Published online Jan 28, 2021. doi: 10.3748/wjg.v27.i4.345
Peer-review started: October 29, 2020
First decision: November 30, 2020
Revised: December 9, 2020
Accepted: December 26, 2020
Article in press: December 26, 2020
Published online: January 28, 2021
Processing time: 87 Days and 22.3 Hours
Studies suggested that remote ischemic preconditioning (RIPC) may effectively lessen the harmful effects of ischemia reperfusion injury during organ transplantation surgery.
To investigate the protective effects of RIPC on living liver donors and recipients following pediatric liver transplantation.
From January 2016 to January 2019 at Renji Hospital Affiliated with Shanghai Jiao Tong University School of Medicine, 208 donors were recruited and randomly assigned to four groups: S-RIPC group (no intervention; n = 55), D-RIPC group (donors received RIPC; n = 51), R-RIPC group (recipients received RIPC, n = 51) and DR-RIPC group (both donors and recipients received RIPC; n = 51). We primarily evaluated postoperative liver function among donors and recipients and incidences of early allograft dysfunction, primary nonfunction and postoperative complications among recipients.
RIPC did not significantly improve alanine transaminase and aspartate aminotransferase levels among donors and recipients or decrease the incidences of early allograft dysfunction, primary nonfunction, and postoperative complications among recipients. Limited protective effects were observed, including a lower creatinine level in the D-RIPC group than in the S-RIPC group on postoperative day 0 (P < 0.05). However, no significant improvements were found in donors who received RIPC. Furthermore, RIPC had no effects on the overall survival of recipients.
The protective effects of RIPC were limited for recipients who received living liver transplantation, and no significant improvement of the prognosis was observed in recipients.
Core Tip: Ischemia reperfusion injury (IRI) has been a well-known underlying cause for inducing or aggravating primary graft nonfunction, vascular complications and biliary complications during liver transplantation (LT). Some studies suggested that remote ischemic preconditioning (RIPC) may effectively lessen the harmful effects of IRI during organ transplantation surgery. However, studies on the effect of RIPC on pediatric LT were rare. The present single-center randomized clinical trial aimed to determine whether RIPC could be beneficial for reducing IRI among both donors and recipients undergoing pediatric LT.
- Citation: Qi B, Wang XQ, Pan ST, Li PY, Chen LK, Xia Q, Yang LQ, Yu WF. Effect of remote ischemic preconditioning among donors and recipients following pediatric liver transplantation: A randomized clinical trial. World J Gastroenterol 2021; 27(4): 345-357
- URL: https://www.wjgnet.com/1007-9327/full/v27/i4/345.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i4.345
Since first performed by Starzl et al[1] in 1963, liver transplantation (LT) has undergone remarkable progress and innovation over the last 50 years. Currently, LT remains the gold standard treatment for patients suffering from end-stage liver disease or metabolic liver disease, with an overall 3-year survival rate exceeding 80% due to advancements in immunosuppressive agents, surgical techniques and perioperative management[2-5]. Approximately 600 pediatric LTs were performed in the United States in 2018[2], with the first pediatric LT successfully performed in 1967 by Starzl et al[6]. Meanwhile, the development of pediatric LT in China has been rapid and prominent, with Renji Hospital becoming the major pediatric LT center, performing more than 400 pediatric LTs in 2019. According to recorded data from Renji Hospital, the overall 3-year survival rate of children in recent years had exceeded 90%.
Despite the outstanding achievements in pediatric LT, some complications, such as primary graft nonfunction (PNF), vascular complications, biliary complications and allograft rejection, still exist[3,5,7,8]. Accordingly, ischemia reperfusion injury (IRI) has been a well-known underlying cause for inducing or aggravating PNF, vascular complications and biliary complications[9-11]. Given that IRIs usually occur when temporarily cutting off and then restoring an organ or tissue’s blood supply, avoiding it during LT is challenging[12]. Although several studies have attempted to ameliorate hepatic IRI[11,13,14], the mechanisms of IRI still remain largely unclear with no definitive therapies having been established.
Remote ischemic preconditioning (RIPC), a simple noninvasive therapy for alleviating the harmful effects of IRI, has shown promise in protecting multiple organs, such as the kidneys, heart and liver[9,15,16]. RIPC is usually performed by inflating and deflating a standard blood pressure cuff placed on the upper arm or thigh to induce transient ischemia and reperfusion, providing systemic multiorgan protection[17]. A number of fundamental and clinical studies have suggested that RIPC can effectively reduce IRI in the liver[9,18-20]. For instance, Abu-Amara et al[19,20] confirmed that RIPC successfully reduced IRI in a mouse model, while Wu et al[18] found that RIPC was able to reduce hepatic IRI among patients undergoing liver resection. Moreover, Jung et al[9] suggested that RIPC might be beneficial for postoperative liver function among recipients after living donor LT. However, other studies have shown no benefits for RIPC in animal models or patients[17,21,22]. Therefore, more studies are needed to validate the effectiveness of RIPC.
Given the current lack of studies on the effect of RIPC on pediatric LT, the present single-center randomized clinical trial aimed to determine whether RIPC could be beneficial for reducing IRI among both donors and recipients undergoing pediatric LT.
This single-center, randomized controlled study had been approved by the ethics committee of the Renji Hospital (2016-002K) and was registered with Clinical-Trails.gov (NCT02830841). Written informed consent was obtained from the donors and families of recipients. The study was conducted in accordance with the Declaration of Helsinki and the CONSORT criteria[23].
Randomization was achieved by using opaque envelopes in which allocations were stored, and random sequence was generated by an independent data manager. Patients who consent to enter this trial were randomly allocated into the S-RIPC group (no intervention to donors and recipients), D-RIPC group (donors received RIPC), R-RIPC group (recipients received RIPC), and DR-RIPC group (both donors and recipients received RIPC) in a 1:1:1:1 fashion. No masking was applied except for data assessors.
Donors and recipients in the S-RIPC group underwent the same procedure without RIPC. Donors in D-RIPC group underwent RIPC in the right upper limb after induction of anesthesia and before abdominal skin incision. The cuff was placed in the upper third of the right upper limb, after which three 5-min cycles each of inflation at a pressure of 200 mmHg and subsequent reperfusion with the cuff deflated were performed. Recipients in the D-RIPC group underwent the same procedure except without RIPC. Recipients in the R-RIPC group underwent RIPC with the cuff being placed on the left lower limb at an inflation pressure of 150 mmHg. Donors in the R-RIPC group underwent the same procedure except without RIPC. Both donors and recipients in the DR-RIPC group underwent RIPC as described.
According to plasma alanine transaminase (ALT) records from 235 children who previously performed LT without RIPC in Renji Hospital, we found that mean natural logarithm of maximum postoperative ALT (ALTmax) was 5.86; assuming the mean logarithm of ALTmax decreases to 5.3 after performing RIPC in recipients, which leads to effect size f = 0.25. Combined with significance level of α = 0.05, and power of 80%, each treatment group had to include at least 32 patients[24]. Considering 10% dropout rate, we decided to include at least 144 patients in total.
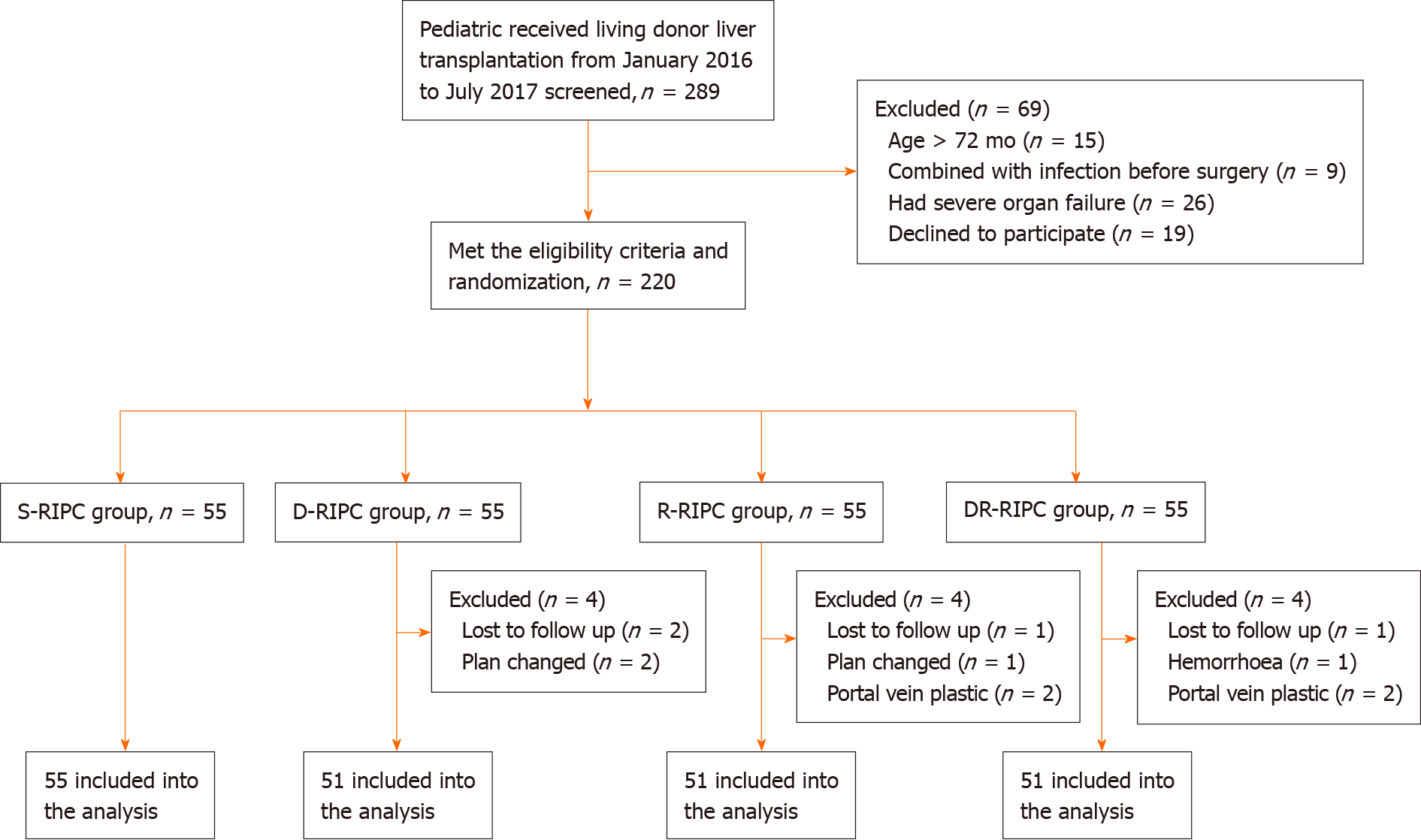
A total of 220 patients with biliary atresia and family liver donors who underwent living pediatric LT from January 2016 to January 2019 at Renji Hospital Affiliated with Shanghai Jiao Tong University School of Medicine were eligible for enrollment. The inclusion criteria were as follows: (1) American society of anesthesiologists score of I-III; (2) Age of 3-72 mo; and (3) Elective living LT surgery. The exclusion criteria were as follows: (1) Peripheral vascular disease; (2) History of thromboembolism; (3) Systemic or local infection before surgery; (4) Autoimmune diseases; (5) Severe congenital heart disease, and (6) History of LT.
Donors and recipient characteristics were obtained from the electronic medical record system. Pediatric end-stage liver disease grade was calculated as described previously[25]. Early allograft dysfunction (EAD), PNF and acute kidney injury were defined according to published studies[26-28]; EAD was defined as in Olthoff et al[29]; and PNF was defined as graft loss, re-transplantation or death due to graft nonfunction within 30 d after surgery (except those induced by hepatic artery embolism, bile duct complications or recurrent liver disease)[30]. Postoperative complications were classified according to the modified Clavien Grading System[31]. Moreover, all recipients were followed up until July 1, 2019, while recipient survival was updated every 3 mo. Two trained research assistants oversaw data collection and recorded them using “Excel” or “Epidata”.
Recipients were monitored through regular electrocardiographic monitoring and underwent initial induction with 8% sevoflurane and 5 L/min of O2. After achieving silence, the peripheral veins of the upper limbs were opened, and tracheal cannulation was performed under induction with 0.05 mg/kg midazolam, 1 mg/kg rocuronium and 1 μg/kg sufentanil. The pressure support ventilation mode was selected, with a respiratory frequency of 16-20 times/min. The end tidal carbon dioxide tension was controlled at 35-40 mmHg. Intraoperative anesthesia was maintained using sevoflurane (anesthesia depth at 0.6 minimum alveolar concentration). Intraoperative analgesia and muscle relaxation were maintained using sufentanil (1 μg/kg/h) and rocuronium (0.15 mg/kg/h). Basic vital signs and circulation capacity were monitored and managed regularly. All recipients were sent to the intensive care unit (ICU) for postoperative care.
Donors were monitored through regular electrocardiographic monitoring. The peripheral veins and radial artery were opened for transfusion and invasive blood pressure measurement. Donors underwent induction with 0.05 mg/kg midazolam, 2 mg/kg propofol, 0.6 mg/kg rocuronium and 0.5 g/kg sufentanil. Endotracheal intubation and mechanical ventilation were performed with a tidal volume of 8 mL/kg, while the end tidal carbon dioxide tension was maintained between 35 mmHg and 45 mmHg. Intraoperative anesthesia was maintained using cisatracurium, sevoflurane and remifentanil. Following the right internal jugular vein puncture, an internal jugular vein catheter with double cavities was inserted for central venous pressure monitoring. After the operation, donors were sent to the anesthesia recovery room for resuscitation and extubation.
Donors were placed in the horizontal position with an inverted L abdominal incision being utilized according to the surgeon’s preference. Intraoperative doppler ultrasonography was used to confirm the anatomical structure of the hepatic portal vein and hepatic vein, while intraoperative cholangiography was performed to verify the division position of the hepatic parenchyma after cholecystectomy. After completing parenchymal dissection, the anesthetists administered intravenous heparin sodium (0.5 mg/kg). After the left hepatic artery and left portal vein were severed, 50 mg of protamine was used to reverse immediately heparinization. The graft was maintained at 4 °C, after which portal vein perfusion was started. After confirming that the color of the perfusate discharged from the hepatic vein had faded, the graft was transferred to the preserving solution for vascular structure examination and weight measurement. Details regarding the liver graft resection have been described previously[5,9,32].
Recipients were placed in the horizontal position with a straight-line abdominal incision being utilized. The original liver was initially resected, after which the surgery entered the anhepatic phase. Thereafter, the hepatic vein, portal vein and hepatic artery were inosculated and successively opened. The velocity and pattern of blood flow in the new hepatic portal vein, hepatic vein and hepatic artery were determined using color doppler ultrasound. Roux-en-Y biliary jejunostomy was then performed to replace the inosculation of recipients and donors’ biliary duct. Recipients were subsequently transferred to the ICU after confirming that all vessels were fluent and vital signs were stable.
Patients were followed up until July 1, 2019. The primary outcomes examined herein included ALTmax and maximum aspartate aminotransferase (ASTmax). Secondary outcomes included EAD, PNF, postoperative complications and overall survival of recipients.
Statistical analyses were conducted using the IBM SPSS Statistics 26.0 (SPSS Inc., Armonk, NY, United States) and R software (Version 3.6.1). Categorical variables are presented as frequency (n) or proportion (%), while continuous variables were expressed as mean ± standard deviation or median (25% interquartile range, 75% interquartile range). Differences were analyzed through repeated measures/block randomized one-way analysis of variance, followed by post-hoc analysis (Tukey’s test) as appropriate. Moreover, non-parametric tests followed by the Kruskal-Wallis test were utilized for multiple groups comparisons. Categorical variables were compared using the 2 test with the Yates correction or Fisher’s exact test (when the total sample was < 40 or the expected frequency was < 1). Overall survival curves were created using Kaplan-Meier survival analysis, while the log-rank t test was used to compare differences between the four groups. Additionally, a post-hoc subgroup analysis was used to investigate possible effect modification of four groups. All statistical tests were two-sided with P values < 0.05 being considered statistically significant.
Patients were randomly assigned to the four groups (n = 55 per group). Twelve patients were excluded from the study owing to unexpected issues or changes in surgical plans (Figure 1). The remaining 208 patients [S-RIPC group (n = 55), D-RIPC group (n = 51), R-RIPC group (n = 51) and DR-RIPC group (n = 51)] were ultimately analyzed.
Demographic and preoperative/intraoperative characteristics of the recipients and donors are presented in Tables 1 and 2, respectively. No significant differences in demographic or preoperative/intraoperative characteristics were observed between recipients and donors. Recipients in all four groups showed good comparability and consistency.
| DR-RIPC, n = 51 | D-RIPC, n = 51 | R-RIPC, n = 51 | S-RIPC, n = 55 | P value | |
| Demographics | |||||
| Sex, male/female | 23/28 | 28/23 | 25/26 | 29/26 | 0.766 |
| Age in mo | 7 (6, 8) | 7 (6, 8) | 7 (6, 9) | 7 (6, 9) | 0.130 |
| Weight in kg | 7.4 ± 1.3 | 7.2 ± 1.0 | 7.6 ± 1.4 | 7.8 ± 1.8 | 0.190 |
| Height in cm | 66.5 ± 5.8 | 66.3 ± 4.6 | 67.3 ± 6.1 | 68.4 ± 6.8 | 0.245 |
| Kasai operation, yes/no | 30/21 | 31/20 | 30/21 | 33/22 | 0.996 |
| PELD grade | 16.7 ± 10.2 | 17.2 ± 8.9 | 14.0 ± 10.1 | 15.3 ± 8.5 | 0.222 |
| Child-Pugh grade, A/B/C | 4/30/17 | 3/30/18 | 5/35/11 | 6/37/12 | 0.599 |
| Preoperative biochemical data | |||||
| ALT in U/L | 140.0 (70.0, 220.0) | 154.5 (93.5, 237.2) | 130.0 (88.67, 201.5) | 140.0 (81.0, 268.6) | 0.774 |
| AST in U/L | 257.7 (166.0, 421.0) | 230.1 (161.0, 339.5) | 239.0 (114.0, 405.5) | 282.0 (153.0, 500.8) | 0.385 |
| TB in mmol/L | 264.4 ± 153.3 | 274.6 ± 166.8 | 243.9 ± 163.0 | 253.9 ± 141.0 | 0.839 |
| ALB in g/L | 34.3 ± 6.3 | 33.3 ± 5.4 | 32.9 ± 6.1 | 34.1 ± 5.5 | 0.604 |
| Cr in μmol/L | 14.8 ± 5.1 | 13.9 ± 3.8 | 15.8 ± 4.8 | 15.2 ± 6.4 | 0.256 |
| WBC as 109/L | 11.6 ± 4.6 | 13.6 ± 10.7 | 12.2 ± 5.9 | 10.4 ± 4.8 | 0.204 |
| N% | 39.4 ± 12.1 | 39.2 ± 14.6 | 34.7 ± 11.9 | 39.9 ± 13.3 | 0.159 |
| Hgb in g/L | 92.4 ± 17.7 | 94.5 ± 15.8 | 96.7 ± 15.9 | 93.3 ± 14.5 | 0.535 |
| PLT as 109/L | 215.0 (148.0, 301.0) | 207.0 (138.0, 315.0) | 202.0 (144.0, 258.0) | 188.0 (129.0, 244.0) | 0.476 |
| Intraoperative characteristics | |||||
| Length of anhepatic phase in min | 32.0 (30.0, 38.0) | 34.0 (29.0, 38.5) | 33.0 (30.25, 39.0) | 34.0 (30.0, 42.5) | 0.714 |
| Time of cold ischemia in min | 65.7 ± 19.2 | 63.4 ± 21.5 | 63.5 ± 17.7 | 66.4 ± 16.8 | 0.477 |
| Weight of donor liver in g | 249.7 ± 36.0 | 238.4 ± 44.5 | 247.8 ± 36.8 | 253.8 ± 54.4 | 0.333 |
| GRWR, % | 3.5 ± 0.7 | 3.4 ± 0.8 | 3.3 ± 0.7 | 3.3 ± 0.9 | 0.674 |
| Time of anesthesia in min | 470.0 (430.0, 502.5) | 441.5 (410.2, 509.8) | 473.5 (420.0, 524.0) | 468.0 (428.5, 503.0) | 0.586 |
| Time of surgery in min | 373.9 ± 55.6 | 366.4 ± 72.2 | 378.4 ± 69.0 | 384.5 ± 63.4 | 0.543 |
| Transfusion volume in mL | 1550.0 (1285.0, 1715.0) | 1485.0 (1210.0, 1750.0) | 1658.0 (1302.0, 1850.0) | 1580.0 (1325.0, 1748.0) | 0.410 |
| ALB transfusion in g | 26.3 ± 7.2 | 24.4 ± 8.2 | 24.8 ± 9.4 | 24.5 ± 7.5 | 0.486 |
| RBC transfusion in U | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 1.9) | 1.0 (1.0, 1.8) | 0.863 |
| Bleeding in mL | 100.0 (100.0, 200.0) | 100.0 (100.0, 200.0) | 100.0 (100.0, 200.0) | 100.0 (100.0, 200.0) | 0.928 |
| Urine in mL | 220.0 (150.0, 300.0) | 210.0 (151.2, 300.0) | 220.0 (152.5, 300.0) | 210.0 (140.0, 305.0) | 0.965 |
| DR-RIPC, n = 51 | D-RIPC, n = 51 | R-RIPC, n = 51 | S-RIPC, n = 55 | P value | |
| Demographics | |||||
| Sex, male/female | 15/36 | 22/29 | 20/31 | 18/37 | 0.463 |
| Age in yr | 31 (27, 36) | 29(26, 32) | 30 (27, 34.5) | 29 (26, 33.5) | 0.299 |
| Weight in kg | 59.0 ± 8.7 | 61.9 ± 10.8 | 58.7 ± 10.8 | 58.8 ± 10.7 | 0.427 |
| Height in cm | 163.0 (159.5, 168.0) | 162.0 (158.0, 171.0) | 162.0 (160.0, 169.5) | 162.0 (160.0, 167.5) | 0.983 |
| Preoperative biochemical data | |||||
| ALT in U/L | 14.0 (12.0, 18.0) | 15.0 (11.0, 21.0) | 15.0 (11.0, 21.0) | 13.0 (11.5, 16.5) | 0.282 |
| AST in U/L | 17.0 (15.5, 20.0) | 18.0 (15.5, 21.0) | 18.0 (14.5, 21.5) | 17.0 (15.0, 20.0) | 0.850 |
| TB in mmol/L | 10.8 (8.9, 13.5) | 11.4 (9.5, 15.7) | 11.0 (8.0, 12.2) | 10.8 (8.8, 12.8) | 0.414 |
| ALB in g/L | 46.6 ± 4.3 | 47.1 ± 3.6 | 46.5 ± 2.7 | 46.8 ± 3.0 | 0.473 |
| Intraoperative characteristics | |||||
| Time of anesthesia in min | 195.0 (164.0, 217.0) | 199.0 (181.0, 218.5) | 190.0 (162.0, 217.0) | 193.0 (180.5, 229.0) | 0.428 |
| Time of surgery in min | 156.5 ± 35.9 | 165.7 ± 43.2 | 156.4 ± 38.0 | 161.5 ± 41.3 | 0.758 |
| Transfusion volume in mL | 1650.0 (1250.0, 2000.0) | 1750.0 (1250.0, 2000.0) | 1750.0 (1250.0, 2000.0) | 1750.0 (1250.0, 1750.0) | 0.936 |
| Bleeding in mL | 50.0 (40.0, 80.0) | 50.0 (50.0, 100.0) | 50.0 (35.0, 100.0) | 50.0 (50.0, 100.0) | 0.138 |
| Urine in mL | 400.0 (300.0, 450.0) | 400.0 (300.0, 500.0) | 400.0 (300.0, 475.0) | 400.0 (300.0, 500.0) | 0.983 |
Recipients were continuously monitored for changes in ALT, AST, total bilirubin, albumin, creatinine (Cr), white blood cell, neutrophil %, hemoglobin and platelet after surgery (0 d) until postoperative day 7, with a portion of the results being presented in Supplementary Table 1. Accordingly, our results found no differences in the aforementioned nine variables except for Cr-D0 (P = 0.029), suggesting a significant reduction in Cr levels at postoperative day 0, which was mainly attributed to the difference between the D-RIPC and S-RIPC group. For donors, no differences were found in all variables (Supplementary Table 2).
Clinical outcomes among recipients are summarized in Table 3. Accordingly, significant differences in ICU duration were observed (P = 0.041). No differences were found for other clinical outcomes. Our results indicated that RIPC did not improve clinical outcomes among recipients or shorten ICU and ventilation duration. On the contrary, those in the DR-RIPC groups seemed to have had longer ICU duration compared to those in the D-RIPC group. In addition, for postoperative complications, no significant differences were observed in donors before discharge (Supplementary Table 3).
| DR-RIPC, n = 51 | D-RIPC, n = 51 | R-RIPC, n = 51 | S-RIPC, n = 55 | P value | |
| Duration in ICU in h | 115.2 (113.6, 126.2) | 113.1a (91.0, 114.8) | 114.6 (91.3, 116.1) | 114.3 (92.5, 138.2) | 0.041 |
| Time of ventilation in h | 17.7 (16.7, 18.7) | 17.8 (16.9, 18.9) | 17.6 (17.1, 19.0) | 17.8 (16.5, 19.2) | 0.941 |
| EAD | 6 | 11 | 5 | 8 | 0.350 |
| PNF | 0 | 0 | 1 | 2 | 0.343 |
| AKI | 0 | 0 | 0 | 0 | / |
| Postoperative complications | 0.870 | ||||
| ≤ 3a | 46 | 50 | 45 | 50 | |
| > 3a | 5 | 1 | 6 | 5 |
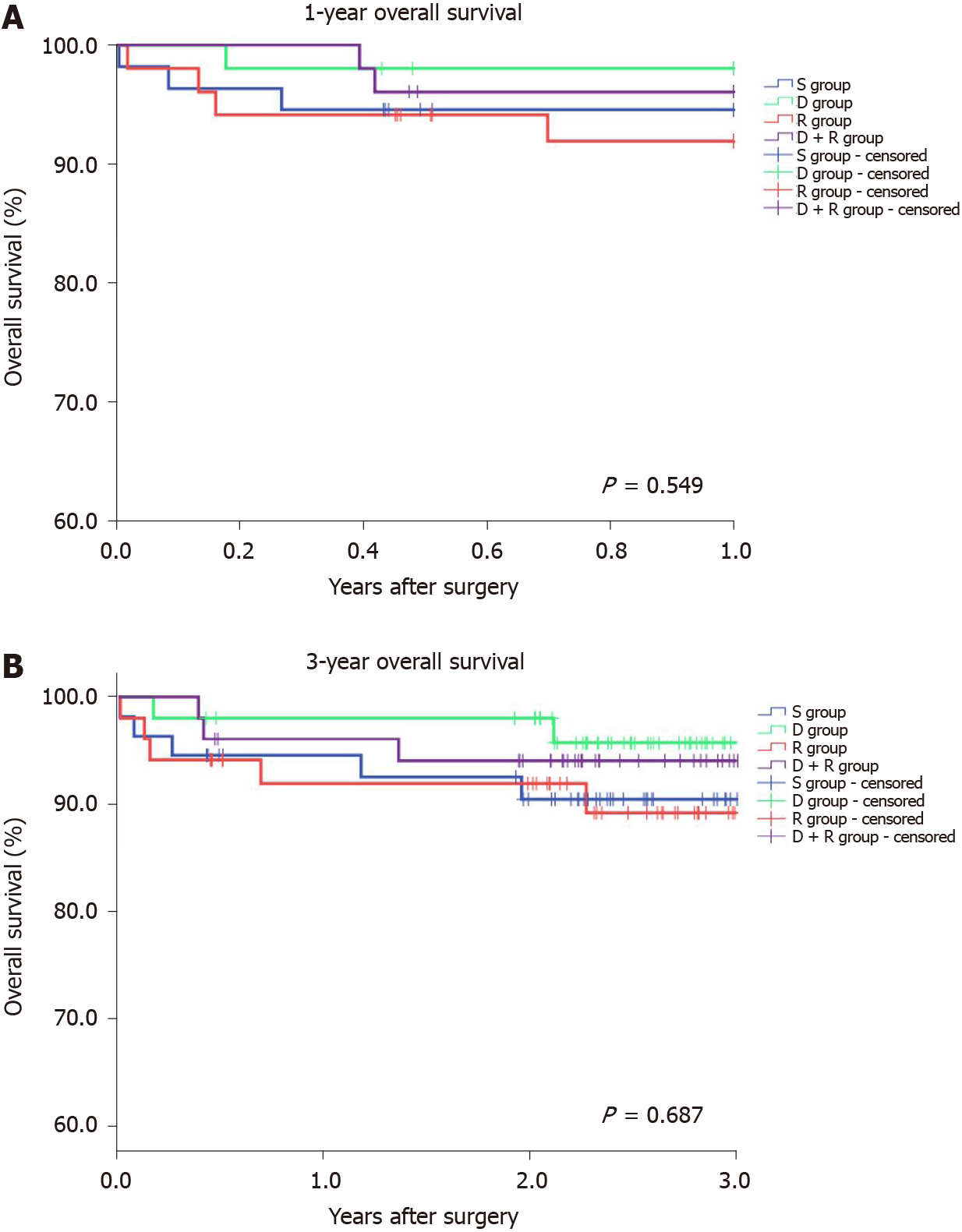
Thereafter, the overall survival among recipients was analyzed. Accordingly, the S-RIPC, D-RIPC, R-RIPC and DR-RIPC groups had a 3-year survival rate of 90.9%, 96.1%, 90.2%, and 92.2%, respectively, with no differences between all four groups (Figure 2).
Lastly, subgroup analyses were performed for recipients, with the results being similar to those for the primary endpoint and clinical outcomes among recipients (Table 4).
| Stratum | Mean difference in postoperative log maximal ALT compared with S-RIPC (95%CI) | Mean difference in postoperative log maximal AST compared with S-RIPC (95%CI) | ||||
| D-RIPC | R-RIPC | DR-RIPC | D-RIPC | R-RIPC | DR-RIPC | |
| Gender | ||||||
| Male | 0.03 (-0.31, 0.38) | 0.11 (-0.24, 0.47) | 0.11 (-0.25, 0.47) | 0.11 (-0.23, 0.44) | 0.20 (-0.14, 0.55) | 0.15 (-0.21, 0.50) |
| Female | -0.06 (-0.50, 0.37) | 0.21 (-0.21, 0.64) | 0.32 (-0.10, 0.73) | -0.06 (-0.41, 0.29) | 0.01 (-0.34, 0.35) | 0.19 (-0.15, 0.53) |
| PELD | ||||||
| ≤ 16 | -0.03 (-0.40, 0.33) | -0.05 (-0.40, 0.31) | 0.11 (-0.27, 0.48) | 0.15 (-0.18, 0.47) | -0.06 (-0.38, 0.27) | 0.17 (-0.16, 0.51) |
| > 16 | 0.02 (-0.40, 0.43) | 0.44 (0.02, 0.86) | 0.33 (-0.07, 0.73) | -0.11 (-0.46, 0.25) | 0.33 (-0.03, 0.69) | 0.17 (-0.17, 0.51) |
| Time of cold ischemia | ||||||
| < 60 | 0.28 (-0.07, 0.63) | 0.29 (-0.07, 0.64) | 0.29 (-0.09, 0.66) | 0.25 (-0.13, 0.63) | 0.27 (-0.11, 0.66) | 0.24 (-0.16, 0.65) |
| ≥ 60 | -0.30 (-0.72, 0.12) | 0.08 (-0.34, 0.50) | 0.18 (-0.22, 0.57) | -0.19 (-0.50, 0.12) | -0.05 (-0.35, 0.26) | 0.13 (-0.16, 0.42) |
| GRWR | ||||||
| 2.5-4 | 0.08 (-0.22, 0.38) | 0.25 (-0.04, 0.53) | 0.28 (-0.01, 0.58) | -0.01 (-0.27, 0.25) | 0.10 (-0.16, 0.35) | 0.19 (-0.07, 0.46) |
| < 2.5 or > 4 | -0.18 (-0.77, 0.40) | 0.07 (-0.62, 0.75) | 0.13 (-0.48, 0.74) | 0.12 (-0.41, 0.64) | 0.16 (-0.45, 0.77) | 0.14 (-0.40, 0.68) |
| Kasai operation | ||||||
| Yes | -0.11 (-0.47, 0.24) | 0.06 (-0.29, 0.42) | 0.18 (-0.18, 0.54) | 0.00 (-0.28, 0.29) | 0.00 (-0.29, 0.29) | 0.19 (-0.10, 0.48) |
| No | 0.15 (-0.29, 0.58) | 0.31 (-0.12, 0.74) | 0.28 (-0.15, 0.72) | 0.07 (-0.35, 0.50) | 0.25 (-0.17, 0.68) | 0.15 (-0.27, 0.57) |
The present randomized clinical trial showed that RIPC did not significantly improve liver functions or decrease incidences of EAD, PNF and postoperative complications among both recipients or donors. The primary end points, ALTmax and ASTmax, did not differ between the four groups regardless of whether donors or recipients received RIPC. Furthermore, no significant differences were found for incidences of EAD, PNF, postoperative complications and the overall survival of recipients. After further analyzing the effects of RIPC on donors, our still results suggested benefits were limited. Nonetheless, some protective effects of RIPC were observed in recipients, including a lower Cr level in the D-RIPC group than the S-RIPC group on postoperative day 0 (P < 0.05), although these were limited to alleviating IRI or improving the prognosis for patients who received LT.
The discovery of possible protective effects of RIPC in reducing IRI and improving organ function highlights a new therapy for clinical treatment[33]. The most important advantages of RIPC include its low cost, ease of performance and almost impeccable safety for patients. Thus, numerous clinical studies have been conducted to explore effects and potential mechanism of RIPC in different areas, such as organ transplantation, cardiac surgery, hepatic surgery and neurosurgery[9,18,34-36]. Studies have demonstrated that RIPC mainly occurs in two “windows,” one of which is the initial period following the preconditioning stimulus, which can last for 1-4 h[37,38], while the other happens at 24 h following preconditioning, which can last for 24-72 h[39]. Therefore, detecting related critical characteristics after surgery (0 d) is necessary until at least postoperative day 3. The present study continuously monitored liver function variables from day 0 to postoperative day 7 among recipients and day 0 to postoperative day 3 among donors. The ample amount of data has certainly helped us understand the dynamic changes in liver function, inflammatory response and kidney function of patients.
Some potential mechanisms whereby RIPC offers protection have been discussed and can be summarized into three components: Triggers, signal transduction and end-effectors[40]. Accordingly, performing RIPC allowed the local release of certain factors, such as adenosine, cytokines and endogenous opioids, termed “triggers,” thereby activating related protein kinase mediators (e.g., phosphoinositol 3-kinase, janus kinase and signal transducer and activator of transcription and protein kinase C) and signaling pathways[41,42]. Signal transduction plays a critical role in exerting the protective effects of RIPC, with some hypotheses having been presented. The two main competing hypotheses regarding the mechanism of signal transduction include “humoral hypothesis” and “neural hypothesis”[43-45]. “Humoral hypothesis” is supported by evidence showing that protection can be transferred by serum transfusion from a rabbit that has undergone ischemic preconditioning (IPC) to one that has not[46]. Meanwhile, “neural hypothesis” is also supported by a series of studies, especially in the cardiovascular and neural field. Lieder et al[44] found that RIPC could activate efferent vagal nerves to stimulate the spleen, which then releases humoral cardioprotective substances. Another study[47] showed that the cardioprotective effects of IPC were completely abolished by denervation of the limbs. To some extent, both the “humoral hypothesis” and “neural hypotheses” are reasonable and interact. After signal transduction, the end-effectors, which could be specific organs, cells or organelles, will finally be activated, and the protective effects induced by RIPC would be transformed into changes in cellular signal pathways[40,43].
A number of studies have focused on the effects of RIPC on graft transplantation. Accordingly, Jung et al[9] found that RIPC might be beneficial for postoperative liver function among recipients after living donor LT. AST level on postoperative day 1 and maximal AST level within 7 postoperative days were significantly lower in recipients who received a preconditioned graft. However, their results did not show any definite beneficial effects among donors. Also, no differences were found in the incidence of EAD or graft failure among recipients. A systematic review[10] that summarized solid organ transplantation and RIPC studies found controversial results, with some studies suggesting improvements in graft function, while others not showing any effects. In another meta-analysis[48], the researchers found that donor IPC promoted a large reduction in recipient mortality and incidence of PNF. However, still, no statistically significant difference had been observed. Overall, studies regarding RIPC and LT have been insufficient, especially for RIPC and pediatric LT. Stronger and more convincing clinical trials are thus needed to clarify the effects of RIPC on adult and pediatric LT.
To our knowledge, this has been the first randomized clinical trial to explore the effects of RIPC on pediatric LT. Moreover, we had discussed the effects of RIPC on recipients and donors simultaneously. Generally, RIPC had been performed on donors, while the grafts treated with RIPC were subsequently transplanted to recipients. However, the grafts were flushed to cleanse the organ of blood before storage and introduction into the recipients which could flush away potential protective “triggers” for alleviating IRI[49]. Therefore, RIPC among recipients seemed to promote better effects compared to RIPC among donors. Our study was designed such that patients were divided into four groups, which allowed us to understand comprehensively the effects of RIPC on both donors and recipients. Accordingly, our findings showed that RIPC might have fairly limited effects for protecting liver function or reducing incidences of EAD, PNF and postoperative complications among both donors and recipients. Though our study led to a negative result, it was of high significance and helped us understand the effects of RIPC in pediatric LT. Some reasons may help us understand these results. First of all, the muscle and neural system are relatively immature and undeveloped in children. As a result, the effects of RIPC may have been weakened when the RIPC was performed in recipients, compared with adults. Second, the potential protective “triggers” for alleviating IRI in grafts may have been flushed away before storage and introduction into the recipients. Given the differences in recipients, samples, interventions and group design, it is reasonable to assume that our findings may be inconsistent with those presented in studies that showed significant protective effects of RIPC[18,36].
Some limitations of the present study are worth noting. First, this was a single-center study. As such, the inclusion of more centers and more samples would strengthen the clinical significance of the study. Second, measuring more indicators of liver function and IRI, such as interleukin-2, interleukin-6, tumor necrosis factor, malonaldehyde and creatine phosphokinase, would provide more useful information. Third, owing to the lack of consensus regarding the optimal RIPC protocols for adults and children, the cycle and time could have been insufficient to yield the best beneficial effects. In future studies, we would like to attempt more intervention methods.
In conclusion, the present study suggested that RIPC may have limited beneficial effects on liver and renal function, overall survival or incidences of EAD, PNF and postoperative complications among recipients undergoing LT, as well as liver function among donors. Nonetheless, more clinical trials regarding the effects of RIPC on pediatric LT are warranted.
Studies suggested that remote ischemic preconditioning (RIPC) may effectively lessen the harmful effects of ischemia reperfusion injury (IRI) during organ transplantation surgery. However, the effect of RIPC on pediatric liver transplantation (LT) was still unknown.
To investigate the protective effects of RIPC on living liver donors and recipients following pediatric LT.
We performed this single-center randomized clinical trial to determine whether RIPC could be beneficial in reducing IRI among both donors and recipients undergoing pediatric living LT.
Two-hundred-eight donors were recruited and randomly assigned to four groups: S-RIPC group (no intervention), D-RIPC group (donors received RIPC), R-RIPC group (recipients received RIPC) and DR-RIPC group (both donors and recipients received RIPC). We primarily evaluated postoperative liver function among donors and recipients and incidences of early allograft dysfunction (EAD), primary nonfunction (PNF) and postoperative complications among recipients.
RIPC did not significantly improve alanine transaminase and aspartate aminotransferase levels among donors and recipients and decrease incidences of EAD, PNF and postoperative complications among recipients. Furthermore, RIPC had no effects on the overall survival of recipients.
The protective effects of RIPC were limited for recipients who received living LT, and no significant improvement of the prognosis was observed in recipients.
Our research suggested that RIPC may have limited beneficial effects for recipients undergoing LT as well as donors. Nonetheless, more clinical trials regarding the effects of RIPC on pediatric LT are warranted.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Carvalho JF, Di Pasqua L, Haruma K S-Editor: Huang P L-Editor: Filipodia P-Editor: Liu JH
| 1. | Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659-676. [PubMed] |
| 2. | Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Foutz J, Miller E, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant. 2020;20 Suppl:193-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 313] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 3. | Bodzin AS, Baker TB. Liver Transplantation Today: Where We Are Now and Where We Are Going. Liver Transpl. 2018;24:1470-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 4. | Meirelles Júnior RF, Salvalaggio P, Rezende MB, Evangelista AS, Guardia BD, Matielo CE, Neves DB, Pandullo FL, Felga GE, Alves JA, Curvelo LA, Diaz LG, Rusi MB, Viveiros Mde M, Almeida MD, Pedroso PT, Rocco RA, Meira Filho SP. Liver transplantation: history, outcomes and perspectives. Einstein (Sao Paulo). 2015;13:149-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Pham YH, Miloh T. Liver Transplantation in Children. Clin Liver Dis. 2018;22:807-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Starzl TE, Marchioro TL, Porter KA, Brettschneider L. Homotransplantation of the liver. Transplantation. 1967;5 Suppl:790-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Benkö T, Belker J, Gallinat A, Treckmann JW, Paul A, Minor T, Hoyer DP. Analysis of Data from the Oxygen Persufflation in Liver Transplantation (OPAL) Study to Determine the Role of Factors Affecting the Hepatic Microcirculation and Early Allograft Dysfunction. Ann Transplant. 2019;24:481-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Kohli R, Cortes M, Heaton ND, Dhawan A. Liver transplantation in children: state of the art and future perspectives. Arch Dis Child. 2018;103:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Jung KW, Kang J, Kwon HM, Moon YJ, Jun IG, Song JG, Hwang GS. Effect of Remote Ischemic Preconditioning Conducted in Living Liver Donors on Postoperative Liver Function in Donors and Recipients Following Liver Transplantation: A Randomized Clinical Trial. Ann Surg. 2020;271:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Farooqui W, Pommergaard HC, Rasmussen A. Remote ischemic preconditioning of transplant recipients to reduce graft ischemia and reperfusion injuries: A systematic review. Transplant Rev (Orlando). 2018;32:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Robertson FP, Fuller BJ, Davidson BR. An Evaluation of Ischaemic Preconditioning as a Method of Reducing Ischaemia Reperfusion Injury in Liver Surgery and Transplantation. J Clin Med. 2017;6:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Cannistrà M, Ruggiero M, Zullo A, Gallelli G, Serafini S, Maria M, Naso A, Grande R, Serra R, Nardo B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int J Surg. 2016;33 Suppl 1:S57-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 13. | Oliveira THC, Marques PE, Proost P, Teixeira MMM. Neutrophils: a cornerstone of liver ischemia and reperfusion injury. Lab Invest. 2018;98:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 14. | Yamada N, Karasawa T, Wakiya T, Sadatomo A, Ito H, Kamata R, Watanabe S, Komada T, Kimura H, Sanada Y, Sakuma Y, Mizuta K, Ohno N, Sata N, Takahashi M. Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: Potential role of ferroptosis. Am J Transplant. 2020;20:1606-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 15. | Lau JK, Roy P, Javadzadegan A, Moshfegh A, Fearon WF, Ng M, Lowe H, Brieger D, Kritharides L, Yong AS. Remote Ischemic Preconditioning Acutely Improves Coronary Microcirculatory Function. J Am Heart Assoc. 2018;7:e009058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Zhang W, Chen C, Jing R, Liu T, Liu B. Remote Ischemic Preconditioning Protects Cisplatin-Induced Acute Kidney Injury through the PTEN/AKT Signaling Pathway. Oxid Med Cell Longev. 2019;2019:7629396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM; ERICCA Trial Investigators. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med. 2015;373:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 560] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 18. | Wu G, Chen M, Wang X, Kong E, Yu W, Sun Y, Wu F. Effect of remote ischemic preconditioning on hepatic ischemia-reperfusion injury in patients undergoing liver resection: a randomized controlled trial. Minerva Anestesiol. 2020;86:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Abu-Amara M, Yang SY, Quaglia A, Rowley P, Tapuria N, Seifalian AM, Fuller BJ, Davidson BR. Effect of remote ischemic preconditioning on liver ischemia/reperfusion injury using a new mouse model. Liver Transpl. 2011;17:70-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Abu-Amara M, Yang SY, Quaglia A, Rowley P, Fuller B, Seifalian A, Davidson B. Role of endothelial nitric oxide synthase in remote ischemic preconditioning of the mouse liver. Liver Transpl. 2011;17:610-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Björnsson B, Winbladh A, Bojmar L, Sundqvist T, Gullstrand P, Sandström P. Conventional, but not remote ischemic preconditioning, reduces iNOS transcription in liver ischemia/reperfusion. World J Gastroenterol. 2014;20:9506-9512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Koneru B, Shareef A, Dikdan G, Desai K, Klein KM, Peng B, Wachsberg RH, de la Torre AN, Debroy M, Fisher A, Wilson DJ, Samanta AK. The ischemic preconditioning paradox in deceased donor liver transplantation-evidence from a prospective randomized single blind clinical trial. Am J Transplant. 2007;7:2788-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2218] [Cited by in RCA: 2793] [Article Influence: 186.2] [Reference Citation Analysis (0)] |
| 24. | Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Routledge, 1988. [DOI] [Full Text] |
| 25. | Hsu EK, Horslen SP, Reyes JD. Pediatric End-stage Liver Disease Scores as a Method of Assessing Mortality Risk or Prioritization to Transplantability: Let Us Save the Children. JAMA Pediatr. 2018;172:1015-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Kurian SM, Fouraschen SM, Langfelder P, Horvath S, Shaked A, Salomon DR, Olthoff KM. Genomic profiles and predictors of early allograft dysfunction after human liver transplantation. Am J Transplant. 2015;15:1605-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Kennedy EM, Wood RP, Shaw BW Jr. Primary nonfunction. Is there a contribution from the back table bath? Transplantation. 1990;49:739-743. [PubMed] |
| 28. | Li PK, Burdmann EA, Mehta RL. World Kidney Day 2013: acute kidney injury-global health alert. Am J Kidney Dis. 2013;61:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 874] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 30. | Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, Pollard S, Lerut J, Paul A, Garcia-Valdecasas JC, Rodríguez FS, Burroughs A; All contributing centers (www. eltr.org); European Liver and Intestine Transplant Association (ELITA). Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 643] [Article Influence: 49.5] [Reference Citation Analysis (2)] |
| 31. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24841] [Article Influence: 1182.9] [Reference Citation Analysis (0)] |
| 32. | Robertson FP, Goswami R, Wright GP, Fuller B, Davidson BR. Protocol for a prospective randomized controlled trial of recipient remote ischaemic preconditioning in orthotopic liver transplantation (RIPCOLT trial). Transplant Res. 2016;5:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic 'preconditioning' protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1003] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 34. | Guo L, Zhou D, Wu D, Ding J, He X, Shi J, Duan Y, Yang T, Ding Y, Ji X, Meng R. Short-term remote ischemic conditioning may protect monkeys after ischemic stroke. Ann Clin Transl Neurol. 2019;6:310-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Flechsig M, Ruf TF, Troeger W, Wiedemann S, Quick S, Ibrahim K, Pfluecke C, Youssef A, Sveric KM, Winzer R, Heinzel FR, Linke A, Strasser RH, Zhang K, Heidrich FM. Remote Ischemic Preconditioning Neither Improves Survival nor Reduces Myocardial or Kidney Injury in Patients Undergoing Transcatheter Aortic Valve Implantation (TAVI). J Clin Med. 2020;9:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Görlich D, Kellum JA, Meersch M; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 317] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 37. | Lawson CS, Downey JM. Preconditioning: state of the art myocardial protection. Cardiovasc Res. 1993;27:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 199] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 38. | Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 322] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 39. | Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 582] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 40. | Veighey K, MacAllister R. Clinical applications of remote ischaemic preconditioning in native and transplant acute kidney injury. Pediatr Nephrol. 2015;30:1749-1759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Hausenloy DJ, Lecour S, Yellon DM. Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxid Redox Signal. 2011;14:893-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 42. | Soares ROS, Losada DM, Jordani MC, Évora P, Castro-E-Silva O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int J Mol Sci. 2019;20:5034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 43. | Basalay MV, Davidson SM, Gourine AV, Yellon DM. Neural mechanisms in remote ischaemic conditioning in the heart and brain: mechanistic and translational aspects. Basic Res Cardiol. 2018;113:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 44. | Lieder HR, Kleinbongard P, Skyschally A, Hagelschuer H, Chilian WM, Heusch G. Vago-Splenic Axis in Signal Transduction of Remote Ischemic Preconditioning in Pigs and Rats. Circ Res. 2018;123:1152-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 45. | Skyschally A, Kleinbongard P, Lieder H, Gedik N, Stoian L, Amanakis G, Elbers E, Heusch G. Humoral transfer and intramyocardial signal transduction of protection by remote ischemic perconditioning in pigs, rats, and mice. Am J Physiol Heart Circ Physiol. 2018;315:H159-H172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Dickson EW, Reinhardt CP, Renzi FP, Becker RC, Porcaro WA, Heard SO. Ischemic preconditioning may be transferable via whole blood transfusion: preliminary evidence. J Thromb Thrombolysis. 1999;8:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, Redington A. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol. 2010;299:H1598-H1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 48. | Robertson FP, Bessell PR, Diaz-Nieto R, Thomas N, Rolando N, Fuller B, Davidson BR. High serum Aspartate transaminase levels on day 3 postliver transplantation correlates with graft and patient survival and would be a valid surrogate for outcome in liver transplantation clinical trials. Transpl Int. 2016;29:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Liu C, Loong CC, Hsia CY, Tsou MY, Tsai HL, Wei C. Retrograde arterial flush of the liver graft in living donor liver transplantation. J Invest Surg. 2009;22:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |