Published online Nov 14, 2021. doi: 10.3748/wjg.v27.i42.7350
Peer-review started: January 30, 2021
First decision: June 4, 2021
Revised: July 12, 2021
Accepted: October 24, 2021
Article in press: October 24, 2021
Published online: November 14, 2021
Processing time: 283 Days and 12.2 Hours
Coronavirus disease 2019 (COVID-19) infection is known to cause abnormal hepatic enzymes. The long term consequences of such elevations are uncertain.
To assessed the prevalence and prognostic value of initial liver enzymes in a large cohort of COVID-19 patients.
We reviewed electronic medical records of 10614 COVID-19 patients without known chronic liver disease who were admitted to our health system from March 1, 2020, to April 30, 2020. We analyzed baseline demographics and liver chemistries. The primary outcome was in-hospital mortality, and the secondary outcome was a composite of in-hospital mortality or need for mechanical ventilation.
Subjects with abnormal liver tests had increased risks of mortality and composite outcome when compared to patients with normal measurements on unadjusted analysis and after adjustment for demographic factors.
In our diverse patient population, liver enzyme abnormalities are associated with increased mortality and the need for mechanical ventilation in subjects without chronic liver disease. Cholestasis patients are at the greatest risk for poor outcomes.
Core Tip: We believe that our paper is an important contribution to the literature for the following reasons: (1) The cohort size is the largest to date; (2) We show the importance of initial liver tests in predicting outcomes; (3) In this large cohort, the finding of initial cholestatic pattern of injury being most predictive of poor outcome has not yet been described; and (4) This is a cohort from a large urban health system in the United States (New York) whose subject demographics reflect more the population seen in the United States. The other publications listed below are more uniform populations not representative of what our practitioners see in daily practice
- Citation: Bernstein D, Roth N, Kim A, Epstein M, Hirschwerk D, Kvasnovsky CL, Satapathy SK. Presentation, patterns and predictive value of baseline liver tests on outcomes in COVID-19 patients without chronic liver disease. World J Gastroenterol 2021; 27(42): 7350-7361
- URL: https://www.wjgnet.com/1007-9327/full/v27/i42/7350.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i42.7350
The outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first reported in Wuhan, China[1], was declared a global health emergency in January 2020 by the World Health Organization (WHO)[2].
Liver enzymes have been reported to be elevated in 15%-76% of patients with COVID-19 infection[3-14]. While most reported liver enzyme abnormalities have been mild, severe acute hepatitis and severe cholestasis has been reported secondary to COVID-19 infection[15,16]. The degree of enzyme elevation and patterns of liver enzymes can indicate patients’ outcomes such as the need for mechanical ventilation and in-hospital mortality[3,12-17] but are not well described in a large, multi-ethnic cohort. Our study reports the results of our analysis of these factors in the largest cohort to date of hospitalized adults with COVID-19 infection without chronic liver disease.
We obtained the medical records and compiled data from our electronic medical record on all patients with documented COVID-19 infection who were admitted to 12 hospitals in New York City, Long Island, and Westchester County, New York, within the Northwell Health system from the period of March 1, 2020, to April 30, 2020. A confirmed case was defined as a positive reverse transcriptase polymerase chain reaction for SARS-CoV-2 on a specimen obtained through nasopharyngeal swabbing, including if an initial test result was negative but repeat testing was positive. We collected the following demographic information: Age, sex, race, ethnicity, presence of co-morbid conditions, and body mass index (BMI). Race and ethnicity data were collected by self-report in pre-specified fixed categories. Baseline laboratory testing was defined as the first measurement available within 24 h of presentation. For this study, we excluded children under 18 years of age, patients missing a baseline value for serum alanine aminotransferase (ALT), and patients with known chronic liver disease. Exclusion for chronic liver disease was based on initial identification of this group of patients using ICD-10 codes and followed by manual chart review to confirm a diagnosis of chronic liver disease.
This study was supported by the Northwell Health COVID-19 Research Consortium and was approved by the Institutional Review Board for the Feinstein Institutes of Medical Research at Northwell Health as minimal-risk research using data collected for routine clinical practice, with the requirement for informed consent waived.
Liver enzymes, namely, serum levels of aspartate aminotransferase (AST), ALT, alkaline phosphatase, and total bilirubin levels were stratified into 4 groups: (1) Within normal limits; (2) Greater than the upper limit of normal (ULN) to less than or equal to 4 times the ULN; (3) Greater than 4 times the ULN to less than or equal to 10 times the ULN; and (4) Greater than 10 times the ULN. As per the American Association for the Study of Liver Diseases expert consensus recommendations, we defined the ULN for ALT as 25 U/L for women and 35 U/L for men[18]. The ULNs of other liver chemistries were defined using standard definitions and based on our health system laboratory’s ULNs, which were: 40 U/L for AST, 125 U/L for alkaline phosphatase, and 1.2 mg/dL for total bilirubin. We classified patients who had more severe liver enzyme abnormalities into three patterns of liver injury: Hepatocellular (defined as ALT > 3 × ULN and alkaline phosphatase ≤ 2 × ULN), cholestatic (defined as alkaline phosphatase > 2 × ULN and ALT ≤ 3 × ULN), or mixed (defined as ALT > 3 × ULN and alkaline phosphatase > 2 × ULN)[7].
The primary outcome of interest was in-hospital mortality. The secondary outcome was a composite outcome of the need for mechanical ventilation or in-hospital mortality.
We summarized each continuous variable using its median and interquartile range (IQR). Categorical variables were summarized using counts and percentages. Comparisons between groups were assessed using Wilcoxon rank sum tests, chi-squared tests, and Fisher exact tests as appropriate. For survival analyses, we censored patients as alive without the event of interest on their date of hospital discharge or at 28 d of follow-up, whichever was earlier. Due to the low numbers of patients with elevations in alkaline phosphatase or bilirubin, we analyzed survival based on normal vs elevated measurements rather than the previously defined four categories. We analyzed survival using Kaplan-Meier survival curves using log-rank tests and estimated hazard ratios (HRs) and 95% confidence intervals (CIs) using univariate and multivariate Cox proportional hazards models. Multivariate models were adjusted for age, sex, race, ethnicity, BMI (< 30 kg/m2 vs ≥ 30 kg/m2), and presence of co-morbid conditions (hypertension, diabetes mellitus, coronary artery disease, chronic obstructive pulmonary disease, heart failure, chronic kidney disease, end-stage renal disease, and malignancy). We checked the proportional hazards assumption for each variable included in our Cox regression models using graphical assessment of the Kaplan-Meier survival curves and log[-log(survival)] vs log(time) graphs to look for parallel curves and by ensuring that Schoenfeld residuals were independent of time. Because the variables of age, sex, and race violated the proportional hazards assumption, we included interaction terms with time for those variables in our multivariate models. We used two-sided tests with alpha = 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
There were 11265 patients with COVID-19 hospitalized from March 1, 2020, to April 30, 2020. After excluding 106 children who were under 18 years old, 300 patients without baseline measurements of AST and ALT, and 245 patients with chronic liver disease, our study population included 10614 patients. Median length of hospital stay was 6 d (range: 0-58 d, IQR: 3-11 d). Baseline characteristics of the study population are described in Table 1. The median age was 65 years (range: 18-107 years, IQR: 54-77 years). The majority of patients were male (59%), white (38%), and non-Hispanic (21%). The most common comorbidities were hypertension (58%), obesity (39%), and diabetes (36%).
| Demographics | |
| Sex | |
| Male | 6243 (58.8%) |
| Female | 4371 (41.2%) |
| Race | |
| White | 4037 (38.0%) |
| Black | 2248 (21.2%) |
| Asian | 914 (8.6%) |
| Other/multiracial | 2942 (27.7%) |
| Unknown | 473 (4.5%) |
| Ethnicity | |
| Non-Hispanic or Latino or other/unknown | 8338 (78.6%) |
| Hispanic or Latino | 2276 (21.4%) |
| Age (yr) | 65 (54-77) |
| Body mass index (kg/m2)1 | 28.3 (24.9-32.6) |
| < 30 | 5120 (61.4%) |
| ≥ 30 | 3220 (38.6%) |
| Presence of co-morbid conditions | |
| Hypertension | 6204 (58.5%) |
| Diabetes mellitus | 3764 (35.5%) |
| Coronary artery disease | 1328 (12.5%) |
| Heart failure | 832 (7.8%) |
| Malignancy | 791 (7.5%) |
| Chronic obstructive pulmonary disease | 608 (5.7%) |
| Chronic kidney disease (stage I-IV) | 391 (3.7%) |
| End-stage renal disease | 434 (4.1%) |
| Liver chemistries (within 24 h) | |
| AST (IU/L)1 | 46 (31-72) |
| Normal | 4377 (41.3%) |
| > 1 to ≤ 4 × ULN | 5713 (53.8%) |
| > 4 to ≤ 10 × ULN | 437 (4.1%) |
| > 10 × ULN | 85 (0.8%) |
| ALT (IU/L) | 33 (21-56) |
| Normal | 4883 (46.0%) |
| > 1 to ≤ 4 × ULN | 5159 (48.6%) |
| > 4 to ≤ 10 × ULN | 484 (4.6%) |
| > 10 × ULN | 88(0.8%) |
| Alkaline phosphatase (IU/L)1 | 75 (59-98) |
| Normal | 9242 (87.1%) |
| > 1 to ≤ 4 × ULN | 1333 (12.6%) |
| > 4 to ≤ 10 × ULN | 35(0.3%) |
| > 10 × ULN | 2 (0.02%) |
| Bilirubin (mg/dL)1 | 0.5 (0.4-0.7) |
| Normal | 10119 (95.4%) |
| > 1 to ≤ 4 × ULN | 486 (4.6%) |
| > 4 to ≤ 10 × ULN | 5 (0.05%) |
| > 10 × ULN | 3 (0.03%) |
More than half of patients had elevations in AST (59%) and ALT (54%) on presentation, whereas alkaline phosphatase and bilirubin levels were elevated for only 13% and 5% of patients, respectively (Table 1). Most transaminase elevations were in group 2. 0.8% had ALT > 10 × ULN. 76% had AST higher than ALT on presentation. 1160 patients (10.95) had severe hepatic enzyme elevations. Of these, 936 (8.9%) had a hepatocellular pattern, 133 (1.3%) a cholestatic pattern, and 91 (0.9%) a mixed pattern. The prevalence of hepatic test elevations differed based on sex, ethnicity, race, and presence of comorbidities (Table 2) Males were more likely to have elevations in AST (64.8% vs 50.1%, P < 0.001) and bilirubin (5.9% vs 2.8%, P < 0.001) but less likely to have elevations in alkaline phosphatase (12.0% vs 14.2%, P = 0.001) than females. There was no difference in ALT levels between males and females (P = 0.34).
| Elevated ALP | P value | Elevated AST | P value | Elevated ALT | P value | Elevated Bilirubin | P value | |
| Sex | 0.001 | < 0.001 | 0.34 | < 0.001 | ||||
| Female | 619 (14.2%) | 2191 (50.1%) | 2336 (53.4%) | 124 (2.8%) | ||||
| Male | 751 (12.0%) | 4044 (64.8%) | 3395 (54.4%) | 370 (5.9%) | ||||
| Race | < 0.001 | < 0.001 | < 0.001 | 0.51 | ||||
| Asian | 134 (14.7%) | 613 (67.1%) | 533 (58.3%) | 43 (4.7%) | ||||
| Black | 228 (10.1%) | 1332 (59.3%) | 1146 (51.0%) | 117 (5.2%) | ||||
| Other/multiracial | 468 (15.9%) | 1845 (62.7%) | 1836 (62.4%) | 139 (4.7%) | ||||
| Unknown | 74 (15.6%) | 300 (63.4%) | 304 (64.3%) | 17 (3.6%) | ||||
| White | 466 (11.6%) | 2145 (53.2%) | 1912 (47.4%) | 178 (4.4%) | ||||
| Ethnicity | < 0.001 | < 0.001 | < 0.001 | 0.51 | ||||
| Hispanic or Latino | 398 (17.5%) | 1410 (62.0%) | 1450 (63.7%) | 97 (4.3%) | ||||
| Non-Hispanic or Latino | 864 (11.3%) | 4384 (57.3%) | 3852 (50.4%) | 361 (4.7%) | ||||
| Unknown or other | 108 (15.7%) | 441 (64.0%) | 429 (62.3%) | 36 (5.2%) | ||||
| BMI (kg/m2) | < 0.001 | 0.08 | < 0.001 | < 0.001 | ||||
| < 30 | 730 (14.3%) | 2996 (58.5%) | 2694 (52.6%) | 269 (5.3%) | ||||
| ≥ 30 | 336 (10.5%) | 1946 (60.4%) | 1885 (58.5%) | 111 (3.5%) | ||||
| Diabetes mellitus | 0.03 | < 0.001 | < 0.001 | < 0.001 | ||||
| No | 920 (13.4%) | 1158 (60.7%) | 3897 (56.9%) | 356 (5.2%) | ||||
| Yes | 450 (12.0%) | 2077 (55.2%) | 1834 (48.7%) | 138 (3.7%) | ||||
| Hypertension | < 0.001 | < 0.001 | < 0.001 | 0.36 | ||||
| No | 642 (14.6%) | 2683 (60.9%) | 2649 (60.1%) | 215 (4.9%) | ||||
| Yes | 728 (11.7%) | 3552 (57.3%) | 3082 (49.7%) | 279 (4.5%) | ||||
| Coronary artery disease | 0.69 | 0.006 | < 0.001 | 0.09 | ||||
| No | 1194 (12.9%) | 5501 (59.3%) | 5184 (55.8%) | 420 (4.5%) | ||||
| Yes | 176 (13.3%) | 734 (55.3%) | 547 (41.2%) | 74 (5.6%) | ||||
| Congestive heart failure | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| No | 1223 (12.5%) | 5811 (59.4%) | 5419 (55.4%) | 432 (4.4%) | ||||
| Yes | 147 (17.7%) | 424 (51.0%) | 312 (37.5%) | 62 (7.5%) | ||||
| COPD | 0.24 | < 0.001 | < 0.001 | 0.46 | ||||
| No | 1301 (13.0%) | 5939 (59.4%) | 5504 (55.0%) | 462 (4.6%) | ||||
| Yes | 69 (11.4%) | 296 (48.7%) | 227 (37.3%) | 32 (5.3%) | ||||
| CKD (stage I-IV) | 0.82 | 0.001 | < 0.001 | 0.59 | ||||
| No | 1321 (12.9%) | 6036 (59.1%) | 5582 (54.6%) | 478 (4.7%) | ||||
| Yes | 49 (12.5%) | 199 (50.9%) | 149 (38.1%) | 16 (4.1%) | ||||
| End-stage renal disease | < 0.001 | < 0.001 | < 0.001 | 0.09 | ||||
| No | 1265 (12.4%) | 6044 (59.4%) | 5621 (55.2%) | 481 (4.7%) | ||||
| Yes | 105 (24.2%) | 191 (44.0%) | 110 (25.4%) | 13 (3.0%) | ||||
| Malignancy | 0.58 | < 0.001 | < 0.001 | 0.049 | ||||
| No | 1273 (13.0%) | 5827 (59.3%) | 5380 (54.8%) | 446 (4.5%) | ||||
| Yes | 97 (12.3%) | 408 (51.7%) | 351 (44.4%) | 48 (6.1%) |
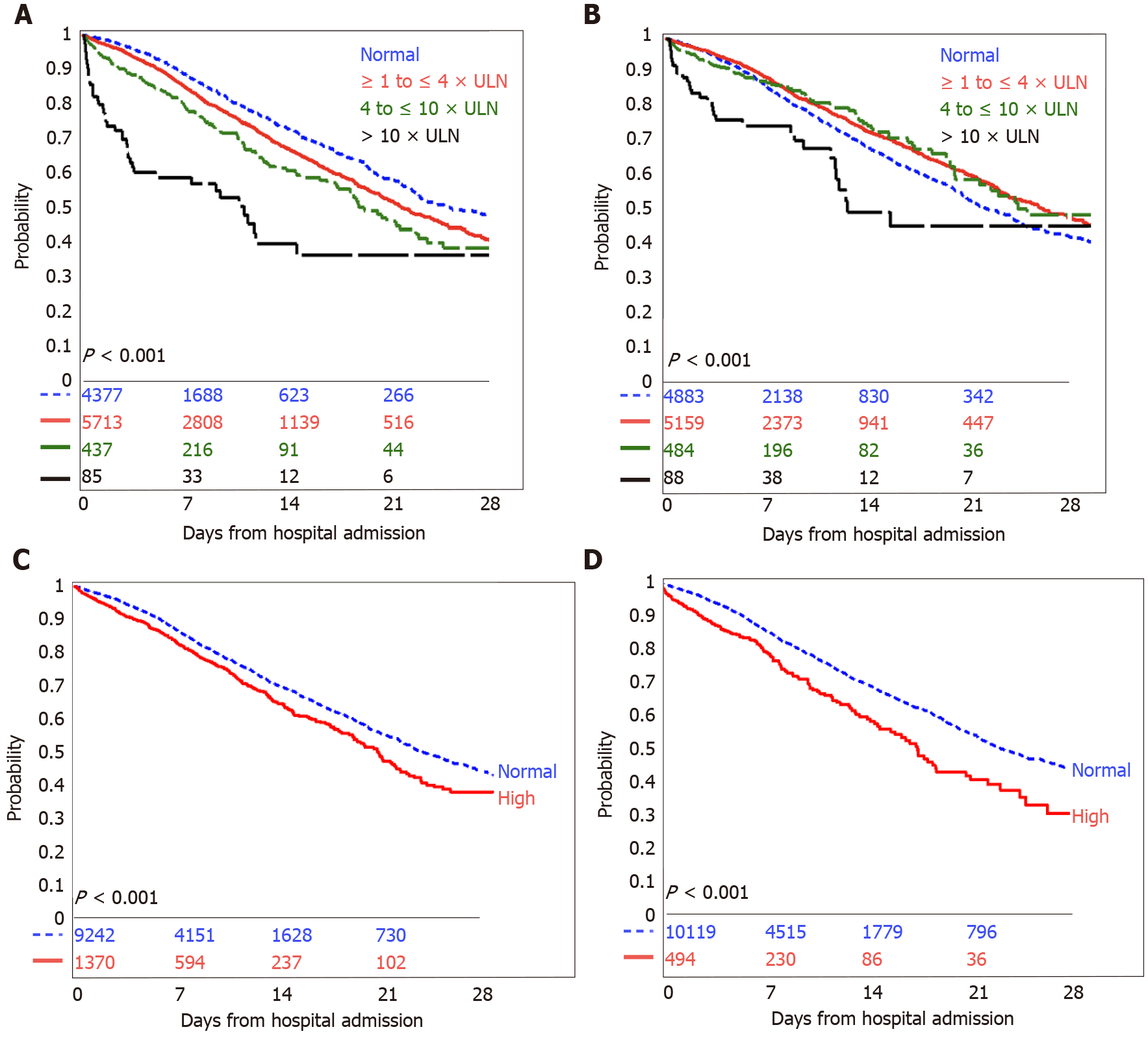
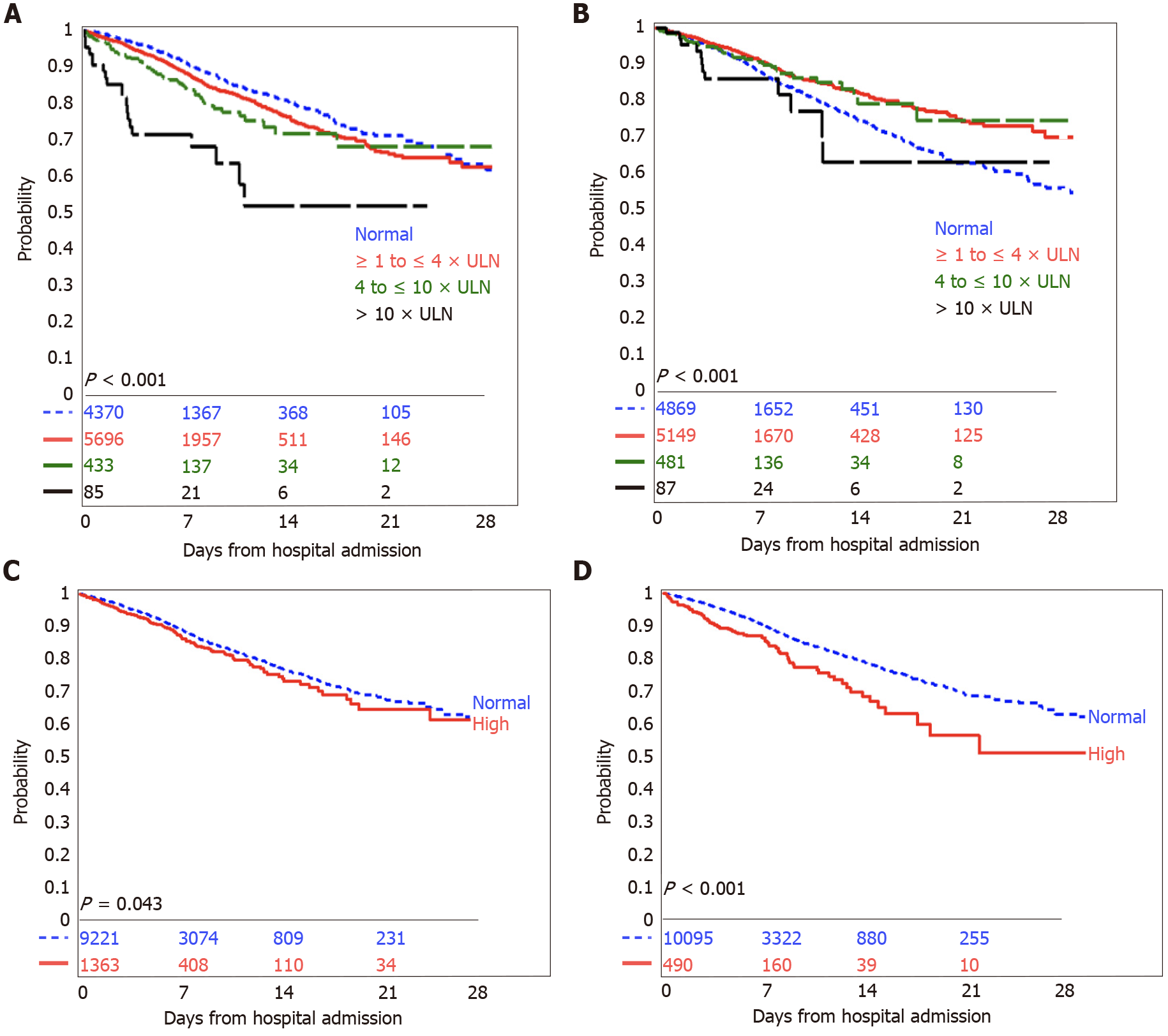
In Kaplan-Meier survival analyses, any elevated liver chemistry was associated with increased mortality (Figure 1) or need for mechanical ventilation (Figure 2).
Having an elevated AST was associated with a higher risk of in-hospital mortality (unadjusted HR 1.34, 95%CI: 1.22-1.47, P < 0.001; adjusted HR 1.67, 95%CI: 1.49-1.86, P < 0.001) and in-hospital mortality or need for mechanical ventilation (unadjusted HR 1.34, 95%CI: 1.18-1.52, P < 0.001; adjusted HR 1.77, 95%CI: 1.51-2.08, P < 0.001). Increasing severity of AST abnormalities was associated with incrementally poor survival and need for mechanical ventilation (Table 3), and the trend remained significant after adjustment for age, sex, race, ethnicity, and the presence of co-morbid conditions. Compared to patients with normal AST, patients with AST > 10 × ULN were three times as likely to have in-hospital mortality (adjusted HR 2.64, 95%CI: 1.73-4.04, P < 0.001) and three times as likely to have in-hospital mortality or need for mechanical ventilation (adjusted HR 3.38, 95%CI: 1.78-6.40, P < 0.001).
| In-hospital mortality | In-hospital mortality or need for mechanical ventilation | |||||||
| Unadjusted analysis-HR (95%CI) | P value | Adjusted analysis1-HR (95%CI) | P value | Unadjusted analysis-HR (95%CI) | P value | Adjusted analysis1-HR (95%CI) | P value | |
| AST | ||||||||
| Normal | Ref. | Ref. | Ref. | Ref. | ||||
| > 1 to ≤ 4 × ULN | 1.29 (1.17-1.42) | < 0.001 | 1.65 (1.47-1.85) | < 0.001 | 1.29 (1.13-1.46) | < 0.001 | 1.73 (1.47-2.04) | < 0.001 |
| > 4 to ≤ 10 × ULN | 1.66 (1.38-1.99) | < 0.001 | 1.69 (1.33-2.14) | < 0.001 | 1.67 (1.27-2.20) | < 0.001 | 2.09 (1.42-3.07) | < 0.001 |
| > 10 × ULN | 3.50 (2.56-4.78) | < 0.001 | 2.64 (1.73-4.04) | < 0.001 | 3.89 (2.48-6.10) | < 0.001 | 3.38 (1.78-6.40) | < 0.001 |
| ALT | ||||||||
| Normal | Ref. | Ref. | Ref. | Ref. | ||||
| > 1 to ≤ 4 × ULN | 0.84 (0.77-0.91) | < 0.001 | 1.21 (1.09-1.34) | < 0.001 | 0.72 (0.63-0.81) | < 0.001 | 1.20 (1.02-1.40) | 0.02 |
| > 4 to ≤ 10 × ULN | 0.88 (0.71-1.09) | 0.24 | 1.22 (0.93-1.61) | 0.15 | 0.79 (0.57-1.09) | 0.15 | 1.40 (0.87-2.26) | 0.16 |
| > 10 × ULN | 1.71 (1.20-2.45) | 0.003 | 1.34 (0.81-2.22) | 0.26 | 1.42 (0.80-2.51) | 0.23 | 1.36 (0.58-3.19) | 0.48 |
| Alkaline phosphatase | ||||||||
| Normal | Ref. | Ref. | Ref. | Ref. | ||||
| Elevated | 1.27 (1.14-1.43) | < 0.001 | 1.29 (1.12-1.48) | < 0.001 | 1.19 (1.01-1.42) | 0.04 | 1.39 (1.12-1.72) | 0.003 |
| Bilirubin | ||||||||
| Normal | Ref. | Ref. | Ref. | Ref. | ||||
| Elevated | 1.55 (1.32-1.82) | < 0.001 | 1.12 (0.92-1.37) | 0.27 | 1.69 (1.34-2.13) | < 0.001 | 0.96 (0.71-1.30) | 0.79 |
| AST:ALT ratio | ||||||||
| ≤ 1 | Ref. | Ref. | Ref. | Ref. | ||||
| > 1 | 2.08 (1.82-2.37) | < 0.001 | 1.72 (1.46-2.02) | < 0.001 | 2.61 (2.13-3.19) | < 0.001 | 1.84 (1.41-2.40) | < 0.001 |
| Pattern of liver injury2 | ||||||||
| Normal or non-severe | Ref. | Ref. | Ref. | Ref. | ||||
| Hepatocellular | 0.93 (0.80-1.09) | 0.39 | 0.90 (0.74-1.11) | 0.33 | 0.84 (0.66-1.07) | 0.15 | 1.18 (0.85-1.65) | 0.33 |
| Mixed | 1.14 (0.74-1.75) | 0.56 | 1.06 (0.65-1.73) | 0.82 | 1.00 (0.52-1.93) | 0.99 | 1.02 (0.44-2.37) | 0.96 |
| Cholestatic | 1.17 (0.85-1.61) | 0.33 | 1.57 (1.07-2.28) | 0.02 | 1.25 (0.81-1.93) | 0.31 | 2.05 (1.17-3.58) | 0.01 |
In unadjusted models, having an elevated ALT appeared to be associated with a lower risk of in-hospital mortality (unadjusted HR 0.85, 95%CI: 0.78-0.92, P < 0.001) and the composite outcome (unadjusted HR 0.73, 95%CI: 0.65-0.83, P < 0.001). However, after adjusting for the confounding factors of age, sex, race, ethnicity and the presence of co-morbid conditions, an elevated ALT was associated with a 1.2 times higher risk of in-hospital mortality (adjusted HR 1.21, 95%CI: 1.09-1.34, P < 0.001) and the composite outcome (adjusted HR 1.21, 95%CI: 1.04-1.41, P = 0.02). Elevation in alkaline phosphatase was associated with a similar modestly increased risk of in-hospital mortality (adjusted HR 1.29, 95%CI: 1.12-1.48, P < 0.001) and in the composite outcome (adjusted HR 1.39, 95%CI: 1.12-1.72, P = 0.003). The associations between elevated bilirubin levels and outcomes became attenuated and lost significance after adjustment for confounding factors (Table 3).
The pattern of liver enzyme abnormalities affected outcomes. Patients with initial AST higher than ALT had a higher risk of in-patient mortality (adjusted HR 1.72, 95%CI: 1.46-2.02, P < 0.001) and the composite outcome of in-patient mortality or need for mechanical ventilation (adjusted HR: 1.84, 95%CI: 1.41-2.40, P < 0.001), compared to patients with AST less than or equal to ALT. Compared to patients with normal or non-severe elevations in ALT and alkaline phosphatase (with ALT < 3 × ULN and alkaline phosphatase ≤ 2 × ULN), patients with a severe cholestatic liver injury had a higher risk of in-patient mortality (adjusted HR 1.57, 95%CI: 1.07-2.28, P = 0.02) and the composite outcome of in-patient mortality or need for mechanical ventilation (adjusted HR 2.05, 95%CI: 1.17-3.58, P = 0.01). A hepatocellular or mixed pattern of liver injury was not associated with outcomes in adjusted models.
Our study shows that initial, abnormal liver enzymes are associated with poor outcomes in patients with COVID-19 infection in a diverse, multi-ethnic patient population. More than half of our patients upon presentation to the hospital had abnormal levels of serum aminotransferases, and a small proportion had elevations of bilirubin and alkaline phosphatase. The predominant pattern of liver injury was hepatocellular. Of those who presented with abnormal transaminases, the overwhelming majority presented with enzymes one-four times the ULN and had an AST greater than ALT. Elevations in either ALT, AST, alkaline phosphatase or bilirubin were associated with increased mortality or need for mechanical ventilation.
Initial AST elevations appear to be the more significant predictor of mortality and the need for mechanical ventilation than elevations in ALT, alkaline phosphatase and total bilirubin with these risks increasing as AST elevations were more severe, even when adjusted for baseline demographics and the presence of co-morbidities. The mechanism behind this is unclear and still needs to be determined. One study noted AST-dominant aminotransferase elevation is common in COVID-19, can mirror disease severity, and appears to reflect true hepatic injury[12]. In this largest cohort of patients reported so far, we have demonstrated that AST predominant hepatic injury is common and associated with survival outcomes.
It has been postulated that abnormal liver enzymes in COVID-19 infection may be related to a direct cytotoxicity from active viral replication of SARS-CoV-2 in the liver, immune-mediated liver damage secondary to the systemic inflammatory response syndrome, hypoxic changes induced by respiratory failure, vascular changes due to COVID-19 induced thrombotic disease, endothelitis, right heart failure or drug induced liver injury[6,7,19-21]. SARS-CoV-2 virions have been detected in portal vein vessel lumens and endothelial cells by in situ hybridization[21]. SARS-CoV-2 enters host cells through the cell receptor, angiotensin converting enzyme 2 (ACE2). Cells with high ACE2 levels are associated with more severe COVID disease. ACE2 is highly expressed in the lung, cholangiocytes and hepatic vessel endothelial cells with some but less expression in hepatocytes. The high ACE2 expression in cholangiocytes may explain the severe cholestatic disease seen in the subset of patients presenting with this liver enzyme pattern[16,21]. Hypoxia is associated with increased expression of ACE2 receptors in hepatocytes and cholangiocytes[21]. While our study is unable to differentiate between a direct viral effect or an immune-induced response, we show that patients with COVID-19 infection have a high prevalence of abnormal liver chemistries prior to the administration of any COVID-19 specific medications.
Previous published smaller studies have reported that the pattern of liver injury, either hepatocellular or mixed hepatocellular-cholestatic, appears to be more common in people with severe COVID-19[6-8,14,22]. Based upon our strict definition of these patterns, we found that the hepatocellular pattern of injury on presentation was the most common type of injury noted and that this pattern was not associated with outcomes in an adjusted model. While an initial presentation of severe cholestasis was uncommon, it was associated with a higher risk of in-patient mortality or the need for mechanical ventilation. This is an important finding and should alert caregivers of the risk of decreased survival with this specific pattern of presentation.
Our study does have several limitations. All data was collected from the electronic health record database, as the large size of the cohort precluded a manual review of all cases. Baseline laboratory tests collected were defined as within 24 h of presentation to the emergency department, but this definition does not take into account the length of patient symptoms prior to presentation. Our study specifically evaluated initial laboratory data prior to the initiation of any multimodal treatments such as patient positioning, supplemental oxygen and medical therapies, both standard of care and experimental as our sites had access to several clinical trials medications. While initiation of therapies during hospital admission may have influenced overall outcomes, it is beyond the scope of this paper to assess the effects of individual therapies on outcomes. The absence of data on patients who remain hospitalized at the final study date may have biased the survival outcomes of the study.
Our study specifically excluded patients with known chronic liver disease. This was done through review of ICD-10 diagnostic codes followed by manual chart review as needed for confirmation. Despite this methodology, it remains a possibility that our cohort did include patients with undiagnosed non-alcoholic fatty liver disease, as the prevalence of obesity and diabetes in our population was 39% and 36%, respectively, and both of these co-morbidities are associated with this condition. Diabetic patients in our study had significantly lower prevalence of AST and ALT elevations than non-diabetics. In contrast, compared to non-obese patients, obese patients had a higher prevalence of ALT elevations but not the other measured liver chemistries. These findings may make it unclear whether the presence of undiagnosed non-alcoholic fatty liver disease affects the risk of liver injury, survival or the need for mechanical ventilation in patients infected with COVID-19 and further study is warranted.
In this largest cohort of hospitalized COVID-19 patients reported so far, we have shown an increased mortality and the need for mechanical ventilation is associated with hepatic test elevations, and in particular those with cholestasis, these findings should be taken into consideration during the initial evaluation of COVID-19 patients, both in the in-patient and out-patient setting and patients with significantly elevated liver enzymes should be prioritized for future treatments as these treatments become available.
Liver enzyme abnormalities are commonly seen in coronavirus disease 2019 (COVID-19) infection. We assessed the prevalence and prognostic value of the initial liver enzymes in patients admitted to hospital with COVID-10 infection.
At the time of the writing of this manuscript, our health system had data on 10614 individual patients admitted with COVID-10 infection. We wanted to assess the prevalence of liver enzyme abnormalities in these patients and determine if any particular enzyme pattern would predict prognosis.
Determine the prevalence of abnormal liver enzymes in patients admitted to the hospital with COVID-19 infection. Determine the prognostic value of initial liver enzymes on mortality and/or the need for mechanical ventilation. Determine if any particular abnormal liver enzyme pattern was most predictive of poor outcome in COVID-19 infection.
Review of electronic medical records of 10614 patients admitted to the hospital with COVID-19 infection.
Elevated liver enzymes are common upon initial hospital presentation of COVID-19 infection.
Increased mortality and the need for mechanical ventilation is associated with elevated hepatic enzymes in COVID-19 patients without chronic liver disease.
This is an important study which highlights the importance of initial liver enzyme patterns in predicting outcomes. Health care workers should be aware of these findings to better triage COVID-19 patients.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases; American Gastroenterological Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hasan M, Lukito AA S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30115] [Article Influence: 6023.0] [Reference Citation Analysis (3)] |
| 2. | World Health Organization. Coronavirus disease (COVID-19) outbreak. Geneva: World Health Organization; 2020. [cited 8 October 2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. |
| 3. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 4. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18877] [Article Influence: 3775.4] [Reference Citation Analysis (7)] |
| 5. | Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4388] [Article Influence: 877.6] [Reference Citation Analysis (1)] |
| 6. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 7. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 8. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 9. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 10. | Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 1617] [Article Influence: 323.4] [Reference Citation Analysis (0)] |
| 11. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6518] [Article Influence: 1303.6] [Reference Citation Analysis (0)] |
| 12. | Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2021;73:890-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 13. | Hajifathalian K, Krisko T, Mehta A, Kumar S, Schwartz R, Fortune B, Sharaiha RZ; WCM-GI research group*. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology. 2020;159:1137-1140.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 14. | Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 15. | Wander P, Epstein M, Bernstein D. COVID-19 Presenting as Acute Hepatitis. Am J Gastroenterol. 2020;115:941-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 16. | Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, Crawford JM. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021;116:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 17. | Goel H, Harmouch F, Garg K, Saraiya P, Daly T, Kumar A, Hippen JT. The liver in COVID-19: prevalence, patterns, predictors, and impact on outcomes of liver test abnormalities. Eur J Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Kim JH. 2018 Korean Association for the Study of the Liver (KASL) Clinical Practice Guidelines of Chronic Hepatitis B: What's Different? Korean J Gastroenterol. 2019;73:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Lee IC, Huo TI, Huang YH. Gastrointestinal and liver manifestations in patients with COVID-19. J Chin Med Assoc. 2020;83:521-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (2)] |
| 20. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 21. | Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 271] [Article Influence: 67.8] [Reference Citation Analysis (2)] |
| 22. | Piano S, Dalbeni A, Vettore E, Benfaremo D, Mattioli M, Gambino CG, Framba V, Cerruti L, Mantovani A, Martini A, Luchetti MM, Serra R, Cattelan A, Vettor R, Angeli P; COVID-LIVER study group. Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19. Liver Int. 2020;40:2394-2406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |