Published online Nov 7, 2021. doi: 10.3748/wjg.v27.i41.7113
Peer-review started: May 20, 2021
First decision: June 12, 2021
Revised: July 4, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: November 7, 2021
Processing time: 169 Days and 11.9 Hours
Non-alcoholic fatty liver disease (NAFLD) is currently considered the most common cause of liver disease. Its prevalence is increasing in parallel with the obesity and type 2 diabetes mellitus (DM2) epidemics in developed countries. Several recent studies have suggested that NAFLD may be the hepatic mani
Core Tip: Non-alcoholic fatty liver disease (NAFLD) is currently considered the most common cause of liver disease. Its prevalence is increasing in parallel with the obesity and type 2 diabetes mellitus epidemics in developed countries. Several recent studies have suggested that NAFLD may be the hepatic manifestation of a systemic inflammatory metabolic disease that also affects other organs. This article reviews the cu
- Citation: Perez-Carreras M, Casis-Herce B, Rivera R, Fernandez I, Martinez-Montiel P, Villena V. Non-alcoholic fatty liver disease in patients with intestinal, pulmonary or skin diseases: Inflammatory cross-talk that needs a multidisciplinary approach. World J Gastroenterol 2021; 27(41): 7113-7124
- URL: https://www.wjgnet.com/1007-9327/full/v27/i41/7113.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i41.7113
Non-alcoholic liver disease (NAFLD) has become the leading cause of liver disease in western countries and has attracted researchers’ and clinicians’ interest in recent years. It affects 20%-30% of the adult population in developed countries and its prevalence rises to 95% among subjects with morbid obesity and 70% among those with type 2 diabetes mellitus (DM2)[1,2]. Close to two-thirds of NAFLD patients have some metabolic factor and one-third have metabolic syndrome (MetS). Although NAFLD has been proposed as the hepatic manifestation of MetS, in actual fact the relationship between these two conditions is complex and reciprocal, mediated by insulin re
NAFLD is a dynamic disease that includes an evolving spectrum of anatomo-clinical lesions, including hepatic steatosis (fat deposition in hepatocytes, usually in the form of macrovacuoles), steatohepatitis (NASH; which adds cell death and inflammatory infiltrates) and cirrhosis. Although most patients have a mild form of the disease, up to 20%-30% may have NASH lesions and one-third may have progressive fibrosis[1,5].
Excluding alcohol consumption has long been the key criterion to diagnosis of NAFLD[6,7]. However, its close association with metabolic factors has recently led some experts to propose replacing the term NAFLD with “MAFLD” (abbreviating for “metabolic dysfunction-associated fatty liver disease”), which would include patients with metabolic conditions, even if they consume alcohol and/or have other causes of fatty liver disease[8,9]. Likewise, “extra-hepatic manifestations” is probably not a suitable designation for the morbidities that coexist with NAFLD in metabolically unhealthy subjects, including inflammatory bowel disease (IBD), obstructive syndro
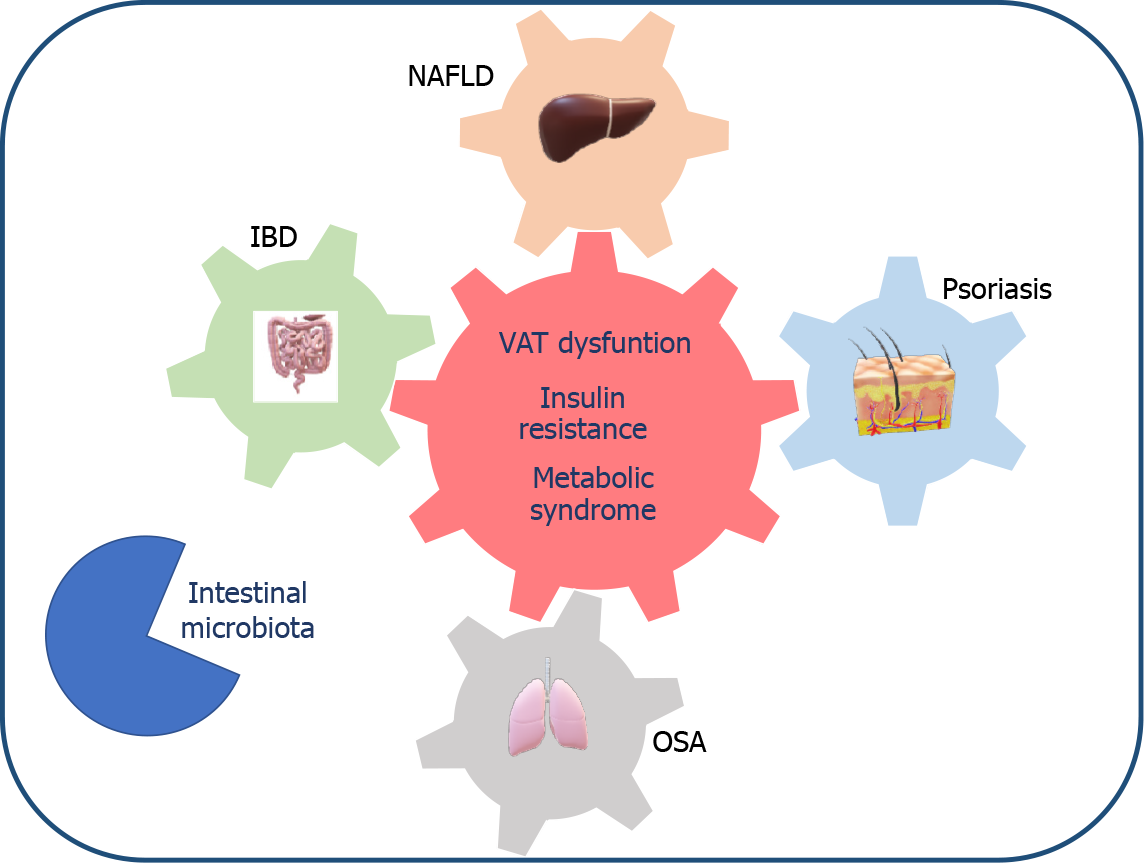
The mechanisms that link NAFLD with these extrahepatic comorbidities are complex and multifactorial. It appears that the imbalance in favor of proinflammatory vs anti-inflammatory cytokines produced by the visceral adipose tissue (VAT) of patients with metabolic dysfunction not only plays a role in the onset of an insulin resistance state, hepatic lipotoxicity and NAFLD but also interacts synergistically with the local production of these same inflammatory mediators in the intestine or skin, as occurs in IBD and psoriasis, or is enhanced by the hypoxemia present in OSA[12-15]. Moreover, there is increasing evidence that changes in the intestinal microbiota and their metabolic interaction with the host also play a role in the multi-hit pathogenesis of NAFLD and related extra-hepatic diseases[12,16].
In this review, our aim was to update the available evidence on the link between NAFLD and three increasingly prevalent diseases: IBD, OSA and psoriasis. Ultimately, we aim to draw attention to the impact that NAFLD may have on the management and prognosis of patients with these extra-hepatic comorbidities in clinical practice.
IBD is a complex and multifactorial gastrointestinal disease that usually presents in the form of acute outbreaks on a chronic immune-mediated inflammatory substrate. Although its main symptoms relate to the intestine, in both types of IBD — Crohn’s disease and ulcerative colitis — extraintestinal symptoms can appear in organs such as the skin, joints, eyes and liver[15]. Hepatobiliary manifestations have been reported in 3%-50% of patients with IBD and elevated transaminases in up to one-third of them, often being transient and attributable to the immunomodulatory drugs used in IBD. NAFLD is now considered the leading cause of liver disease among IBD patients[17,18].
Since the link between ulcers in the intestine and fatty hepatomegaly was first described in 1873[19], multiple cases, series and observational cohort studies have been published that analyze the prevalence of NAFLD in patients with IBD and the factors that may link the two diseases, both metabolic (e.g., DM2, obesity, arterial hypertension, dyslipemia, MetS) and relating to the IBD itself (e.g., type, duration, inflammatory activity, extension, drugs, intestinal surgery)[20-30].
Zou et al[31] conducted a systematic review and meta-analysis covering 19 observational studies published up to 2018, involving a total of 5620 patients with IBD. Although NAFLD prevalence varied significantly (8%-40%), in the most recent studies they found an increase in the frequency of this liver disease among IBD patients, to the point that it now exceeds the level in the general population (33% vs 25%, res
The liver-intestine interaction in IBD and NAFLD patients could be explained by mechanisms such as the synergism between inflammatory mediators produced by hypertrophic adipocytes in the VAT and the increase of proinflammatory cytokines from the intestine[12,15]. It could also be explained by the involvement of intestinal microbiota, including changes in its diversity, interaction of metabolites produced by intestinal microorganisms with the host’s lipogenesis and host hydrocarbon me
It has recently been recognized that patients with IBD, mainly those with Crohn’s disease, have a specific type of mesenteric adipose tissue, located in the areas of inflamed bowel, called “creeping fat”. This is an immunologically active tissue that behaves similarly to VAT, promoting inflammation of the intestinal mucosa and perhaps playing a role in the metabolic changes involved in the onset of NAFLD[36,37].
There are some practical issues of particular interest to IBD gastroenterologists, such as: (1) Identifying IBD patients with NAFLD risk; (2) The impact of a NAFLD diagnosis on the treatment and prognosis of patients with IBD; and (3) When to refer the patient with IBD to a hepatologist.
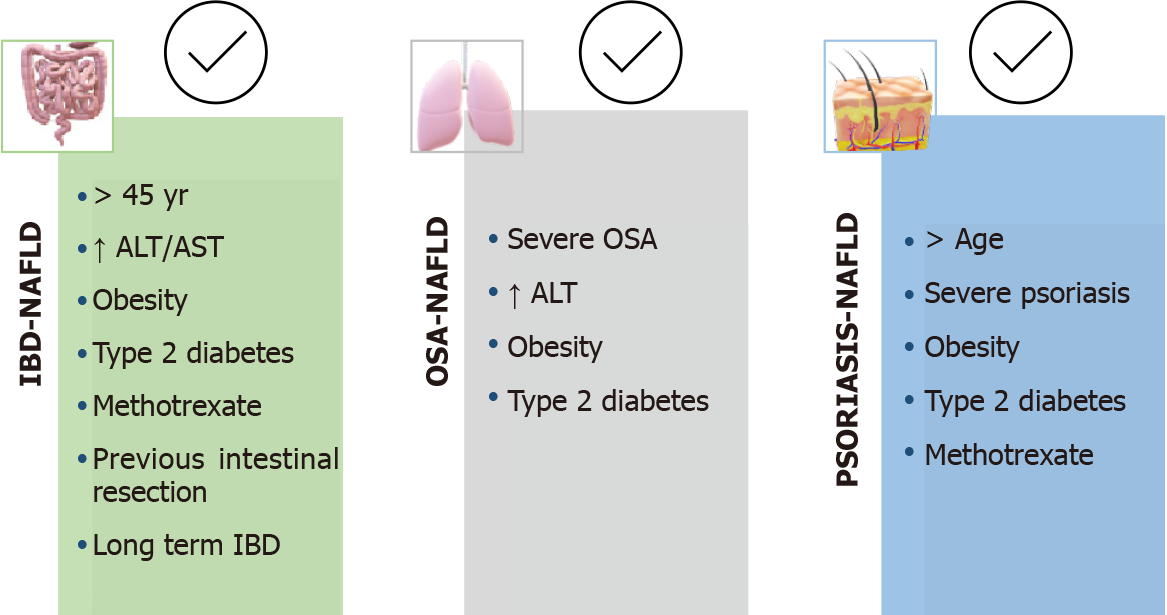
NAFLD should generally be suspected in older IBD patients with metabolic conditions, previous bowel surgery, or long-standing bowel disease[31,36]. However, comparing NAFLD patients with and without IBD, it appears that patients with NAFLD and IBD are younger and have less metabolic factors than those NAFLD patients without bowel disease. This is why some authors have proposed defining two phenotypes of patients with IBD and NAFLD: “classic or metabolic” and “IBD-specific”. The first group included subjects > 45-years-old with elevated transaminases, obesity, DM2 or arterial hypertension, and a later onset of bowel disease, while the second group included younger individuals with normal transaminases and less metabolic factors[23,25] (Figure 2).
We do not have prospective studies analyzing whether there are differences in natural history and prognosis between these two phenotypes. Sartini et al[25] have associated NAFLD severity, measured by the degree of ultrasound steatosis, with fewer metabolic conditions and a “severe” IBD phenotype (i.e. more than one annual inflammatory bowel flare, more extensive IBD and previous intestinal surgery). These data suggest that in addition to metabolic conditions, other IBD-specific factors are likely to be involved in the onset and progression of NAFLD.
Based on animals and in vitro experimental studies, it has been speculated that tumor necrosis factor-alpha inhibitors may protect against developing NAFLD and also that glucocorticoids and immunomodulators increase the risk of liver disease progression[38,39]. However, few clinical studies have adequately collected the time and dose of treatments and thus conclusive information is not available at present. A synergistic effect of NAFLD and methotrexate treatment has been suggested, favoring liver toxicity and progression of NAFLD to more severe forms, especially in patients with obesity or DM2[40,41]. Lapumnuaypol et al[27] found a very high prevalence of NAFLD (54%) in their series of 80 patients with IBD treated only with biologics (i.e. infliximab, adalimumab, certolizumab or goligumab), most of whom were male and obese. In the multivariate analysis, they found an association between NAFLD and the clinical activity of IBD but not with drugs.
Considering that liver fibrosis is the main prognostic marker in NAFLD patients, it is important for gastroenterologists to use non-invasive markers of fibrosis in patients with IBD and NAFLD. These markers consist of mathematical algorithms that include clinical and analytical variables whose result enables the identification and stratification of patients with liver fibrosis. Among them, FIB-4 and NFS (non-alcoholic fatty liver disease score) have been validated in patients with NAFLD[4,6,7]. Retrospective and longitudinal studies that include the calculation of both serological markers of fibrosis found that only 2.2% of subjects with IBD and NAFLD have advanced fibrosis, and that it remains stable during a 3-5 year follow-up in most patients. Age, metabolic factors and duration of IBD appear to increase the risk of fibrosis progression[26,42,43].
Palumbo et al[42] were the authors of the only prospective study designed as part of a screening program for NAFLD and fibrosis involving 384 patients with IBD using control attenuation parameter (CAP) and hepatic elastography available on the FibroScan device probe (Echosens, France). They found any grade NAFLD in 32.8% of patients (CAP ≥ 248 dB/m), severe NAFLD (CAP > 300 dB/m), significant fibrosis in 24.6% (> 7 kPa) and advanced fibrosis in 18% (> 8.7 kPa). These NAFLD prevalence data should be taken with caution as there is evidence that higher CAP cutoffs than those used by these authors improve the diagnostic accuracy of this method[44,45]. The authors compared the presence of significant and advanced fibrosis in patients with NAFLD vs non-NAFLD and found a higher prevalence in the NAFLD group (24.6% vs 6.2% and 18.3% vs 3.1%, respectively; P < 0.001). Age, obesity, plasma triglycerides and methotrexate use were factors associated with liver fibrosis. In this way, they stratified the patients and referred a third of them to the hepatologist. In addition, they highlighted the presence of chronic kidney disease and cardiovascular disease among patients with IBD and NAFLD, in many cases related to subclinical atherosclerosis, information which supports the multidisciplinary approach to at-risk patients.
Although we now have scientific evidence to establish the suspicion of NAFLD in patients with IBD, prospective, longitudinal and control group studies are needed to identify those phenotypes at risk of developing fibrosis and advanced liver disease before we can establish an appropriate algorithm for screening, diagnosis and follow-up of patients with IBD and NAFLD.
OSA is a clinical condition usually considered a respiratory disease, which has begun to be recognized as a multisystemic disease in the last two decades. It consists of recurrent nocturnal episodes of complete (apnea) and incomplete (hypopnea) ob
Over the last decade, at least 20 clinical studies have been published linking NAFLD and OSA, including systematic reviews and meta-analyses[49]. Although most include short series of patients and histological information is usually obtained from liver biopsies during morbid obesity surgery, OSA is considered to be an independent risk factor for the development and progression of NAFLD. In a recent meta-analysis and systematic review, Jin et al[50] found that patients with severe OSA (number of apnea-hypopnea episodes > 30/h) have higher aspartate aminotransferase values and a greater degree of steatosis, inflammation, ballooning degeneration and fibrosis. Trzepizur et al[51] found in their cohort of almost 1300 patients that those with severe OSA had a 2.5-fold increased risk of liver fibrosis; although, this association was not independent of other factors when logistic regression analysis was performed. When determining the epidemiological and pathogenic relationship between NAFLD and OSA, it is difficult to avoid the impact of obesity and other metabolic conditions that are so frequent in both diseases. Evidence from experimental models shows that chronic intermittent ischemia and increased sympathetic tone triggered by nocturnal hypoxemia phenomena are the main causes of cardiometabolic manifestations linked to OSA (i.e. DM2, dyslipemia, arterial hypertension, atherosclerosis) and of the onset and progression of NAFLD lesions[13,46].
The decrease in oxygen tension that occurs during nocturnal apnea-hypopnea episodes primarily affects hepatocytes in zone 3 of the hepatic lobule, where NAFLD lesions predominate, and results in the release of ischemia-induced factors (commonly known as HIF)[52,53]. These HIF favor the expression of genes involved in lipogenesis, with the consequent excess of triglycerides in the hepatocytes (steatosis), free fatty acids and hepatokines. This state of liver lipotoxicity leads to inflammation, mito
At present, we do not have prospective studies that allow us to determine the prevalence of NAFLD in patients with OSA or vice versa. However, considering that chronic intermittent ischemia can promote and aggravate liver damage, some authors propose screening for OSA in patients with NAFLD[47,49]. Taking into account the available information, hepatologists should always ask NAFLD patients about OSA-related symptoms and consider referring to the pneumologist those with elevated transaminases and/or advanced liver lesions, and/or associated metabolic factors, especially DM2 and obesity (Figure 2).
The use of a continuous positive airway pressure (CPAP) device and lifestyle changes to diet and exercise, especially in obesity/overweight patients, comprise the standard treatment of OSA patients. CPAP increases pharyngeal intraluminal pneu
Psoriasis is one of the diseases that dermatologists are most concerned about because of its prevalence (it affects 2%-3% of the adult population in developed countries), the gaps in our knowledge of its pathogenesis, and its relationship with extracutaneous pathologies[14]. Currently, psoriasis is considered a systemic immune-mediated chronic inflammatory disease, since psoriasis patients not only present skin lesions but also frequently have comorbidities that can condition their prognosis and treatment, such as MetS and its hepatic manifestation, NAFLD[60-63].
There is epidemiological evidence and pathogenic hypotheses linking psoriasis to NAFLD[64,65]. In the last 10 years, multiple controlled cross-sectional observational studies have been published, some in large populations, studying the prevalence of NAFLD in subjects with psoriasis and the specific characteristics of the subpopulation of patients with psoriasis and NAFLD[66-69]. Van der Voort et al[66] analyzed these data in a Dutch cohort of 2292 individuals > 55 years of age and found that the prevalence of NAFLD among the 118 psoriasis patients was significantly higher than among the 2174 healthy controls (46.2% vs 33.3%; P < 0.005). Furthermore, after adjusting for confounders, including MetS, they determined that elderly patients with psoriasis are 70% more likely to have NAFLD than those without psoriasis. Gisondi et al[67] found that 44% of the 124 psoriasis patients included in their study had NAFLD vs 26% of the 79 healthy controls (P < 0.001). Comparing patients with psoriasis and NAFLD vs no-NAFLD, they found that those included in the first group were more frequently male and had a higher body mass index, transaminase values (Alanine aminotransferase and aspartate aminotransferase), and psoriasis severity [measured according to Psoriasis Area Severity Index (PASI) score. It combines the assessment of the severity of the lesion and affected area into a single figure between the values of 0-no disease to 72-maximum disease].
In 2015, Candia et al[68] published the first meta-analysis and systematic review of seven case-control studies evaluating the psoriasis-NAFLD association in populations from different continents (n = 267761). Their combined analysis indicated that pso
Approximately one-third of psoriasis patients have joint involvement and this has been linked to increased inflammatory burden, severity of skin lesions and risk of NAFLD[14,70]. However, not all authors found an association between arthropathy and liver disease[71]. The systematic review and meta-analysis by Candia et al[68] found that patients with psoriatic arthritis had double the risk of NAFLD when compared to those without arthropathy (OR: 2.25, 95%CI: 1.4-3.7; P < 0.05). Although this information would suggest considering patients with psoriasis and joint in
Although most studies found an independent link between psoriasis and NAFLD, those with metabolic factors appear to be particularly at risk[61,62]. The high pre
Most studies have assessed NAFLD using ultrasound, while few have involved liver biopsy. Roberts et al[76] found NAFLD in half of psoriasis patients (n = 103) and NASH histological lesions in 22% (liver biopsy in 52/103), one-third of them with significant advanced fibrosis. Furthermore, they linked NASH lesions with higher PASI scores, obesity and higher transaminase levels. More authors have analyzed the severity of liver lesions in psoriasis using non-invasive markers of fibrosis, serological or technological (FibroScan®)[67,77,78]. Using these scores it was found that 7%-8% of psoriasis patients have advanced liver fibrosis. This risk is multiplied by 4 when NAFLD is associated with this skin disease. Although fibrosis is more frequent in older subjects with metabolic factors, psoriasis appears to be an independent risk factor for fibrosis when adjusting in the multivariate logistic regression analysis, including metabolic conditions and drugs used to treat psoriasis[78].
From a practical perspective, the psoriasis-NAFLD association not only has an impact on the severity of skin and liver lesions, but may also influence dermatologists’ decisions when selecting systemic treatment for psoriasis[62,79].
Although some studies associate methotrexate liver toxicity with cumulative dose and treatment time, most find that the risk of liver damage is primarily related to the presence of risk conditions such as obesity, DM2, alcohol consumption, and NAFLD[80,81]. Cyclosporine and acitretin, a retinoid derived from vitamin A, are considered to be diabetogenic and to promote atherogenic dyslipemia and arterial hypertension[79,82].
It has been speculated that new biologic drugs could be beneficial in patients with psoriasis and NAFLD by acting on the proinflammatory cytokines involved in both diseases[63]. Although controlled studies with some tumor necrosis factor-alpha inhibitors have demonstrated their ability to decrease insulin resistance and the risk of DM2 (etanercept), others appear to favor weight gain and dyslipemia (infliximab, adalimumab)[79]. Recent research studies in animals have shown that IL-17 is in
Considering that being overweight or obesity significantly increases psoriasis risk and severity, recent clinical trials have shown that weight loss, both with a hypocaloric diet and physical exercise or with bariatric surgery, improves psoriasis activity and also favors the response to systemic treatment. These interventions could be of particular importance in patients with psoriasis and risk of NAFLD[61,74].
Aware of the impact of NAFLD on psoriasis patients, experts from the European and American academies of dermatology have recently published recommendations for the management of psoriasis comorbidities, including MetS and NAFLD[82,85]. According to the available evidence, they consider NAFLD screening to be indicated in patients with a risk phenotype (i.e. moderate-severe psoriasis and metabolic factors) by means of transaminase measures and ultrasound, and propose a monitoring and follow-up algorithm that includes evaluation by the hepatologist in patients with suspected liver disease (Figure 2). In addition, they recommend that a diagnosis of NAFLD be taken into account when selecting psoriasis treatment. However, these recommendations do not appear to be implemented universally in dermatologists’ clinical practice, nor has a specific hepatologist referral protocol for patients with psoriasis and NAFLD been established in most hospitals.
NAFLD should be considered more than a liver disease and be taken into account not only by hepatologists but also by clinicians caring for patients with other related diseases, such as IBD, OSA and psoriasis. The scientific evidence shows that these comorbidities share an inflammatory background that synergistically impacts the severity and management of these patients. It is essential that screening and referral algorithms for NAFLD subjects are developed from a multidisciplinary perspective in which not only liver, intestinal, respiratory or skin lesions are analyzed but also the risk of morbidity and mortality from metabolic and cardiovascular causes.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Sociedad Española De Patologia Digestiva.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferraioli G, Grefhorst A S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY
| 1. | Tanaka N, Kimura T, Fujimori N, Nagaya T, Komatsu M, Tanaka E. Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World J Gastroenterol. 2019;25:163-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (4)] |
| 2. | Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 994] [Article Influence: 198.8] [Reference Citation Analysis (0)] |
| 3. | Kim D, Touros A, Kim WR. Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Clin Liver Dis. 2018;22:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 4. | Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J Gastroenterol. 2018;24:3361-3373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 412] [Cited by in RCA: 393] [Article Influence: 56.1] [Reference Citation Analysis (7)] |
| 5. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 793] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 6. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 505] [Article Influence: 56.1] [Reference Citation Analysis (2)] |
| 7. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2612] [Article Influence: 200.9] [Reference Citation Analysis (1)] |
| 8. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2828] [Article Influence: 565.6] [Reference Citation Analysis (1)] |
| 9. | Shiha G, Korenjak M, Eskridge W, Casanovas T, Velez-Moller P, Högström S, Richardson B, Munoz C, Sigurðardóttir S, Coulibaly A, Milan M, Bautista F, Leung NWY, Mooney V, Obekpa S, Bech E, Polavarapu N, Hamed AE, Radiani T, Purwanto E, Bright B, Ali M, Dovia CK, McColaugh L, Koulla Y, Dufour JF, Soliman R, Eslam M. Redefining fatty liver disease: an international patient perspective. Lancet Gastroenterol Hepatol. 2021;6:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 10. | Rosato V, Masarone M, Dallio M, Federico A, Aglitti A, Persico M. NAFLD and Extra-Hepatic Comorbidities: Current Evidence on a Multi-Organ Metabolic Syndrome. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 11. | Kumar R, Priyadarshi RN, Anand U. Non-alcoholic Fatty Liver Disease: Growing Burden, Adverse Outcomes and Associations. J Clin Transl Hepatol. 2020;8:76-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 12. | Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 918] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 13. | Ahmed MH, Byrne CD. Obstructive sleep apnea syndrome and fatty liver: association or causal link? World J Gastroenterol. 2010;16:4243-4252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Ganzetti G, Campanati A, Offidani A. Non-alcoholic fatty liver disease and psoriasis: So far, so near. World J Hepatol. 2015;7:315-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 15. | Chao CY, Battat R, Al Khoury A, Restellini S, Sebastiani G, Bessissow T. Co-existence of non-alcoholic fatty liver disease and inflammatory bowel disease: A review article. World J Gastroenterol. 2016;22:7727-7734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (34)] |
| 16. | Zhou D, Fan JG. Microbial metabolites in non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25:2019-2028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 17. | Rojas-Feria M, Castro M, Suárez E, Ampuero J, Romero-Gómez M. Hepatobiliary manifestations in inflammatory bowel disease: the gut, the drugs and the liver. World J Gastroenterol. 2013;19:7327-7340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Restellini S, Chazouillères O, Frossard JL. Hepatic manifestations of inflammatory bowel diseases. Liver Int. 2017;37:475-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Thomas CH. Ulceration of the colon with a much enlarged fatty liver. Trans Pathol Soc Phil. 2021;4:87-88. |
| 20. | Glassner K, Malaty HM, Abraham BP. Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease Among Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Principi M, Iannone A, Losurdo G, Mangia M, Shahini E, Albano F, Rizzi SF, La Fortezza RF, Lovero R, Contaldo A, Barone M, Leandro G, Ierardi E, Di Leo A. Nonalcoholic Fatty Liver Disease in Inflammatory Bowel Disease: Prevalence and Risk Factors. Inflamm Bowel Dis. 2018;24:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Sourianarayanane A, Garg G, Smith TH, Butt MI, McCullough AJ, Shen B. Risk factors of non-alcoholic fatty liver disease in patients with inflammatory bowel disease. J Crohns Colitis. 2013;7:e279-e285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (2)] |
| 23. | Sartini A, Gitto S, Bianchini M, Verga MC, Di Girolamo M, Bertani A, Del Buono M, Schepis F, Lei B, De Maria N, Villa E. Non-alcoholic fatty liver disease phenotypes in patients with inflammatory bowel disease. Cell Death Dis. 2018;9:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 24. | Carr RM, Patel A, Bownik H, Oranu A, Kerner C, Praestgaard A, Forde KA, Reddy KR, Lichtenstein GR. Intestinal Inflammation Does Not Predict Nonalcoholic Fatty Liver Disease Severity in Inflammatory Bowel Disease Patients. Dig Dis Sci. 2017;62:1354-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Sartini A, Gitto S, Villa E. Does Metabolic Syndrome and Not the Inflammatory Load Predict Nonalcoholic Fatty Liver Disease Severity in Inflammatory Bowel Disease Patients? Dig Dis Sci. 2017;62:2604-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Bessissow T, Le NH, Rollet K, Afif W, Bitton A, Sebastiani G. Incidence and Predictors of Nonalcoholic Fatty Liver Disease by Serum Biomarkers in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:1937-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 27. | Lapumnuaypol K, Kanjanahattakij N, Pisarcik D, Thongprayoon C, Wijarnpreecha K, Cheungpasitporn W. Effects of inflammatory bowel disease treatment on the risk of nonalcoholic fatty liver disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Karaivazoglou K, Konstantakis C, Tourkochristou E, Assimakopoulos SF, Triantos C. Non-alcoholic fatty liver disease in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol. 2020;32:903-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Sagami S, Ueno Y, Tanaka S, Fujita A, Hayashi R, Oka S, Hyogo H, Chayama K. Significance of non-alcoholic fatty liver disease in Crohn's disease: A retrospective cohort study. Hepatol Res. 2017;47:872-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Magrì S, Paduano D, Chicco F, Cingolani A, Farris C, Delogu G, Tumbarello F, Lai M, Melis A, Casula L, Fantini MC, Usai P. Nonalcoholic fatty liver disease in patients with inflammatory bowel disease: Beyond the natural history. World J Gastroenterol. 2019;25:5676-5686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Zou ZY, Shen B, Fan JG. Systematic Review With Meta-analysis: Epidemiology of Nonalcoholic Fatty Liver Disease in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:1764-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 32. | Moran C, Sheehan D, Shanahan F. The Changing Phenotype of Inflammatory Bowel Disease. Gastroenterol Res Pract. 2016;2016:1619053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Spagnuolo R, Montalcini T, De Bonis D, Ferro Y, Cosco C, Mazza E, Romeo S, Doldo P, Pujia A. Weight Gain and Liver Steatosis in Patients with Inflammatory Bowel Diseases. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Verdugo-Meza A, Ye J, Dadlani H, Ghosh S, Gibson DL. Connecting the Dots Between Inflammatory Bowel Disease and Metabolic Syndrome: A Focus on Gut-Derived Metabolites. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Kwon J, Lee C, Heo S, Kim B, Hyun CK. DSS-induced colitis is associated with adipose tissue dysfunction and disrupted hepatic lipid metabolism leading to hepatosteatosis and dyslipidemia in mice. Sci Rep. 2021;11:5283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 36. | Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 302] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 37. | Michalak A, Mosińska P, Fichna J. Common links between metabolic syndrome and inflammatory bowel disease: Current overview and future perspectives. Pharmacol Rep. 2016;68:837-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Barbuio R, Milanski M, Bertolo MB, Saad MJ, Velloso LA. Infliximab reverses steatosis and improves insulin signal transduction in liver of rats fed a high-fat diet. J Endocrinol. 2007;194:539-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 39. | Dolinsky VW, Douglas DN, Lehner R, Vance DE. Regulation of the enzymes of hepatic microsomal triacylglycerol lipolysis and re-esterification by the glucocorticoid dexamethasone. Biochem J. 2004;378:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Likhitsup A, Dundulis J, Ansari S, Patibandla S, Hutton C, Kennedy K, Helzberg JH, Chhabra R. High prevalence of non-alcoholic fatty liver disease in patients with inflammatory bowel disease receiving anti-tumor necrosis factor therapy. Ann Gastroenterol. 2019;32:463-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | McGowan CE, Jones P, Long MD, Barritt AS 4th. Changing shape of disease: nonalcoholic fatty liver disease in Crohn's disease-a case series and review of the literature. Inflamm Bowel Dis. 2012;18:49-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Saroli Palumbo C, Restellini S, Chao CY, Aruljothy A, Lemieux C, Wild G, Afif W, Lakatos PL, Bitton A, Cocciolillo S, Ghali P, Bessissow T, Sebastiani G. Screening for Nonalcoholic Fatty Liver Disease in Inflammatory Bowel Diseases: A Cohort Study Using Transient Elastography. Inflamm Bowel Dis. 2019;25:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 43. | Ritaccio G, Stoleru G, Abutaleb A, Cross RK, Shetty K, Sakiani S, Wong U. Nonalcoholic Fatty Liver Disease Is Common in IBD Patients However Progression to Hepatic Fibrosis by Noninvasive Markers Is Rare. Dig Dis Sci. 2021;66:3186-3191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V, Bedossa P, Newsome PN. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 965] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 45. | Petroff D, Blank V, Newsome PN, Shalimar, Voican CS, Thiele M, de Lédinghen V, Baumeler S, Chan WK, Perlemuter G, Cardoso AC, Aggarwal S, Sasso M, Eddowes PJ, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Cobbold JF, Naveau S, Lupsor-Platon M, Mueller S, Krag A, Irles-Depe M, Semela D, Wong GL, Wong VW, Villela-Nogueira CA, Garg H, Chazouillères O, Wiegand J, Karlas T. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:185-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 168] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 46. | Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 47. | Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 575] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 48. | Parikh MP, Gupta NM, McCullough AJ. Obstructive Sleep Apnea and the Liver. Clin Liver Dis. 2019;23:363-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Mesarwi OA, Loomba R, Malhotra A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am J Respir Crit Care Med. 2019;199:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 50. | Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sleep Breath. 2018;22:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 51. | Trzepizur W, Boursier J, Mansour Y, Le Vaillant M, Chollet S, Pigeanne T, Bizieux-Thaminy A, Humeau MP, Alizon C, Goupil F, Meslier N, Priou P, Calès P, Gagnadoux F; Institut de Recherche en Santé Respiratoire des Pays de la Loire Sleep Cohort Group. Association Between Severity of Obstructive Sleep Apnea and Blood Markers of Liver Injury. Clin Gastroenterol Hepatol. 2016;14:1657-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring). 2011;19:2167-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 53. | Shin MK, Drager LF, Yao Q, Bevans-Fonti S, Yoo DY, Jun JC, Aja S, Bhanot S, Polotsky VY. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1α. PLoS One. 2012;7:e46562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Lin QC, Chen LD, Chen GP, Zhao JM, Chen X, Huang JF, Wu LH. Association between nocturnal hypoxia and liver injury in the setting of nonalcoholic fatty liver disease. Sleep Breath. 2015;19:273-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Kuvat N, Tanriverdi H, Armutcu F. The relationship between obstructive sleep apnea syndrome and obesity: A new perspective on the pathogenesis in terms of organ crosstalk. Clin Respir J. 2020;14:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 56. | Ko CY, Liu QQ, Su HZ, Zhang HP, Fan JM, Yang JH, Hu AK, Liu YQ, Chou D, Zeng YM. Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin Sci (Lond). 2019;133:905-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 57. | Tan X, Saarinen A, Mikkola TM, Tenhunen J, Martinmäki S, Rahikainen A, Cheng S, Eklund N, Pekkala S, Wiklund P, Munukka E, Wen X, Cong F, Wang X, Zhang Y, Tarkka I, Sun Y, Partinen M, Alen M. Effects of exercise and diet interventions on obesity-related sleep disorders in men: study protocol for a randomized controlled trial. Trials. 2013;14:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Labarca G, Cruz R, Jorquera J. Continuous Positive Airway Pressure in Patients With Obstructive Sleep Apnea and Non-Alcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. J Clin Sleep Med. 2018;14:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Sundaram SS, Halbower AC, Klawitter J, Pan Z, Robbins K, Capocelli KE, Sokol RJ. Treating Obstructive Sleep Apnea and Chronic Intermittent Hypoxia Improves the Severity of Nonalcoholic Fatty Liver Disease in Children. J Pediatr. 2018;198:67-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Tula E, Ergun T, Seckin D, Ozgen Z, Avsar E. Psoriasis and the liver: problems, causes and course. Australas J Dermatol. 2017;58:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Ganzetti G, Campanati A, Molinelli E, Offidani A. Psoriasis, non-alcoholic fatty liver disease, and cardiovascular disease: Three different diseases on a unique background. World J Cardiol. 2016;8:120-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Romero-Pérez D, Belinchón-Romero I, Bellot P, Francés R, Marco F, Ramos-Rincón JM. Nonalcoholic fatty liver disease puts patients with psoriasis at greater cardiovascular risk. Australas J Dermatol. 2019;60:e304-e310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Mantovani A, Gisondi P, Lonardo A, Targher G. Relationship between Non-Alcoholic Fatty Liver Disease and Psoriasis: A Novel Hepato-Dermal Axis? Int J Mol Sci. 2016;17:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 64. | Prussick RB, Miele L. Nonalcoholic fatty liver disease in patients with psoriasis: a consequence of systemic inflammatory burden? Br J Dermatol. 2018;179:16-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 65. | Olveira A, Herranz P, Montes ML. Psoriasis and fatty liver: a harmful synergy. Rev Esp Enferm Dig. 2019;111:314-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | van der Voort EA, Koehler EM, Dowlatshahi EA, Hofman A, Stricker BH, Janssen HL, Schouten JN, Nijsten T. Psoriasis is independently associated with nonalcoholic fatty liver disease in patients 55 years old or older: Results from a population-based study. J Am Acad Dermatol. 2014;70:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 67. | Gisondi P, Barba E, Girolomoni G. Non-alcoholic fatty liver disease fibrosis score in patients with psoriasis. J Eur Acad Dermatol Venereol. 2016;30:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Candia R, Ruiz A, Torres-Robles R, Chávez-Tapia N, Méndez-Sánchez N, Arrese M. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 69. | Phan K, Onggo J, Charlton O, Smith SD. Relationship between psoriasis and non-alcoholic fatty liver disease - Updated systematic review and adjusted meta-analysis. Australas J Dermatol. 2019;60:e352-e355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Perez-Chada LM, Merola JF. Comorbidities associated with psoriatic arthritis: Review and update. Clin Immunol. 2020;214:108397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 71. | Ogdie A, Grewal SK, Noe MH, Shin DB, Takeshita J, Chiesa Fuxench ZC, Carr RM, Gelfand JM. Risk of Incident Liver Disease in Patients with Psoriasis, Psoriatic Arthritis, and Rheumatoid Arthritis: A Population-Based Study. J Invest Dermatol. 2018;138:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 72. | Carrascosa JM, Bonanad C, Dauden E, Botella R, Olveira-Martín A; en nombre del Grupo de Trabajo en Inflamación Sistémica en Psoriasis. Psoriasis and Nonalcoholic Fatty Liver Disease. Actas Dermosifiliogr. 2017;108:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 73. | Rivera R, Vanaclocha F. [Nonalcoholic fatty liver disease and psoriasis]. Actas Dermosifiliogr. 2010;101:657-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | Gisondi P, Fostini AC, Fossà I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clin Dermatol. 2018;36:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 75. | Benhadou F, Mintoff D, Schnebert B, Thio HB. Psoriasis and Microbiota: A Systematic Review. Diseases. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 76. | Roberts KK, Cochet AE, Lamb PB, Brown PJ, Battafarano DF, Brunt EM, Harrison SA. The prevalence of NAFLD and NASH among patients with psoriasis in a tertiary care dermatology and rheumatology clinic. Aliment Pharmacol Ther. 2015;41:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Ortolan A, Lorenzin M, Tadiotto G, Russo FP, Oliviero F, Felicetti M, D'Incà R, Favero M, Piaserico S, Doria A, Ramonda R. Metabolic syndrome, non-alcoholic fatty liver disease and liver stiffness in psoriatic arthritis and psoriasis patients. Clin Rheumatol. 2019;38:2843-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 78. | Pongpit J, Porntharukchareon S, Kaewduang P, Promson K, Stitchantrakul W, Petraksa S, Thakkinstian A, Kositchaiwat C, Rajatanavin N, Sobhonslidsuk A. Liver Stiffness Measurement in Psoriasis: Do Metabolic or Disease Factors Play the Important Role? Biomed Res Int. 2016;2016:7963972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Klujszo EH, Parcheta P, Witkowska AB, Krecisz B. Non-alcoholic fatty liver disease in patients with psoriasis: therapeutic implications. Postepy Dermatol Alergol. 2020;37:468-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Cheng HS, Rademaker M. Monitoring methotrexate-induced liver fibrosis in patients with psoriasis: utility of transient elastography. Psoriasis (Auckl). 2018;8:21-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Rivera R, Vilarrasa E, Ribera M, Roe E, Kueder-Pajares T, Zayas AI, Martínez-Molina L, Mataix Díaz J, Rodríguez-Nevado IM, Usero-Bárcena T, de la Mano D, García-Donoso C, Olveira A, Guinea G, Martín-Vázquez V, Ferran M. Unmet needs in patients with moderate-to-severe plaque psoriasis treated with methotrexate in real world practice: FirST study. J Dermatolog Treat. 2020;1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Elmets CA, Leonardi CL, Davis DMR, Gelfand JM, Lichten J, Mehta NN, Armstrong AW, Connor C, Cordoro KM, Elewski BE, Gordon KB, Gottlieb AB, Kaplan DH, Kavanaugh A, Kivelevitch D, Kiselica M, Korman NJ, Kroshinsky D, Lebwohl M, Lim HW, Paller AS, Parra SL, Pathy AL, Prater EF, Rupani R, Siegel M, Stoff B, Strober BE, Wong EB, Wu JJ, Hariharan V, Menter A. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 297] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 83. | Vasseur P, Serres L, Jégou JF, Pohin M, Delwail A, Petit-Paris I, Levillain P, Favot L, Samson M, Yssel H, Morel F, Silvain C, Lecron JC. High-Fat Diet-Induced IL-17A Exacerbates Psoriasiform Dermatitis in a Mouse Model of Steatohepatitis. Am J Pathol. 2016;186:2292-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | D'Adamio S, Silvaggio D, Lombardo P, Bianchi L, Talamonti M, Galluzzo M. The safety of anti-interleukins monoclonal antibodies for the treatment of psoriasis. Expert Opin Drug Saf. 2019;18:1031-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Dauden E, Blasco AJ, Bonanad C, Botella R, Carrascosa JM, González-Parra E, Jodar E, Joven B, Lázaro P, Olveira A, Quintero J, Rivera R. Position statement for the management of comorbidities in psoriasis. J Eur Acad Dermatol Venereol. 2018;32:2058-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |