Published online Oct 21, 2021. doi: 10.3748/wjg.v27.i39.6715
Peer-review started: February 25, 2021
First decision: April 18, 2021
Revised: May 12, 2021
Accepted: September 2, 2021
Article in press: September 2, 2021
Published online: October 21, 2021
Processing time: 236 Days and 19.6 Hours
Dementia is a chronic progressive neurological disease affecting millions of people worldwide, and represents a relevant economic burden for healthcare systems. Although its pathogenesis is still unknown, recent findings have reported that a dysregulated gut-brain axis communication, a fundamental relationship mediated by several host and microbial molecules, is associated with cognitive disorders. In addition, gut microbiota manipulation reduces neuroinflammation, improving cognitive function by restoring the functional gut-brain axis.
To better define the effects of probiotics, prebiotics, synbiotics, and fecal microbiota transplant (FMT) on cognitive function.
We performed a literature search of human randomized clinical trials to examine the effects of the administration of probiotics, prebiotics, synbiotics, or FMT on cognition outcomes in healthy or sick people of every age, sex, and nationality. We systematically searched Embase, Medline/PubMed, Cochrane Library, central and clinicaltrials.gov databases with a combination of comprehensive terms related to cognition and gut microbiota manipulation. Then we carefully reviewed and synthesized the data by type of study design and setting, characteristics of the studied population, kind of intervention (strain type or mixture type, dosage, and frequency of administration), control treatment, inclusion and exclusion criteria, follow-up duration, and cognitive or memory outcomes.
After examining the titles and abstracts, the initial literature screening identified 995 articles, but we added 23 papers in our systematic review. The analyses of these selected studies highlighted that both probiotic supplementation and FMT improved cognitive function regardless of the type and posology of administration and the adopted cognitive tests and questionnaires. We found that most of the studies conducted in healthy people showed a significant positive effect of the intervention on at least one of the performed cognitive tests. Regarding unhealthy subjects, while FMT and especially probiotic administration had multiple beneficial effects on different cognitive functions, supplementation with prebiotics did not provide any cognitive improvement.
Probiotic supplementation and FMT may represent a promising strategy to restore gut eubiosis and enhance the cognitive functions of healthy people and patients with neurological disorders.
Core Tip: Dementia and cognitive impairment are age-related conditions that are on the rise worldwide. Recent studies have demonstrated the existence of a gut-brain axis and that the manipulation of gut microbiota composition can exert positive effects on cognition. The administration of probiotics, prebiotics, and fecal microbiota transplant may represent a good strategy to counteract gut dysbiosis and ameliorate cognitive dysfunction by reducing neuroinflammation and brain damage.
- Citation: Baldi S, Mundula T, Nannini G, Amedei A. Microbiota shaping — the effects of probiotics, prebiotics, and fecal microbiota transplant on cognitive functions: A systematic review. World J Gastroenterol 2021; 27(39): 6715-6732
- URL: https://www.wjgnet.com/1007-9327/full/v27/i39/6715.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i39.6715
Global population ageing, defined as the increasing proportion of older people around the globe, represents a deep shift in society and a considerable challenge for the sustainability of healthcare systems due to the rise of geriatric illnesses[1,2]. Currently, the prevalence of cognition impairment, particularly dementia, is estimated worldwide in 50 million people with an economic burden of 818 billion dollars in 2016 and a forecast of about 115 million people by 2050[3,4].
Dementia is an acquired, gradual, and progressive disorder involving multiple adverse neurocognitive changes that can affect learning processes, memory, executive function, language, complex attention, mood, perceptual-motor function or social cognition. Moreover, although its detailed pathological mechanism is still not well understood, dementia often occurs in association with advanced age or the presence of contributing causes, usually Alzheimer’s disease (AD), Parkinson’s disease (PD), or cerebrovascular pathology[5-7]. Unfortunately, current therapies are only sympto
Recent studies have shown that that the gut microbiota, with more than 100 trillion microorganisms carrying three times of human genes, plays a pivotal role in human health; manipulation of the intestinal microbiota can modify the release of neuroactive metabolites, which affect brain health[9,10]. This role can be further explained by the documented existence of the gut-brain axis, a complex bidirectional system in which communication occurs through three parallel and interplaying pathways that involve nervous, endocrine, and immune signals[11]. Therefore, different preclinical and observational studies have demonstrated that the gut dysbiosis is responsible for increased intestinal permeability, which correlates with both neuroinflammation and a decline of cognitive abilities[12-15].
Dietary interventions (in nutritional supplements or specific diets) have often been applied in clinical practice to restore intestinal eubiosis and prevent and treat cognitive disorders. For example, a Mediterranean diet and/or a healthy diet based on fruits, vegetables, and fish seems to stabilize or slow cognitive decline[16].
Nevertheless, the most promising strategy to counteract gut dysbiosis and to maintain cognitive function seems represented by the administration of probiotics, prebiotics, and fecal microbiota transplant (FMT).
Interestingly, administration in animal models of an adequate posology of multistrain probiotics reduces both Firmicutes/Bacteroidetes ratio and intestinal permeability, slowing cognitive decline and reducing neuroinflammation[17,18]. Moreover, using specific prebiotics seems to ameliorate cognitive performance with a direct effect on gut microbiota[19].
Moreover, even FMT has demonstrated remarkable efficacy in healthy subjects and people affected by various diseases caused by gut microbiota perturbation, particularly Clostridium difficile infection. It could represent a promising therapy for cognitive impairment improvement because of its capability to re-establish a healthy gut microbial community[20,21].
Therefore, since the evidence derived from human randomized clinical trials (RCTs) is currently limited, this systematic review identified the available RCTs and better defined the effects of probiotics, prebiotics, synbiotics, and FMT on cognitive functions.
Our study followed the PRISMA statement guidelines. A computerized search of the articles published until 24 October 2019 was conducted in Embase, Medline/PubMed, Cochrane Library, central and clinicaltrials.gov databases and other individual journal sources, using the following search string: (memory OR cognition OR dementia) AND (lactobacillus OR bifidobacteria OR streptococcus OR enterococcus OR probiotic OR prebiotic OR symbiotic OR fecal, transplantation). In the PubMed database, we activated the filter “Humans”; in Embase, we selected the filter “Research articles”; in Cochrane Library, we activated the filter “Trials”; and in clinicaltrials.gov, we selected the filter “recruitment: terminated or completed.” The search did not apply filters for language, country, duration of follow-up, and participants’ characteristics (age and sex).
Two authors independently reviewed the titles and abstracts of the collected articles, applying predefined inclusion/exclusion criteria. The inclusion criteria were as follows: RCTs; availability of full text; patients regardless of age, nationality, sex, and health status; comparison between oral intake of probiotics, prebiotics, or symbiotic and control treatment or placebo; and outcome as cognitive or memory evaluation. The adopted exclusion criteria were as follows: Studies with fewer than 10 participants; reviews, articles, and case reports; or studies with incomplete outcomes.
The same two authors performed analyses of the full text and data extraction with the intervention of a third author in case of poor agreement or discrepancies. Each reviewer independently recorded the data in a predefined data extraction form. The following data, if reported, were obtained from each selected trial: First author name, year of publication, study design, setting (institution, city, and country), characteristics of the studied population (mainly age and health status), number of total participants and their gender, number of subjects in both treatment and control groups, characteristics of the intervention (strain type or mixture type, dosage and frequency of administration), control treatment, inclusion and exclusion criteria, follow-up duration, cognitive or memory outcomes and compliance data.
For each selected study, cognitive functions were assessed through specific tests which evaluated the eight main cognitive skills: sustained attention, speed of information processing, cognitive flexibility and control, multiple simultaneous attention, working memory (short-term memory), category formation, pattern recognition, and response inhibition[22]. A detailed description of all cognitive tests performed in the selected papers for this systematic review is annexed in Supplementary material.
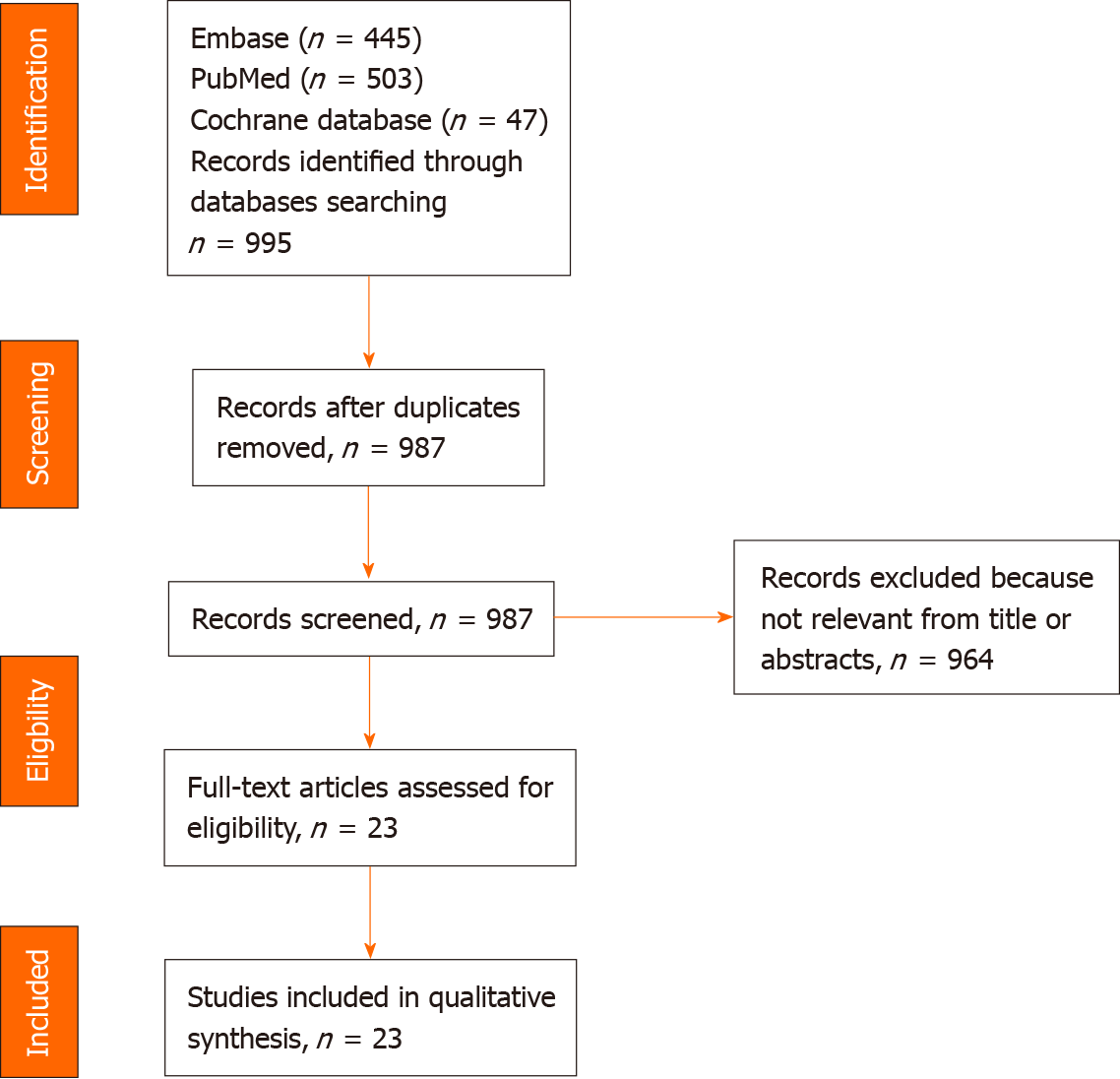
The initial literature screening identified 995 papers. Eight studies were excluded for duplication and another 964 papers were removed after the title and abstract screening because they did not respect inclusion criteria. The selection process, in accordance with the PRISMA statement 2009, is illustrated in Figure 1.
An overview of the 23 studies included in this systematic review is reported in Table 1. All 23 included papers were RCTs published from 2007 to 2019[23-45]. The total number of participants was 1285 (491 males and 650 females); unfortunately, both articles published by Tamtaji et al[44,45] did not report the gender of the participants. Regarding the age of the enrolled subjects, one study was conducted in healthy scholars (7-9 years)[31], four studies enrolled young adults (19-30 years)[25,36,40,43], and most of the studies involved adults or older people (48-95 years)[23,24,26-30,32-35,37-39,40,42,44,45]. Most studies (four) were performed in Iran[22,24,44,45]; three in the United States[26-28] and Japan[34,37,39]; two in the United Kingdom[29,31], South Korea[33,35], and Spain[30,41]; and one in Austria[25], Italy[32], Ireland[36], Malaysia[38], Poland[42], Wales[43] and the Netherlands[40].
| Ref. | Study design | Setting | Characteristics of the studied population | Number of participants (M/F) | Intervention | Comparison | Duration of intervention | Outcomes | Compliance |
| Agahi et al[23], 2018 | RCT | Cities: Emam Ali, Golabchi, Miad, Barekat; Country: Iran | Patients with Alzheimer disease; Age: 65-90 yr; Control group: 80.57 ± 1.79 yr; Intervention group: 79.70 ± 1.72 yr | Total: 48; Control group = 23 (10/13); Intervention group = 25 (7/18) | 1 capsule with L. fermentum, L. plantarum, B. lactis and 1 capsule with L. acidophilus, B. bifidum, and B. longum (3 × 109 CFU) | Placebo | 12 wk | TYM | - |
| Akbari et al[24], 2016 | RCT | Cities: Golabchi, Sadeghyeh; Country: Iran | Patients with Alzheimer disease; Age: 60-95 yr; Control group: 82.00 ± 1.69 yr; Intervention group: 77.67 ± 2.62 yr | Total: 60; Control group = 30 (24/6); Intervention group = 30 (24/6) | 200 mL/d probiotic milk containing L. acidophilus, L. casei, B. bifidum, and L. fermentum (2 × 109 CFU each) | Placebo | 12 wk | MMSE | - |
| Bagga et al[25], 2018 | RCT | City: Graz Country: Austria | Healthy volunteers; Age: 20-40 yr; Control group (placebo): 27.25 ± 5.78 yr; No intervention group: 23.87 ± 4.97 yr; Intervention group: 28.27 ± 4.2 yr | Total: 45; Control group = 15 (9/6); No intervention group = 15 (7/8); Intervention group = 15 (7/8) | 1 sachet/d with 3 g freeze-dried powder containing L. casei W56, L. acidophilus W22, L. paracasei W20, B. lactis W51, L. salivarius W24, L. lactis W19, B. lactis W52, L. plantarum W62 and B. bifidum W23(7.5 × 106 CFU/g) | Placebo or no intervention | 4 wk | PANAS, SCL-90, ADS, LEIDS, RM task, ED task | - |
| Bajaj et al[26], 2014 | RCT | City: Richmond, Virginia Country: United States | Patients with hepatic encephalopathy; Age: 18-65 yr; Control group: 58.5 ± 4.5 yr; Intervention group: 58.4 ± 3.8 yr | Total: 30; Control group = 16 (12/4); Intervention group = 14 (10/4) | L. rhamnosus GG (ATCC 53103)(> 50 billion CFU/gm) | Placebo | 8 wk | NCT-A, NCT-B, DS, BDT | - |
| Bajaj et al[27], 2017 | RCT | City: Richmond, Virginia Country: United States | Patients with hepatic encephalopathy; Mean age: 62 yr; Control group: 62.9 ± 9.8 yr; Intervention group: 64.5 ± 5.1 yr | Total: 20; Control group = 10 (10/0); Intervention group = 10 (10/0) | FMT units (90 mL total) instilled by enema and retained for 30 min | Standard of care | 20 wk | EncephalApp-Stroop, PHES | - |
| Bajaj et al[28], 2019 | RCT | City: Richmond, Virginia Country: United States | Patients with hepatic encephalopathy; Control group: 64.2 ± 6.2 yr; Intervention group: 63.3 ± 4.2 yr | Total: 20; Control group = 10 (8/2); Intervention group = 10 (8/2) | FMT capsules (550 μL of stool and buffer solution) | Placebo | 20 wk | EncephalApp-Stroop, PHES | - |
| Benton et al[29], 2007 | RCT | City: Swansea Country: Wales | Healthy volunteers; Age: 48-79 yr; Average age 61.8 ±7.3 yr | Total: 126 (51/75) | 65 mL of milk drink containing L. casei Shirota (108/mL) | Placebo | 3 wk | POMS, WMS, VFT, NART, Ability to recall the capital cities of countries | - |
| Buigues et al[30], 2016 | RCT | City: Valencia Country: Spain | People with frailty syndrome; Age: 66-90 yr; Control group: 73.4 ± 1.8 yr; Intervention group: 74.2 ± 1.6 yr | Total: 50; Control group = 22 (6 /16); Intervention group = 28 (9/19) | 7.5 g/d of Darmocare Pre® (Inulin 3375 mg, FOS 3488) | Placebo | 13 wk | MMSE | - |
| Capitão et al[31], 2020 | RCT | Cities: Swindon, Milton Keynes, London Country: United Kingdom | Healthy scholars; Age: 7-9 yr; Control group: 9.12 ± 1.02 yr; Intervention group: 8.54 ± 0.79 yr | Total: 35; Control group = 18 (12/6); Intervention group = 17 (12/5) | 5.5 g/d of Bimuno (B-GOS, Lactose, Glucose, Galactose) | Placebo | 12 wk | BAS-III, CogTrackTM battery, STAIC, MFQ | High (> 80%) |
| Ceccarelli et al[32], 2017 | RCT | City: Rome Country: Italy | HIV-1 infected individuals; Median age: 48 (IQR: 38-54) yr; Intervention group: 45 (35-52.5) yr; Control group: 43 (38.2-53) yr | Total: 35; Control group = 26 (24/2); Intervention group = 9 (9/0) | Sachet containing L. plantarum DSM 24730 S. thermophilus DSM 24731, B. breve DSM 24732, L. paracasei DSM 24733, L. delbrueckii subsp. bulgaricus DSM 24734, L. acidophilus DSM 24735 B. longum DSM 24736, and B. infantis DSM 24737(450 × 109 bacteria) | Control group | 24 wk | ROCF, RAVLT, STEP, VST, PVF, SVF, SPM, DS, CBTT, AAT, TMT A, TMT B | - |
| Chung et al[33], 2014 | RCT | City: Jeonju Country: Korea | Healthy volunteers; Age: 60-75 yr; Control group: 64.50 ± 4.84 yr; Intervention group (500 mg): 64.50 ± 2.17 yr; Intervention group (1000 mg): 64.43 ± 4.47 yr; Intervention group (2000 mg): 66.56 ± 4.98 yr | Total: 36; Control group = 10 (4/6); Intervention group (500 mg) = 10 (9/1); Intervention group (1000 mg) = 7 (2/5); Intervention group (2000 mg) = 9 (5/4) | Daily doses of 500, 1000, or 2000 mg. of tablet containing L. helveticus IDCC3801 | Placebo | 12 wk | DS, SRT, VLT, RVIP, SCWT | > 70% |
| Inoue et al[34], 2018 | RCT | City; Hyogo prefecture, Country: Japan | Healthy volunteers; Average age: 70.3 ± 3.1 yr; Control group: 70.9 ± 3.2 yr; Intervention group: 69.9 ± 3.0 yr | Total: 38; Control group = 18 (7/11); Intervention group = 20 (7/13) | Sachet containing lyophilised powder of B. longum BB536, B. infantis M-63, B. breve M-16V and B.breve B-3 (1.25 × 1010 CFU each) | Placebo | 12 wk | MoCA, Modified flanker task, PHQ-9, GAD-7 | > 99% |
| Hwang et al[35], 2019 | RCT | City: Jeonju Country: South Korea | People with mild cognitive impairment; Age: 55-85 yr; Control group: 69.2 ± 7.00 yr; Intervention group: 68.0 ± 5.12 yr | Total: 100; Control group = 50 (14/36); Intervention group = 50 (20/30) | Mixture of fermented soybean powder and L. plantarum C29 (1.25 × 1010 CFU/g) | Placebo | 12 wk | VLT, DS, ACPT | > 90% |
| Kelly et al[36], 2017 | RCT | City: Cork Country: Ireland | Healthy volunteers; Age: 20-33 yr; Placebo/Probiotic group: 23.6 ± 0.97 yr; Probiotic/Placebo group: 25.64 ± 1.14 yr | Total: 29; Placebo/Probiotic group = 15 (15/0); Probiotic/Placebo group = 14 (14/0) | Active capsules contained corn starch, magnesium stearate, silicon dioxide and L. Rhamnosus(1 × 109 CFU) | Placebo | 8 wk | MOT, PAL, AST, RVIP, ERT, Emotional Stroop | - |
| Kobayashi et al[37], 2019 | RCT | City: Tokyo Country: Japan | People with memory complaints; Age: 50-80 yr; Control group: 61.6 ± 6.37 yr; Intervention group: 61.5 ± 6.83 yr | Total: 117; Control group = 58 (29/29); Intervention group = 59 (29/30) | 1 capsule per day with B. breve A1 (> 2 × 1010 CFU) | Placebo | 12 wk | RBANS, MMSE | - |
| Lew et al[38], 2019 | RCT | Cities: Penang, Kubang Kerian Country: Malaysia | Stressed adults; Age: 18-60 yr; Control group: 32.1 ± 11.4 yr; Intervention group: 31.3 ± 10.8 yr | Total: 103; Control group = 51 (12/39); Probiotic group = 52 (12/40) | L. plantarum P8 (1010 CFU/sachet per day) | Placebo | 12 wk | PSS-10, DASS-42, CBB | - |
| Ohsawa et al[39], 2018 | RCT | Country: Japan | People with forgetfulness; 50-70 yr; Control group: 57.8 ± 5.9 yr; Intervention group: 58.5 ± 6.5 yr | Total: 60; Control group = 29 (13/16); Intervention group = 31 (13/18) | One bottle per day (190 g per bottle) of a L. helveticus-fermented milk contained 2.4 mg of lactononadecapeptide | Placebo | 8 wk | RBANS, POMS | - |
| Papalini et al[40], 2019 | RCT | City: Nijmegen Country: The Netherlands | Healthy volunteers; Age:18-40 yr; Control group: 22 yr (SE = 0.5); Intervention group: 21 yr (SE = 0.4) | Total: 58; Control group = 29 (0/29); Intervention group = 29 (0/29) | 2 g/d of powder diluted in water or milk containing B. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, L. lactis W19 L. lactis W58(5 × 109 CFU) | Placebo | 4 wk | BDI, LEIDS-r, Emotional face-word Stroop task, Emotional face-matching paradigm, SCWT, DS, SECPT | - |
| Roman et al[41], 2018 | RCT | City: Almerìa Country: Spain | Fibromyalgia patients; Control group: 50.27 ± 2.03 yr; Intervention group: 55.00 ± 2.09 yr | Total: 31; Control group = 15 (2/13); Intervention group = 16 (1/15) | 4 pills/d containing L. Rhamnosus GG®, L. casei, L. acidophilus, and B. Bifidus (6 × 106 bacteria per capsule) | Placebo | 8 wk | MMSE, BDI, IGT, Two-choice Task | - |
| Rudzki et al[42], 2019 | RCT | City: Bialystok Country: Poland | People with major depression; Control group: 38.90 (12) yr (SD); Intervention group: 39.13 (9.96) yr | Total: 60; Control group = 30 (10/20); Intervention group = 30 (7/23) | 2 capsules/d containing L. plantarum 299v (10 × 109 CFU per capsule) | Placebo | 8 wk | HAM-D 17, SCL-90, PSS-10, APT, RFFT, TMT A, TMT B, CVLT Stroop Test parts A and B | - |
| Smith et al[43], 2015 | RCT | City: Cardiff Country: Galles | Healthy volunteers; Age: 19-30 yr; Mean age 23.0 yr | Total: 47 (19/28) | One sachet of Inulin per day (5 mg) | Placebo | 4 h | Mood, Performance Tasks, Memory Tasks, Psychomotor Tasks, Selective Attention Tasks, Sustained Attention Task | - |
| Tamtaji et al[44], 2019 | RCT | City: Kashan, Shahrekord Country: Iran | Patients with Alzheimer disease; Age: 55-100 yr; Control group: 78.5 ± 8.0 yr; Intervention group (Selenium): 78.8 ± 10.2 yr; Intervention group (Selenium + probiotic): 76.2 ± 8.1 yr | Total: 79; Control group = 26; Intervention group (Selenium) = 26; Intervention group (Selenium + probiotic) = 27 | Selenium (200 μg/d) and probiotic containing L. acidophilus, | Placebo or only selenium (200 μg/d) | 12 wk | MMSE | 100% |
| Tamtaji et al[45], 2019 | RCT | City: Kashan Country: Iran | Patients with Parkinson disease; Age: 50-90 yr; Control group: 67.7 ± 10.2 yr; Intervention group: 68.2 ± 7.8 yr | Total: 60; Control group = 30; Intervention group = 30 | Probiotic containing L. acidophilus, B. bifidum, L. reuteri, and L. fermentum (each 2 × 109 CFU/g) | Placebo | 12 wk | MDS-UPDRS | 90% |
Concerning the patients’ health state, most studies enrolled healthy people[25,29,31,33-36,40,43], whereas three studies involved patients with AD[23,24,44] or cirrhotic subjects with recurrent encephalopathy[26-28]. The other studies were focused on stressed adults[38], patients with PD[45], human immunodeficiency virus (HIV)-1-infected individuals[32], subjects affected by fibromyalgia syndrome[41], people with major depression[42], elderly with frailty syndrome[30], and adults with forgetfulness[39] and mild cognitive impairment[37].
In the trials, subjects were administered probiotics[22-25,26,29,32-42,44,45], prebiotics[30,31,43], or FMT[27,28] and its duration lasted a maximum of 24 wk[32] and a minimum of 4 h[43]; however, the trials continued for 12 wk for most of the studies[23,24,31,33-35,37,38,44,45]. No studies have reported the administration of synbiotics. In the studies examining the effects of probiotics, a total of 21 different bacterial species were administered (alone or in combination) in a dosage ranging from 1 × 109 CFU/mL to 2.5 × 1010 CFU/mL; the most represented species were Lactobacillus plantarum, L. acidophilus, and Bifidobacterium bifidum. On the other side, the administered prebiotics was composed of inulin or galacto-oligosaccharides (GOS), in a dosage that ranged from 5 g/d to 7.5 g/d. In FMT studies, subjects were admin
Regarding the healthy subjects, three studies showed no significant difference between probiotic and placebo groups[29,31,36]. In comparison, five studies showed a significant positive effect of the intervention on at least one of the performed cognitive tests[25,33,35,40,43].
In Benton et al[29], the healthy enrolled subjects ingested fermented milk containing L. casei Shirota daily for 3 wk. However, no significant differences between the probiotic and placebo groups were reported regarding episodic and long-term memory, assessed with the Wechsler Memory Scale test and the ability to remember the capitals of 30 countries. Moreover, the healthy people treated with L. rhamnosus supplement in Kelly et al[36] did not report any cognitive improvement, as assessed with the Paired Associates Learning, Attention Switching Task, Rapid Visual Information-Processing task (RVIP), Emotion Recognition Task and electroencephalography tests.
Considering the five studies reporting a significant cognitive improvement, Bagga et al[25] found that 4 wk administration of a multistrain probiotic increased Positive and Negative Affect Schedule (PANAS) score (paired with the response accuracy to unpleasant stimuli test) and showed the activation of the cingulum, pre-cuneum and cerebellum areas, involved in decision making and memory process.
Papalini et al[40] tested a probiotic multistrain mixture in women who underwent a stressful condition for 4 wk. The results showed that the trial reduced the unfavorable stress effect on working memory performance measured by the DS backward test. In addition, Chung et al[33] demonstrated a significant improvement in Verbal Learning Test, Story Recall Test, RVIP and Stroop Color and Word Test after 12 wk administration of L. helveticus in healthy subjects compared to placebo. Finally, Inoue et al[34] demonstrated that intervention with Bifidobacterium spp. for 12 wk, added to resistance training, significantly improved response accuracy and reaction time tests in healthy elderly subjects. Regarding the cognitive effects of prebiotic administration, the study conducted in healthy children by Capitão et al[31] reported that 12 wk GOS supplement only improved memory retrieval speed assessed with the CogTrackTM test battery. By contrast, Smith et al[43] investigated the acute effects of inulin intake on healthy volunteers and reported improving memory tasks, especially immediate free and delayed recall. No FMT intervention has been carried out in healthy subjects. Hence, although three of eight studies conducted in healthy subjects showed no significant difference between intervention and placebo groups, probiotics resulted were more effective in improving cognitive function than prebiotics.
The effects of probiotics, prebiotics, and FMT on cognitive functions were also assessed in different diseases, of which the most represented were hepatic encephalopathy (HE) and AD. Bajaj et al[26] conducted three studies on HE. In particular, the authors first investigated the effect of Lactobacillus GG administration on HE but did not report changes in cognition. However, they also treated HE patients with FMT via enema and reported a significant improvement in PHES and EncephalApp Stroop tests[27]. Moreover, Bajaj et al[28] evaluated the treatment with FMT capsules effects on HE patients, and they reported only a significant improvement in the EncephalApp Stroop test.
Regarding AD, Agahi et al[23] administered two different multistrain probiotic capsules to patients affected by severe disease for 12 wk, but no effect on TYM cognitive tests were reported. By contrast, Akbari et al[24] found that daily administration of probiotic milk enriched with Lactobacillus spp. led to a decline in Mini Mental State Evaluation (MMSE) score in AD patients compared to placebo. Moreover, Tamtaji et al[44] found that a probiotic and selenium co-supplement in AD patients was responsible for a significant increase in MMSE score. In addition, Hwang et al[35] found that people with mild cognitive impairment showed an improvement in a battery of tests related to verbal memory and attention domains after ingesting a mixture of L. plantarum C29 and fermented soybean powder. Finally, Lew et al[38] reported that daily administration of L. plantarum P8 for 12 wk in stressed adults led to a reduction of stress score and enhanced cognition and verbal learning memory, assessed through the CBB. Another study, conducted by Roman et al[41], explored the effect of a multispecies probiotic on fibromyalgia patients and found a significantly reduced number of impulsive choices. Moreover, Kobayashi et al[37] carried out a 12-wk treatment with Bifidobacterium breve A1 in elderly subjects with memory complaints, documenting a significant decline of total scores of both Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and MMSE tests. Regarding patients affected by PD, Tamtaji et al[45] highlighted a favorable reduction of the Movement Disorders Society-Unified PD Rating Scale (MDS-UPDRS) after ingesting a probiotic mixture for 12 wk. In addition, Ohsawa et al[39] reported improved attention, coding, and delayed memory scores (assessed with RBANS) in people with forgetfulness after 8 wk intake of a L. helveticus fermented milk drink. Also, Rudzki et al[42] reported a significant improvement in CVLT and APT in people with major depression treated with L. plantarum 299v. Moreover, Ceccarelli et al[32] demonstrated a significant improvement in several cognitive functions in HIV-1 infected patients ingesting a multistrain probiotic for 24 wk (primarily in the following neurocognitive tests: Rey-Osterrieth Complex Figure, Rey Auditory Verbal Learning Test, Test of Time and Weights Estimation, Phonological Verbal Fluency Test, Corsi Block Tapping Test and Trail Making Test A). Finally, the only study which assessed the effectiveness of a prebiotic intake (inulin and fructooligosaccharides), conducted by Buigues et al[30] on elderly affected by frailty syndrome, the MMSE did not report significant cognitive improvement. As a result, while the FMT and especially probiotics played multiple beneficial effects on different cognitive functions of unhealthy subjects, the prebiotics’ supplementation did not provide any cognitive improvement, maybe because of their short-term administration.
Dementia is a chronic, gradual, and progressive neurological disease that affects millions of people in both industrialized and rural countries. Cognitive decline and daily activities impairment limits patients’ self-care and causes a severe burden to parents, friends, caregivers, and especially to the healthcare systems[46,47]. Increasing evidence suggests that the prevalence of dementia rises with age and is strongly associated with other comorbidities, including AD and cardiovascular risk factors such as hypertension and hypercholesterolaemia[48].
Although the specific dementia pathogenesis is not yet understood and current therapies only attempt to counterbalance the disturbance, several studies recently highlighted the central role of the gut microbiota in brain health and the onset and persistence of neurodegenerative diseases[49,50]. Nevertheless, our systematic review of human RCTs reported contradictory results due to the diverse type, posology, and duration of interventions and the different responses of healthy or diseased people to the treatment.
In general, supplementation with probiotics and prebiotics determined the positive effects on healthy subjects. Five (63%) out[25,33,34,40,43] of the eight studies conducted in volunteers reported beneficial effects on the cognitive functions, while the other three[29,31,36] studies did not find any difference between the intervention and control groups.
Regarding the different evaluated patients, only 3 (20%)[23,26,30] of the 15 studies did not report an amelioration of cognitive functions for other possible reasons. For example, the probiotics’ administration performed by Bajaj et al[26] probably did not last for a sufficient time to obtain cognitive improvement in patients with HE. In contrast, with 13 wk of prebiotics’ supplementation, Buigues et al[30] did not observe effects on cognitive behaviour because MMSE does not represent a sensitive tool to detect the small changes in cognition that may occur after inulin and FOS supplementation. In addition, in the study conducted by Agahi et al[23], the 12 wk probiotic administration did not lead to cognitive amelioration in patients with AD; a probable explanation could be the enrollment of only patients with advanced disease.
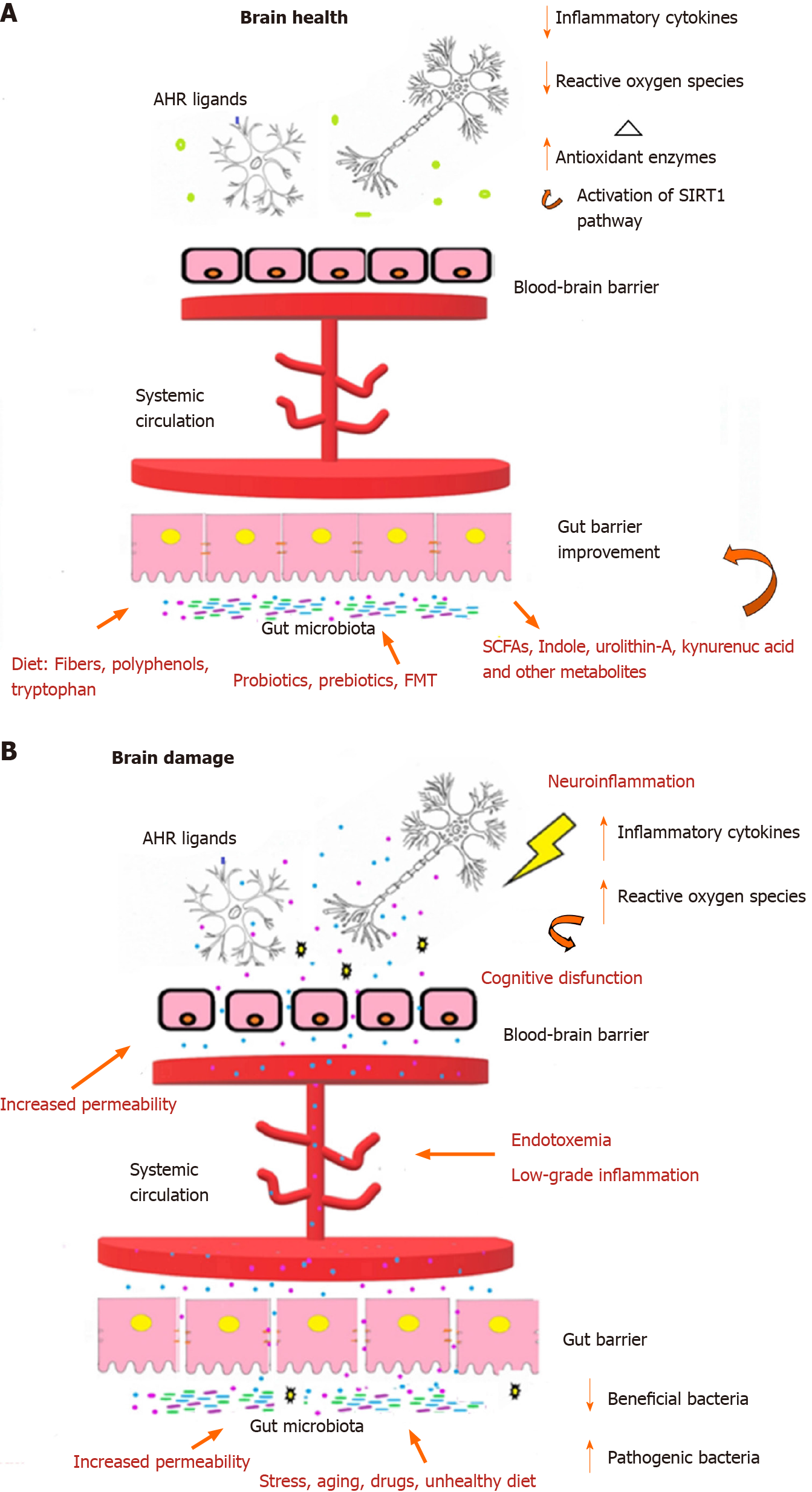
Probiotic supplementation improves cognitive functions in many different diseases such as HIV[32], PD[45], fibromyalgia, major depression[42], AD[24,44], and other mild cognitive deficits[35,37-39]. Furthermore, studies evaluating the effects of FMT on patients with HE highlighted a significant amelioration in cognitive functionality[27,28]. It is well established that a balanced gut microbiota composition (eubiosis condition) plays a crucial role in our health; a dysbiotic status (meaning a reduced gut microbiota diversity) is related to many human gastrointestinal, immunological, and neurodegenerative diseases[51]. Concerning neurological impairments, recent findings elucidated the importance of the gut microbiota in the bidirectional communication between the central and enteric nervous systems, called the gut-brain axis[52]. Hence, the main factors responsible for intestinal dysbiosis such as stress, unbalanced diet, and drug abuse also determine an alteration of the gut-brain axis by causing a loss of epithelial integrity. The loss of this barrier functionality allows microbial-derived molecules to enter the systemic circulation, promoting endotoxemia, oxidative stress and low-grade inflammation responsible for the blood-brain barrier disruption[53,54] (Figure 2); these factors represent a signature for neurodegenerative disorders, especially AD. Consequently, given the importance of the intestinal barrier integrity for the prevention of neuroinflammation and brain damage, gut microbiota modulation by psychobiotics, namely beneficial bacteria (probiotics) or support for such bacteria (prebiotics) and FMT, represent an excellent strategy to restore the intestinal permeability and prevent the consequences of a leaky gut[55-57].
However, although several studies have highlighted the local beneficial effects of probiotics, prebiotics and FMT (e.g., modification of the gut microbiota composition, strengthening of the gut epithelial barrier and modulation of the local (mucosal) immune system), they also exerted systemic effects, in particular on the central nervous system[58,60]. More specifically, recent studies have reported that the intestinal microbiota affects neurodevelopment and diverse brain functions by regulating the gut-brain axis, for example, by acting on the electrophysiological thresholds of the enteric nervous system neurons, which interact via neurotransmitters (adrenaline, noradrenaline, and acetylcholine) with the central nervous system[61].
Another important neuronal pathway in gut-brain communication involves the vagus nerve, and many effects of probiotics strains influence its activity[62]. Furthermore, since the gut houses the most extensive collection of lymphoid tissues in the human body and various intestinal immune cells can cross the blood-brain barrier, gut microbiota manipulation represents a key indirect route for communication between the gut microbiota and the central nervous system[63]. Intriguingly, specific probiotic formulations have also been shown an ability to stimulate the production of neurotransmitters (e.g., GABA, serotonin, and dopamine) or are even microbially neuroactive. These microbial metabolites can trigger epigenetic signals on human brain genes involved in various complex networks or act as a ligand for specific human receptors[64].
For instance, the probiotic activated Sirtuin 1 pathway, which regulates the brain antioxidant enzymes such as superoxide dismutase and glutathione peroxidase, could favor cognitive improvements by preventing oxidative stress and deposition of beta-amyloid in the brain[65]. Even the modulation of kynurenine metabolism, the primary route for tryptophan catabolism, which is closely related to the structural and functional dynamics of the gut microbiota, could positively affect brain health[66]. Indeed, in vivo L. plantarum administration demonstrated a beneficial reduction of kynurenines as most act as neurotoxic compounds[42,67,68]. Notably, although L. plantarum was administered to healthy or ill people in most of our selected RCTs, its positive effects have been probably underestimated because of the unknown impact of the other components of the probiotic formula that include it.
By contrast, indole-3-lactic acid (ILA) is an interesting neuroprotective tryptophan metabolite mainly produced by Bifidobacterium spp. acting as an aryl hydrocarbon receptor (AhR) agonist, expressed by intestinal and neuronal cells[69,70]. In detail, microbial agonists produced by L. bulgaricus and L. reuteri could activate microglia and astrocytes AhRs, suppressing pro-inflammatory signals and preventing neuronal damage[71-73]. Furthermore, the administration of some probiotics (especially L. helveticus, L. casei and L. rhamnosus) and prebiotics could also improve cognitive functions by stimulating the production of short-chain fatty acids as they enhance the transcription of the brain-derived neurotrophic factor that stimulates neuronal plasticity, protecting against neuroinflammation and neuronal apoptosis[74-78]. Moreover, the FMT represents a very promising strategy to re-establish gut eubiosis and improve cognitive functions. For instance, in transgenic mice, FMT significantly improved cognitive deficits, beta-amyloid accumulation, and neuroinflammation while reducing UPDRS score and tremor in people with Parkinson disease[79].
Finally, our recent study demonstrated that age-associated shifts of the microbiota have a detrimental impact on the central nervous system’s protein expression and critical functions. Still, FMT represents an excellent strategy to restore a young-like microbiota and improve cognitive functions[80]. Therefore, although the modulation of intestinal microbiota represents a new precious therapeutic opportunity, it also shows some restrictions and risks. In particular, even if probiotics are generally considered safe and have many advantages such as a tolerated mode of administration (orally) and the possibility to integrate them with other pharmacological/non-pharmacological approaches, they displayed some limitations mainly due to potential side effects, especially in some patients (including immunocompromised people), or to their long-term safety[81]. Besides, even if probiotics can promote the production of several compounds such as lactic acid, bioamines, bile salts and other molecules that could play detrimental effects on the host, most of them are sold as dietetic supplements, and the regulatory agencies do not require safety studies in humans before their commercialization[82,83].
Although reported to be fairly safe in most clinical trials, FMT can be responsible for acute or prolonged adverse effects such as diarrhea, abdominal pain, nausea, headaches, and fatigue[84]. In particular, immunological concerns have been raised regarding safety assessments for both probiotics and the FMT because either indigenous or transient microorganisms could impact the immune system’s functionality. Hence, the FMT application or the administration of probiotics to specific vulnerable populations and stressed or aged people, immunocompromised patients, newborns or pregnant women must be well evaluated to prevent microbial translocation and sepsis[85-87]. Moreover, the current literature lacks information about the long-term administration of probiotics; therefore, the possible horizontal transfer of antibiotic resistance genes favored by their supplementation cannot be excluded. Likewise, because stool contains thousands of microorganisms and a vast number of metabolites, FMT represents a constant risk of pathogens or commensals transfer to donors that may harmfully affect them[88].
As a final note, the different defects found in the evaluated studies highlighted some methodical limitations such as small sample sizes, the limited sampling time and the wide range of other cognitive tests. Supplementation of probiotics and FMT could represent a non-invasive successful strategy to restore gut eubiosis and enhance cognitive functions in healthy people and patients with different neuro
Due to the global population aging, cognitive impairments will affect approximately 115 million people by 2050. Since current therapies only attempt to counterbalance cognitive disorders, many recent studies recently highlighted the central role of the gut microbiota in brain health.
The pathogenesis of several cognitive disorders is still not fully understood; however, it has been recently established that a dysregulated gut-brain axis communication is associated with the onset and persistence of neurodegenerative diseases. Thus, gut microbiota manipulation could restore a functional gut-brain axis improving cognitive functions.
Since the evidence derived from human randomized clinical trials (RCTs) is currently limited, the main purpose of this systematic review was to detect the currently available RCTs, to define better the effects of probiotics, prebiotics, and fecal microbiota transplant (FMT) on cognitive functions.
We systematically searched Embase, Medline/PubMed, Cochrane Library, central and clinicaltrials.gov databases with a combination of comprehensive terms related to cognition and gut microbiota manipulation. Then, we carefully reviewed and synthesized the data by types of study design and setting, characteristics of the studied population, kind of the intervention (strain type or mixture type, dosage and frequency of administration), control treatment, inclusion and exclusion criteria, follow-up duration, and cognitive or memory outcomes.
The analysis of the 23 included in our systematic review highlighted that, although the different type and posology of administration and the various cognitive tests and questionnaires adopted, both probiotics supplementation and FMT improved the cognitive functions in most of healthy people and patients affected by different neurological pathologies.
The gut microbiota manipulation could represent a good strategy to counteract gut dysbiosis and so ameliorate cognitive dysfunction.
The supplementation of probiotics and FMT could represent a non-invasive successful strategy to restore gut eubiosis and enhance cognitive functions in healthy people and patients with different neurological/neurodegenerative diseases.
We thank Dr. Stroobant M for the language editing of the manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: García-Mena J, Qi R, Sales-Campos H S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | Land KC, Lamb VL. Demography of Aging. In: Heggenhougen K, Stella Q. International Encyclopedia of Public Health. San Diego: Academic Press, 2008: 89-95. |
| 2. | Brayne C, Miller B. Dementia and aging populations-A global priority for contextualized research and health policy. PLoS Med. 2017;14:e1002275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Tomaskova H, Kuhnova J, Cimler R, Dolezal O, Kuca K. Prediction of population with Alzheimer's disease in the European Union using a system dynamics model. Neuropsychiatr Dis Treat. 2016;12:1589-1598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O'Donnell M, Sullivan R, Yusuf S. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1317] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 5. | Sacuiu SF. Dementias. Handb Clin Neurol. 2016;138:123-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement. 2015;11:710-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 458] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 7. | Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016;36:172-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 314] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 8. | Yiannopoulou KG, Papageorgiou SG. Current and future treatments for Alzheimer's disease. Ther Adv Neurol Disord. 2013;6:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 425] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 9. | Boem F, Amedei A. Healthy axis: Towards an integrated view of the gut-brain health. World J Gastroenterol. 2019;25:3838-3841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front Neuroendocrinol. 2018;51:80-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 11. | Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2403] [Cited by in RCA: 2966] [Article Influence: 228.2] [Reference Citation Analysis (1)] |
| 12. | Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A. Gut microbiota in autism and mood disorders. World J Gastroenterol. 2016;22:361-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 248] [Cited by in RCA: 275] [Article Influence: 30.6] [Reference Citation Analysis (10)] |
| 13. | Noble EE, Hsu TM, Kanoski SE. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front Behav Neurosci. 2017;11:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 204] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 14. | Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, Meschi T. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. 2018;13:1497-1511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 15. | Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C, Fiorini D, Boarelli MC, Rossi G, Eleuteri AM. Microbiota modulation counteracts Alzheimer's disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7:2426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 325] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 16. | Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: World Health Organization; 2019 . [PubMed] |
| 17. | Chen D, Yang X, Yang J, Lai G, Yong T, Tang X, Shuai O, Zhou G, Xie Y, Wu Q. Prebiotic Effect of Fructooligosaccharides from Morinda officinalis on Alzheimer's Disease in Rodent Models by Targeting the Microbiota-Gut-Brain Axis. Front Aging Neurosci. 2017;9:403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 18. | Larsen N, Vogensen FK, Gøbel RJ, Michaelsen KF, Forssten SD, Lahtinen SJ, Jakobsen M. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin Nutr. 2013;32:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini G, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Högenauer C, Malfertheiner P, Mattila E, Milosavljević T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A; European FMT Working Group. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 853] [Cited by in RCA: 784] [Article Influence: 98.0] [Reference Citation Analysis (1)] |
| 20. | Sun J, Xu J, Ling Y, Wang F, Gong T, Yang C, Ye S, Ye K, Wei D, Song Z, Chen D, Liu J. Fecal microbiota transplantation alleviated Alzheimer's disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry. 2019;9:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 333] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 21. | Goloshchapov OV, Olekhnovich EI, Sidorenko SV, Moiseev IS, Kucher MA, Fedorov DE, Pavlenko AV, Manolov AI, Gostev VV, Veselovsky VA, Klimina KM, Kostryukova ES, Bakin EA, Shvetcov AN, Gumbatova ED, Klementeva RV, Shcherbakov AA, Gorchakova MV, Egozcue JJ, Pawlowsky-Glahn V, Suvorova MA, Chukhlovin AB, Govorun VM, Ilina EN, Afanasyev BV. Long-term impact of fecal transplantation in healthy volunteers. BMC Microbiol. 2019;19:312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Neisser U. Cognitive psychology. Englewood Cliffs: Prentice-Hall ll, Inc., 1967. |
| 23. | Agahi A, Hamidi GA, Daneshvar R, Hamdieh M, Soheili M, Alinaghipour A, Esmaeili Taba SM, Salami M. Does Severity of Alzheimer's Disease Contribute to Its Responsiveness to Modifying Gut Microbiota? Front Neurol. 2018;9:662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 24. | Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, Hamidi GA, Salami M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer's Disease: A Randomized, Double-Blind and Controlled Trial. Front Aging Neurosci. 2016;8:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 610] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 25. | Bagga D, Reichert JL, Koschutnig K, Aigner CS, Holzer P, Koskinen K, Moissl-Eichinger C, Schöpf V. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes. 2018;9:486-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 26. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK, Luketic V, Stravitz RT, Siddiqui MS, Fuchs M, Thacker LR, Wade JB, Daita K, Sistrun S, White MB, Noble NA, Thorpe C, Kakiyama G, Pandak WM, Sikaroodi M, Gillevet PM. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther. 2014;39:1113-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 222] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 27. | Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 453] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 28. | Bajaj JS, Salzman NH, Acharya C, Sterling RK, White MB, Gavis EA, Fagan A, Hayward M, Holtz ML, Matherly S, Lee H, Osman M, Siddiqui MS, Fuchs M, Puri P, Sikaroodi M, Gillevet PM. Fecal Microbial Transplant Capsules Are Safe in Hepatic Encephalopathy: A Phase 1, Randomized, Placebo-Controlled Trial. Hepatology. 2019;70:1690-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 29. | Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 395] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 30. | Buigues C, Fernández-Garrido J, Pruimboom L, Hoogland AJ, Navarro-Martínez R, Martínez-Martínez M, Verdejo Y, Mascarós MC, Peris C, Cauli O. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 31. | Capitão LP, Baião R, Baek HK, Kappelmann N, Sharman R, Harvey CJ, Montgomery P, Burnet PW. Prebiotic supplementation does not affect reading and cognitive performance in children: A randomised placebo-controlled study. J Psychopharmacol. 2020;34:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Ceccarelli G, Brenchley JM, Cavallari EN, Scheri GC, Fratino M, Pinacchio C, Schietroma I, Fard SN, Scagnolari C, Mezzaroma I, Vullo V, d'Ettorre G. Impact of High-Dose Multi-Strain Probiotic Supplementation on Neurocognitive Performance and Central Nervous System Immune Activation of HIV-1 Infected Individuals. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Chung YC, Jin HM, Cui Y, Kim DS, Jung JM, Park JI, Jung ES, Choi EK, Chae SW. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J Funct Foods. 2014;10:465-474. [RCA] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 34. | Inoue T, Kobayashi Y, Mori N, Sakagawa M, Xiao JZ, Moritani T, Sakane N, Nagai N. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef Microbes. 2018;9:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Hwang YH, Park S, Paik JW, Chae SW, Kim DH, Jeong DG, Ha E, Kim M, Hong G, Park SH, Jung SJ, Lee SM, Na KH, Kim J, Chung YC. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 36. | Kelly JR, Allen AP, Temko A, Hutch W, Kennedy PJ, Farid N, Murphy E, Boylan G, Bienenstock J, Cryan JF, Clarke G, Dinan TG. Lost in translation? Brain Behav Immun. 2017;61:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 242] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 37. | Kobayashi Y, Kuhara T, Oki M, Xiao JZ. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Benef Microbes. 2019;10:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 38. | Lew LC, Hor YY, Yusoff NAA, Choi SB, Yusoff MSB, Roslan NS, Ahmad A, Mohammad JAM, Abdullah MFIL, Zakaria N, Wahid N, Sun Z, Kwok LY, Zhang H, Liong MT. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin Nutr. 2019;38:2053-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 39. | Ohsawa K, Nakamura F, Uchida N, Mizuno S, Yokogoshi H. Lactobacillus helveticus-fermented milk containing lactononadecapeptide (NIPPLTQTPVVVPPFLQPE) improves cognitive function in healthy middle-aged adults: a randomised, double-blind, placebo-controlled trial. Int J Food Sci Nutr. 2018;69:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | Papalini S, Michels F, Kohn N, Wegman J, van Hemert S, Roelofs K, Arias-Vasquez A, Aarts E. Stress matters: Randomized controlled trial on the effect of probiotics on neurocognition. Neurobiol Stress. 2019;10:100141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 41. | Roman P, Estévez AF, Miras A, Sánchez-Labraca N, Cañadas F, Vivas AB, Cardona D. A Pilot Randomized Controlled Trial to Explore Cognitive and Emotional Effects of Probiotics in Fibromyalgia. Sci Rep. 2018;8:10965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 42. | Rudzki L, Ostrowska L, Pawlak D, Małus A, Pawlak K, Waszkiewicz N, Szulc A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 321] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 43. | Smith AP, Sutherland D, Hewlett P. An Investigation of the Acute Effects of Oligofructose-Enriched Inulin on Subjective Wellbeing, Mood and Cognitive Performance. Nutrients. 2015;7:8887-8896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, Tajabadi-Ebrahimi M, Asemi Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: A randomized, double-blind, controlled trial. Clin Nutr. 2019;38:2569-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 45. | Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, Oryan S, Mafi A, Asemi Z. Clinical and metabolic response to probiotic administration in people with Parkinson's disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38:1031-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 257] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 46. | Lee MT, Jang Y, Chang WY. How do impairments in cognitive functions affect activities of daily living functions in older adults? PLoS One. 2019;14:e0218112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 47. | Bunn F, Burn AM, Goodman C, Rait G, Norton S, Robinson L, Schoeman J, Brayne C. Comorbidity and dementia: a scoping review of the literature. BMC Med. 2014;12:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 300] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 48. | Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11:111-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 548] [Cited by in RCA: 701] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 49. | Vuotto C, Battistini L, Caltagirone C, Borsellino G. Gut Microbiota and Disorders of the Central Nervous System. Neuroscientist. 2020;26:487-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016;39:763-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 646] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 51. | Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99:1877-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 2786] [Article Influence: 464.3] [Reference Citation Analysis (2)] |
| 52. | Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol. 2018;6:133-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 796] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 53. | Liu S, Gao J, Zhu M, Liu K, Zhang HL. Gut Microbiota and Dysbiosis in Alzheimer's Disease: Implications for Pathogenesis and Treatment. Mol Neurobiol. 2020;57:5026-5043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 289] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 54. | Cerovic M, Forloni G, Balducci C. Neuroinflammation and the Gut Microbiota: Possible Alternative Therapeutic Targets to Counteract Alzheimer's Disease? Front Aging Neurosci. 2019;11:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 55. | Rao RK, Samak G. Protection and Restitution of Gut Barrier by Probiotics: Nutritional and Clinical Implications. Curr Nutr Food Sci. 2013;9:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 56. | Cheng S, Ma X, Geng S, Jiang X, Li Y, Hu L, Li J, Wang Y, Han X. Fecal Microbiota Transplantation Beneficially Regulates Intestinal Mucosal Autophagy and Alleviates Gut Barrier Injury. mSystems. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 57. | Li LL, Wang YT, Zhu LM, Liu ZY, Ye CQ, Qin S. Inulin with different degrees of polymerization protects against diet-induced endotoxemia and inflammation in association with gut microbiota regulation in mice. Sci Rep. 2020;10:978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 58. | Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61:160-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 921] [Cited by in RCA: 692] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 59. | Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of Action of Probiotics. Adv Nutr. 2019;10:S49-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 689] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 60. | Ma Q, Xing C, Long W, Wang HY, Liu Q, Wang RF. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflammation. 2019;16:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 467] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 61. | Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 545] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 62. | Bonaz B, Bazin T, Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front Neurosci. 2018;12:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 771] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 63. | Kim N, Yun M, Oh YJ, Choi HJ. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. J Microbiol. 2018;56:172-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 64. | Qin Y, Wade PA. Crosstalk between the microbiome and epigenome: messages from bugs. J Biochem. 2018;163:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 65. | Bonfili L, Cecarini V, Cuccioloni M, Angeletti M, Berardi S, Scarpona S, Rossi G, Eleuteri AM. SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model. Mol Neurobiol. 2018;55:7987-8000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 66. | Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 1810] [Article Influence: 301.7] [Reference Citation Analysis (1)] |
| 67. | Birner A, Platzer M, Bengesser SA, Dalkner N, Fellendorf FT, Queissner R, Pilz R, Rauch P, Maget A, Hamm C, Herzog-Eberhard S, Mangge H, Fuchs D, Moll N, Zelzer S, Schütze G, Schwarz M, Reininghaus B, Kapfhammer HP, Reininghaus EZ. Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PLoS One. 2017;12:e0172699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 68. | Bansal Y, Singh R, Parhar I, Kuhad A, Soga T. Quinolinic Acid and Nuclear Factor Erythroid 2-Related Factor 2 in Depression: Role in Neuroprogression. Front Pharmacol. 2019;10:452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 69. | Wong CB, Tanaka A, Kuhara T, Xiao JZ. Potential Effects of Indole-3-Lactic Acid, a Metabolite of Human Bifidobacteria, on NGF-induced Neurite Outgrowth in PC12 Cells. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 70. | Cuartero MI, Ballesteros I, de la Parra J, Harkin AL, Abautret-Daly A, Sherwin E, Fernández-Salguero P, Corbí AL, Lizasoain I, Moro MA. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation. 2014;130:2040-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 71. | Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19:184-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 797] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 72. | Takamura T, Harama D, Fukumoto S, Nakamura Y, Shimokawa N, Ishimaru K, Ikegami S, Makino S, Kitamura M, Nakao A. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol Cell Biol. 2011;89:817-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 73. | Lamas B, Hernandez-Galan L, Galipeau HJ, Constante M, Clarizio A, Jury J, Breyner NM, Caminero A, Rueda G, Hayes CL, McCarville JL, Bermudez Brito M, Planchais J, Rolhion N, Murray JA, Langella P, Loonen LMP, Wells JM, Bercik P, Sokol H, Verdu EF. Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation. Sci Transl Med. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (1)] |
| 74. | Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 467] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 75. | Gu F, Wu Y, Liu Y, Dou M, Jiang Y, Liang H. Lactobacillus casei improves depression-like behavior in chronic unpredictable mild stress-induced rats by the BDNF-TrkB signal pathway and the intestinal microbiota. Food Funct. 2020;11:6148-6157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 76. | Orlando A, Chimienti G, Lezza AMS, Pesce V, Gigante I, D'Attoma B, Russo F. Lactobacillus Rhamnosus GG Affects the BDNF System in Brain Samples of Wistar Rats with Pepsin-Trypsin-Digested Gliadin (PTG)-Induced Enteropathy. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | McLoughlin RF, Berthon BS, Jensen ME, Baines KJ, Wood LG. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106:930-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 78. | Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020;11:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 1608] [Article Influence: 321.6] [Reference Citation Analysis (0)] |
| 79. | Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, Ducarmon QR, Keller JJ, Kuijper EJ, Contarino MF. Fecal Microbiota Transplantation in Neurological Disorders. Front Cell Infect Microbiol. 2020;10:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 80. | D'Amato A, Di Cesare Mannelli L, Lucarini E, Man AL, Le Gall G, Branca JJV, Ghelardini C, Amedei A, Bertelli E, Regoli M, Pacini A, Luciani G, Gallina P, Altera A, Narbad A, Gulisano M, Hoyles L, Vauzour D, Nicoletti C. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome. 2020;8:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 81. | Sanders ME, Akkermans LM, Haller D, Hammerman C, Heimbach J, Hörmannsperger G, Huys G, Levy DD, Lutgendorff F, Mack D, Phothirath P, Solano-Aguilar G, Vaughan E. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 450] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 82. | Lerner A, Shoenfeld Y, Matthias T. Probiotics: If It Does Not Help It Does Not Do Any Harm. Really? Microorganisms. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 83. | Zawistowska-Rojek A, Tyski S. Are Probiotic Really Safe for Humans? Pol J Microbiol. 2018;67:251-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 84. | Sbahi H, Di Palma JA. Faecal microbiota transplantation: applications and limitations in treating gastrointestinal disorders. BMJ Open Gastroenterol. 2016;3:e000087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 85. | Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol. 2001;21:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 175] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 86. | Snydman DR. The safety of probiotics. Clin Infect Dis. 2008;46 Suppl 2:S104-11; discussion S144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 87. | Wardill HR, Secombe KR, Bryant RV, Hazenberg MD, Costello SP. Adjunctive fecal microbiota transplantation in supportive oncology: Emerging indications and considerations in immunocompromised patients. EBioMedicine. 2019;44:730-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 88. | Merrick B, Allen L, Masirah M Zain N, Forbes B, Shawcross DL, Goldenberg SD. Regulation, risk and safety of Faecal Microbiota Transplant. Infect Prev Pract. 2020;2:100069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |