Published online Nov 7, 2021. doi: 10.3748/wjg.v27.i41.7159
Peer-review started: March 16, 2021
First decision: May 1, 2021
Revised: May 8, 2021
Accepted: October 18, 2021
Article in press: October 18, 2021
Published online: November 7, 2021
Processing time: 234 Days and 19.8 Hours
Laparoscopic ileocolic resection (LICR) is the preferred surgical approach for primary ileocolic Crohn’s disease (CD) because it has greater recovery benefits than open ICR (OICR).
To compare short- and long-term outcomes in patients who underwent LICR and OICR.
Patients who underwent ICR for primary CD from 2006 to 2017 at a single tertiary center specializing in CD were included. Patients who underwent LICR and OICR were subjected to propensity-score matching analysis. Patients were propensity-score matched 1:1 by factors potentially associated with 30-d perioperative morbidity. These included demographic characteristics and disease- and treat
During the study period, 348 patients underwent ICR, 211 by the open approach and 137 laparoscopically. Propensity-score matching yielded 102 pairs of patients. The rate of postoperative complication was significantly lower (14% versus 32%, P = 0.003), postoperative hospital stay significantly shorter (8 d versus 13 d, P = 0.003), and postoperative pain on day 7 significantly lower (1.4 versus 2.3, P < 0.001) in propensity-score matched patients who underwent LICR than in those who underwent OICR. Multivariate analysis showed that postoperative complications were significantly associated with preoperative treatment with biologics [odds ratio (OR): 3.14, P = 0.01] and an open approach to surgery (OR: 2.86, P = 0.005). The 5- and 10-year SRFS rates in the matched pairs were 92.9% and 83.3%, respectively, with SRFS rates not differing significantly between the OICR and LICR groups. The performance of additional procedures was an independent risk factor for surgical recurrence [hazard ratio (HR): 3.28, P = 0.02].
LICR yielded better short-term outcomes and postoperative recovery than OICR, with no differences in long-term outcomes. LICR may provide greater benefits in selected patients with primary CD.
Core Tip: The laparoscopic approach to ileocolic resection can be safely performed in patients with primary Crohn’s disease (CD), resulting in fewer postoperative complications, faster postoperative recovery, and non-inferior surgical recurrence rate when compared with open surgery. Postoperative complications were significantly associated with preoperative use of biologics and open ileocolic resection. Additional procedures were found to be independent risk factors for surgical recurrence in patients with CD.
- Citation: Pak SJ, Kim YI, Yoon YS, Lee JL, Lee JB, Yu CS. Short-term and long-term outcomes of laparoscopic vs open ileocolic resection in patients with Crohn's disease: Propensity-score matching analysis. World J Gastroenterol 2021; 27(41): 7159-7172
- URL: https://www.wjgnet.com/1007-9327/full/v27/i41/7159.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i41.7159
Ileocolic resection (ICR) is the most frequently performed operation for patients with abdominal Crohn’s disease (CD) with involvement of the terminal ileum. Since the introduction of laparoscopic colectomy in 1991, experience with laparoscopic ICR (LICR) for CD has increased[1,2]. LICR has become the preferred surgical approach for primary ileocolic CD because it shows greater recovery benefits than open ICR (OICR). These benefits include reduced pain, lower rates of overall morbidity, shorter hospital stay, earlier return to full activity, lower costs, and improved quality of life and cosmesis compared with OICR[1-7].
Conventionally, patients with penetrating type or complex CD have not been candidates for laparoscopic surgery, with open surgery remaining the generally accepted approach for these patients. The laparoscopic approach is regarded as more technically challenging than open surgery in CD patients with complex features, including huge phlegmons, multiple enteric fistulas, and dense adhesions, as well as those requiring repeated surgery[8]. In addition, patients with complex CD have higher rates of host related risks, such as homeostasis disturbance, infection, and severe malnutrition prior to surgery caused by external or internal fistulas[9]. Therefore, utilization of LICR in patients with complex CD remains problematic.
In South Korea, the number of laparoscopic operations in CD patients has increased dramatically, from 11.6% in 2009 to more than 31% in 2015[10]. Although our institution is the highest volume center for CD in South Korea, laparoscopic surgery for CD was conservative, with performance increasing since 2014.
The present study compared the short- and long-term outcomes of LICR and OICR in patients with primary CD over a 12-year period. To overcome possible selection bias, patients in the two groups were analyzed after propensity-score matching.
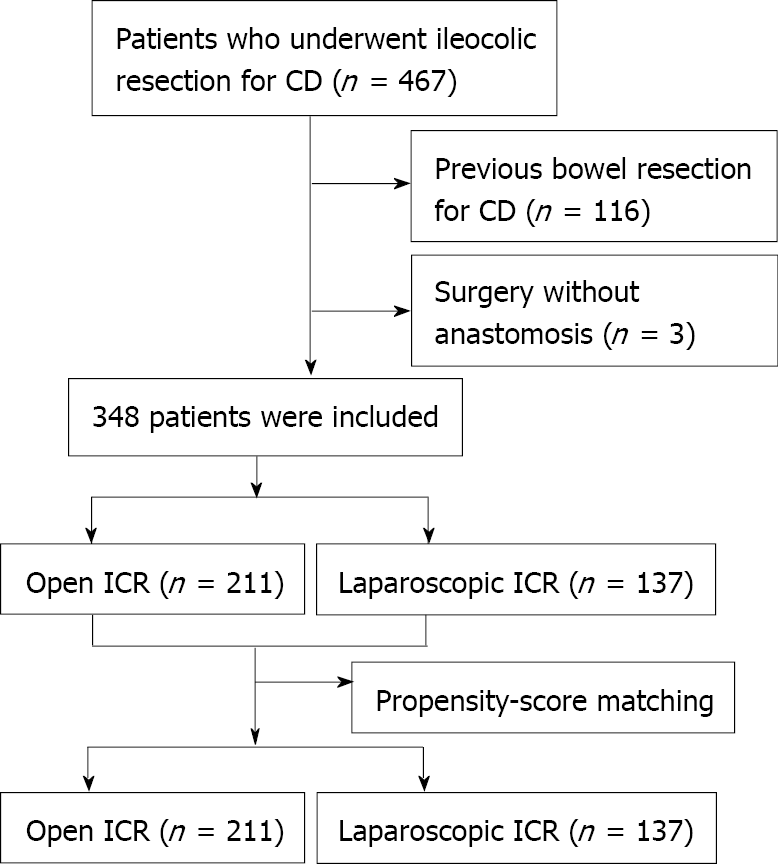
Patients who underwent LICR or OICR for primary CD at Asan Medical Center in Seoul, Korea, from January 2006 to December 2017, were retrospectively identified. Ileocolic resection included patients undergoing resection of the ileum and colon with ileocolic anastomosis within the right/transverse colon. Patients who underwent previous bowel resection for CD, those without anastomosis, those with ileocolic anastomosis distal to the transverse colon, and patients with missing data or loss to follow-up were excluded (Figure 1). Patients who initially underwent laparoscopic surgery but required conversion to open surgery were included in the intention-to-treat analysis. Factors recorded from patients’ electronic medical records and charts included demographic characteristics, preoperative disease characteristics, operative details, and perioperative outcomes. Demographic characteristics included patient sex, age at time of surgery, body mass index (BMI), duration of disease from the time of diagnosis, smoking history, and comorbidities. Preoperative disease characteristics included Montreal classification at the time of surgery[11], extra-intestinal manifestations of disease, family history of CD, history of perianal CD, previous history of abdominal surgery, and specific history of intestinal resection for CD. Operative details included intraoperative findings from operation reports (adhesions, strictures, intestinal fistulas, abscesses, phlegmons), indications for surgical intervention, conversion to open surgery, diverting stoma, estimated blood loss, intraoperative red blood cell (RBC) transfusion, operation time, urgent surgery, and American Society of Anesthesiologists (ASA) score. Perioperative outcomes included intraoperative and postoperative morbidity and mortality within 30 d after surgery, readmission, reoperation, length of hospital stay, pain scale, and time to recovery of bowel function[12]. Additional variables included preoperative hemoglobin and albumin levels, preoperative CD medications, anastomosis configuration (side-to-side, end-to-side, side-to-end, or end-to-end), type of anastomosis (stapled or hand-sewn), and synchronous additional surgical procedures. Postoperative morbidity was graded according to the Clavien-Dindo classification. The study protocol was approved by the Institutional Review Board of Asan Medical Center (approval number: 2019-0972).
Comorbidities included hypertension, diabetes mellitus, and others. Major intraoperative bleeding was defined as intraoperative hemorrhage reported in operation notes and requirement for transfusion of packed RBCs. However, postoperative transfusion without a need for surgical intervention was not regarded as a postoperative compli
Laparoscopic or open surgery was selected for each patient according to surgeon preference. A history of previous abdominal surgery with or without bowel resection was an important consideration when choosing the surgical method. Because patients with previous bowel resection were excluded, multiple factors such as age, general condition, and disease extent were taken into consideration. Anastomosis configurations were classified as side-to-side, end-to-side, side-to-end, and end-to-end. Anastomosis materials were categorized as stapled and hand-sewn. ICR involves the removal of the ileocecal valve and was categorized as right colectomy or ileocecal resection depending on the involvement of the hepatic flexure of the colon.
Preoperative CD medications were divided into four categories: Systemic steroids, biologics [infliximab (Remicade®, Janssen Biotech, Inc., Horsham, PA, United States) or adalimumab (Humira®, AbbVie Inc. Chicago, IL, United States)], immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate), and anti-inflammatory agents (5-aminosalicylate acid or budesonide). Preoperative treatment with steroids and anti-inflammatory agents was defined as the administration of each medication within 1 mo before surgery. Treatment with biologics was defined as the administration of at least one infusion of anti-TNF agents within 3 mo before surgery, whereas treatment with immunomodulators was defined as administration within 2 mo before surgery[5]. Immunosuppressive medications included steroids, biologics, and immuno
Complications within the first 30 postoperative days included inadvertent intraoperative injury, anastomosis leak, fistula formation, prolonged postoperative ileus, wound or intra-abdominal infection requiring antibiotics or drainage, readmission, or return to the operating room. Septic complications included anastomosis leakage, abdominal abscess, and resulting sepsis or septic shock. Ileus was defined as the absence of bowel function by postoperative day 5 and/or the need for nasogastric tube insertion due to abdominal distension, nausea, or vomiting, without evidence of mechanical bowel obstruction.
Surgical recurrence was defined as a repeat operation on any part of the bowel for pathologically confirmed CD or for pathologically confirmed anastomotic disease, including manifestations of the small bowel at the anastomosis or stoma site. Operations not performed to treat CD exacerbations (e.g., adhesiolysis or stoma closure only) were not considered reoperations for surgical recurrence.
To minimize the impact of selection bias for the surgical approach and potential confounding in this observational study, patients who underwent LICR and OICR were subjected to propensity-score matching, with rigorous adjustment for significant differences in patient characteristics. Propensity scores were estimated by multiple logistic regression analysis. All pre-specified covariates were included in the full non-parsimonious models. The covariates included demographic characteristics (age, gender, BMI, smoking history, previous history of abdominal surgery, previous history of comorbidity, and ASA score), disease-related variables (Montreal classification, disease duration, perianal CD, family history of CD and extra-intestinal CD manifestations), and treatment-related variables (preoperative hemoglobin and albumin concentrations, preoperative RBC transfusions, preoperative medications, and indications for surgery). These variables were selected because they can affect the choice of surgical approach and perioperative outcomes. The operative approach was entered into the regression model as a dependent variable. A 1:1 “nearest neighbor”, case-control match without replacement was used. The discrimination and calibration abilities of the propensity-score model were 0.7332 by C-statistics and P = 0.1219 by Hosmer-Lemeshow statistics. Following propensity-score matching, short- and long-term results were compared in the two groups.
Continuous variables were reported as mean ± standard deviation (SD) or as median (min, max) and compared by t-tests or Wilcoxon rank sum tests, whereas categorical variables were reported as frequency (%) and compared by Pearson’s χ2 test or Fisher’s exact test.
Univariate analyses were performed to assess the risk factors associated with postoperative complications and surgical recurrence. Multivariable models were created to identify factors independently associated with postoperative complications. Surgical recurrence-free survival (SRFS) was calculated using the Kaplan-Meier method and compared using log-rank tests. Multivariate analyses to assess the risk factors associated with SRFS were performed using Cox proportional hazards model with 95% confidence interval (CI). All statistical analyses were performed using the Statistical Package for the Social Sciences, version 24.0 for Windows (SPSS, IBM Corp., Armonk, NY, United States), with P values < 0.05 considered statistically significant.
A total of 467 patients underwent ICR for CD during the study period. Of these, 119 were excluded, including three patients without anastomosis or with ileocolic anastomosis distal to the transverse colon and 116 patients who had previously undergone bowel resection for CD. Of the 348 eligible patients, 211 underwent OICR and 137 underwent LICR. Patient characteristics before and after propensity-score matching are summarized in Table 1. Compared with patients who underwent OICR for CD, those who underwent LICR had significantly lower rates of penetrating behavior (P < 0.001) and comorbidities (P = 0.036), longer disease duration (P = 0.003), a higher rate of surgical indication of obstruction (P < 0.001), and a lower rate of fistula as a surgical indication (P = 0.022). Crohn’s Disease Activity Index (CDAI) scores were compared between the two groups. LICR and OICR groups had moderate CDAI scores (230.8 ± 9.5 and 269.1 ± 10.8, respectively). Because the demographic data differed between the OICR and LICR groups, these patients were subjected to 1:1 propensity-score matching to reduce selection bias. A total of 102 pairs was therefore included in the propensity-score matched population.
| All patients | Propensity-score matched patients | |||||
| Open (n = 211) | Laparoscopy (n = 137) | P | Open (n = 102) | Laparoscopy (n = 102) | SMD | |
| Age (yr) | 29.2 ± 9.7 | 29.2 ± 9.0 | 0.976 | 28.8 ± 9.0 | 28.6 ± 8.7 | -0.026 |
| Gender, male | 153 (72.5) | 95 (69.3) | 0.546 | 78 (76.5) | 72 (70.6) | -0.134 |
| BMI (kg/m2) | 18.8 ± 3.2 | 18.9 ± 2.8 | 0.822 | 19.0 ± 3.3 | 18.9 ± 2.8 | -0.041 |
| Any smoking history | 41 (19.4) | 33 (24.1) | 0.348 | 21 (20.6) | 20 (19.6) | -0.025 |
| Previous abdominal surgery | 23 (10.9) | 10 (7.3) | 0.349 | 10 (9.8) | 8 (7.8) | -0.069 |
| Montreal classification | ||||||
| Behavior | < 0.001 | -0.044 | ||||
| Inflammatory (B1) | 2 (0.1) | 2 (1.5) | 2 (2.0) | 2 (2.0) | ||
| Stricturing (B2) | 38 (17.5) | 52 (38.0) | 30 (29.4) | 26 (25.5) | ||
| Penetrating (B3) | 172 (82.0) | 83 (60.6) | 70 (68.6) | 74 (72.5) | ||
| Location | 0.218 | -0.060 | ||||
| Terminal ileal (L1) | 68 (32.2) | 50 (36.5) | 36 (35.3) | 37 (36.3) | ||
| Colonic (L2) | 10 (4.7) | 2 (1.5) | 6 (5.9) | 2 (2.0) | ||
| Ileocolic (L3) | 133 (63.0) | 85 (62.0) | 60 (58.8) | 63 (61.8) | ||
| Disease duration (mo) | 49.8 ± 50.9 | 68.1 ± 59.3 | 0.003 | 61.5 ± 57.4 | 67.0 ± 57.8 | 0.095 |
| Perianal CD | 103 (48.8) | 57 (41.6) | 0.226 | 45 (44.1) | 45 (44.1) | 0.000 |
| Family history of CD | 5 (2.4) | 5 (3.6) | 0.523 | 4 (3.9) | 4 (3.9) | 0.000 |
| Extra-intestinal CD manifestation | 31 (14.7) | 18 (13.1) | 0.754 | 13 (12.7) | 10 (9.8) | -0.093 |
| Comorbidity | 21 (10.0) | 5 (3.6) | 0.036 | 2 (2.0) | 4 (3.9) | 0.116 |
| Hypertension | 4 (1.9) | 0 (0.0) | 0.157 | 1 (1.0) | 0 (0.0) | |
| Diabetes mellitus | 2 (0.9) | 1 (0.7) | 1.000 | 0 (0.0) | 1 (1.0) | |
| Others | 15 (7.1) | 5 (3.6) | 0.239 | 1 (1.0) | 4 (3.9) | |
| ASA score, 3-4 | 7 (3.3) | 2 (1.5) | 0.397 | 3 (2.9) | 2 (2.0) | 2 |
| Emergency | 17 (8.1) | 9 (6.6) | 0.680 | 6 (5.9) | 7 (6.9) | 0.040 |
| Preoperative data | ||||||
| Hemoglobin (g/dL) | 11.5 ± 1.5 | 11.5 ± 1.9 | 0.780 | 11.4 ± 1.4 | 11.4 ± 1.8 | 0.037 |
| Albumin (g/dL) | 3.1 ± 0.5 | 3.2 ± 0.5 | 0.140 | 3.1 ± 0.5 | 3.2 ± 0.5 | 0.075 |
| Transfusion | 40 (19.0) | 26 (19.0) | 1.000 | 22 (21.6) | 20 (19.6) | 2 |
| Preoperative medications | ||||||
| Steroids | 44 (20.9) | 25 (18.2) | 0.584 | 22 (21.6) | 15 (14.7) | -0.179 |
| Immuno-modulators | 96 (45.5) | 67 (48.9) | 0.583 | 48 (47.1) | 50 (49.0) | 0.039 |
| Biologics | 29 (13.7) | 26 (19.0) | 0.229 | 17 (16.7) | 16 (15.7) | -0.027 |
| Indication for surgery | < 0.001 | |||||
| 1Fistula versus others | 83 (39.3) | 34 (24.8) | 0.022 | 33 (32.4) | 32 (31.4) | 0.021 |
| 2Obstruction versus others | 39 (18.5) | 54 (39.4) | < 0.001 | 31 (30.4) | 26 (25.5) | -0.042 |
A comparison of the propensity-score matched groups showed that the rate of intraoperative transfusion was significantly lower (P = 0.017), and the length of small bowel resection significantly shorter (P < 0.001), in the LICR than in the OICR group. Three patients (2.9%) in the OICR group, but none in the LICR group, underwent a diverting ileostomy. Although most patients in both groups underwent side-to-side anastomosis, end-to-side anastomosis was more frequently performed in the LICR than in the OICR group (P = 0.014). The open conversion rate in the LICR group was 4.9%. The reasons for open conversion were adhesions in two patients, huge phleg
| Open (n = 102) | Laparoscopy (n = 102) | P | |
| Operation time (min) | 136.6 ± 4.5 | 130.4 ± 2.7 | 0.241 |
| Estimated blood loss (mL) | 201.8 ± 19.2 | 145.7 ± 22.1 | 0.057 |
| Intraoperative transfusion | 13 (12.7) | 3 (2.9) | 0.017 |
| Diverting ileostomy | 3 (2.9) | 0 (0.0) | 0.246 |
| Anastomosis configuration | 0.014 | ||
| Side-to-side | 89 (87.3) | 74 (72.5) | |
| End-to-side | 9 (8.8) | 26 (25.5) | |
| Side-to-end | 3 (2.9) | 1 (1.0) | |
| End-to-end | 1 (1.0) | 1 (1.0) | |
| Stapled anastomosis | 100 (98.0) | 101 (99.0) | 1.000 |
| Operation type | 0.322 | ||
| Right colectomy | 48 (47.1) | 40 (39.2) | |
| Ileocecal resection | 54 (52.9) | 62 (60.8) | |
| Additional procedure | 40 (39.2) | 30 (29.4) | 0.184 |
| Strictureplasty | 16 (15.7) | 11 (10.8) | |
| Small bowel resection | 20 (19.6) | 19 (18.6) | |
| Colon resection | 4 (3.9) | 0 (0.0) | |
| Length of small bowel resected (cm) | 60.6 ± 4.2 | 43.9 ± 2.8 | 0.001 |
Of the 348 CD patients who underwent ICR, 75 (21.6%) experienced complications within 30 d, but none died. Of the 204 matched patients, 47 (23%) experienced postoperative complications, with 15 cases being classified as class III Clavien-Dindo complications. All six patients who required reoperation had undergone OICR, including three for anastomotic leakage and one each for an intra-abdominal abscess, luminal bleeding, and a surgical wound complication.
The overall complication rate was significantly lower in the LICR than in the OICR group (13.7% versus 32.4%, P = 0.003). The rates of septic complications, including intra-abdominal abscess (P = 0.035) and/or anastomosis leakage (P = 0.014), were also lower in the LICR group. In addition, the rates of reoperation (P = 0.029) and blood transfusion (P = 0.021) were significantly lower, hospital stay (P = 0.003) significantly shorter, and postoperative pain on postoperative day 7 significantly lower (P < 0.001) in the LICR than in the OICR group (Table 3).
| Open (n = 102) | Laparoscop (n = 102) | P | OR | 95%CI | P | |
| Total complications | 33 (32.4) | 14 (13.7) | 0.003 | 0.379 | 0.189-0.759 | 0.006 |
| Intra-abdominal abscess | 8 (7.8) | 1 (1.0) | 0.035 | |||
| Anastomotic leakage | 7 (6.9) | 0 (0.0) | 0.014 | |||
| Wound complication | 12 (11.8) | 4 (3.9) | 0.065 | |||
| Ileus | 2 (2.0) | 7 (6.9) | 0.170 | |||
| Bleeding | 7 (6.9) | 2 (2.0) | 0.170 | |||
| Other | 0 (0.0) | 2 (2.0) | 0.498 | |||
| Septic complications1 | 15 (14.7) | 1 (1.0) | < 0.001 | 0.091 | 0.012-0.704 | 0.006a |
| Reoperation | 6 (5.9) | 0 (0.0) | 0.029 | 0.107 | 0.006-2.039 | 0.121a |
| Readmission | 4 (3.9) | 2 (2.0) | 0.689 | 0.250 | 0.053-1.177 | 0.109a |
| Recovery of bowel movement (d) | 2.97 ± 1.0 | 2.82 ± 1.0 | 0.295 | 0.971 | 0.694-1.359 | 0.864 |
| Duration of NPO (d) | 4.2 ± 1.9 | 3.5 ± 2.2 | 0.026 | 0.573 | 0.400-0.820 | 0.002 |
| Total hospital stay (d) | 20.7 ± 17.3 | 16.1 ± 8.3 | 0.016 | 0.594 | 0.404-0.875 | 0.008 |
| Postoperative hospital stay (d) | 13.1 ± 16.3 | 8.2 ± 3.3 | 0.003 | 0.443 | 0.298-0.660 | < 0.001 |
| Pain scale (NRS) | ||||||
| POD#1 | 5.1 ± 2.0 | 4.9 ± 1.9 | 0.458 | 0.386 | -0.183-0.956 | 0.181 |
| POD#2 | 3.9 ± 1.8 | 3.9 ± 2.1 | 0.756 | 0.159 | -0.392-0.710 | 0.567 |
| POD#3 | 3.8 ± 2.1 | 3.3 ± 1.8 | 0.057 | 0.602 | 0.00-1.198 | 0.048 |
| POD#7 | 2.3 ± 1.9 | 1.4 ± 1.4 | < 0.001 | 0.628 | 0.156-1.100 | 0.010 |
| Postoperative transfusion | 23 (22.5) | 10 (9.8) | 0.021 | 0.278 | 0.103-0.748 | 0.011 |
Univariate analysis showed that an open approach (P = 0.003), previous abdominal surgery (P = 0.037), preoperative use of biologics (P = 0.023), additional procedures (P = 0.050), and longer operation time (> 135 min) (P = 0.016) were associated with postoperative complications. In multivariate analysis, an open approach [odds ratio (OR): 2.86; 95%CI: 0.17-0.73; P = 0.005], previous abdominal surgery (OR: 3.61; 95%CI: 1.23-10.60, P = 0.020), preoperative use of biologics (OR: 3.14; 95%CI: 1.33-7.40; P = 0.009), and longer operation time (> 135 min) (OR: 2.38; 95%CI: 1.17-4.84; P = 0.017) were found to be independent risk factors for postoperative complications (Table 4).
| Univariate analysis | Multivariate analysis | |||||
| No complication (n = 157, %) | Complication (n = 47, %) | P | OR | 95%CI | P | |
| Demographics | ||||||
| Male | 115 (73.2) | 35 (74.5) | 1.000 | |||
| Family history | 6 (3.8) | 2 (4.3) | 1.000 | |||
| Smoking history | 32 (20.4) | 9 (19.1) | 1.000 | |||
| Comorbidity | 6 (3.8) | 0 (0.0) | 0.340 | |||
| Fistula-in-ano | 73 (46.5) | 17 (36.2) | 0.243 | |||
| Previous abdominal surgery | 10 (6.4) | 8 (17.0) | 0.037 | 3.61 | 1.23-10.60 | 0.020 |
| Penetrating type | 112 (71.3) | 34 (72.3) | 1.000 | |||
| Open approach | 69 (43.9) | 14 (70.2) | 0.003 | 2.86 | 0.17-0.73 | 0.005 |
| Preoperative medications | ||||||
| Biologics | 20 (12.7) | 13 (27.7) | 0.023 | 3.14 | 1.33-7.40 | 0.009 |
| Steroid | 24 (15.3) | 13 (27.7) | 0.082 | |||
| Immunomodulators | 77 (49.0) | 21 (44.7) | 0.622 | |||
| Operation details | ||||||
| Indications | ||||||
| Fistula | 53 (33.8) | 20 (42.6) | 0.300 | |||
| Obstruction | 52 (33.1) | 16 (34.0) | 1.000 | |||
| Anastomosis configuration | ||||||
| Side-to-side | 126 (80.3) | 37 (78.7) | 0.837 | |||
| End-to-side | 29 (18.5) | 6 (12.8) | 0.508 | |||
| Stapled anastomosis | 155 (99.7) | 46 (2.1) | 0.546 | |||
| Additional procedures | 45 (28.7) | 21 (44.7) | 0.050 | 1.38 | 0.61-3.14 | 0.444 |
| Operation time1 > 135 min | 52 (33.1) | 25 (53.2) | 0.016 | 2.38 | 1.17-4.84 | 0.017 |
Sixteen patients experienced septic complications, 15 in the OICR and one in the LICR group. Preoperative use of steroids (OR: 4.19; 95%CI: 1.19-14.71; P = 0.025) and fistula as a surgical indication (OR: 4.03; 95%CI: 1.23-13.22; P = 0.021) were signi
The median follow-up duration was 74.8 mo for all patients, 88.13 mo in the OICR group, and 61.45 mo in the LICR group. By the end of the study period, 21 patients (10.3%) in the propensity-score matched cohort had experienced surgical recurrence of CD, including 14 (13.7%) who underwent OICR and seven (6.9%) who underwent LICR; these included 12 patients with fistula/abscess, four with perforation, and five with stricture. The median time to surgical recurrence was 70.3 mo for all patients, 81.7 mo in the OICR group, and 58.9 mo in the LICR group.
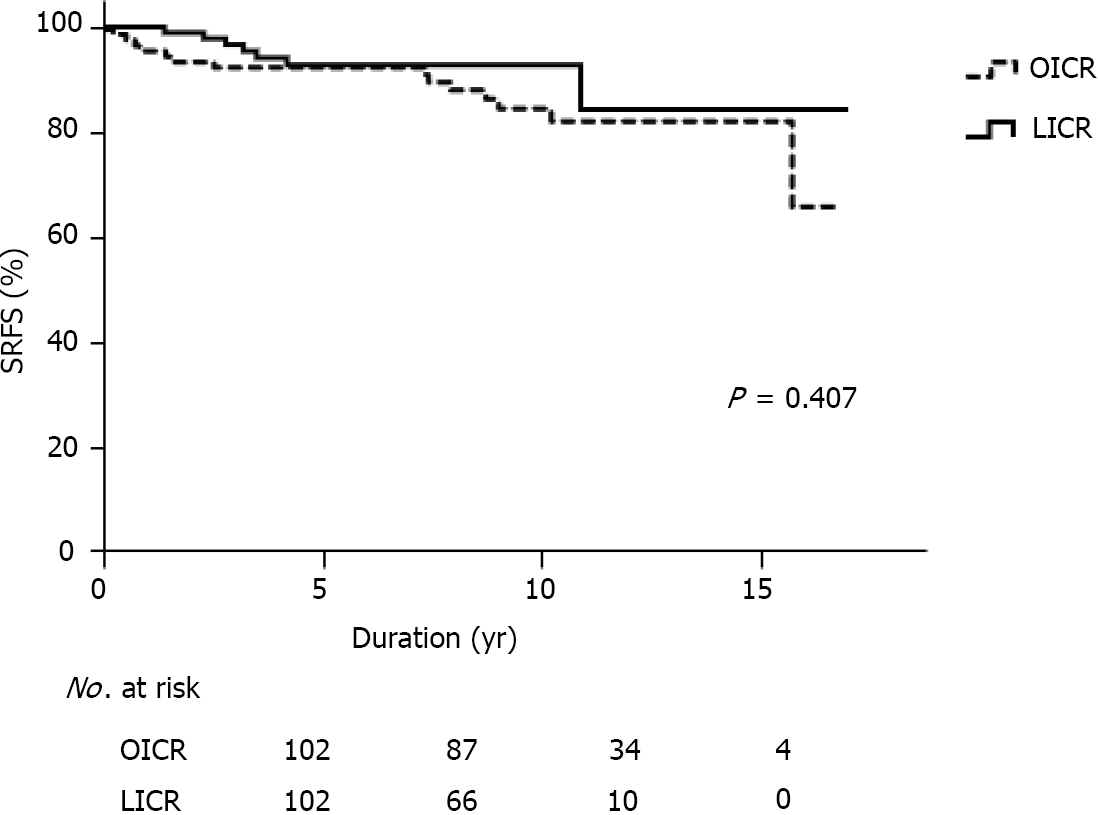
The overall 5- and 10-year SRFS rates were 92.9% and 83.3%, respectively, in the 204 propensity-score matched patients, 92.6% and 82.4%, respectively, in the OICR group, and 92.8% and 84.3%, respectively, in the LICR group. Kaplan-Meier analysis showed that the difference between the two groups was not statistically significant (P = 0.407, Figure 2). Univariate analysis showed that preoperative treatment with biologics (P = 0.024), side-to-side anastomosis (P = 0.042), and additional procedures (P = 0.049) were associated with surgical recurrence. Multivariate analysis revealed that the perfor
| Univariate analysis | Multivariate analysis | |||||
| No recurrence (n = 183) | Recurrence (n = 21) | P | HR | 95%CI | P | |
| Demographics | ||||||
| Male | 134 (73.2) | 16 (76.2) | 1.000 | |||
| Family history | 8 (4.4) | 0 (0.0) | 1.000 | |||
| Smoking history | 38 (20.8) | 3 (14.3) | 0.579 | |||
| Comorbidity | 6 (3.3) | 0 (0.0) | 1.000 | |||
| Fistula-in-ano | 81 (44.3) | 9 (42.9) | 1.000 | |||
| Previous abdominal surgery | 15 (8.2) | 3 (14.3) | 0.407 | |||
| Penetrating type | 129 (70.5) | 17 (81.0) | 0.445 | |||
| Open approach | 95 (48.1) | 14 (66.7) | 0.166 | |||
| Preoperative medications | ||||||
| Biologics | 26 (14.2) | 7 (33.3) | 0.024 | 2.64 | 0.89-7.86 | 0.081 |
| Steroid | 31 (16.9) | 6 (28.6) | 0.229 | |||
| Immunomodulators | 88 (48.1) | 10 (47.6) | 1.000 | |||
| Operation details | ||||||
| Indication | ||||||
| Fistula | 69 (37.7) | 4 (19.0) | 0.099 | |||
| Obstruction | 62 (33.9) | 6 (28.6) | 0.808 | |||
| Anastomosis configuration | ||||||
| Side-to-side | 150 (82.0) | 13 (61.9) | 0.042 | 3.82 | 1.24-11.78 | 0.078 |
| End-to-side | 30 (16.4) | 5 (23.8) | 0.370 | |||
| Stapled anastomosis | 180 (98.4) | 21 (100.0) | 1.000 | |||
| Additional procedures | 55 (30.1) | 11 (52.4) | 0.049 | 3.28 | 1.20-8.91 | 0.020 |
| Operation time > 135 min | 66 (36.1) | 11 (52.4) | 0.159 | |||
This propensity-score matched case-control study compared short and long-term outcomes of LICR and OICR for primary CD at a single tertiary center specializing in CD. The results confirmed that LICR can be safely performed in these patients, resulting in a lower rate of postoperative complications, faster postoperative recovery, and non-inferior surgical recurrence rate compared with open surgery.
The laparoscopic approach is the preferred surgical approach for simple CD, as it is associated with a lower postoperative morbidity rate, a shorter hospital stay, earlier return to full activity, and improved quality of life[1-4]. Laparoscopic surgery is being performed more frequently in patients with complex or recurrent CD, with ran
Operation time was comparable in the LICR and OICR groups. Although several studies have reported that operation time is longer for laparoscopic than for open surgery[1,13,14], other studies have found that operation time is shorter for LICR than for OICR[15,16]. Our finding, of no difference in operation time between laparoscopic and open surgery, may have been due to lack of calibration of selection, even after propensity-score matching. For example, the resected length of the small bowel differed between the LICR and OICR groups, and additional procedures were more frequent in patients who underwent open surgery.
The present study found that preoperative use of corticosteroids was associated with higher rates of intra-abdominal abscess and anastomosis leakage. Moreover, preoperative treatment with biologics was associated with a higher risk of short-term complications. These results are similar to those of the large observational TREAT registry (n = 6273), in which use of infliximab and corticosteroid increased the risks of serious infection[17-19]. In addition, our study found that a history of previous abdominal surgery was associated with postoperative complications. The specific impact of previous intestinal resection on postoperative complications in patients with CD has not been determined. One study reported that 47.6% of postoperative complications occurred in patients with a history of previous abdominal surgery (n = 10, P = 0.310), although the difference was not statistically significant[20]. Other studies have found that previous abdominal surgery is a significant risk factor for postoperative complications[21,22]. Moreover, patients who had previous surgery were more inc
In our study, the rate of conversion from laparoscopic to open surgery was about 5%. Pooled conversion rates have been found to range from 0% to 21.5%[13,24]. Recurrent disease with dense adhesions, pelvic sepsis with fistulizing disease, large inflammatory mass, and thickened mesentery are all conditions predisposing to conversion open surgery[24]. The low conversion rate in the present study may have been due to the relatively mild complexities and complications in patients who underwent laparoscopic surgery. Laparoscopy can be attempted in patients with CD, even if they have risk factors for open conversion. For safety reasons, however, patients should be converted to open surgery without delay.
Prevention of long-term postoperative recurrence in CD patients is a major challenge, especially as 10-15 year post-surgical recurrence rates are approximately 45% to 50%[25]. The long-term effects of laparoscopy have not been determined, as few studies have compared long-term outcomes, especially surgical recurrence, in CD patients who have undergone laparoscopic and open surgery. A meta-analysis reported that laparoscopic surgery is associated with a lower rate of late reoperations for CD recurrence (OR: 0.46; 95%CI: 0.27-0.80)[14], and laparoscopy was found to protect against surgical recurrence (HR: 0.24; 95%CI: 0.10-0.53; P = 0.04)[26]. Another study, however, reported no difference in surgical recurrence rates between surgical techniques (OR: 0.78; 95%CI: 0.54-1.11; P = 0.17)[27]. The present study also showed no difference in long-term outcomes between the LICR and OICR groups. Because this study was a retrospective analysis of a small number of patients, future large randomized-controlled trials are needed to assess the impact of laparoscopy on surgical recurrence.
Simultaneous surgery at the time of ICR was also associated with a higher risk of surgical recurrence. The extent of disease at diagnosis had an impact on recurrence, with higher recurrence rates in patients with small bowel and continuous ileocolonic CD than in patients with ileocecal and colorectal disease[28]. Disease extent > 50 cm is considered a risk factor for postoperative recurrence of CD[29]. An additional surgical procedure may be related to surgical recurrence because these patients may have more severe disease. Prospective clinical studies in larger numbers of patients are needed to further evaluate the results of the present study.
Since the introduction of the first anti-TNF agent in the late 1990s, biologic therapy has revolutionized the medical treatment of patients with CD[30]. Although administering biologics prior to surgery has been reported to reduce clinical/endoscopic recurrence rates[31-33], most of these trials had small sample sizes and limited follow-up, and focused on endoscopic findings and clinical scores rather than repeat ope
Stapled side-to-side anastomosis has been associated with lower rates of leakage and surgical recurrence than other types of anastomosis[36-39]. Side-to-side anas
The present study had several limitations. First, this study was a retrospective evaluation of patients at a single center. Randomized-controlled trials are required to specifically evaluate the ability of a laparoscopic approach to minimize postoperative complications. Although propensity-score matching can reduce selection bias, resulting in a situation similar to a randomized-controlled trial, our propensity-score matching models could not eliminate all selection biases. For example, the most frequent reasons for conversion to open surgery, such as adhesions and huge phlegmons, could not be calibrated by propensity-score matching analysis. Also, although the CDAI scores for both groups were moderate, the laparoscopic group had a significantly lower CDAI score (230.8) than the open group (269.1) (P = 0.008)[42]. Inevitably, a randomized controlled trial will be required to evaluate the role of the laparoscopic approach with more reliable evidence. Second, the study included only East Asian patients from a single country, thus not representing a global population. Korean and western CD patients differ in gender distribution, disease location, and perianal fistula occurrence[43]. Third, the use of biologics in this study was less frequent than in western studies because the health insurance reimbursement policy of the Korean government was strict during the study period. This study period was dominated by ‘step-up treatment’. Although ‘top-down treatment’ has been more frequent in recent years, its use in Korea is limited.
LICR yielded better short-term outcomes, including more rapid postoperative recovery, than open surgery. Long-term outcomes, however, did not differ between the two groups. Laparoscopic surgery might be a better surgical option in selected patients with CD.
Ileocolic resection (ICR) is the most frequently performed operation for patients with abdominal Crohn’s disease (CD) with involvement of the terminal ileum. Laparoscopic ICR (LICR) has become the preferred surgical approach for primary ileocolic CD because it has greater recovery benefits than open ICR (OICR).
The laparoscopic approach is regarded as more technically challenging than open surgery in CD patients with complex features, including huge phlegmons, multiple enteric fistulas, and dense adhesions, as well as those requiring repeated surgery. Utilization of LICR in patients with complex CD remains problematic.
This study aimed to compare the short- and long-term outcomes of LICR and OICR in patients with primary CD.
A total of 348 eligible patients who underwent LICR or OICR for primary CD at Asan Medical Center in Seoul, Korea, from January 2006 to December 2017, were retro
During the study period, 348 patients underwent ICR, 211 by the open approach and 137 by the laparoscopic approach. Propensity-score matching yielded 102 pairs of patients. The rate of postoperative complications was significantly lower, postope
LICR yielded better short-term outcomes and postoperative recovery than OICR, with no differences in long-term outcomes. LICR may provide greater benefits in selected patients with primary CD.
The laparoscopic approach to ileocolic resection can be safely performed in patients with primary CD, resulting in fewer postoperative complications, faster postoperative recovery, and non-inferior surgical recurrence rate when compared with open surgery.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brisinda G, Shi Y S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Maartense S, Dunker MS, Slors JF, Cuesta MA, Pierik EG, Gouma DJ, Hommes DW, Sprangers MA, Bemelman WA. Laparoscopic-assisted vs open ileocolic resection for Crohn's disease: a randomized trial. Ann Surg. 2006;243:143-149; discussion 150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Neumann PA, Rijcken EJ, Bruewer M. Current status of laparoscopic surgery for patients with Crohn's disease. Int J Colorectal Dis. 2013;28:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Stocchi L, Milsom JW, Fazio VW. Long-term outcomes of laparoscopic vs open ileocolic resection for Crohn's disease: follow-up of a prospective randomized trial. Surgery. 2008;144:622-627; discussion 627-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Canedo J, Pinto RA, Regadas S, Regadas FS, Rosen L, Wexner SD. Laparoscopic surgery for inflammatory bowel disease: does weight matter? Surg Endosc. 2010;24:1274-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Beyer-Berjot L, Mancini J, Bege T, Moutardier V, Brunet C, Grimaud JC, Berdah S. Laparoscopic approach is feasible in Crohn's complex enterovisceral fistulas: a case-match review. Dis Colon Rectum. 2013;56:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Kristo I, Stift A, Argeny S, Mittlböck M, Riss S. Minimal-invasive approach for penetrating Crohn's disease is not associated with increased complications. Surg Endosc. 2016;30:5239-5244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Yu ZL, Lin DZ, Hu JC, Chen YF, Cai ZR, Zou YF, Ke J, Guo XF, Lan P, Wu XJ. Laparoscopic Surgery for Complex Crohn's Disease: A Meta-Analysis. J Laparoendosc Adv Surg Tech A. 2019;29:1397-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Geltzeiler CB, Hart KD, Lu KC, Deveney KE, Herzig DO, Tsikitis VL. Trends in the Surgical Management of Crohn's Disease. J Gastrointest Surg. 2015;19:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Ren J, Liu S, Wang G, Gu G, Ren H, Hong Z, Li J. Laparoscopy improves clinical outcome of gastrointestinal fistula caused by Crohn's disease. J Surg Res. 2016;200:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Baek SJ, Lee KY, Song KH, Yu CS. Current Status and Trends in Inflammatory Bowel Disease Surgery in Korea: Analysis of Data in a Nationwide Registry. Ann Coloproctol. 2018;34:299-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2356] [Article Influence: 124.0] [Reference Citation Analysis (2)] |
| 12. | Beaupel N, Brouquet A, Abdalla S, Carbonnel F, Penna C, Benoist S. Preoperative oral polymeric diet enriched with transforming growth factor-beta 2 (Modulen) could decrease postoperative morbidity after surgery for complicated ileocolonic Crohn's disease. Scand J Gastroenterol. 2017;52:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Maggiori L, Panis Y. Laparoscopy in Crohn's disease. Best Pract Res Clin Gastroenterol. 2014;28:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Rosman AS, Melis M, Fichera A. Metaanalysis of trials comparing laparoscopic and open surgery for Crohn's disease. Surg Endosc. 2005;19:1549-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Lee Y, Fleming FJ, Deeb AP, Gunzler D, Messing S, Monson JR. A laparoscopic approach reduces short-term complications and length of stay following ileocolic resection in Crohn's disease: an analysis of outcomes from the NSQIP database. Colorectal Dis. 2012;14:572-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Alizadeh RF, Chaudhry HH, Li S, Jafari MD, Mills SD, Carmichael JC, Pigazzi A, Monson JRT, Stamos MJ. Ileocolic Resection for Crohn's Disease: A Minimally Invasive Approach Claims Its Place. Am Surg. 2018;84:1639-1644. [PubMed] [DOI] [Full Text] |
| 17. | Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Chen DM, Pritchard ML, Sandborn WJ. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 635] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 18. | Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Price S, Langholff W, Londhe A, Sandborn WJ. Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 2012;107:1409-1422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 536] [Cited by in RCA: 599] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 19. | Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Safdi M, Popp JW Jr, Langholff W, Sandborn WJ. Infliximab for Crohn's Disease: More Than 13 Years of Real-world Experience. Inflamm Bowel Dis. 2018;24:490-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Melo-Pinto D, Santos JV, Barbosa E. Risk factors for postoperative complications in Crohn disease: Analysis of 173 patients. J Coloproctol. 2018;38:214-220. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Yamamoto T, Spinelli A, Suzuki Y, Saad-Hossne R, Teixeira FV, de Albuquerque IC, da Silva RN, de Barcelos IF, Takeuchi K, Yamada A, Shimoyama T, da Silva Kotze LM, Sacchi M, Danese S, Kotze PG. Risk factors for complications after ileocolonic resection for Crohn's disease with a major focus on the impact of preoperative immunosuppressive and biologic therapy: A retrospective international multicentre study. United European Gastroenterol J. 2016;4:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Huang W, Tang Y, Nong L, Sun Y. Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn's disease: A meta-analysis of observational studies. J Crohns Colitis. 2015;9:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 23. | Duan Y, Liu Y, Li Y. Previous Intestinal Resection Is Associated with Postoperative Complications in Crohn's Disease: A Cohort Study. Gastroenterol Res Pract. 2020;2020:2194382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Mege D, Michelassi F. Laparoscopy in Crohn Disease: Learning Curve and Current Practice. Ann Surg. 2020;271:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Hasegawa H, Watanabe M, Nishibori H, Okabayashi K, Hibi T, Kitajima M. Laparoscopic surgery for recurrent Crohn's disease. Br J Surg. 2003;90:970-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Zhou J, Li Y, Gong J, Zhu W. Frequency and risk factors of surgical recurrence of Crohn's disease after primary bowel resection. Turk J Gastroenterol. 2018;29:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Patel SV, Patel SV, Ramagopalan SV, Ott MC. Laparoscopic surgery for Crohn's disease: a meta-analysis of perioperative complications and long term outcomes compared with open surgery. BMC Surg. 2013;13:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn's disease. Ann Surg. 2000;231:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 475] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 29. | Gionchetti P, Dignass A, Danese S, Magro Dias FJ, Rogler G, Lakatos PL, Adamina M, Ardizzone S, Buskens CJ, Sebastian S, Laureti S, Sampietro GM, Vucelic B, van der Woude CJ, Barreiro-de Acosta M, Maaser C, Portela F, Vavricka SR, Gomollón F; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis. 2017;11:135-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 532] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 30. | Wong DJ, Roth EM, Feuerstein JD, Poylin VY. Surgery in the age of biologics. Gastroenterol Rep (Oxf). 2019;7:77-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M, Harrison J, Plevy SE. Infliximab prevents Crohn's disease recurrence after ileal resection. Gastroenterology. 2009;136:441-50.e1; quiz 716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 432] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 32. | Uchino M, Ikeuchi H, Hata K, Minagawa T, Horio Y, Kuwahara R, Nakamura S, Watanabe K, Saruta M, Fujii T, Kobayashi T, Sugimoto K, Hirai F, Esaki M, Hiraoka S, Matsuoka K, Shinzaki S, Matsuura M, Inoue N, Nakase H, Watanabe M. Does anti-tumor necrosis factor alpha prevent the recurrence of Crohn's disease? J Gastroenterol Hepatol. 2021;36:864-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Bakouny Z, Yared F, El Rassy E, Jabbour R, Hallit R, Khoury N, Honein K, Bou Jaoude J. Comparative Efficacy of Anti-TNF Therapies For The Prevention of Postoperative Recurrence of Crohn's Disease: A Systematic Review and Network Meta-Analysis of Prospective Trials. J Clin Gastroenterol. 2019;53:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Kristo I, Stift A, Bergmann M, Riss S. Surgical recurrence in Crohn's disease: Are we getting better? World J Gastroenterol. 2015;21:6097-6100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Ministry of Health and Welfare. Partial revision of "Details on the Standards and Methods of Application of Medical Care Benefits". [cited 16 Feb 2021]. Available from: http://www.mohw.go.kr/react/jb/sjb0406vw.jsp?PAR_MENU_ID=03&MENU_ID=030406&CONT_SEQ=361375. |
| 36. | Scott NA, Sue-Ling HM, Hughes LE. Anastomotic configuration does not affect recurrence of Crohn's disease after ileocolonic resection. Int J Colorectal Dis. 1995;10:67-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Yamamoto T, Allan RN, Keighley MR. Strategy for surgical management of ileocolonic anastomotic recurrence in Crohn's disease. World J Surg. 1999;23:1055-1060; discussion 1060-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Yamamoto T, Bain IM, Mylonakis E, Allan RN, Keighley MR. Stapled functional end-to-end anastomosis vs sutured end-to-end anastomosis after ileocolonic resection in Crohn disease. Scand J Gastroenterol. 1999;34:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | He X, Chen Z, Huang J, Lian L, Rouniyar S, Wu X, Lan P. Stapled side-to-side anastomosis might be better than handsewn end-to-end anastomosis in ileocolic resection for Crohn's disease: a meta-analysis. Dig Dis Sci. 2014;59:1544-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Anuj P, Yoon YS, Yu CS, Lee JL, Kim CW, Park IJ, Lim SB, Kim JC. Does Anastomosis Configuration Influence Long-term Outcomes in Patients With Crohn Disease? Ann Coloproctol. 2017;33:173-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Guo Z, Li Y, Zhu W, Gong J, Li N, Li J. Comparing outcomes between side-to-side anastomosis and other anastomotic configurations after intestinal resection for patients with Crohn's disease: a meta-analysis. World J Surg. 2013;37:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn's disease. Dis Mon. 2018;64:20-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 329] [Article Influence: 41.1] [Reference Citation Analysis (1)] |
| 43. | Ye BD, Yang SK, Cho YK, Park SH, Yang DH, Yoon SM, Kim KJ, Byeon JS, Myung SJ, Yu CS, Kim JH. Clinical features and long-term prognosis of Crohn's disease in Korea. Scand J Gastroenterol. 2010;45:1178-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |