Published online Oct 14, 2021. doi: 10.3748/wjg.v27.i38.6453
Peer-review started: March 4, 2021
First decision: April 5, 2021
Revised: April 15, 2021
Accepted: August 23, 2021
Article in press: August 23, 2021
Published online: October 14, 2021
Processing time: 222 Days and 3.4 Hours
Acute kidney injury (AKI) is one of the most common acute pancreatitis (AP)-associated complications that has a significant effect on AP, but the factors affecting the AP patients’ survival rate remains unclear.
To assess the influences of AKI on the survival rate in AP patients.
A total of 139 AP patients were included in this retrospective study. Patients were divided into AKI group (n = 72) and non-AKI group (n = 67) according to the occurrence of AKI. Data were collected from medical records of hospitalized patients. Then, these data were compared between the two groups and further analysis was performed.
AKI is more likely to occur in male AP patients (P = 0.009). AP patients in AKI group exhibited a significantly higher acute physiologic assessment and chronic health evaluation II score, higher Sequential Organ Failure Assessment score, lower Glasgow Coma Scale score, and higher demand for mechanical ventilation, infusion of vasopressors, and renal replacement therapy than AP patients in non-AKI group (P < 0.01, P < 0.01, P = 0.01, P = 0.001, P < 0.01, P < 0.01, respectively). Significant differences were noted in dose of norepinephrine and adrenaline, duration of mechanical ventilation, maximum and mean values of intra-peritoneal pressure (IPP), maximum and mean values of procalcitonin, maximum and mean serum levels of creatinine, minimum platelet count, and length of hospitalization. Among AP patients with AKI, the survival rate of surgical intensive care unit and in-hospital were only 23% and 21% of the corresponding rates in AP patients without AKI, respectively. The factors that influenced the AP patients’ survival rate included body mass index (BMI), mean values of IPP, minimum platelet count, and hospital day, of which mean values of IPP showed the greatest impact.
AP patients with AKI had a lower survival rate and worse relevant clinical outcomes than AP patients without AKI, which necessitates further attention to AP patients with AKI in surgical intensive care unit.
Core Tip: Acute pancreatitis (AP) has become a common gastrointestinal disorder in surgical intensive care unit, and excessive secretion and/or poor drainage of pancreatic juice are the essence of AP onset. Acute kidney injury (AKI) is a common compli
- Citation: Shi N, Sun GD, Ji YY, Wang Y, Zhu YC, Xie WQ, Li NN, Han QY, Qi ZD, Huang R, Li M, Yang ZY, Zheng JB, Zhang X, Dai QQ, Hou GY, Liu YS, Wang HL, Gao Y. Effects of acute kidney injury on acute pancreatitis patients’ survival rate in intensive care unit: A retrospective study. World J Gastroenterol 2021; 27(38): 6453-6464
- URL: https://www.wjgnet.com/1007-9327/full/v27/i38/6453.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i38.6453
Acute pancreatitis (AP) is an excessive inflammatory response caused by digestion of the pancreas itself, which can further lead to local and distant organ damage, or even single or multiple organ failure. In the wake of continuous in-depth understanding of pathophysiological mechanism of AP and improvement of treatment measures, AP-related mortality rate is annually declining, whereas AP-associated hospitalization rises year-by-year worldwide[1,2]. In clinical practice, about 80% of the common etiologies are attributed to gallstones and alcohol consumption[3], however, the proportion of different etiologies significantly varies among different countries. In addition, hypertriglyceridemia is a high-risk factor for AP, and may lead to direct damage to pancreas and pancreatic exocrine function[4,5]. Thus, identification of etiologies is highly essential to manage better AP patients. AP can be categorized into mild acute pancreatitis (MAP), moderately severe acute pancreatitis (MSAP), and severe acute pancreatitis (SAP) according to the presence and duration of organ failure presented in the revised Atlanta classification (2013)[6]. The mortality rate is high among SAP patients, reaching 15%-30% or even higher[7], due to persistent organ failure. Therefore, earlier identification and appropriate use of intensive care support can be conducive to prevent further disease progression, thereby improving AP patients’ prognosis[8].
Acute kidney injury (AKI) is one of the most common AP-associated complications, resulting from uncontrolled inflammatory response, release of pancreatic amylase, hypovolemia, insufficient renal perfusion, micro-circulatory disturbance, intra-abdominal hypertension, and reactive oxygen species[9]. At present, AKI is mainly diagnosed based on the criteria presented by the Kidney Disease Improving Global Outcomes (KIDGO) guidelines[10,11]. The incidence of AKI has gradually, while steadily, increased year-by-year worldwide[12]. AKI can deteriorate AP patient’s medical status and is an independent risk factor for increased mortality and development of chronic kidney disease (CKD). When AKI and AP occur simultaneously, a worse clinical prognosis is expected[13,14], involving longer period of hospitalization and higher mortality rate. However, in clinical practice, there is no an effective therapeutic approach for AKI except for renal replacement therapy (RRT)[15]. AKI has imposed a huge medical burden in China as well as in the world. Therefore, development of early detection and prevention measures is highly significant to avoid adverse outcomes associated with AKI[16].
Although previous studies have confirmed that concurrent AKI is associated with a poor prognosis[17], there is no reliable research assessing the influences of AKI on Chinese AP patients’ survival rate who were hospitalized at surgical intensive care unit (SICU). Hence, to address this scientific gap, the current retrospective study was conducted.
This retrospective study enrolled 139 AP patients who were admitted to the SICU of The Second Affiliated Hospital of Harbin Medical University (Harbin, Heilongjiang Province, China) between January 2014 and March 2019. Baseline and clinical data were collected during hospitalization. The enrolled AP patients were divided into AKI group (n = 72) and non-AKI group (n = 67) according to occurrence of AKI. This study was approved by the Ethics Committee of The Second Affiliated Hospital of Harbin Medical University.
The inclusion criteria for this retrospective study were as follows: Patients who were admitted to the SICU of The Second Affiliated Hospital of Harbin Medical University; patients who were diagnosed with AP; patients’ age > 18-years-old. The exclusion criteria were as follows: Pregnant or breastfeeding women; patients with CKD; patients with recurrent pancreatitis; patients who received renal transplantation; incomplete medical data.
A combination of medical history, symptoms and physical examinations, laboratory tests, and radiographic examinations (e.g., abdominal ultrasound, contrast-enhanced computed tomography, or magnetic resonance imaging) was applied to confirm the diagnosis of AP. A limited number of diagnosed AP patients were eventually confirmed by undergoing exploratory laparotomy.
Diagnosis and classification of AKI were conducted based on the criteria presented by the KIDGO guidelines (2012)[18], in which serum creatinine criteria was defined as an increased absolute value in serum creatinine level of ≥ 0.3 mg/dL (≥ 26.4 μmol/L) or a percentage increase in serum creatinine level of ≥ 50% within 48 h. Baseline serum creatinine level was defined as the lowest serum creatinine level measured within 2 d prior to admission to SICU. If no serum creatinine level was measured, the serum creatinine level recorded in the first measurement within 2 d after admission to SICU was considered as baseline serum creatinine level.
In the present study, all AP patients who needed to undergo RRT were managed with continuous veno-venous hemofiltration (CVVH), anti-coagulation with heparin, and fixed pre- and post-dilution strategies. Blood flow rates, amount of substitute fluid, and dehydration volume were individually adjusted according to each patient’s medical status.
Serum procalcitonin (PCT) level was intermittently measured after AP patients’ admission to SICU through Mini-VIDAS (Hain Lifescience GmbH; Nehren, Germany). Intra-peritoneal pressure (IPP) was indirectly reflected via measuring intravesical pressure with Freund's catheter.
Baseline and clinical data, including patients’ age, gender, body mass index (BMI), Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, Glasgow Coma Scale (GCS) score, duration of mechanical ventilation (MV), abdominal puncture drainage, gallbladder puncture drainage, infusion of vasopressors, demand of RRT, IPP, body temperature, PCT, creatinine, platelet count, hospital day, and prognosis were collected from medical records of hospitalized patients. APACHE II score and SOFA score were calculated by the first 24 h clinical data after SICU admission.
Variables conforming to normal distribution were described as mean ± SD, while those abnormally distributed variables were expressed as median (range). Normality analysis was applied to continuous data. SPSS 22.0 software (IBM, Armonk, NY, United Sates) was used to carry out statistical analyses. Independent-samples t-test was used to perform inter-group comparison for normally distributed data, while Mann-Whitney U test was employed for inter-group comparison for abnormally distributed data. Classification data were expressed by the number of samples, and χ2test was adopted. The prognosis of AP patients who were admitted to SICU was analyzed by binary logistic regression analysis, while the remaining indicators were analyzed by the t-test or Mann-Whitney U test. P value < 0.05 was considered statistically significant.
A total of 139 AP patients were enrolled in this retrospective study. The enrolled AP patients were divided into AKI group (n = 72) and non-AKI group (n = 67) according to occurrence of AKI. As shown in Table 1, AKI was more likely to occur in male AP patients (P = 0.009). AP patients with AKI exhibited a significantly higher APACHE II score, higher SOFA score, lower GCS score, and higher demand for MV, infusion of vasopressors, and RRT than non-AKI AP patients (P < 0.01, P < 0.01, P = 0.01, P = 0.001, P < 0.01, P < 0.01, respectively). No significant difference was observed in the remaining baseline and clinical data, including patients’ age, BMI, proportion of abdominal puncture drainage, and gallbladder puncture drainage.
| AKI (n = 72) | Non-AKI (n = 67) | Z/χ2 | P value | |
| Age | 45.00 (17.50) | 44.00 (21.00) | -0.07 | 0.94 |
| Gender | 6.745 | 0.009 | ||
| Male | 50 | 32 | ||
| Female | 22 | 35 | ||
| BMI | 24.45 (2.38) | 23.80 (2.60) | -1.20 | 0.23 |
| APACHE II Score | 18.00 (7.75) | 9.00 (7.00) | -7.47 | < 0.01 |
| SOFA Score | 9.00 (6.00) | 4.00 (4.00) | -6.50 | < 0.01 |
| GCS Score | 15.00 (3.00) | 15.00 (0.00) | -2.71 | 0.01 |
| MV | 10.94 | 0.001 | ||
| Yes | 46 | 24 | ||
| No | 26 | 43 | ||
| Abdominal puncture drainage | 0.19 | 0.66 | ||
| Yes | 36 | 36 | ||
| No | 36 | 31 | ||
| Gallbladder puncture drainage | 2.36 | 0.12 | ||
| Yes | 17 | 9 | ||
| No | 55 | 58 | ||
| Vasopressor infusion | 26.84 | < 0.01 | ||
| Yes | 21 | 18 | ||
| No | 51 | 49 | ||
| RRT | ||||
| Yes | 44 | 20 | 13.651 | < 0.01 |
| No | 28 | 47 |
There were significant differences in types of vasopressors and proportion of norepinephrine, adrenaline, and vasopressor infusion between the two groups (Table 2).
| AKI, n = 72 | Non-AKI, n = 67 | χ2 | P value | |
| Types of vasopressors | 27.44 | < 0.01 | ||
| No | 21 | 49 | ||
| 1 | 34 | 14 | ||
| ≥ 2 | 17 | 4 | ||
| Norepinephrine infusion | 35.35 | < 0.01 | ||
| Yes | 49 | 17 | ||
| No | 23 | 50 | ||
| Adrenaline infusion | 12.75 | < 0.01 | ||
| Yes | 15 | 1 | ||
| No | 57 | 66 | ||
| Vasopressor infusion | 26.84 | < 0.01 | ||
| Yes | 51 | 18 | ||
| No | 21 | 49 |
Significant differences were noted in dose of norepinephrine and adrenaline, duration of MV, maximum and mean values of IPP, maximum and mean values of PCT, maximum and mean serum levels of creatinine, minimum platelet count, prognosis of AP patients admitted to SICU, and hospital day between the two groups (Table 3). Among AP patients with AKI, the survival rates of SICU and in-hospital were only 23% and 21% of the corresponding rates in AP patients without AKI, respectively.
| AKI, n = 72 | Non-AKI, n = 67 | Z/t/Wald | P value | OR | |
| Prognosis of SICU | 9.54 | 0.002 | 0.23 | ||
| cure | 48 | 60 | |||
| other | 24 | 7 | |||
| Prognosis of in-hospital | 11.33 | 0.001 | 0.21 | ||
| cure | 46 | 60 | |||
| other | 26 | 7 | |||
| Duration of MV | 34.00 (87.75) | 0.00 (18.00) | -3.92 | < 0.01 | |
| Maximum values of IPP | 24.50 (10.00) | 18.00 (10.00) | -5.39 | < 0.01 | |
| Mean values of IPP | 21.40 (6.82) | 15.29 (4.73) | 6.175 | < 0.01 | |
| Maximum values of body temperature | 37.80 (1.20) | 37.50 (1.00) | -2.00 | 0.05 | |
| Mean values of body temperature | 37.00 (0.50) | 37.00 (0.30) | -0.59 | 0.56 | |
| Maximum values of PCT | 16.28 (21.12) | 2.87 (9.28) | -5.96 | < 0.01 | |
| Mean values of PCT | 8.52 (11.52) | 1.58 (4.20) | -6.42 | < 0.01 | |
| Maximum serum levels of creatinine | 284.35 (215.73) | 87.60 (47.80) | -9.94 | < 0.01 | |
| Mean serum levels of creatinine | 197.45 (172.10) | 65.60 (29.60) | -9.48 | < 0.01 | |
| Minimum platelet count | 93.00 (87.75) | 137.00 (73.00) | -3.18 | < 0.01 | |
| Mean platelet count | 174.40 (112.15) | 198.00 (103.60) | -1.64 | 0.10 | |
| Hospital day | 9.00 (12.75) | 8.00 (11.00) | -0.07 | 0.94 | |
| Dose of norepinephrine | 16.00 (52.00) | 0.00 (4.00) | -5.11 | < 0.01 | |
| Dose of adrenaline | 0.00 (0.00) | 0.00 (0.00) | -3.58 | < 0.01 |
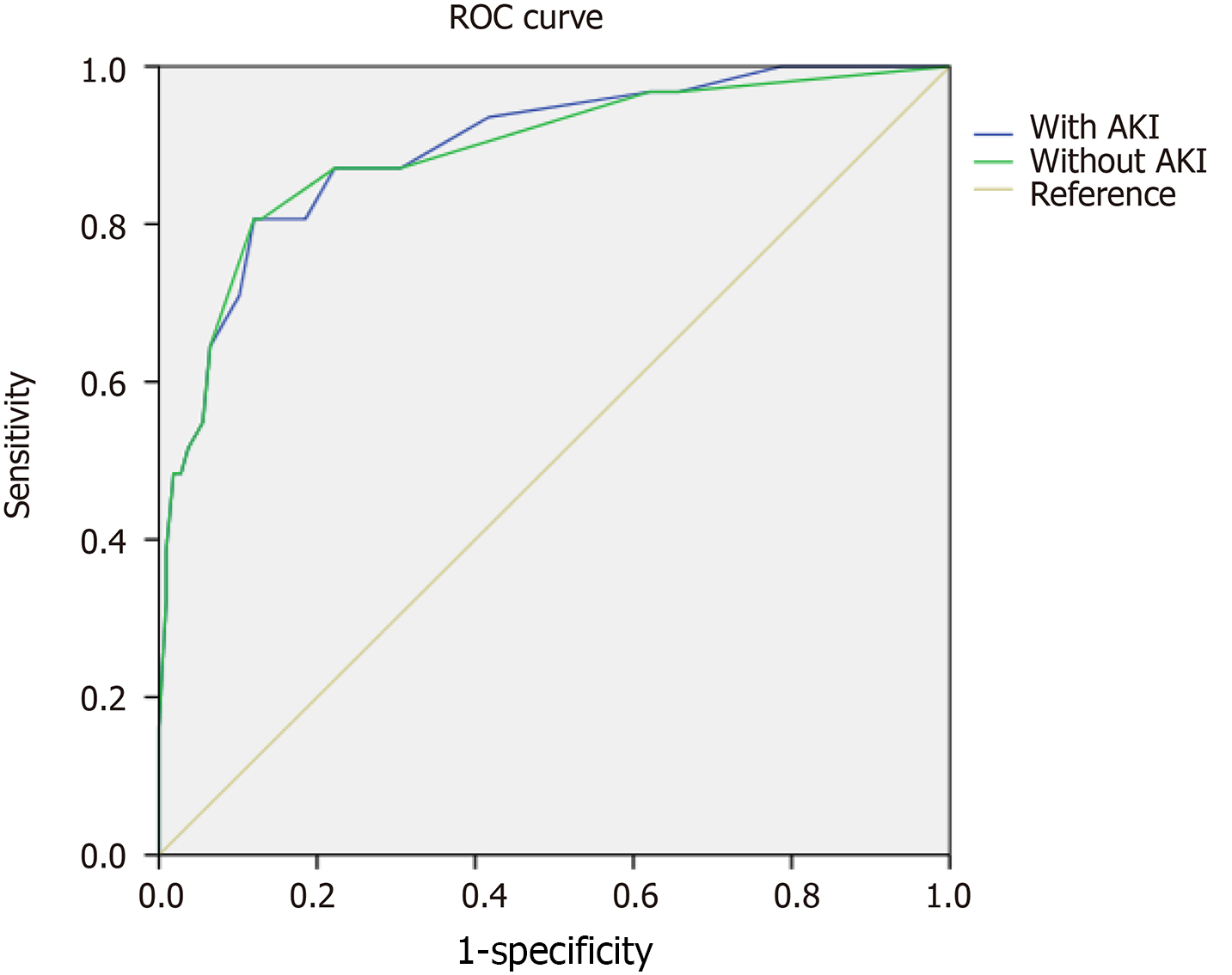
The factors influencing the AP patients’ survival rate who were hospitalized at SICU included BMI, mean values of IPP, minimum platelet count, and hospital day. Among these factors, mean values of IPP showed the greatest effect (Table 4). The values of area under the receiver operating characteristic (ROC) curve for the four factors related to patients with or without AKI were calculated to predict the AP patients’ survival rate, and were 0.896 and 0.891, respectively (Figure 1 and Table 5; P > 0.5). However, there was no significant different in values of the area under the two ROC curves. The sensitivity and specificity of the two ROC curves were approximately the same [sensitivity (80.6%) vs specificity (88.0%)].
| B | SE | Wald | df | P value | Exp (B) | |
| BMI | 1.4904 | 0.6467 | 5.3105 | 1.0000 | 0.0212 | 4.4387 |
| Mean values of IPP | 2.6477 | 0.6217 | 18.1355 | 1.0000 | 0.0000 | 14.1211 |
| Minimum platelet count | -1.2285 | 0.6125 | 4.0237 | 1.0000 | 0.0449 | 0.2927 |
| Hospital day | -1.8571 | 0.5965 | 9.6913 | 1.0000 | 0.0019 | 0.1561 |
| Constant | -0.9863 | 0.8689 | 1.2883 | 1.0000 | 0.2564 | 0.3730 |
| Variables | Area | SE | Asymptotic, P value | Asymptotic 95%CI | |
| Lower bound | Upper bound | ||||
| With AKI | 0.896 | 0.033 | 0 | 0.831 | 0.961 |
| Without AKI | 0.891 | 0.037 | 0 | 0.819 | 0.963 |
Risk thresholds of BMI, mean values of IPP, minimum platelet count, and hospital day were ≥ 24, ≥ 23, ≤ 77, and ≤ 4, respectively, which indicated that BMI ≥ 24, mean values of IPP ≥ 23, minimum platelet count ≤ 77, and hospital day ≤ 4 were risk factors for AP patients’ survival rate who were hospitalized at SICU.
Excessive secretion and/or poor drainage of pancreatic juice are the essence of AP onset. With the increasing incidence of gallstones and alcohol abuse, AP had become a common gastrointestinal disorder. The majority of cases with AP are MAP characterized by no new onset of organ dysfunction, whereas SAP not only leads to new onset of organ dysfunction, but also lasts for more than 48 h[6]. With aggravation of the condition from MAP to SAP, the length of stay at SICU and mortality rate significantly increased. Hence, SAP patients should be managed in SICU for multiple organ support therapy and surgical interventions to avoid further disease progression[19]. When AP happens, hemodynamic status should be assessed immediately to avoid hypovolemia or volume overload caused by excessive fluid resuscitation[20], which might cause detrimental influences[21]. Routine use of prophylactic antibiotics in SAP or necrotizing pancreatitis was not clinically recommended in the guidelines[22], because of no mortality benefit or reduction in the incidence of infected necrosis[23]. The ability to penetrate pancreatic necrotic tissue is of great significance in the selection of antibiotics. Less than 50% of necrotizing pancreatitis patients need surgical interventions, in which step-up approach and minimally invasive strategies were advocated[24-27], involving endoscopic nasobiliary drainage, percutaneous transhepatic gallbladder drainage, percutaneous transhepatic biliary drainage, endoscopic retrograde cholangiopancreatography, endoscopic transgastric/ transduodenal drainage, and video-assisted retroperitoneal debridement.
During AP, kidney is the most vulnerable organ and is typically sacrificed to protect other important organs, such as heart, lung, brain, and liver. As a consequence, AKI is a common complication of AP, which is ordinarily associated with adverse outcomes, including risk of subsequent CKD, end-stage renal disease, RRT dependence, in-hospital and post-discharge mortality, and even healthcare cost-containment concerns[28-30]. It was generally believed that the incidence of AKI was about 20% in critically ill adult patients[31]. More than 50% of SAP patients would develop into AKI according to previous literature[3,17,32]. Different organs interact with each other in the whole body, and the deteriorated kidney function can inevitably cause or aggravate damage to other organs without early intervention. Diuretics had been demonstrated to be an independent risk factor for AKI[33], and thus, were no longer recommended for routine use in clinical practice. When RRT is required, the mortality rate may even exceed 75%[34]. Compared with developed countries, AKI is typically substantial underdiagnoses and undertreatment in developing countries[35], especially in African countries, which may be partially explained as seriously inadequate repeated serum creatinine assay[36].
At present, about 20% of AKI patients require to undergo RRT[37], and this rate continues to increase worldwide. Some eligible patients for RRT may decline to undergo this intervention because of resource constraints, high costs of therapy, or severe comorbidities[38], which significantly increase mortality rate compared with those cases who received it[39]. RRT involves different treatment modalities, such as hemodialysis, hemofiltration, hemodiafiltration, and peritoneal dialysis. It has been suggested that continuous RRT has several advantages over intermittent RRT, including better hemodynamic stability (blood pressure control and blood circulation), improved survival, and greater likelihood of renal recovery[38,40,41]. Thus, CVVH had occupied the mainstream of RRT, particularly for AP patients who were hospitalized at SICU, because of its outstanding effectiveness and safety. Although it has been suggested that early application of RRT in patients with severe sepsis, irrespective of the presence of renal failure, might be beneficial, CVVH did not limit further organ damage and even prolonged the need for organ support. RRT is a non-selective method, which can simultaneously remove detrimental and beneficial substances. In clinical practice, in spite of great advances in RRT development, there is still a lack of uniform standards for RRT-associated strategies, such as optimal timing for initiation of RRT, anti-coagulation, and optimal dosage[42,43].
Among several scoring systems, APACHE II score and SOFA score are commonly used to reflect timely and accurately illness severity and predict prognosis of AP patients[44]. It is well known that GCS score is a simple and valuable tool for indication of nervous system function and prediction of short- and long-term mortality[45], in which its discriminative power is similar to other more complex scoring systems[46]. Therefore, these three scoring systems were selected to assess the severity of AP in the present research. A number of scholars pointed out that demand for MV, infusion of vasopressors, and RRT could be risk factors for increased mortality in AP patients[47]. PCT level may contribute to earlier and better stratification of septic patients who are at risk of death and admitted to SICU[48]. To our knowledge, the increased IPP and decreased platelet count are closely associated with the severity and prognosis of AP in clinical practice[49]. Hence, the above-mentioned indicators were employed in the current study.
The present retrospective study provided a comprehensive description about the influences of AKI on AP patients’ survival rate who were hospitalized at SICU in accordance with the criteria presented by the KIDGO guidelines. Our findings revealed that AP patients with AKI had more severe degree of illness than AP patients without AKI, as evidenced by higher APACHE II score, higher SOFA score, and lower GCS score, and the survival rates of SICU and in-hospital were only 23% and 21% of the corresponding rates in AP patients without AKI, respectively, which were roughly consistent with previously reported rates[10,31]. These gaps can be partially explained by the increased emphasis placed on AP and improvement of diagnostic methods and standardized therapeutic bundles. For AP patients with AKI, other influences were found to be associated with demand for MV, infusion of vasopressors and RRT, dosages of norepinephrine and adrenaline, duration of MV, maximum and mean values of IPP, maximum and mean values of PCT, maximum and mean serum levels of creatinine, and minimum platelet count. It was revealed that AKI negatively and seriously affected AP patients who were hospitalized at SICU.
The factors that influenced AP patients’ survival rate who were hospitalized at SICU included BMI, mean values of IPP, minimum platelet count, and hospital day. With every unit change in BMI, mean values of IPP, minimum platelet count, and hospital day, the AP patients’ survival rate was 4.4387, 14.1211, 0.1561, and 0.3730 of the original rates, respectively. Therefore, mean values of IPP had the greatest influence on the AP patients’ survival rate who were hospitalized at SICU. Risk thresholds of BMI, mean values of IPP, minimum platelet count, and hospital day were ≥ 24, ≥ 23, ≤ 77, and ≤ 4, respectively, which indicated that BMI ≥ 24, mean values of IPP ≥ 23, minimum platelet count ≤ 77, and hospital day ≤ 4 were risk factors for the AP patients’ survival rate who were hospitalized at SICU.
The present study contains a number of limitations. First, although significant differences were detected, this was only a small sample-size, single-center, retrospective study, which might reduce the reliability of our conclusion and its application to AP patients in clinical practice. Second, clinical data were obtained only on the basis of the medical records during hospitalization, which might lead to research bias. Last but not least, the etiology of AP had not been further differentiated. It is noteworthy that different etiologies may induce different clinical manifestations and prognosis, necessitating conducting further studies in the future.
In conclusion, this study attempted to clarify the influence of AKI on AP patients’ survival rate who were hospitalized at SICU. Results showed that AP patients with AKI exhibited lower survival rate and worse relevant clinical outcomes than AP patients without AKI. Besides, BMI, mean values of IPP, minimum platelet count, and hospital day may play significant roles in predicting the AP patients’ survival rate who were hospitalized at SICU. Our findings suggest that prevention of AKI is clinically important.
Acute kidney injury (AKI) is common in acute pancreatitis (AP) patients, but the risk factors are not clear.
This study tried to explore the associated risk factors of AKI in AP patients.
This study aimed to assess the influences of AKI on the survival rate in AP patients.
Patients were divided into two groups based on AKI status, and comparisons between the groups were calculated for diverse variables.
AKI is more likely to occur in male AP patients.
AP patients with AKI exhibited lower survival rate and worse relevant clinical outcomes than AP patients without AKI in SICU.
This study provided clinical evidence for prevention of AKI in AP patients.
We highly appreciate the contribution of participants and co-workers from surgical intensive care unit of The Second and First Affiliated Hospital of Harbin Medical University to this research.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Deepak P, Zimmerman M S-Editor: Yan JP L-Editor: Filipodia P-Editor: Xing YX
| 1. | Krishna SG, Kamboj AK, Hart PA, Hinton A, Conwell DL. The Changing Epidemiology of Acute Pancreatitis Hospitalizations: A Decade of Trends and the Impact of Chronic Pancreatitis. Pancreas. 2017;46:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 2. | Russell PS, Mittal A, Brown L, McArthur C, Phillips AJR, Petrov M, Windsor JA. Admission, management and outcomes of acute pancreatitis in intensive care. ANZ J Surg. 2017;87:E266-E270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Pavlidis P, Crichton S, Lemmich Smith J, Morrison D, Atkinson S, Wyncoll D, Ostermann M. Improved outcome of severe acute pancreatitis in the intensive care unit. Crit Care Res Pract. 2013;2013:897107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Weiss FU, Laemmerhirt F, Lerch MM. Etiology and Risk Factors of Acute and Chronic Pancreatitis. Visc Med. 2019;35:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 5. | Kopecky K, Moreland A, Hebert C, Colbert GB. Plasmapheresis for recurrent acute pancreatitis from hypertriglyceridemia. Proc (Bayl Univ Med Cent). 2017;30:358-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4323] [Article Influence: 360.3] [Reference Citation Analysis (45)] |
| 7. | Karakayali FY. Surgical and interventional management of complications caused by acute pancreatitis. World J Gastroenterol. 2014;20:13412-13423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (2)] |
| 8. | Jacob AO, Stewart P, Jacob O. Early surgical intervention in severe acute pancreatitis: Central Australian experience. ANZ J Surg. 2016;86:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Petejova N, Martinek A. Acute kidney injury following acute pancreatitis: A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Luo X, Jiang L, Du B, Wen Y, Wang M, Xi X; Beijing Acute Kidney Injury Trial (BAKIT) workgroup. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care. 2014;18:R144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 11. | Pan HC, Chien YS, Jenq CC, Tsai MH, Fan PC, Chang CH, Chang MY, Tian YC, Fang JT, Yang CW, Chen YC. Acute Kidney Injury Classification for Critically Ill Cirrhotic Patients: A Comparison of the KDIGO, AKIN, and RIFLE Classifications. Sci Rep. 2016;6:23022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M; CDC Prevention Epicenter Program. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA. 2017;318:1241-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 1288] [Article Influence: 161.0] [Reference Citation Analysis (0)] |
| 13. | Lin HY, Lai JI, Lai YC, Lin PC, Chang SC, Tang GJ. Acute renal failure in severe pancreatitis: A population-based study. Ups J Med Sci. 2011;116:155-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Li H, Qian Z, Liu Z, Liu X, Han X, Kang H. Risk factors and outcome of acute renal failure in patients with severe acute pancreatitis. J Crit Care. 2010;25:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Gao Y, Kang K, Liu H, Kong W, Han Q, Zhang X, Huang R, Qu J, Wang H, Wang S, Liu R, Liu Y, Yu K. GTS-21 attenuates LPS-induced renal injury via the cholinergic anti-inflammatory pathway in mice. Am J Transl Res. 2017;9:4673-4681. [PubMed] |
| 16. | Li PK, Burdmann EA, Mehta RL; World Kidney Day Steering Committee 2013. Acute kidney injury: global health alert. Kidney Int. 2013;83:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Zhou J, Li Y, Tang Y, Liu F, Yu S, Zhang L, Zeng X, Zhao Y, Fu P. Effect of acute kidney injury on mortality and hospital stay in patient with severe acute pancreatitis. Nephrology (Carlton). 2015;20:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1192] [Cited by in RCA: 1847] [Article Influence: 153.9] [Reference Citation Analysis (0)] |
| 19. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-15; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1380] [Article Influence: 115.0] [Reference Citation Analysis (3)] |
| 20. | Ekinci C, Karabork M, Siriopol D, Dincer N, Covic A, Kanbay M. Effects of Volume Overload and Current Techniques for the Assessment of Fluid Status in Patients with Renal Disease. Blood Purif. 2018;46:34-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | de-Madaria E, Soler-Sala G, Sánchez-Payá J, Lopez-Font I, Martínez J, Gómez-Escolar L, Sempere L, Sánchez-Fortún C, Pérez-Mateo M. Influence of fluid therapy on the prognosis of acute pancreatitis: a prospective cohort study. Am J Gastroenterol. 2011;106:1843-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 22. | Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 559] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 23. | Bai Y, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2008;103:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Hollemans RA, Bakker OJ, Boermeester MA, Bollen TL, Bosscha K, Bruno MJ, Buskens E, Dejong CH, van Duijvendijk P, van Eijck CH, Fockens P, van Goor H, van Grevenstein WM, van der Harst E, Heisterkamp J, Hesselink EJ, Hofker S, Houdijk AP, Karsten T, Kruyt PM, van Laarhoven CJ, Laméris JS, van Leeuwen MS, Manusama ER, Molenaar IQ, Nieuwenhuijs VB, van Ramshorst B, Roos D, Rosman C, Schaapherder AF, van der Schelling GP, Timmer R, Verdonk RC, de Wit RJ, Gooszen HG, Besselink MG, van Santvoort HC; Dutch Pancreatitis Study Group. Superiority of Step-up Approach vs Open Necrosectomy in Long-term Follow-up of Patients With Necrotizing Pancreatitis. Gastroenterology. 2019;156:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 25. | Rashid MU, Hussain I, Jehanzeb S, Ullah W, Ali S, Jain AG, Khetpal N, Ahmad S. Pancreatic necrosis: Complications and changing trend of treatment. World J Gastrointest Surg. 2019;11:198-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Jones JD, Clark CJ, Dyer R, Case LD, Mishra G, Pawa R. Analysis of a Step-Up Approach Versus Primary Open Surgical Necrosectomy in the Management of Necrotizing Pancreatitis: Experience in a Cohort of Patients at a US Academic Medical Center. Pancreas. 2018;47:1317-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Koutroumpakis E, Slivka A, Furlan A, Dasyam AK, Dudekula A, Greer JB, Whitcomb DC, Yadav D, Papachristou GI. Management and outcomes of acute pancreatitis patients over the last decade: A US tertiary-center experience. Pancreatology. 2017;17:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 29. | Lewington AJ, Cerdá J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84:457-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 530] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 30. | Horkan CM, Purtle SW, Mendu ML, Moromizato T, Gibbons FK, Christopher KB. The association of acute kidney injury in the critically ill and postdischarge outcomes: a cohort study*. Crit Care Med. 2015;43:354-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL; Acute Kidney Injury Advisory Group of the American Society of Nephrology. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1025] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 32. | Wajda J, Dumnicka P, Maraj M, Ceranowicz P, Kuźniewski M, Kuśnierz-Cabala B. Potential Prognostic Markers of Acute Kidney Injury in the Early Phase of Acute Pancreatitis. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Nisula S, Kaukonen KM, Vaara ST, Korhonen AM, Poukkanen M, Karlsson S, Haapio M, Inkinen O, Parviainen I, Suojaranta-Ylinen R, Laurila JJ, Tenhunen J, Reinikainen M, Ala-Kokko T, Ruokonen E, Kuitunen A, Pettilä V; FINNAKI Study Group. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 34. | Nassar TI, Qunibi WY. AKI Associated with Acute Pancreatitis. Clin J Am Soc Nephrol. 2019;14:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 35. | Singh TB, Rathore SS, Choudhury TA, Shukla VK, Singh DK, Prakash J. Hospital-acquired acute kidney injury in medical, surgical, and intensive care unit: A comparative study. Indian J Nephrol. 2013;23:24-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, He Q, Chen J, Chen M, Liu X, Zhu Z, Yang L, Lian X, Ding F, Li Y, Wang H, Wang J, Wang R, Mei C, Xu J, Li R, Cao J, Zhang L, Wang Y, Bao B, Liu B, Chen H, Zha Y, Luo Q, Chen D, Shen Y, Liao Y, Zhang Z, Wang X, Zhang K, Liu L, Mao P, Guo C, Li J, Wang Z, Bai S, Shi S, Liu Z, Wang F, Huang D, Wang S, Ge S, Shen Q, Zhang P, Wu L, Pan M, Zou X, Zhu P, Zhao J, Zhou M, Hu W, Zhang T, Han J, Wen T, Zhao M; ISN AKF 0by25 China Consortiums. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 332] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 37. | Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2063] [Cited by in RCA: 1827] [Article Influence: 182.7] [Reference Citation Analysis (0)] |
| 38. | Clark WR, Ding X, Qiu H, Ni Z, Chang P, Fu P, Xu J, Wang M, Yang L, Wang J, Ronco C. Renal replacement therapy practices for patients with acute kidney injury in China. PLoS One. 2017;12:e0178509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Wang F, Hong D, Wang Y, Feng Y, Wang L, Yang L; ISN AKF 0 by 25 China Consortium. Renal replacement therapy in acute kidney injury from a Chinese cross-sectional study: patient, clinical, socioeconomic and health service predictors of treatment. BMC Nephrol. 2017;18:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Schneider AG, Bellomo R, Bagshaw SM, Glassford NJ, Lo S, Jun M, Cass A, Gallagher M. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2013;39:987-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 41. | Heung M, Yessayan L. Renal Replacement Therapy in Acute Kidney Injury: Controversies and Consensus. Crit Care Clin. 2017;33:365-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Gao Y, Qi ZD, Liu RJ, Liu HT, Han QY, Zhang X, Huang R, Li M, Yang ZY, Zheng JB, Qu JD, Wang SC, Liu YS, Wang HL, Yu KJ. A multi-center cross-sectional study on blood purification among adult patients in intensive care unit in China: a study protocol. Chin Med J (Engl). 2019;132:1208-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Zhang X, Cao Y, Pan CK, Han QY, Guo YQ, Song T, Qi ZD, Huang R, Li M, Yang ZY, Zheng JB, Hou GY, Li JY, Wang SC, Liu YS, Liu RJ, Gao Y, Wang HL. Effect of initiation of renal replacement therapy on mortality in acute pancreatitis patients. Medicine (Baltimore). 2020;99:e23413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Mikó A, Vigh É, Mátrai P, Soós A, Garami A, Balaskó M, Czakó L, Mosdósi B, Sarlós P, Erőss B, Tenk J, Rostás I, Hegyi P. Computed Tomography Severity Index vs. Other Indices in the Prediction of Severity and Mortality in Acute Pancreatitis: A Predictive Accuracy Meta-analysis. Front Physiol. 2019;10:1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 45. | Handschu R, Haslbeck M, Hartmann A, Fellgiebel A, Kolominsky-Rabas P, Schneider D, Berrouschot J, Erbguth F, Reulbach U. Mortality prediction in critical care for acute stroke: Severity of illness-score or coma-scale? J Neurol. 2005;252:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Huang KB, Ji Z, Wu YM, Wang SN, Lin ZZ, Pan SY. The prediction of 30-day mortality in patients with primary pontine hemorrhage: a scoring system comparison. Eur J Neurol. 2012;19:1245-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Fischer AJ, Andreottola F, Lenz P, Lebiedz P. [Acute pancreatitis in intensive care medicine: Which risk score is useful? Med Klin Intensivmed Notfmed. 2017;112:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Jain S, Sinha S, Sharma SK, Samantaray JC, Aggrawal P, Vikram NK, Biswas A, Sood S, Goel M, Das M, Vishnubhatla S, Khan N. Procalcitonin as a prognostic marker for sepsis: a prospective observational study. BMC Res Notes. 2014;7:458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Kefeli A, Basyigit S, Özgür Yeniova A, Küçükazman M, Nazligül Y, Aktas B. Platelet Number and Indexes during Acute Pancreatitis. Euroasian J Hepatogastroenterol. 2014;4:67-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |