Published online Nov 7, 2021. doi: 10.3748/wjg.v27.i41.7190
Peer-review started: July 4, 2021
First decision: July 14, 2021
Revised: July 27, 2021
Accepted: August 31, 2021
Article in press: August 31, 2021
Published online: November 7, 2021
Processing time: 124 Days and 22 Hours
Despite the popularity of immune checkpoint inhibitors (ICIs) in the treatment of advanced cancer, patients often develop gastrointestinal (GI) and non-GI im
To evaluate the clinical characteristics of GI-irAEs and their impact on survival in patients treated with ICIs.
In this single-center, retrospective, observational study, we reviewed the records of 661 patients who received ICIs for various cancers at Nagoya University Hospital from September 2014 to August 2020. We analyzed the clinical characteristics of patients who received ICI treatment. We also evaluated the correlation between GI-irAE development and prognosis in non-small cell lung cancer (LC) and malignant melanoma (MM). Kaplan-Meier analysis was used to compare the median overall survival (OS). Multivariate Cox proportional hazards models were used to identify prognostic factors. A P value < 0.05 was considered statistically significant.
GI-irAEs occurred in 34 of 605 patients (5.6%) treated with an anti-programmed cell death-1/programmed death-ligand 1 (anti-PD-1/PD-L1) antibody alone and in nine of 56 patients (16.1%) treated with an anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody alone or a combination of anti-PD-1 and anti-CTLA-4 antibodies. The cumulative incidence and median daily diarrhea frequency were significantly higher in patients receiving anti-CTLA-4 antibodies (P < 0.05). In 130 patients with MM, OS was significantly prolonged in the group that continued ICI treatment despite the development of GI-irAEs compared to the group that did not experience GI-irAEs (P = 0.035). In contrast, in 209 patients with non-small cell LC, there was no significant difference in OS between the groups. The multi
Patients receiving anti-CTLA-4 antibodies develop GI-irAEs more frequently and with higher severity than those receiving anti-PD-1/PD-L1 antibodies. Conti
Core Tip: We compared the clinical characteristics of gastrointestinal immune-related adverse events (GI-irAEs) in patients receiving anti-programmed cell death-1/pro
- Citation: Yamada K, Sawada T, Nakamura M, Yamamura T, Maeda K, Ishikawa E, Iida T, Mizutani Y, Kakushima N, Ishikawa T, Furukawa K, Ohno E, Honda T, Kawashima H, Ishigami M, Furune S, Hase T, Yokota K, Maeda O, Hashimoto N, Akiyama M, Ando Y, Fujishiro M. Clinical characteristics of gastrointestinal immune-related adverse events of immune checkpoint inhibitors and their association with survival. World J Gastroenterol 2021; 27(41): 7190-7206
- URL: https://www.wjgnet.com/1007-9327/full/v27/i41/7190.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i41.7190
In recent years, the prognosis of various malignancies has been improved with the introduction of immune checkpoint inhibitors (ICIs)[1-4]. Monoclonal antibodies targeting programmed cell death-1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen-4 (CTLA-4) exhibit antitumor activity by specifically activating T-cells and suppressing the inhibition of the immune system.
Adverse events (AEs) specific to ICIs, which are different from those of conventional chemotherapy, have been observed in patients receiving ICIs. These AEs are called immune-related AEs (irAEs) and include skin disorders, gastrointestinal (GI) di
These clinical features reportedly differ between anti-CTLA-4 antibodies, anti-PD-1/anti-PD-L1 antibodies, and their combinations[7-10]. However, there are only a few coherent reports, and the clinical features of GI-irAEs for each ICI have not yet been fully clarified.
The development of irAEs is associated with an improved prognosis in different malignancies[11-14]. In contrast, a previous study[15] showed a poor prognosis with the development of irAEs; hence, a consensus has not yet been reached. In addition, few studies observed prolongation of overall survival (OS) with GI-irAE development[16-18].
Therefore, this study aimed to investigate differences in the clinical characteristics of GI-irAEs associated with anti-PD-1/PD-L1 antibodies, anti-CTLA-4 antibodies, and combination therapy with anti-PD-1 and anti-CTLA-4 antibodies. Further, we exa
This single-center, retrospective, observational study included consecutive patients who received ICIs (i.e. nivolumab, pembrolizumab, atezolizumab, avelumab, durva
Eligible patients were those treated with ICIs at standard doses. Nivolumab was administered at 3 mg/kg or 240 mg/body every 2 wk. However, some MM patients were administered 2 mg/kg nivolumab every 3 wk. Pembrolizumab was administered at 2 mg/kg or 200 mg/body every 3 wk. Atezolizumab was administered at 1200 mg/body every 3 wk. Avelumab was administered at 10 mg/kg every 2 wk. Durva
GI-irAEs were defined as diarrhea or bloody stools after ICI administration in patients in whom infectious enteritis could be excluded. Infectious enteritis (Clostridium difficile, other bacterial infections, or viral pathogens, such as cytomegalovirus) was ruled out using blood tests and stool samples. The National Cancer Institute Common Terminology Criteria for AEs (version 5.0) was used to assess the severity of enterocolitis and diarrhea.
Treatment of GI-irAEs was based on the strategy of the attending physician, but in most cases was based on the American Society of Clinical Oncology clinical practice guideline[19]. In the case of Grade 1 GI-irAEs, ICIs were continued or were stopped temporarily and resumed if toxicity did not exceed Grade 1. In the case of Grade 2 or higher GI-irAEs, ICIs were discontinued until the patient’s symptoms recovered to Grade 1 or lower, at which point restarting ICIs was considered depending on the patient’s condition.
We divided the patients into those who received an anti-PD-1/PD-L1 antibody alone (PD-1/PD-L1 group) and those who received an anti-CTLA-4 antibody alone or a combination of anti-PD-1 and anti-CTLA-4 antibodies (CTLA-4 group). The clinical characteristics of GI-irAEs [incidence, cumulative incidence, severity, computed tomography (CT) findings, endoscopic findings, and treatments] were compared between the PD-1/PD-L1 and CTLA-4 groups.
We examined the correlation between the incidence of GI-irAEs and the prognosis of patients with NSCLC and MM, which included more cases of ICI administration than other cancers. Patients who developed GI-irAEs were categorized into two groups: The ICI continuation group in which ICI administration was continued or resumed after improvement of GI-irAEs and the ICI discontinuation group in which ICI administration was permanently discontinued after the development of GI-irAEs. Prognosis was assessed in three groups: Non-GI-irAE, ICI continuation, and ICI discontinuation. Prognosis was also examined by cancer stage (stage III or IV).
To compare each group, the Mann–Whitney U test was used for continuous variables and Fisher’s exact test was used for categorical variables. The Kaplan–Meier method and log-rank tests were used to compare the cumulative incidence and median OS among the groups. Univariate and multivariate Cox proportional hazards models were used to identify prognostic factors associated with GI-irAEs. SPSS Statistics software (version 27.0; IBM Corp., Armonk, NY, United States) was used for analysis. For all analyses, a P value < 0.05 was considered statistically significant.
Overall, there were 605 patients in the PD-1/PD-L1 group and 56 patients in the CTLA-4 group. The clinical characteristics of the PD-1/PD-L1 and CTLA-4 groups are described in Table 1. The median ages were 69 (range: 22–87) and 65 (range: 21–85) years in the PD-1/PD-L1 and CTLA-4 groups, respectively. Patients in the PD-1/PD-L1 group were significantly older than those in the CTLA-4 group (P = 0.039). There were no significant differences in sex, body mass index (BMI), or Eastern Cooperative Oncology Group performance status (ECOG PS) between the groups. The PD-1/PD-L1 group included patients with LC, MM, RCC, GC, and other cancers (head and neck cancer, urothelial cancer, gynecological cancer, breast cancer, and colorectal cancer); the CTLA-4 group included patients with MM and RCC.
| Characteristic | PD-1/PD-L1, n = 605 | CTLA-4, n = 56 | P value |
| Age, yr, median (range) | 69 (22-87) | 65 (21-85) | 0.039 |
| Sex, n (%) | 0.228 | ||
| Male | 419 (69.3) | 34 (60.7) | |
| Female | 186 (30.7) | 22 (39.3) | |
| BMI, kg/m2 | 21.3 (12.0-37.0) | 21.6 (13.9-43.0) | 0.532 |
| ECOG PS, n (%) | 0.073 | ||
| 0-1 | 534 (88.3) | 54 (96.4) | |
| 2-3 | 71 (11.7) | 2 (3.6) | |
| Cancer type, n (%) | |||
| NSCLC | 241 (39.8) | 0 (0.0) | |
| MM | 110 (18.2) | 39 (69.6) | |
| RCC | 52 (8.6) | 17 (30.4) | |
| GC | 49 (8.1) | 0 (0.0) | |
| Others | 153(25.3) | 0 (0.0) | |
| Drugs, n (%) | |||
| Nivolumab | 317 (52.4) | 0 (0.0) | |
| Pembrolizumab | 180 (29.8) | 0 (0.0) | |
| Atezolizumab | 74 (12.2) | 0 (0.0) | |
| Durvalumab | 32 (5.3) | 0 (0.0) | |
| Avelumab | 2 (0.3) | 0 (0.0) | |
| Ipilimumab | 0 (0.0) | 28 (50.0) | |
| Nivolumab + ipilimumab | 0 (0.0) | 28 (50.0) |
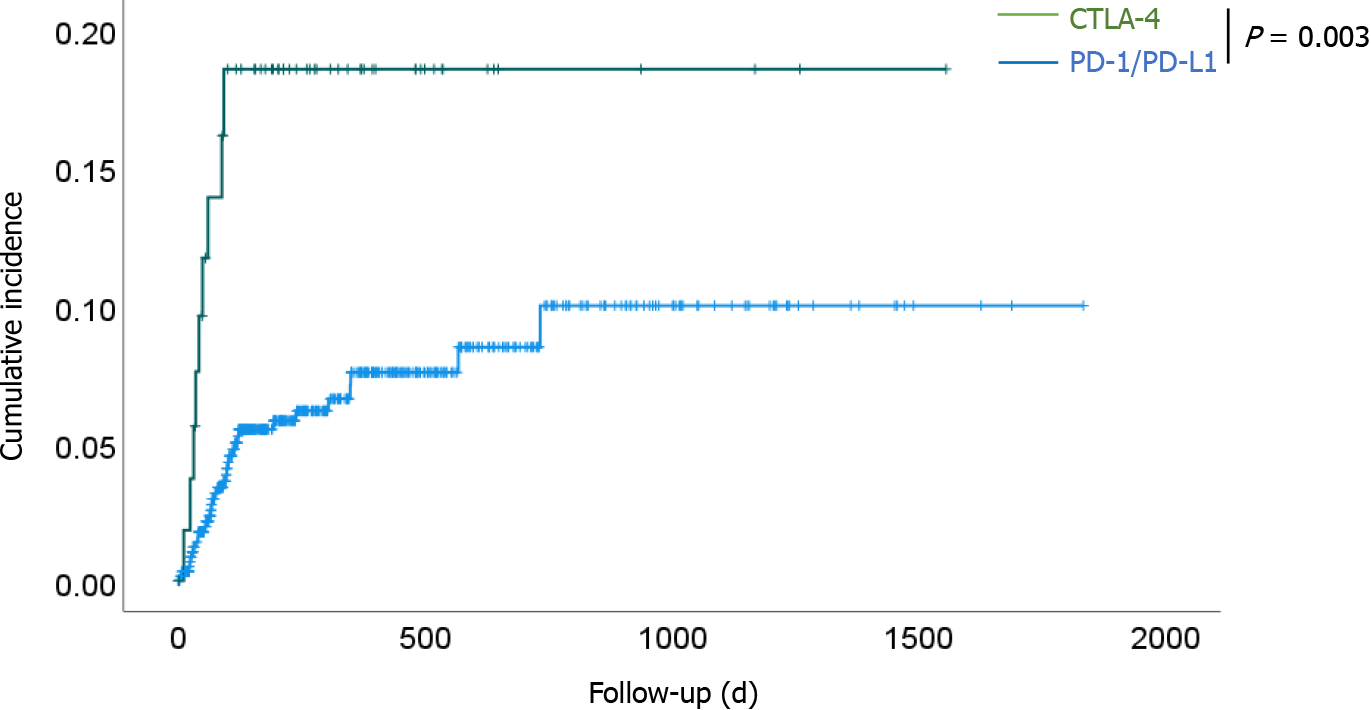
Among 47 patients who developed diarrhea or bloody stools after ICI administration, 39 had diarrhea, one had bloody stools, and seven had diarrhea and bloody stools. Forty-three patients (excluding four patients diagnosed with infectious enteritis) were included in the analysis. GI-irAEs occurred in 34 of 605 patients (5.6%) in the PD-1/PD-L1 group and 9 of 56 patients (16.1%) in the CTLA-4 group. The incidence of GI-irAEs in the CTLA-4 group was significantly higher than that in the PD-1/PD-L1 group (P = 0.008). We compared the cumulative incidence of GI-irAEs in the PD-1/PD-L1 group with that in the CTLA-4 group for all cancers (Figure 1). The cumulative incidence was significantly higher in the CTLA-4 group than in the PD-1/PD-L1 group (P = 0.003). The median observation periods until the development of GI-irAEs were 1683.1 ± 28.3 d [95% confidence interval (CI): 1627.6-1738.5] in the PD-1/PD-L1 group and 1299.7 ± 77.7 d (95%CI: 1147.5-1452.0) in the CTLA-4 group.
The clinical characteristics of patients with GI-irAEs in the PD-1/PD-L1 and CTLA-4 groups are shown in Table 2. There were no differences in age, sex, or median ICI duration before the development of GI-irAEs between the groups. In the PD-1/PD-L1 group, nine patients (26%) had Grade 1 GI-irAEs, 18 (53%) had Grade 2 GI-irAEs, and seven (21%) had Grade 3 GI-irAEs. In the CTLA-4 group, no patients had Grade 1 GI-irAEs, five (55.6%) developed Grade 2 GI-irAEs, and four (44.4%) had Grade 3 GI-irAEs.
| Characteristic | PD-1/PD-L1, n = 34 | CTLA-4, n = 9 | P value |
| Age, yr, median (range) | 69 (37-86) | 56 (46-80) | 0.187 |
| Sex, n (%) | 0.427 | ||
| Male | 29 (85.3) | 7 (77.8) | |
| Female | 5 (14.7) | 2 (22.2) | |
| Drugs, n (%) | |||
| Nivolumab | 12 (35.3) | 0 (0.0) | |
| Pembrolizumab | 16 (47.0) | 0 (0.0) | |
| Atezolizumab | 2 (5.9) | 0 (0.0) | |
| Durvalumab | 4 (11.8) | 0 (0.0) | |
| Ipilimumab | 0 (0.0) | 6 (66.7) | |
| Nivolumab + ipilimumab | 0 (0.0) | 3 (33.3) | |
| Median ICI duration before GI-irAE onset (d), median (range) | 77 (4-733) | 42 (11-92) | 0.127 |
| Diarrhea frequency per day, times (range) | 5.0 (0-10) | 6.5 (4-15) | 0.031 |
| CTCAE Grade, n (%) | 0.288 | ||
| 1 | 9 (26.5) | 0 (0.0) | |
| 2-3 | 25 (73.5) | 9 (100) | |
| GI-irAE treatment, n (%) | |||
| Improvement without medication | 13 (38.2) | 1 (11.1) | 0.017 |
| Corticosteroids | 11 (32.4) | 8 (88.9) | 0.006 |
| Loperamide | 8 (23.5) | 1 (11.1) | 0.657 |
Grade 2 or 3 GI-irAEs were more frequent in the CTLA-4 group than in the PD-1/PD-L1 group, but there was no statistically significant difference (P = 0.166). The median daily diarrhea frequencies were 5.0 (range: 0-10) times and 6.5 (range: 4-15) times in the PD-1/PD-L1 and CTLA-4 groups, respectively. The median daily diarrhea frequency was significantly higher in the CTLA-4 group than in the PD-1/PD-L1 group (P = 0.03).
Thirteen patients (38.2%) in the PD-1/PD-L1 group and one (11.1%) in the CTLA-4 group improved without medication (Table 2). More patients in the CTLA-4 group than in the PD-1/PD-L1 group improved without medication (P = 0.017). Corticosteroids were required in 11 patients (32.4%) in the PD-1/PD-L1 group and eight (88.9%) in the CTLA-4 group. More patients in the CTLA-4 group than in the PD-1/PD-L1 group required steroid therapy (P = 0.006). All patients in this study improved with observation or treatment with steroids, and no patient was treated with biological agents or surgery.
Twenty-three patients in the PD-1/PD-L1 group underwent abdominal CT, which showed many inflammation sites, especially between the descending colon and rectum. Eight patients in the CTLA-4 group had diffused CT findings between the jejunum and rectum (Table 3). There was no statistically significant difference between the PD-1/PD-L1 group and the CTLA-4 group in each segment from the jejunum to the rectum.
| Site of inflammation | PD-1/PD-L1, n = 23 | CTLA-4, n = 8 |
| Jejunum, n (%) | 1 (4.3) | 2 (25.0) |
| Ileum, n (%) | 2 (8.7) | 2 (25.0) |
| Cecum, n (%) | 2 (8.7) | 2 (25.0) |
| Ascending colon, n (%) | 3 (13.0) | 4 (50.0) |
| Transverse colon, n (%) | 3 (13.0) | 3 (37.5) |
| Descending colon, n (%) | 6 (26.1) | 3 (37.5) |
| Sigmoid colon, n (%) | 7 (30.4) | 2 (25.0) |
| Rectum, n (%) | 8 (34.8) | 2 (25.0) |
| No findings, n (%) | 9 (39.1) | 2 (25.0) |
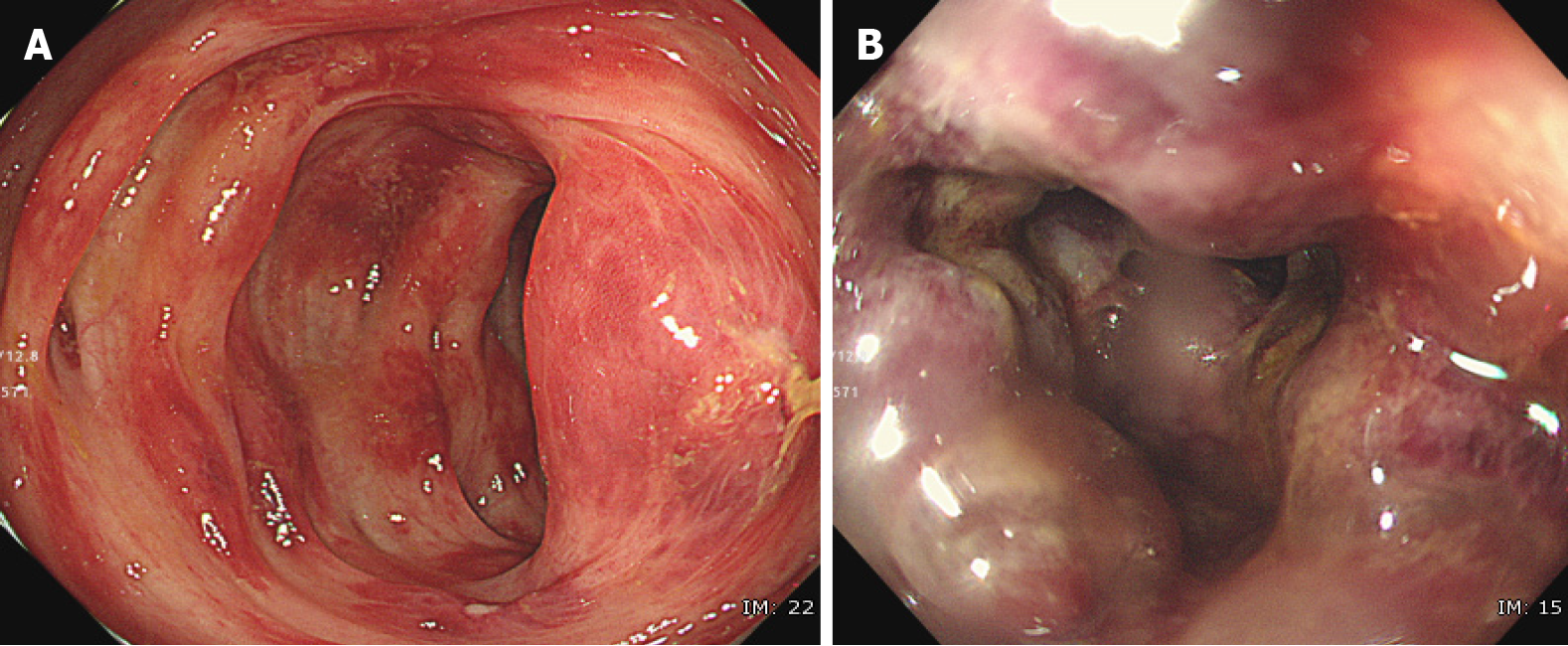
Lower GI endoscopy was performed in 16 patients (12 in the PD-1/PD-L1 group and four in the CTLA-4 group). Endoscopic findings were classified as follows, according to a previous report[6]: (1) Large deep ulcer [PD-1/PD-L1 group (n = 1)]; (2) Diffuse erythema with exudate [PD-1/PD-L1 group (n = 2); CTLA-4 group (n = 1)]; (3) Patchy erythema [CTLA-4 group (n = 1)]; (4) Aphtha [PD-1/PD-L1 group (n = 2)]; (5) Edema [PD-1/PD-L1 group (n = 4); CTLA-4 group (n = 2)]; and (6) Normal [PD-1/PD-L1 group (n = 2)]. There was no specific trend in endoscopic findings based on treatment group. There was an ischemic change [PD-1/PD-L1 group (n = 1)] that could not be classified using the classifications above (Figure 2).
The study included 209 patients with NSCLC and 130 patients with MM, excluding 30 patients who received durvalumab as maintenance therapy after curative chemoradiation for unresectable advanced NSCLC and 19 patients with MM who received ICIs as postoperative adjuvant therapy.
Twelve patients with NSCLC and 13 with MM developed GI-irAEs. The clinical characteristics of all patients and those who developed GI-irAEs are shown in Tables 4 and 5, respectively.
| Characteristic | NSCLC, n = 209 | MM, n = 130 |
| Age, yr | 66 ± 11 | 66 ± 13 |
| Sex, n (%) | ||
| Male | 143 (68.4) | 75 (57.7) |
| Female | 66 (31.6) | 55 (42.3) |
| BMI, kg/m2 | 21.7 ± 3.4 | 22.4 ± 4.3 |
| ECOG PS, n | ||
| 0-1 | 184 | 119 |
| 2-3 | 25 | 11 |
| Drugs, n (%) | ||
| Nivolumab | 61 (29.2) | 58 (44.6) |
| Pembrolizumab | 87 (41.6) | 35 (26.9) |
| Atezolizumab | 61 (29.2) | 0 (0.0) |
| Ipilimumab | 0 (0.0) | 27 (20.8) |
| Nivolumab + ipilimumab | 0 (0.0) | 10 (7.7) |
| History of ICI use, n (%) | 11 (5.3) | 34 (26.2) |
| Follow-up, d | 365 ± 335 | 466 ± 419 |
| Total irAEs, n (%) | ||
| GI-irAEs | 9 (4.3) | 13 (10.0) |
| Liver-irAEs | 7 (3.3) | 13 (10.0) |
| Lung-irAEs | 10 (4.8) | 11 (8.5) |
| Skin-irAEs | 9 (4.3) | 9 (6.9) |
| Thyroid-irAEs | 12 (5.7) | 9 (6.9) |
| Characteristic | NSCLC, n = 12 | MM, n = 13 |
| Age, yr | 67 ± 11 | 67 ± 12 |
| Sex, n | ||
| Male | 10 | 9 |
| Female | 2 | 4 |
| BMI, kg/m2 | 22.2 ± 3.7 | 22.1 ± 4.4 |
| ECOG PS, n | ||
| 0-1 | 12 | 13 |
| 2-3 | 0 | 0 |
| Stage, n | ||
| III | 1 | 2 |
| IV | 11 | 11 |
| Latest ICI, n | ||
| Nivolumab | 2 | 5 |
| Pembrolizumab | 8 | 3 |
| Atezolizumab | 2 | 0 |
| Ipilimumab | 0 | 5 |
| Nivolumab + ipilimumab | 0 | 0 |
| Diarrhea frequency | 4.3 ± 1.8 | 5.5 ± 2.5 |
| CTCAE Grade, n | ||
| 1 | 4 | 3 |
| 2 | 7 | 8 |
| 3 | 1 | 2 |
| Median ICI duration before GI-irAE onset (d), median (range) | 60 (7-567) | 75 (24-733) |
| Treatment with ICIs after the onset of GI-irAEs | ||
| Continued or resumed | 8 | 10 |
| Discontinued | 4 | 3 |
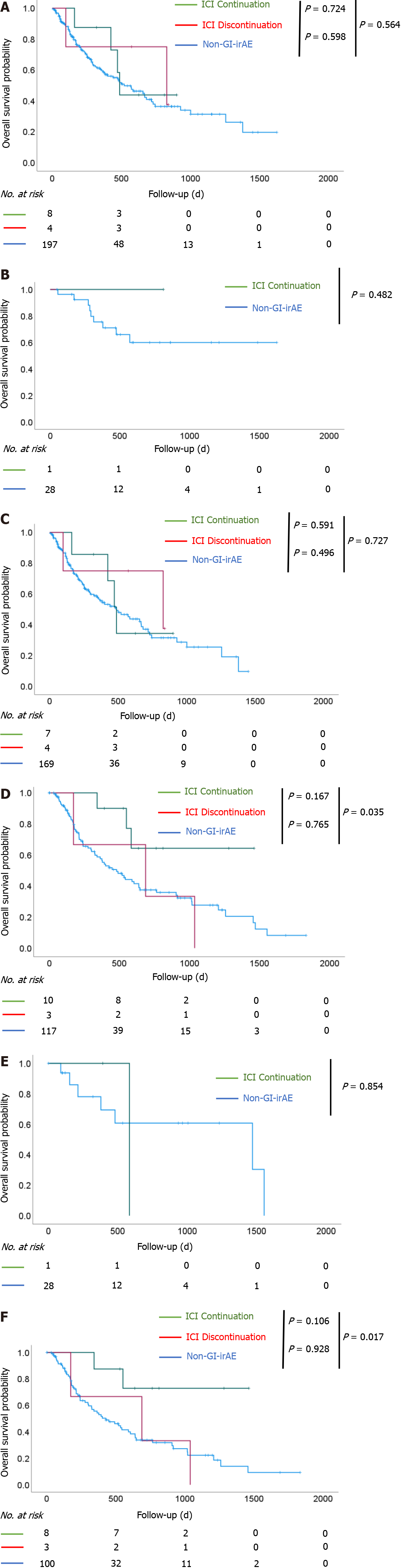
There were eight and four patients with NSCLC in the ICI continuation and ICI discontinuation groups, respectively, and 10 and three patients with MM in the ICI continuation and ICI discontinuation groups, respectively. Among patients with NSCLC, the median OS was 488.0 ± 20.6 d (95%CI: 447.6-528.4) in the ICI continuation group, 829.0 ± 558.3 d (95%CI: 0-1923.3) in the ICI discontinuation group, and 521.0 ± 102.4 d (95%CI: 320.2-721.8) in the non-GI-irAE group. There was no significant difference in OS among the three groups. The results were similar when patients were stratified by disease stage (stage III or IV). Among patients with MM, there was a significant prolongation of OS in the ICI continuation group compared to the non-GI-irAE group [not reached vs 481.0 d (95%CI: 329.1-632.9); P = 0.035]. There was no significant difference in OS based on the development of GI-irAEs among patients with stage III disease. Among patients with stage IV disease, there was a significant prolongation of OS in the ICI continuation group compared to the non-GI-irAE group [not reached vs 427.0 d (95%CI: 248.5-605.5); P = 0.017] (Figure 3).
Prognostic factors for OS in NSCLC and MM were examined using Cox proportional hazards models (Tables 6 and 7). The following factors were considered: Age (< 75 vs ≥ 75 years), sex, BMI [underweight (< 18.5 kg/m2) vs normal weight (18.5−24.9 kg/m2) vs overweight (≥ 25.0 kg/m2)], stage (III vs IV), ECOG PS (0-1 vs 2-3), ICIs (PD-1/PD-L1 vs CTLA-4), and GI-irAEs (ICI continuation vs ICI discontinuation vs non-GI-irAE groups). In NSCLC, the univariate analysis showed significant associations of OS with age, BMI, stage, and ECOG PS. In the multivariate analysis of these factors, stage IV disease [hazard ratio (HR): 2.182; 95%CI: 1.085–4.387; P = 0.029] and a ECOG PS of 2-3 (HR: 12.772; 95%CI: 7.067-23.085; P < 0.001) were identified as independent predictors of OS. Similarly, in MM, the univariate analysis showed significant associations of OS with age, sex, ECOG PS, and GI-irAEs. In the multivariate analysis of these factors, only ECOG PS (HR: 2.406; 95%CI: 1.125-5.147; P = 0.024) was identified as an independent predictor of OS.
| Factor | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, yr | ||||||
| < 75 | 1 | 1 | ||||
| ≥ 75 | 0.520 | 0.277-0.976 | 0.042 | 0.658 | 0.345-1.253 | 0.203 |
| Sex | ||||||
| Male | 1 | |||||
| Female | 1.301 | 0.845-2.003 | 0.233 | |||
| BMI, kg/m2 | ||||||
| Underweight (< 18.5) | 1 | 1 | ||||
| Normal (18.5-24.9) | 0.527 | 0.316-0.878 | 0.014 | 0.635 | 0.377-1.067 | 0.086 |
| Overweight (> 25.0) | 0.394 | 0.195-0.795 | 0.009 | 0.506 | 0.250-1.040 | 0.064 |
| Stage | ||||||
| III | 1 | 1 | ||||
| IV | 2.447 | 1.227-4.881 | 0.011 | 2.182 | 1.085-4.387 | 0.029 |
| ECOG PS | ||||||
| 0-1 | 1 | 1 | ||||
| 2-3 | 15.197 | 8.486-27.214 | < 0.001 | 12.772 | 7.067-23.085 | < 0.001 |
| GI-irAE | ||||||
| Continued administration of ICIs | 1 | |||||
| Discontinued administration of ICIs | 0.904 | 0.165-4.945 | 0.907 | |||
| Non-GI-irAEs | 1.334 | 0.489-3.642 | 0.574 | |||
| Factor | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, yr | ||||||
| < 75 | 1 | 1 | ||||
| ≥ 75 | 1.717 | 1.067-2.761 | 0.026 | 1.474 | 0.816-2.663 | 0.199 |
| Sex | ||||||
| Male | 1 | 1 | ||||
| Female | 0.593 | 0.354-0.993 | 0.047 | 0.793 | 0.418-1.506 | 0.479 |
| BMI, kg/m2 | ||||||
| Underweight (< 18.5) | 1 | |||||
| Normal (18.5-24.9) | 1.252 | 0.671-2.336 | 0.48 | |||
| Overweight (> 25.0) | 1.044 | 0.510-2.137 | 0.906 | |||
| Stage | ||||||
| III | 1 | |||||
| IV | 1.758 | 0.838-3.686 | 0.135 | |||
| ECOG PS | ||||||
| 0-1 | 1 | 1 | ||||
| 2-3 | 3.014 | 1.427-6.366 | 0.004 | 2.406 | 1.125-5.147 | 0.024 |
| GI-irAE | ||||||
| Continued administration of ICIs | 1 | 1 | ||||
| Discontinued administration of ICIs | 3.818 | 0.767-18.996 | 0.102 | 4.079 | 0.779-21.368 | 0.096 |
| Non-GI-irAEs | 3.25 | 1.020-10.360 | 0.046 | 3.081 | 0.963-9.861 | 0.058 |
| ICI | ||||||
| Anti PD-1/PD-L1 antibody | 1 | |||||
| Anti CTLA-4 antibody | 1.366 | 0.837-2.228 | 0.212 | |||
In this study, patients in the CTLA-4 group had a higher incidence of GI-irAEs and a higher frequency of diarrhea than those in the PD-1/PD-L1 group. In addition, treatment of GI-irAEs required more steroids in the CTLA-4 group than in the PD-1/PD-L1 group. In a previous report[7], the onset of GI-irAEs has been reported to occur 6-7 wk after the initiation of ipilimumab, an anti-CTLA-4 antibody. Patients who received anti-PD-1 monotherapy developed GI-irAEs at a median of 25.4 wk (range: 0.6-119.9), whereas those who received combination therapy with anti-PD-1 and anti-CTLA-4 antibodies developed GI-irAEs earlier, at a median of 7.2 wk (range: 0.7-51)[8]. Regarding incidence, a meta-analysis by Wang et al[20] reported overall incidences of colitis of 9.1% among patients treated with anti-CTLA-4 antibody monotherapy, 1.3% among those treated with anti-PD-1/PD-L1 antibody monotherapy, and 13.6% among those treated with a combination of anti-PD-1 and anti-CTLA-4 antibodies. Similarly, our study showed that the incidence of GI-irAEs in the CTLA-4 group was indeed higher than that in the PD-1/PD-L1 group.
There are several possible explanations for the higher incidence, severity, and use of steroids among patients treated with anti-CTLA-4 antibodies compared to those treated with anti-PD-1/PD-L1 antibodies. CTLA-4 is required for the accumulation of regulatory T cells in the bowel[21], and is thought to play an essential role in the maintenance of bowel homeostasis, even in the absence of inflammation. In contrast, PD-1 depends on PD-L1/PD-L2 expression to regulate T-cell activation, and PD-L1 and PD-L2 are upregulated by inflammation, inflammatory cytokines, and chemokines[22-25]. When this mechanism is blocked by anti-PD-1/PD-L1 antibodies, it may be difficult to control inflammation, given that blocking with ICIs leads to a partial block of the mechanisms that trigger inflammation. Some reports suggest that these me
There were no reported differences in the CT or endoscopic findings of enteritis caused by anti-CTLA-4 and anti-PD-1/PD-L1 antibodies. There was no specific trend, and a variety of findings were found in this study. In one case in the PD-1/PD-L1 group, an ischemic colitis-like endoscopic finding, which is rarely reported as a GI-irAE, was observed. Since necrotizing gastritis has been previously reported as an AE in the upper GI tract[9], it was included as a GI-irAE in this study. When GI-irAEs are suspected, CT and endoscopy should be considered, as they are useful for confirming the extent of the lesion and excluding other diseases[19].
In this study, OS was prolonged in the ICI continuation group compared to the non-GI-irAE group among patients with MM, but not among those with NSCLC. Ac
Conversely, patients who discontinued ICIs after developing GI-irAEs did not have prolonged OS compared to those who did not develop GI-irAEs in this study. We consider this result to be caused by the difficulty in continuing ICIs in patients with no antitumor effects at the time of developing GI-irAEs. We also consider the possibility that OS may have been shortened by not administering ICIs for a sufficient period.
ICI administration often cannot be continued depending on the extent of irAEs. According to consensus guidelines[19], careful follow-up after the onset of irAEs or the administration of steroids and continuation of ICIs after improvement of irAEs to Grade 1 can be considered. However, severe irAEs (Grade 3 or higher) may result in permanent discontinuation of ICIs, necessitating hospitalization or surgery, and may be fatal in some cases.
As irAEs are life-threatening, depending on severity, it is difficult to determine whether to continue ICIs in patients with Grade 1 irAEs or to resume ICIs after recovery from Grade 2 or higher irAEs. However, it is expected that the number of cases in which resuming ICIs is considered will increase in the future when irAEs occur and no other alternative treatments are available. IrAEs that occur during ICI reintroduction are less severe than initial irAEs and can be tolerated if monitored appropriately[31,32]. GI-irAE recurrence in cases of ICI re-administration after GI-irAE onset has been observed in about one-third of patients[33], and GI-irAEs occurring at re-administration were less frequent and less severe than the initial GI-irAEs[34].
This study showed that the continuation of ICI administration after GI-irAEs in MM may contribute to enhanced patient prognosis. However, which patients with severe GI-irAEs (Grade ≥ 3) should continue ICIs is a topic for future studies. In addition, we believe that the decision to reinitiate ICI treatment after irAEs should be based on the clinical judgment of the treating physician, considering the physical health of the patient, and appropriate informed consent.
Once the mechanism of irAE development and predictive markers for irAE development are identified, it will be possible to continue ICI treatment for a longer period while monitoring patients prone to irAE development and preventing irAEs. Since this may improve the prognosis of patients, further research to clarify the pathogenesis is desirable.
There are several limitations to this study. First, it is a single-center, retrospective study. Second, abdominal CT scans and lower GI endoscopy were not performed in all patients in this study, and the number of patients who had abdominal CT scans and lower GI endoscopy was not enough. More cases are expected in the future. Third, the OS analysis for each ICI regimen was not statistically significant, probably due to the small sample size. Therefore, patients who received ICI monotherapy and those who received multiple ICIs sequentially were included in the analysis.
GI-irAEs tended to occur more frequently and with higher severity in patients treated with anti-CTLA-4 antibodies or combination therapy than in those treated with anti-PD-1/PD-L1 antibodies. Abdominal CT and lower GI endoscopy did not show any characteristic findings or trends. We found evidence that the clinical characteristics and pathologies may be different between the groups, but a further investigation with a larger sample size is necessary.
In terms of prognosis, OS was not prolonged in NSCLC patients with or without GI-irAEs; however, in MM patients, OS was significantly prolonged in patients who developed GI-irAEs and continued ICI treatment compared with that in other patients.
Immune checkpoint inhibitors (ICIs) are gaining popularity as a treatment for advanced cancer. However, among immune-related adverse events (irAEs), gastro
There are only a few coherent reports on the clinical characteristics of GI-irAEs for each ICI. In addition, the correlation between the development of GI-irAEs and patient prognosis has not been fully elucidated.
We aimed to evaluate the clinical characteristics of GI-irAEs and its influence on survival in patients treated with ICI.
We retrospectively reviewed the records of 661 patients who received ICIs at Nagoya University Hospital from September 2014 to August 2020. We analyzed the clinical characteristics of patients who received an anti-programmed cell death-1/program
GI-irAEs occurred in 34 of 605 patients (5.6%) treated with an anti-PD-1/PD-L1 antibody alone and in nine of 56 patients (16.1%) treated with an CTLA-4 antibody alone or a combination of anti-PD-1 and anti-CTLA-4 antibodies. The cumulative incidence and median daily diarrhea frequency were significantly higher in patients receiving anti-CTLA-4 antibodies (P < 0.05). In 130 patients with malignant melanoma (MM), overall survival was significantly prolonged in the group that continued ICI treatment despite the development of GI-irAEs compared to the group that did not experience GI-irAEs (P = 0.035).
GI-irAEs occurred more frequently and with a higher severity in patients using anti-CTLA-4 antibodies than in those using anti-PD-1/PD-L1 antibodies. In patients with MM who developed GI-irAEs and continued treatment with ICIs, overall survival was significantly prolonged.
Multicenter studies with large samples are expected to evaluate clinical characteristics of GI-irAEs and their association to long-term survival outcomes.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soldera J, Yu F S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1801] [Cited by in RCA: 1826] [Article Influence: 166.0] [Reference Citation Analysis (0)] |
| 2. | Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3964] [Cited by in RCA: 4334] [Article Influence: 433.4] [Reference Citation Analysis (0)] |
| 3. | Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1826] [Cited by in RCA: 2613] [Article Influence: 435.5] [Reference Citation Analysis (0)] |
| 4. | Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Plimack ER, Procopio G, McDermott DF, Castellano D, Choueiri TK, Donskov F, Gurney H, Oudard S, Richardet M, Peltola K, Alva AS, Carducci M, Wagstaff J, Chevreau C, Fukasawa S, Tomita Y, Gauler TC, Kollmannsberger CK, Schutz FA, Larkin J, Cella D, McHenry MB, Saggi SS, Tannir NM. Nivolumab vs everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126:4156-4167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 5. | Geukes Foppen MH, Rozeman EA, van Wilpe S, Postma C, Snaebjornsson P, van Thienen JV, van Leerdam ME, van den Heuvel M, Blank CU, van Dieren J, Haanen JBAG. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3:e000278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 6. | Wang Y, Abu-Sbeih H, Mao E, Ali N, Qiao W, Trinh VA, Zobniw C, Johnson DH, Samdani R, Lum P, Shuttlesworth G, Blechacz B, Bresalier R, Miller E, Thirumurthi S, Richards D, Raju G, Stroehlein J, Diab A. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm Bowel Dis. 2018;24:1695-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (2)] |
| 7. | Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1107] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 8. | Wang DY, Mooradian MJ, Kim D, Shah NJ, Fenton SE, Conry RM, Mehta R, Silk AW, Zhou A, Compton ML, Al-Rohil RN, Lee S, Voorhees AL, Ha L, McKee S, Norrell JT, Mehnert J, Puzanov I, Sosman JA, Chandra S, Gibney GT, Rapisuwon S, Eroglu Z, Sullivan R, Johnson DB. Clinical characterization of colitis arising from anti-PD-1 based therapy. Oncoimmunology. 2019;8:e1524695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Collins M, Michot JM, Danlos FX, Mussini C, Soularue E, Mateus C, Loirat D, Buisson A, Rosa I, Lambotte O, Laghouati S, Chaput N, Coutzac C, Voisin AL, Soria JC, Marabelle A, Champiat S, Robert C, Carbonnel F. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol. 2017;28:2860-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Tandon P, Bourassa-Blanchette S, Bishay K, Parlow S, Laurie SA, McCurdy JD. The Risk of Diarrhea and Colitis in Patients With Advanced Melanoma Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis. J Immunother. 2018;41:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Abu-Sbeih H, Ali FS, Qiao W, Lu Y, Patel S, Diab A, Wang Y. Immune checkpoint inhibitor-induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol Immunother. 2019;68:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Masuda K, Shoji H, Nagashima K, Yamamoto S, Ishikawa M, Imazeki H, Aoki M, Miyamoto T, Hirano H, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Boku N. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer. 2019;19:974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 13. | Xing P, Zhang F, Wang G, Xu Y, Li C, Wang S, Guo Y, Cai S, Wang Y, Li J. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer. 2019;7:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 14. | Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 770] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 15. | Fukihara J, Sakamoto K, Koyama J, Ito T, Iwano S, Morise M, Ogawa M, Kondoh Y, Kimura T, Hashimoto N, Hasegawa Y. Prognostic Impact and Risk Factors of Immune-Related Pneumonitis in Patients With Non-Small-Cell Lung Cancer Who Received Programmed Death 1 Inhibitors. Clin Lung Cancer. 2019;20:442-450.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, Lum P, Raju G, Shuttlesworth G, Stroehlein J, Diab A. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 17. | Maillet D, Corbaux P, Stelmes JJ, Dalle S, Locatelli-Sanchez M, Perier-Muzet M, Duruisseaux M, Kiakouama-Maleka L, Freyer G, Boespflug A, Péron J. Association between immune-related adverse events and long-term survival outcomes in patients treated with immune checkpoint inhibitors. Eur J Cancer. 2020;132:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Matsuoka H, Hayashi T, Takigami K, Imaizumi K, Shiroki R, Ohmiya N, Sugiura K, Kawada K, Sawaki A, Maeda K, Ando Y, Uyama I. Correlation between immune-related adverse events and prognosis in patients with various cancers treated with anti PD-1 antibody. BMC Cancer. 2020;20:656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2245] [Cited by in RCA: 2566] [Article Influence: 366.6] [Reference Citation Analysis (0)] |
| 20. | Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology. 2017;6:e1344805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 21. | Barnes MJ, Griseri T, Johnson AM, Young W, Powrie F, Izcue A. CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol. 2013;6:324-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 503] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 23. | Lee I, Wang L, Wells AD, Ye Q, Han R, Dorf ME, Kuziel WA, Rollins BJ, Chen L, Hancock WW. Blocking the monocyte chemoattractant protein-1/CCR2 chemokine pathway induces permanent survival of islet allografts through a programmed death-1 ligand-1-dependent mechanism. J Immunol. 2003;171:6929-6935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O'Shea MA, Fauci AS. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738-6746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 407] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 25. | Gong AY, Zhou R, Hu G, Li X, Splinter PL, O'Hara SP, LaRusso NF, Soukup GA, Dong H, Chen XM. MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes. J Immunol. 2009;182:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, Ettinger DS, Hann CL, Brahmer JR, Ricciuti B, Owen D, Toi Y, Walker P, Otterson GA, Patel SH, Sugawara S, Naidoo J. Multisystem Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Treatment of Non-Small Cell Lung Cancer. JAMA Oncol. 2020;6:1952-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 327] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 27. | Ando T, Ueda A, Ogawa K, Motoo I, Kajiura S, Nakajima T, Hirano K, Okumura T, Tsukada K, Hara T, Suzuki N, Nakada N, Horikawa N, Fujii T, Yasuda I. Prognosis of Immune-related Adverse Events in Patients With Advanced Gastric Cancer Treated With Nivolumab or Pembrolizumab: A Multicenter Retrospective Analysis. In Vivo. 2021;35:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? BMC Med. 2020;18:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 243] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 29. | Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr, Anders RA, Sosman JA, Moslehi JJ. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375:1749-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1305] [Cited by in RCA: 1694] [Article Influence: 188.2] [Reference Citation Analysis (0)] |
| 30. | Läubli H, Koelzer VH, Matter MS, Herzig P, Dolder Schlienger B, Wiese MN, Lardinois D, Mertz KD, Zippelius A. The T cell repertoire in tumors overlaps with pulmonary inflammatory lesions in patients treated with checkpoint inhibitors. Oncoimmunology. 2018;7:e1386362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, Cengizalp G, Vozy A, Laparra A, Varga A, Hollebecque A, Champiat S, Marabelle A, Massard C, Lambotte O. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol. 2019;5:1310-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 272] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 32. | Allouchery M, Lombard T, Martin M, Rouby F, Sassier M, Bertin C, Atzenhoffer M, Miremont-Salame G, Perault-Pochat MC, Puyade M; French Network of Regional Pharmacovigilance Centers. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 33. | Abu-Sbeih H, Ali FS, Naqash AR, Owen DH, Patel S, Otterson GA, Kendra K, Ricciuti B, Chiari R, De Giglio A, Sleiman J, Funchain P, Wills B, Zhang J, Naidoo J, Philpott J, Gao J, Subudhi SK, Wang Y. Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. J Clin Oncol. 2019;37:2738-2745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 34. | de Malet A, Antoni G, Collins M, Soularue E, Marthey L, Vaysse T, Coutzac C, Chaput N, Mateus C, Robert C, Carbonnel F. Evolution and recurrence of gastrointestinal immune-related adverse events induced by immune checkpoint inhibitors. Eur J Cancer. 2019;106:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |