Published online Nov 7, 2021. doi: 10.3748/wjg.v27.i41.7080
Peer-review started: May 15, 2021
First decision: June 22, 2021
Revised: July 2, 2021
Accepted: September 30, 2021
Article in press: September 30, 2021
Published online: November 7, 2021
Processing time: 174 Days and 11.1 Hours
The aberrant use of alcohol is a major factor in cancer progression and metastasis. Contributing mechanisms include the systemic effects of alcohol and the exchange of bioactive molecules between cancerous and non-cancerous cells along the brain-gut-liver axis. Such interplay leads to changes in molecular, cellular, and biological functions resulting in cancer progression. Recent investigations have examined the role of extracellular vesicles (EVs) in cancer mechanisms in addition to their contribution as diagnostic biomarkers. Also, EVs are emerging as novel cell-free mediators in pathophysiological scenarios including alcohol-mediated gut microbiome dysbiosis and the release of nanosized EVs into the circulatory system. Interestingly, EVs in cancer patients are enriched with oncogenes, miRNA, lipids, and glycoproteins whose delivery into the hepatic microenvironment may be enhanced by the detrimental effects of alcohol. Proof-of-concept studies indicate that alcohol-associated liver disease is impacted by the effects of exosomes, including altered immune responses, reprogramming of stromal cells, and remodeling of the extracellular matrix. Moreover, the culmination of alcohol-related changes in the liver likely contributes to enhanced hepatic metastases and poor outcomes for cancer patients. This review summarizes the numerous aspects of exosome communications between organs with emphasis on the relationship of EVs in alcohol-associated diseases and cancer metastasis. The potential impact of EV cargo and release along a multi-organ axis is highly relevant to the promotion of tumorigenic mechanisms and metastatic disease. It is hypothesized that EVs target recipient tissues to initiate the formation of prometastatic niches and cancer progression. The study of alcohol-associated mechanisms in metastatic cancers is expected to reveal a better understanding of factors involved in the growth of secondary malignancies as well as novel approaches for therapeutic interventions.
Core Tip: Alcohol consumption is an independent risk factor for cancer development as well as the promotion of metastatic disease, a major cause of morbidity and mortality in cancer patients. The identification of mechanisms and potential therapeutic targets for metastases remains to be determined for many cancers. Interorgan communication involving extracellular vesicles (EVs) is considered a vital process in the promotion of tumorigenic pathways and the spread of disease. Understanding the role of EVs in organ-organ communication networks will likely contribute to the development of future opportunities to combat cancer metastasis.
- Citation: Kuracha MR, Thomas P, Tobi M, McVicker BL. Role of cell-free network communication in alcohol-associated disorders and liver metastasis. World J Gastroenterol 2021; 27(41): 7080-7099
- URL: https://www.wjgnet.com/1007-9327/full/v27/i41/7080.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i41.7080
The consumption of alcohol in chronic and/or aberrant drinking patterns correlates with a substantial burden of disease worldwide. A recent study conducted by the National Survey on Drug Use and Health stated that in the United States alone, 73.1% of adults regularly use alcohol and nearly 15 million people have an alcohol use disorder[1]. Based on World Health Organization reports, alcohol use has a negative impact on health and quality of life, creating more than 5% of global disease burden and premature deaths[2,3]. The processing of alcohol in the body significantly affects multiple organs including the liver, gut, lungs, heart and brain[4-7]. A prominent alcohol-related disorder is alcohol-associated liver disease (AALD) that is initially facilitated by ethanol metabolism in the liver[8]. However, AALD is a complex disease with factors from other organs also contributing to its development and progression. Notable contributing factors include cells of the innate immune system and bacteria of the alcohol-altered gut microbiota[9,10]. Overall, the interplay between alcohol-affected organs clearly plays a role in the outcomes of AALD as well as additional adverse consequences such as alcohol-related cancer development and metastatic disease.
Alcohol is an identified carcinogenic factor in several cancers including head and neck, esophageal, liver, breast, pancreatic, and colorectal[11,12]. Recent reports indicate that alcohol consumption is the third and fourth largest contributor of all primary cancers in women and men, respectively[3]. Further, studies have shown that alcohol associates with an increased risk of secondary cancers of the upper aerodigestive tract (i.e. oral cavity, pharynx and esophagus) as well as metastases of colorectal cancers[13,14]. Multiple mechanisms are attributed to alcohol-induced cancer risk including toxic products and reactive oxygen species generated by ethanol metabolism. Additionally, cellular factors produced in response to injury such as protein, lipids and microRNAs can be packaged and released in extracellular vesicles (EVs)[15]. The EVs can migrate to modulate neighboring cells and/or distant tissues, acting in many cases as tumorigenic signaling molecules. Multiple cell types including endothelial cells, epithelial cells, neuronal cells, immune cells, and cancer cells can secrete nanosized EVs as part of their normal physiology, as well as during the pathophysiology of disease[16]. Recent studies have suggested that during patho
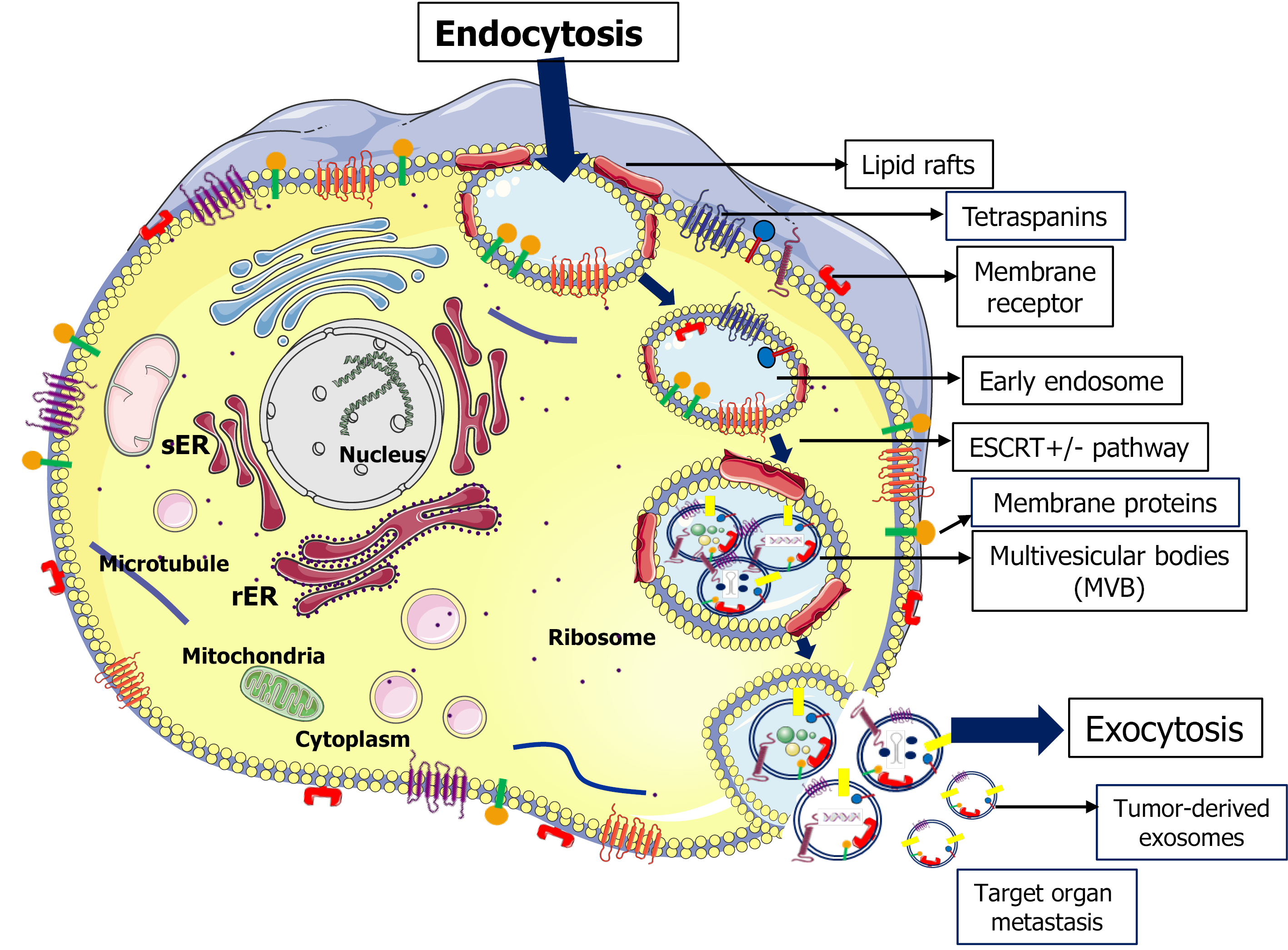
Exosomes were first identified in 1981 as cell-derived, membrane-bound enzymatic vesicles[19]. Subsequently, it was demonstrated that exosomes are nano-sized (30 to 150 nm) lumen vesicles that originate from the endosomal system[20]. Further, it was elucidated that EV biogenesis is a sequential process in which multivesicular bodies (MVBs) form following membrane invagination of intraluminal vesicles[18,20]. A small fraction of MVBs fuse with the plasma membrane and are released into the extracellular milieu[21]. The regulation of MVB fusion and release can involve cho
Endosome pathways identified in the regulation of exosome biogenesis include endosomal sorting complex required for transport (ESCRT)-dependent and inde
A significant feature of exosomes is the morphological and size profile of the vesicles. Based on size, EVs are classified into large exosome vesicles (90-120 nm), small exosome vesicles (60-80 nm), or non-membranous nanoparticles called exomeres (35 nm)[34]. While both large and small size exosomes can respond to signaling pathways such as IL-2/STAT5, density gradient centrifugation studies revealed differences in lipid compositions between various sized EVs[34]. Moreover, subpopulations of low-density and high-density exosomes can have differential effects on gene expression profiles.
In addition to EV size, the characterization of exosome cargo is important to the understanding of EV effects in healthy and pathophysiological scenarios. Exosomes contain distinct ratios of molecular constituents such as nucleic acids, proteins, lipids, and metabolites that vary depending upon their cellular conditions, cells of origin, epigenetic changes, and metabolomic stages[35]. Moreover, studies have described various RNA species that are components of exosome cargo including microRNAs (miRNAs), rRNAs, tRNAs, or long noncoding RNAs (lncRNAs)[36]. The role of miRNAs as EV cargo is an emerging area of study, especially in oncology. In cancer cells, exosomes are highly enriched in miRNAs compared to parent cells indicating that miRNAs are sorting into the exosome cargo[37-39]. Several studies have identified exosomal miRNAs as serum biomarkers for the prediction of cancer progression and metastasis[40-42]. Significantly, the differential expression of exosomal miRNA was noted to have a role in the regulation of tumor progression and metastasis in various cancer models[43-45]. However, the mechanisms involved in the loading and sorting of molecules into exosome vesicles remain to be elucidated. Towards those efforts, Villarroya-Beltri et al[46], have identified a sequence motif that controls the miRNA loading into exosomes. In addition, Kirsten rat sarcoma (KRAS) oncogene-dependent miRNA sorting into exosomes was found to play a key role in colorectal cancer cell (CRC) since CRC cells expressing mutant KRAS have distinct miRNA profiles compared to wild-type cells[47]. In another study, it was shown that the hyper
Another important aspect of exosome cargo and sorting mechanisms is the lipid content of exosome membranes such as cholesterol, sphingomyelin, and glycosphingolipids that have specific roles in protein sorting into exosomes[33,56]. Data indicates that subdomains of the plasma membrane (lipid rafts) enriched with distinct proteins on exosome membranes mediate exosome signaling as well as molecule sorting into exosomes[57,58]. Further, mechanistic studies demonstrated the release of factors such as flotillin-1 and stomatin into the external medium via EVs associated with lipid microdomains[59]. Another study showed a positive regulation of sphingosine 1-phosphate (S1P) by sphingosine kinases that enabled S1P receptors to be continuously active on EVs[31]. The continuous activation of S1P has been shown to regulate CD63, CD81, and flotillin-mediated sorting into exosomes through inhibitory G protein-coupled S1P receptors located on MVBs[31]. This suggests that G protein receptor-mediated S1P signaling on MVEs is mainly involved in the ESCRT-independent exosome cargo. Collectively, these studies suggest that distinct molecular constituents such as proteins, lipids, and nucleic acids play an essential role in exosome maturation culminating in effective sorting and extracellular release of EV cargo. The molecular, cellular, and biological functions that result from the released EVs is a critical area of research, especially in the evolving era to understand the mechanisms of alcohol-associated diseases including cancer.
Clinical manifestations of AALD include steatosis, steatohepatitis, fibrosis, and cirrhosis[8,60]. The liver is sensitized to triggers such as oxidative stress and en
Alcohol-induced impairments to the intestinal epithelial barrier result in increased gut permeability and release of bacterial products into the circulation[9,64]. The released products can perpetuate gut-barrier dysfunction, as well as contribute to hepatic injury, as the liver is the primary organ to receive and detoxify gut-derived factors. The translocation of intestinal products to the liver is involved in several diseases including obesity, metabolic syndrome, and non-alcoholic and alcoholic liver diseases. In the setting of alcohol, the gut-liver axis sustains bilateral communications between the intestine and the liver leading to gut-dysbiosis and progression of liver injury[65,66]. Notably, the transfer of gut-derived toxins to the liver due to alcohol consumption is considered a pivotal event in the development and severity of AALD. Clinical data indicates that drinking patterns correlate with processes of the gut-liver axis as changes in intestinal permeability increase with the degree of alcohol consumption[64]. Next-generation sequencing data further confirmed the association between chronic alcohol consumption and altered gut microbiome functions in mice and humans[67,68]. Overall, alcohol consumption is linked to multiple changes in the gut including intestinal epithelial barrier dysfunction, alterations in gut epithelial and mucosal cells, and changes to the intestinal microbiota. As a result, bacterial products (i.e. endotoxin and other pathogen-associated molecular patterns) translocate to the liver and contribute to the production of proinflammatory pathways. Despite the current understanding of alcohol’s effects on the gut microbiome, the role of EVs in the transfer of gut-derived products is not defined. However, emerging data indicates the EVs significantly contribute to alcohol-related liver inflammation.
The effects of alcohol on the intestinal microbiome and the translocation of injurious factors to the liver is an area of extensive research. It is well characterized that alcohol consumption results in the dysbiosis of bacterial and fungal intestinal species and the release of products including lipopolysaccharide (LPS) from the leaky gut[69,70]. In search of contributing mechanisms, studies have described alcohol-induced reductions in the expression of tight junction proteins as well as direct injury to gut epithelial cells[71,72]. The overexpression miRNA has been implicated in tight junction alterations as the knockdown of miRNA-21 prevented ethanol-induced disruption of tight junctions through the restoration of associated transmembrane proteins such as occludin and zonula occludens-1 (ZO-1)[71,73]. Additionally, the blockade of miRNA-122a was found to be protective against tight junction alterations in Caco-2 cells[74]. It is suggested that EVs generated during alcohol-induced changes to the intestinal barrier contain cargo such as miRNAs, LPS, and bacterial products that target the liver and contribute to AALD. Indeed, a recent study by Lamas-Paz et al[75] demonstrated that EVs derived from alcohol-affected intestinal epithelial cells contributed to hepatocellular injury. Further, it is likely that ethanol-mediated changes in intestinal barrier and microbiome composition result in the release of bacterial EVs. For example, in addition to its role as a soluble factor, LPS can also be packaged into EVs for transport from the injured gut. This is supported by a recent report indicating the presence and activity of bacterial EVs in patients with intestinal barrier dysfunction[76]. The role of bacterial EVs in alcohol consuming patients remains to be characterized along with the therapeutic potential of targeting such EVs.
The mechanistic role of bacterial products in the progression of alcohol-associated diseases has led to the study of the gut microbiota as a therapeutic target in patients with alcohol use disorders[77]. Currently, probiotics (living bacterial cultures), pre
It is well described that manifestations of AALD lead to a spectrum of symptoms in the brain such as cerebral edema and hepatic encephalopathy. Of notable involvement, ammonia and other harmful substances produced by the alcohol-injured liver can reach the brain causing injury and neuroinflammation. However, mechanisms related to exosome communication networks of the liver-brain axis remain to be characterized. Reports to date indicate that the coadministration of alcohol and LPS result in altered profiles of cytokines such as TNF-α, MCP-1, IL-1β in the gut, liver, and brain[84]. Other studies demonstrate that the lack of the tumor necrosis factor receptor 1 results in the accumulation of TNF-α in mouse serum, gut, and liver; and that alcohol intake potentiates long-lasting levels of proinflammatory cytokines in the brain[85]. A recent study demonstrated that TNF-α inhibition reduced systemic inflammation and improved symptoms[86]. Additionally, chronic alcohol consumption not only in
Besides chronic alcohol addiction, the loss of gut barrier integrity is a causative factor of endotoxin transport during sepsis and brain inflammation. Alcohol-induced gut dysbiosis is thought to not only play a role in alcohol dependency but also in the regulation of effects including neuro and endocrine signaling and immune system alterations[64,68]. However, a connective factor such as EVs in the gut-liver-brain axis has yet to be identified. Interestingly, the blood-brain barrier (BBB) serves as a defensive barrier against the extravasation of tumor cells and pathogens[88]. However, cancer cells can destruct the BBB structure to mediate migration during brain me
Excessive alcohol use is a major factor in the enhanced risk of acute respiratory distress syndrome (ARDS)[90]. Chronic alcohol exposure in the liver-lung axis is linked to hepatopulmonary syndrome, bacterial infection, and increased mortality from ARDS[90,91]. Recently, Siore et al[92] reported that pulmonary edema and acute lung damage occur through the activation of inflammatory responses and oxidative stress involving liver-lung axis communications. It was shown that alcohol administration results in elevated levels of the TNF-α responsive chemokines, macrophage inflammatory protein, and keratinocyte chemoattractant. Further, the enhanced chemokine expression is associated with the recruitment of pulmonary neutrophils. Additional studies indicated that the liver-lung axis is bidirectional for the com
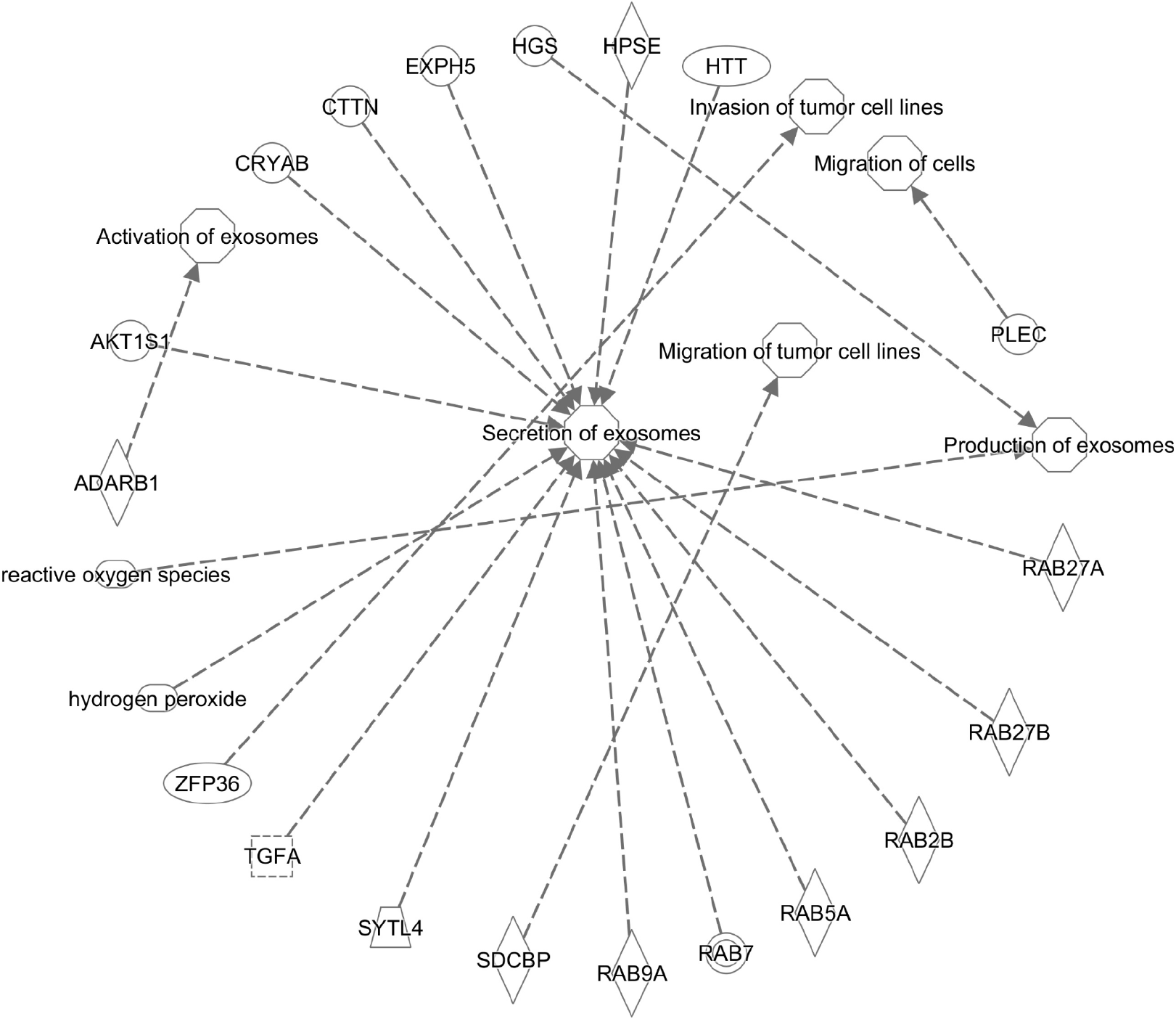
The interorgan communication mediated by EVs is clearly a factor of pathophysiology in various disease states. The role of alcohol-induced EV communication in the development and progression of cancer is not defined and is an area of clinical importance due to the prevalence of alcohol consumption and associated risk of cancers. Thus, investigations into the role of EVs in the initiation and severity of cancers aims to gain insight into the relationship of comorbid conditions related to the effects of alcohol consumption. Moreover, realization of the importance of EVs in cancer progression and metastasis has increased exponentially, as have their potential application in therapy and diagnosis[99]. The contribution of EVs in pathological processes is far reaching since tumor-secreted exosomes can mediate angiogenesis, modulate the immune system, and facilitate the generation of pre-metastatic niches[96,100]. Indeed, EVs have been identified as key mediators of communication networks within and between organ systems, highlighting the clinical importance of exosome function[18,101,102]. Existing web-based online bioinformatic tools including high-throughput techniques (i.e. ExoCarta, EVpedia, Vesiclepedia catalog, and Ingenuity Pathway Analysis, IPA) are beneficial to the scientific community in EV research[103,104]. These resources assist in the characterization of EV molecular and pathophy
The clinical assessment of EVs in body fluids provides another measure towards the understanding of exosomes as diagnostic biomarkers and therapeutic targets. Biomolecule-loaded EVs from blood are stable for more than 90 days under normal storage conditions making EV analyses more useful compared to other less-stable measures of cell-free DNAs and circulating tumor cells that are used as liquid biopsies[105,106]. Examples of exosome-related identification in serum samples include prostate cancer-derived exosomes[107]; and exosome cargo containing an androgen receptor variant that is a biomarker of metastatic prostate cancer[108]. Several studies have also reported the sensitivity of EV miRNA composition as biomarkers in disease identification that can be isolated from various body fluids including blood, saliva, and urine[109,110]. A noted example is the oncogenic signature of miR-21 as a biomarker for various cancers including colorectal[111], breast[112], brain[113], and liver[114]. Concerning diseases of the liver, it has been shown that the concentration of EVs in the circulation is enhanced in the setting of AALD, nonalcoholic fatty liver disease, viral hepatitis, and hepatocellular carcinoma indicating the clinical signi
Mechanisms of tumor development and progression are dynamic, multi-step processes that occur in response to the accumulation of genetic alterations in damaged cells. An integral component of tumor development is thought to be the communication between cancerous and non-cancerous cells that is mediated by nanosized vesicles[115]. Research to date indicates that cancer cell microvesicles actively transfer oncogenic molecules from primary cancer cells to intercellular populations. Indeed, tumor-derived exosomes can regulate cancer progression by stimulating oncogene overexpression, stromal cell remodeling, immune system modulation, and angio
The knowledge that exosomes are potential stimulators in cancer progression indicates that EVs can promote angiogenesis and changes in the microenvironment[117]. In this regard, tumor-derived exosomes can influence mesenchymal stem cell differentiation facilitating cancer cell proliferation and disease progression[118]. Moreover, the exosome-mediated transfer of lncRNAs as tumor-promoting material has been shown during the transformation of non-malignant cells[119]. The role of EVs enriched with miRNAs has also been shown in cell-cell communications and con
| Exosomal miRNA | Expression profile | Mode of action | Type of cancer | Ref. |
| miR-320d | Upregulated | Predicts metastasis | CRC | Tang et al[41] |
| miR-106b-3p | Upregulated | Promotes metastasis | CRC | Liu et al[163] |
| miR-6803-5p | Upregulated | Prognosis marker | CRC | Yan et al[42] |
| miR-874 | Upregulated | Prognosis marker | GC | Zhang et al[164] |
| miR-30a-5p | Downregulated | Diagnostic tool | CRC | Sun et al[40] |
| miR-21 | Upregulated | Diagnostic tool | CRC | Bastaminejad et al[111] |
| miR-135b-5p | Upregulated | Metastatic marker | CRC | Li et al[122] |
| miR-150-5p | Downregulated | Prognosis, marker | CRC | Zou et al[123] |
| miR-183-5p | Upregulated | Angiogenesis | CRC | Shang et al[124] |
| miR-155 | Upregulated | Diagnostic tool | CRC | Lv et al[125] |
| miR-16-5p | Upregulated | Regulation of ITGA2 | CRC | Xu et al[165] |
| miR-497 | Downregulated | Prognosis marker | CRC | Zou et al[126] |
| miR-4461 | Downregulated | Regulation of COPB2 | CRC | Chen et al[43] |
| miR-146a | Upregulated | Invasion and metastasis | BC | Yang et al[45] |
| miR-125a-3p, miR-320c | Upregulated | Stage I marker | CC | Wang et al[166] |
| miR-4772-3p | Upregulated | Stage II & III marker | CC | Liu et al[167] |
| miR-21, miR-10b | Upregulated | Metastatic marker | HCC | Tian et al[44] |
| miR-1290, miR-375 | Upregulated | Prognostic marker | CRPC | Huang et al[129] |
| miR-373, miR-200a, miR-200b, miR-200c | Upregulated | Tumor progression | EOC | Meng et al[131] |
| mir-181b-5p | Downregulated | Diagnostic tool | GC | Yun et al[127] |
Another component identified in cancer progression is the release of cancer-associated fibroblasts (CAFs) from exosomes. CAF-derived EVs can play a key role in tumor progression by enabling the transfer of oncogenic molecules such as amino acids, lipids, and TCA-cycle intermediates to confer glycolysis modulation and carboxylation in cancer cells[132]. Tumor-derived exosomes have also been shown to be involved in the stimulation of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 (ICAM-1) enhancing the process of neovascularization in en
Metastasis is one of the most common causative factors in cancer-related death. Cancer metastasis is a multi-step process for the development of secondary cancers. In 1889, Stephen Paget described the “seed and soil” theory, in which metastasis depends on the interaction between primary cancer cells as the seed and secondary host microenvironments designated as the soil[138]. Involved mechanisms were found to include changes to the extracellular matrix architecture and associated reprograming of normal cells. Clinically significant interactions between cancer cells and the cells of secondary organ sites have been shown to involve hepatocytes, bone marrow pro
| EV cargo | Type of molecule | Action on recipient cells/tissue | Type of cancer | Ref. |
| CEA | Protein | Inflammation | Colorectal | Yokoyama et al[162] |
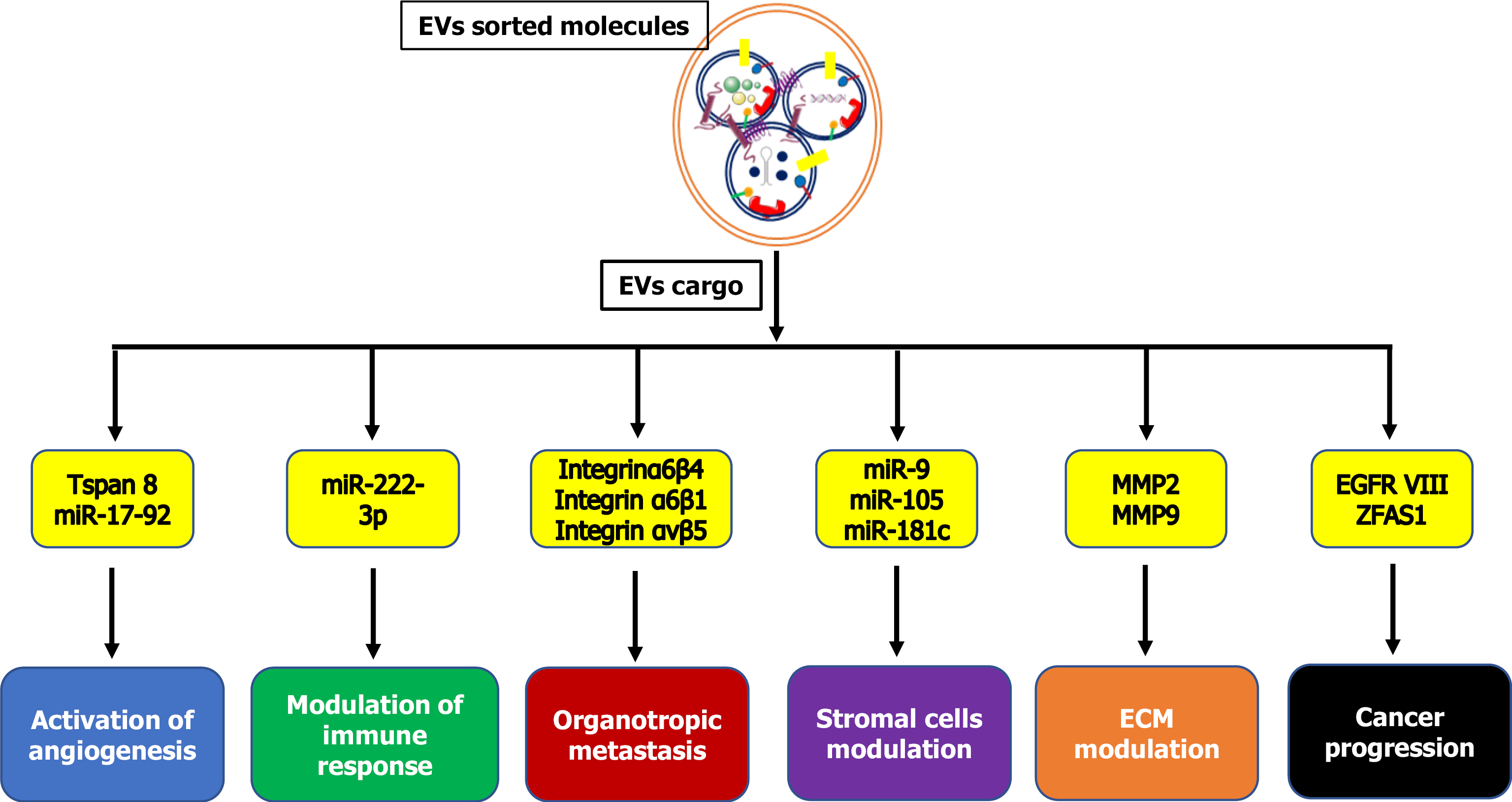
| KRAS | Protein | Invasiveness in recipient cells | Colorectal | Beckler et al[152] |
| ITG | Protein | Metastatic organotropism | Breast | Hoshino et al[96] |
| TNC | ECM protein | Stem cell niche formation | Breast | Oskarsson et al[97] |
| MIF | Protein | Liver premetastatic niche formation | Pancreatic | Costa-Silva et al[168] |
| ZFAS1 | lncRNA | Cancer growth/metastasis | Gastric | Pan et al[119] |
| Amino acids, lipids, TCA-cycle intermediates | Metabolites | Cancer growth | Prostate | Zhao et al[132] |
Recent studies indicate an alarming increased rate of morbidity and mortality from alcohol use disorders in the United States[141]. Of particular clinical significance is the disease burden related to alcohol use in colorectal cancer and associated liver me
The development of CRC is a multi-step process involving the malignant trans
EVs represent a new form of communication in colorectal cancer progression and liver metastasis. The fact that EVs can deliver cargo (i.e. RNAs, lipids, proteins) between cells and organs indicates the potential of playing a key role in metastatic disease[146,147]. CRC proliferation and migration can induce the release of EVs and other tumor-derived factors that can promote prometastatic niche formation, vascular changes, inflammation, and immunosuppression in host microenvironments. Several studies have recently described the contributions of EV cargo as prime mechanisms of CRC metastasis. For example, proteomic data revealed a distinct profile of metastatic factors, signal transduction molecules, and lipid raft-associated components in EVs obtained from metastatic CRC cells[148]. The contribution of mRNA components from CRC-derived EVs in cancer progression has also been shown for miRNAs (i.e. miR-21, miR-192 and miR-221) as well as natural antisense RNAs such as Leucine Rich Repeat Containing 24, MDM2 Proto-Oncogene, and Cyclin Dependent Kinase Inhibitor 1A[149]. Moreover, the role of genetic mutations in CRC patients are of interest. In particular, KRAS mutations are frequently associated with CRC metastasis and the regulation of exosome composition and release in CRC cells[150,151]. In addition, many oncogenic proteins (e.g. KRAS, Src family kinases, integrins) are highly enriched in mutant KRAS-derived exosomes indicating a role in CRC progression and metastasis[152]. Together, these observations provide novel insight into the role of EVs and the therapeutic potential of targeting the CRC-generated EVs during metastatic disease.
There is a growing body evidence suggesting that tumor-derived exosomes are crucial factors that influence differentiation in the microenvironment through particular signaling pathways[153]. For example, CRC cell-derived EVs have been shown to promote angiogenesis and tumor growth in the host microenvironment through the hyper-activation of Wnt/β-catenin signaling. As a result, hypoxic me
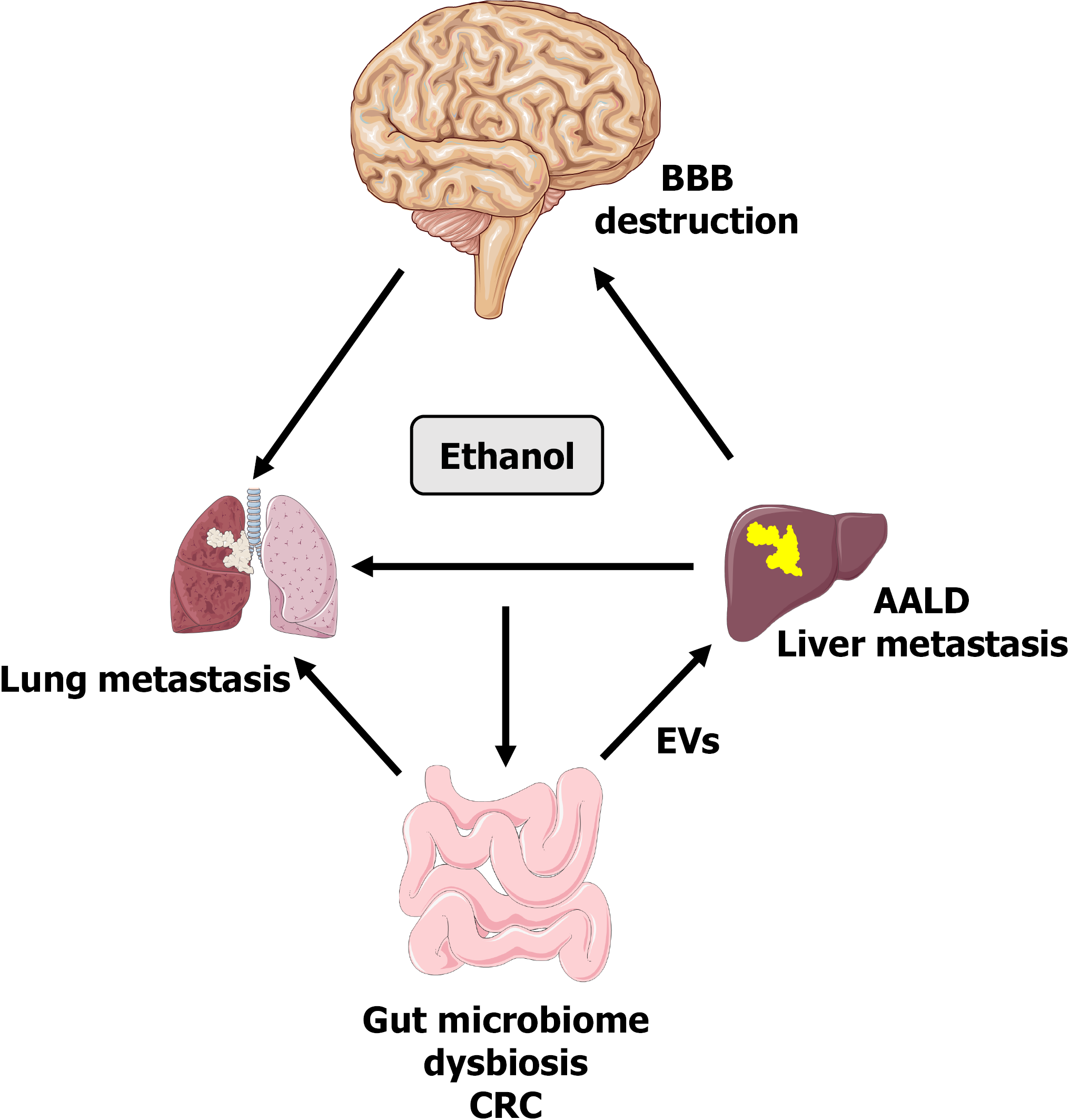
In recent years, investigations into the role of EVs in cancer progression and AALD have increased in a remarkable manner. The elucidation of EV communication networks to date have indicated the powerful role of EVs as metastatic cancer markers and inducers of varied biological effects. Extensive work is ongoing to characterize the biogenesis and effects of distinct EV populations generated from different cell types and diseases. The unique features of EV size and cargo contents can produce hallmark effects on recipient cells. Therefore, the heterogeneity of exosome populations will dictate studies on the role and outcomes of exosome networks during disease states. For example, understanding the diversity of EVs released during gut microbiome dysbiosis, migration, and organ-organ communication aims to reveal the association of AALD and hepatic CRC metastasis. The complexity of interorgan communication and the involvement of mediators such as EVs, cytokines, and chemokines is the ongoing focus of translational research. Related to alcohol-associated diseases, it is proposed that EV-mediated communication affects multi-organ damage as well as cancer metastasis along the liver/gut/lung/brain axis (Figure 4). Future studies will likely focus on the characterization of exosomal components involved in alcohol’s effects and cancer cell metastasis to secondary organs. Moreover, further investigation is needed to explore the role of exosome-mediated cell-free networks in the detection of alcohol-related tumors and microenvironment interactions for the development of targeted therapeutics.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases.
Specialty type: Biochemistry and Molecular Biology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang Z S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, Giovino GA, West R, Hall W, Griffiths P, Ali R, Gowing L, Marsden J, Ferrari AJ, Grebely J, Farrell M, Degenhardt L. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction. 2018;113:1905-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 660] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 2. | Wallace AE, Weeks WB. Substance abuse intensive outpatient treatment: does program graduation matter? J Subst Abuse Treat. 2004;27:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Global status report on alcohol and health 2018. [cited 15 May 2021]. Available from: https://apps.who.int/iris/handle/10665/274603. |
| 4. | Adachi J, Asano M, Ueno Y, Niemelä O, Ohlendieck K, Peters TJ, Preedy VR. Alcoholic muscle disease and biomembrane perturbations (review). J Nutr Biochem. 2003;14:616-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Barritt AS 4th, Jiang Y, Schmidt M, Hayashi PH, Bataller R. Charges for Alcoholic Cirrhosis Exceed All Other Etiologies of Cirrhosis Combined: A National and State Inpatient Survey Analysis. Dig Dis Sci. 2019;64:1460-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 420] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 7. | Guidot DM, Roman J. Chronic ethanol ingestion increases susceptibility to acute lung injury: role of oxidative stress and tissue remodeling. Chest. 2002;122:309S-314S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, Tsukamoto H. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 808] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 9. | Sarin SK, Pande A, Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. J Hepatol. 2019;70:260-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 10. | Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12:387-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 464] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 11. | Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pelucchi C, Galeone C, Bellocco R, Negri E, Corrao G, Boffetta P, La Vecchia C. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112:580-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 875] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 12. | IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum. 2010;96:3-1383. [PubMed] |
| 13. | Rossi M, Jahanzaib Anwar M, Usman A, Keshavarzian A, Bishehsari F. Colorectal Cancer and Alcohol Consumption-Populations to Molecules. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 14. | Seitz HK, Stickel F, Homann N. Pathogenetic mechanisms of upper aerodigestive tract cancer in alcoholics. Int J Cancer. 2004;108:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Urabe F, Kosaka N, Ito K, Kimura T, Egawa S, Ochiya T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am J Physiol Cell Physiol. 2020;318:C29-C39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 16. | Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1383] [Article Influence: 125.7] [Reference Citation Analysis (0)] |
| 17. | Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30:836-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1487] [Article Influence: 165.2] [Reference Citation Analysis (0)] |
| 18. | Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4900] [Cited by in RCA: 6158] [Article Influence: 513.2] [Reference Citation Analysis (0)] |
| 19. | Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 797] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 20. | Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481-3500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1521] [Cited by in RCA: 1801] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 21. | Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-9420. [PubMed] |
| 22. | Möbius W, Ohno-Iwashita Y, van Donselaar EG, Oorschot VM, Shimada Y, Fujimoto T, Heijnen HF, Geuze HJ, Slot JW. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J Histochem Cytochem. 2002;50:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 230] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Chen Q, Takada R, Noda C, Kobayashi S, Takada S. Different populations of Wnt-containing vesicles are individually released from polarized epithelial cells. Sci Rep. 2016;6:35562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 554] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 25. | Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013;12:587-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 352] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 26. | Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 794] [Cited by in RCA: 874] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 27. | Baixauli F, López-Otín C, Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol. 2014;5:403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 28. | Hessvik NP, Øverbye A, Brech A, Torgersen ML, Jakobsen IS, Sandvig K, Llorente A. PIKfyve inhibition increases exosome release and induces secretory autophagy. Cell Mol Life Sci. 2016;73:4717-4737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 29. | Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol. 2013;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 345] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 30. | Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2186] [Cited by in RCA: 2685] [Article Influence: 157.9] [Reference Citation Analysis (0)] |
| 31. | Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013;4:2712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 246] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 32. | Christ L, Raiborg C, Wenzel EM, Campsteijn C, Stenmark H. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem Sci. 2017;42:42-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 337] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 33. | Skotland T, Sandvig K, Llorente A. Lipids in exosomes: Current knowledge and the way forward. Prog Lipid Res. 2017;66:30-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 753] [Article Influence: 94.1] [Reference Citation Analysis (1)] |
| 34. | Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KE, Sadik M, Alaarg A, Smith CI, Lehtiö J, El Andaloussi S, Wood MJ, Vader P. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016;6:22519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 724] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 35. | Tai YL, Chen KC, Hsieh JT, Shen TL. Exosomes in cancer development and clinical applications. Cancer Sci. 2018;109:2364-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 309] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 36. | Pefanis E, Wang J, Rothschild G, Lim J, Kazadi D, Sun J, Federation A, Chao J, Elliott O, Liu ZP, Economides AN, Bradner JE, Rabadan R, Basu U. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell. 2015;161:774-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 37. | Hessvik NP, Phuyal S, Brech A, Sandvig K, Llorente A. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim Biophys Acta. 2012;1819:1154-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 38. | Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, Bernad A, Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1233] [Cited by in RCA: 1433] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 39. | Nolte-'t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH, 't Hoen PA. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40:9272-9285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 584] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 40. | Sun Y, Yang B, Lin M, Yu H, Chen H, Zhang Z. Identification of serum miR-30a-5p as a diagnostic and prognostic biomarker in colorectal cancer. Cancer Biomark. 2019;24:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Tang Y, Zhao Y, Song X, Niu L, Xie L. Tumor-derived exosomal miRNA-320d as a biomarker for metastatic colorectal cancer. J Clin Lab Anal. 2019;33:e23004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 42. | Yan S, Jiang Y, Liang C, Cheng M, Jin C, Duan Q, Xu D, Yang L, Zhang X, Ren B, Jin P. Exosomal miR-6803-5p as potential diagnostic and prognostic marker in colorectal cancer. J Cell Biochem. 2018;119:4113-4119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 43. | Chen HL, Li JJ, Jiang F, Shi WJ, Chang GY. MicroRNA-4461 derived from bone marrow mesenchymal stem cell exosomes inhibits tumorigenesis by downregulating COPB2 expression in colorectal cancer. Biosci Biotechnol Biochem. 2020;84:338-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Tian XP, Wang CY, Jin XH, Li M, Wang FW, Huang WJ, Yun JP, Xu RH, Cai QQ, Xie D. Acidic Microenvironment Up-Regulates Exosomal miR-21 and miR-10b in Early-Stage Hepatocellular Carcinoma to Promote Cancer Cell Proliferation and Metastasis. Theranostics. 2019;9:1965-1979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 45. | Yang SS, Ma S, Dou H, Liu F, Zhang SY, Jiang C, Xiao M, Huang YX. Breast cancer-derived exosomes regulate cell invasion and metastasis in breast cancer via miR-146a to activate cancer associated fibroblasts in tumor microenvironment. Exp Cell Res. 2020;391:111983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 46. | Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1165] [Cited by in RCA: 1514] [Article Influence: 137.6] [Reference Citation Analysis (0)] |
| 47. | Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, Demory Beckler M, Weaver AM, Vickers K, Prasad N, Levy S, Zhang B, Coffey RJ, Patton JG. KRAS-dependent sorting of miRNA to exosomes. Elife. 2015;4:e07197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 289] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 48. | McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, Patton JG, Weaver AM. KRAS-MEK Signaling Controls Ago2 Sorting into Exosomes. Cell Rep. 2016;15:978-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 339] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 49. | Batagov AO, Kurochkin IV. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3'-untranslated regions. Biol Direct. 2013;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 50. | Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Ströbel T, Erkan EP, Fan JB, Breakefield XO, Saydam O. miR-1289 and "Zipcode"-like Sequence Enrich mRNAs in Microvesicles. Mol Ther Nucleic Acids. 2012;1:e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 51. | Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289:3869-3875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 786] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 52. | Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, Williams C, Rodriguez-Barrueco R, Silva JM, Zhang W, Hearn S, Elemento O, Paknejad N, Manova-Todorova K, Welte K, Bromberg J, Peinado H, Lyden D. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 997] [Cited by in RCA: 1257] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 53. | Buschow SI, Liefhebber JM, Wubbolts R, Stoorvogel W. Exosomes contain ubiquitinated proteins. Blood Cells Mol Dis. 2005;35:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Smith VL, Jackson L, Schorey JS. Ubiquitination as a Mechanism To Transport Soluble Mycobacterial and Eukaryotic Proteins to Exosomes. J Immunol. 2015;195:2722-2730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 55. | Cheng Y, Schorey JS. Targeting soluble proteins to exosomes using a ubiquitin tag. Biotechnol Bioeng. 2016;113:1315-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 56. | Record M, Poirot M, Silvente-Poirot S. Emerging concepts on the role of exosomes in lipid metabolic diseases. Biochimie. 2014;96:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 883] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 58. | Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3136] [Cited by in RCA: 3469] [Article Influence: 231.3] [Reference Citation Analysis (0)] |
| 59. | de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102:4336-4344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 501] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 60. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1495] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 61. | Eguchi A, Feldstein AE. Extracellular vesicles in non-alcoholic and alcoholic fatty liver diseases. Liver Res. 2018;2:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Rahman MA, Patters BJ, Kodidela S, Kumar S. Extracellular Vesicles: Intercellular Mediators in Alcohol-Induced Pathologies. J Neuroimmune Pharmacol. 2020;15:409-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14:455-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 219] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 64. | Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, Windey K, Tremaroli V, Bäckhed F, Verbeke K, de Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485-E4493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 701] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 65. | Dasarathy S, Brown JM. Alcoholic Liver Disease on the Rise: Interorgan Cross Talk Driving Liver Injury. Alcohol Clin Exp Res. 2017;41:880-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Stärkel P, Schnabl B. Bidirectional Communication between Liver and Gut during Alcoholic Liver Disease. Semin Liver Dis. 2016;36:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 67. | Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J, Kong M, Barker D, McClain C, Barve S. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8:e53028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 427] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 68. | Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966-G978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 595] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 69. | Gao B, Lang S, Duan Y, Wang Y, Shawcross DL, Louvet A, Mathurin P, Ho SB, Stärkel P, Schnabl B. Serum and Fecal Oxylipins in Patients with Alcohol-Related Liver Disease. Dig Dis Sci. 2019;64:1878-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 70. | Zhou R, Fan X, Schnabl B. Role of the intestinal microbiome in liver fibrosis development and new treatment strategies. Transl Res. 2019;209:22-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 71. | Zhao H, Zhao C, Dong Y, Zhang M, Wang Y, Li F, Li X, McClain C, Yang S, Feng W. Inhibition of miR122a by Lactobacillus rhamnosus GG culture supernatant increases intestinal occludin expression and protects mice from alcoholic liver disease. Toxicol Lett. 2015;234:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 72. | Tang Y, Zhang L, Forsyth CB, Shaikh M, Song S, Keshavarzian A. The Role of miR-212 and iNOS in Alcohol-Induced Intestinal Barrier Dysfunction and Steatohepatitis. Alcohol Clin Exp Res. 2015;39:1632-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 73. | Pettinelli P, Videla LA. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab. 2011;96:1424-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 278] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 74. | Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 75. | Lamas-Paz A, Morán L, Peng J, Salinas B, López-Alcántara N, Sydor S, Vilchez-Vargas R, Asensio I, Hao F, Zheng K, Martín-Adrados B, Moreno L, Cogolludo A, Gómez Del Moral M, Bechmann L, Martínez-Naves E, Vaquero J, Bañares R, Nevzorova YA, Cubero FJ. Intestinal Epithelial Cell-Derived Extracellular Vesicles Modulate Hepatic Injury via the Gut-Liver Axis During Acute Alcohol Injury. Front Pharmacol. 2020;11:603771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 76. | Tulkens J, Vergauwen G, Van Deun J, Geeurickx E, Dhondt B, Lippens L, De Scheerder MA, Miinalainen I, Rappu P, De Geest BG, Vandecasteele K, Laukens D, Vandekerckhove L, Denys H, Vandesompele J, De Wever O, Hendrix A. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut. 2020;69:191-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 77. | Temko JE, Bouhlal S, Farokhnia M, Lee MR, Cryan JF, Leggio L. The Microbiota, the Gut and the Brain in Eating and Alcohol Use Disorders: A 'Ménage à Trois'? Alcohol Alcohol. 2017;52:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 78. | Sung H, Kim SW, Hong M, Suk KT. Microbiota-based treatments in alcoholic liver disease. World J Gastroenterol. 2016;22:6673-6682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 79. | Tian F, Chi F, Wang G, Liu X, Zhang Q, Chen Y, Zhang H, Chen W. Lactobacillus rhamnosus CCFM1107 treatment ameliorates alcohol-induced liver injury in a mouse model of chronic alcohol feeding. J Microbiol. 2015;53:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 80. | Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ, Cave M. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 388] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 81. | Tang Y, Forsyth CB, Banan A, Fields JZ, Keshavarzian A. Oats supplementation prevents alcohol-induced gut leakiness in rats by preventing alcohol-induced oxidative tissue damage. J Pharmacol Exp Ther. 2009;329:952-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Zhang X, Wang H, Yin P, Fan H, Sun L, Liu Y. Flaxseed oil ameliorates alcoholic liver disease via anti-inflammation and modulating gut microbiota in mice. Lipids Health Dis. 2017;16:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 83. | Kalambokis GN, Mouzaki A, Rodi M, Tsianos EV. Rifaximin improves thrombocytopenia in patients with alcoholic cirrhosis in association with reduction of endotoxaemia. Liver Int. 2012;32:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 420] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 85. | Mayfield J, Ferguson L, Harris RA. Neuroimmune signaling: a key component of alcohol abuse. Curr Opin Neurobiol. 2013;23:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 86. | Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 162] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 87. | Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641-1653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 88. | Lee TH, Avraham HK, Jiang S, Avraham S. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem. 2003;278:5277-5284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 89. | Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8246] [Cited by in RCA: 9792] [Article Influence: 544.0] [Reference Citation Analysis (0)] |
| 90. | Afshar M, Smith GS, Terrin ML, Barrett M, Lissauer ME, Mansoor S, Jeudy J, Netzer G. Blood alcohol content, injury severity, and adult respiratory distress syndrome. J Trauma Acute Care Surg. 2014;76:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 91. | Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 92. | Siore AM, Parker RE, Stecenko AA, Cuppels C, McKean M, Christman BW, Cruz-Gervis R, Brigham KL. Endotoxin-induced acute lung injury requires interaction with the liver. Am J Physiol Lung Cell Mol Physiol. 2005;289:L769-L776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 93. | Patterson EK, Yao LJ, Ramic N, Lewis JF, Cepinskas G, McCaig L, Veldhuizen RA, Yamashita CM. Lung-derived mediators induce cytokine production in downstream organs via an NF-κB-dependent mechanism. Mediators Inflamm. 2013;2013:586895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2149] [Cited by in RCA: 2289] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 95. | Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, Ponomarev V, Gerald WL, Blasberg R, Massagué J. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 508] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 96. | Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2697] [Cited by in RCA: 3757] [Article Influence: 375.7] [Reference Citation Analysis (0)] |
| 97. | Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massagué J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 690] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 98. | Sousa B, Pereira J, Paredes J. The Crosstalk Between Cell Adhesion and Cancer Metabolism. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 99. | Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1613] [Cited by in RCA: 1746] [Article Influence: 249.4] [Reference Citation Analysis (0)] |
| 100. | Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2866] [Cited by in RCA: 2945] [Article Influence: 226.5] [Reference Citation Analysis (0)] |
| 101. | Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1946] [Article Influence: 129.7] [Reference Citation Analysis (0)] |
| 102. | Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841:108-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 617] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 103. | Keerthikumar S, Gangoda L, Gho YS, Mathivanan S. Bioinformatics Tools for Extracellular Vesicles Research. Methods Mol Biol. 2017;1545:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2902] [Cited by in RCA: 4208] [Article Influence: 350.7] [Reference Citation Analysis (0)] |
| 105. | Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, Hulett MD, Mathivanan S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013;13:3354-3364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 485] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 106. | Qin Z, Ljubimov VA, Zhou C, Tong Y, Liang J. Cell-free circulating tumor DNA in cancer. Chin J Cancer. 2016;35:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 107. | McKiernan J, Donovan MJ, O'Neill V, Bentink S, Noerholm M, Belzer S, Skog J, Kattan MW, Partin A, Andriole G, Brown G, Wei JT, Thompson IM Jr, Carroll P. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol. 2016;2:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 460] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 108. | Del Re M, Biasco E, Crucitta S, Derosa L, Rofi E, Orlandini C, Miccoli M, Galli L, Falcone A, Jenster GW, van Schaik RH, Danesi R. The Detection of Androgen Receptor Splice Variant 7 in Plasma-derived Exosomal RNA Strongly Predicts Resistance to Hormonal Therapy in Metastatic Prostate Cancer Patients. Eur Urol. 2017;71:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 109. | Eguchi A, Lazaro RG, Wang J, Kim J, Povero D, Willliams B, Ho SB, Stärkel P, Schnabl B, Ohno-Machado L, Tsukamoto H, Feldstein AE. Extracellular vesicles released by hepatocytes from gastric infusion model of alcoholic liver disease contain a MicroRNA barcode that can be detected in blood. Hepatology. 2017;65:475-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 110. | Lin J, Wang Y, Zou YQ, Chen X, Huang B, Liu J, Xu YM, Li J, Zhang J, Yang WM, Min QH, Sun F, Li SQ, Gao QF, Wang XZ. Differential miRNA expression in pleural effusions derived from extracellular vesicles of patients with lung cancer, pulmonary tuberculosis, or pneumonia. Tumour Biol. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 111. | Bastaminejad S, Taherikalani M, Ghanbari R, Akbari A, Shabab N, Saidijam M. Investigation of MicroRNA-21 Expression Levels in Serum and Stool as a Potential Non-Invasive Biomarker for Diagnosis of Colorectal Cancer. Iran Biomed J. 2017;21:106-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 112. | Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL, Zhao ZJ, Blick KE, Dooley WC, Ding WQ. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 462] [Cited by in RCA: 437] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 113. | Santangelo A, Imbrucè P, Gardenghi B, Belli L, Agushi R, Tamanini A, Munari S, Bossi AM, Scambi I, Benati D, Mariotti R, Di Gennaro G, Sbarbati A, Eccher A, Ricciardi GK, Ciceri EM, Sala F, Pinna G, Lippi G, Cabrini G, Dechecchi MC. A microRNA signature from serum exosomes of patients with glioma as complementary diagnostic biomarker. J Neurooncol. 2018;136:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 114. | Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int. 2014;2014:864894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 115. | Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front Cell Dev Biol. 2018;6:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 505] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 116. | Raimondo S, Saieva L, Corrado C, Fontana S, Flugy A, Rizzo A, De Leo G, Alessandro R. Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism. Cell Commun Signal. 2015;13:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 117. | Hood JL, Pan H, Lanza GM, Wickline SA; Consortium for Translational Research in Advanced Imaging and Nanomedicine (C-TRAIN). Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89:1317-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 118. | Zhou J, Tan X, Tan Y, Li Q, Ma J, Wang G. Mesenchymal Stem Cell Derived Exosomes in Cancer Progression, Metastasis and Drug Delivery: A Comprehensive Review. J Cancer. 2018;9:3129-3137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 119. | Pan L, Liang W, Fu M, Huang ZH, Li X, Zhang W, Zhang P, Qian H, Jiang PC, Xu WR, Zhang X. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143:991-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 264] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 120. | Baroni S, Romero-Cordoba S, Plantamura I, Dugo M, D'Ippolito E, Cataldo A, Cosentino G, Angeloni V, Rossini A, Daidone MG, Iorio MV. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016;7:e2312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 245] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 121. | Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32:2747-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 370] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 122. | Li L, Wang A, Cai M, Tong M, Chen F, Huang L. Identification of stool miR-135b-5p as a non-invasive diaognostic biomarker in later tumor stage of colorectal cancer. Life Sci. 2020;260:118417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 123. | Zou SL, Chen YL, Ge ZZ, Qu YY, Cao Y, Kang ZX. Downregulation of serum exosomal miR-150-5p is associated with poor prognosis in patients with colorectal cancer. Cancer Biomark. 2019;26:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 124. | Shang A, Wang X, Gu C, Liu W, Sun J, Zeng B, Chen C, Ji P, Wu J, Quan W, Yao Y, Wang W, Sun Z, Li D. Exosomal miR-183-5p promotes angiogenesis in colorectal cancer by regulation of FOXO1. Aging (Albany NY). 2020;12:8352-8371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 125. | Lv ZC, Fan YS, Chen HB, Zhao DW. Investigation of microRNA-155 as a serum diagnostic and prognostic biomarker for colorectal cancer. Tumour Biol. 2015;36:1619-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 126. | Zou G, Wang R, Wang M. Clinical response and prognostic significance of serum miR-497 expression in colorectal cancer. Cancer Biomark. 2019;25:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 127. | Yun J, Han SB, Kim HJ, Go SI, Lee WS, Bae WK, Cho SH, Song EK, Lee OJ, Kim HK, Yang Y, Kwon J, Chae HB, Lee KH, Han HS. Exosomal miR-181b-5p Downregulation in Ascites Serves as a Potential Diagnostic Biomarker for Gastric Cancer-associated Malignant Ascites. J Gastric Cancer. 2019;19:301-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 128. | Andreu Z, Otta Oshiro R, Redruello A, López-Martín S, Gutiérrez-Vázquez C, Morato E, Marina AI, Olivier Gómez C, Yáñez-Mó M. Extracellular vesicles as a source for non-invasive biomarkers in bladder cancer progression. Eur J Pharm Sci. 2017;98:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 129. | Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, Wang D, See W, Costello BA, Quevedo F, Tan W, Nandy D, Bevan GH, Longenbach S, Sun Z, Lu Y, Wang T, Thibodeau SN, Boardman L, Kohli M, Wang L. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 507] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 130. | Endzeliņš E, Berger A, Melne V, Bajo-Santos C, Soboļevska K, Ābols A, Rodriguez M, Šantare D, Rudņickiha A, Lietuvietis V, Llorente A, Linē A. Detection of circulating miRNAs: comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer. 2017;17:730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 201] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 131. | Meng X, Müller V, Milde-Langosch K, Trillsch F, Pantel K, Schwarzenbach H. Diagnostic and prognostic relevance of circulating exosomal miR-373, miR-200a, miR-200b and miR-200c in patients with epithelial ovarian cancer. Oncotarget. 2016;7:16923-16935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 132. | Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, Alvarez H, Gupta S, Maiti SN, Cooper L, Peehl D, Ram PT, Maitra A, Nagrath D. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5:e10250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 724] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 133. | Taverna S, Flugy A, Saieva L, Kohn EC, Santoro A, Meraviglia S, De Leo G, Alessandro R. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2012;130:2033-2043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 134. | Ying X, Wu Q, Wu X, Zhu Q, Wang X, Jiang L, Chen X. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. 2016;7:43076-43087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 291] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 135. | Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Théry C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920-4930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 504] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 136. | Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans. 2013;41:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 137. | Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290-9298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 393] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 138. | Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98-101. [PubMed] |
| 139. | Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18:533-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 1017] [Article Influence: 145.3] [Reference Citation Analysis (1)] |
| 140. | Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C, Wang W, Wang G, Wang H, Yuan W, Ji Z, Sun Z. Effects of exosomes on pre-metastatic niche formation in tumors. Mol Cancer. 2019;18:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 315] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 141. | Bataller R, Arteel GE, Moreno C, Shah V. Alcohol-related liver disease: Time for action. J Hepatol. 2019;70:221-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 142. | Paschos KA, Majeed AW, Bird NC. Natural history of hepatic metastases from colorectal cancer--pathobiological pathways with clinical significance. World J Gastroenterol. 2014;20:3719-3737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (2)] |
| 143. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3269] [Article Influence: 653.8] [Reference Citation Analysis (2)] |
| 144. | Cai S, Li Y, Ding Y, Chen K, Jin M. Alcohol drinking and the risk of colorectal cancer death: a meta-analysis. Eur J Cancer Prev. 2014;23:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 145. | Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Høyer-Hansen G, Eefsen RL, Reynolds AR, Brodt P. The multifaceted role of the microenvironment in liver metastasis: biology and clinical implications. Cancer Res. 2013;73:2031-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 146. | Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1226] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 147. | Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4100] [Cited by in RCA: 3943] [Article Influence: 231.9] [Reference Citation Analysis (0)] |
| 148. | Ji H, Greening DW, Barnes TW, Lim JW, Tauro BJ, Rai A, Xu R, Adda C, Mathivanan S, Zhao W, Xue Y, Xu T, Zhu HJ, Simpson RJ. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics. 2013;13:1672-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 149. | Chiba M, Kimura M, Asari S. Exosomes secreted from human colorectal cancer cell lines contain mRNAs, microRNAs and natural antisense RNAs, that can transfer into the human hepatoma HepG2 and lung cancer A549 cell lines. Oncol Rep. 2012;28:1551-1558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 150. | Camp ER, Ellis LM. CCR 20th Anniversary Commentary: RAS as a Biomarker for EGFR--Targeted Therapy for Colorectal Cancer-From Concept to Practice. Clin Cancer Res. 2015;21:3578-3580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 151. | Kuracha MR, Thomas P, Loggie BW, Govindarajan V. Bilateral blockade of MEK- and PI3K-mediated pathways downstream of mutant KRAS as a treatment approach for peritoneal mucinous malignancies. PLoS One. 2017;12:e0179510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 152. | Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12:343-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 417] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 153. | Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, Wymant J, Jones AT, Kynaston H, Mason MD, Tabi Z, Clayton A. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34:290-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 154. | Huang Z, Feng Y. Exosomes Derived From Hypoxic Colorectal Cancer Cells Promote Angiogenesis Through Wnt4-Induced β-Catenin Signaling in Endothelial Cells. Oncol Res. 2017;25:651-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 155. | Talwar H, McVicker B, Tobi M. p38γ Activation and BGP (Biliary Glycoprotein) Induction in Primates at Risk for Inflammatory Bowel Disease and Colorectal Cancer-A Comparative Study with Humans. Vaccines (Basel). 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 156. | Aldulaymi B, Byström P, Berglund A, Christensen IJ, Brünner N, Nielsen HJ, Glimelius B. High plasma TIMP-1 and serum CEA levels during combination chemotherapy for metastatic colorectal cancer are significantly associated with poor outcome. Oncology. 2010;79:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 157. | Mohr AM, Gould JJ, Kubik JL, Talmon GA, Casey CA, Thomas P, Tuma DJ, McVicker BL. Enhanced colorectal cancer metastases in the alcohol-injured liver. Clin Exp Metastasis. 2017;34:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 158. | Tobi M, Chintalapani S, Kithier K, Clapp N. Carcinoembryonic antigen family of adhesion molecules in the cotton top tamarin (Saguinus oedipus). Cancer Lett. 2000;157:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 159. | Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013;32:643-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 371] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 160. | Gangopadhyay A, Lazure DA, Thomas P. Adhesion of colorectal carcinoma cells to the endothelium is mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp Metastasis. 1998;16:703-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 161. | Thomas P, Forse RA, Bajenova O. Carcinoembryonic antigen (CEA) and its receptor hnRNP M are mediators of metastasis and the inflammatory response in the liver. Clin Exp Metastasis. 2011;28:923-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 162. | Yokoyama S, Takeuchi A, Yamaguchi S, Mitani Y, Watanabe T, Matsuda K, Hotta T, Shively JE, Yamaue H. Clinical implications of carcinoembryonic antigen distribution in serum exosomal fraction-Measurement by ELISA. PLoS One. 2017;12:e0183337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 163. | Liu H, Liu Y, Sun P, Leng K, Xu Y, Mei L, Han P, Zhang B, Yao K, Li C, Bai J, Cui B. Colorectal cancer-derived exosomal miR-106b-3p promotes metastasis by down-regulating DLC-1 expression. Clin Sci (Lond). 2020;134:419-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 164. | Zhang N, Zhang PP, Huang JJ, Wang ZY, Zhang ZH, Yuan JZ, Ma EM, Liu X, Bai J. Reduced serum exosomal miR-874 expression predicts poor prognosis in colorectal cancer. Eur Rev Med Pharmacol Sci. 2020;24:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 165. | Xu Y, Shen L, Li F, Yang J, Wan X, Ouyang M. microRNA-16-5p-containing exosomes derived from bone marrow-derived mesenchymal stem cells inhibit proliferation, migration, and invasion, while promoting apoptosis of colorectal cancer cells by downregulating ITGA2. J Cell Physiol. 2019;234:21380-21394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 166. | Wang J, Yan F, Zhao Q, Zhan F, Wang R, Wang L, Zhang Y, Huang X. Circulating exosomal miR-125a-3p as a novel biomarker for early-stage colon cancer. Sci Rep. 2017;7:4150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 167. | Liu C, Eng C, Shen J, Lu Y, Takata Y, Mehdizadeh A, Chang GJ, Rodriguez-Bigas MA, Li Y, Chang P, Mao Y, Hassan MM, Wang F, Li D. Serum exosomal miR-4772-3p is a predictor of tumor recurrence in stage II and III colon cancer. Oncotarget. 2016;7:76250-76260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 168. | Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1554] [Cited by in RCA: 2061] [Article Influence: 206.1] [Reference Citation Analysis (0)] |