic manifestation of immunoglobulin (Ig)G4-related disease and is often associated with extrapancreatic disorders, especially IgG4-associated cholangitis[2].
AIP can be divided into diffuse type and focal type based on the image findings[3]. With endoscopic ultrasound (EUS) as an effective tool to find parenchymal and main pancreatic ductal (MPD) changes, typical AIP features can be observed for diffuse type AIP, such as diffuse enlargement of the whole pancreas with diffuse hypoechoic parenchyma and hypoechoic peripancreatic margin[4-6]. But the typical EUS findings for AIP may not be as common for the focal type and differences between the diffuse and focal type need to be demonstrated.
As a special form of chronic pancreatitis (CP), almost all CP features could be recognized in AIP cases during EUS examination[4-6]. After recurrent attack or prolonged inflammatory injury from AIP, advanced CP features such as parenchymal calcification/atrophy and MPD calculi will emerge from the diffuse enlarged hypoechoic pancreas[7-9]. So, from newly diagnosed AIP to advanced CP, we need a measure to describe the level of CP change.
Therefore, we conducted this single center retrospective study for a detailed description of the EUS features of newly diagnosed AIP patients and demonstration of the difference between diffuse and focal AIP, and we tried to compare the CP change level in both groups via the Rosemont criteria based on all CP features.
Patients diagnosed with AIP undergoing EUS before the initiation of corticosteroid treatment at the Peking Union Medical College Hospital from January 2012 to August 2018 were included in this retrospective study, and patients who had a history of alcoholism or recent acute pancreatitis onset (within 3 mo) were excluded. All diagnoses was made following the International Consensus Diagnostic Criteria (ICDC; see Supplement Figure)[10]. The study was approved by the Ethics Committee of Peking Union Medical College Hospital (approval number S-K1613).
All EUS procedures were performed by experienced endoscopists (XW, DSW, TG, QWJ; all with over 5 years EUS experience) with radial or linear echoendoscopes (frequency 5-7.5 MHz; GF-UCT260 or GF-UE260, Olympus, Tokyo, Japan) and ultrasound workstations (EM-2, Olympus, Tokyo, Japan, and Aloka ProSound F75, Hitachi, Tokyo, Japan). EUS images and videos were stored as digital data, and the image analysis was completed by SYZ and YLF in a blinded manner independently, in which LZ selected all image videos for each case from the database, removed the patient’s information and diagnosis and showed them to SYZ and YLF. If disagreement existed between the two investigators, the third one (QW) would decide the image interpreting results.
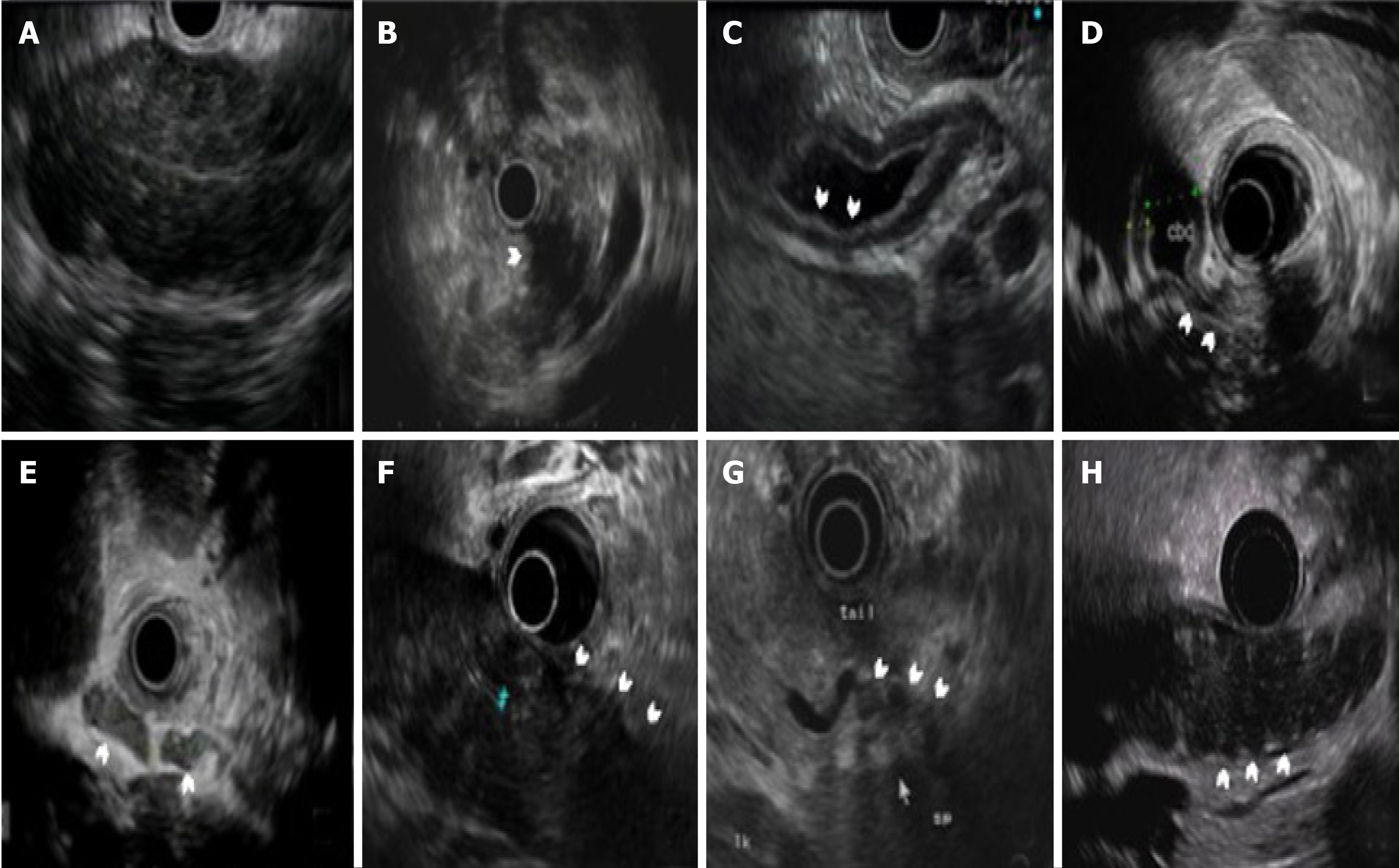
We defined the typical EUS features of AIP, including diffuse enlargement (the pancreas is divided into three parts: head, body, and tail; cases with more than one part of the pancreas enlarged were defined as “diffuse enlargement” and grouped as the “diffuse type”), focal enlargement (cases with less than one part of the pancreas enlarged were defined as “focal enlargement” and grouped as the “focal type”), diffuse hypoechoic area (DHA), focal hypoechoic areas, bile duct wall thickening, extrahepatic bile duct dilation, intrapancreatic bile stenosis, peripancreatic lymphadenopathy, peripancreatic hypoechoic margins, lobular outer margins and peripancreatic vessel involvement (Figure 1).
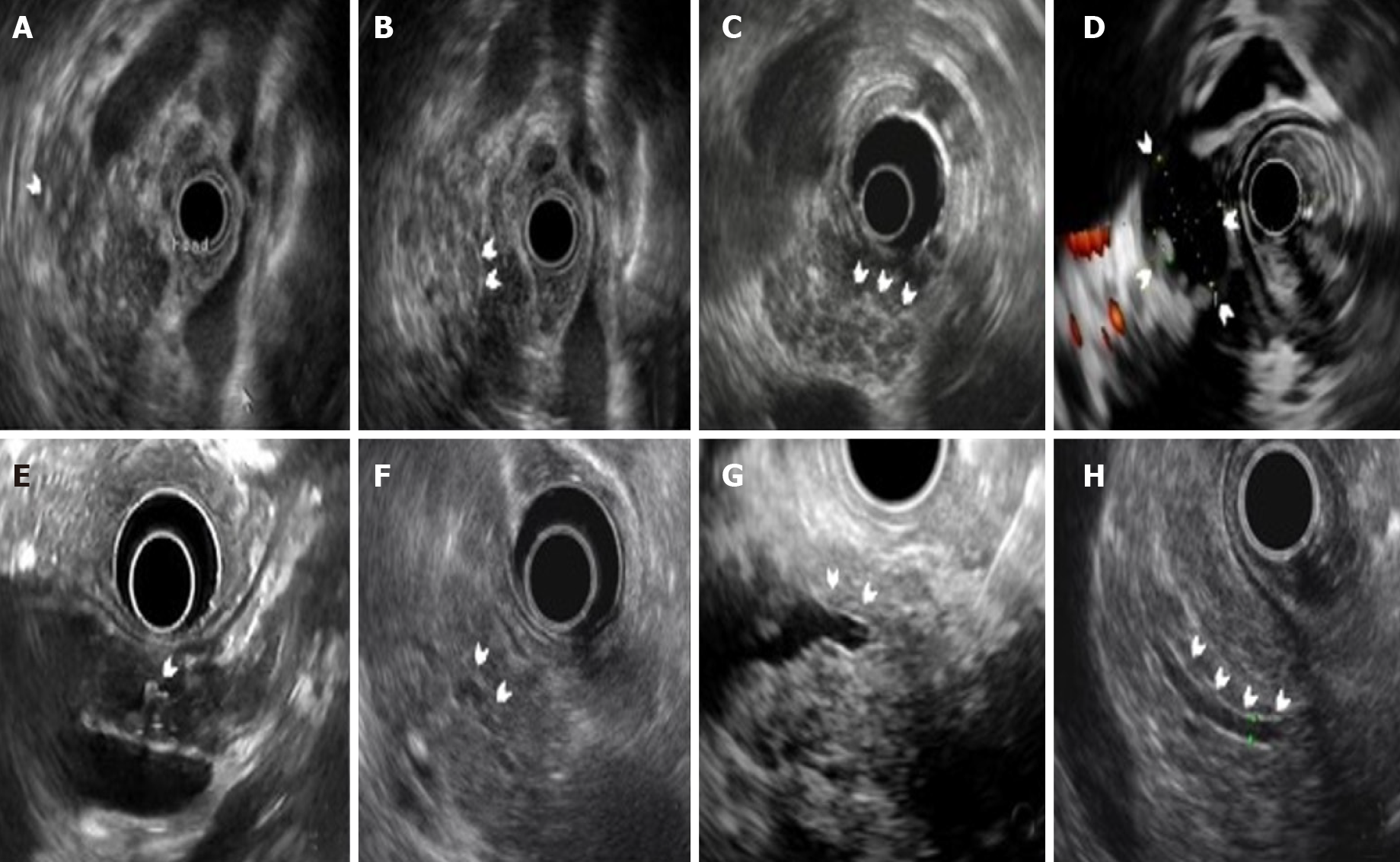
We used the Rosemont criteria to describe the CP features in AIP[11]. The parenchymal changes included hyperechoic foci (HF), hyperechoic strand (HS), lobularity with/without honeycombing, cystic lesions and calcifications (Figure 2). The MPD changes included MPD stones, duct irregularity, dilation and hyperechoic duct margins (Figure 2).
All definitions are interpreted in detail in Supplementary Table 1.
We applied the Rosemont criteria to describe the level of CP change in newly diagnosed AIP patients of both diffuse and focal types.
Descriptive analysis was used to describe the clinical and EUS features of AIP patients. Categorical variables were presented as n (%), and continuous variables were presented as the mean ± SD or the median and the interquartile range, depending on the distribution. The distribution of the continuous variables was checked using a visual inspection of the histogram. Statistical analysis was accomplished with SPSS 23.0 (IBM, NY, United States). The differences in EUS features and clinical factors between the diffuse and focal AIP cases were compared using Student’s t test/Mann-Whitney U test (for continuous variables, depending on distribution) or χ2 test (for categorical variables). Multivariate analysis using the binary logistic regression including variables identified to be significant (P ≤ 0.10) at univariate analysis. A two-tailed P value of less than 0.05 was considered to be statistically significant.
A total of 285 patients were included in this study, with 230 male patients (80.7%) and a median age of 62 [interquartile range 54, 68] years. The mean follow-up duration was 26 [interquartile range 12, 51] mo. All patients were diagnosed with type 1 AIP according to the ICDC criteria. The clinical data (including symptoms and laboratory tests) are shown in Table 1. All items were comparable between the diffuse and focal group, except for alanine transaminase and carbohydrate antigen 19-9, which were higher in the diffuse group.
| All (n = 285) | Diffuse type (n = 214) | Focal type (n = 71) | P value | |
| Sex | 230 (80.7) | 179 (83.6) | 51 (71.8) | 0.03 |
| Age | 62 (54, 68) | 62 (55, 68) | 59 (53, 68) | 0.12 |
| Follow-up time (mo) | 26 (12, 51) | 28 (13, 51) | 21 (9, 51) | 0.13 |
| Symptoms | ||||
| Abdominal pain | 73 (26.0) | 51 (23.8) | 22 (31.0) | 0.23 |
| Jaundice | 106 (37.2) | 81 (37.8) | 25 (35.2) | 0.69 |
| Number of involved organs1 | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 0.47 |
| Laboratory tests | ||||
| ALT (U/L) (9-50) | 47 (18, 133) | 64 (18, 184) | 30 (15, 83) | 0.01 |
| TBil (μmol/L) (5.1-22.2) | 20.6 (12.2, 55.6) | 21.4 (12.9, 68.7) | 14.5 (11.6, 45.2) | 0.08 |
| IgG (mg/dL) (700-1700) | 1590 (1140, 2070) | 1630 (1130, 2140) | 1510 (1130, 1760) | 0.37 |
| IgG4 (mg/dL) (8-140) | 558.0 (280.5, 1270.0) | 605.5 (253.5, 1457.5) | 458.0 (301.0, 1050.0) | 0.26 |
| CA 19-9 (U/L) (0-34.0) | 20.3 (7.9, 61.0) | 23.4 (10.5, 74.1) | 12.6 (6.7, 36.5) | 0.01 |
The EUS features of all patients were shown, and the differences between the diffuse and focal types of AIP were compared (Table 2).
| EUS findings | All (n = 285) | Diffuse type (n = 214) | Focal type (n = 71) | P value |
| Typical findings | ||||
| DHA | 213 (74.7) | 197 (92.1) | 16 (22.5) | < 0.001 |
| FHA | 59 (20.7) | 0 (0) | 59 (83.1) | < 0.001 |
| Bile duct changes | ||||
| Bile duct wall thickening | 195 (68.4) | 158 (73.4) | 37 (52.1) | 0.001 |
| Intrapancreatic bile duct stenosis | 165 (57.9) | 131 (61.2) | 34 (47.9) | 0.05 |
| Extrahepatic bile duct dilation | 122 (42.8) | 97 (45.3) | 25 (35.2) | 0.14 |
| Peripancreatic changes | ||||
| Peripancreatic lymphadenopathy | 89 (31.2) | 72 (33.6) | 17 (23.9) | 0.13 |
| Peripancreatic hypoechoic margin | 81 (28.4) | 76 (35.5) | 5 (7.0) | < 0.001 |
| Lobular outer margin | 40 (14.0) | 34 (15.9) | 6 (8.5) | 0.12 |
| Peripancreatic vessel involvement | 21 (7.4) | 16 (7.5) | 5 (7.0) | 0.90 |
| Chronic pancreatitis changes | ||||
| Parenchymal changes | ||||
| HF | 271 (95.1) | 202 (94.4) | 69 (97.2) | 0.531 |
| HS | 174 (61.1) | 131 (61.2) | 43(60.6) | 0.92 |
| Cystic lesion | 18 (6.3) | 14 (6.5) | 4 (5.6) | 1.001 |
| Parenchymal calcification | 3 (1.1) | 2 (0.9) | 1 (1.4) | 1.001 |
| Lobularity with honeycombing | 26 (9.1) | 19 (8.9) | 7 (9.9) | 0.80 |
| Lobularity without honeycombing | 48 (16.8) | 36 (16.8) | 12 (16.9) | 0.99 |
| Main pancreatic duct changes | ||||
| MPD calculi | 1 (0.4) | 0 (0) | 1 (1.4) | 0.561 |
| MPD dilation | 48 (16.8) | 30 (14.0) | 18 (25.3) | 0.03 |
| Diffuse stenosis/irregularity | 29 (10.2) | 20 (9.3) | 9 (12.7) | 0.42 |
| Focal stenosis | 11 (3.9) | 6 (2.8) | 5 (7.0) | 0.151 |
| Hyperechoic duct margin | 119 (41.8) | 91 (42.5) | 28 (39.4) | 0.65 |
There were 214 cases of diffuse type and 71 cases of focal type AIP; among focal cases, more lesions were located in the pancreatic head (50 cases, 70.4%), while less in the body (8 cases, 11.3%) and tail (13 cases, 18.3%).
For the typical AIP features, there were significantly more patients with DHA in the diffuse group (197 of 214 cases, 92.1% vs 16 of 71 cases, 22.5%, P < 0.001), while there were significantly more patients with focal hypoechoic areas in the focal group (0 of 214 cases, 0% vs 59 of 71 cases, 83.1%, P < 0.001), which was consistent with the original definitions. For cholangitis-like changes, there were significantly more patients with bile duct wall thickening (158 of 214 cases, 73.4% vs 37 of 71 cases, 52.1%, P = 0.001) in the diffuse group. For peripancreatic changes, there were significantly more patients with peripancreatic hypoechoic margins (76 of 214 cases, 35.5% vs 5 of 71 cases, 7.0%, P < 0.001) in the diffuse group.
In the focal group, the cholangitis-like changes (bile duct wall thickening, intrapancreatic bile duct stenosis and extrahepatic bile duct dilation) were more prevalent in cases with pancreatic head involvement compared to those with body or tail involvement (P < 0.001, P = 0.01 and P = 0.02, respectively) (Supplementary Table 2).
For the CP features, parenchymal changes were all comparable in the diffuse and focal group. For MPD changes, there were significantly more patients with MPD dilation in the focal group than in the diffuse group (30 of 214 cases, 14.0% vs 18 of 71 cases, 25.3%, P = 0.03).
In the logistic regression analysis for the diffuse AIP with the EUS findings, the DHA [odds ratio (OR) = 11.23, 95% confidence index (CI): 3.07–41.03; P < 0.001], bile duct wall thickening (OR = 4.44, 95%CI 2.49–7.93; P < 0.001) and peripancreatic hypoechoic margin (OR = 4.34, 95%CI: 1.93–9.80; P < 0.001) were all predictors of the diffuse AIP (Table 3).
| Effect | Odds ratio (95%CI) | P value |
| DHA | 11.23 (3.07, 41.03) | < 0.001 |
| Bile duct wall thickening | 4.44 (2.49, 7.93) | < 0.001 |
| Peripancreatic hypoechoic margin | 4.34 (1.93, 9.80) | < 0.001 |
The Rosemont criteria were applied to describe the CP change level. Only a small portion of patients was diagnosed as “suggestive of CP” (12.1% in the diffuse group vs 15.5% in the focal group), and patients with advanced CP change (“consistent with CP”) were even more rare (0.9% vs 1.4%) (Table 4). The CP change level was similar for newly diagnosed AIP cases in the diffuse and focal groups.
| Rosemont criteria | Diffuse type (n = 14) | Focal type (n = 71) | P value |
| Consistent with CP | 2 (0.9) | 1 (1.4) | 0.45 |
| Suggestive of CP | 26 (12.1) | 11 (15.5) | |
| Indeterminate of CP | 174 (81.3) | 58 (81.7) | |
| Normal | 12 (5.6) | 1 (1.4) |
Type 1 AIP is a special form of CP that is pathologically characterized by an abundance of lymphoplasmacytic infiltrates, fibrosis and obliterative phlebitis[2]. EUS can detect abnormalities in the parenchyma and pancreatic duct that are possibly not visible by other modalities. To date, this is the largest single center retrospective study demonstrating EUS features in newly diagnosed type 1 AIP patients, which not only describes the typical findings and the CP features of AIP but also figures out the difference between the diffuse and focal type.
We demonstrated the typical EUS features in AIP, including diffuse or focal hypoechoic area, bile duct changes due to IgG4-associated cholangitis and peripancreatic changes. In fact, the typical EUS findings (such as diffuse enlargement of the pancreas, DHA, bile duct wall thickening or stenosis and peripancreatic hypoechoic margin) were still prominent manifestations for AIP patients, which was consistent with previous studies (Supplement Table 3). More patients in the diffuse group showed typical features, especially for bile duct wall thickening and peripancreatic hypoechoic margin due to the profound inflammatory change[6]. In the multivariate regression analysis for the diffuse AIP, we demonstrated the predictors of the diffuse AIP: the DHA, bile duct wall thickening and peripancreatic hypoechoic margin (all P < 0.001), which are all typical EUS findings of AIP rather than CP features.
It is also notable that despite hypoechoic parenchyma in the enlarged part of the focal type the rest of the parenchyma also showed hypoechogenicity and hyperechoic foci/strands under EUS, which implied the dynamic change of the parenchyma possibly caused by the spontaneous remission of AIP[12]. But we did not define these cases as “diffuse type” to avoid confusion with the ICDC, which emphasizes the diffuse or focal “enlargement” of the pancreas.
Bile duct changes often happen in AIP, as shown in this study. Bile duct wall thickening (73.4% in the diffuse group, 66.0% in the focal cases with pancreatic head involved and 19.0% in the focal cases without pancreatic head involved) resulting from IgG4-associated cholangitis and intrapancreatic bile duct stenosis (61.2% in the diffuse group, 58.0% in the focal cases with pancreatic head involved and 23.8% in the focal cases without pancreatic head involved) that possibly caused by both bile duct wall thickening and extrinsic pancreatic compression[13] are both more common in the diffuse group (almost all cases have head involvement) and focal AIP cases with pancreatic head involved.
The CP changes of AIP were also fully explored in this study. All parenchymal changes were comparable in the diffuse and focal groups. In previous studies, Farrell et al[4] showed that lobularity existed in 7.1% (1/14) of patients[7]. Hoki et al[5] demonstrated that the occurrence of HF, HS, lobularity, cystic lesions and calcifications were 32% (8/25), 56% (14/25), 8% (2/25), 16% (4/25) and 16% (4/25), respectively. Okabe et al[6] found that HF existed in all patients (32/32), with a lower incidence rate of HS and lobularity (81.3% and 53.1%, respectively). As for MPD changes, there was no difference between the two groups except for MPD dilation, which was more frequently seen in the focal group (14.0% vs 25.3%, P = 0.03) and seemed to be more prevalent in the focal cases with pancreatic head involved (32.0% in head involved cases vs 9.5% in non-head involved cases, P = 0.05). Previous studies reported that MPD dilation was present in 12%-37% of AIP patients, which was often located proximally to the AIP affected area where the MPD or surrounding parenchyma was involved, while hyperechoic duct margins and diffuse stenosis/irregularity were present in approximately 12% (3/25) and 40% (10/25) of patients, respectively[8,9] (Supplementary Table 3). The reason for the different incidence rate of parenchymal and MPD changes may be that AIP patients are possibly at different clinical stages (early or advanced) in these studies[14]. After the recurrent attack or prolonged inflammatory damage, pancreatic duct stones or parenchymal calcifications may be formed, which will make the shape of AIP more similar to advanced CP[7-9].
The focal AIP accompanied by MPD dilation, sometimes also by peripancreatic lymphadenopathy and vessel involvement, is difficult to differentiate from pancreatic carcinoma. There are some EUS features, like bile duct wall thickening and peripancreatic hypoechoic margin that are relatively specific for focal AIP patients[5]. Several noninvasive EUS methods have been developed for differential diagnosis but without satisfactory sensitivity or specificity[15,16]. A prediction model with multiple EUS features could help to differentiate focal AIP from pancreatic carcinoma[17]. The EUS-guided fine-needle aspiration (EUS-FNA) procedure should be considered as the first choice to diagnosis focal AIP or rule out malignancy[18-21]. The diagnostic accuracy of EUS-FNA is between 45%–78%[22]. In this study, 92 initial “non-diagnostic” AIP cases received EUS-FNA procedures, among whom 36 cases (39.1%) got level 1 and 2 histological evidence (Supplementary Figure). All cases receiving EUS-FNA got the final diagnosis of “definite” AIP according to the ICDC.
To date, endoscopic retrograde pancreatography is included as part of the diagnosis of AIP in the Japanese guideline and the ICDC[10,23]. Exploring quantitative and qualitative parenchymal and ductal change, EUS is a reliable method to diagnose CP, which is statistically comparable to the endoscopic retrograde pancreatography and the Cambridge criteria (the gold standard in the past)[24-26]. So, we tried to describe the CP change level of newly diagnosed AIP cases with the CP features in the Rosemont criteria and found that only a small portion of patients was diagnosed as “suggestive of CP” (12.1% in the diffuse group vs 15.5% in the focal group), and patients with more advanced CP change (“consistent with CP”) were even more rare (0.9% vs 1.4%). Therefore, the CP change is relatively limited for most newly diagnosed AIP cases that were probably in the early stage of disease, while advanced CP findings may happen in the long-term recurrent attacks[8,14]. EUS can detect the early parenchymal fibrosis of CP (like HF and HS) in AIP cases, which changes dynamically after corticosteroid therapy[6]. As the tool for accessing the fibrosis degree of the pancreas, the EUS findings of CP may be used for predicting the pancreatic atrophy and diabetes exacerbation, which needs further investigation[27].
This study had limitations. First, this was a single center retrospective study, and all AIP patients included in this study were diagnosed with type 1 AIP. Therefore, selection bias inevitably existed. Second, the EUS-FNA diagnosis accuracy in this study was somehow lower than previously reported (about 39.1% for level 1 and 2 histological evidence), which might be because the fine needle biopsy needles were used sparingly[22] (Supplementary Table 4). The long time period of the study (our center did not have fine needle biopsy needles until 2015) might explain the reason that we did use the fine needle biopsy needles (22G Procore and 20G Procore, COOK, United States) except in a low proportion (12.0% in diffuse AIP patients and 29.9% in focal AIP cases). However, the FNA needles still have the clinical significance of ruling out malignancy in AIP patients[21]. Third, MPD dilation was defined as “> 3 mm in the head, > 2 mm in the body, >1 mm in the tail” in the Rosemont criteria, but in the elderly population of AIP patients, the normal range of MPD diameter might be larger[28]. Therefore, we might have overestimated the incidence rate of MPD dilation. Lastly, the “dilated side branches” of the pancreatic duct in the Rosemont criteria were relatively difficult to evaluate without endoscopic retrograde pancreatography, so they were not included in this study.
In conclusion, this study demonstrated the EUS features of newly diagnosed AIP and the difference in the typical AIP features and CP features between diffuse and focal AIP on the basis of the largest number of cases and indicated the relatively limited CP change in newly diagnosed AIP cases via the Rosemont criteria.
Few studies have fully described the endoscopic ultrasound (EUS) features of newly diagnosed autoimmune pancreatitis (AIP) involving both typical findings and chronic pancreatitis (CP) features. The typical EUS findings are prevalent in diffuse AIP but may not be as common for the focal type, and the differences between diffuse and focal AIP need to be specified. The EUS typical features of AIP (especially the cholangiopathy-like features) can help to differentiate diffuse AIP from classic CP and differentiate focal AIP from pancreatic cancer.
This is the largest single center retrospective study demonstrating EUS features in newly diagnosed type 1 AIP patients that not only describes the typical findings and the CP features of AIP but also figures out the difference between the diffuse and focal types.
The authors conducted this single center retrospective study for a detailed description of the EUS features of newly diagnosed AIP patients and demonstration of the difference between diffuse and focal AIP, and we tried to compare the CP change level in both groups via the Rosemont criteria based on all CP features.
This retrospective single center study included 285 patients of newly diagnosed type 1 AIP following the international consensus diagnostic criteria, with the EUS procedures accomplished before corticosteroid initiation. We explored the EUS features and compared the typical AIP and CP features between the diffuse and focal AIP cases. The Rosemont criteria were employed for CP features definition and CP change level comparison.
For the typical AIP features, there were significantly more patients in the diffuse group with bile duct wall thickening and peripancreatic hypoechoic margin. In the multivariate regression analysis for diffuse AIP, we demonstrated the predictors of diffuse AIP: the DHA, bile duct wall thickening and peripancreatic hypoechoic margin (all P < 0.001), which are all typical EUS findings of AIP rather than CP features. For the CP features, there were significantly more patients in the focal group with main pancreatic duct dilation. The cholangitis-like changes were more prevalent in the focal cases with pancreatic head involvement. The CP change level was relatively limited for newly diagnosed AIP cases in the diffuse and focal groups.
This study demonstrated the EUS features of newly diagnosed AIP and the difference in the typical AIP features and CP features between the diffuse and focal AIP cases on the basis of the largest number of cases. It indicated the relatively limited CP change in newly diagnosed AIP cases via the Rosemont criteria.
EUS can detect the early parenchymal fibrosis of CP in AIP cases, which changes dynamically after corticosteroid therapy. As the tool for accessing the fibrosis degree of the pancreas, the EUS findings of CP may be used for predicting pancreatic atrophy and diabetes exacerbation, which needs further investigation.
The authors would like to thank Yue-Lun Zhang, PhD (Central Research Laboratory, Peking Union Medical College Hospital) for his professional assistance in statistical analysis and all the nurses for their expert assistance during the EUS procedures.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Arcidiacono PG, Emmanuel J, Fujimori N, Nakai Y S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 925] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 2. | Miyabe K, Zen Y, Cornell LD, Rajagopalan G, Chowdhary VR, Roberts LR, Chari ST. Gastrointestinal and Extra-Intestinal Manifestations of IgG4-Related Disease. Gastroenterology. 2018;155:990-1003.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Kwon JH, Kim JH, Kim SY, Byun JH, Kim HJ, Lee MG, Lee SS. Differentiating focal autoimmune pancreatitis and pancreatic ductal adenocarcinoma: contrast-enhanced MRI with special emphasis on the arterial phase. Eur Radiol. 2019;29:5763-5771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Farrell JJ, Garber J, Sahani D, Brugge WR. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc. 2004;60:927-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Hoki N, Mizuno N, Sawaki A, Tajika M, Takayama R, Shimizu Y, Bhatia V, Yamao K. Diagnosis of autoimmune pancreatitis using endoscopic ultrasonography. J Gastroenterol. 2009;44:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Okabe Y, Ishida Y, Kaji R, Sugiyama G, Yasumoto M, Naito Y, Toyonaga A, Tsuruta O, Sata M. Endoscopic ultrasonographic study of autoimmune pancreatitis and the effect of steroid therapy. J Hepatobiliary Pancreat Sci. 2012;19:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Takayama M, Hamano H, Ochi Y, Saegusa H, Komatsu K, Muraki T, Arakura N, Imai Y, Hasebe O, Kawa S. Recurrent attacks of autoimmune pancreatitis result in pancreatic stone formation. Am J Gastroenterol. 2004;99:932-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Kanai K, Maruyama M, Kameko F, Kawasaki K, Asano J, Oguchi T, Watanabe T, Ito T, Muraki T, Hamano H, Matsumoto A, Arakura N, Kawa S. Autoimmune Pancreatitis Can Transform Into Chronic Features Similar to Advanced Chronic Pancreatitis With Functional Insufficiency Following Severe Calcification. Pancreas. 2016;45:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Ito T, Kawa S, Matsumoto A, Kubota K, Kamisawa T, Okazaki K, Hirano K, Hirooka Y, Uchida K, Masuda A, Ohara H, Shimizu K, Arakura N, Masamune A, Kanno A, Sakagami J, Itoi T, Ito T, Ueki T, Nishino T, Inui K, Mizuno N, Yoshida H, Sugiyama M, Iwasaki E, Irisawa A, Shimosegawa T, Chiba T. Risk Factors for Pancreatic Stone Formation in Type 1 Autoimmune Pancreatitis: A Long-term Japanese Multicenter Analysis of 624 Patients. Pancreas. 2019;48:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, Notohara K, Okazaki K, Schneider A, Zhang L; International Association of Pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1058] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 11. | Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, Brugge W, Freeman M, Yamao K, Canto M, Hernandez LV. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2009;69:1251-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 345] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 12. | Kubota K, Iida H, Fujisawa T, Yoneda M, Inamori M, Abe Y, Kirikoshi H, Saito S, Ohshiro H, Kakuta Y, Nakajima A. Clinical factors predictive of spontaneous remission or relapse in cases of autoimmune pancreatitis. Gastrointest Endosc. 2007;66:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Hirano K, Tada M, Isayama H, Yamamoto K, Mizuno S, Yagioka H, Yashima Y, Sasaki T, Kogure H, Togawa O, Arizumi T, Matsubara S, Nakai Y, Sasahira N, Tsujino T, Kawabe T, Omata M. Endoscopic evaluation of factors contributing to intrapancreatic biliary stricture in autoimmune pancreatitis. Gastrointest Endosc. 2010;71:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Suda K, Nishimori I, Takase M, Oi I, Ogawa M. Autoimmune pancreatitis can be classified into early and advanced stages. Pancreas. 2006;33:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Cho MK, Moon SH, Song TJ, Kim RE, Oh DW, Park DH, Lee SS, Seo DW, Lee SK, Kim MH. Contrast-Enhanced Endoscopic Ultrasound for Differentially Diagnosing Autoimmune Pancreatitis and Pancreatic Cancer. Gut Liver. 2018;12:591-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Imazu H, Kanazawa K, Mori N, Ikeda K, Kakutani H, Sumiyama K, Hino S, Ang TL, Omar S, Tajiri H. Novel quantitative perfusion analysis with contrast-enhanced harmonic EUS for differentiation of autoimmune pancreatitis from pancreatic carcinoma. Scand J Gastroenterol. 2012;47:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Guo T, Xu T, Zhang S, Lai Y, Wu X, Wu D, Feng Y, Jiang Q, Wang Q, Qian J, Yang A. The role of EUS in diagnosing focal autoimmune pancreatitis and differentiating it from pancreatic cancer. Endosc Ultrasound. 2021;10:280-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Kanno A, Masamune A, Fujishima F, Iwashita T, Kodama Y, Katanuma A, Ohara H, Kitano M, Inoue H, Itoi T, Mizuno N, Miyakawa H, Mikata R, Irisawa A, Sato S, Notohara K, Shimosegawa T. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: a prospective multicenter study. Gastrointest Endosc. 2016;84:797-804.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Cao L, Wang Y, Wang J, Guo Q, Chen Q, Wu X, Tang SJ, Cheng B. The role of EUS-guided fine needle aspiration in autoimmune pancreatitis: a single center prospective study. Scand J Gastroenterol. 2018;53:1604-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Kurita A, Yasukawa S, Zen Y, Yoshimura K, Ogura T, Ozawa E, Okabe Y, Asada M, Nebiki H, Shigekawa M, Ikeura T, Eguchi T, Maruyama H, Ueki T, Itonaga M, Hashimoto S, Shiomi H, Minami R, Hoki N, Takenaka M, Itokawa Y, Uza N, Hashigo S, Yasuda H, Takada R, Kamada H, Kawamoto H, Kawakami H, Moriyama I, Fujita K, Matsumoto H, Hanada K, Takemura T, Yazumi S. Comparison of a 22-gauge Franseen-tip needle with a 20-gauge forward-bevel needle for the diagnosis of type 1 autoimmune pancreatitis: a prospective, randomized, controlled, multicenter study (COMPAS study). Gastrointest Endosc. 2020;91:373-381.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 21. | Sugimoto M, Takagi T, Suzuki R, Konno N, Asama H, Watanabe K, Nakamura J, Kikuchi H, Waragai Y, Takasumi M, Sato Y, Hikichi T, Ohira H. Endoscopic Ultrasonography-Guided Fine Needle Aspiration Can Be Used to Rule Out Malignancy in Autoimmune Pancreatitis Patients. J Ultrasound Med. 2017;36:2237-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Chhoda A, Rustagi T. EUS-guided needle biopsy for autoimmune pancreatitis. Clin J Gastroenterol. 2020;13:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Okazaki K, Kawa S, Kamisawa T, Ito T, Inui K, Irie H, Nishino T, Notohara K, Nishimori I, Tanaka S, Nishiyama T, Suda K, Shiratori K, Tanaka M, Shimosegawa T; Working Committee of the Japan Pancreas Society and the Research Committee for Intractable Pancreatic Disease supported by the Ministry of Health, Labour and Welfare of Japan. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 I. Concept and diagnosis of autoimmune pancreatitis. J Gastroenterol. 2014;49:567-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Axon AT, Classen M, Cotton PB, Cremer M, Freeny PC, Lees WR. Pancreatography in chronic pancreatitis: international definitions. Gut. 1984;25:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 296] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Sahai AV, Zimmerman M, Aabakken L, Tarnasky PR, Cunningham JT, van Velse A, Hawes RH, Hoffman BJ. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 201] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Stevens T, Conwell DL, Zuccaro G Jr, Vargo JJ, Dumot JA, Lopez R. Comparison of endoscopic ultrasound and endoscopic retrograde pancreatography for the prediction of pancreatic exocrine insufficiency. Dig Dis Sci. 2008;53:1146-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Yamada Y, Masuda A, Sofue K, Ueshima E, Shiomi H, Sakai A, Kobayashi T, Ikegawa T, Tanaka S, Nakano R, Tanaka T, Kakihara M, Ashina S, Tsujimae M, Yamakawa K, Abe S, Gonda M, Masuda S, Inomata N, Kutsumi H, Itoh T, Murakami T, Kodama Y. Prediction of pancreatic atrophy after steroid therapy using equilibrium-phase contrast computed tomography imaging in autoimmune pancreatitis. JGH Open. 2020;4:677-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Hastier P, Buckley MJ, Dumas R, Kuhdorf H, Staccini P, Demarquay JF, Caroli-Bosc FX, Delmont JP. A study of the effect of age on pancreatic duct morphology. Gastrointest Endosc. 1998;48:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |