Published online Nov 14, 2021. doi: 10.3748/wjg.v27.i42.7362
Peer-review started: March 21, 2021
First decision: April 29, 2021
Revised: May 12, 2021
Accepted: October 24, 2021
Article in press: October 24, 2021
Published online: November 14, 2021
Processing time: 233 Days and 20.3 Hours
Chronic liver disease, particularly cirrhosis, is associated with worse outcomes in patients infected with coronavirus disease 2019 (COVID-19).
To assess outcomes of COVID-19 infection among patients with pre-existing hepatitis C with or without liver cirrhosis.
This multicenter, retrospective cohort study included all cases of confirmed co-infection of severe acute respiratory syndrome coronavirus 2 and chronic hepatitis C with or without liver cirrhosis who were admitted to six hospitals (Al-Sahel Hospital, Al-Matareya Hospital, Al-Ahrar Hospital, Ahmed Maher Teaching Hospital, Al-Gomhoreya Hospital, and the National Hepatology and Tropical Medicine Research Institute) affiliated with the General Organization for Teaching Hospitals and Institutes in Egypt. Patients were recruited from May 1, 2020, to July 31, 2020. Demographic, laboratory, imaging features, and outcomes were collected. Multivariate regression analysis was performed to detect factors affecting mortality.
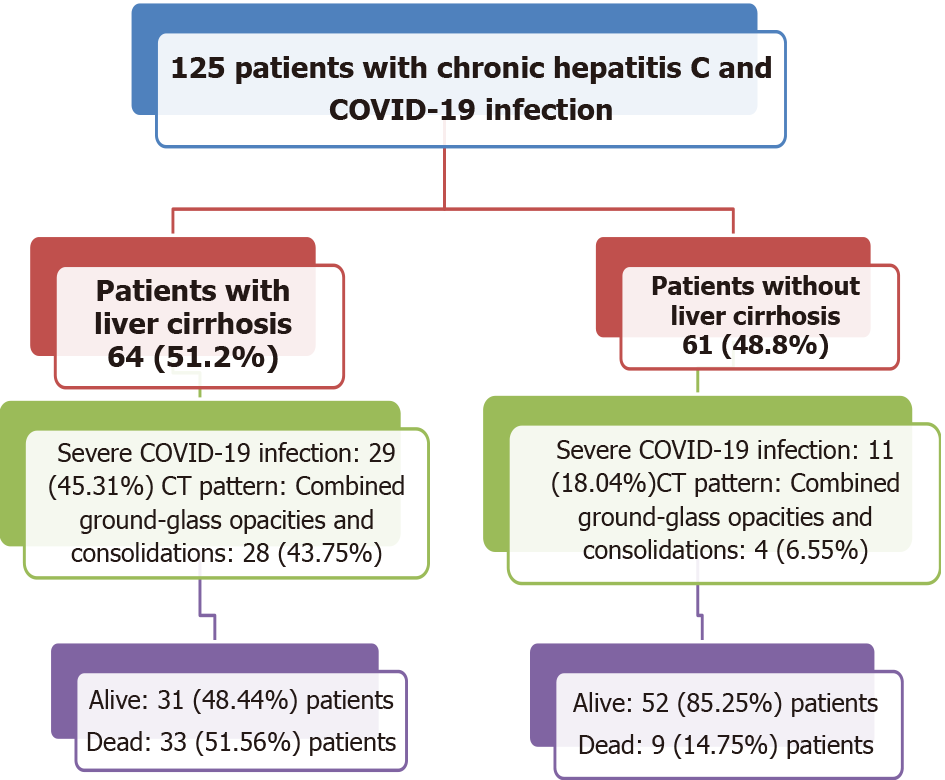
This retrospective cohort study included 125 patients with chronic hepatitis C and COVID-19 co-infection, of which 64 (51.20%) had liver cirrhosis and 40 (32.00%) died. Fever, cough, dyspnea, and fatigue were the most frequent symptoms in patients with liver cirrhosis. Cough, sore throat, fatigue, myalgia, and diarrhea were significantly more common in patients with liver cirrhosis than in non-cirrhotic patients. There was no difference between patients with and without cirrhosis regarding comorbidities. Fifteen patients (23.40%) with liver cirrhosis presented with hepatic encephalopathy. Patients with liver cirrhosis were more likely than non-cirrhotic patients to have combined ground-glass opacities and consolidations in CT chest scans: 28 (43.75%) vs 4 (6.55%), respectively (P value < 0.001). These patients also were more likely to have severe COVID-19 infection, compared to patients without liver cirrhosis: 29 (45.31%) vs 11 (18.04%), respectively (P value < 0.003). Mortality was higher in patients with liver cirrhosis, compared to those with no cirrhosis: 33 (51.56%) vs 9 (14.75%), respectively (P value < 0.001). All patients in Child-Pugh class A recovered and were discharged. Cirrhotic mortality occurred among decompensated patients only. A multivariate regression analysis revealed the following independent factors affecting mortality: Male gender (OR 7.17, 95%CI: 2.19–23.51; P value = 0.001), diabetes mellitus (OR 4.03, 95%CI: 1.49–10.91; P value = 0.006), and liver cirrhosis (OR 1.103, 95%CI: 1.037–1.282; P value < 0.0001). We found no differences in liver function, COVID-19 disease severity, or outcomes between patients who previously received direct-acting antiviral therapy (and achieved sustained virological response) and patients who did not receive this therapy.
Patients with liver cirrhosis are susceptible to higher severity and mortality if infected with COVID-19. Male gender, diabetes mellitus, and liver cirrhosis are independent factors associated with increased mortality risk.
Core Tip: Chronic liver disease, particularly cirrhosis, is associated with worse outcomes in patients infected with coronavirus disease 2019 (COVID-19). This study examined the impact of COVID-19 infection on patients with chronic hepatitis C during the first COVID-19 peak in Egypt. This retrospective cohort study was performed in six Egyptian hospitals. We found that cirrhotic patients had higher rates of pneumonia, severe COVID-19, and mortality. Cirrhotic mortality was observed among decompensated patients only. Male gender, diabetes mellitus, and liver cirrhosis were independent factors associated with increased mortality risk in Egyptian patients with COVID-19 and chronic hepatitis C.
- Citation: Afify S, Eysa B, Hamid FA, Abo-Elazm OM, Edris MA, Maher R, Abdelhalim A, Abdel Ghaffar MM, Omran DA, Shousha HI. Survival and outcomes for co-infection of chronic hepatitis C with and without cirrhosis and COVID-19: A multicenter retrospective study. World J Gastroenterol 2021; 27(42): 7362-7375
- URL: https://www.wjgnet.com/1007-9327/full/v27/i42/7362.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i42.7362
On March 11, 2020, WHO declared severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), also known as coronavirus disease 2019 (COVID-19), as the sixth pandemic of the 21st century[1]. As of January 2021, the virus has caused more than 85 million confirmed infections and about 2 million deaths worldwide[2]. In Egypt, about 140000 confirmed infections and about 8000 deaths were recorded by the Egyptian Ministry of Health and Population as of January 2021[3]. Lung lesions can cause major damage in those infected with COVID-19, and liver injury also has been reported[4]. Studies conducted in Wuhan early in the epidemic outbreak found that up to 50% of infected patients had abnormal liver enzymes. Zhang et al[5] showed that SARS-CoV-2 infection occurs in 2%–11% of patients with pre-existing liver conditions.
COVID-19’s adverse effects on the liver could be explained by both direct cytopathic injury and indirect effects of the virus[4]. It is well-established that SARS-CoV-2 uses angiotensin-converting enzyme receptors to gain entry into cells. These receptors are much more abundant in cholangiocytes (59.70%) than in hepatocytes (2.60%)[6]. The bile duct epithelium also plays an important role in regeneration after injury and immune response. Thus, the indirect effects may be due to exposure to multiple insults. For example, in severe cases admitted to intensive care, hemodynamic instability can lead to hypoperfusion and ischemic liver injury. Pneumonitis-associated hypoxia can lower mean arterial pressure, causing a synergistic effect contributing to this ischemic insult[4]. Another contributing factor could be toxic effects of medications (e.g., steroids, non-steroid anti-inflammatory drugs, antibiotics, anticoagulants, antivirals), which are associated predominantly with hepatocellular rather than cholestatic liver injury[6,7]. Finally, immune dysregulation can lead to systemic inflammatory response syndrome, or cytokine storm, which is the release of inflammatory mediators (e.g., interleukins IL-6 and IL-1, TNF- α, and interferon) that can cause and exacerbate liver injury[8].
Hepatitis C virus remains the most common etiology of overt and occult chronic liver diseases, liver cirrhosis, and risk of hepatocellular carcinoma in Egypt[9]. Chronic liver disease is associated with immune dysregulation and multiple system involvement (e.g., cardiomyopathy, hepatopulmonary syndrome, and coagulopathy)[9]. Patients in Child-Pugh classes B and C and those with higher MELD scores have much higher mortality rates than patients in class A or with lower MELD scores[10]. Importantly, the cause of death in most of these patients is respiratory failure rather than acute on top of chronic liver failure[10].
Transaminitis, which is abnormal levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), is the most frequent and direct cause of disease severity in patients without cirrhosis[4]. Decompensation with worsening liver symptoms (e.g., ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, and variceal hemorrhage) has been reported in patients with chronic liver disease and is associated with a high risk of death. Interestingly, decompensation occurs without respiratory symptoms[11]. This study aimed to demonstrate the impact of COVID-19 infection on patients with pre-existing hepatitis C with or without liver cirrhosis during the first COVID-19 peak in Egypt.
This multicenter, retrospective cohort study included all cases of confirmed co-infection of SARS-CoV-2 and chronic hepatitis C with or without liver cirrhosis who were admitted to six hospitals (Al-Sahel Hospital, Al-Matareya Hospital, Al-Ahrar Hospital, Ahmed Maher Teaching Hospital, Al-Gomhoreya Hospital, and the National Hepatology and Tropical Medicine Research Institute) in the General Organization for Teaching Hospitals and Institutes in Egypt. Patients were recruited from May 1, 2020, to July 31, 2020. The diagnosis of COVID-19 was based on a positive RT-PCR from nasopharyngeal swabs. Patients with negative RT-PCR results or those who did not undergo the swab were excluded.
The authors assert that all procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects and patients were approved by the research ethics committee of the General Organization for Teaching Hospitals and Institutes. Written informed consent was obtained from all patients.
Baseline demographic data collected included age, gender, cigarette smoking status, and comorbidities. Other information recorded included general respiratory and gastrointestinal symptoms, chest CT scans, and laboratory results (complete blood count, liver and renal function, coagulation, D-dimer, ferritin, and C-reactive protein). We also included treatments administered to the patients and COVID-19 disease classification and outcome. COVID-19 severity was categorized as mild, moderate, or severe, according to the management protocol of the Egyptian Ministry of Health and Population[12]. Mild cases were symptomatic with lymphopenia or leucopenia and no radiological lung affection by pneumonia. Moderate cases were symptomatic with radiological features of pneumonia with or without leucopenia and lymphopenia. Severe and critical cases included any of the following: respiratory rate > 30 per minute; SaO2 < 92 in room air; PaO2/FiO2 ratio < 300; chest radiology showing > 50% lung affection or progressive lung affection within 24 to 48 h; or critically ill at SaO2 < 92, respiratory rate > 30 per minute, or PaO2/FiO2 ratio < 200 despite oxygen therapy. Severe and critical cases were indicated for intensive care unit (ICU) admission. Treatments were applied according to the protocol[12].
Data were analyzed using SPSS version 25 (SPSS Inc., Chicago, IL, United States). Numerical data are expressed as mean and standard deviation or median and range, as appropriate. Qualitative data are expressed as frequency and percentage. The Chi-square test (Fisher’s exact test) was used to examine the relation between qualitative variables. For quantitative data, Student’s t-test or the Mann-Whitney test (non-parametric t-test) was used to compare groups, as appropriate. A P value ≤ 0.05 was considered significant. To study possible associations between selected variables (gender, liver cirrhosis, and diabetes mellitus) and mortality, we fitted multiple logistic regression models. The results are expressed as the odds ratio (OR) with 95% confidence interval (CI).
This study included 125 patients infected with chronic hepatitis C virus and COVID-19 (Figure 1). Of those, 64 (51.2%) patients had liver cirrhosis: 25 (39.06%) were classified as Child-Pugh class A, 22 (34.38%) as class B, and 17 (26.56%) as class C. Patient residences included five Egyptian governorates: Cairo, Giza, Al-Behera, Al-Qalubya, and Al-Menofeya. Over half (61.2%) were older than 60 years, and most (68.8%) were men. Table 1 presents the baseline demographic features. Regarding COVID-19 symptoms, five (4.0%) patients were asymptomatic. The most common symptoms were dyspnea (80 patients, 64.0%), fever (78 patients, 62.4%), and cough (55 patients, 44.0%), and five (4.0%) had diarrhea. Table 2 summarizes the patient symptoms. Table 3 summarizes the baseline laboratory results and treatment protocols among the studied patients.
| Item | n (%) |
| Egyptian governorate | |
| Cairo | 78 (62.4) |
| Al-Menofeya | 17 (13.6) |
| Giza | 14 (11.2) |
| Al-Qalubya | 9 (7.2) |
| Al-Behera | 7 (5.6) |
| Age (yr) | |
| 20 to 30 | 1 (0.8) |
| 30 to 40 | 6 (4.8) |
| 40 to 50 | 10 (8.1) |
| 50 to 60 | 31 (25.0) |
| 60 to 70 | 35 (28.2) |
| 70 to 80 | 35 (28.2) |
| 80 to 90 | 6 (4.8) |
| Gender | |
| Male | 86 (68.8) |
| Female | 39 (31.2) |
| Cigarette smoking | 10 (8.0) |
| History of contact with COVID-19 case | 32 (25.6) |
| Diabetes mellitus | 52 (41.6) |
| Hypertension | 52 (41.6) |
| Direct-acting antiviral therapy treatment | |
| Not previously treated | 108 (86.4) |
| Sustained virological response | 17 (13.6) |
| COPD | 1 (0.8) |
| Coronary artery disease | 12 (9.6) |
| Acute kidney injury | 1 (0.8) |
| Chronic renal insufficiency | 8 (6.4) |
| Heart failure | 2 (1.6) |
| Bronchial asthma | 1 (0.8) |
| CT pattern | 1 (0.8) |
| Consolidations and ground-glass opacities | 31 (24.8) |
| Ground-glass opacities | 94 (75.2) |
| Lesion distribution on CT | |
| Bilateral | 122 (97.6) |
| Unilateral | 3 (2.4) |
| COVID-19 case severity | |
| Moderate | 86 (68.8) |
| Severe | 39 (31.2) |
| Admission zone | |
| ICU | 24 (19.2) |
| Intermediate care | 6 (4.8) |
| Ward | 95 (76.0) |
| Symptom | n (%) |
| Dyspnea | 80 (64.0) |
| Fever | 78 (62.4) |
| Cough | 55 (44.0) |
| Fatigue | 26 (20.8) |
| Disturbed consciousness | 22 (17.6) |
| Sore throat | 15 (12.0) |
| Newly developed hepatic encephalopathy | 15 (12.0) |
| Hepatocellular carcinoma | 15 (12.0) |
| Myalgia | 12 (9.6) |
| Current ascites | 9 (7.2) |
| History of hematemesis from esophageal varices | 9 (7.2) |
| Asymptomatic | 5 (4.0) |
| Diarrhea | 5 (4.0) |
| Arthralgia | 5 (4.0) |
| Anorexia | 4 (3.2) |
| Nausea | 3 (2.4) |
| Vomiting | 3 (2.4) |
| Loss of taste | 2 (1.6) |
| Hemoptysis | 1 (0.8) |
| Abdominal pain | 1 (0.8) |
| Loss of smell | 1 (0.8) |
| Jaundice | 1 (0.8) |
| Rhinorrhea | 1 (0.8) |
| mean ± SD/n (%) | |
| Pulse rate | 92 ± 17 |
| Temperature | 37 ± 1 |
| Respiratory rate | 23 ± 5 |
| Oxygen saturation | 93 ± 5 |
| Hemoglobin (gm/dL) | 12 ± 2 |
| Platelet count (× 1000/cmm) | 183 ± 107 |
| Total leucocyte count (× 1000/cmm) | 10 ± 8 |
| Neutrophil : Lymphocyte ratio | 1 ± 0 |
| INR | 1.16 ± 0.29 |
| PTT | 33.9 ± 13.2 |
| Serum creatinine (mg/dL) | 1.39 ± 1.43 |
| Serum sodium (mEq/L) | 137 ± 9 |
| Serum potassium (mEq/L) | 4.18 ± 0.77 |
| Total bilirubin (mg/dL) | 3 ± 5 |
| Direct bilirubin (mg/dL) | 2.1 ± 3.8 |
| Serum albumin (g/dL) | 3.2 ± 0.7 |
| Alanine Transaminase (U/L) | 58 ± 53 |
| Aspartate Transaminase (U/L) | 92 ± 123 |
| Alkaline phosphatase (U/L) | 262 ± 226 |
| Serum ferritin (ng/mL) | 667 ± 483 |
| D-dimer (mg/mL) | 1560 ± 2503 |
| Fibrinogen (mg/dL) × 100 if presented in g/L | 77 |
| Azithromycin | 72 (57.6) |
| Paracetamol | 45 (36.0) |
| Supplementary vitamin C | 85 (68.0) |
| Zinc | 69 (55.2) |
| Colchicine | 2 (1.6) |
| Lactoferrin | 18 (14.4) |
| Other antibiotics | 71 (56.8) |
| Steroids | 38 (30.4) |
| Hydroxyl-chloroquine | 29 (23.2) |
| Low-molecular weight heparin | 56 (44.8) |
| Warfarin | 3 (2.4) |
We compared the characteristics of patients with and without liver cirrhosis. Fever, cough, dyspnea, and fatigue were the most frequent symptoms in patients with liver cirrhosis. Cough, sore throat, fatigue, myalgia, and diarrhea were significantly more common in patients with liver cirrhosis than non-cirrhotic patients. There was no difference between patients with and without cirrhosis regarding comorbidities. Fifteen (23.4%) patients with liver cirrhosis presented with hepatic encephalopathy (Table 4).
| Variable | No liver cirrhosis61 (48.8%) | Liver cirrhosis 64 (51.2%) | P value | |
| Gender | Male | 42 (68.9%) | 44 (68.8%) | 0.57 |
| Female | 19 (31.1%) | 20 (31.3%) | ||
| Age (yr) | 20 to 30 | 0 (0.0%) | 1 (1.6%) | 0.004 |
| 30 to 40 | 3 (4.9%) | 3 (4.8%) | ||
| 40 to 50 | 0 (0.0%) | 10 (15.9%) | ||
| 50 to 60 | 19 (31.1%) | 12 (19.0%) | ||
| 60 to 70 | 14 (23.0%) | 21 (33.3%) | ||
| 70 to 80 | 21 (34.4%) | 14 (22.2%) | ||
| 80 to 90 | 4 (6.6%) | 2 (3.2%) | ||
| Cigarette smoking | 2 (3.2%) | 8 (11.5%) | 0.06 | |
| Diabetes mellitus | 24 (39.3%) | 28 (43.8%) | 0.71 | |
| Hypertension | 23 (37.7%) | 29 (45.3%) | 0.46 | |
| COPD | 0 (0.0%) | 1 (1.6%) | 1 | |
| Coronary artery disease | 5 (8.2%) | 7(10.9%) | 0.76 | |
| Chronic renal insufficiency | 4 (6.6%) | 4 (6.3%) | 1 | |
| Heart failure | 0 | 2 (3.1%) | 0.49 | |
| Hepatocellular carcinoma | 3 (4.9%) | 12 (18.8%) | 0.026 | |
| Esophageal varices | 0 (0.0%) | 9 (14.1%) | 0.003 | |
| Asymptomatic | 1 (1.6%) | 4 (6.3%) | 0.36 | |
| Fever | 35 (57.4%) | 43 (67.2%) | 0.27 | |
| Cough | 18 (29.5% | 37 (57.8%) | 0.002 | |
| Dyspnea | 44 (72.1%) | 36 (56.3%) | 0.09 | |
| Sore throat | 2 (3.3%) | 13 (20.3%) | 0.005 | |
| Hemoptysis | 0 (0.0%) | 1 (1.6%) | 1 | |
| Fatigue | 5 (8.2%) | 21 (32.8%) | 0.001 | |
| Anorexia | 1 (1.6%) | 3 (4.7%) | 0.62 | |
| Diarrhea | 0 (0.0%) | 5 (7.8%) | 0.05 | |
| Nausea | 1 (1.6%) | 2 (3.1%) | 1 | |
| Vomiting | 1 (1.6%) | 2 (3.1%) | 1 | |
| Abdominal pain | 1 (1.6%) | 0 (0.0%) | 0.48 | |
| Arthralgia | 1 (1.6%) | 4 (6.3%) | 0.36 | |
| Myalgia | 1 (1.6%) | 11 (17.2%) | 0.004 | |
| Loss of taste | 0 (0.0%) | 2 (3.1%) | 0.49 | |
| Loss of smell | 0 (0.0%) | 1 (1.6%) | 1 | |
| Disturbed consciousness | 3 (4.9%) | 19 (29.7%) | 0.001 | |
| Hepatic encephalopathy | 0 (0.0%) | 15 (23.4%) | 0.033 | |
| Direct-acting antiviral therapy with sustained virological response before COVID-19 infection | 7 (11.5%) | 10 (15.6%) | 0.61 | |
Patients with liver cirrhosis were more likely to show combined ground-glass opacities and consolidations in their chest CT scans: 28 (43.75%) vs 4 (6.55%), respectively (P value < 0.001). These patients also were more likely to present with severe COVID-19 infection: 29 (45.31%) vs 11 (18.04%), respectively (P-value 0.003), compared to patients without liver cirrhosis. Mortality was higher in patients with liver cirrhosis: 33 (51.56%) vs 9 (14.75%), respectively (P value < 0.001) (Table 5). All patients classified as Child-Pugh class A recovered and were discharged, whereas 18 (45%) mortalities occurred among patients considered class B and 15 (37.5%) among those considered class C.
| No liver cirrhosis 61 (48.8%) | Liver cirrhosis 64 (51.2%) | P value | |||
| Hemoglobin (mg/dL) | 12 ± 2 | 12 ± 2 | 0.85 | ||
| Platelet count (× 1000/cmm) | 193 ± 107 | 171 ± 107 | 0.36 | ||
| TLC (× 1000/cmm) | 12 ± 10 | 9 ± 5 | 0.31 | ||
| Serum creatinine (mg/dL) | 1.58 ± 1.89 | 1.21 ± 0.76 | 0.59 | ||
| Total bilirubin (mg/dL) | 3 ± 4 | 3 ± 5 | 0.79 | ||
| Serum albumin (g/dL) | 3.3 ± 0.7 | 3.1 ± 0.6 | 0.36 | ||
| Alanine transaminase (U/L) | 67 ± 67 | 50 ± 40 | 0.21 | ||
| Aspartate transaminase (U/L) | 60 ± 37 | 101 ± 137 | 0.89 | ||
| CT pattern | Consolidations and ground-glass opacities | 4 (6.55%) | 28 (43.75%) | < 0.001 | |
| Ground-glass opacities | 57 (93.45%) | 36 (56.25%) | |||
| Lesion distribution on CT | Bilateral | 58 (95.1%) | 64 (100.0%) | 0.11 | |
| Unilateral | 3 (4.9%) | 0 | |||
| COVID-19 severity | Moderate | 50 (81.96%) | 35 (54.69%) | 0.003 | |
| Severe | 11 (18.04%) | 29 (45.31%) | |||
| Azithromycin | 33 (54.1%) | 39 (60.9%) | 0.47 | ||
| Paracetamol | 16 (26.2%) | 29 (45.3%) | 0.04 | ||
| Supplementary vitamin C | 37 (60.7%) | 48 (75.0%) | 0.12 | ||
| Supplementary zinc | 33 (54.1%) | 36 (56.3%) | 0.85 | ||
| Lactoferrin | 3 (4.9%) | 15 (23.4%) | 0.004 | ||
| Other antibiotics | 28 (45.9%) | 43 (67.2%) | 0.019 | ||
| Anticoagulants | LMWH | 28 (45.9%) | 28 (43.8%) | 1 | |
| Warfarin | 1 (1.6%) | 2 (3.1%) | 1 | ||
| Steroids | 16 (26.2%) | 22 (34.4%) | 0.339 | ||
| Alive (discharged) | 52 (85.25%) | 31 (48.44%) | < 0.001 | ||
| Died at the hospital | 9 (14.75%) | 33 (51.56%) | |||
A multivariate logistic regression revealed that male patients were more likely than female patients to die, after adjusting for liver cirrhosis and diabetes mellitus status (OR 7.166, 95%CI: 2.185–23.506; P value 0.001). Mortality was four times more likely among patients with diabetes mellitus, after adjusting for other factors in the model (OR 4.029, 95%CI: 1.488–10.906; P value 0.006). Patients with liver cirrhosis also were more likely to die (OR 1.103, 95%CI: 1.037–1.282; P value 0.0001), compared to those without cirrhosis (Table 6).
| B | S.E. | P value | Odds ratio | 95%CI | ||
| Lower | Upper | |||||
| Male | 1.969 | 0.606 | 0.001 | 7.166 | 2.185 | 23.506 |
| Diabetes mellitus | 1.393 | 0.508 | 0.006 | 4.029 | 1.488 | 10.906 |
| Liver cirrhosis | 2.274 | 0.515 | 0.0001 | 1.103 | 1.037 | 1.282 |
| Constant | -1.879 | 0.604 | 0.002 | 0.153 | ||
Within our cohort, 17 (13.6%) patients received direct-acting antiviral therapy (DAA) and achieved sustained virological response before acquiring COVID-19 infection. Among them, 10 (15.6%) patients had liver cirrhosis and seven (11.5%) did not. Three (17.6%) DAA recipients had severe COVID-19 disease, and the rest had moderate COVID-19 disease on admission. Regarding liver function, COVID-19 disease severity, and outcome, we found no difference between patients who previously received DAA and those who did not (Table 7).
| Did not receive previous DAA (n = 108) | Received previous DAA (n = 17) | P value | ||
| COVID-19 severity | Moderate | 72 (66.7%) | 14 (82.4%) | 0.26 |
| Severe | 36 (33.3%) | 3 (17.6%) | ||
| Admission zone | ICU | 22 (20.4%) | 2 (11.8%) | 0.52 |
| Intermediate care | 6 (5.6%) | 0 (0.0%) | ||
| Ward | 80 (74.1%) | 15 (88.2%) | ||
| Vital status | Alive | 73 (67.6%) | 12 (70.6%) | 1 |
| Dead | 35 (32.4%) | 5 (29.4%) | ||
| Total bilirubin (mg/dL) | 1.00 (1-20) | 1.30 (1-6) | 0.84 | |
| Direct bilirubin (mg/dL) | 0.9 (0.2-15.6) | 0.9 (0.1-2.7) | 0.93 | |
| Serum albumin (g/dL) | 3.1 (1.7-5) | 3.15 (2.4-3.8) | 0.45 | |
| Alanine transaminase (U/L) | 41.5 (13-324) | 49 (18-175) | 0.54 | |
| Aspartate transaminase (U/L) | 46.5 (10- 549) | 53 (16-415) | 0.42 | |
Little research has assessed outcomes of patients co-infected with chronic hepatitis C virus and COVID-19, and existing studies include either a small number of patients or report data from patients with chronic liver disease and multiple underlying etiologies (viral and non-viral). This is the first Egyptian study reporting the outcome of SARS-CoV-2 infection in patients with isolated chronic hepatitis C as the etiology of their underlying chronic liver disease. We found a substantially increased incidence (23.43%) of hepatic encephalopathy in our cirrhotic patients infected with COVID-19. The 1-year cumulative incidence of hepatic encephalopathy in liver cirrhosis ranges from 0% to 21%[13]. We also found that the severity of COVID-19 symptoms and mortality rates were significantly higher in patients with liver cirrhosis. All patients assigned to Child-Pugh class A recovered and were discharged, but mortalities occurred among patients assigned to Child-Pugh class B or class C on admission. Male gender, diabetes mellitus, and liver cirrhosis were independent factors affecting mortality in our cohort.
In our study, fever (67.2%), cough (57.8%), dyspnea (56.3%), and fatigue (32.8%) were the most frequent symptoms in patients with liver cirrhosis, followed by diarrhea (7.8%). Among patients with cirrhosis, 6.3% were asymptomatic. Iavarone et al[14] studied 50 patients with liver cirrhosis with hepatitis C, hepatitis B, non-viral (e.g., alcoholic), or multiple etiologies and reported fever in 64%, fatigue in 60%, dyspnea in 42%, and cough in 36%, followed by diarrhea in 10% and no symptoms in 12% of patients. In our study, 15 (23.4%) patients with liver cirrhosis presented with hepatic encephalopathy, compared with 11 (22%) of patients in Iavarone et al[14]. For ALT levels in our cohort, 58 (46.4%) were normal, 40 (32%) were elevated 1-2 times above the upper limit of normal, 7 (5.6%) were elevated 2-3 times above the upper limit, 20 (16%) were three times above normal, and 1 patient was five times over the upper limit. Iavarone et al[14] define ALT elevation at five times over the upper limit of normal as hepatic flare.
Our results revealed higher mortality among male patients, compared to female patients. Nasiri et al[15] similarly reported COVID-19-related mortality to be higher among males, and cohorts from China, Italy, Denmark, and the United States confirmed these findings[16-20]. Underlying sex-related mechanisms could include chromosomal immunological response, lifestyle (alcohol, smoking, and obesity), and comorbidities[19]. We also found that mortality was significantly higher in patients with liver cirrhosis, particularly those with decompensated cirrhosis. Boettler et al[21] reported that chronic viral hepatitis did not seem to raise the risk of a severe COVID-19 in a study by Guan et al[22], which included patients in China with chronic hepatitis B only. Shalimar et al[23] found no difference in mortality among patients with and without cirrhosis, but their sample size was small. An Italian study by Mangia et al[24] found that cirrhosis of metabolic origin, older age, leucopenia, and lymphopenia were risk factors for mortality. They also suggested that the very low prevalence of SARS-CoV-2 infection in patients with chronic hepatitis C infection could play a protective role against SARS-CoV-2 infection.
A meta-analysis by Váncsa et al[25], which included mainly studies of chronic hepatitis B in China, reported that liver failure and platelet count could predict in-hospital mortality with high specificity and lactate dehydrogenase with moderate specificity. Singh et al[26] found that patients with cirrhosis had a higher relative risk of mortality and a higher risk of hospitalization, compared to patients without liver disease. Another study by Galiero et al[27] described outcomes in 35 patients with liver cirrhosis, though they did not compare cirrhotic vs non-cirrhotic patients, they found that male sex, chronic liver disease, and malignancies were independent factors of poor prognosis in hospitalized patients with COVID-19. They also reported that patients with advanced chronic liver disease had worse clinical conditions compared to patients with no liver disease[27].
In a retrospective multicenter study from 16 hospitals in China that included 21 patients with COVID-19 and hepatitis B virus-related liver cirrhosis (Child-Pugh classes A, B, and C in 16, 3, and 2 cases, respectively), mortalities occurred in patients assigned to class A (3; 60.0%) and C (2; 40.0%)[10]. In another multinational cohort study of 745 patients from 29 countries, Marjot et al[28] found that liver cirrhosis was present in 386 patients: 171 (44%) in Child-Pugh class A, 124 (32%) in class B, and 91 (24%) in class C. Mortality rates significantly increased with worsening scores: 33 (19%) in class A, 44 (35%) in class B, and 46 (51%) in class C died. Age, Child-Pugh class, and alcoholic liver disease were the independent factors affecting mortality in their study, and hepatic encephalopathy occurred in 104 (27%) of their patients[28]. Another study from 13 Asian countries studied 43 patients with liver cirrhosis and reported worsening liver disease and increased hepatic complications in patients with COVID-19 infection (P value < 0.05); they found that a baseline Child-Pugh score ≥ 9 was associated with higher mortality (area under the ROC 0.94, hazard ratio 19.2, 95%CI: 2.3–163.3; P value < 0.001). The independent factors affecting mortality in their study were increased serum bilirubin and AST/ALT ratio[29].
The exact mechanism causing poor outcomes among patients with COVID-19 and preexisting liver disease remains unknown; however, interactions between local liver injury and systemic disturbances seem likely culprits, especially in patients with cirrhosis. Patients with liver cirrhosis are theoretically more susceptible to poor outcomes from COVID-19-related liver injury. Moreover, patients with advanced liver disease exhibit immune deficiency and systemic inflammation, as reflected by activated circulating immune cells and increased serum levels of pro-inflammatory cytokines. These factors can predispose this population to cytokine storms[26]. Furthermore, some cirrhotic patients may have an underlying hepato-pulmonary syndrome, portopulmonary hypertension, or hydrothorax, which can increase the risk of respiratory failure, as indicated in a study by Oyelade et al[30].
Sun et al[31] suggested SARS-CoV-2-related direct cytotoxicity (i.e., severe inflammatory response leading to immune-mediated liver damage), hypoxic hepatitis due to anoxia (particularly in patients with severe COVID-19), drug‐induced liver injury (especially related to the use of antiviral agents such as lopinavir, ritonavir, remdesivir, chloroquine, tocilizumab, umifenovir, and traditional Chinese preparations), and reactivation of pre-existing chronic liver disease as possible mechanisms of hepatic injury. They also found that liver biopsy specimens from deceased patients with severe COVID-19 showed moderate microvascular steatosis and mild lobular and portal activity, indicating that liver injury could have been caused by either SARS-CoV-2 infection or drug-induced liver injury[31].
A limitation to our study is its retrospective nature and the limited number of patients. It remains unknown whether liver injuries and mortalities among patients with chronic hepatitis C and COVID-19 co-infection are due to patients’ pre-existing chronic conditions or the impact of SARS-CoV-2 infection. We found that patients with decompensated hepatitis C-related cirrhosis were at higher risk of COVID-19-related mortality. Hepatic encephalopathy could be the presentation of underlying SARS-CoV-2 infection in patients with liver cirrhosis. The underlying etiology of chronic liver disease also could impact COVID-19 disease course and outcome. Further studies comparing outcomes and prognostic factors in patients with isolated underlying etiologies of chronic liver disease are therefore encouraged, rather than combining them into one group, which could obscure relevant risk factors for disease severity and outcomes.
In Egypt, among patients with chronic hepatitis C who also were infected with COVID-19, those with cirrhosis had higher rates of pneumonia, severe COVID-19, and mortality, though cirrhotic mortality occurred among decompensated patients only. Male gender, diabetes mellitus, and liver cirrhosis were the independent factors affecting mortality in these patients.
Coronavirus disease 2019 (COVID-19) disease severity and outcomes are affected by pre-existing chronic liver disease, particularly cirrhosis. Patients with decompensated liver cirrhosis (Child-Pugh classes B and C) are severely affected, with higher mortality rates than patients with compensated disease.
Comprehensive research on the outcome of COVID-19 in patients with isolated etiology of pre-existing chronic liver disease is needed to understand the clinical presentations and outcomes.
This study aimed to demonstrate the impact of COVID-19 factors affecting mortality among patients with pre-existing hepatitis C with or without liver cirrhosis during the first peak of the pandemic in Egypt.
This multicenter retrospective cohort study included 125 patients with COVID-19 at six quarantine hospitals in Egypt from May 1, 2020, to July 31, 2020. Clinical, laboratory features, COVID-19 severity, and outcomes were recorded. A regression analysis was performed to detect factors affecting mortality.
Fever, cough, dyspnea, and fatigue were the most frequent symptoms in patients with liver cirrhosis. Cough, sore throat, fatigue, myalgia, and diarrhea were significantly more common in patients with liver cirrhosis than in non-cirrhotic patients. Fifteen (23.4%) patients with liver cirrhosis presented with hepatic encephalopathy. Patients with liver cirrhosis were more likely to exhibit combined ground-glass opacities and consolidations in their chest CT scans and more likely to present with severe COVID-19 infection, compared to patients without liver cirrhosis. Mortality was higher among patients with liver cirrhosis: 33 (51.56%) vs 9 (14.75%), respectively (P value < 0.001. All patients in Child-Pugh class A recovered and were discharged, and mortalities occurred among patients in Child-Pugh classes B and C. A multivariate logistic regression revealed that male gender, diabetes mellitus, and liver cirrhosis were independent factors affecting mortality. Regarding liver function, COVID-19 disease severity, and outcomes, we found no difference between patients who previously received direct acting antiviral therapy (and achieved sustained virological response) and patients who did not receive such therapy.
Patients with decompensated hepatitis C virus-related liver cirrhosis are at higher risk of severe COVID-19 disease and mortality. Male gender, diabetes mellitus, and liver cirrhosis are the independent factors affecting mortality.
Male gender, diabetes mellitus, and liver cirrhosis significantly increased mortality in patients with COVID-19 and isolated hepatitis C virus-related chronic liver disease. Previous achievement of sustained virological response after direct acting antiviral therapy for chronic hepatitis C does not impact COVID-19 disease severity, outcome, or the results of liver function tests.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Egyptian Association for Research and Training in Hepatogastroenterology, Shimaa Afify; United European Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beraldo RF, Gupta T S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | World Health Organization. [cited March 22, 2020] Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020. |
| 2. | WorldOmeter. World / Countries / Egypt. Last updated: September 28, 2021. Available from: https://www.worldometers.info/coronavirus/country/egypt/. |
| 3. | Anirvan P, Bharali P, Gogoi M, Thuluvath PJ, Singh SP, Satapathy SK. Liver injury in COVID-19: The hepatic aspect of the respiratory syndrome - what we know so far. World J Hepatol. 2020;12:1182-1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 4. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30112] [Article Influence: 6022.4] [Reference Citation Analysis (3)] |
| 5. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 6. | Kumar P, Sharma M, Kulkarni A, Rao PN. Pathogenesis of Liver Injury in Coronavirus Disease 2019. J Clin Exp Hepatol. 2020;10:641-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Cabibbo G, Rizzo GEM, Stornello C, Craxì A. SARS-CoV-2 infection in patients with a normal or abnormal liver. J Viral Hepat. 2021;28:4-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 9. | Strickland GT, Elhefni H, Salman T, Waked I, Abdel-Hamid M, Mikhail NN, Esmat G, Fix A. Role of hepatitis C infection in chronic liver disease in Egypt. Am J Trop Med Hyg. 2002;67:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Qi X, Liu Y, Wang J, Fallowfield JA, Li X, Shi J, Pan H, Zou S, Zhang H, Chen Z, Li F, Luo Y, Mei M, Liu H, Wang Z, Li J, Yang H, Xiang H, Liu T, Zheng MH, Liu C, Huang Y, Xu D, Kang N, He Q, Gu Y, Zhang G, Shao C, Liu D, Zhang L, Kawada N, Jiang Z, Wang F, Xiong B, Takehara T, Rockey DC; COVID-Cirrhosis-CHESS Group. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut. 2021;70:433-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 11. | Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73:705-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 12. | Ministry of Health and Population, Egypt. Management protocol for COVID-19 Patients. Version 1.4 / 30th May 2020. Available from: https://www.elwatannews.com/data/iframe/pdf/17175200761591035127.pdf. |
| 13. | Elsaid MI, Rustgi VK. Epidemiology of Hepatic Encephalopathy. Clin Liver Dis. 2020;24:157-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 14. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 15. | Galbadage T, Peterson BM, Awada J, Buck AS, Ramirez DA, Wilson J, Gunasekera RS. Systematic Review and Meta-Analysis of Sex-Specific COVID-19 Clinical Outcomes. Front Med (Lausanne). 2020;7:348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 16. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6517] [Article Influence: 1303.4] [Reference Citation Analysis (0)] |
| 17. | Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1653] [Cited by in RCA: 2124] [Article Influence: 424.8] [Reference Citation Analysis (0)] |
| 18. | Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1590] [Cited by in RCA: 1820] [Article Influence: 364.0] [Reference Citation Analysis (1)] |
| 19. | Kragholm K, Andersen MP, Gerds TA, Butt JH, Østergaard L, Polcwiartek C, Phelps M, Andersson C, Gislason GH, Torp-Pedersen C, Køber L, Schou M, Fosbøl EL. Association between male sex and outcomes of Coronavirus Disease 2019 (Covid-19) - a Danish nationwide, register-based study. Clin Infect Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 20. | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2289] [Cited by in RCA: 2550] [Article Influence: 510.0] [Reference Citation Analysis (2)] |
| 21. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 22. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18875] [Article Influence: 3775.0] [Reference Citation Analysis (7)] |
| 23. | Shalimar, Elhence A, Vaishnav M, Kumar R, Pathak P, Soni KD, Aggarwal R, Soneja M, Jorwal P, Kumar A, Khanna P, Singh AK, Biswas A, Nischal N, Dar L, Choudhary A, Rangarajan K, Mohan A, Acharya P, Nayak B, Gunjan D, Saraya A, Mahapatra S, Makharia G, Trikha A, Garg P. Poor outcomes in patients with cirrhosis and Corona Virus Disease-19. Indian J Gastroenterol. 2020;39:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Mangia A, Cenderello G, Verucchi G, Ciancio A, Fontana A, Piazzolla V, Minerva N, Squillante MM, Copetti M. Is positivity for hepatitis C virus antibody predictive of lower risk of death in COVID-19 patients with cirrhosis? World J Clin Cases. 2020;8:5831-5834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 25. | Váncsa S, Hegyi PJ, Zádori N, Szakó L, Vörhendi N, Ocskay K, Földi M, Dembrovszky F, Dömötör ZR, Jánosi K, Rakonczay Z Jr, Hartmann P, Horváth T, Erőss B, Kiss S, Szakács Z, Németh D, Hegyi P, Pár G. Pre-existing Liver Diseases and On-Admission Liver-Related Laboratory Tests in COVID-19: A Prognostic Accuracy Meta-Analysis With Systematic Review. Front Med (Lausanne). 2020;7:572115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 27. | Galiero R, Pafundi PC, Simeon V, Rinaldi L, Perrella A, Vetrano E, Caturano A, Alfano M, Beccia D, Nevola R, Marfella R, Sardu C, Coppola C, Scarano F, Maggi P, De Lucia Sposito P, Vocciante L, Rescigno C, Sbreglia C, Fraganza F, Parrella R, Romano A, Calabria G, Polverino B, Pagano A, Bologna C, Amitrano M, Esposito V, Coppola N, Maturo N, Adinolfi LE, Chiodini P, Sasso FC; COVOCA Study Group. Impact of chronic liver disease upon admission on COVID-19 in-hospital mortality: Findings from COVOCA study. PLoS One. 2020;15:e0243700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 29. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 30. | Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis. Trop Med Infect Dis. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 31. | Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |