Published online Jan 28, 2021. doi: 10.3748/wjg.v27.i4.358
Peer-review started: November 22, 2020
First decision: November 30, 2020
Revised: December 11, 2020
Accepted: December 28, 2020
Article in press: December 28, 2020
Published online: January 28, 2021
Processing time: 64 Days and 1.6 Hours
A previous study showed that irrigation with 100 mL saline reduced residual common bile duct (CBD) stones, which potentially cause recurrent stones after endoscopic retrograde cholangiopancreatography.
To determine whether saline irrigation can improve CBD clearance after lithotripsy.
This prospective self-controlled study enrolled patients receiving mechanical lithotripsy for large (> 1.2 cm) CBD stones. After occlusion cholangiography confirmed CBD stone clearance, peroral cholangioscopy (POC) was performed to determine clearance scores based on the number of residual stones. The amounts of residual stones spotted via POC were graded on a 5-point scale (score 1, worst; score 5, best). Scores were documented after only stone removal (control) and after irrigation with 50 mL and 100 mL saline, respectively. The stone composition was analyzed using infrared spectroscopy.
Between October 2018 and January 2020, 47 patients had CBD clearance scores of 2.4 ± 1.1 without saline irrigation, 3.5 ± 0.7 with 50 mL irrigation, and 4.6 ± 0.6 with 100 mL irrigation (P < 0.001). Multivariate analysis showed that CBD diameter > 15 mm [odds ratio (OR) = 0.08, 95% confidence interval (CI): 0.01-0.49; P = 0.007] and periampullary diverticula (PAD) (OR = 6.51, 95%CI: 1.08-39.21; P = 0.041) were independent risk factors for residual stones. Bilirubin pigment stones constituted the main residual stones found in patients with PAD (P = 0.004).
Irrigation with 100 mL of saline may not clear all residual CBD stones after lithotripsy, especially in patients with PAD and/or a dilated (> 15 mm) CBD. Pigment residual stones are soft and commonly found in patients with PAD. Additional saline irrigation may be required to remove retained stones.
Core Tip: This is a prospective self-controlled study with 47 patients seeking to determine whether saline irrigation can also improve common bile duct (CBD) clearance after mechanical lithotripsy. Irrigation with 100 mL of saline may not clear all residual CBD stones after mechanical lithotripsy, especially in patients with periampullary diverticula (PAD) and/or a dilated (> 15 mm) CBD. Pigment residual stones are soft and commonly found in patients with PAD. Additional saline irrigation may be required to remove the retained stones.
- Citation: Lin YY, Wang YD, Yue P, Zhang XZ, Leung JW, Jiao PP, Yang M, Wang HP, Bai B, Liu Y, Zhang JD, Chen HB, Meng WB, Li X. Could saline irrigation clear all residual common bile duct stones after lithotripsy? A self-controlled prospective cohort study. World J Gastroenterol 2021; 27(4): 358-370
- URL: https://www.wjgnet.com/1007-9327/full/v27/i4/358.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i4.358
Endoscopic retrograde cholangiopancreatography (ERCP) is an effective and relatively minimally invasive technique for common bile duct (CBD) stones[1-3]. It has been reported that the recurrence rate of CBD stones after ERCP has increased from 4% to 24%[4-6]. The incidence of residual stones after mechanical lithotripsy for intractable CBD stones is 24% to 40%[7-10]. A growing number of studies suggest that an important reason for the recurrence of bile duct stones is the presence of stone debris after lithotripsy[11-13]. During ERCP, occluded cholangiography (OC) is often performed after stone removal to determine whether the stone is removed completely, but OC lacks accuracy. Even if no obvious stones are found on cholangiography, the presence of contrast can obscure small stone debris in the bile duct[8,14]. Complete bile duct clearance is necessary to decrease recurrent bile duct stones.
Some studies[15-17] reported that irrigation of the bile duct with saline after stone extraction further improves the clearance of the bile duct and has the advantages of being a simple, low-cost procedure with rare complications. Ang et al[16] showed that a mean of 48 mL of saline solution could irrigate and flush out residual stones after the endoscopic removal of CBD stones. Ahn et al[17] found that irrigation with 100 mL of saline can flush out residual stone fragments from the bile duct into the duodenum after stone extraction. However, intraductal ultrasound (IDUS) has a high sensitivity and accuracy in diagnosing bile duct stones/debris[11,16]. This modality yields only indirect images of the debris.
The lack of direct evidence to support the efficacy of saline irrigation after lithotripsy prompted us to use peroral cholangioscopy (POC) to examine the bile duct and detect any residual stones/debris. The results of no irrigation after stone extraction were confirmed by OC and were compared to the effectiveness of irrigation with 50 mL or 100 mL saline. To evaluate whether irrigation with 100 mL of saline is more effective in achieving complete clearance of the bile duct after mechanical lithotripsy, we conducted this prospective self-controlled study.
Between October 2018 and January 2020, 47 patients with CBD stones were enrolled in a prospective clinical trial conducted in the surgical endoscopy center of The First Hospital of Lanzhou University (Figure 1). All eligible patients or their legal representatives gave informed consent before the treatment. The inclusion criteria were patients with CBD stones undergoing ERCP, able to provide informed consent, with a stone size larger than 1.2 cm and requiring mechanical lithotripsy for stone removal. The exclusion criteria included pre-ERCP acute suppurative cholangitis, acute pancreatitis, gastrointestinal (GI) tract hemorrhage and/or perforation, previous history of ERCP, prior Bilroth II gastrectomy and Roux-en-Y or cholangiojejunostomy, pregnancy or breastfeeding, coagulopathy with international normalized ratio > 1.5 and low platelet count (< 50 × 109/L) or active use of anticoagulation drugs, severe liver disease including decompensated liver cirrhosis or liver failure, septic shock, biliary-duodenal fistula confirmed before ERCP cannulation, the presence of intrahepatic duct stones and malignancy, and patient unwillingness or inability to give informed consent.
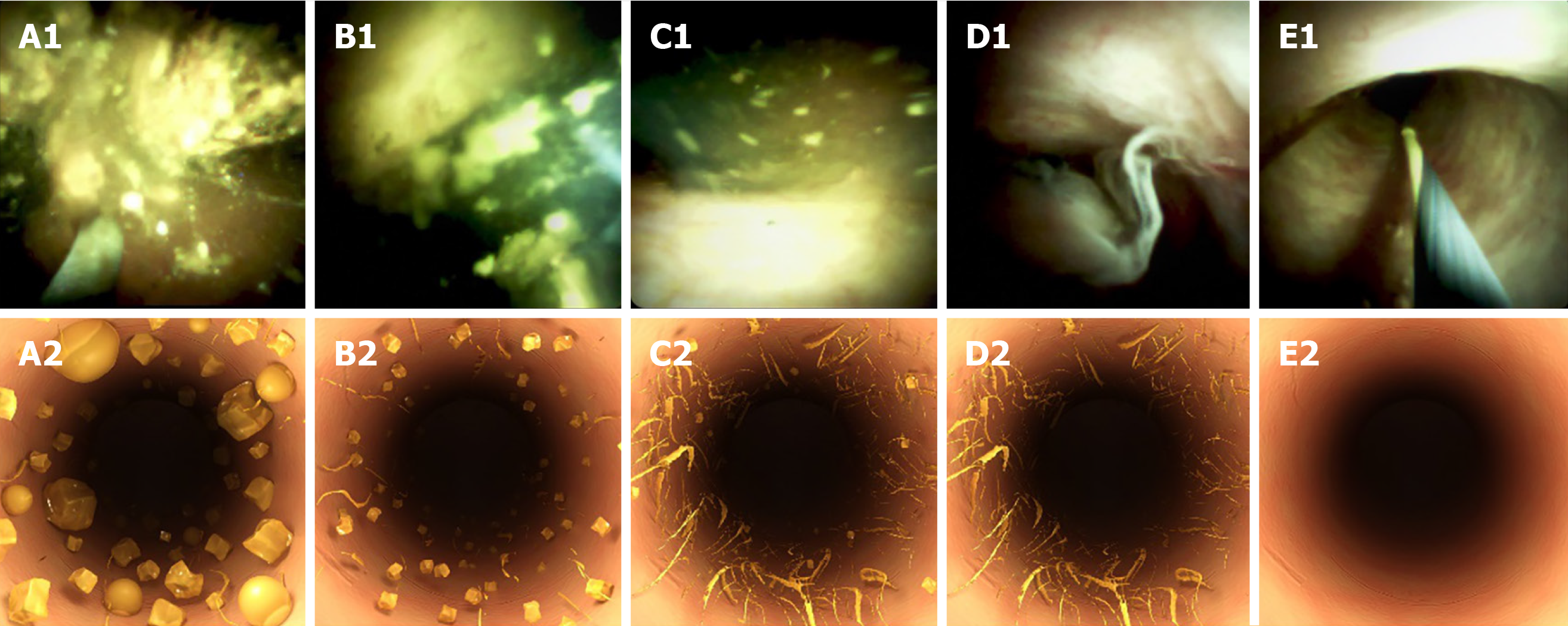
To assess the number of residual stones, we created a CBD clearance score as follows: (1) A large number of stone fragments and biliary sludge; (2) A moderate number of stone fragments and biliary sludge; (3) A small number of stone fragments and biliary sludge; (4) Presence of biliary sludge without any stones; and (5) Completely cleared CBD without any biliary sludge. The scores were determined by two endoscopists independently (Figure 2).
The study was approved by the ethics committee of The First Hospital of Lanzhou University (No. LDYYMENG2018-1028) and conducted in accordance with the ethical principles of the Declaration of Helsinki. All authors had access to the study data and reviewed and approved the final manuscript.
All endoscopic procedures were performed by two experienced endoscopists (each has performed > 1000 ERCPs). Routine prophylactic antibiotics were given in all cases. ERCP was performed with a standard duodenoscope (TJF-260V, Olympus, Tokyo, Japan). The patients received propofol anesthesia without tracheal intubation.
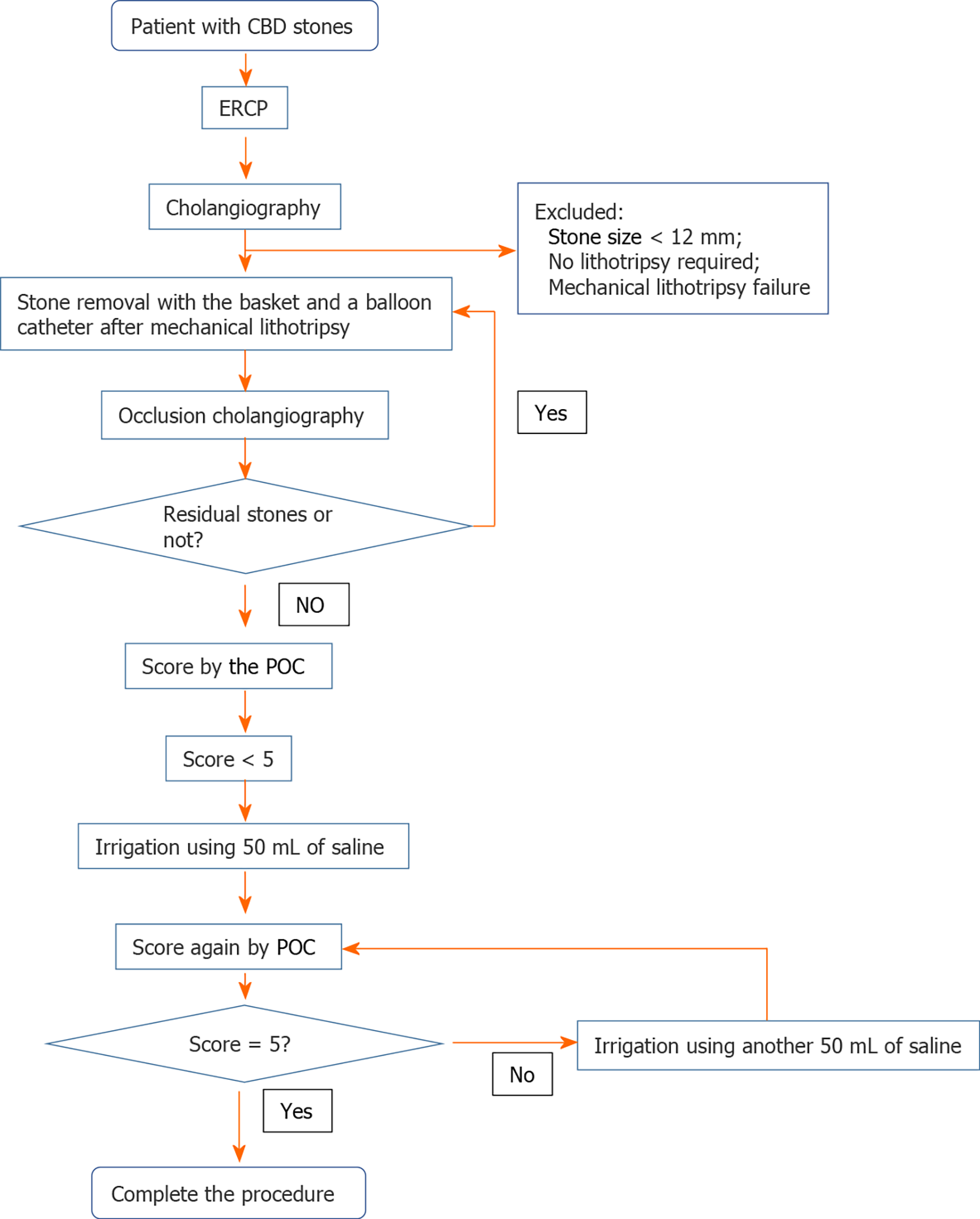
During ERCP, cannulation was performed with a wire-guided sphincterotome. After successful cannulation, contrast was injected to determine the stone size (more or less than 12 mm) and the need for mechanical lithotripsy. All patients underwent a small sphincterotomy with an average length of 3-5 mm using the ENDO CUT mode (power setting 90-120 W; Erbe Elektromedizin, Tuebingen, Germany) followed by balloon sphincteroplasty using a controlled radial expansion (CRE) balloon (10-12 mm in diameter; Boston Scientific, Cork, Ireland). Lithotripsy was performed using an endoscopic lithotripter-compatible basket (Boston Scientific, Marlborough, MA, United States), and stones were removed using the basket and a balloon catheter. Samples of CBD stones were collected using an EndoRetrieval bag (Micro-Tech, Nanjing, China) and placed in a container for subsequent analysis. Occlusion cholangiography was performed by injecting contrast with a balloon catheter. Fluoroscopic assessment using a C-arm X-ray (OEC 9900 Elite, Salt Lake City, Utah, United States) was applied to confirm complete removal of CBD stones and the existence of stone residuals was determined by both endoscopist and radiologist. Confirmed stone residues by applying occlusion cholangiography were then followed by repeated extraction attempts. If complete stone removal was confirmed, a CBD clearance score was generated by further applying SpyGlass DS (Boston Scientific Corporation, Marlborough, Massachusetts, United States) examination. If the CBD clearance score was less than 5, the bile duct was irrigated intermittently with 50 mL of normal saline using a basket. The basket was shaken during irrigation with slight suction applied to the duodenoscope to promote drainage. The bile duct was then examined again using the SpyGlass DS to detect any residual stones/sludge and document the clearance score. If the clearance score was still less than 5, repeat irrigation of the CBD was performed using another 50 mL of normal saline. The final CBD clearance score was obtained one more time using SpyGlass DS examination. CBD stones that were not irrigated out after double 50 mL-saline irrigations continued to be irrigated with saline until they were fully cleared (Figure 3). Endoscopic nasobiliary drainage (ENBD) or biliary stenting was performed if necessary. The CBD clearance score was assessed by two blinded endoscopists who were masked to the treatment.
The composition of the removed CBD stones was analyzed using infrared ray (IR) spectroscopy. One milligram (mg) of stone samples was mixed with 150 mg of potassium bromide in a mortar and ground into powder. The stone mixture was pressed into a mold and then placed into an automatic IR spectrum analyzer for automated detection to determine the IR spectrogram of the CBD stones. The IR spectrogram showed characteristic absorption peaks at a wavelength of 1460 cm-1, indicating cholesterol-based stones, and those at a wavelength of 1680 cm-1 indicated pigment-based stones.
Most of the post-ERCP adverse events were detected within 24 h after the procedure. We routinely assessed these adverse events, including cholangitis, oozing/bleeding, pancreatitis, cholecystitis, and perforation, at 24 and 48 h after the ERCP procedure by symptoms, signs, laboratory tests, and imaging examinations if necessary[18,19]. Post-ERCP adverse events were defined as follows: (1) The diagnosis of acute cholangitis was based on Tokyo Guidelines 2018/2013 (TG18/TG13) diagnostic criteria[20]; (2) Oozing was defined as bleeding that was slight and stopped spontaneously; (3) Pancreatitis was described as new or worsening abdominal pain along with an increase in serum amylase levels (> 3 × higher than normal upper limit measured 24 h after surgery); (4) Cholecystitis was defined as pain in the epigastrium or RUQ accompanied by a positive Murphy sign, and abdominal ultrasonography showing a thickened gallbladder wall; and (5) Perforation was defined as sudden abdominal pain accompanied by retroperitoneal air and fluid.
Mechanical lithotripsy was performed by the same assistant for all patients. Stones that were fragmented successfully with only one attempt were defined as soft stones. Stones that required multiple fragmentation attempts or needed to be broken again were defined as hard stones.
The sample size calculation was based on the rate of residual stone clearance. According to a previous study[16] and our prior experience, we assumed a success rate of 84.5% for endoscopic extraction of CBD stones and an increase of up to 97% with saline irrigation. We estimated that 47 patients were needed in this analysis to obtain a power of 80% at the 5% level.
Categorical variables are expressed as numbers or percentages (%). Continuous variables are presented as the mean ± SD or median and interquartile range, as appropriate. Continuous variables with a normal distribution were analyzed by paired t-test or signed-rank test. Categorical variables were analyzed using the chi-square test or Fisher’s exact test. Logistic regression was used to predict risk factors for complications, and the results are presented as odds ratios (ORs) with 95% confidence intervals (Cis). Variables with a P value of < 0.2 in the univariate analysis were included in the multivariate analysis. Linear mixed-effects models were conducted to assess the effect of saline irrigation on the clearance score with a random intercept for each patient and an unstructured covariance structure. A two-sided P value of less than 0.05 was considered statistically significant. The analyses were conducted with statistical software (SAS version 9.4, SAS Institute, Inc., NC, United States).
The patients' average age was 61 ± 16.5 years, and 23 (48.9%) were male. Comorbidities included coronary disease in three (6.4%) patients, hypertension in 14 (29.8%), diabetes in two (4.3%), liver cirrhosis in three (2.1%), and portal hypertension in one (6.4%). The stone analysis showed that 17 (36.2%) cases had cholesterol-based stones, and 30 (63.8%) had pigment-based stones.
Procedure-related adverse events occurred in 11 (23.4%) of 47 patients, with cholangitis in four patients, bleeding in two, pancreatitis in four, and cholecystitis in one. No mortality or perforation occurred. The mean time for ERCP was 40.3 ± 15.4 min (Table 1).
| Patients | n = 47 |
| Age (yr, mean ± SD) | 61 ± 16.5 |
| Male | 23 (48.9%) |
| Stone size (mm) | 14 (14-15) |
| Multiple CBD stones | 36 (76.6%) |
| Diameter of CBD (mm) | 15 (12-18) |
| Gallbladder stones | 21 (44.7%) |
| Previous cholecystectomy | 24 (51.1%) |
| Periampullary diverticulum | 15 (31.9%) |
| Total bilirubin (µmol/L) | 71.1 (64.9-107.9) |
| Comorbidities | |
| Coronary disease | 3 (6.4%) |
| Hypertension | 14 (29.8%) |
| Diabetes | 2 (4.3%) |
| Liver cirrhosis | 3 (2.1%) |
| Portal hypertension | 1 (6.4%) |
| ENBD | 40 (85.1%) |
| Procedure time, min (mean ± SD) | 40.3 ± 15.4 |
| Stone texture | |
| Hard stones | 16 (34.0%) |
| Soft stones | 31 (66.0%) |
| Stone compositions | |
| Cholesterol-based stones | 17 (36.2%) |
| Pigment-based stones | 30 (63.8%) |
| Procedure-related adverse events | |
| Cholangitis | 4 (8.5%) |
| Oozing | 2 (4.3%) |
| Pancreatitis | 4 (8.5%) |
| Cholecystitis | 1 (2.1%) |
| Perforation | 0 |
| Death | 0 |
After endoscopic stone extraction, occlusion cholangiography showed that there were no residual CBD stones. Seven patients had a clearance score of 4 identified by POC with the SpyGlass DS, and no patients had a score of 5 before saline irrigation. After irrigation with 50 mL saline, CBD clearance improved to 4 in 28 patients, but none achieved a score of 5. After a total of 100 mL of saline irrigation, there was further improvement in the clearance scores: 12 (26%) patients had a score of 4, and 32 (68%) had a score of 5 identified by the SpyGlass DS. The respective CBD stone clearance rates for the control (no irrigation), 50 mL saline irrigation, and 100 mL saline irrigation were 15%, 60%, and 94%, respectively, and the difference among them was statistically significant (P < 0.001) (Table 2). The CBD clearance score (mean ± SD) was 2.4 ± 1.1 in the control, 3.5 ± 0.7 in the 50 mL saline irrigation, and 4.6 ± 0.6 in the 100 mL saline irrigation (P < 0.001) (Supplementary Figure 1).
| Score | No irrigation | 50 mL | 100 mL | P value |
| Score 1 | 13 (28%) | 0 (0%) | 0 (0%) | |
| Score 2 | 8 (17%) | 4 (8%) | 0 (0%) | |
| Score 3 | 19 (40%) | 15 (32%) | 3 (6%) | |
| Score 4 | 7 (15%) | 28 (60%) | 12 (26%) | |
| Score 5 | 0 (0%) | 0 (0%) | 32 (68%) | |
| Clear | 7 (15%) | 28 (60%) | 44 (94%) | < 0.001 |
After ERCP, 13 patients had a score of 1 (a large amount of residual stone/sludge) before saline irrigation. Only 15 patients did not reach a score of 5 (complete clearance) after 100 mL of saline irrigation. A dilated CBD diameter > 15 mm (OR = 4.93, 95%CI: 1.13-21.57; P = 0.034) was an independent risk factor for significant residual CBD stones (score = 1). Multivariate analysis revealed that CBD diameter > 15 mm (OR = 0.08, 95%CI: 0.01-0.49; P = 0.007) and the presence of periampullary diverticula (PAD) (OR = 6.51, 95%CI: 1.08-39.21; P = 0.041) were independent risk factors for failed CBD clearance despite irrigation with 100 mL saline (Table 3).
| Residual massive stones (score = 1) before saline irrigation | Biliary clearance (score = 5) after 100 mL saline irrigation | |||||||||
| n/N | Univariable analysis | Multivariable analysis | n/N | Univariable analysis | Multivariable analysis | |||||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |||
| Age | ||||||||||
| ≤ 65 yr | 6/27 | 1.00 | 19/27 | 19/27 | 1.00 | - | ||||
| > 65 yr | 7/20 | 1.89 (0.52-6.86) | 0.34 | 13/20 | 13/20 | 0.78 (0.23-2.69) | 0.70 | - | - | |
| Gender | - | |||||||||
| Female | 9/24 | 1.00 | 16/24 | 16/24 | 1.00 | - | ||||
| Male | 4/23 | 0.35 (0.09-1.37) | 0.13 | 16/23 | 0.17 | 16/23 | 1.14 (0.34-3.90) | 0.83 | - | - |
| CBD diameter | ||||||||||
| ≤ 15 mm | 5/32 | 1.00 | 26/32 | 26/32 | 1.00 | 1.00 | ||||
| > 15 mm | 8/15 | 6.17 (1.53-24.84) | 0.01 | 6/15 | 0.034 | 6/15 | 0.15 (0.04-0.60) | 0.007 | 0.08 (0.01-0.49) | 0.007 |
| Stone number | ||||||||||
| Single | 3/11 | 1.00 | 10/11 | 10/11 | 1.00 | 1.00 | ||||
| Multiple | 10/36 | 1.03 (0.23-4.66) | 0.97 | 22/36 | - | 22/36 | 0.16 (0.02-1.37) | 0.09 | 0.08 (0.01-1.09) | 0.06 |
| Stone diameter | ||||||||||
| ≤ 15 mm | 8/36 | 1.00 | 26/36 | 26/36 | 1.00 | - | ||||
| > 15 mm | 5/11 | 2.92 (0.70-12.11) | 0.14 | 6/11 | 0.34 | 6/11 | 0.46 (0.12-1.86) | 0.28 | - | - |
| Pigment-based stones | ||||||||||
| No | 4/17 | 1.00 | 14/17 | 14/17 | 1.00 | 1.00 | ||||
| Yes | 9/30 | 1.39 (0.36-5.46) | 0.63 | 18/30 | - | 18/30 | 0.32 (0.08-1.36) | 0.12 | 0.43 (0.05-3.60) | 0.44 |
| PAD | ||||||||||
| No | 4/15 | 1.00 | 26/32 | 26/32 | 1.00 | 1.00 | ||||
| Yes | 9/32 | 1.08 (0.27-4.28) | 0.92 | 6/15 | - | 6/15 | 6.50 (1.67-25.38) | 0.007 | 6.51 (1.08-39.21) | 0.041 |
Further analysis showed that the volume of saline used for irrigation[20] (P < 0.001) was an important factor determining CBD clearance (Supplementary Table 1).
We used IR spectroscopy to analyze the components of stones in the CBD of all patients. IR spectroscopy analysis showed that the proportion of pigment-based stones was significantly higher in patients with PAD (93.3% vs 50.0%, P = 0.04), and these stones were mostly soft stones (77.4% vs 22.6%, P = 0.01) (Table 4).
| Variable | Pigment-based stones | Cholesterol-based stones | P value | |
| PAD | ||||
| Yes | 14 (93.3%) | 1 (6.7%) | 8.31 | 0.004 |
| No | 16 (50.0%) | 16 (50.0%) | ||
| Diameter of the CBD | ||||
| ≤ 15 mm | 21 (65.6%) | 11 (34.4%) | 0.14 | 0.75 |
| > 15 mm | 9 (60.0%) | 6 (40.0%) | ||
| Diameter of the stone | ||||
| ≤ 15 mm | 25 (69.4%) | 11 (30.6%) | 2.10 | 0.17 |
| > 15 mm | 5 (45.5%) | 6 (54.5%) | ||
| Stone texture | ||||
| Hard | 6 (37.5%) | 10 (62.5%) | 7.28 | 0.01 |
| Soft | 24 (77.4%) | 7 (22.6%) |
Fluoroscopic cholangiographic imagery is currently the main method to determine the successful clearance of CBD stones[20]. However, studies involving the use of IDUS have identified residual biliary sludge within the bile duct despite the absence of filling defects on cholangiography. Mechanical lithotripsy produces a large number of stone fragments, and these minor residual CBD stones may lead to recurrent stone formation[21].
It has been reported that residual small CBD stone fragments can be flushed out of the bile duct using saline irrigation with a balloon catheter with a side hole[15]. A study reported that an average of 48 mL of saline could remove residual stones[16]. However, another study found that at least 100 mL of saline was needed to reduce residual stones[17]. Therefore, the effectiveness of saline irrigation on the clearance of residual bile duct stones after lithotripsy is not clear. This study's purpose differs from previous ones in that all of the patients had large stones and were post lithotripsy. In this study, we found that after ERCP and an additional round of lithotripsy were performed to remove CBD stones, POC using the SpyGlass DS showed that only 15% of the patients were relatively cleared of bile duct stones despite a negative occlusion cholangiogram. After irrigation with 50 mL of normal saline, 60% of the patients had relatively cleared bile ducts. After a total of 100 mL saline irrigation, 94% of the patients achieved complete clearance of the bile duct. The results showed that irrigation with 50 mL of saline was not enough to clear the bile duct of residual stones/sludge.
In this series, all patients received mechanical lithotripsy for stone fragmentation, which generates substantial stone fragments/debris, thus increasing the difficulty of clearing the bile duct. Our results showed that a good CBD clearance rate could be achieved with a larger saline irrigation volume. The clearance rate of bile duct stones could be higher for those without lithotripsy.
Residual stones have been found in approximately 1/3 of cases after stone retrieval using endoscopic ultrasound (EUS)[22,23]. In addition to stone detection, the EUS approach might also offer an alternative for treating bile duct stones. However, the use of EUS is very challenging[24-27]. IDUS has also been used to detect small residual stones in 14/59 patients (23.7%) with residual stones[11]. However, the ultrasonic probe is costly and can be easily damaged. In addition, the method is highly operator dependent and often produces poor quality images, and the presence of air in the bile duct can affect the detection of residual stones. Many studies have shown that POC has a high sensitivity in the detection of residual CBD stones, ranging from 25.3% to 34%, where residual stones are missed by standard cholangiography[8,9,28].
In our study, we used the SpyGlass DS system to determine the CBD clearance score. The SpyScope has a 4-way tip deflection and is much smaller (10 French) than conventional choledochoscopes. More importantly, it has a separate and independent irrigation channel that allows intermittent or continuous irrigation of the biliary system. It also offers a direct examination of the bile duct lumen and is more accurate in detecting and diagnosing residual bile duct stones/sludge.
Residual CBD stones can lead to recurrent stone formation. Itoi et al[7] reported that PAD and intraoperative lithotripsy were closely related to residual stones (P < 0.05), as we observed in our study. We also found that a dilated CBD with a diameter > 15 mm was an independent risk factor for failed CBD clearance and a large number of residual stones. Despite irrigation with 100 mL saline, PAD and a dilated CBD remained independent risk factors for incomplete bile duct clearance. This may be due to the presence of an air-filled duodenum/diverticulum that compresses the distal CBD, making it difficult to wash away the residual stones/sludge[29,30]. CBD clearance can be improved by increasing the volume of saline irrigation.
Our results showed that PAD not only increased the difficulty of bile duct clearance but can also affect stone composition. We found that pigment-based stones constituted the majority of CBD stones in our patients with PAD (P = 0.004). Song et al[31] reported that recurrent bile duct stones were more likely to be brown pigmented stones than cholesterol-based stones. This may be because pigment-based stones are often related to bacterial infection, and the formation of biliary sludge will contribute to recurrent stone formation over time[32-34]. The brown pigmented stones that form as a result of bacterial infection are soft and easy to fragment and generate abundant debris. Our experience showed that soft stones/sludge require more saline irrigation to clear the bile duct. In this study, 26% of cases with biliary sludge were found even with 100 mL saline irrigation. If an effective bile duct cleaning device is used to remove biliary sludge, it may reduce the CBD stone recurrence rate after ERCP.
Without adequate biliary drainage, saline irrigation can increase the intraductal pressure, causing cholangitis because of contaminated bile[35]. Proper drainage methods can mitigate this stress[36,37]. No serious adverse events were reported in our study except for 10% of patients with cholangitis secondary possibly to saline irrigation. Intermittent irrigation and endoscopic suction to promote biliary drainage may lower the risk in addition to antibiotic coverage. Differences between this study and previous studies include the following: We studied the effect of flushing with saline after lithotripsy; our evaluation method was different from that of previous studies (we used SpyGlass DS examination); and we performed a component analysis related to diverticula. Our study has several methodological advantages. First, it was a prospective study using a self-controlled design, and SpyGlass DS was used to assess the presence of residual stones in the bile ducts. All of the patients showed no stones by imaging even though small residual bile duct stones were observed by SpyGlass DS, indicating that the evaluation of residual bile duct stones using SpyGlass DS is more sensitive than imaging alone. If we observe that there are still stones, it would be unethical not to continue flushing after using 50 mL of saline. Second, this study used objective definitions to assess residual stones identified by POC. Third, this study included objective stone composition analysis using IR spectroscopy.
The study has limitations. This study was not a randomized controlled trial. The use of direct cholangioscopy by the SpyGlass DS increases the procedure time with additional cost. Therefore, we do not recommend routine use of SpyGlass DS to rule out residual stones.
In conclusion, in patients with large CBD stones undergoing mechanical lithotripsy, routine irrigation of the bile duct with at least 100 mL of saline is recommended to improve CBD clearance, especially for patients with a dilated bile duct and/or those with PAD. Saline irrigation is simple, inexpensive, and easy to perform to improve bile duct clearance, and it may avoid recurrent stone formation.
Endoscopic retrograde cholangiopancreatography (ERCP) is an effective and relatively minimally invasive technique for common bile duct (CBD) stones. The recurrence rate of CBD stones after ERCP has increased from 4% to 24%. The incidence of residual stones after mechanical lithotripsy for intractable CBD stones is 24% to 40%. An important reason for the recurrence of CBD stones is the presence of stone debris after lithotripsy.
It has been suggested that saline irrigation of the bile duct after stone removal can increase the cleaning of the bile duct. Complete bile duct clearance is necessary to decrease recurrent bile duct stones. However, the efficacy and dosage of saline are still unclear.
To determine whether saline irrigation can improve CBD clearance after lithotripsy and how much saline solution will remove residual stones/debris.
This prospective self-controlled study enrolled patients receiving mechanical lithotripsy for large (> 1.2 cm) CBD stones. After occlusion cholangiography confirmed CBD stone clearance, peroral cholangioscopy (POC) was performed to determine clearance scores based on the number of residual stones. The number of residual stones spotted via POC was graded on a 5-point scale (score 1, worst; score 5, best). Scores were documented after only stone removal (control) and after irrigation with 50 mL and 100 mL saline, respectively. The stone composition was analyzed using infrared spectroscopy.
Between October 2018 and January, 47 patients had CBD clearance scores of 2.4 ± 1.1 without saline irrigation, 3.5 ± 0.7 with 50 mL irrigation, and 4.6 ± 0.6 with 100 mL irrigation. Multivariate analysis showed that CBD diameter > 15 mm and periampullary diverticula (PAD) were independent risk factors for residual stones. Bilirubin pigment stones constituted the main residual stones found in patients with PAD.
Irrigation with 100 mL of saline may not clear all residual CBD stones after lithotripsy, especially in patients with PAD and/or a dilated (> 15 mm) CBD. Pigment residual stones are soft and commonly found in patients with PAD. Additional saline irrigation may be required to remove retained stones.
In the future, prospective, large sample, multi-center, well-designed studies are needed to validate the amount of saline needed to completely remove the residual stones/debris of different types of CBD stones after mechanical lithotripsy.
We thank the clinical and research teams at the Department of General Surgery for providing ongoing support.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cascella M, Maetani I S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Cheng CL, Tsou YK, Lin CH, Tang JH, Hung CF, Sung KF, Lee CS, Liu NJ. Poorly expandable common bile duct with stones on endoscopic retrograde cholangiography. World J Gastroenterol. 2012;18:2396-2401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Cotton PB. Fifty years of ERCP: a personal review. Gastrointest Endosc. 2018;88:393-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Frossard JL, Morel PM. Detection and management of bile duct stones. Gastrointest Endosc. 2010;72:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Baiu I, Visser B. Endoscopic Retrograde Cholangiopancreatography. JAMA. 2018;320:2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Talukdar R. Complications of ERCP. Best Pract Res Clin Gastroenterol. 2016;30:793-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Cai JS, Qiang S, Bao-Bing Y. Advances of recurrent risk factors and management of choledocholithiasis. Scand J Gastroenterol. 2017;52:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Itoi T, Sofuni A, Itokawa F, Shinohara Y, Moriyasu F, Tsuchida A. Evaluation of residual bile duct stones by peroral cholangioscopy in comparison with balloon-cholangiography. Dig Endosc. 2010;22 Suppl 1:S85-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Lee YN, Moon JH, Choi HJ, Min SK, Kim HI, Lee TH, Cho YD, Park SH, Kim SJ. Direct peroral cholangioscopy using an ultraslim upper endoscope for management of residual stones after mechanical lithotripsy for retained common bile duct stones. Endoscopy. 2012;44:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Sejpal DV, Trindade AJ, Lee C, Miller LS, Benias PC, Inamdar S, Singh G, Stewart M, George BJ, Vegesna AK. Digital cholangioscopy can detect residual biliary stones missed by occlusion cholangiogram in ERCP: a prospective tandem study. Endosc Int Open. 2019;7:E608-E614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Manzoor Ul Haque M, Hassan Luck N, Ali Tasneem A, Mudassir Laeeq S, Mandhwani R, Hanif FM, Ullah Lail G. Safety and Efficacy of Extracorporeal Shock Wave Lithotripsy for Difficult-to-retrieve Common Bile Duct Stones: A Ten-year Experience. J Transl Int Med. 2020;8:159-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Tsuchiya S, Tsuyuguchi T, Sakai Y, Sugiyama H, Miyagawa K, Fukuda Y, Ando T, Saisho H, Yokosuka O. Clinical utility of intraductal US to decrease early recurrence rate of common bile duct stones after endoscopic papillotomy. J Gastroenterol Hepatol. 2008;23:1590-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Konstantakis C, Triantos C, Theopistos V, Theocharis G, Maroulis I, Diamantopoulou G, Thomopoulos K. Recurrence of choledocholithiasis following endoscopic bile duct clearance: Long term results and factors associated with recurrent bile duct stones. World J Gastrointest Endosc. 2017;9:26-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Jang SE, Ahn DW, Lee SH, Lee BS, Jeong JB, Hwang JH, Ryu JK, Kim YT, Lee KH, Kim YH. Preventive saline irrigation of the bile duct after the endoscopic removal of common bile duct stones. Dig Dis Sci. 2013;58:2353-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Huang SW, Lin CH, Lee MS, Tsou YK, Sung KF. Residual common bile duct stones on direct peroral cholangioscopy using ultraslim endoscope. World J Gastroenterol. 2013;19:4966-4972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Komatsu Y, Toda N, Isayama H, Tsujino T, Tateishi K, Yamagata M, Ohashi M, Tada M, Yoshida H, Shiratori Y, Kawabe T, Omata M. Washout of small stones in the bile duct by saline infusion using a side-holed balloon catheter in patients undergoing endoscopic papillary balloon dilation. Gastrointest Endosc. 1999;49:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Ang TL, Teo EK, Fock KM, Lyn Tan JY. Are there roles for intraductal US and saline solution irrigation in ensuring complete clearance of common bile duct stones? Gastrointest Endosc. 2009;69:1276-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Ahn DW, Lee SH, Paik WH, Song BJ, Park JM, Kim J, Jeong JB, Hwang JH, Ryu JK, Kim YT. Effects of Saline Irrigation of the Bile Duct to Reduce the Rate of Residual Common Bile Duct Stones: A Multicenter, Prospective, Randomized Study. Am J Gastroenterol. 2018;113:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Cotton PB, Garrow DA, Gallagher J, Romagnuolo J. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009;70:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 467] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 19. | Sugiura R, Kuwatani M, Hirata K, Kato S, Kawamoto Y, Kawakubo K, Mitsuhashi T, Asano T, Hirano S, Sakamoto N. Synchronous multiple pancreatic cancers developed long after severe postendoscopic retrograde cholangiopancreatography pancreatitis. Endosc Ultrasound. 2019;8:213-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Huang WS, Kim MH, Okamoto K, Belli G, Dervenis C, Chan ACW, Lau WY, Endo I, Gomi H, Yoshida M, Mayumi T, Baron TH, de Santibañes E, Teoh AYB, Hwang TL, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Higuchi R, Kitano S, Inomata M, Deziel DJ, Jonas E, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 424] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 21. | ASGE Standards of Practice Committee, Maple JT, Ikenberry SO, Anderson MA, Appalaneni V, Decker GA, Early D, Evans JA, Fanelli RD, Fisher D, Fisher L, Fukami N, Hwang JH, Jain R, Jue T, Khan K, Krinsky ML, Malpas P, Ben-Menachem T, Sharaf RN, Dominitz JA. The role of endoscopy in the management of choledocholithiasis. Gastrointest Endosc. 2011;74:731-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Endo R, Satoh A, Tanaka Y, Shimoda F, Suzuki K, Takahashi K, Okata H, Hiramoto K, Kimura O, Asonuma S, Umemura K, Shimosegawa T. Saline Solution Irrigation of the Bile Duct after Stone Removal Reduces the Recurrence of Common Bile Duct Stones. Tohoku J Exp Med. 2020;250:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Kim YJ, Chung WC, Jo IH, Kim J, Kim S. Efficacy of endoscopic ultrasound after removal of common bile duct stone. Scand J Gastroenterol. 2019;54:1160-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Kaspy MS, Hassan GM, Paquin SC, Sahai AV. An assessment of the yield of EUS in patients referred for dilated common bile duct and normal liver function tests. Endosc Ultrasound. 2019;8:318-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Ogura T, Okuda A, Higuchi K. Intrahepatic bile duct stone removal using peroral transluminal cholangioscopy (with videos). Endosc Ultrasound. 2019;8:131-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Cazacu IM, Singh BS, Saftoiu A, Bhutani MS. Recent developments in hepatopancreatobiliary EUS. Endosc Ultrasound. 2019;8:146-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Wang G, Liu X, Wang S, Ge N, Guo J, Sun S. Endoscopic Ultrasound-guided Gastroenterostomy: A Promising Alternative to Surgery. J Transl Int Med. 2019;7:93-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Theerasuwipakorn N, Tasneem AA, Kongkam P, Angsuwatcharakon P, Ridtitid W, Navicharern P, Kitisin K, Wangrattanapranee P, Rerknimitr R, Kullavanijaya P. Walled-off Peripancreatic Fluid Collections in Asian Population: Paradigm Shift from Surgical and Percutaneous to Endoscopic Drainage. J Transl Int Med. 2019;7:170-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Yang JJ, Liu XC, Chen XQ, Zhang QY, Liu TR. Clinical value of DPOC for detecting and removing residual common bile duct stones (video). BMC Gastroenterol. 2019;19:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Yue P, Zhu KX, Wang HP, Meng WB, Liu JK, Zhang L, Zhu XL, Zhang H, Miao L, Wang ZF, Zhou WC, Suzuki A, Tanaka K, Li X. Clinical significance of different periampullary diverticulum classifications for endoscopic retrograde cholangiopancreatography cannulation. World J Gastroenterol. 2020;26:2403-2415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (4)] |
| 31. | Song ME, Chung MJ, Lee DJ, Oh TG, Park JY, Bang S, Park SW, Song SY, Chung JB. Cholecystectomy for Prevention of Recurrence after Endoscopic Clearance of Bile Duct Stones in Korea. Yonsei Med J. 2016;57:132-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Mu P, Yue P, Li F, Lin Y, Liu Y, Meng W, Zhou W, Li X. Does periampullary diverticulum affect ERCP cannulation and post-procedure complications? Turk J Gastroenterol. 2020;31:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Sung JY, Leung JW, Shaffer EA, Lam K, Costerton JW. Bacterial biofilm, brown pigment stone and blockage of biliary stents. J Gastroenterol Hepatol. 1993;8:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Leung JW, Ling TK, Chan RC, Cheung SW, Lai CW, Sung JJ, Chung SC, Cheng AF. Antibiotics, biliary sepsis, and bile duct stones. Gastrointest Endosc. 1994;40:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Meng W, Leung JW, Zhang K, Zhou W, Wang Z, Zhang L, Sun H, Xue P, Liu W, Wang Q, Zhang J, Wang X, Wang M, Shao Y, Cai K, Hou S, Li Q, Zhang L, Zhu K, Yue P, Wang H, Zhang M, Sun X, Yang Z, Tao J, Wen Z, Wang Q, Chen B, Shao Q, Zhao M, Zhang R, Jiang T, Liu K, Zhang L, Chen K, Zhu X, Zhang H, Miao L, Wang Z, Li J, Yan X, Wang F, Zhang L, Suzuki A, Tanaka K, Nur U, Weiderpass E, Li X. Optimal dilation time for combined small endoscopic sphincterotomy and balloon dilation for common bile duct stones: a multicentre, single-blinded, randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Sethi A, Chen YK, Austin GL, Brown WR, Brauer BC, Fukami NN, Khan AH, Shah RJ. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: a single-center experience. Gastrointest Endosc. 2011;73:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 37. | Mu P, Lin Y, Zhang X, Lu Y, Lu Y, Yang M, Da Z, Gao L, Mi N, Li T, Liu Y, Wang H, Wang F, Leung J, Yue P, Meng W, Zhou W, Li X. The evaluation of ENGBD versus PTGBD in high-risk acute cholecystitis: A single-center prospective randomized controlled trial. EClinicalMedicine. 2021;31. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |