Published online Oct 28, 2021. doi: 10.3748/wjg.v27.i40.6861
Peer-review started: February 22, 2021
First decision: May 13, 2021
Revised: May 30, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 28, 2021
Processing time: 246 Days and 14.5 Hours
Chronic viral hepatitis is one of the leading causes of cirrhosis worldwide. Chronic hepatitis B is more common in the Asia-Pacific region due to the larger population and lower screening availability. Hepatitis C predominates in the west due to injection drug abuse. The discovery of (oral) direct-acting antiviral agents (DAAs) has changed the landscape of chronic hepatitis C (CHC) management. Nucleos(t)ide analogs (NUCs) have also changed the approach to the treatment of chronic hepatitis B (CHB). Oral NUCs and DAAs have excellent efficacy and patient acceptance as well as a lower risk of resistance. However, certain popu
Core Tip: Hepatitis B and hepatitis C are leading causes of liver disease and pose significant burdens on healthcare and the economy, especially in developing countries. The management of chronic hepatitis B and C in special populations is less known. In this review, we discuss the indications, timing of treatment, and safety of drugs in special populations infected with hepatitis B or C. The special populations discussed herein are those with chronic kidney disease, pregnant women, coinfected patients, healthcare workers, and patients undergoing chemotherapy.
- Citation: Kulkarni AV, Duvvuru NR. Management of hepatitis B and C in special population. World J Gastroenterol 2021; 27(40): 6861-6873
- URL: https://www.wjgnet.com/1007-9327/full/v27/i40/6861.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i40.6861
Hepatitis B and C together constitute a major etiology of cirrhosis of the liver, especially in the Asia-Pacific region[1-3]. Nucleos(t)ide analogs and direct-acting antivirals (DAAs) are breakthrough treatments for chronic hepatitis B (CHB) and chronic hepatitis C (CHC), respectively. The management of Hepatitis B and C in an adult population without comorbidities is well known. Special population groups are those who are less often studied, and the drugs cannot be tested in such populations due to ethical reasons. The data on management strategies are still evolving for special populations. Such populations include patients with chronic kidney disease (CKD), patients on hemodialysis (HD), pregnant women, coinfected patients, healthcare workers, and patients undergoing chemotherapy[4]. In this review, we discuss the indications and safety of antiviral agents in special populations.
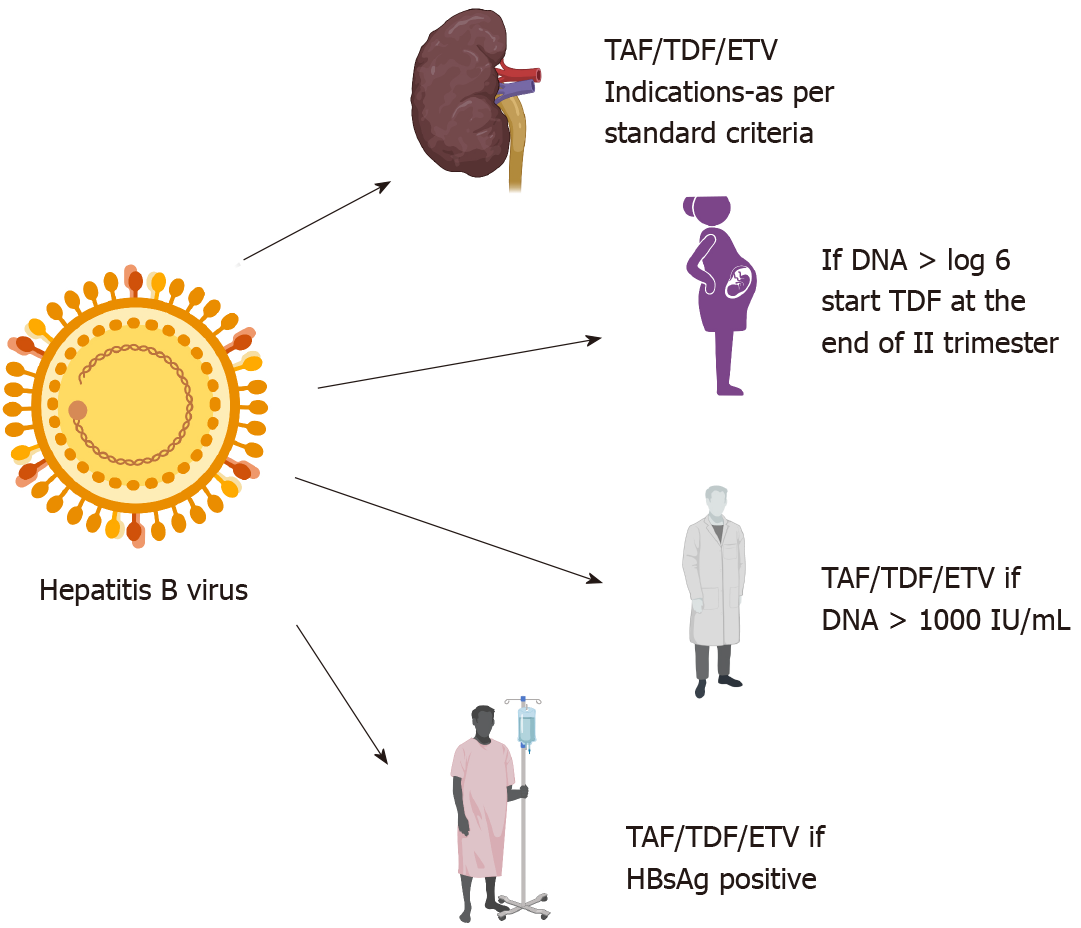
Hepatitis B is associated with proteinuria and a higher risk of CKD[5,6]. Hepatitis B virus (HBV)-infected treatment naïve patients have a higher incidence of hematuria, glycosuria, and leukocyturia[7]. Nearly 28%-40% of CHB patients have a glomerular filtration rate (GFR) < 90 mL/min/1.73 m2[7,8]. The prevalence of CKD in CHB patients is 3%-8%, with increasing prevalence with age[9]. This translates into an enormous burden of CKD due to the higher prevalence of CHB in the Asia-Pacific region. The presence of hypertension, diabetes mellitus, and cirrhosis further increases the risk of CKD in CHB[9]. Smoking and physical inactivity can also increase the risk of CKD[10]. The presence of CKD can also increase the risk of HBV infection due to immuno
| HBV and CKD | |
| Prevalence of CKD in HBV patients | 8% |
| Pathogenesis | Direct cytopathic effect of the HBV on cells of the kidney; Glomerular deposition of immune complexes; Virus-induced specific immunological effector mechanisms (specific T lymphocyte or antibody); CHB induced cytokine toxicity on renal tissue |
| Risk factors | Smoking, diabetes mellitus, hypertension, cirrhosis. |
| Common type of renal injury | Membranous GN; Membranoproliferative GN; Polyarteritis nodosa; IgA nephropathy |
| Treatment indication | HBV DNA 2000 IU/mL with or without elevated ALT; Liver biopsy-chronic hepatitis with > F1 fibrosis; If planned for renal transplant, initiate NUCs 2 wk before transplant even if DNA ≤ 2000 IU/mL |
| Safe drugs | TAF (no dose adjustment till eGFR < 15 mL); ETV and TDF (If GFR > 50: ETV 0.5 mg/d or TDF 300 mg/d; GFR 30-49: ETV 0.5 mg alternate day or TDF 300 mg alternate day; GFR 10-29: ETV 0.5 mg once in 3 d and TDF 300 mg once in 3 d; on HD-ETV 0.5 mg or TDF 300 mg after every dialysis or every 7 d) |
| Prevention | Regular screening; Vaccination (double dose); Serology should be performed every year, and a booster dose should be given if antibody titers are below 10 mIU/mL. |
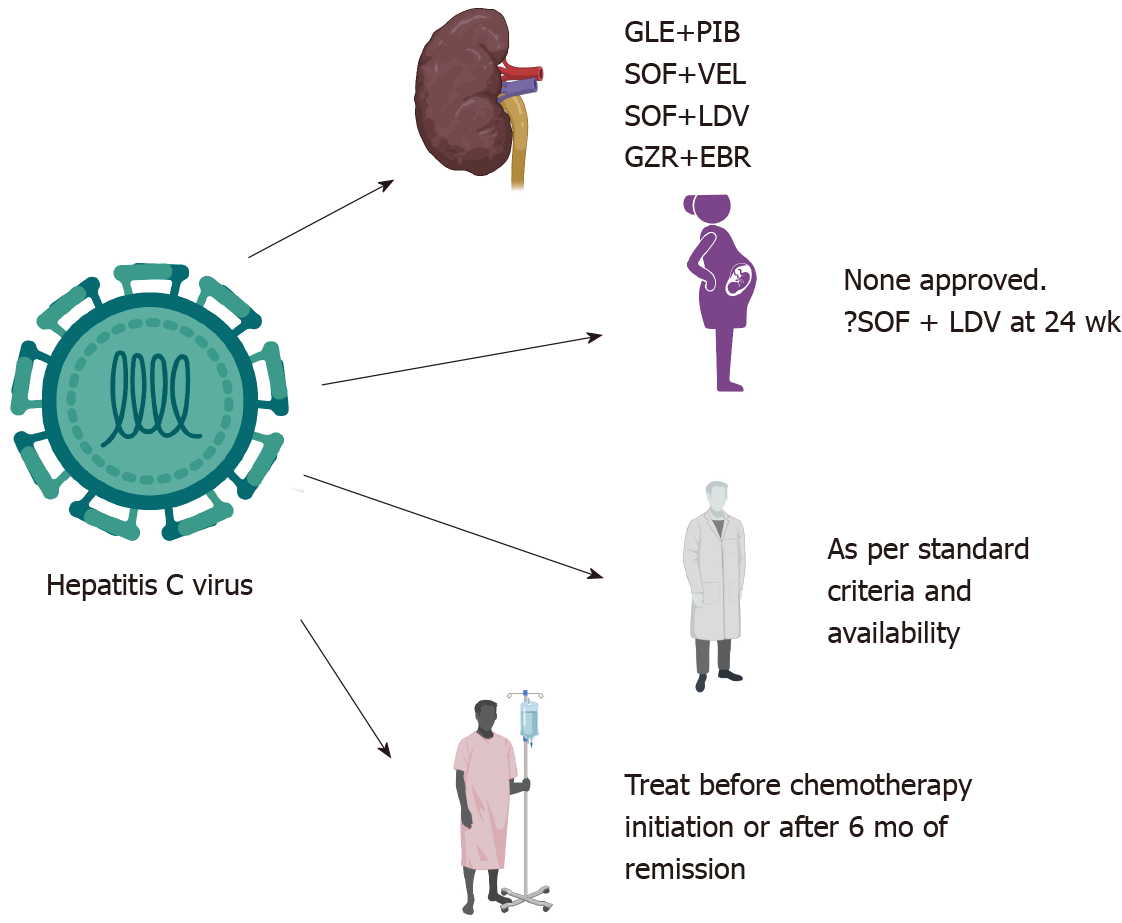
Extrahepatic manifestations are more common in hepatitis C virus (HCV) than HBV[14]. CHC patients may have renal failure even in the absence of liver disease[15]. HCV-infected individuals have a 23% higher risk of developing CKD than non-HCV-infected individuals[16]. Hepatitis C is a leading cause of liver disease among patients with CKD, particularly those on dialysis. The seroprevalence of HCV in the Asian population in patients on dialysis ranges between 1%-18%, with higher a prevalence in those on HD than in those on peritoneal dialysis, i.e., 8 ± 5.5%[17]. Due to a higher number of adverse effects, the use of pegylated IFN plus ribavirin is not recommended[18]. Glecaprevir and pibrentasvir are NS3/4A protease and NS5A inhibitors, respectively, which have pangenotypic activity. Glecapravir 300 mg and pibrentasvir 120 mg in a fixed-dose combination are the treatments of choice for patients with chronic hepatitis C and stage 4 or 5 CKD (including those on HD)[19,20]. In patients with stage 1-3 CKD, similar dosing of DAA without dose adjustments is recom
| HCV and CKD | |
| Prevalence of HCV in CKD patients | 10%-14% |
| Pathogenesis | Pronounced leucocyte infiltration of glomerular capillaries and the precipitation of immunoglobulins, immune complexes/cryoglobulins; Glomerular deposition of HCV protein |
| Risk factors | Age, male gender, lack of HCV treatment, concomitant HAV/HBV infection; Diabetes mellitus |
| Common types of renal injury | Membranous GN; Membranoproliferative GN; Essential mixed cryoglobulinemia (type II); IgA nephropathy; Polyarteritis nodosa |
| Treatment indication | Viremia |
| Safe drugs | Glecapravir + Pibrentasvir; Sofofbuvir + Velpatasvir; Sofosbuvir + Ledipasvir; Grazoprevir + Elbasvir |
| Prevention | Regular screening and strict infection control procedures; Effective dialysis machine decontamination |
Pregnancy is an opportunity to diagnose chronic viral hepatitis. Screening for hepatitis B and C is recommended for all individuals to prevent mother-to-child transmission (MTCT) and prevent transmission to health care professionals[29].
HBV and pregnancy: Nearly 10% of childbearing women in the Asia-Pacific region are infected with HBV[30]. There can be four situations in pregnancy: (1) First time incidentally diagnosed CVH during pregnancy; (2) Individuals are infected and are already on treatment with antiviral therapy; (3) Individuals are under surveillance for CVH and are contemplating pregnancy; and (4) Cirrhosis due to viral hepatitis.
The risk of transmission to the newborn is as high as 90% if the mother is HBeAg (e antigen)-positive and 10%-12% if the mother is HBeAg-negative and HBeAb (e antibody)-positive[31]. The risk of transmission is also directly proportional to viral load. The risk of progression to chronicity is nearly 90% if the newborn becomes infected[30]. Hence, preventing MTCT is of utmost importance. Screening for HBsAg is mandatory for all pregnant individuals in the first trimester. It is prudent to test HBeAg, HBeAb, and DNA levels by the end of the second trimester. Treatment is indicated if HBV DNA > 200000 IU/mL or HBsAg levels > 4 Log10 IU/mL. TDF can be started at 24-32 wk of gestation and must be continued for up to 12 wk after delivery[4]. Currently, TAF has no data to support its use during pregnancy. However, an initial review of data on TAF in pregnancy is encouraging[32]. If a woman is infected and is already on treatment with antivirals, the treatment must be continued. TDF is the recommended drug of choice during pregnancy, and if the individual is on ETV or any other antivirals, she should be switched to TDF[29]. If the individual is infected and is contemplating pregnancy, it is advised to postpone the treatment until childbirth unless the woman fulfills CHB treatment criteria (presence of advanced fibrosis or high viral load)[29]. Cirrhosis patients who wish to becomes pregnant should be counseled about the risk of decompensations during pregnancy. Pregnancy-induced volume disturbances are similar to cirrhosis, i.e., reduction in systemic vascular resistance and rise in blood volume and splanchnic vasodilation, which may exacerbate pre-existing portal hypertension[29]. Newborns of HBV-positive mothers should receive active and passive immunization. The newborn should be tested for HBsAb 3 mo after complete immunization (i.e., > 9 mo of age)[33]. Chronic HBV infection may not influence pregnancy outcomes; however, postpregnancy, there may be a flare of HBV due to immune restoration[29,30]. Breastfeeding is not contraindicated, and cesarean section is not indicated for HBV-infected mothers.
HCV and pregnancy: The prevalence of HCV infection is high in Western countries due to injection drug abuse; however, the Asia-Pacific region has a lower prevalence than the west. Due to the large population, the incidence of HCV is considered high in the Asia-Pacific region. The prevalence of HCV in the Asia-Pacific region is 0.1%-5%[34]. The lack of sensitive universal testing in developing countries is a major hindrance to diagnose HCV infection. Further transient elastography is not recommended in pregnant individuals to stage fibrosis.
HCV can affect pregnancy outcomes. HCV, a cytopathic virus, has been linked to intrahepatic cholestasis of pregnancy[35]. HCV can also downregulate multidrug resistance protein 2 (MRP2), which would induce a failure to transport toxic substances and subsequent defects in bile transport; high estrogen and progesterone levels would further compound this effect on MRP2 during pregnancy[35,36]. HCV-infected women also have a higher risk of preterm birth[37]. In contrast, pregnancy being immunosuppressed does not affect HCV. However, in the postpartum period, women may clear the virus spontaneously due to immune reconstitution and HCV-specific T-cell response development[36]. The pooled incidence of MTCT is 6%[38]. Concomitant human immunodeficiency virus (HIV) infection, high HCV viral load, and injection drug abusers (due to acute hepatitis) are at higher risk of transmitting the infection to the newborn[38,39]. MTCT occurs most often in late stages, either during intrauterine or intrapartum transmission[29]. There is no added benefit of cesarean section and hence is not indicated for patients with HCV infection to prevent transmission. Breastfeeding is not contraindicated[40]. Prolonged rupture of membranes (> 6 h) and invasive tests such as internal fetal monitoring, amniocentesis, and chorionic villous sampling have the potential risk of transmission[41]. Episiotomy should also be avoided, if possible, in HCV-infected pregnant individuals[41]. None of the DAAs have been approved to be used in pregnancy yet. DAAs are category B drugs in pregnancy. Sofosbuvir with ledipasvir has been shown to be effective and safe in pregnancy[42]. The drug was initiated in the 2nd or early third trimester. There are two trials underway assessing DAAs in pregnancy. The first trial evaluating sofosbuvir plus ledipasvir initiated at 24 wk of gestation (ClinicalTrials.gov Identifier: NCT02683005). Another trial assessing sofosbuvir plus velpatasvir commenced six months postpartum (ClinicalTrials.gov Identifier: NCT03570112). The results of these trials are expected soon. Differences in HBV and HCV management are shown in Table 3.
| HBV | HCV | |
| MTCT | 90% if HBeAg+; 10% if HBeAg-; Directly proportional to viral load | 6%; Higher risk with concomitant HIV infection, higher viral load, IV drug abuse; Higher risk with PROM and CVS |
| Treatment | TDF is safe; Can be initiated in third trimester | DAAs are not approved; Treat prior to pregnancy or 6 mo postpartum |
| Effect on pregnancy outcome | None | Preterm birth, ICP |
| Effect of pregnancy on virus | None | None |
| Effect of postpartum (immune restoration) on virus | Risk of HBV flares | Higher chance of viral clearance |
| Timing of transmission | Intrapartum > intrauterine | Intrapartum > intrauterine |
| C-section for all | Not indicated | Not indicated |
| Breastfeeding | Not contraindicated | Not contraindicated |
| Prevention | Active and passive immunization to child prevents 90% of transmission; Failure is nearly 15% if the viral load in mother is > log6 | None |
| Confirming the perinatal transmission | Persistence of HBsAg in newborn for > 6 mo | Anti-HCV positive at 18 mo of age HCV RNA positive after 2 mo on 2 different samples |
| Confirming the protection | Anti-HBs titers at 9 mo | Negative Anti-HCV at 18 mo |
Healthcare workers (HCWs) are attending clinicians, surgeons, employees, students, contractors, public-safety workers, or volunteers whose activities involve contact with patients or with blood or other body fluids from patients in healthcare, laboratory, or public-safety settings[43]. HCWs are at risk of becoming infected and can also be a potential source of transmission to patients. The risk of transmission is, although rare from providers to patients, it is recommended to treat HCWs even with a low viral load (HBV DNA < 1000 IU/mL)[44]. Prior to universal vaccination, the risk of transmission was 5%-13% from HBeAg-positive HCWs to patients and 0.8%-3.5% from HBeAg-negative HCWs to patients[45]. The prevalence of HBV among HCWs ranges between 5%-7% in developing countries[46,47]. The presence of HBV infection does not preclude the HCWs from practicing medicine (dentistry or surgery, or any allied health care work) as per the Centers for Disease control and Prevention (CDC) [44]. The European Association for the Study of the Liver recommends treating HCWs if DNA levels are more than 200 IU/mL[4]. The CDC recommends treating HCWs if DNA >1000 IU/mL and prohibits HCWs from performing exposure-prone procedures if DNA >1000 IU/mL[44]. In contrast, transmission of HCV from HCWs to patients is rare. HCWs are at higher risk of becoming infected by patients. A meta-analysis reported higher odds of infection among HCWs than controls[48]. HCWs with viremia should be treated.
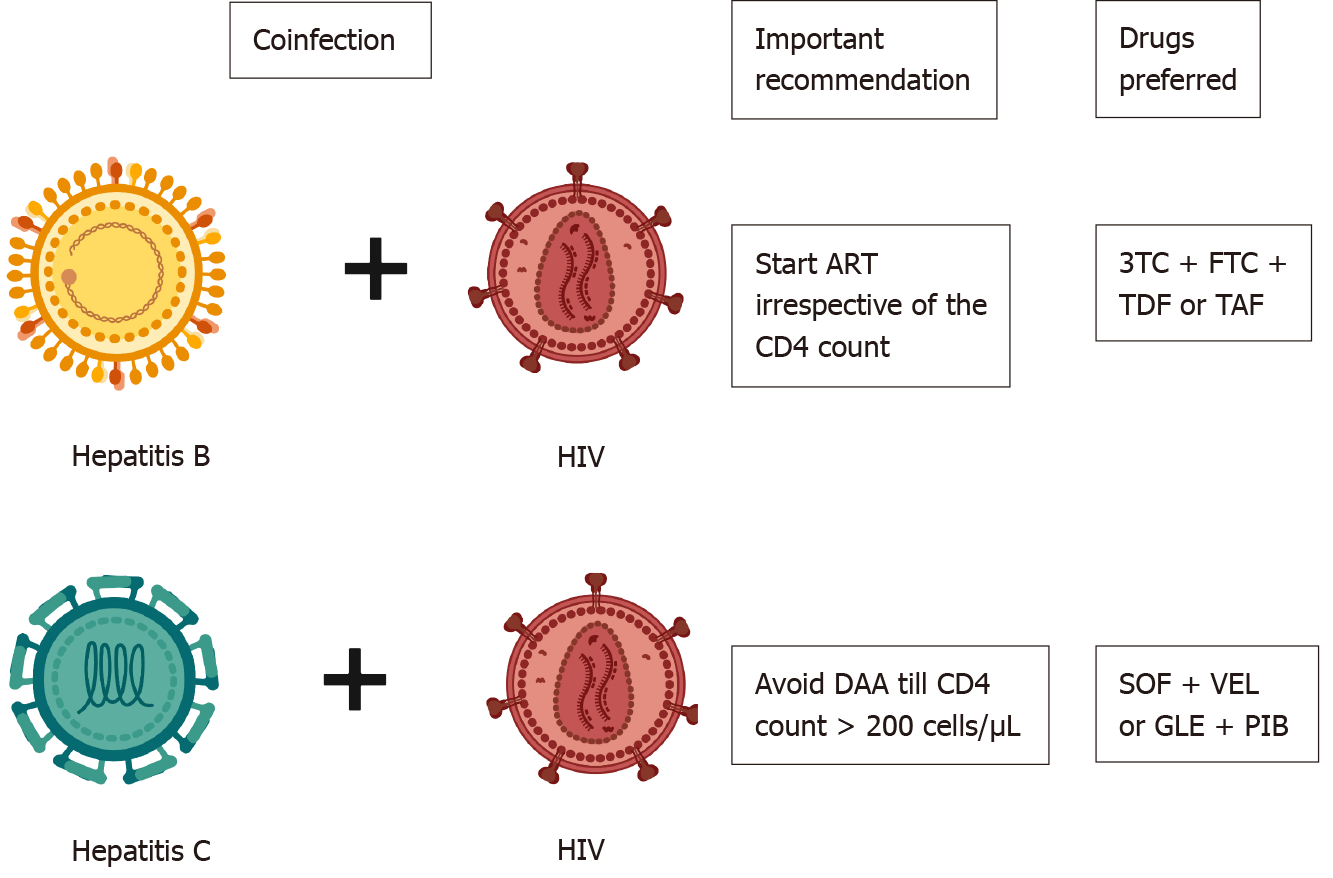
HBV with HIV: The prevalence of HBV HIV coinfection ranges between 5%-20%, with a higher prevalence among injection drug abusers[49]. The risk of liver-related mortality is twice as high in patients with coinfection as in those with HIV monoinfection[50]. HBV also increases overall mortality and hepatocellular carcinoma in HIV-infected patients[51,52]. Coinfected patients have higher levels of HBV viremia and lower rates of HBeAg clearance[53]. HIV-infected patients are prone to drug-induced liver injuries (especially from nevirapine-based regimens) and hepatitis flares[54]. HBV is reported to increase the risk of acquired immunodeficiency syndrome development, but this report was contraindicated in later studies[55]. All coinfected patients should be treated with ART (antiretroviral therapy) irrespective of the CD4 count[4,56]. ART should contain tenofovir as a part of the regimen. Lamivudine + emtricitabine and tenofovir (TDF or TAF) should be used for treating coinfected patients. TDF + lamivudine+ efavirenz or nevirapine combination has a lower attrition rate and is associated with lower mortality in HIV/HBV infected patients[57]. A fixed drug combination of elvitegravir, cobicistat, emtricitabine, and TAF has excellent efficacy in HIV/HBV coinfected patients[58]. Dolutegravir, emtricitabine and TDF/TAF fixed dose combinations may also be considered for patients with HIV/HBV coinfection[59]. All HBsAg patients should be screened for HIV infection prior to initiating tenofovir-based therapy (to prevent resistance), and HIV-infected patients should be screened for HBsAg prior to initiation of ART[56]. If negative, it is recommended to vaccinate the individual to achieve an anti-HBs of ≥ 10 mIU/mL.
HCV and HIV coinfection is common in injection drug abusers. HCV does not alter the natural history of HIV; however, HIV infection significantly alters the natural history of HCV. HIV leads to a rapid worsening of liver disease, shortens survival, and increases the risk of decompensations in HCV-related cirrhosis patients[60]. HIV also hastens fibrosis progression in CHC patients. Spontaneous clearance of HCV is noted in 5%-10% of patients with HIV infection, whereas nearly 15%-30% of HIV noninfected patients clear HCV spontaneously[61]. The indication for treating HCV and HIV is similar to those infected with either alone. However, it is recommended to avoid HCV treatment until the CD4 count > 200/μL in coinfected patients[62]. Sofosbuvir (400 mg) plus velpatasvir (100 mg) is the recommended drug of choice for HCV for 12 wk in cirrhosis and noncirrhosis[63]. Glecapravir and pibrentasvir are other options for HIV and HCV coinfected patients for 8 wk (no cirrhosis) or 12 wk (cirrhosis)[20] (Figure 1).
HBV with HCV coinfection: The prevalence of HBV/HCV coinfection varies across the globe. The prevalence of HBV coinfection in HCV-positive individuals ranges from 0.7%-5.8%, and the prevalence of HCV coinfection in HBsAg-positive individuals is between 3.4%-23.0%[64,65]. The presence of HCV coinfection leads to rapid progression of liver disease, fibrosis and accelerates the development of hepatocellular carcinoma (HCC)[65]. Patients with HCV viremia should be treated with DAAs, and those satisfying criteria for treatment for HBV should be treated with NUCs. However, if the patient is HBsAg-positive (not satisfying the treatment criteria for HBV) but requires DAA therapy for HCV, NUCs should be initiated[4]. NUCs should be continued for 12 wk post DAA to prevent reactivation of HBV[4]. HCV core protein strongly inhibits HBV replication, and post DAA lack of HCV core poses a risk for HBV reactivation. The incidence of HBV reactivation is around 12%-14% after HCV treatment[66].
Patients planned for chemotherapy or immunosuppression should be evaluated for HBV and HCV infection[4]. HBV and HCV are strongly associated with non-Hodgkin’s lymphoma[67-69]. Approximately 7%-23% of NHL patients have HBV infection, and 3%-10% harbor HCV infection[69-71]. The risk of HBV reactivation in HBsAg-positive patients undergoing chemotherapy ranges from 26%-53%[72]. HCV reactivation is noted in 11% of patients undergoing chemotherapy[73]. If the surface antigen is positive (irrespective of viremia), it is recommended to start NUCs (TDF, TAF, or ETV)[4,72]. Therapy should be continued for 12 mo (18 mo for those on rituximab) after the cessation of immunosuppressive therapy. The stopping rules for those satisfying the standard treatment criteria are the same as those not on immunosuppression.
Patients with HCV viremia should be treated with DAA therapy; however, the timing of therapy is still controversial. If malignancy treatment is deemed urgent, then DAA can be initiated six months after malignancy remission. However, the patient should be monitored for flares. If the malignancy is suspected to be related to the virus itself, then DAAs should be initiated, and viral suppression should be documented prior to chemotherapy initiation[74].
Chronicity in children is common and depends on the age of acquisition of infection. Perinatally infected patients have a 90% chance of progression to chronicity, while patients infected before 5 years of age have a 25%-50% chance of progression to chronicity. Data on the treatment of the pediatric population are limited. The global prevalence of HBsAg positivity of among children < 5 years of age is 1.3%[75]. The indication for treatment is slightly different from that in the adult population. The presence of cirrhosis (regardless of decompensation status) requires treatment[56]. However, it is recommended to assess the inflammation and stage of fibrosis via liver biopsy in all patients prior to therapy. Children with persistently elevated ALT levels (> 1.5 times the upper limit) for ≥ 6 mo who are HBeAg-positive (or ≥ 1 year for HBeAg-negative) with a DNA > 2000 IU/mL and biopsy evidence of moderate to severe necroinflammation or fibrosis require treatment[76]. For patients with a family history of HCC treatment should be initiated even if necroinflammation/fibrosis is mild or absent provided that DNA is > 2000 IU/mL and the ALT levels are elevated[56,76]. TAF (25 mg/d) and ETV (0.015 mg/kg; maximum of 0.5 mg/d) are drugs with high genetic barriers to resistance that are approved for patients aged ≥ 12 years and ≥ 2 years, respectively[75,77]. Last, interferon α-2b (6 million IU/m2 thrice weekly) is also approved for children aged ≥ 1 year[75]. For HBeAg-positive children, the drug can be stopped 1 year after HBeAg seroconversion[77]. However, the duration of therapy for HBeAg-negative patients is unknown.
HCV and Children: Nearly 0.15% of the global population aged < 18 years has HCV viremia, which corresponds to 3.26 million (2.07-3.9) children. The seropositivity ranges between 0.2% and 0.4% of the population aged < 18 years[78,79]. Interestingly, 25%-40% of children with perinatal transmission spontaneously achieve viral clearance, usually by age 2, and an additional 6%-12% of those with chronic hepatitis C infection may clear the virus before adulthood[80]. Hence, children born to HCV-positive mothers should undergo antibody testing after 18 mo of age[36]. If they are antibody-positive, then their HCV RNA should be assessed after the age of 3 years to confirm the chronicity of infection. The AASLD/IDSA approved drugs widely available are sofosbuvir/Ledipasvir for children aged ≥ 3 years and sofo
Although there are recommendations for each of the above conditions described, further research is required in many areas. The role of TAF in pregnancy is unknown. Since newborns have a higher risk of progression to chronicity if infected, is it prudent to treat all the pregnant mothers' pre-emptively needs to be assessed. Furthermore, in countries in which vertical transmission is the most common mode of infection and testing resources (or ability to provide passive immunization to newborns) are limited, is it beneficial to treat all pregnant women infected with HBV needs to be evaluated. There are some data on the safety of concomitant DAA therapy in patients undergoing chemotherapy without any adverse drug-drug interactions[81]. However, prospective trials are still lacking. The most important aspects of managing HBV and HCV in CKD, pregnant patients, HCWs, and immunosuppressed patients are depicted in Figure 2 and 3.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases, No. 173063.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ikegami T S-Editor: Wu YXJ L-Editor: A P-Editor: Wu RR
| 1. | Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, Jia J, Tian Q, Aggarwal R, Muljono DH, Omata M, Ooka Y, Han KH, Lee HW, Jafri W, Butt AS, Chong CH, Lim SG, Pwu RF, Chen DS. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2020;5:167-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 371] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 2. | Razavi H. Global Epidemiology of Viral Hepatitis. Gastroenterol Clin North Am. 2020;49:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 3. | Jefferies M, Rauff B, Rashid H, Lam T, Rafiq S. Update on global epidemiology of viral hepatitis and preventive strategies. World J Clin Cases. 2018;6:589-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 171] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (20)] |
| 4. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3801] [Article Influence: 475.1] [Reference Citation Analysis (1)] |
| 5. | Hong YS, Ryu S, Chang Y, Caínzos-Achirica M, Kwon MJ, Zhao D, Shafi T, Lazo M, Pastor-Barriuso R, Shin H, Cho J, Guallar E. Hepatitis B virus infection and development of chronic kidney disease: a cohort study. BMC Nephrol. 2018;19:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Du Y, Zhang S, Hu M, Wang Q, Liu N, Shen H, Zhang Y, Yan D, Zhang M. Association between hepatitis B virus infection and chronic kidney disease: A cross-sectional study from 3 million population aged 20 to 49 years in rural China. Medicine (Baltimore). 2019;98:e14262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Amet S, Bronowicki JP, Thabut D, Zoulim F, Bourliere M, Mathurin P, de Ledinghen V, Benhamou Y, Larrey DG, Janus N, Deray G, Launay-Vacher V, Pol S. Prevalence of renal abnormalities in chronic HBV infection: the HARPE study. Liver Int. 2015;35:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Ha NB, Ha NB, Garcia RT, Trinh HN, Vu AA, Nguyen HA, Nguyen KK, Levitt BS, Nguyen MH. Renal dysfunction in chronic hepatitis B patients treated with adefovir dipivoxil. Hepatology. 2009;50:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Ning L, Lin W, Hu X, Fan R, Liang X, Wu Y, Shen S, Yu R, Sun J, Hou J. Prevalence of chronic kidney disease in patients with chronic hepatitis B: A cross-sectional survey. J Viral Hepat. 2017;24:1043-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Si J, Yu C, Guo Y, Bian Z, Qin C, Yang L, Chen Y, Yin L, Li H, Lan J, Chen J, Chen Z, Lv J, Li L; China Kadoorie Biobank Collaborative Group. Chronic hepatitis B virus infection and risk of chronic kidney disease: a population-based prospective cohort study of 0.5 million Chinese adults. BMC Med. 2018;16:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Guidelines for vaccination in patients with chronic kidney disease. Indian J Nephrol. 2016;26:S15-18. |
| 13. | Cholongitas E, Tziomalos K, Pipili C. Management of patients with hepatitis B in special populations. World J Gastroenterol. 2015;21:1738-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Chacko EC, Surrun SK, Mubarack Sani TP, Pappachan JM. Chronic viral hepatitis and chronic kidney disease. Postgrad Med J. 2010;86:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Radhakrishnan J, Uppot RN, Colvin RB. Case records of the Massachusetts General Hospital. Case 5-2010. A 51-year-old man with HIV infection, proteinuria, and edema. N Engl J Med. 2010;362:636-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Park H, Adeyemi A, Henry L, Stepanova M, Younossi Z. A meta-analytic assessment of the risk of chronic kidney disease in patients with chronic hepatitis C virus infection. J Viral Hepat. 2015;22:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Johnson DW, Dent H, Yao Q, Tranaeus A, Huang CC, Han DS, Jha V, Wang T, Kawaguchi Y, Qian J. Frequencies of hepatitis B and C infections among haemodialysis and peritoneal dialysis patients in Asia-Pacific countries: analysis of registry data. Nephrol Dial Transplant. 2009;24:1598-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Gordon CE, Berenguer MC, Doss W, Fabrizi F, Izopet J, Jha V, Kamar N, Kasiske BL, Lai CL, Morales JM, Patel PR, Pol S, Silva MO, Balk EM, Earley A, Di M, Cheung M, Jadoul M, Martin P. Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C Virus Infection in Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2018 Clinical Practice Guideline. Ann Intern Med. 2019;171:496-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Bräu N, Brown A, Pol S, Leroy V, Persico M, Moreno C, Colombo M, Yoshida EM, Nelson DR, Collins C, Lei Y, Kosloski M, Mensa FJ. Glecaprevir and Pibrentasvir in Patients with HCV and Severe Renal Impairment. N Engl J Med. 2017;377:1448-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 306] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 20. | European Association for the Study of the Liver. Clinical Practice Guidelines Panel: Chair; EASL Governing Board representative; Panel members:. EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020;73:1170-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 779] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 21. | Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H Jr, Martin P, Pol S, Londoño MC, Hassanein T, Zamor PJ, Zuckerman E, Wan S, Jackson B, Nguyen BY, Robertson M, Barr E, Wahl J, Greaves W. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 533] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 22. | Cox-North P, Hawkins KL, Rossiter ST, Hawley MN, Bhattacharya R, Landis CS. Sofosbuvir-based regimens for the treatment of chronic hepatitis C in severe renal dysfunction. Hepatol Commun. 2017;1:248-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Borgia SM, Dearden J, Yoshida EM, Shafran SD, Brown A, Ben-Ari Z, Cramp ME, Cooper C, Foxton M, Rodriguez CF, Esteban R, Hyland R, Lu S, Kirby BJ, Meng A, Markova S, Dvory-Sobol H, Osinusi AO, Bruck R, Ampuero J, Ryder SD, Agarwal K, Fox R, Shaw D, Haider S, Willems B, Lurie Y, Calleja JL, Gane EJ. Sofosbuvir/velpatasvir for 12 weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J Hepatol. 2019;71:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 24. | Lawitz E, Landis CS, Flamm SL, Bonacini M, Ortiz-Lasanta G, Huang J, Zhang J, Kirby BJ, De-Oertel S, Hyland RH, Osinusi AO, Brainard DM, Robson R, Maliakkal BJ, Gordon SC, Gane EJ. Sofosbuvir plus ribavirin and sofosbuvir plus ledipasvir in patients with genotype 1 or 3 hepatitis C virus and severe renal impairment: a multicentre, phase 2b, non-randomised, open-label study. Lancet Gastroenterol Hepatol. 2020;5:918-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 991] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 26. | Nguyen DB, Bixler D, Patel PR. Transmission of hepatitis C virus in the dialysis setting and strategies for its prevention. Semin Dial. 2019;32:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Page K, Melia MT, Veenhuis RT, Winter M, Rousseau KE, Massaccesi G, Osburn WO, Forman M, Thomas E, Thornton K, Wagner K, Vassilev V, Lin L, Lum PJ, Giudice LC, Stein E, Asher A, Chang S, Gorman R, Ghany MG, Liang TJ, Wierzbicki MR, Scarselli E, Nicosia A, Folgori A, Capone S, Cox AL. Randomized Trial of a Vaccine Regimen to Prevent Chronic HCV Infection. N Engl J Med. 2021;384:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 28. | Roth D, Bloom RD, Molnar MZ, Reese PP, Sawinski D, Sise ME, Terrault NA. KDOQI US Commentary on the 2018 KDIGO Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C. Am J Kidney Dis. 2020;75:665-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Sarkar M, Brady CW, Fleckenstein J, Forde KA, Khungar V, Molleston JP, Afshar Y, Terrault NA. Reproductive Health and Liver Disease: Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:318-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 30. | Trehanpati N, Hissar S, Shrivastav S, Sarin SK. Immunological mechanisms of hepatitis B virus persistence in newborns. Indian J Med Res. 2013;138:700-710. [PubMed] |
| 31. | Borgia G, Carleo MA, Gaeta GB, Gentile I. Hepatitis B in pregnancy. World J Gastroenterol. 2012;18:4677-4683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 32. | Eke AC, Brooks KM, Gebreyohannes RD, Sheffield JS, Dooley KE, Mirochnick M. Tenofovir alafenamide use in pregnant and lactating women living with HIV. Expert Opin Drug Metab Toxicol. 2020;16:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Nelson NP, Jamieson DJ, Murphy TV. Prevention of Perinatal Hepatitis B Virus Transmission. J Pediatric Infect Dis Soc. 2014;3 Suppl 1:S7-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Lim SG, Aghemo A, Chen PJ, Dan YY, Gane E, Gani R, Gish RG, Guan R, Jia JD, Lim K, Piratvisuth T, Shah S, Shiffman ML, Tacke F, Tan SS, Tanwandee T, Win KM, Yurdaydin C. Management of hepatitis C virus infection in the Asia-Pacific region: an update. Lancet Gastroenterol Hepatol. 2017;2:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Wijarnpreecha K, Thongprayoon C, Sanguankeo A, Upala S, Ungprasert P, Cheungpasitporn W. Hepatitis C infection and intrahepatic cholestasis of pregnancy: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Kushner T, Terrault NA. Hepatitis C in Pregnancy: A Unique Opportunity to Improve the Hepatitis C Cascade of Care. Hepatol Commun. 2019;3:20-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 37. | Huang QT, Huang Q, Zhong M, Wei SS, Luo W, Li F, Yu YH. Chronic hepatitis C virus infection is associated with increased risk of preterm birth: a meta-analysis of observational studies. J Viral Hepat. 2015;22:1033-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 39. | Syriopoulou V, Nikolopoulou G, Daikos GL, Theodoridou M, Pavlopoulou I, Nicolaidou P, Manolaki N. Mother to child transmission of hepatitis C virus: rate of infection and risk factors. Scand J Infect Dis. 2005;37:350-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Cottrell EB, Chou R, Wasson N, Rahman B, Guise JM. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 41. | Society for Maternal-Fetal Medicine (SMFM), Hughes BL, Pag CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 42. | Yattoo N. Treatment of chronic hepatitis C with ledipasvir/sofosbuvir combination during pregnancy. Hepatol Int. 2018;12:S292-S293. |
| 43. | Singhal V, Bora D, Singh S. Hepatitis B in health care workers: Indian scenario. J Lab Physicians. 2009;1:41-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Centers for Disease Control and Prevention (CDC). Updated CDC recommendations for the management of hepatitis B virus-infected health-care providers and students. MMWR Recomm Rep. 2012;61:1-12. [PubMed] |
| 45. | Gerlich WH. Reduction of infectivity in chronic hepatitis B virus carriers among healthcare providers and pregnant women by antiviral therapy. Intervirology. 2014;57:202-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Ganju SA, Goel A. Prevalence of HBV and HCV infection among health care workers (HCWs). J Commun Dis. 2000;32:228-230. [PubMed] |
| 47. | Mueller A, Stoetter L, Kalluvya S, Stich A, Majinge C, Weissbrich B, Kasang C. Prevalence of hepatitis B virus infection among health care workers in a tertiary hospital in Tanzania. BMC Infect Dis. 2015;15:386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 48. | Westermann C, Peters C, Lisiak B, Lamberti M, Nienhaus A. The prevalence of hepatitis C among healthcare workers: a systematic review and meta-analysis. Occup Environ Med. 2015;72:880-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS. 2017;31:2035-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 50. | van Griensven J, Phirum L, Choun K, Thai S, De Weggheleire A, Lynen L. Hepatitis B and C co-infection among HIV-infected adults while on antiretroviral treatment: long-term survival, CD4 cell count recovery and antiretroviral toxicity in Cambodia. PLoS One. 2014;9:e88552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 52. | Pinchoff J, Tran OC, Chen L, Bornschlegel K, Drobnik A, Kersanske L, Fuld J. Impact of hepatitis B on mortality and specific causes of death in adults with and without HIV co-infection in NYC, 2000-2011. Epidemiol Infect. 2016;144:3354-3364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Thio CL. Hepatitis B in the human immunodeficiency virus-infected patient: epidemiology, natural history, and treatment. Semin Liver Dis. 2003;23:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Yang R, Gui X, Xiong Y, Gao SC, Yan Y. Impact of hepatitis B virus infection on HIV response to antiretroviral therapy in a Chinese antiretroviral therapy center. Int J Infect Dis. 2014;28:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Rockstroh JK. Influence of viral hepatitis on HIV infection. J Hepatol. 2006;44:S25-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1959] [Article Influence: 217.7] [Reference Citation Analysis (0)] |
| 57. | Zhu J, Yang W, Feng Y, Lo C, Chen H, Zhu Q, Shen Z, Lan G, Chen Y, Tang Z, Xing H, Shao Y, Ruan Y, Li L. Treatment effects of the differential first-line antiretroviral regimens among HIV/HBV coinfected patients in southwest China: an observational study. Sci Rep. 2019;9:1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Gallant J, Brunetta J, Crofoot G, Benson P, Mills A, Brinson C, Oka S, Cheng A, Garner W, Fordyce M, Das M, McCallister S; GS-US-292-1249 Study Investigators. Brief Report: Efficacy and Safety of Switching to a Single-Tablet Regimen of Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide in HIV-1/Hepatitis B-Coinfected Adults. J Acquir Immune Defic Syndr. 2016;73:294-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, Masenya M, Serenata C, Akpomiemie G, Qavi A, Chandiwana N, Norris S, Chersich M, Clayden P, Abrams E, Arulappan N, Vos A, McCann K, Simmons B, Hill A. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N Engl J Med. 2019;381:803-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 504] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 60. | Pineda JA, Romero-Gómez M, Díaz-García F, Girón-González JA, Montero JL, Torre-Cisneros J, Andrade RJ, González-Serrano M, Aguilar J, Aguilar-Guisado M, Navarro JM, Salmerón J, Caballero-Granado FJ, García-García JA; Grupo Andaluz para el Estudio de las Enfermedades Infecciosas; Grupo Andaluz para el Estudio del Hígado. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 211] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 61. | Thomas DL. Hepatitis C and human immunodeficiency virus infection. Hepatology. 2002;36:S201-S209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Omata M, Kanda T, Wei L, Yu ML, Chuang WL, Ibrahim A, Lesmana CR, Sollano J, Kumar M, Jindal A, Sharma BC, Hamid SS, Dokmeci AK, Mamun-Al-Mahtab, McCaughan GW, Wasim J, Crawford DH, Kao JH, Yokosuka O, Lau GK, Sarin SK. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int. 2016;10:702-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 63. | Wyles D, Bräu N, Kottilil S, Daar ES, Ruane P, Workowski K, Luetkemeyer A, Adeyemi O, Kim AY, Doehle B, Huang KC, Mogalian E, Osinusi A, McNally J, Brainard DM, McHutchison JG, Naggie S, Sulkowski M; ASTRAL-5 Investigators. Sofosbuvir and Velpatasvir for the Treatment of Hepatitis C Virus in Patients Coinfected With Human Immunodeficiency Virus Type 1: An Open-Label, Phase 3 Study. Clin Infect Dis. 2017;65:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 64. | Holmes JA, Yu ML, Chung RT. Hepatitis B reactivation during or after direct acting antiviral therapy - implication for susceptible individuals. Expert Opin Drug Saf. 2017;16:651-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 65. | Caccamo G, Saffioti F, Raimondo G. Hepatitis B virus and hepatitis C virus dual infection. World J Gastroenterol. 2014;20:14559-14567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 66. | Chen G, Wang C, Chen J, Ji D, Wang Y, Wu V, Karlberg J, Lau G. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: A systematic review and meta-analysis. Hepatology. 2017;66:13-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 67. | Li M, Gan Y, Fan C, Yuan H, Zhang X, Shen Y, Wang Q, Meng Z, Xu D, Tu H. Hepatitis B virus and risk of non-Hodgkin lymphoma: An updated meta-analysis of 58 studies. J Viral Hepat. 2018;25:894-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 68. | Rattotti S, Ferretti VV, Rusconi C, Rossi A, Fogazzi S, Baldini L, Pioltelli P, Balzarotti M, Farina L, Ferreri AJM, Laszlo D, Speziale V, Varettoni M, Sciarra R, Morello L, Tedeschi A, Frigeni M, Defrancesco I, Zerbi C, Flospergher E, Nizzoli ME, Morra E, Arcaini L; “Rete Ematologica Lombarda” (REL - Hematology Clinical Network of Lombardy - Lymphoma Workgroup). Lymphomas associated with chronic hepatitis C virus infection: A prospective multicenter cohort study from the Rete Ematologica Lombarda (REL) clinical network. Hematol Oncol. 2019;37:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Kang J, Cho JH, Suh CW, Lee DH, Oh HB, Sohn YH, Chi HS, Park CJ, Jang SS, Lee KH, Lee JH, Lee SW, Chung YH, Kim TH, Shin HR, Huh J. High prevalence of hepatitis B and hepatitis C virus infections in Korean patients with hematopoietic malignancies. Ann Hematol. 2011;90:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 70. | Takai S, Tsurumi H, Ando K, Kasahara S, Sawada M, Yamada T, Hara T, Fukuno K, Takahashi T, Oyama M, Onishi H, Tomita E, Takami T, Imawari M, Moriwaki H. Prevalence of hepatitis B and C virus infection in haematological malignancies and liver injury following chemotherapy. Eur J Haematol. 2005;74:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 71. | Chen MH, Hsiao LT, Chiou TJ, Liu JH, Gau JP, Teng HW, Wang WS, Chao TC, Yen CC, Chen PM. High prevalence of occult hepatitis B virus infection in patients with B cell non-Hodgkin's lymphoma. Ann Hematol. 2008;87:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 72. | Huang H, Li X, Zhu J, Ye S, Zhang H, Wang W, Wu X, Peng J, Xu B, Lin Y, Cao Y, Li H, Lin S, Liu Q, Lin T. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA. 2014;312:2521-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 73. | Mahale P, Kontoyiannis DP, Chemaly RF, Jiang Y, Hwang JP, Davila M, Torres HA. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol. 2012;57:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 74. | Torres HA, McDonald GB. How I treat hepatitis C virus infection in patients with hematologic malignancies. Blood. 2016;128:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Indolfi G, Easterbrook P, Dusheiko G, Siberry G, Chang MH, Thorne C, Bulterys M, Chan PL, El-Sayed MH, Giaquinto C, Jonas MM, Meyers T, Walsh N, Wirth S, Penazzato M. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019;4:466-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 76. | Sokal EM, Paganelli M, Wirth S, Socha P, Vajro P, Lacaille F, Kelly D, Mieli-Vergani G; European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Management of chronic hepatitis B in childhood: ESPGHAN clinical practice guidelines: consensus of an expert panel on behalf of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Hepatol. 2013;59:814-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 77. | Lai MW, Chang MH. Updates in the management of hepatitis B in children. Expert Rev Gastroenterol Hepatol. 2019;13:1065-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Schmelzer J, Dugan E, Blach S, Coleman S, Cai Z, DePaola M, Estes C, Gamkrelidze I, Jerabek K, Ma S, Montoya S, Razavi-Shearer D, Razavi-Shearer K, Robbins-Scott S, Razavi H, El Sayed MH. Global prevalence of hepatitis C virus in children in 2018: a modelling study. Lancet Gastroenterol Hepatol. 2020;5:374-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 79. | Pawlowska M, Sobolewska-Pilarczyk M, Domagalski K. Hepatitis C virus infection in children in the era of direct-acting antiviral. World J Gastroenterol. 2018;24:2555-2566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Squires JE, Balistreri WF. Hepatitis C virus infection in children and adolescents. Hepatol Commun. 2017;1:87-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 81. | Economides MP, Mahale P, Kyvernitakis A, Turturro F, Kantarjian H, Naing A, Hosry J, Shigle TL, Kaseb A, Torres HA. Concomitant use of direct-acting antivirals and chemotherapy in hepatitis C virus-infected patients with cancer. Aliment Pharmacol Ther. 2016;44:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |