Published online Oct 28, 2021. doi: 10.3748/wjg.v27.i40.6985
Peer-review started: June 16, 2021
First decision: July 14, 2021
Revised: July 25, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: October 28, 2021
Processing time: 132 Days and 20.5 Hours
Despite the improvement in the endoscopic hemostasis of non-variceal upper gastrointestinal bleeding (NVUGIB), rebleeding remains a major concern.
To assess the role of prophylactic transcatheter arterial embolization (PTAE) added to successful hemostatic treatment among NVUGIB patients.
We searched three databases from inception through October 19th, 2020. Randomized controlled trials (RCTs) and observational cohort studies were eligible. Studies compared patients with NVUGIB receiving PTAE to those who did not get PTAE. Investigated outcomes were rebleeding, mortality, reintervention, need for surgery and transfusion, length of hospital (LOH), and intensive care unit (ICU) stay. In the quantitative synthesis, odds ratios (ORs) and weighted mean differences (WMDs) were calculated with the random-effects model and interpreted with 95% confidence intervals (CIs).
We included a total of 3 RCTs and 9 observational studies with a total of 1329 patients, with 486 in the intervention group. PTAE was associated with lower odds of rebleeding (OR = 0.48, 95%CI: 0.29–0.78). There was no difference in the 30-d mortality rates (OR = 0.82, 95%CI: 0.39–1.72) between the PTAE and control groups. Patients who underwent PTAE treatment had a lower chance for reintervention (OR = 0.48, 95%CI: 0.31–0.76) or rescue surgery (OR = 0.35, 95%CI: 0.14–0.92). The LOH and ICU stay was shorter in the PTAE group, but the difference was non-significant [WMD = -3.77, 95%CI: (-8.00)–0.45; WMD = -1.33, 95%CI: (-2.84)–0.18, respectively].
PTAE is associated with lower odds of rebleeding and any reintervention in NVUGIB. However, further RCTs are needed to have a higher level of evidence.
Core Tip: Rebleeding remains a significant concern in patients with non-variceal upper gastrointestinal bleeding (NVUGIB), despite the improvements in endoscopic and pharmacologic treatments. Our systematic review and meta-analysis indicate that prophylactic transcatheter arterial embolization (PTAE) compared to standard of care is accompanied by lower odds of rebleeding, need for rescue surgery, and reinterventions NVUGIB. However, we could not justify a beneficial effect of PTAE on mortality rates compared with the standard of care. In line with our results, we suggest using PTAE in selected cases, where risk stratification predicts high rebleeding risk or the anatomical situation makes the secure and permanent endoscopic hemostasis impossible.
- Citation: Boros E, Sipos Z, Hegyi P, Teutsch B, Frim L, Váncsa S, Kiss S, Dembrovszky F, Oštarijaš E, Shawyer A, Erőss B. Prophylactic transcatheter arterial embolization reduces rebleeding in non-variceal upper gastrointestinal bleeding: A meta-analysis. World J Gastroenterol 2021; 27(40): 6985-6999
- URL: https://www.wjgnet.com/1007-9327/full/v27/i40/6985.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i40.6985
Acute upper gastrointestinal bleeding (UGIB) remains a common medical emergency with an incidence of 47-90/100000[1-3]. The age-standardized incidence of ulcer bleeding decreased by 41.6% between 1983 and 2004 in a prospective observational study; notable the decrease occurred only in people younger than 70 years of age[1]. Mortality in UGIB ranges between 1.1%–11%[4], although it has significantly decreased due to improvements and innovation in both endoscopic and pharmacologic treatments[1,4]. The UGIB population characteristics have changed considerably, the mean age of the patients has increased, the prevalence of co-morbidities is higher than before, and the use of nonsteroidal anti-inflammatory, antiplatelet and anticoagulant drugs is more widespread[1,5,6].
Rebleeding is a significant concern in patients with UGIB, occurring in 7%–16% of cases despite endoscopic therapy[6]. As published in a study from the United Kingdom in 2007, rebleeding is associated with a higher mortality rate, potentially induces more extended hospital stay and need for reintervention[5]. Thus, preventing rebleeding is a critical factor from the patients' and a healthcare economic view. The management of non-variceal UGIB (NVUGIB) is well established in guidelines based on a high level of evidence. In the post-endoscopy care of NVUGIB, a strong recommendation is to use high-dose proton pump inhibitors and eradicate Helicobacter pylori (H. pylori) if presence is established[7]. On the other hand, if rebleeding happens, the patient should receive a repeat upper gastrointestinal endoscopy[7]. In the case of failure of this second attempt with endoscopic hemostasis, either transcatheter angiographic embolization (TAE) or rescue surgery are indicated, as they provide the same level of efficacy[8,9].
In contrast, there are only a few studies about the potential role of prophylactic transcatheter arterial embolization (PTAE) in the management of NVUGIB. A recent randomized controlled trial suggested that PTAE may reduce the incidence of recurrent bleeding (10.2% vs 11.4%, P = 0.745), but they could not show a clear benefit in adding angiographic embolization to endoscopic hemostasis in NVUGIB patients[10]. Investigating a subset of patients with ulcers 15mm or more in size, PTAE significantly reduced the risk of rebleeding (23.1% vs 4.5%, P = 0.027)[10]. A similar improvement in the rebleeding rate after PTAE was observed in some cohort studies[11-13].
Our study aimed to assess the role of PTAE among NVUGIB patients. We hypothesized that PTAE could reduce the risk of rebleeding and even the mortality rate among NVUGIB patients and improve other outcomes.
We are reporting our systematic review and meta-analysis in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Statement[14] (Supplementary Table 1) and also in line with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines[15] because we included both RCTs and observational cohort studies. Methods of the analysis and inclusion criteria were established in advance, and the protocol was documented on the International Prospective Register of Systematic Reviews (PROSPERO, registration number CRD42021223726).
Three databases, MEDLINE (via PubMed), EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL), were searched from inception through October 19th, 2020. We applied the following search key: (embolization OR embolisation) AND (peptic OR ulcer OR "gastrointestinal bleeding" OR nonvariceal OR non-variceal OR "gastrointestinal hemorrhage" OR "gastrointestinal haemorrhage"). We did not use any restrictions or filters during the search. We provide the complete search strategy in Supplementary Appendix 1.
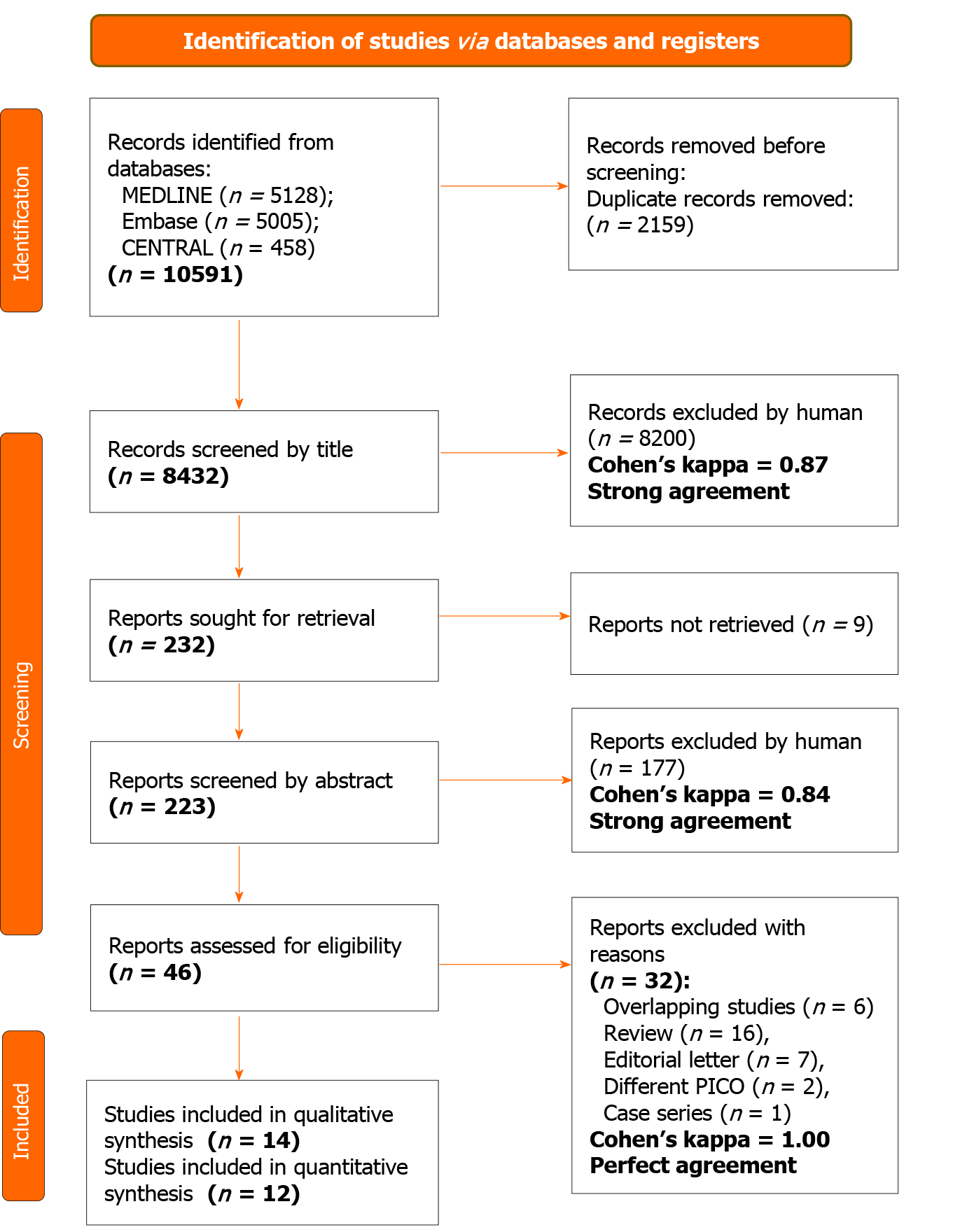
After the automatic and manual removal of duplicates with a reference manager software (EndNote X9, Clarivate Analytics, Philadelphia, PA, United States), screening and selection for the title, abstract, and full text were undertaken independently by two review authors. Cohen's kappa coefficient (κ) was calculated after each step to measure inter-reviewer reliability[16]. Disagreements were resolved through discu
To identify eligible studies, we used the population-intervention-control-outcome (PICO) framework. Our investigated population (P) consisted of adult patients (age > 18 years) with endoscopy, digital subtraction angiography, or computed tomography angiography proved NVUGIB source, who either underwent successful endoscopic hemostasis or during angiography, there was no detectable contrast extravasation described. Studies compared the outcomes of patients who received PTAE (I) to patients who did not receive PTAE (C). The primary outcomes (O) were rebleeding and 30-d mortality rate, and the secondary outcomes were in-hospital or overall mortality rate, reintervention, need for salvage surgery, need of transfusion, length of hospital (LOH), and intensive care unit (ICU) stay. We pooled studies with different measurement time points regarding rebleeding (such as in-hospital, 28- and 30-d). If articles did not provide a precise measurement time point for mortality, we referred to that as "overall mortality". We defined reintervention as any repeated invasive treatment of rebleeding such as embolization, endoscopy, or surgery. Randomized controlled trials, prospective and retrospective observational cohort studies were eligible. Case reports, case series with less than ten patients, and review articles that did not report original research were excluded. In the case of publications using data with overlapping study populations, we used the one with the bigger sample size.
Two independent review authors extracted data from eligible studies into a predesigned data collection form. The following data were collected from each study: first author, year of publication, study design, study period, study site (country), demographic features of the study population, the number of participants with PTAE, the number of patients without PTAE, bleeding etiology, type of embolic agents, data on outcomes (rebleeding, surgery, mortality, reintervention, LOH stay, ICU stay, and blood transfusion) in the intervention and control groups. In the case of the RCTs, intention-to-treat and per-protocol data were collected separately.
A biomedical statistician performed the statistical analysis of the study. Calculations were made by Stata 16 data analysis and statistical software (Stata Corp LLC, College Station, TX, United States). In the case of dichotomous categorical outcomes (mortality, rebleeding rate, etc.), we determined odds ratios (ORs) with 95% confidence intervals (95%CIs) from two-by-two tables (intervention vs control, outcome present and absent). For continuous variables, weighted mean difference (WMD) with 95%CIs were calculated. A P value less than 0.05 was considered a statistically significant difference. The random-effects model, according to the method of DerSimonian-Laird[17], was used to calculate the pooled estimates. We used forest plots to present the results of the meta-analyses.
I² and χ² tests were performed to assess heterogeneity. I2 values were described as “minimal” (0%–40%), “moderate” (30%–60%), “substantial” (50%–90%), and “considerable” (75%–100%) heterogeneity, with a P value < 0.1 considered significant, as suggested by the Cochrane Handbook[18]. For the outcome of rebleeding, publication bias was assessed by visual inspection of a funnel plot and Egger's test. As for the other outcomes, we were unable to determine the presence of publication bias because of the low number of studies included in each analysis.
Sensitivity analysis was carried out by removing each trial analysis in turn in the case of rebleeding, reintervention, surgery, and need of transfusion outcome (the leave-one-out-method).
We performed a subgroup analysis for rebleeding and compared randomized controlled studies (RCTs) with non-randomized studies. For RCTs, we analyzed the intention-to-treat and per-protocol analysis results separately. Trial Sequential Analysis (TSA 0.9.5.10.) was performed for the RCTs regarding rebleeding to control random errors and estimate the optimal information size.
Two independent review authors carried out the risk of bias assessment. Discrepancies were resolved by third-party arbitration. We followed the recommendations of the Cochrane Prognosis Methods group, and we used the revised Risk of Bias (RoB) 2 tool for randomized and the Risk of Bias In Non-randomized Studies - of Interventions (ROBINS-I) tool for non-randomized studies[19,20]. We used the Risk-of-bias VISualization (robvis) web-based tool[21].
Two independent review authors assessed the overall quality of evidence following the recommendation of the "Grades of Recommendation, Assessment, Development, and Evaluation (GRADE)" workgroup[22]. A third author resolved disagreements. Summary of Findings table and the additional tables were prepared with the GRADE profiler (GRADEpro) tool [GRADEpro Guideline Development Tool (Software)]. McMaster University, 2020 (developed by Evidence Prime, Inc.).
We identified 10591 records in three databases for evaluation. After removing duplicates and careful selection by title and abstract, 46 articles were eligible for full-text assessment. Altogether, 14 papers were retrieved for qualitative and 12 for quantitative synthesis. Two studies[23,24] were excluded from the quantitative synthesis due to major differences in intervention or outcome compared to other included articles. In the study of Ying et al[23], the intervention group got not only PTAE but also superior mesenteric arterial hypophysin infusion, which was too much alteration from our PICO and inclusion criteria. In the publication of Yonemoto et al[24], different outcomes (statistical analysis for laboratory data, number of endoscopic treatments) were presented than we were assessing in our meta-analysis. The selection process is detailed in Figure 1.
The 14 included studies are summarized in Table 1. We included in the quantitative synthesis three randomized controlled trials[10,25,26], two prospective[11,12], and seven retrospective cohort studies[13,27-32]. The source of bleeding was peptic ulcer lesions in eight studies[10-13,25,29,30,32], while in four studies[26-28,31], NVUGIB lesion was used as a generic term for various bleeding sources (e.g., angiodysplasia, solid tumors, peptic ulcers). Each study had a relatively small sample size, with similar characteristics between intervention groups. The eligibility criteria of the studies included are presented in Supplementary Table 2. Most of the studies did not report any adverse events during or after PTAE. Information about the reported endoscopic treatments, technical success rate of PTAE, adverse events, and standard of care are summarized in Supplementary Table 3.
| Ref. | Country | Study design | Study period | Number of patients/ intervention group | Female % | Age,1 (yr) | Bleeding etiology | Outcome(s) | Embolic agents |
| Arrayeah et al[27] | United States, Israel | Retrospective cohort | 1997–2009 | 73/56 | 40 | 61.1 | NVUGIB | Rebleeding, 30-d mortality, reintervention, surgery | Microcoils, gelatin sponge, polyvinyl alcohol particles |
| Dixon et al[28] | United Kingdom | Retrospective cohort | 05.2008–11.2010 | 27/20 | 18.5 | 66 | NVUGIB of duodenal origin | Rebleeding, 30-d mortality, reintervention | Microcoils alone or combined with gelatin/gelfoam sponge, polyvinyl alcohol particles |
| Kaminskis et al[11] | Latvia | Prospective cohort | 2014–2018 | 399/58 | 44.4 | 67 | High-risk peptic ulcer | Rebleeding, in-hospital mortality, surgery, hospital stay, ICU stay, transfused blood units | ND |
| Kaminskis et al[12] | Latvia | Prospective cohort | 2010–2013 | 75/25 | 66.6 | 64 | High-risk peptic ulcer | Rebleeding, in-hospital mortality, surgery, hospital stay, ICU stay, transfused blood units | Coil or sandwich technique |
| Lau et al[10] | China | Randomized controlled trial | 2010–2014 | 241/118 | 24.9 | 66 | High-risk peptic ulcer | Rebleeding, 30-d mortality, reintervention, surgery, hospital stay, ICU stay, transfused blood units | Sandwich technique: Coils and gel foam particles |
| Laursen et al[25] | Denmark | Randomized controlled trial | 11.2009–05.2012 | 105/49 | 44 | 73 | High-risk peptic ulcer | Rebleeding, in-hospital and 30-d mortality, hospital stay, readmission | Coils |
| Lebedev et al[29] | Russia | Retrospective cohort | 1991–2016 | 90/30 | ND | ND | Peptic ulcer | Rebleeding, mortality | Microcoils, polyvinyl alcohol |
| Mille et al[30] | Germany | Retrospective cohort | 2008–2012 | 102/55 | 31.4 | 70.7 | Duodenal ulcer | Rebleeding, 30-d mortality, surgery, transfused blood units | Coils, cyanoacrylate glue or both |
| Sildiroglu et al[31] | United States | Retrospective cohort | 10.2001–11.2011 | 43/18 | ND | 60.1 | NVUGIB | Rebleeding, mortality | Coils, gelfoam, polyvinyl alcohol |
| Tong et al[13] | China | Retrospective cohort | 2014–2016 | 74/16 | 23 | 57.2 | High-risk peptic ulcer | Rebleeding | ND |
| Wu et al[32] | Australia | Retrospective cohort | 01.2008–12.2012 | 34/8 | ND | 70.1 | Peptic ulcer | Rebleeding | ND |
| Ying et al[26] | China | Randomized controlled trial | 05.2012–06.2013 | 66/33 | 25 | 51.5 | Upper GIB | Rebleeding | Gelatin sponge particles |
| Ying et al[23] | China | Randomized controlled trial | 06.2010–06.2014 | 78/39 | 46.2 | 46.5 | Upper GIB | Short term haemostasis, long term haemostasis, hospital stay, transfusion | Coils, gelatin sponge |
| Yonemoto et al[24] | Japan | Retrospective cohort | 04.2005–12.2017 | 141/11 | 22.7 | 62.8 | Duodenal ulcer | Laboratory data at initial diagnosis, the amount of blood transfusion, 30-d mortality | ND |
In our meta-analysis, we included a total of twelve studies with 1329 patients evaluating the clinical effect of PTAE on various outcomes. In the intervention group, 486 patients received PTAE in addition to standard of care. There was a total of 843 patients in the control group.
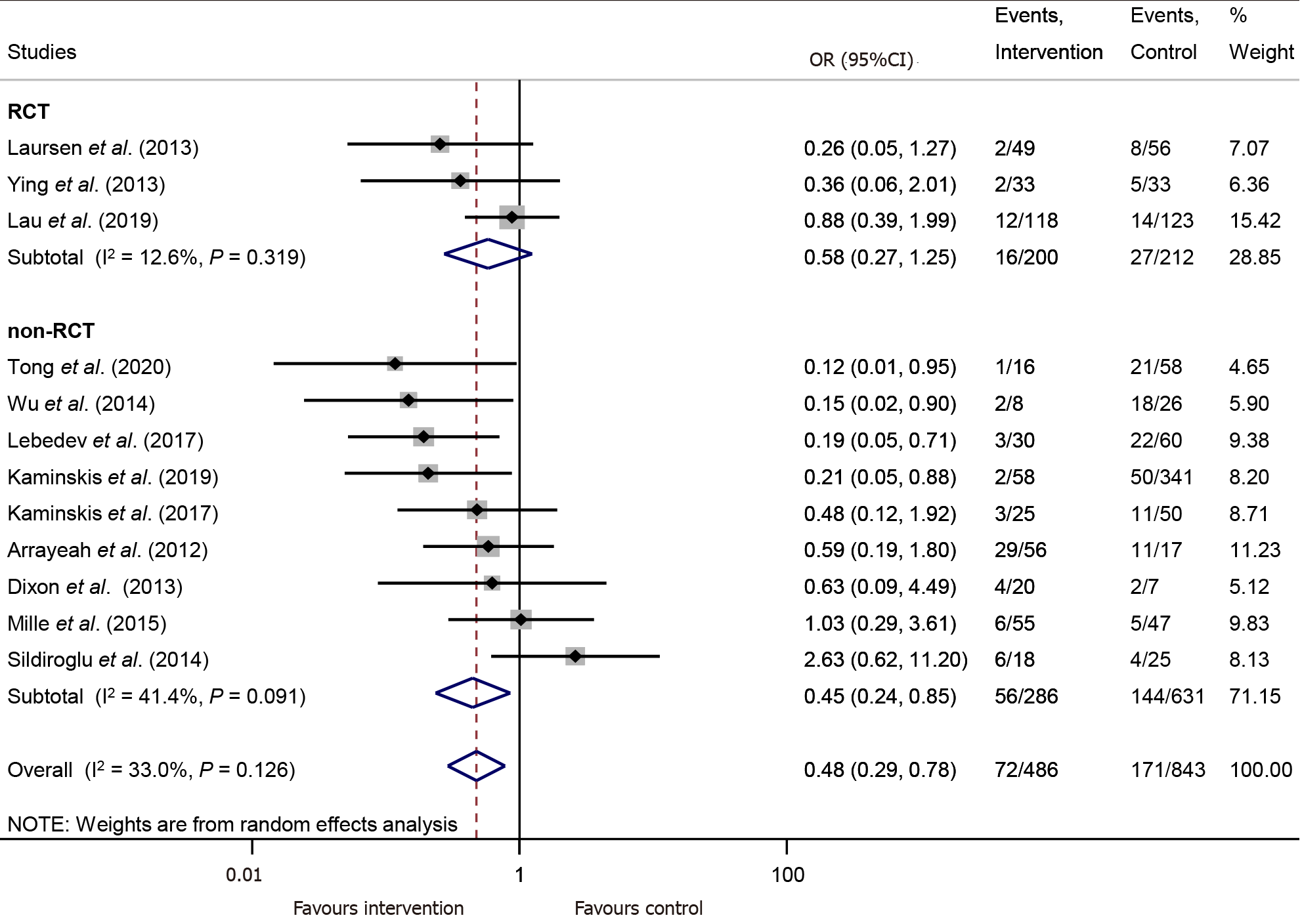
PTAE is connected with significantly lower chance for rebleeding compared to the control group (OR = 0.48, 95%CI: 0.29–0.78, P = 0.003; in a mildly heterogenous dataset, I2 = 33.0%, P = 0.126) (Figure 2).
For this comparison, publication bias assessment by visual inspection of a funnel-plot and Egger's test was carried out, suggesting a likelihood for publication bias. (Supplementary Figure 1). Leave-one-out analysis showed no significant change in the overall odds for rebleeding (Supplementary Figure 2).
According to the RCT subgroup analysis with intention-to-treat data, there was no significant difference between the PTAE and control group in the rate of rebleeding (OR = 0.58, 95%CI: 0.27–1.25, P = 0.165; in a mildly heterogeneous dataset, I2 = 12.6%, P = 0.319) (Figure 2). However, with available per-protocol analysis results, the odds of rebleeding were significantly lower in the PTAE group, compared to the control group even according to the RCT subgroup analysis (OR = 0.42, 95%CI: 0.19–0.93, P = 0.033; [I2 = 0.0%, P = 0.712]) (Supplementary Figure 3). The performed TSA showed that the required information size was reached neither in the intention-to-treat nor in the per-protocol calculation (Supplementary Figure 4A and 4B).
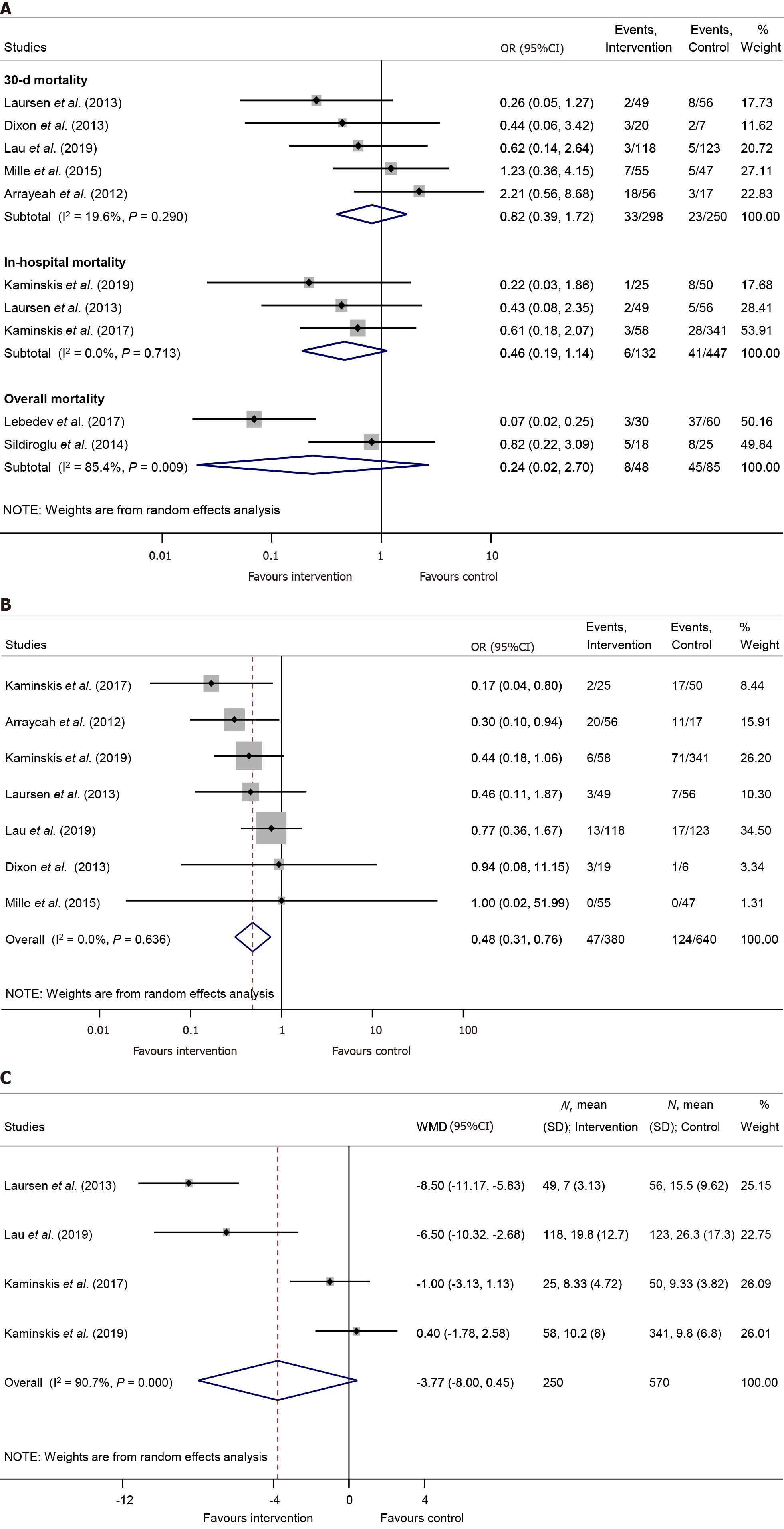
There was no significant difference neither in 30-day mortality [OR = 0.82, 95%CI: 0.39–1.72, P = 0.594; (I2 = 19.6%, P = 0.290)], nor in in-hospital mortality rates [OR = 0.46, 95%CI: 0.19–1.14, P = 0.092; (I2 = 0.0%, P = 0.713), respectively] between the PTAE and control group (Figure 3A).
Patients who underwent PTAE treatment were less likely to need any kind of reintervention caused by rebleeding, compared to those without PTAE (OR = 0.48, 95%CI: 0.31–0.76, P = 0.002; in a homogenous dataset, I2 = 0.0%, P = 0.636) (Figure 3B). We found that PTAE group has significantly lower odds of rescue surgery in contrast with the control group [OR = 0.35, 95%CI: 0.14–0.92, P = 0.033; (I2 = 44.1%, P = 0.128)] (Supplementary Figure 5). Leave-one-out analysis showed no major change in the overall odds of reintervention or rescue surgery (Supplementary Figure 6 and 7).
The length of hospital stay was reported in four studies[10-12,25]. Our meta-analysis established no statistically significant difference between the intervention and the control group in the length of hospital stay [WMD = -3.77 days, 95%CI: (-8.00)–0.45, P = 0.08; considerable heterogenous dataset, I2 = 90.7%, P < 0.001] (Figure 3C). In parallel, three publications[10-12] reported the length of ICU stay, however the difference between the PTAE and control group was non-significant [WMD = -1.33 days, 95%CI: (-2.84)–0.18, P = 0.084; I2 = 84.8%, P = 0.001] (Supplementary Figure 8).
Lastly, the PTAE group needed significantly more units of blood transfusion, than the control group [WMD = 1.49 units, 95%CI: 0.05–2.94, P = 0.042; (I2 = 90.8%, P < 0.001)] (Supplementary Figure 9). Applying the leave-one-out method, the study of Mille et al[30] proved to be influential as the difference was no longer statistically significant between the two groups without their results (Supplementary Figure 10).
Among the included studies, four[10-12,25] were of low overall risk of bias, two[29,30] were of high overall risk of bias, all the other studies were rated to carry moderate overall risk of bias. The summary of the risk of bias assessment is shown in Supplementary Figure 11-18.
We included all outcomes in the "Summary of findings table" (SupplementaryTable 4). The certainty of the evidence is very low for every outcome because our meta-analysis mainly contained observational studies. We calculated the quality of evidence among the RCT subgroup in rebleeding, and we found a moderate certainty of the evidence for PTAE, lowering the chance of rebleeding.
In this study, we assessed the effect of PTAE in addition to the successful endoscopic treatment of NVGUIB or PTAE as the first treatment option in case of not actively bleeding patients with NVUGIB lesions. Based on our findings, PTAE is associated with lower odds of rebleeding, need for additional reintervention, and rescue surgery compared to standard of care. We found a roughly 50% lower rate of the outcomes mentioned above, which is a considerable proportion, especially considering the prevalence of NVUGIB.
A recent meta-analysis of Chang et al[33] attempted to evaluate the role of PTAE in the management of patients with high-risk peptic ulcer bleeding. They included only 2 RCTs and 3 observational studies in their meta-analysis while narrowing their search to patients with a high risk of rebleeding. We designed our systematic search for NVUGIB without restriction to high-risk peptic ulcer bleeding because of the various NVUGIB bleeding etiologies that could be treated with TAE[9].
We found three eligible RCTs[10,25,26], which provide the core of our meta-analysis and the highest level of evidence. When we performed a subgroup analysis of the RCTs for the rebleeding outcome, we found no significant difference between PTAE and the control group. We think that the main reason why with intention-to-treat data, we could not demonstrate the clear beneficial effect of PTAE is that in Lau et al[10]'s study, 22 patients out of 118 (18.6%), and Laursen et al[25], 18 out of 49 (36.7%) did not receive embolization at all despite being in the embolization arm of the studies. When we used per-protocol data, also in the RCT subgroup, there was a significantly lower risk for rebleeding in the PTAE group. However, the TSA calculation showed that the required information size was not reached, and our results are inconclusive.
It is important to note that in Laursen et al[25]'s study, 9% of the potentially eligible patients were excluded because they were admitted to the hospital on weekends. These patients could not receive the allocated intervention due to the lack of staff in interventional radiology. This highlights that PTAE could be the most beneficial treatment option in centers with well-established interventional radiology units.
According to Lau et al[10], the chance of recurrent bleeding was significantly reduced in ulcers 15 mm in size or greater. Unfortunately, there was not enough data on the size of the culprit lesions in the other included studies to perform a meta-analysis. Nevertheless, risk stratification is essential before choosing between multiple possible treatment modalities in the case of NVUGIB. Several studies[10-13,25] used the Forrest classification, Rockall score, or the American Society of Anesthesiologists score to assess the baseline risk of rebleeding or mortality among the patients, and it was approximately the same between the PTAE and the control group.
The publication of Mille et al[30] described that only high rebleeding risk patients got PTAE, and in the control group were only low-risk patients. We consider this a selection bias, causing confounding of the measured outcomes, which could explain why they did not manage to find any difference in the rebleeding, reintervention, or rescue surgery rate between the intervention and the control group. Applying the leave-one-out method in the case of the study of Mille et al[30], there was no major change in the overall odds for rebleeding, reintervention, and rescue surgery.
The study of Sildiroglu et al[31] was the only one where the PTAE group had a worse rebleeding rate than the control group. There was no data on the baseline characteristics of the two treatment groups, so we cannot explain this contradicting result.
We included in our analyses four studies[26-28,31], with a definition of NVUGIB including various sources such as peptic ulcers, tumors, Dieulafoy lesion, Mallory-Weiss tear. Arrayeh et al[27] carried out a retrospective analysis that compared three therapeutic options: angiography without embolization, PTAE, and TAE with an abnormal angiogram. They published the interesting observation that patients with duodenal bleeding due to a mass (various types of malignant) lesion had a greater primary hemostasis rate 30 d after angiography compared with patients with nonmass (different types of benign) sources of duodenal bleeding (100% vs 54%; P = 0.008). This difference was not detectable between mass and nonmass lesions in the case of gastric bleeding. We did not find data in other studies about the investigated outcomes separated by the type of the bleeding lesion.
The rebleeding rate could also depend on whether NVUGIB has a gastric or duodenal source. According to Arrayeh et al[27], PTAE may be advantageous in patients with a duodenal source of bleeding but not in patients with gastric hemorrhage. We did not identify enough evidence to support this inference because none of the other included studies reported gastric and duodenal bleeding separately.
Our results indicated that PTAE does not improve the mortality rates of NVUGIB significantly. We could not draw a clear conclusion about this outcome because of the different time-frames for mortality assessment used between the different studies: 30-d mortality was used in five studies[10,25,27,28,30], in-hospital mortality in three[11,12,25] and two studies[29,31] did not report any time-frame for mortality. Mortality is strongly associated with pre-endoscopy and complete Rockall score, according to Hearnshaw et al[5] We can only speculate that numerous major confounding factors affect the mortality of NVUGIB patients, and only one of them is the chosen treatment modality.
We had predicted a shorter hospital stay and ICU stay in the PTAE group, but our findings could not prove a significant difference between the two groups. Interestingly, if we analyzed only the data coming from RCTs, there was an apparent reduction in the length of hospital and ICU stay in the PTAE group compared with the control group. This result highlights the possible bias of the observational cohort studies.
The PTAE group needed slightly more red blood cell transfusion than the control group, although the heterogeneity between the studies suggests a careful interpre
There are a few therapies, which are already proved to reduce rebleeding from NVUGIB. Proton pump inhibitors (PPI) significantly decrease the recurrence of bleeding compared to control (placebo or histamine type 2 receptor antagonists); pooled rates were 10.6% with PPI vs 17.3% with control treatment (OR = 0.49; 95%CI: 0.37-0.65) in a Cochrane review comprising 24 RCTs[34]. Our recent meta-analysis showed that PPIs given either orally or intravenously are equally efficacious in preventing rebleeding[35].
After peptic ulcer bleeding, investigation for the presence of H. pylori should be mandatory. Our network meta-analysis[36] demonstrated that none of the individual tests or the strategy of combined tests is superior in detecting H. pylori. Gisbert et al[37] reported that rebleeding did not occur in patients with complicated ulcers after H. pylori eradication; moreover, maintenance of anti-ulcer therapy is unnecessary if eradication was achieved.
Endoscopic Doppler probe guided hemostasis significantly reduced the 30-d rates of rebleeding compared with standard visually guided hemostasis in an RCT (11.1% vs 26.3%), and the use of the endoscopic Doppler probe was suggested for risk stratification in the management of NVUGIB[38].
Another promising endoscopic technique to reduce the rebleeding rate of peptic ulcers is over-the-scope clipping (OTSC), which is superior to standard therapy with through-the-scope clips in preventing further bleeding according to the prospective RCT of Schmidt et al[39].
A recent meta-analysis[40] showed that the routine second-look endoscopy was not superior to a single endoscopy with complete endoscopic hemostasis in reducing the risk of recurrent bleeding, mortality, or need for surgery in patients with acute UGIB due to peptic ulcer disease. In contrast, according to our results, PTAE added to the standard of care could decrease the probability of rebleeding. Moreover, PTAE might reduce the need for surgery and any reintervention.
Our work is assessing the potential effects of PTAE compared to the standard of care in the treatment of NVUGIB. We used a rigorous methodology and followed a transparent protocol, combined with a comprehensive statistical analysis as possible. We collected a total of 486 patients who received PTAE, which is an infrequent therapeutic choice so far.
The main limitation of our meta-analysis is that we collected our data mostly from observational cohort studies, and we found only three RCTs comparing PTAE to the standard of care. Thus, the quality of evidence for every outcome in our meta-analysis is very low based on the GRADE framework. When we assessed the quality of evidence regarding rebleeding in the RCT subgroup, we found moderate evidence for the risk reduction with PTAE. The diversity of the NVUGIB population in some studies could also limit our results and explain the statistical heterogeneity in some cases. Significant differences are present in the embolic agents utilized for PTAE among publications. There was very restricted data on the endoscopic treatment before PTAE, which could also influence the outcomes. The included studies contain only limited information on secondary outcomes, so our conclusions about these are less certain.
In selected cases, where the previous risk stratification suggests high rebleeding risk or the anatomical situation makes the secure and permanent endoscopic hemostasis impossible, we can consider the routine use of PTAE. Considering the demographic trends of NVUGIB, we predict that elderly, high-risk patients with co-morbidities could benefit the most from PTAE as a therapeutic approach.
Further RCTs are warranted to achieve a higher level of evidence about the potentially beneficial effects of PTAE. Developing an accurate risk stratification system would be crucial to select the ideal candidates for PTAE. Clinical trials investigating the use of existing risk scores or creating a new risk stratification tool of NVUGIB could help clinicians choose between the emerging number of treatment options.
PTAE is accompanied by lower odds of rebleeding, need for surgery, and reinterventions in NVUGIB. However, our meta-analysis could not justify a beneficial effect of PTAE on mortality rates compared with the standard of care in NVUGIB.
The prevention of rebleeding is one of the main goals in managing non-variceal upper gastrointestinal bleeding (NVUGIB). Prophylactic transcatheter arterial embolization (PTAE) can be used in NVUGIB as second-line therapy.
The results of the individual studies about the beneficial effects of PTAE among NVUGIB patients were contradictory.
The authors aimed to carry out a comprehensive systematic review and meta-analysis. The authors compared the PTAE to no embolization as a second line, prophylactic treatment among NVUGIB patients.
The authors conducted a systematic search in three databases (MEDLINE, EMBASE, CENTRAL). The eligible studies compared patients with NVUGIB receiving PTAE to those who did not get PTAE. The authors calculated odds ratios (ORs) with 95% confidence intervals (CI) for rebleeding, mortality, reintervention, need for surgery, and weighted mean differences (WMDs) of need for transfusion, length of hospital (LOH), and intensive care unit (ICU) stay.
PTAE was associated with significantly lower odds of rebleeding, reintervention and rescue surgery (OR = 0.48, 95%CI: 0.29–0.78; OR = 0.48, 95%CI: 0.31–0.76; OR = 0.35, 95%CI: 0.14–0.92; respectively). There was no significant difference in the mortality rates, LOH, and ICU stays between the PTAE and control groups. The quality of evidence for every outcome in our meta-analysis is very low based on the GRADE framework.
The results suggest that PTAE is a reasonable therapeutic choice to prevent rebleeding or reintervention in NVUGIB, although it did not improve the mortality rates of NVUGIB.
Further randomized controlled trials are needed about the use of PTAE. We also propose a clinical trial that could recommend a new risk stratification tool of NVUGIB, helping clinicians choose between treatment options.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu XM, Papadopoulos VP, Xiong A, Ziogas IA S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Loperfido S, Baldo V, Piovesana E, Bellina L, Rossi K, Groppo M, Caroli A, Dal Bò N, Monica F, Fabris L, Salvat HH, Bassi N, Okolicsanyi L. Changing trends in acute upper-GI bleeding: a population-based study. Gastrointest Endosc. 2009;70:212-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Oakland K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2019;42-43:101610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Wuerth BA, Rockey DC. Changing Epidemiology of Upper Gastrointestinal Hemorrhage in the Last Decade: A Nationwide Analysis. Dig Dis Sci. 2018;63:1286-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 4. | Jairath V, Martel M, Logan RF, Barkun AN. Why do mortality rates for nonvariceal upper gastrointestinal bleeding differ around the world? Can J Gastroenterol. 2012;26:537-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 431] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 6. | van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:209-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 259] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 7. | Gralnek IM, Stanley AJ, Morris AJ, Camus M, Lau J, Lanas A, Laursen SB, Radaelli F, Papanikolaou IS, Cúrdia Gonçalves T, Dinis-Ribeiro M, Awadie H, Braun G, de Groot N, Udd M, Sanchez-Yague A, Neeman Z, van Hooft JE. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy. 2021;53:300-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 266] [Article Influence: 66.5] [Reference Citation Analysis (1)] |
| 8. | Nykänen T, Peltola E, Kylänpää L, Udd M. Bleeding gastric and duodenal ulcers: case-control study comparing angioembolization and surgery. Scand J Gastroenterol. 2017;52:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Tarasconi A, Baiocchi GL, Pattonieri V, Perrone G, Abongwa HK, Molfino S, Portolani N, Sartelli M, Di Saverio S, Heyer A, Ansaloni L, Coccolini F, Catena F. Transcatheter arterial embolization versus surgery for refractory non-variceal upper gastrointestinal bleeding: a meta-analysis. World J Emerg Surg. 2019;14:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Lau JYW, Pittayanon R, Wong KT, Pinjaroen N, Chiu PWY, Rerknimitr R, Holster IL, Kuipers EJ, Wu KC, Au KWL, Chan FKL, Sung JJY. Prophylactic angiographic embolisation after endoscopic control of bleeding to high-risk peptic ulcers: a randomised controlled trial. Gut. 2019;68:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Kaminskis A, Ivanova P, Kratovska A, Ponomarjova S, Ptašņuka M, Demičevs J, Demičeva R, Boka V, Pupelis G. Endoscopic hemostasis followed by preventive transarterial embolization in high-risk patients with bleeding peptic ulcer: 5-year experience. World J Emerg Surg. 2019;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Kaminskis A, Kratovska A, Ponomarjova S, Tolstova A, Mukans M, Stabiņa S, Gailums R, Bernšteins A, Ivanova P, Boka V, Pupelis G. Preventive transarterial embolization in upper nonvariceal gastrointestinal bleeding. World J Emerg Surg. 2017;12:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Tong H, Lan T, Tang CW. Prophylactic angiographic embolisation after endoscopic treatment of bleeding for high-risk peptic ulcers: what are the more appropriate indications? Gut. 2020;69:1897-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40406] [Article Influence: 10101.5] [Reference Citation Analysis (2)] |
| 15. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 16793] [Article Influence: 671.7] [Reference Citation Analysis (0)] |
| 16. | McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22:276-282. [PubMed] |
| 17. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30408] [Article Influence: 779.7] [Reference Citation Analysis (0)] |
| 18. | Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from: https://training.cochrane.org/handbook. |
| 19. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10841] [Article Influence: 1204.6] [Reference Citation Analysis (2)] |
| 20. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15219] [Article Influence: 2536.5] [Reference Citation Analysis (0)] |
| 21. | McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 2547] [Article Influence: 509.4] [Reference Citation Analysis (0)] |
| 22. | Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schunemann H, Guyatt G. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 576] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 23. | Ying Y, Luo JF, He X, Zeng FL, Xie YL. Clinical effects of preventive interventional therapy in gastrointestinal bleeding patients with negative digital subtraction angiography findings. Shijie Huaren Xiaohua Zazhi. 2014;22:5556-5560. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Yonemoto Y, Fukami Y, Hara H, Mochida T, Machida T, Sugiyama Y, Watanabe A, Kaneshiro M, Ikemiyagi H, Yoshino K, Sakita S. The statistical comparison of endoscopic procedure and transarterial embolization for hemorrhage caused by duodenal ulcer. United European Gastroenterol J. 2018;6:A496-A497. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 25. | Laursen SB, Hansen JM, Andersen PE, Schaffalitzky de Muckadell OB. Supplementary arteriel embolization an option in high-risk ulcer bleeding--a randomized study. Scand J Gastroenterol. 2014;49:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Ying Y, Luo JF, Zhang WH, Wang XN, He X. Effects of vasopressin infusion aided prophylactic gastroduodenal artery embolization in DSA-negative gastrointestinal bleeding patients. Shijie Huaren Xiaohua Zazhi. 2013;21:4180‐4184. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Arrayeh E, Fidelman N, Gordon RL, LaBerge JM, Kerlan RK Jr, Klimov A, Bloom AI. Transcatheter arterial embolization for upper gastrointestinal nonvariceal hemorrhage: is empiric embolization warranted? Cardiovasc Intervent Radiol. 2012;35:1346-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Dixon S, Chan V, Shrivastava V, Anthony S, Uberoi R, Bratby M. Is there a role for empiric gastroduodenal artery embolization in the management of patients with active upper GI hemorrhage? Cardiovasc Intervent Radiol. 2013;36:970-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Lebedev NV, Belozerov GE, Klimov AE, Sokolova PY, Spasskiy AA, Barkhudarov AA. [Transcatheter embolization in prevention of recurrent bleeding from stomach ulcers]. Khirurgiia (Mosk). 2017;31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Mille M, Huber J, Wlasak R, Engelhardt T, Hillner Y, Kriechling H, Aschenbach R, Ende K, Scharf JG, Puls R, Stier A. Prophylactic Transcatheter Arterial Embolization After Successful Endoscopic Hemostasis in the Management of Bleeding Duodenal Ulcer. J Clin Gastroenterol. 2015;49:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Sildiroglu O, Muasher J, Arslan B, Sabri SS, Saad WE, Angle JF, Matsumoto AH, Turba UC. Outcomes of patients with acute upper gastrointestinal nonvariceal hemorrhage referred to interventional radiology for potential embolotherapy. J Clin Gastroenterol. 2014;48:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Wu P, Szczesniak MM, Craig PI, Choo L. A novel predictor of rebleeding in high risk peptic ulcer disease selects patients who would benefit most from prophylactic arterial embolisation. Gastroenterology. 2014;146:S-183. [DOI] [Full Text] |
| 33. | Chang JHE, Lye TJY, Zhu HZ, Syn NL, Tang SS, Gogna A, Chan WH, Ong HS, Tan JTH, Lim CH. Systematic Review and Meta-Analysis of Prophylactic Transarterial Embolization for High-Risk Bleeding Peptic Ulcer Disease. J Vasc Interv Radiol. 2021;32:576-584.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev. 2006;CD002094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Csiki E, Szabó H, Hanák L, Szakács Z, Kiss S, Vörhendi N, Pécsi D, Hegyi E, Hegyi P, Erőss B. Oral Proton Pump Inhibitors May Be as Effective as Intravenous in Peptic Ulcer Bleeding: A Systematic Review and Meta-analysis. Clin Transl Gastroenterol. 2021;12:e00341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Vörhendi N, Soós A, Anne Engh M, Tinusz B, Szakács Z, Pécsi D, Mikó A, Sarlós P, Hegyi P, Eröss B. Accuracy of the Helicobacter pylori diagnostic tests in patients with peptic ulcer bleeding: a systematic review and network meta-analysis. Therap Adv Gastroenterol. 2020;13:1756284820965324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Gisbert JP, Calvet X, Cosme A, Almela P, Feu F, Bory F, Santolaria S, Aznárez R, Castro M, Fernández N, García-Grávalos R, Benages A, Cañete N, Montoro M, Borda F, Pérez-Aisa A, Piqué JM; H. pylori Study Group of the Asociación Española de Gastroenterología (Spanish Gastroenterology Association). Long-term follow-up of 1,000 patients cured of Helicobacter pylori infection following an episode of peptic ulcer bleeding. Am J Gastroenterol. 2012;107:1197-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Jensen DM, Kovacs TOG, Ohning GV, Ghassemi K, Machicado GA, Dulai GS, Sedarat A, Jutabha R, Gornbein J. Doppler Endoscopic Probe Monitoring of Blood Flow Improves Risk Stratification and Outcomes of Patients With Severe Nonvariceal Upper Gastrointestinal Hemorrhage. Gastroenterology. 2017;152:1310-1318.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Schmidt A, Gölder S, Goetz M, Meining A, Lau J, von Delius S, Escher M, Hoffmann A, Wiest R, Messmann H, Kratt T, Walter B, Bettinger D, Caca K. Over-the-Scope Clips Are More Effective Than Standard Endoscopic Therapy for Patients With Recurrent Bleeding of Peptic Ulcers. Gastroenterology. 2018;155:674-686.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 40. | Kamal F, Khan MA, Lee-Smith W, Sharma S, Imam Z, Henry C, Jowhar D, Khan Z, Petryna E, Iqbal U, Tombazzi C, Ismail MK, Howden CW. Role of routine second-look endoscopy in patients with acute peptic ulcer bleeding: meta-analysis of randomized controlled trials. Gastrointest Endosc. 2021;93:1228-1237.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |