Published online Oct 14, 2021. doi: 10.3748/wjg.v27.i38.6489
Peer-review started: April 4, 2021
First decision: May 27, 2021
Revised: June 10, 2021
Accepted: August 27, 2021
Article in press: August 27, 2021
Published online: October 14, 2021
Processing time: 190 Days and 14.3 Hours
Acute pancreatitis (AP) is an inflammatory disease in which the regulatory pathway is complex and not well understood. Soluble suppression of tumorigenicity 2 (sST2) protein receptor functions as a decoy receptor for interleukin (IL)-33 to prevent IL-33/suppression of tumorigenicity 2L (ST2L)-pathway-mediated T helper (Th)2 immune responses.
To investigate the role of sST2 in AP.
We assessed the association between sST2 and severity of AP in 123 patients enrolled in this study. The serum levels of sST2, C-reactive protein (CRP) and Th1- and Th2-related cytokines, including interferon (IFN)-γ, tumor necrosis factor (TNF)-α, IL-2, IL-4, IL-5 and IL-13, were measured by highly sensitive ELISA, and the severity of AP in patients was evaluated by the 2012 Atlanta Classification Criteria.
Serum sST2 levels were significantly increased in AP patients, and further, these levels were significantly elevated in severe AP (SAP) patients compared to moderately severe AP (MSAP) and mild AP (MAP) patients. Logistic regression showed sST2 was a predictor of SAP [odds ratio (OR): 1.003 (1.001–1.006), P = 0.000]. sST2 cutoff point was 1190 pg/mL, and sST2 above this cutoff was associated with SAP. sST2 was also a predictor of any organ failure and mortality during AP [OR: 1.006 (1.003–1.009), P = 0.000, OR: 1.002 (1.001–1.004), P = 0.012, respectively]. Additionally, the Th1-related cytokines IFN-γ and TNF-α in the SAP group were higher and the Th2-related cytokine IL-4 in the SAP group was significantly lower than those in MSAP and MAP groups.
sST2 may be used as a novel inflammatory marker in predicting AP severity and may regulate the function and differentiation of IL-33/ST2-mediated Th1 and Th2 Lymphocytes in AP homeostasis.
Core Tip: Acute pancreatitis (AP) is an inflammatory disease in which the regulatory pathway is complex and not well understood. The interleukin (IL)-33/ suppression of tumorigenicity 2L (ST2L) functional pathway is involved in the pathological process of AP. Soluble suppression of tumorigenicity 2 protein (sST2) is a soluble receptor, which is released in the circulation acts as a decoy receptor by binding IL-33. However, sST2 as one of the most promising disease biomarker, has not been studied in the development of AP. In this study we studied the role of sST2 as an inflammatory marker for predicting the severity of acute pancreatitis.
- Citation: Zhang Y, Cheng B, Wu ZW, Cui ZC, Song YD, Chen SY, Liu YN, Zhu CJ. Serum soluble suppression of tumorigenicity 2 as a novel inflammatory marker predicts the severity of acute pancreatitis. World J Gastroenterol 2021; 27(38): 6489-6500
- URL: https://www.wjgnet.com/1007-9327/full/v27/i38/6489.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i38.6489
Acute pancreatitis (AP) is caused by activation of pancreatic enzymes induced by a variety of etiologies, resulting in autodigestion, hemorrhage, edema, and even necrosis of pancreatic tissue, with or without other organ function changes. The clinical severity of AP is related to its prognosis. Severe AP (SAP) has a poor prognosis, and can cause severe disorders of multiple organ functions, and the mortality can reach 30%[1]. Excessive activation of inflammatory cells and their cytokines is one of the main mechanisms of pathogenesis of AP. The development and outcome of AP are closely related to immune function[2]. Research on changes in inflammatory mediators in patients with different clinical severity of AP is important clinically. A better understanding of the regulatory inflammatory pathways in AP is beneficial for discovery of new therapeutic targets.
Suppression of tumorigenicity 2 protein (ST2) is a member of the interleukin (IL)-1 receptor family with transmembrane (ST2L) and soluble (sST2) isoforms. The ligand of ST2 is IL-33, which can produce nuclear signal transduction and immunomodulatory functions in various cells when combined with ST2L. IL-33 is mainly produced by epithelial and endothelial cells. After exposure to pathogens, stress or necrosis caused by injury, IL-33 can signal the presence of tissue damage to local immune cells, thereby acting as a danger signal or alarm protein[3]. ST2L can form a heterodimer with IL-1R-related protein, which is widely present in the membranes of mast cells, T helper (Th)2 cells, dendritic cells, basophils, and macrophages[4,5]. The importance and role of the IL-33/ST2L axis have been evaluated and confirmed in several inflammatory and cardiac diseases and cancer. sST2, as a soluble receptor that is released in the circulation, acts as a decoy receptor by binding IL-33, and, thus, sST2 is involved as the counterbalance/response on IL-33/ST2L axis activation by inhibiting its signal transduction through ST2L.
The IL-33/ST2L functional pathway is involved in coxsackievirus B5 (CVB5)-induced pancreatitis[6]. Mice deficient in ST2L develop significantly more severe pancreatitis. Conversely, wild-type mice treated with recombinant IL-33 develop significantly lower viral titers, and pancreatitis is attenuated, indicating that IL-33/ST2L is involved in the pathological process of AP. SAP can cause severe disorders of multiple organ, including AP-related myocardial, kidney, or lung injury. In clinical studies, sST2 is recognized as an important marker for monitoring treatment in heart failure patients and higher sST2 is associated with worse right ventricular dysfunction and higher mean pulmonary and right atrial pressures[7-9]. sST2 is also recognized as an important prognostic marker in patients with kidney injury, where specific characteristics of sST2 enable better assessment of the risk of end-stage renal disease patients on dialysis[10]. It has been also found to be relevant in pulmonary diseases, sepsis, trauma, and gastrointestinal diseases[11-14]. sST2 can be used as a biomarker in heart, lung and kidney injury, and may also be served as biomarker in the severity of AP. However, sST2, as one of the most promising disease biomarkers, has not been studied in the development of AP.
The IL-33/ST2L pathway promotes CD4+ T-cell differentiation to an atypical Th2 phenotype[15]. CD4+ T cells play a major role in the pathogenesis of pancreatitis[16]. In this study, we investigated whether and how the IL-33/ST2L pathway was involved in AP in patients with different clinical severity of AP.
A total of 123 hospitalized AP patients were recruited between January 2018 and August 2020 in the Emergency Surgery Department, Emergency Internal Medicine Department, and Comprehensive Intensive Care Unit (ICU) in the First Affiliated Hospital of Zhengzhou University. Inclusion criteria for subject enrollment included: (1) age 18–90 years; (2) clinically and radiographically confirmed AP; and (3) syndrome onset < 24 h prior to study enrollment. Exclusion criteria were: (1) Pregnancy or patients with immunodeficiency, acute and chronic hepatitis, end-stage liver and kidney disease, malignant tumors, or trauma; (2) Patients who have used hormones or immunosuppressive agents within the past 3 mo; and (3) Patients with chronic pancreatitis. This study complied with medical ethics standards and was approved by the hospital Ethics Committee. All participants provided written informed consent.
Demographic and clinical data were collected, including age, sex, ethnicity, body mass index (BMI), etiology, and comorbidities. According to the 2012 Atlanta Classification Criteria, patients were divided into: (1) Mild AP (MAP): No organ failure and local and systemic complications; (2) Moderately severe AP (MSAP): Local and/or systemic complications and/or transient organ failure (< 48 h); and (3) SAP: Persistent organ failure (> 48 h), with or without local complications.
For serum preparation, peripheral venous blood samples (4 mL) were collected from each patient within 24 h after symptom onset and allowed to clot for 2 h at room temperature prior to centrifugation at 1500 rpm for 20 min. Serum was aliquoted and stored at -80 °C until further testing. The laboratory technicians were blinded to the baseline data and AP severity of the patients. Serum sST2 Levels were measured using a human sST2 ELISA kit (Elabscience, Wuhan, China). Serum C-reactive protein (CRP) and levels of Th1-cell-related inflammatory factors interferon (IFN)-γ, tumor necrosis factor (TNF)-α and IL-2 and Th2-cell-related inflammatory factors IL-4, IL-5 and IL-13 were measured in duplicate using an ELISA kit from Elabscience.
The Shapiro–Wilk test was used to test the normality of the data, and the Levene test was used to analyze the homogeneity of the data. Normally distributed measurement data are expressed as mean ± SE, and data were compared between groups by Student’s t test and analysis of variance. Non-normally distributed measurement data are represented by median and interquartile range [M (IQR)], and comparison of data between groups used the Kruskal–Wallis test, and the nonparametric Mann–Whitney U test was used for comparison between the two groups. The Z value was used for the statistic. Categorical variables were described as frequencies with percentages, and chi-square test was used to examine significant differences between categorical variables. The influence of serum sST2 levels on severity was assessed using univariate and multivariate binary logistic regression analysis, with significant confounding factors tested in the adjusted univariate analysis. Results were expressed as adjusted odds ratios (ORs) with the corresponding 95% confidence intervals (CIs). Optimal sST2 cutoff points were obtained using receiver operating characteristic (ROC) curve analysis. Additionally, IFN-γ, TNF-α, IL-2, IL-4, IL-5, and IL-13 data were logarithmically transformed for analysis. The relationships between sST2, IFN-γ, TNF-α, IL-2, IL-4, IL-5, and IL-13 and total length of hospital stay and ICU hospital stay were analyzed using Spearman’s rank correlation. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 21.0.
We enrolled 123 patients: 55 (45%) with MAP, 37 (30%) with MSAP, and 31 (25%) with SAP. The etiology of AP was biliary stones (74.8%), alcohol (8.9%), laparoscopic retro
The BMI of the healthy control group was lower than that of the MAP, MSAP, and SAP groups, and the difference was significant (Z = -3.15, P = 0.002, Z = -3.16, P = 0.002, Z = -5.046, P = 0.000). There was no significant difference in BMI between the MAP and MSAP groups (Z = -2.27, P = 0.820). The BMI of the MAP and MSAP groups was lower than that of the SAP group (Z = -2.767, P = 0.006, Z = -2.452, P = 0.014, respectively).
In the MAP group, three patients had accompanying hypertension, three were complicated by diabetes, and three were complicated by coronary heart disease. In the MSAP group, two patients had hypertension, three were complicated by diabetes, and one was complicated by coronary heart disease. Two patients in the SAP group had hypertension, five were complicated with diabetes, and one with coronary heart disease. There was no significant difference between the groups for co-morbidity, hypertension, diabetes, and coronary heart disease. None of the 123 patients were taking immunosuppressive agents. After statistical analysis, there was no significant difference in age, gender, or ethnicity between the AP groups. Patients in the AP groups had no significant difference in age, gender, or ethnicity compared with the healthy control group (Table 1).
| MAP (n = 55) | MSAP (n = 37) | SAP (n = 31) | Control (n = 42) | χ2/t | P value | |
| Age, yr | 45.16 ± 1.74 | 50.35 ± 2.54 | 51.94 ± 2.42 | 45.60 ± 1.75 | 2.173 | 0.093 |
| Gender | 4.418 | 0.220 | ||||
| Male | 34 (61.8) | 21 (56.8) | 14 (45.2) | 18 (42.9) | ||
| Female | 21 (38.2) | 16 (43.2) | 17 (54.8) | 24 (57.1) | ||
| BMI, kg/m2 | 25.63 (2.15) | 25.81 (1.97) | 26.54 (1.22) | 24.61 (2.09) | 28.518 | 0.000 |
| Ethnicity | 1.7 | 0.721 | ||||
| Han nationality | 52 (94.5) | 36 (97.3) | 31 (100.0) | 41 (97.6) | ||
| Minority | 3 (5.5) | 1 (2.7) | 0 (0.0) | 1 (2.4) | ||
| Etiology | 9.374 | 0.677 | ||||
| Biliary stones | 45 (81.8) | 27 (73.0) | 20 (64.5) | |||
| Alcoholic | 4 (7.3) | 4 (10.8) | 3 (9.7) | |||
| ERCP | 1 (1.8) | 0 (0.0) | 1 (3.2) | |||
| Metabolic | 1 (1.8) | 1 (2.7) | 1 (3.2) | |||
| Mixed (alcohol + biliary stones) | 2 (3.7) | 3 (8.1) | 5 (16.2) | |||
| Drug-induced idiopathic | 1 (1.8) | 0 (0.0) | 0 (0.0) | |||
| Co-morbidity | 1 (1.8) | 2 (5.4) | 1 (3.2) | |||
| Hypertension | 3 (5.5) | 2 (5.4) | 2 (6.5) | 0.044 | 0.978 | |
| Diabetes | 3 (5.5) | 3 (8.1) | 5 (16.1) | 2.691 | 0.283 | |
| Coronary heart disease | 3 (5.5) | 1 (2.7) | 1 (3.2) | 0.515 | 0.860 | |
| Cholecystitis | 3 (5.5) | 1 (2.7) | 2 (6.5) | 0.582 | 0.747 |
To identify the involvement of the IL-33/ST2L pathway in AP, we examined expression of IL-33 and sST2 in the serum after AP. The level of IL-33 was extremely low in the blood of all AP patients. Almost all samples were under the detection limit of the IL-33 ELISA, and only seven patients showed higher values. In addition, the low, almost undetectable levels of IL-33 were unlikely to be associated with worsening clinical outcomes. Nevertheless, we tested the levels of sST2 at the same time.
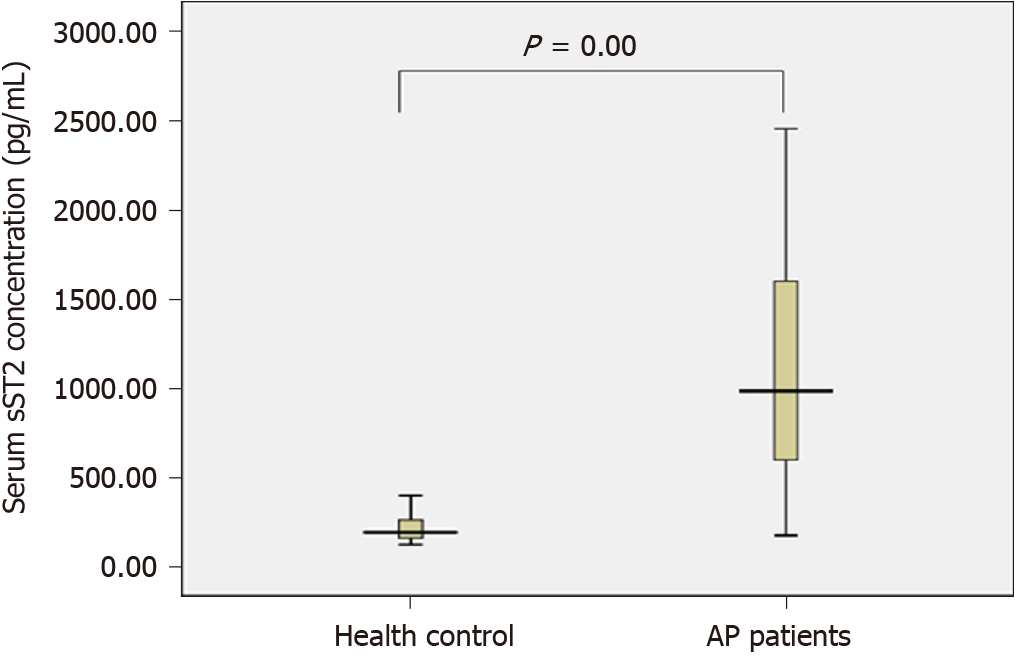
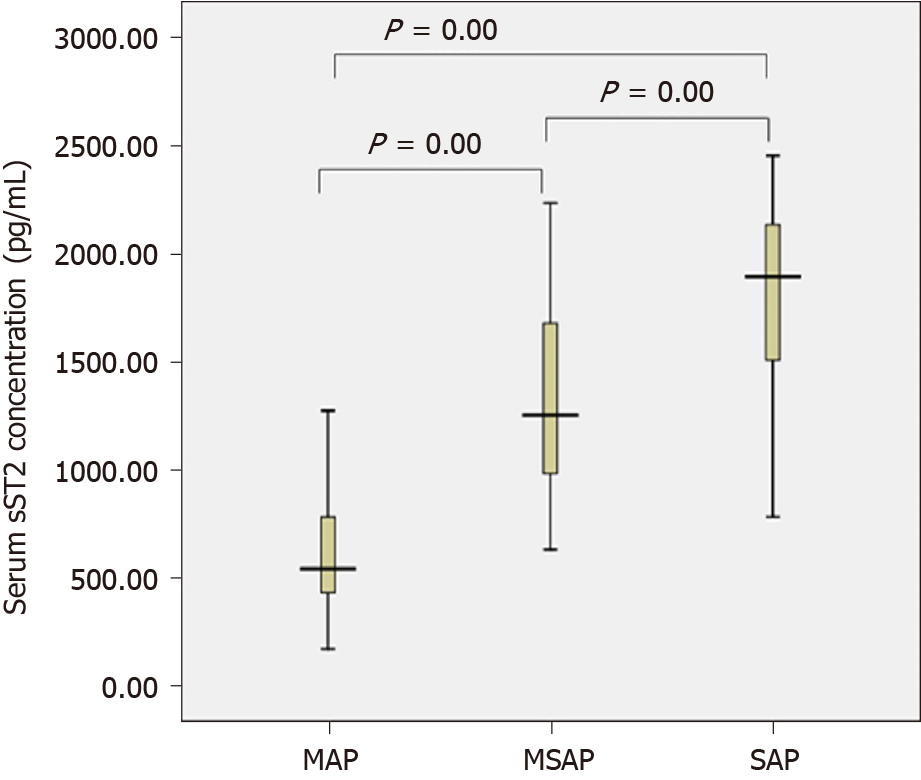
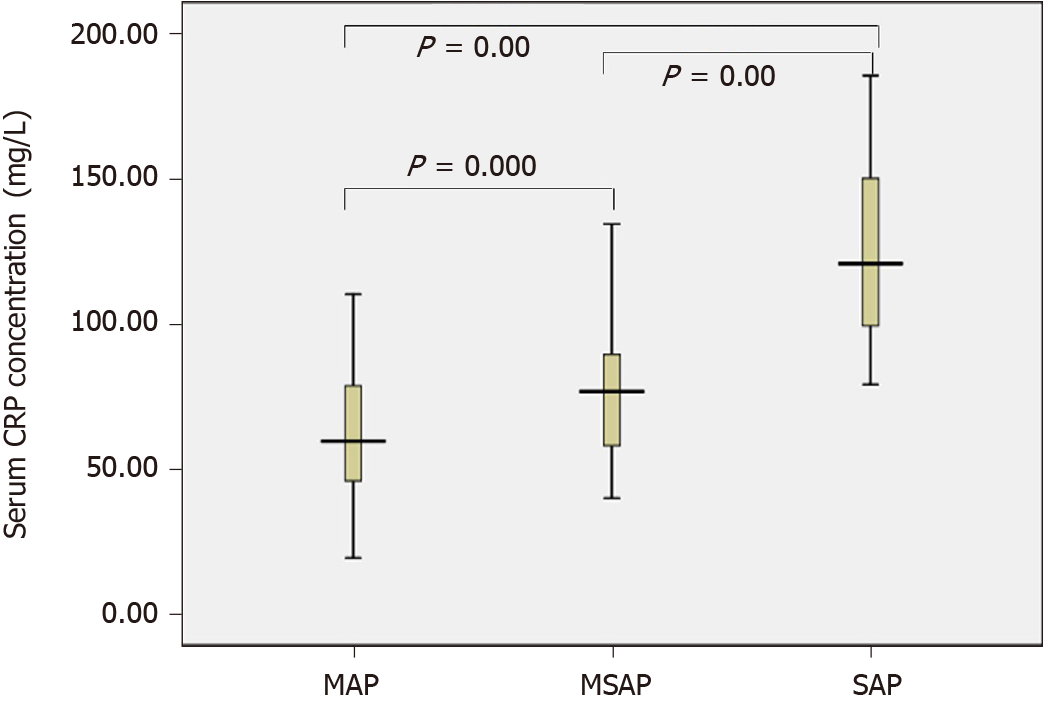
The sST2 content of the AP group and the healthy control group conformed to a normal distribution and uniformity of variance. We observed increased levels of sST2 in AP patients compared to healthy controls (1115.4 vs 211.1 pg/mL, t = 9.355, P = 0.000) (Figure 1). The sST2 value of the three AP groups did not conform to a normal distribution, and the nonparametric Kruskal–Wallis H test was used for comparison among multiple groups. The level of sST2 was significantly higher in the SAP group compared with MSAP and MAP groups (Z = -3.510, P = 0.000, Z = -7.305, P = 0.000, respectively). The level of sST2 in the MSAP group was significantly higher than in the MAP group (Z = -6.489, P = 0.000). The data are shown in Figure 2 and Table 2. The level of CRP in the SAP group was significantly higher than in the MSAP and MAP groups (Z = -5.634, P = 0.000, Z = -7.150, P = 0.000, respectively). The level of CRP in the MSAP group was higher than in the MAP group (Z = -2.891, P = 0.000) (Figure 3). This suggested that sST2 expression was increased in the SAP group, and that IL-33 signaling played a role in regulating the inflammatory response during AP.
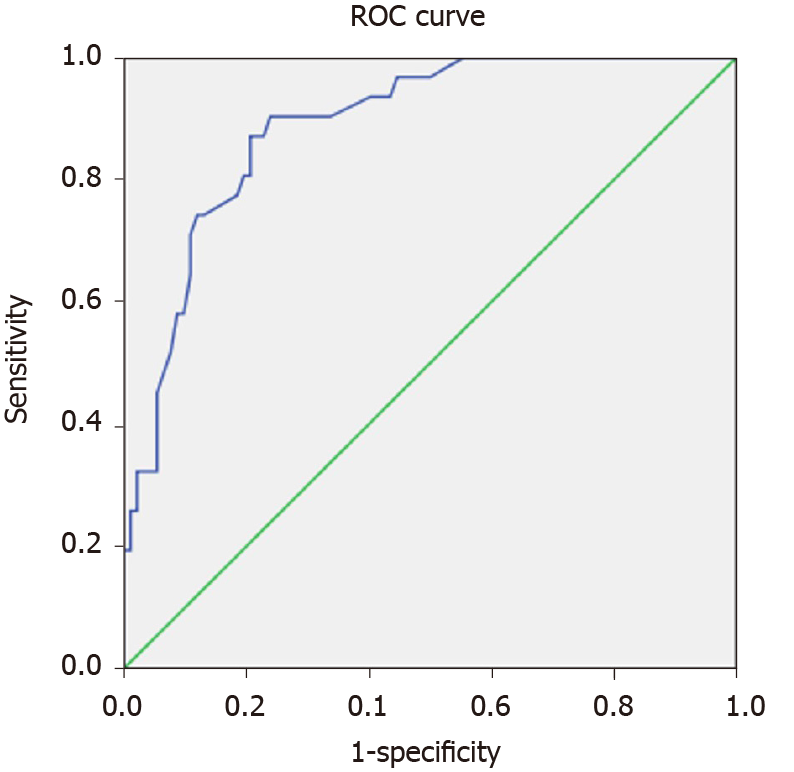
We conducted a multivariate analysis to evaluate whether the relationship of sST2 with SAP was really independent. Logistic regression showed that sST2 levels at admission [OR: 1.003 (1.001–1.006), P = 0.000] was a predictor of SAP. With the ROC curve, the optimal cut-off value of serum sST2 levels as an indicator for prediction of SAP was projected to be 1190 pg/mL, which yielded a sensitivity of 90.3% and specificity of 76.1%, with the area under the curve 0.889 (95%CI: 0.829–0.949; P = 0.000) (Figure 4).
sST2 was also a predictor of any organ failure and mortality during AP [OR: 1.006 (1.003–1.009), P = 0.000, OR: 1.002 (1.000–1.004), P = 0.012, respectively]. BMI and CRP were also predictors of SAP at admission [OR: 2.629 (1.075–6.429), P = 0.034, OR: 1.066 (1.031–1.101), P =0.002, respectively]. However, there was no value of BMI and CRP for predicting organ failure and mortality. sST2, CRP, age, BMI, and gender were not predictive of necrosis. The data are shown in Table 3. Furthermore, there was a significant correlation between the level of sST2 and total hospital stay and length of stay in ICU (r = 0.463, P = 0.000, r = 0.673, P = 0.000) (Table 4).
| Variables | OR (95%CI) | P value | Variables | OR (95%CI) | P value |
| MAP/MSAP and SAP | Necrosis | ||||
| Age | 1.031 (0.969-1.097) | 0.330 | Age | 1.019 (0.990-1.049) | 0.209 |
| BMI | 2.629 (1.075-6.429) | 0.034 | BMI | 0.973 (0.695-1.362) | 0.847 |
| Gender | 1.096 (1.001-1.006) | 0.910 | Gender | 0.529 (0.234-1.198) | 0.127 |
| CRP | 1.066 (1.031-1.101) | 0.002 | CRP | 1.009 (0.995-1.023) | 0.209 |
| sST2 | 1.003 (1.001-1.006) | 0.000 | sST2 | 1.000 (0.999-1.001) | 0.837 |
| Any organ failure | Mortality | ||||
| Age | 1.017 (0.963-1.074) | 0.546 | Age | 1.024 (0.967-1.085) | 0.411 |
| BMI | 1.407 (0.788-2.511) | 0.248 | BMI | 1.685 (0.731-3.883) | 0.220 |
| Gender | 0.246 (0.050-1.202) | 0.083 | Gender | 0.249 (0.040-1.563) | 0.138 |
| CRP | 1.030 (0.991-1.071) | 0.128 | CRP | 1.009 (0.987-1.032) | 0.415 |
| sST2 | 1.006 (1.003-1.009) | 0.000 | sST2 | 1.002 (1.000-1.004) | 0.012 |
| sST2/LN (Inflammatory factors, pg/mL) | Total hospital stay | Total ICU stay | ||
| r | P value | r | P value | |
| sST2 | 0.463b | 0.000 | 0.673b | 0.000 |
| LN (IFN-γ) | 0.430b | 0.000 | 0.700b | 0.000 |
| LN (TNF-α) | 0.341b | 0.000 | 0.652b | 0.000 |
| LN (IL-2) | -0.043 | 0.638 | -0.082 | 0.366 |
| LN (IL-4) | -0.483b | 0.000 | -0.440b | 0.000 |
| LN (IL-5) | -0.123 | 0.174 | 0.062 | 0.494 |
| LN (IL-13) | -0.006 | 0.946 | -0.139 | 0.126 |
To further explore the biological mechanism of AP, we examined the production of Th1-related cytokines IFN-γ, TNF-α and IL-2 and Th2-related cytokines IL-4, IL-5 and IL-13 in the serum to clarify the role of the inflammatory process in the pathogenesis of AP. The concentration of inflammatory factors measured after logarithmic conversion was in accordance with the normal distribution. Statistical analysis showed that the serum levels of IFN-γ and TNF-α in the SAP and MSAP groups were higher than those in the MAP group, and the difference was significant. There was no significant difference in IFN-γ and TNF-α between the SAP and MSAP groups. IL-4 expression in the SAP group was significantly lower than that in the MAP and MSAP groups, but there was no significant difference between the MAP and MSAP groups. There was no significant difference in the expression of IL-2, IL-5 and IL-13 between the MAP, MSAP and SAP groups (Table 5).
| LN (inflammatory factors pg/mL) | MAP, mean ± SE | MSAP, mean ± SE | SAP, mean ± SE | F | P value |
| LN (IFN-γ) | 1.98 ± 0.07 | 2.57 ± 0.08b | 3.08 ± 0.07b | 53.393 | 0.000 |
| LN (TNF-α) | 1.63 ± 0.04 | 2.07 ± 0.05b | 2.33 ± 0.05b,d | 45.369 | 0.000 |
| LN (IL-2) | 1.06 ± 0.05 | 1.05 ± 0.06 | 0.99 ± 0.05 | 0.362 | 0.697 |
| LN (IL-4) | 1.02 ± 0.03 | 0.99 ± 0.04 | 0.64 ± 0.03b,d | 23.195 | 0.000 |
| LN (IL-5) | 0.10 ± 0.05 | 0.10 ± 0.07 | 0.13 ± 0.08 | 0.043 | 0.958 |
| LN (IL-13) | 0.76 ± 0.03 | 0.68 ± 0.06 | 0.63 ± 0.07 | 1.703 | 0.187 |
Expression of Th1-cell-associated inflammatory factor IFN-γ and TNF-α was positively correlated with the length of total hospital stay and total ICU stay, while expression of Th2-cell-associated inflammatory factor IL-4 was negatively correlated with the length of total hospital stay and total ICU stay (Table 4).
The results of our study showed that the levels of sST2 were significantly increased in SAP patients, and there were significant increases in Th1-related cytokines IFN-γ and TNF-α, and a decrease in Th2-related cytokine IL-4. This suggested an insufficient IL-33-driven Th2-type inflammatory response was involved in the pathophysiological process of AP. sST2 is a secreted form of the ST2 receptor and acts as a decoy receptor for IL-33, thus inactivating the functions of IL-33. sST2 expression increases in response to proinflammatory cytokines[17]. Our data were in line with a previous study showing elevated levels of sST2 in AP[18]. Importantly, in our study the levels of sST2 predicted the total hospital stay and length of stay in the ICU, as well as predicted organ failure and mortality during AP. These data support the findings of our study showing that SAP patients more frequently had higher serum levels of sST2 compared with MAP and MSAP patients, and that the early elevated levels of sST2 correlated with worsened outcome of AP. Thus, the normal function of IL-33 may be inhibited in AP.
SAP can cause severe systemic inflammation and multiple organ dysfunction. It is reported that endogenous danger signals, such as tissue damage or necrosis, and exogenous danger signals, such as microbial pathogens and endotoxins, can enhance the production of sST2 and stimulate the secretion of inflammatory cytokines, thereby weakening the immune response of organs exposed to the danger signals and leading to adverse outcomes[19]. It has been suggested that serum levels of sST2 are significantly increased in inflammatory diseases and serve as a prognostic biomarker of multiple organ dysfunction syndrome (MODS), cardiovascular events, heart failure, acute hypoxemic respiratory failure, and progression of kidney failure. A study based on cardiopulmonary resuscitation showed that plasma levels of sST2 were associated with higher risk of MODS and early death[20]. A role of sST2 in acute hypoxemic respiratory failure indicates that using sST2 concentrations to guide ventilator management may more accurately reflect underlying lung injury and outperform traditional measures of readiness for ventilator liberation[21]. Higher sST2 Levels predict mortality in severe sepsis[12]. These findings agree with our results showing that sST2 was a predictor of any organ failure and mortality during AP.
The IL-33/ST2L pathway has been shown to mediate the modulation of inflammation in different diseases by promoting Th2 response and inhibiting Th1 response[22,23]. Mice that lack IL-33 signaling develop significantly more severe pancreatitis[6]. Based on the regulatory effect of the IL-33/ST2 signaling pathway on Th1 and Th2 cells, we further measured the function of Th1 and Th2 cells in patients with AP. An important finding in our study was the relationship between Th1- and Th2-related cytokines and the severity of AP. Cytokine levels in Th1 cells in patients with SAP were significantly higher than those in patients with MAP and MSAP, and cytokines in Th2 cells in patients with MAP and MSAP were significantly increased compared with patients with SAP. In our study, we observed that both sST2 and the Th1 cytokines IFN-γ and TNF-α were increased and the Th2 cytokine IL-4 was decreased in SAP. We speculate that increased sST2 in SAP might cause increases in IFN-γ and TNF-α, and decreases in IL-4 by antagonizing the IL-33/ST2 signaling pathway, whereas this inference still needs to be verified by further animal research in the future.
The serum levels of Th1-related cytokines IFN-γ and TNF-α in the SAP group were higher than those in the MAP and MSAP groups, and expression of Th2-related cytokine IL-4 in the SAP group was significantly lower than in the MAP and MSAP groups. The expression of Th1-related inflammatory factors IFN-γ and TNF-α was positively correlated with the length of total hospital stay and total ICU stay, and Th2-cell-associated inflammatory factor IL-4 expression was negatively correlated with the length of hospital and ICU stays. The results suggested that Th1-cell-related immune response was an important factor that aggravated the development of AP, and Th2 cells might have a protective role in the development of AP. Our findings indicate that Th1 and Th2 cells were involved in cytokine-dependent pathways in severity of AP. The results proved that a change in immune response played an important role in the pathogenesis of AP.
In this study, we found an immune imbalance in patients with SAP. Th1-cell-related cytokines IFN-γ and TNF-α in the serum of patients with SAP were higher than those in patients with MSAP. We also found that the expression levels of Th1-cell-associated inflammatory factors IFN-γ and TNF-α were positively correlated with the length of total hospital stay and total ICU stay. This finding is consistent with the latest research showing that inflammatory factors play a consistent role in the pathogenesis of AP[24-26]. IFN-γ and TNF-α could stimulate B cells to produce antibodies, activate macrophages and CD8+ T cells, promote cell-mediated immunity and cytotoxic T cell responses[27], and could induce Th1 cell differentiation and inhibit Th2 cell proliferation. Previous research findings and the present study suggest that Th1 cells are involved in the pathogenesis of AP, especially in the development of SAP. Th1 cells exacerbate the pathophysiological process of AP by releasing Th1-related inflammatory factors.
In humans, serum concentrations of sST2 are increased in several diseases, such as heart disease, pulmonary disease, burn injury, and graft-versus-host disease[28-33]. Few studies have reported the influence of the IL-33/ST2 signaling pathway on the development of AP[6,34-36] and less attention has been paid to Th1 and Th2 cells in the pathogenesis of AP[24]. In this study, we found that the expression of the Th2-related cytokine IL-4 in SAP was significantly lower than that in MAP and MSAP. Expression of the Th2-related inflammatory factor IL-4 was negatively correlated with the length of hospital and ICU hospital stays, suggesting that Th2 cells are involved in the pathogenesis of AP with the tendency to improve symptoms of acute pancreatitis. Collectively, Th2 cells might have a protective effect in the development of AP, tending to delay its development.
Our research had some limitations. Firstly, the main limitation was the small sample size, which may have reduced the statistical power, the accuracy, and effectiveness of identifying true positive results. In future, the results need to be confirmed with larger sample sizes. Secondly, this study was a single-center study, which might have resulted in selection bias. Thirdly, in cases of pancreatitis combined with cholecystitis, cholecystitis might affect the results of the study. Fourthly, we did not examine the numbers of Th1 and Th2 cells. Finally, establishing the initial relationship between AP and elevated sST2 concentrations in our study is novel and useful, but it is necessary to determine the long-term predictive value of sST2 for the prognosis of AP in subsequent longitudinal studies.
In conclusion, sST2 may be used as a novel inflammatory marker in predicting AP severity and may regulate the function and differentiation of IL-33/ST2-mediated Th1 and Th2 Lymphocytes in AP homeostasis.
The clinical severity of acute pancreatitis (AP) is related to its prognosis. Excessive activation of inflammatory cells and their cytokines is one of the main mechanisms of pathogenesis of AP. Research on changes in inflammatory mediators in patients with different clinical severity of AP is important clinically. The interleukin (IL)-33/ST2L functional pathway is involved in the pathological process of AP. Soluble suppression of tumorigenicity 2 (sST2) is a secreted form of the ST2 receptor and acts as a decoy receptor for IL-33. In this study, we investigated whether the sST2 could serve as a novel inflammatory marker predicting the severity of acute pancreatitis.
In this study, the authors focused on the function of sST2 in predicting the severity of AP. The key issue to be solved is the association between IL-33/ST2L pathway and AP. The significance of solving these problems may constitute a new therapeutic target for regulating immune activation during the AP inflammatory storm.
The objective of this study was to investigate the role of sST2 in AP.
The authors assessed the association between sST2 and severity of AP in 123 patients enrolled in this study. The serum levels of sST2, C-reactive protein (CRP) and Th1- and Th2-related cytokines interferon (IFN)-γ, tumor necrosis factor (TNF)-α ,IL-2, IL-4, IL-5 and IL-13 were measured by highly sensitive ELISA and the severity of AP patients was evaluated by the 2012 Atlanta Classification Criteria.
The serum sST2 Level was significantly increased in AP patients and significantly elevated in severe acute pancreatitis (SAP) patients compared to moderate severe acute pancreatitis and mild acute pancreatitis patients. The cutoff point of sST2 at 1190pg/mL was associated with SAP.
This study suggests that sST2 may be used as a novel inflammatory marker in predicting the severity of acute pancreatitis and that sST2 might regulate the function and differentiation of IL-33/ST2L mediated Th1 and Th2 lymphocytes in the homeostasis of acute pancreatitis.
It is necessary to determine the long-term predictive value of sST2 for the prognosis of AP in subsequent longitudinal studies. Additionally, the role of IL-33/ST2L in acute pancreatitis needs to be further verified in both in vivo and in vitro experiments.
We thank the study participants and the clinical staff for their contribution to this study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barauskas G, Pezzilli R S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Liu JH
| 1. | Muniraj T, Gajendran M, Thiruvengadam S, Raghuram K, Rao S, Devaraj P. Acute pancreatitis. Dis Mon. 2012;58:98-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Minkov GA, Halacheva KS, Yovtchev YP, Gulubova MV. Pathophysiological mechanisms of acute pancreatitis define inflammatory markers of clinical prognosis. Pancreas. 2015;44:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Pascual-Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol. 2015;115:3B-7B. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 4. | Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2564] [Cited by in RCA: 2892] [Article Influence: 144.6] [Reference Citation Analysis (0)] |
| 5. | Barksby HE, Lea SR, Preshaw PM, Taylor JJ. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007;149:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Sesti-Costa R, Silva GK, Proença-Módena JL, Carlos D, Silva ML, Alves-Filho JC, Arruda E, Liew FY, Silva JS. The IL-33/ST2 pathway controls coxsackievirus B5-induced experimental pancreatitis. J Immunol. 2013;191:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Emdin M, Aimo A, Vergaro G, Bayes-Genis A, Lupón J, Latini R, Meessen J, Anand IS, Cohn JN, Gravning J, Gullestad L, Broch K, Ueland T, Nymo SH, Brunner-La Rocca HP, de Boer RA, Gaggin HK, Ripoli A, Passino C, Januzzi JL Jr. sST2 Predicts Outcome in Chronic Heart Failure Beyond NT-proBNP and High-Sensitivity Troponin T. J Am Coll Cardiol. 2018;72:2309-2320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 8. | Aimo A, Januzzi JL Jr, Bayes-Genis A, Vergaro G, Sciarrone P, Passino C, Emdin M. Clinical and Prognostic Significance of sST2 in Heart Failure: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:2193-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 9. | O'Meara E, Prescott MF, Claggett B, Rouleau JL, Chiang LM, Solomon SD, Packer M, McMurray JJV, Zile MR. Independent Prognostic Value of Serum Soluble ST2 Measurements in Patients With Heart Failure and a Reduced Ejection Fraction in the PARADIGM-HF Trial (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ Heart Fail. 2018;11:e004446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Homsak E, Ekart R. ST2 as a novel prognostic marker in end-stage renal disease patients on hemodiafiltration. Clin Chim Acta. 2018;477:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Shieh JM, Tseng HY, Jung F, Yang SH, Lin JC. Elevation of IL-6 and IL-33 Levels in Serum Associated with Lung Fibrosis and Skeletal Muscle Wasting in a Bleomycin-Induced Lung Injury Mouse Model. Mediators Inflamm. 2019;2019:7947596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Hoogerwerf JJ, Tanck MW, van Zoelen MA, Wittebole X, Laterre PF, van der Poll T. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med. 2010;36:630-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Billiar IM, Guardado J, Abdul-Malak O, Vodovotz Y, Billiar TR, Namas RA. Elevations in Circulating sST2 Levels Are Associated With In-Hospital Mortality and Adverse Clinical Outcomes After Blunt Trauma. J Surg Res. 2019;244:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Boga S, Alkim H, Koksal AR, Ozagari AA, Bayram M, Tekin Neijmann S, Sen I, Alkim C. Serum ST2 in inflammatory bowel disease: a potential biomarker for disease activity. J Investig Med. 2016;64:1016-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Lu J, Kang J, Zhang C, Zhang X. The role of IL-33/ST2L signals in the immune cells. Immunol Lett. 2015;164:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Schmidt AI, Kühlbrey C, Lauch R, Wolff-Vorbeck G, Chikhladze S, Hopt UT, Wittel UA. The predominance of a naive T helper cell subset in the immune response of experimental acute pancreatitis. Pancreatology. 2017;17:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Mildner M, Storka A, Lichtenauer M, Mlitz V, Ghannadan M, Hoetzenecker K, Nickl S, Dome B, Tschachler E, Ankersmit HJ. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc Res. 2010;87:769-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Ouziel R, Gustot T, Moreno C, Arvanitakis M, Degré D, Trépo E, Quertinmont E, Vercruysse V, Demetter P, Le Moine O, McKenzie AN, Delhaye M, Devière J, Lemmers A. The ST2 pathway is involved in acute pancreatitis: a translational study in humans and mice. Am J Pathol. 2012;180:2330-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Griesenauer B, Paczesny S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front Immunol. 2017;8:475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 452] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 20. | Ristagno G, Varpula T, Masson S, Greco M, Bottazzi B, Milani V, Aleksova A, Sinagra G, Assandri R, Tiainen M, Vaahersalo J, Kurola J, Barlera S, Montanelli A, Latini R, Pettilä V, Bendel S, Skrifvars MB; FINNRESUSCI Study Group. Elevations of inflammatory markers PTX3 and sST2 after resuscitation from cardiac arrest are associated with multiple organ dysfunction syndrome and early death. Clin Chem Lab Med. 2015;53:1847-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Alladina J, Levy SD, Cho JL, Brait KL, Rao SR, Camacho A, Hibbert KA, Harris RS, Medoff BD, Januzzi JL, Thompson BT, Bajwa EK. Plasma Soluble Suppression of Tumorigenicity-2 Associates with Ventilator Liberation in Acute Hypoxemic Respiratory Failure. Am J Respir Crit Care Med. 2021;203:1257-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Akimoto M, Maruyama R, Takamaru H, Ochiya T, Takenaga K. Soluble IL-33 receptor sST2 inhibits colorectal cancer malignant growth by modifying the tumour microenvironment. Nat Commun. 2016;7:13589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Mahmutovic Persson I, Menzel M, Ramu S, Cerps S, Akbarshahi H, Uller L. IL-1β mediates lung neutrophilia and IL-33 expression in a mouse model of viral-induced asthma exacerbation. Respir Res. 2018;19:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 24. | Rodriguez-Nicolas A, Martínez-Chamorro A, Jiménez P, Matas-Cobos AM, Redondo-Cerezo E, Ruiz-Cabello F. TH1 and TH2 Cytokine Profiles as Predictors of Severity in Acute Pancreatitis. Pancreas. 2018;47:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Nieminen A, Maksimow M, Mentula P, Kyhälä L, Kylänpää L, Puolakkainen P, Kemppainen E, Repo H, Salmi M. Circulating cytokines in predicting development of severe acute pancreatitis. Crit Care. 2014;18:R104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Gunjaca I, Zunic J, Gunjaca M, Kovac Z. Circulating cytokine levels in acute pancreatitis-model of SIRS/CARS can help in the clinical assessment of disease severity. Inflammation. 2012;35:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Romagnani S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunology. 2004;112:352-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 267] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 28. | Jiang M, Tao S, Zhang S, Wang J, Zhang F, Li F, Ding J. Type 2 innate lymphoid cells participate in IL-33-stimulated Th2-associated immune response in chronic obstructive pulmonary disease. Exp Ther Med. 2019;18:3109-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Kriechbaum SD, Wiedenroth CB, Peters K, Barde MA, Ajnwojner R, Wolter JS, Haas M, Roller FC, Guth S, Rieth AJ, Rolf A, Hamm CW, Mayer E, Keller T, Liebetrau C. Galectin-3, GDF-15, and sST2 for the assessment of disease severity and therapy response in patients suffering from inoperable chronic thromboembolic pulmonary hypertension. Biomarkers. 2020;25:578-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Kula AJ, Katz R, Zelnick LR, Soliman E, Go A, Shlipak M, Deo R, Ky B, DeBoer I, Anderson A, Christenson R, Seliger SL, Defilippi C, Feldman HI, Wolf M, Kusek J, Shafi T, He J, Bansal N. Association of circulating cardiac biomarkers with electrocardiographic abnormalities in chronic kidney disease. Nephrol Dial Transplant. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Li X, Chen T, Gao Q, Zhang W, Xiao Y, Zhu W, Zeng L, Li Z, Yang S, Wang R, Wang X, Feng Y, Zhang X. A panel of 4 biomarkers for the early diagnosis and therapeutic efficacy of aGVHD. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Watson CJ, Gallagher J, Wilkinson M, Russell-Hallinan A, Tea I, James S, O'Reilly J, O'Connell E, Zhou S, Ledwidge M, McDonald K. Biomarker profiling for risk of future heart failure (HFpEF) development. J Transl Med. 2021;19:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Zhang Y, Xiao Y, Liu Y, Fang Q, Tian Z, Li J, Zhou D, Xie Z, Dong R, Zhang S. Prognostic Value of Circulating sST2 for the Prediction of Mortality in Patients With Cardiac Light-Chain Amyloidosis. Front Cardiovasc Med. 2020;7:597472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Kempuraj D, Twait EC, Williard DE, Yuan Z, Meyerholz DK, Samuel I. The novel cytokine interleukin-33 activates acinar cell proinflammatory pathways and induces acute pancreatic inflammation in mice. PLoS One. 2013;8:e56866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Hara A, Watanabe T, Minaga K, Yoshikawa T, Kamata K, Kudo M. Biomarkers in autoimmune pancreatitis and immunoglobulin G4-related disease. World J Gastroenterol. 2021;27:2257-2269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Minaga K, Watanabe T, Hara A, Kamata K, Omoto S, Nakai A, Otsuka Y, Sekai I, Yoshikawa T, Yamao K, Takenaka M, Chiba Y, Kudo M. Identification of serum IFN-α and IL-33 as novel biomarkers for type 1 autoimmune pancreatitis and IgG4-related disease. Sci Rep. 2020;10:14879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |