Published online Nov 7, 2021. doi: 10.3748/wjg.v27.i41.7173
Peer-review started: April 2, 2021
First decision: June 24, 2021
Revised: June 26, 2021
Accepted: September 3, 2021
Article in press: September 3, 2021
Published online: November 7, 2021
Processing time: 217 Days and 13.6 Hours
Combined hepatocellular carcinoma (HCC) and cholangiocarcinoma (cHCC-CCA) is defined as a single nodule showing differentiation into HCC and intrahepatic cholangiocarcinoma and has a poor prognosis.
To develop a radiomics nomogram for predicting post-resection survival of patients with cHCC-CCA.
Patients with pathologically diagnosed cHCC-CCA were randomly divided into training and validation sets. Radiomics features were extracted from portal venous phase computed tomography (CT) images using the least absolute shrinkage and selection operator Cox regression and random forest analysis. A nomogram integrating the radiomics score and clinical factors was developed using univariate analysis and multivariate Cox regression. Nomogram perfor
CT and clinical data of 118 patients were included in the study. The radiomics score, vascular invasion, anatomical resection, total bilirubin level, and satellite lesions were found to be independent predictors of overall survival (OS) and were therefore included in an integrative nomogram. The nomogram was more strong
This nomogram may predict survival of cHCC-CCA patients after hepatectomy and therefore help identify those more likely to benefit from surgery.
Core Tip: Combined hepatocellular carcinoma (HCC) and cholangiocarcinoma (cHCC-CCA) is defined as a single nodule showing differentiation into HCC and intrahepatic cholangiocarcinoma. Studies vary regarding the prognosis of cHCC-CCA patients after potentially curative hepatectomy, with 5-year postoperative overall survival rates ran
- Citation: Tang YY, Zhao YN, Zhang T, Chen ZY, Ma XL. Comprehensive radiomics nomogram for predicting survival of patients with combined hepatocellular carcinoma and cholangiocarcinoma. World J Gastroenterol 2021; 27(41): 7173-7189
- URL: https://www.wjgnet.com/1007-9327/full/v27/i41/7173.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i41.7173
Combined hepatocellular carcinoma (HCC) and cholangiocarcinoma (cHCC-CCA), which arises in hepatic progenitor cells, accounts for 0.8%-6.5% of primary liver carcinoma cases[1-5]. The World Health Organization defines the condition as the presence of a single nodule showing differentiation into HCC and intrahepatic cholangiocarcinoma (ICC)[6,7]. There is disagreement in the literature on whether the prognosis of cHCC-CCA patients is worse or similar to that of patients with only HCC. Several studies concur that the prognosis of cHCC-CCA patients is comparable to that of patients with only ICC[8-11]. Studies vary regarding the prognosis of cHCC-CCA patients after potentially curative hepatectomy, with 5-year postoperative overall survival (OS) rates ranging from 8% to 63%[12-15]. A reliable method to predict prognosis after resection may help select cHCC-CCA patients more likely to benefit from surgery.
Radiomics is a promising comprehensive analysis to predict the prognosis of liver cancer patients after hepatectomy, which is a post-processing method to quantitatively evaluate imaging features in order to assess cancer heterogeneity non-invasively and objectively[16,17]. Radiomics features have proven effective in predicting the survival of patients with HCC or ICC alone[18-21]. Radiomics can also differentiate cHCC-CCA from common HCC or ICC[18,22], although no radiomics models have been estab
The predictive performance of radiomics features may improve when combined with clinical factors, as demonstrated for patients with ICC[23-25]. Therefore, the current study aimed to construct and validate a nomogram based on radiomics and clinical features for predicting postoperative survival of cHCC-CCA patients. This prognostic model may help guide treatment decisions for these patients.
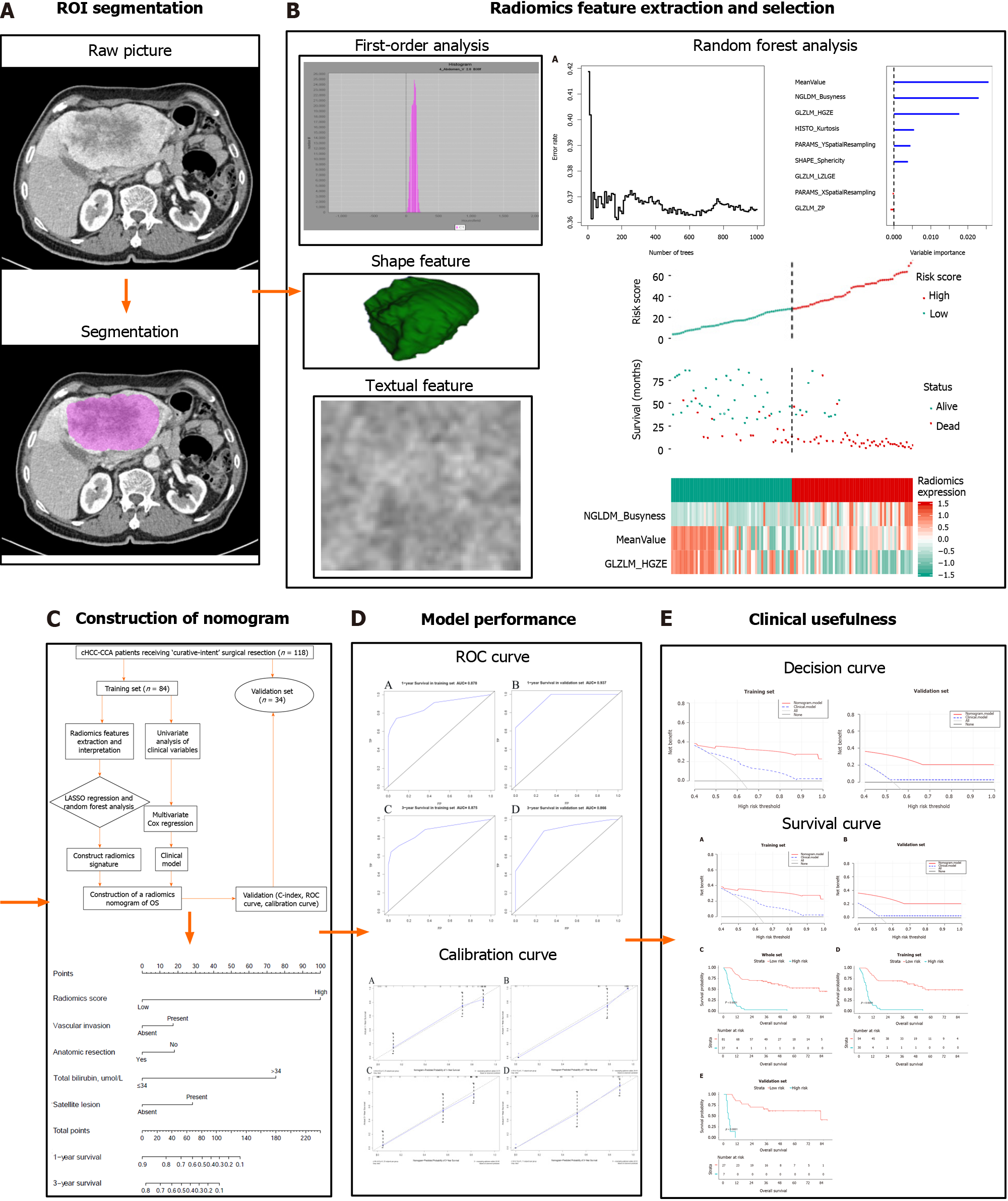
This retrospective study was approved by the West China Hospital Ethics Committee, and the requirement for informed consent was waived. All patients agreed to undergo medical examination and were informed that their anonymized medical data would be analyzed and published for the purposes of medical research. We retrospectively reviewed the data of all patients: (1) Who were diagnosed with cHCC-CCA based on the 2019 guidelines of the World Health Organization which defined cHCC-CCA as a single nodule showing differentiation into HCC and ICC; (2) Who underwent hepatectomy with curative intent at West China Hospital between February 2012 and May 2017; and (3) For whom complete medical records were available during hospitalization and during follow-up, as well as computed tomography (CT) data within 2 wk before surgery.
Patients were excluded if they were diagnosed with morphologically typical HCC or ICC based on the expression of markers for cholangiocytes, hepatocytes, or progenitor cells (e.g., keratins 7 and 19 based on immunostaining). Patients were considered to have common HCC if they showed trabecular growth (often accom
Patients were also excluded if they had received transcatheter arterial chemoembolization or any other type of chemotherapy before CT, or if they had other malignancies simultaneously with cHCC-CCA. The primary endpoint of this study was OS, defined as the time from the date of surgery until the date of all-cause death or last follow-up. Patients were routinely followed at 1 mo after surgery and then every 3-6 mo there
Enhanced CT of the abdomen was performed with a single 64-detector row scanner (Brilliance 64, Philips Medical Systems, Eindhoven, The Netherlands) in all the patients. The scan parameters were as follows: Beam pitch, 0.891; tube voltage, 120 kV; tube current, 200 mA; detector collimation, 0.75 mm; slice thickness, 1.0 mm; re
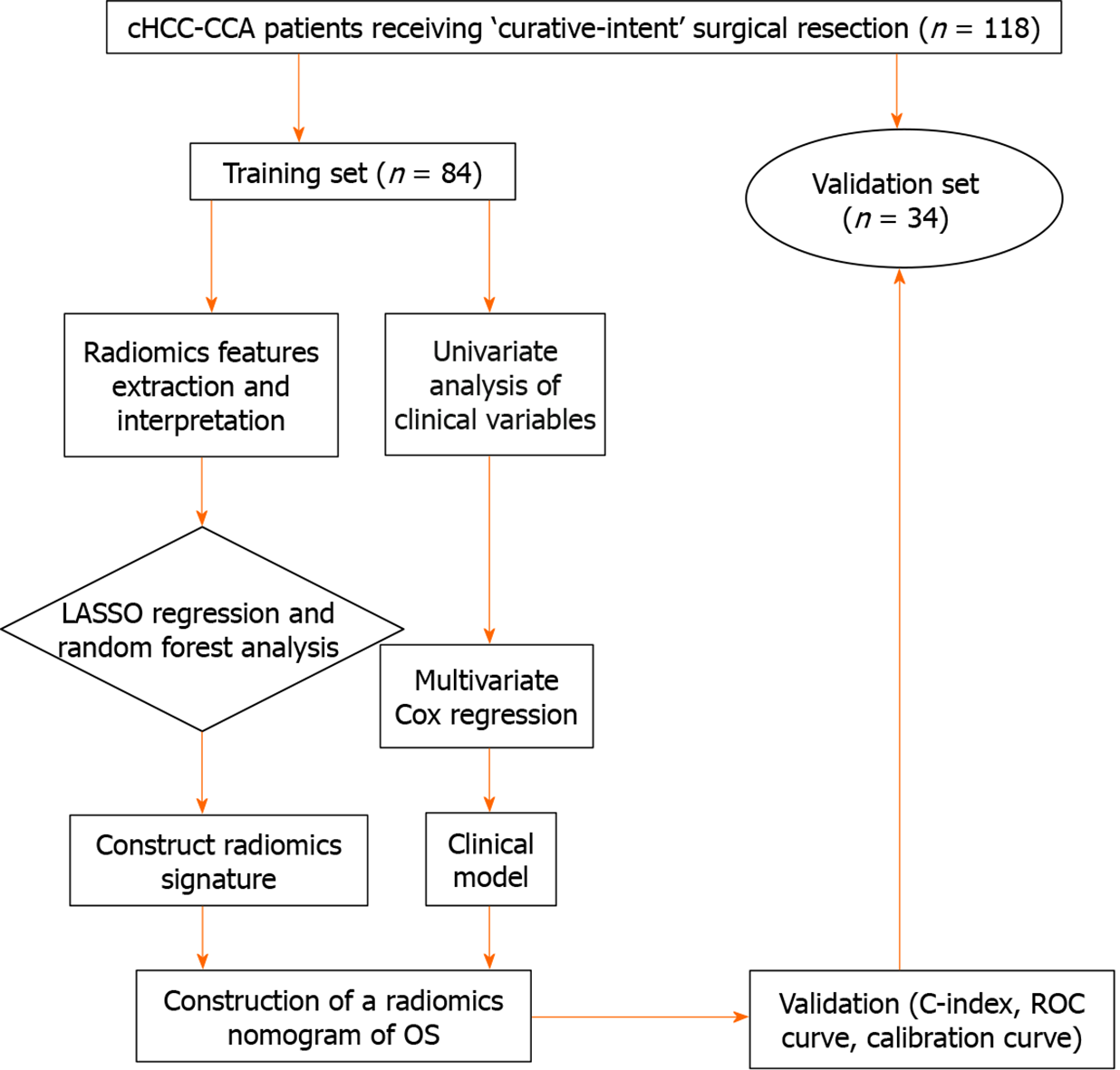
All patients were randomly divided into a training set and validation set at a ratio of 7:3. All CT images from portal venous phase scanning were loaded into LIFEx software (version 3.74; CEA-SHFJ, Orsay, France)[28]. Working independently, two radiologists manually drew regions of interest for each patient within the hepatic neoplasm in all portal venous phase CT images. Radiomics features in the CT images were screened using the Least Absolute Shrinkage and Selection Operator (LASSO) and Cox regression, followed by random forest analysis[29]. The selected radiomics features were linearly combined with their own weighting coefficients, generating a radiomics score for each patient.
All clinical variables in the training set were subjected to univariate analysis followed by multivariate Cox analysis with step-wise selection in order to identify independent predictors of OS. In these analyses, total bilirubin level was converted into a cate
To develop the nomogram, radiomics scores were categorized as “high” or “low” based on whether they were greater or smaller than the median score. Then the nomogram was constructed based on the radiomics score and the clinical risk factors identified in multivariate Cox regression. Within the nomogram, each variable was scored ranging from 0 to 100, and the variable associated with the greatest hazard ratio (HR) was assigned 100 points[30]. Using the nomogram, we classified patients as being at high or low risk based on the maximum Youden index[31].
The performance of the nomogram was assessed in terms of a calibration curve related to the predicted and observed OS, the C-index used to assess model discrimination, and receiver operating characteristic (ROC) curve[32]. The clinical usefulness of the nomogram was assessed using decision curve analysis[33].
Differences in continuous variables were assessed for significance using the Wilcoxon rank-sum test if the data were skewed, or Student’s t test if the data showed a normal distribution. Differences in categorical variables were assessed using the χ2 or Fisher’s exact test. OS was plotted using the Kaplan-Meier method, and groups were compared using the log-rank test. All statistical analyses were performed with EmpowerStats (version 2.20; 2011 X&Y Solutions) and R software (version 4.0.0; The R Foundation). The following packages in R were used: glmnet, cmprsk, rms, survival, rmda, and devtools. Differences with P < 0.05 were considered statistically significant.
A total of 118 eligible patients (86.4% men) were enrolled (Table 1). Their mean age was 51.6 years, and 90 patients had been diagnosed when they were younger than 60 years. Follow-up data were complete for 110 patients, who were followed for a median of 25.1 mo (95% confidence interval [CI]: 17.3-59.7 mo). Median OS was 21.6 mo, and OS rates were 61.0% at 1 year, 48.3% at 3 years, and 37.4% at 5 years.
| Variable | Entire cohort (n = 118) | Training set (n = 84) | Validation set (n = 34) | P value |
| Male sex | 102 (86.4) | 73 (86.9) | 29 (85.3) | 0.817 |
| Age, yr | 51.6 ± 10.5 | 51.2 ± 10.5 | 52.7 ± 10.6 | 0.484 |
| Hypertension | 11 (9.3) | 7 (8.3) | 4 (11.8) | 0.561 |
| Diabetes mellitus | 7 (5.9) | 6 (7.1) | 1 (2.9) | 0.382 |
| Hepatitis B/C | 61 (51.7) | 40 (47.6) | 21 (61.8) | 0.164 |
| Child-Pugh, A/B | 116/2 | 83/1 | 33/1 | 0.495 |
| Liver cirrhosis | 47 (39.8) | 35 (41.7) | 12 (35.3) | 0.522 |
| Hypersplenia | 15 (12.7) | 11 (13.1) | 4 (11.8) | 0.844 |
| ALT (U/L) | 55.2 ± 100.4 | 46.1 ± 29.3 | 77.6 ± 181.1 | 0.807 |
| AST (U/L) | 59.8 ± 136.6 | 48.1 ± 28.8 | 88.6 ± 250.7 | 0.513 |
| ALB (g/L) | 42.1 ± 4.6 | 42.3 ± 4.0 | 41.5 ± 5.7 | 0.643 |
| TB (mmol/L) | 15.9 ± 10.1 | 15.7 ± 10.1 | 16.5 ± 10.0 | 0.597 |
| AFP (ng/mL) | 285.2 ± 475.1 | 256.3 ± 454.6 | 356.5 ± 522.4 | 0.156 |
| CA19-9 (U/mL) | 106.8 ± 251.2 | 109.7 ± 258.3 | 99.6 ± 236.2 | 0.184 |
| CA125 (U/mL) | 117.0 ± 624.6 | 152.9 ± 727.5 | 18.3 ± 11.9 | 0.541 |
| CEA (ng/mL) | 6.4 ± 30.3 | 7.5 ± 35.5 | 3.4 ± 3.2 | 0.444 |
| Liver fibrosis | 0.871 | |||
| No significant fibrosis | 15 (13.8) | 11 (13.8) | 4 (13.8) | |
| Significant fibrosis | 37 (33.9) | 26 (32.5) | 11 (37.9) | |
| Advanced fibrosis | 57 (52.3) | 43 (53.8) | 14 (48.3) | |
| Not mentioned | 8 (6.8) | 3 (3.6) | 5 (14.7) | |
| Tumor size, ≤ 5 cm | 38 (32.2) | 20 (23.8) | 18 (52.9) | 0.002 |
| Tumor number, ≥ 2 | 67 (56.8) | 52 (61.9) | 15 (44.1) | 0.077 |
| Satellite lesions | 42 (35.6) | 29 (34.5) | 13 (38.2) | 0.703 |
| Vascular invasion | 46 (39.0) | 35 (41.7) | 11 (32.4) | 0.347 |
| Lymph node infiltration | 15 (12.7) | 10 (11.9) | 5 (14.7) | 0.679 |
| Differentiation | 0.578 | |||
| Well | 44 (37.3) | 30 (35.7) | 14 (41.2) | |
| Moderate | 22 (18.6) | 18 (21.4) | 4 (11.8) | |
| Poor | 1 (0.8) | 1 (1.2) | 0 (0.0) | |
| Undifferentiated | 51 (43.2) | 35 (41.7) | 16 (47.1) | |
| 8th AJCC stage | 0.027 | |||
| I | 9 (7.6) | 7 (8.3) | 2 (5.9) | |
| II | 28 (23.7) | 14 (16.7) | 14 (41.2) | |
| III | 66 (55.9) | 53 (63.1) | 13 (38.2) | |
| IV | 15 (12.7) | 10 (11.9) | 5 (14.7) | |
| T stage | 0.042 | |||
| T1 | 13 (11.0) | 9 (10.7) | 4 (11.8) | |
| T2 | 29 (24.6) | 15 (17.9) | 14 (41.2) | |
| T3 | 45 (38.1) | 37 (44.0) | 8 (23.5) | |
| T4 | 31 (26.3) | 23 (27.4) | 8 (23.5) | |
| N stage | 0.762 | |||
| N0 | 103 (87.3) | 74 (88.1) | 29 (85.3) | |
| N1 | 15 (12.7) | 10 (11.9) | 5 (14.7) | |
| Transfusion | 17 (14.4) | 14 (16.7) | 3 (8.8) | 0.388 |
| Blood loss ≤ 400 mL | 71 (60.2) | 49 (58.3) | 22 (64.7) | 0.522 |
| Margin, R1 | 13 (11.0) | 9 (10.7) | 4 (11.8) | 0.869 |
| Surgical method | 0.285 | |||
| Major resection | 57 (48.3) | 44 (52.4) | 13 (38.2) | |
| Minor resection | 50 (42.4) | 32 (38.1) | 18 (52.9) | |
| Resection + ablation | 11 (9.3) | 8 (9.5) | 3 (8.8) | |
| Anatomical resection | 50 (43.9) | 39 (48.1) | 11 (33.3) | 0.148 |
| Postoperative TACE | 35 (29.7) | 28 (33.3) | 7 (20.6) | 0.17 |
| Hospital stay (d) | 12.2 ± 4.5 | 12.3 ± 4.4 | 11.9 ± 5.0 | 0.608 |
| Overall survival (mo) | 30.8 ± 26.3 | 29.6 ± 26.2 | 33.6 ± 26.9 | 0.462 |
Patients were randomly assigned to either the training or validation set, and the two sets did not differ significantly in terms of clinical features, except for tumor size, American Joint Committee on Cancer stage and T stage. OS rates at 1 and 3 years were 58.3% and 46.4% in the training set, compared to 67.7% and 52.9% in the validation set.
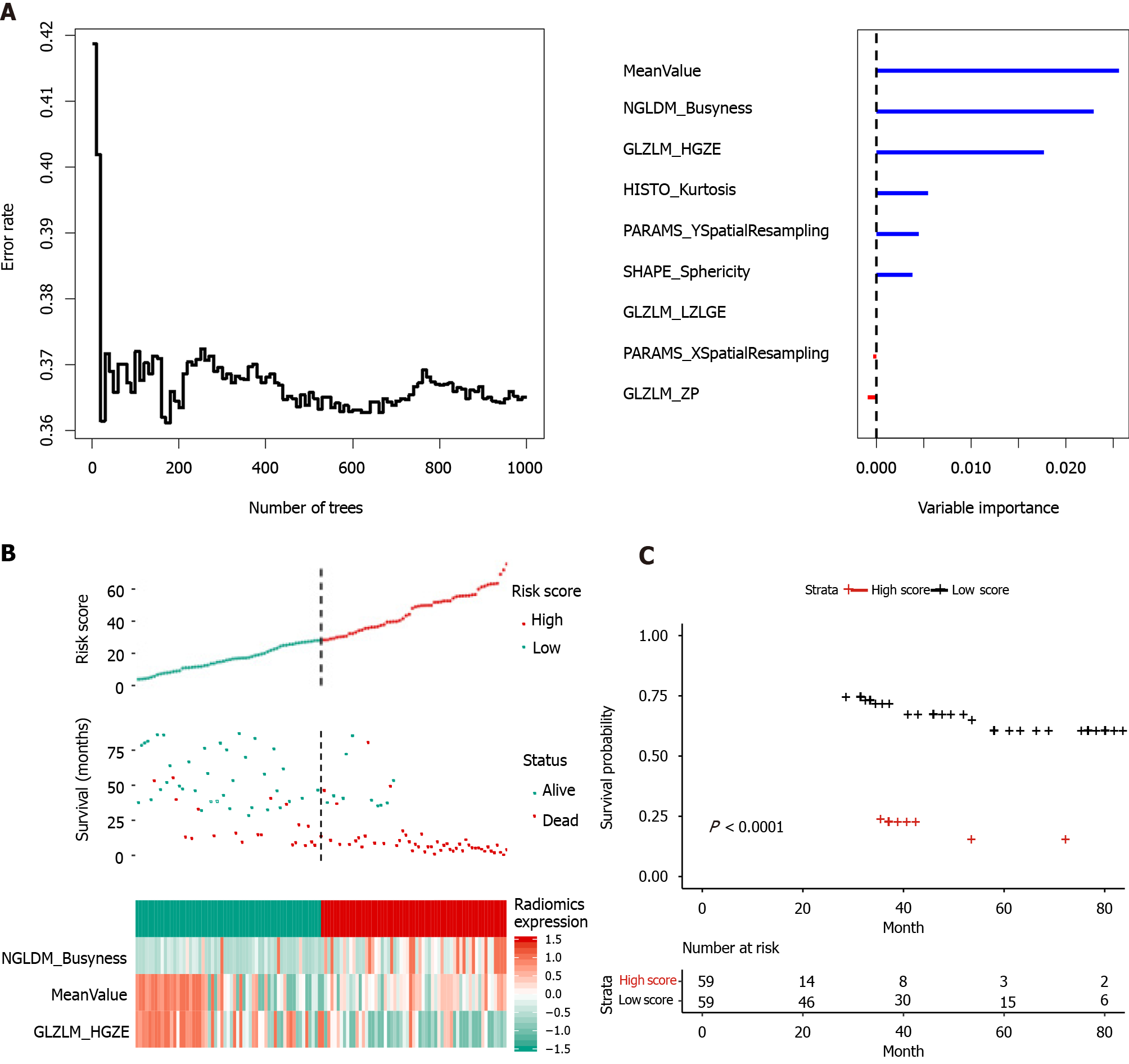
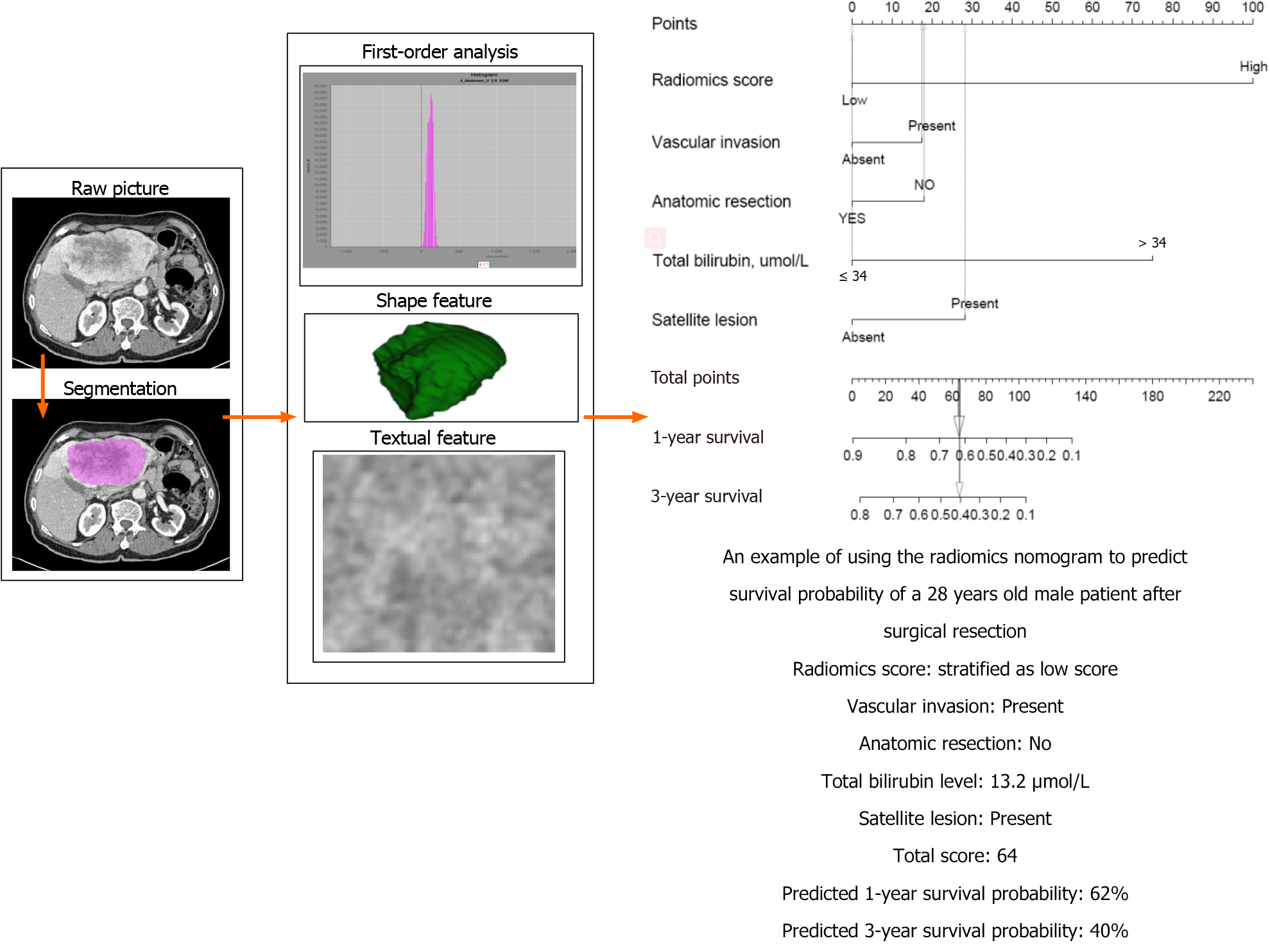
The integrative nomogram flow chart is depicted in Figures 1 and 2. For each patient, data on 49 radiomics features were extracted from portal venous phase CT images. Among these 49 features, LASSO regression selected nine with non-zero coefficients, of which random forest analysis selected three (MeanValue, NGLDM Busyness and GLZLM HGZE) (Supplementary Table 1) that showed the highest prediction values (variable importance > 0.01, Figure 3A). Radiomics scores were calculated based on these three features, and scores were subsequently categorized into “high” or “low” based on whether they were lower or higher than the median score (Figure 3).
In total, 31 clinical variables were initially considered in the univariate analysis; and seven variables with P < 0.1 were then entered into the multivariate Cox analysis (Table 2). The multivariate analysis identified four predictors of OS: Vascular invasion, anatomical resection, total bilirubin level, and satellite lesions. Total bilirubin level (> 17.1 μmol/L) resulted in a larger HR (13.94) than the other three risk factors. Ne
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Male sex | 0.470 (0.203-1.088) | 0.078 | 1.767 (0.244-1.316) | 0.186 |
| Age, yr | ||||
| ≤ 60 | Ref. | |||
| > 60 | 1.173 (0.644-2.139) | 0.602 | ||
| Liver cirrhosis | ||||
| Absent | Ref. | |||
| Present | 1.370 (0.852-2.203) | 0.194 | ||
| AFP (ng/mL) | 0.990 (0.597-1.643) | 0.970 | ||
| CA 19-9 (U/mL) | 0.987 (0.586-1.662) | 0.960 | ||
| Albumin (g/L) | 2.496 (0.997-6.244) | 0.051 | 1.025 (0.968-1.085) | 0.403 |
| TB (μmol/L) | ||||
| ≤ 34 | Ref. | Ref. | ||
| > 34 | 17.994 (4.726-68.509) | < 0.001 | 13.943 (3.561-54.602) | < 0.001 |
| Tumor number, multiple | 0.766 (0.473-1.240) | 0.277 | ||
| Satellite lesions | ||||
| Absent | Ref. | Ref. | ||
| Present | 2.037 (1.267-3.268) | 0.003 | 1.762 (1.079-2.877) | 0.024 |
| Vascular invasion | ||||
| Absent | Ref. | Ref. | ||
| Present | 2.009 (1.247-3.239) | 0.004 | 1.725 (1.049-2.834) | 0.032 |
| T stage | ||||
| T1 | Ref. | |||
| T2 | 1.171 (0.705-1.942) | 0.542 | ||
| T3 | 2.424 (0.704-8.348) | 0.161 | ||
| T4 | 3.823 (1.158-12.615) | 0.028 | ||
| Anatomy resection | ||||
| Yes | Ref. | Ref. | ||
| No | 2.011 (1.344-3.006) | 0.006 | 1.731 (1.083-2.767) | 0.028 |
| Margin | ||||
| R0 | Ref. | |||
| R1 | 1.032 (0.446-2.387) | 0.941 | ||
| Postoperative TACE | ||||
| Yes | Ref. | |||
| No | 1.597 (0.924-2.759) | 0.093 | 1.6051 (0.3546-1.0947) | 0.100 |
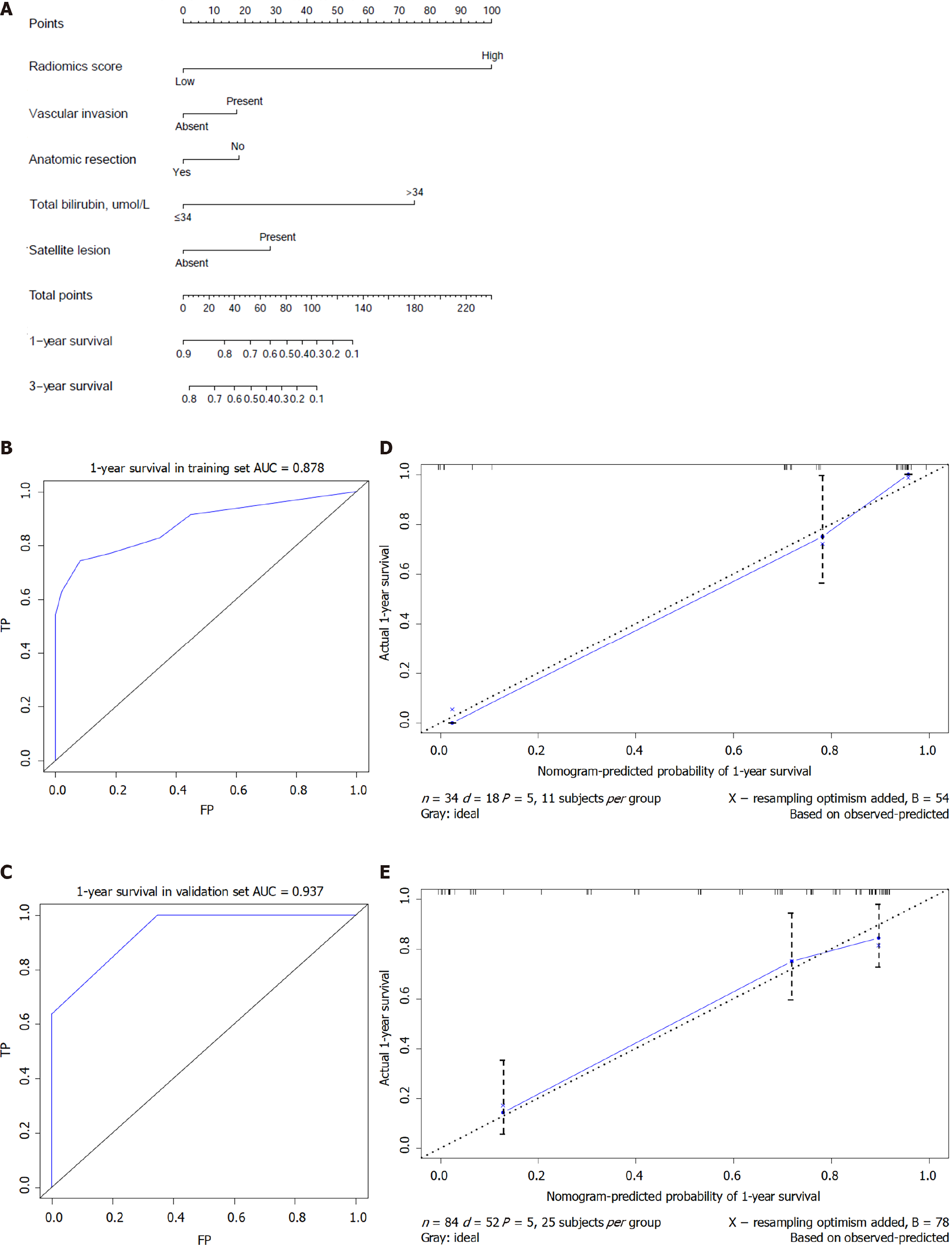
Based on the above-mentioned four clinical factors and the radiomics score, we developed a comprehensive integrative nomogram to predict 1-year and 3-year OS of cHCC-CCA patients after surgical resection with curative intent (Figure 4A). The area under the ROC curve (AUC) for 1-year OS was 0.878 in the training set and 0.937 in the validation set (Figure 4B). The calibration curve of 1-year OS showed good agreement between predicted and observed values in both the training and validation sets (Figure 4C). The AUC for 3-year OS was 0.875 in the training set and 0.866 in the validation set. The C-index was 0.807 (95%CI: 0.756-0.858) in the training set and 0.820 (95%CI: 0.723-0.917) in the validation set. An example of predicting 1- and 3-year OS using the nomogram is shown in Figure 5.
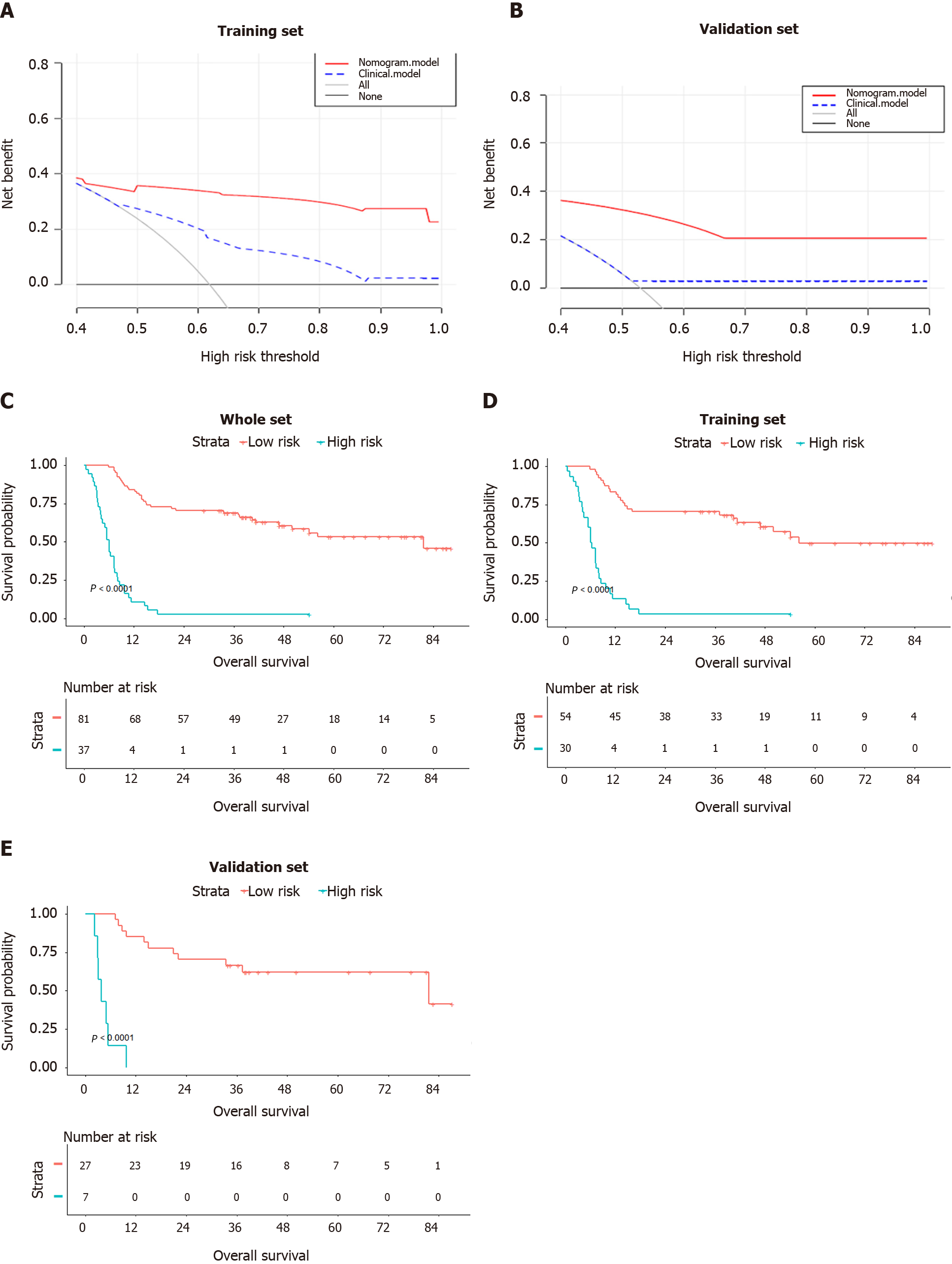
In decision curve analysis, the nomogram showed higher “net benefit” than a model based only on the four clinical factors or models based on “treat-all-patients” or “treat-no-patients” approaches. These results were observed at nearly all threshold probabilities in the training set (Figure 6A) and validation set (Figure 6B).
A total risk score was calculated for each patient by summing the scores for each variable in the nomogram. The maximum Youden index of 105 points in the nomo
Table 3 compares HRs obtained with the integrated nomogram, the radiomics score alone, or a model based only on clinical factors. The model based only on the four clinical risk factors resulted in an HR of 2.65 (95%CI: 1.53-4.60), even though total bilirubin level resulted in an HR of 13.94 (95%CI: 3.56-54.60) in multivariate analysis. The nomogram HR was higher than that provided by models based on the radiomics score or on clinical factors alone.
| Model | HR (95%CI) | P value |
| Radiomics score | < 0.001 | |
| Low risk | Ref. | |
| High risk | 5.908 (3.285-10.626) | |
| Clinical model | < 0.001 | |
| Low risk | Ref. | |
| High risk | 2.653 (1.532-4.595) | |
| Radiomics nomogram | < 0.001 | |
| Low risk | Ref. | |
| High risk | 8.155 (4.498-14.785) |
In the present study, we developed a comprehensive integrative nomogram that takes into account CT radiomics scores and four clinical risk factors that independently predict OS (vascular invasion, anatomical resection, total bilirubin, and satellite lesions), and we showed that this nomogram can predict OS in cHCC-CCA patients following potentially curative hepatectomy. The AUC for 1-year OS was 0.878 in the training set and 0.937 in the validation set. To our knowledge, this is the first CT-based radiomics model to predict postoperative survival of cHCC-CCA patients.
Our results extend the number of situations in which radiomics has shown potential in predicting the survival of patients with liver tumors[34,35]. The patients in our study who were assigned a high radiomics score had a 5.91-fold higher risk of death than those with a low score, consistent with a previously reported association between high radiomics score and risk of recurrence in patients with HCC or ICC[24,36]. These findings imply that radiomics scores may be able to identify patients preoperatively who are more likely to benefit from surgical resection.
Our results further support previous work indicating that combining clinical variables with radiomics features may predict prognosis better than either the va
The rate of vascular invasion in our patients was 39.0%, similar to previous studies and within the prevalence of 9%-89.5% reported for cHCC-CCA[3,39,40]. As shown in Supplementary Figure 1, the OS rate at 3 years was 56.8% among our patients without vascular invasion, compared to only 36.8% among those with invasion, consistent with the association between vascular invasion and worse postoperative prognosis[2,13,41]. Indeed, vascular invasion has been shown to be an independent predictor of postoperative survival in patients with combined hepatocellular-cholangiocarcinoma and it increases the risk of death in these patients by 1.6- fold to 5.2-fold[42,43].
In addition, elevated total bilirubin level (> 34 μmol/L) and no anatomic surgical resection were considered to be independent risk factors related to the poor prognosis of cHCC-CCA patients. Total bilirubin level is one element of the Child-Pugh classification which plays a remarkable role in survival prediction of liver malignancy. In a previous study, Chen et al[44] revealed that elevated total bilirubin level (> 17.1 μmol/L) was an independent risk factor resulting in poor prognosis in advanced HCC patients. Peak postoperative bilirubin > 7.0 mg/dL was significantly related to liver-related death and worse outcomes after major hepatectomy. The group of patients with a total bilirubin level higher than the cut-off value (22.7 μmol/L) was also associated with a poorer OS in another study[45]. Moreover, Chantajitr et al[46] found that dilation of the intrahepatic bile duct was related to a poor prognosis in cHCC-CCA patients, and Lee et al[47] suggested that an increased Child-Pugh score (mean score: 5.8) was related to early death in cHCC-CCA patients. The role of anatomical hepatectomy in the prognosis of cHCC-CCA patients has rarely been evaluated, and some studies have reported that anatomical hepatectomy can prolong the survival time of HCC, but had no benefit in ICC patients[48,49]. These findings imply that the impact of anatomical hepatectomy on OS in cHCC-CCA is unclear and further large scale studies with a prospective design should be conducted to verify the results of this study.
Studies have suggested that anatomical hepatectomy can prolong survival in HCC but not ICC patients[48,49]; however, we are unaware of studies that have examined this issue in cHCC-CCA patients. The impact of anatomical hepatectomy on OS of cHCC-CCA patients after resection should be explored in large, prospective studies.
The present study has some limitations. First, its retrospective nature may be associated with a greater risk of selection bias and loss to follow-up, although only eight (6.8%) patients were lost to follow-up. Second, we validated the nomogram internally, not externally; nevertheless, AUCs were > 0.85 for both training and validation sets. Third, the study involved a small sample; thus, the nomogram described here should be validated and optimized using larger samples.
This study established a nomogram which combined the CT radiomics score with clinical risk factors to predict OS in patients with cHCC-CCA after resection with curative intent. The radiomics score was strongly associated with postoperative prognosis, and the integrative nomogram predicted OS well: High-risk patients showed a significantly shorter OS than low-risk patients. This integrative nomogram may aid in predicting the prognosis of cHCC-CCA patients after resection, and may support clinical decision-making.
Combined hepatocellular carcinoma (HCC) and cholangiocarcinoma (cHCC-CCA) arises in hepatic progenitor cells and are defined as a single nodule showing differentiation into HCC and intrahepatic cholangiocarcinoma (ICC) with 5-year postoperative overall survival (OS) rates ranging from 8% to 63%. There are different opinions in the literature on whether the prognosis of patients with cHCC-CCA is worse than that of patients with simple HCC or similar ICC.
Due to the poor prognosis of cHCC-CCA and absence of a promising way to predict prognosis of cHCC-CCA, the authors aimed to construct a radiomics nomogram for predicting postoperative survival of cHCC-CCA patients. This prognostic model may help guide treatment decisions for these patients.
The purpose of this study was to construct and validate a nomogram based on radiomics and clinical characteristics to predict the postoperative survival rate of patients with cHCC-CCA.
We collected the clinical data and computed tomography (CT) imaging data of patients with cHCC-CCA. Radiomics features were extracted from portal venous phase CT images using the least absolute shrinkage and selection operator Cox regression and random forest analysis. A nomogram integrating radiomics score and clinical factors was developed using multivariate Cox regression and each patient got a risk score. And patients were categorized as being at “high” or “low” risk based on their risk scores.
A total of five factors, which were Radiomics score, vascular invasion, anatomical resection, total bilirubin level, and satellite lesions, were independent predictors of prognosis and the nomogram was associated with OS more strongly than a model based on radiomics score or only clinical factors. Patients stratified as being at high risk showed a significantly shorter median OS than those stratified as being at low risk (6.1 vs 81.6 mo, P < 0.001).
This nomogram have potential usefulness in predicting postoperative survival of cHCC-CCA patients and may therefore help identify those more likely to benefit from it, which may facilitate clinical decision-making.
Considering the high AUC of this radiomics nomogram in predicting prognosis of cHCC-CCA, this prognostic model may help guide treatment decisions for these patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Antwi SO, Farid K S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 258] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, Rhee JC, Cho JW, Park CK, Kim HJ. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Yin X, Zhang BH, Qiu SJ, Ren ZG, Zhou J, Chen XH, Zhou Y, Fan J. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol. 2012;19:2869-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, Giardini V. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014;20:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Chu KJ, Lu CD, Dong H, Fu XH, Zhang HW, Yao XP. Hepatitis B virus-related combined hepatocellular-cholangiocarcinoma: clinicopathological and prognostic analysis of 390 cases. Eur J Gastroenterol Hepatol. 2014;26:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system, 2010. Available form: https://www.researchgate.net/publication/312628194_WHO_classification_of_tumours_of_the_digestive_system. |
| 7. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2439] [Article Influence: 487.8] [Reference Citation Analysis (3)] |
| 8. | Bergquist JR, Groeschl RT, Ivanics T, Shubert CR, Habermann EB, Kendrick ML, Farnell MB, Nagorney DM, Truty MJ, Smoot RL. Mixed hepatocellular and cholangiocarcinoma: a rare tumor with a mix of parent phenotypic characteristics. HPB (Oxford). 2016;18:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Lee CH, Hsieh SY, Chang CJ, Lin YJ. Comparison of clinical characteristics of combined hepatocellular-cholangiocarcinoma and other primary liver cancers. J Gastroenterol Hepatol. 2013;28:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Spolverato G, Bagante F, Tsilimigras D, Ejaz A, Cloyd J, Pawlik TM. Management and outcomes among patients with mixed hepatocholangiocellular carcinoma: A population-based analysis. J Surg Oncol. 2019;119:278-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Yoon YI, Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, Song GW, Jung DH, Lee JW, Hong SM, Yu ES, Lee SG. Postresection Outcomes of Combined Hepatocellular Carcinoma-Cholangiocarcinoma, Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Gastrointest Surg. 2016;20:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Zuo HQ, Yan LN, Zeng Y, Yang JY, Luo HZ, Liu JW, Zhou LX. Clinicopathological characteristics of 15 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2007;6:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Li DB, Si XY, Wang SJ, Zhou YM. Long-term outcomes of combined hepatocellular-cholangiocarcinoma after hepatectomy or liver transplantation: A systematic review and meta-analysis. Hepatobiliary Pancreat Dis Int. 2019;18:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Jung DH, Hwang S, Song GW, Ahn CS, Moon DB, Kim KH, Ha TY, Park GC, Hong SM, Kim WJ, Kang WH, Kim SH, Yu ES, Lee SG. Longterm prognosis of combined hepatocellular carcinoma-cholangiocarcinoma following liver transplantation and resection. Liver Transpl. 2017;23:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Allen RA, LISA JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647-655. [PubMed] |
| 16. | Wakabayashi T, Ouhmich F, Gonzalez-Cabrera C, Felli E, Saviano A, Agnus V, Savadjiev P, Baumert TF, Pessaux P, Marescaux J, Gallix B. Radiomics in hepatocellular carcinoma: a quantitative review. Hepatol Int. 2019;13:546-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 17. | Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 3564] [Article Influence: 445.5] [Reference Citation Analysis (0)] |
| 18. | Huang X, Long L, Wei J, Li Y, Xia Y, Zuo P, Chai X. Radiomics for diagnosis of dual-phenotype hepatocellular carcinoma using Gd-EOB-DTPA-enhanced MRI and patient prognosis. J Cancer Res Clin Oncol. 2019;145:2995-3003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Sun Y, Bai H, Xia W, Wang D, Zhou B, Zhao X, Yang G, Xu L, Zhang W, Liu P, Xu J, Meng S, Liu R, Gao X. Predicting the Outcome of Transcatheter Arterial Embolization Therapy for Unresectable Hepatocellular Carcinoma Based on Radiomics of Preoperative Multiparameter MRI. J Magn Reson Imaging. 2020;52:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Wang XH, Long LH, Cui Y, Jia AY, Zhu XG, Wang HZ, Wang Z, Zhan CM, Wang ZH, Wang WH. MRI-based radiomics model for preoperative prediction of 5-year survival in patients with hepatocellular carcinoma. Br J Cancer. 2020;122:978-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 21. | Zheng BH, Liu LZ, Zhang ZZ, Shi JY, Dong LQ, Tian LY, Ding ZB, Ji Y, Rao SX, Zhou J, Fan J, Wang XY, Gao Q. Radiomics score: a potential prognostic imaging feature for postoperative survival of solitary HCC patients. BMC Cancer. 2018;18:1148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 22. | Zhang J, Huang Z, Cao L, Zhang Z, Wei Y, Zhang X, Song B. Differentiation combined hepatocellular and cholangiocarcinoma from intrahepatic cholangiocarcinoma based on radiomics machine learning. Ann Transl Med. 2020;8:119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Yuan C, Wang Z, Gu D, Tian J, Zhao P, Wei J, Yang X, Hao X, Dong D, He N, Sun Y, Gao W, Feng J. Prediction early recurrence of hepatocellular carcinoma eligible for curative ablation using a Radiomics nomogram. Cancer Imaging. 2019;19:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 24. | Ji GW, Zhu FP, Zhang YD, Liu XS, Wu FY, Wang K, Xia YX, Jiang WJ, Li XC, Wang XH. A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur Radiol. 2019;29:3725-3735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 25. | Zhu HB, Zheng ZY, Zhao H, Zhang J, Zhu H, Li YH, Dong ZY, Xiao LS, Kuang JJ, Zhang XL, Liu L. Radiomics-based nomogram using CT imaging for noninvasive preoperative prediction of early recurrence in patients with hepatocellular carcinoma. Diagn Interv Radiol. 2020;26:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Lee SD, Park SJ, Han SS, Kim SH, Kim YK, Lee SA, Ko YH, Hong EK. Clinicopathological features and prognosis of combined hepatocellular carcinoma and cholangiocarcinoma after surgery. Hepatobiliary Pancreat Dis Int. 2014;13:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Wu ZF, Wu XY, Zhu N, Xu Z, Li WS, Zhang HB, Yang N, Yao XQ, Liu FK, Yang GS. Prognosis after resection for hepatitis B virus-associated intrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21:935-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Nioche C, Orlhac F, Boughdad S, Reuzé S, Goya-Outi J, Robert C, Pellot-Barakat C, Soussan M, Frouin F, Buvat I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018;78:4786-4789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 753] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 29. | Breiman L. Random forests, machine learning 45. J Clin Microbiol. 2001;45:5-32. |
| 30. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2396] [Article Influence: 239.6] [Reference Citation Analysis (0)] |
| 31. | Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 126] [Reference Citation Analysis (0)] |
| 32. | Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35:1925-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1269] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 33. | Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA. 2015;313:409-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 532] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 34. | Saini A, Breen I, Pershad Y, Naidu S, Knuttinen MG, Alzubaidi S, Sheth R, Albadawi H, Kuo M, Oklu R. Radiogenomics and Radiomics in Liver Cancers. Diagnostics (Basel). 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Kim J, Choi SJ, Lee SH, Lee HY, Park H. Predicting Survival Using Pretreatment CT for Patients With Hepatocellular Carcinoma Treated With Transarterial Chemoembolization: Comparison of Models Using Radiomics. AJR Am J Roentgenol. 2018;211:1026-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 36. | Peng J, Zhang J, Zhang Q, Xu Y, Zhou J, Liu L. A radiomics nomogram for preoperative prediction of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma. Diagn Interv Radiol. 2018;24:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 37. | Xu X, Zhang HL, Liu QP, Sun SW, Zhang J, Zhu FP, Yang G, Yan X, Zhang YD, Liu XS. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019;70:1133-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 494] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 38. | Lewis S, Hectors S, Taouli B. Radiomics of hepatocellular carcinoma. Abdom Radiol (NY). 2021;46:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 39. | Zhan Q, Shen BY, Deng XX, Zhu ZC, Chen H, Peng CH, Li HW. Clinical and pathological analysis of 27 patients with combined hepatocellular-cholangiocarcinoma in an Asian center. J Hepatobiliary Pancreat Sci. 2012;19:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Shibahara J, Hayashi A, Misumi K, Sakamoto Y, Arita J, Hasegawa K, Kokudo N, Fukayama M. Clinicopathologic Characteristics of Hepatocellular Carcinoma With Reactive Ductule-like Components, a Subset of Liver Cancer Currently Classified as Combined Hepatocellular-Cholangiocarcinoma With Stem-Cell Features, Typical Subtype. Am J Surg Pathol. 2016;40:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Zhou YM, Sui CJ, Zhang XF, Li B, Yang JM. Influence of cirrhosis on long-term prognosis after surgery in patients with combined hepatocellular-cholangiocarcinoma. BMC Gastroenterol. 2017;17:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Holzner ML, Tabrizian P, Parvin-Nejad FP, Fei K, Gunasekaran G, Rocha C, Facciuto ME, Florman S, Schwartz ME. Resection of Mixed Hepatocellular-Cholangiocarcinoma, Hepatocellular Carcinoma, and Intrahepatic Cholangiocarcinoma. Liver Transpl. 2020;26:888-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Chi CT, Chau GY, Lee RC, Chen YY, Lei HJ, Hou MC, Chao Y, Huang YH. Radiological features and outcomes of combined hepatocellular-cholangiocarcinoma in patients undergoing surgical resection. J Formos Med Assoc. 2020;119:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Chen ZH, Zhang XP, Lu YG, Li LQ, Chen MS, Wen TF, Jia WD, Zhou D, Li J, Yang DH, Zhen ZJ, Xia YJ, Fan RF, Huang YQ, Zhang Y, Wu XJ, Hu YR, Tang YF, Lin JH, Zhang F, Zhong CQ, Guo WX, Shi J, Lau J, Cheng SQ. Actual long-term survival in HCC patients with portal vein tumor thrombus after liver resection: a nationwide study. Hepatol Int. 2020;14:754-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 45. | Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM, Vauthey JN. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854-862; discussion 862-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 517] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 46. | Chantajitr S, Wilasrusmee C, Lertsitichai P, Phromsopha N. Combined hepatocellular and cholangiocarcinoma: clinical features and prognostic study in a Thai population. J Hepatobiliary Pancreat Surg. 2006;13:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Lee JH, Chung GE, Yu SJ, Hwang SY, Kim JS, Kim HY, Yoon JH, Lee HS, Yi NJ, Suh KS, Lee KU, Jang JJ, Kim YJ. Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol. 2011;45:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 48. | Jiao S, Li G, Zhang D, Xu Y, Liu J. Anatomic vs non-anatomic resection for hepatocellular carcinoma, do we have an answer? Int J Surg. 2020;80:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 49. | Li B, Song JL, Aierken Y, Chen Y, Zheng JL, Yang JY. Nonanatomic resection is not inferior to anatomic resection for primary intrahepatic cholangiocarcinoma: A propensity score analysis. Sci Rep. 2018;8:17799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |