Published online Oct 14, 2021. doi: 10.3748/wjg.v27.i38.6442
Peer-review started: May 24, 2021
First decision: June 22, 2021
Revised: July 17, 2021
Accepted: September 10, 2021
Article in press: September 10, 2021
Published online: October 14, 2021
Processing time: 140 Days and 17.7 Hours
We hypothesized that thermal damage accumulation during endoscopic sub
To determine the association between Joule heat and the onset of PECS.
We performed a retrospective cohort study in patients who underwent colorectal ESD from May 2013 to March 2021 in Japan. We developed a novel device that measures swift coagulation time with a sensor adjacent to the electrosurgical coagulation unit foot switch, which enabled us to calculate total Joule heat. PECS was defined as localized abdominal pain (visual analogue scale ≥ 30 mm during hospitalization or increased by ≥ 20 mm from the baseline) and fever (temperature ≥ 37.5 degrees or white blood cell count ≥ 10000 µ/L). Patients exposed to more or less than the median Joule heat value were assigned to the high and low Joule heat groups, respectively. Statistical analyses included Mann-Whitney U and chi-square tests and logistic regression and receiver operating characteristic curve (ROC) analyses.
We evaluated 151 patients. The PECS incidence was 10.6% (16/151 cases), and all patients were followed conservatively and discharged without severe complications. In multivariate analysis, high Joule heat was an independent PECS risk factor. The area under the ROC curve showing the correlation between PECS and total Joule heat was high [0.788 (95% confidence interval: 0.666-0.909)].
Joule heat accumulation in the gastrointestinal wall is involved in the onset of PECS. ESD-related thermal damage to the peeled mucosal surface is probably a major component of the mechanism underlying PECS.
Core Tip: We investigated the association between Joule heat and the onset of post- endoscopic submucosal dissection electrocoagulation syndrome (PECS), using ori
- Citation: Ochi M, Kawagoe R, Kamoshida T, Hamano Y, Ohkawara H, Ohkawara A, Kakinoki N, Yamaguchi Y, Hirai S, Yanaka A, Tsuchiya K. High total Joule heat increases the risk of post-endoscopic submucosal dissection electrocoagulation syndrome after colorectal endoscopic submucosal dissection. World J Gastroenterol 2021; 27(38): 6442-6452
- URL: https://www.wjgnet.com/1007-9327/full/v27/i38/6442.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i38.6442
Colorectal endoscopic submucosal dissection (ESD), a minimally invasive operation, is the best endoscopic procedure for en bloc resection of superficial colorectal tumors[1-4]. However, ESD is associated with severe complications, with rates of perforation and bleeding of 2% to 14% and 0.7% to 3.1%, respectively[4-10]. Post-ESD electrocoagulation syndrome (PECS) is another notable adverse event that can occur after colorectal ESD. The incidence of PECS is 9% to 40%, although most cases improve with conservative therapy[11-15]. In addition, PECS sometimes manifests as a delayed perforation. Therefore, physicians require clinically useful PECS predictors to identify patients who are at risk[12,16].
Currently, two main hypotheses explain the mechanism underlying PECS. One suggests that ESD exposes the mucosa, which is then infected by intestinal bacteria, resulting in inflammation[13,17]. The other proposes that the peeled mucosal surface is inflamed by thermal damage during ESD[13,18]. Interestingly, a clipping closure method performed to prevent intestinal bacteria from infecting the exposed mucosa did not reduce the onset of PECS[19]. In contrast, few studies have examined the impact of thermal damage during ESD on the onset of PECS.
We have often encountered patients with PECS who underwent a lengthy colorectal ESD, and previous studies of PECS risk factors implicated prolonged ESD procedures[20,21]. We hypothesized that electrocoagulation is associated with Joule heat capable of causing thermal damage to the gastrointestinal wall during long ESD operations. Therefore, we aimed to determine the association between Joule heat and the onset of PECS.
We performed a retrospective study in patients who underwent colorectal ESD at Hitachi General Hospital in Japan from May 2013 to March 2021. Case selection was according to the indications for colorectal ESD established by the Japan Gastroenterological Endoscopy Society guidelines[22]. Therefore, our inclusion criteria were as follows: (1) Laterally spreading tumors of the non-granular type with the Vi-type pit pattern, carcinomas with shallow T1 submucosal (SM) invasion, large depressed-type tumors, and large protruded-type tumors that are difficult to remove en bloc by endoscopic mucosal resection; and (2) Mucosal tumors with SM fibrosis. Patients with multiple colorectal neoplasms or apparent deeply invasive T1 SM carcinoma were excluded. R0 resection was defined as no cancer cells seen microscopically at the primary tumor site. We converted the swift-coagulation mode time, measured by an electrosurgical-coagulation unit with a high-frequency generator (VIO 300D, ERBE Co. Ltd., Tubingen, Germany), to Joule heat.
The Hitachi General Hospital Institutional Review Board approved the study (No. 2019-97, 2020-1), and our research was performed according to the ethical guidelines of the 1964 Declaration of Helsinki and its later amendments. The study is registered on the University Hospital Medical Information Network (ID: UMIN000038704, UMIN000041580). Although our ethics committee waived the requirement for informed consent from each patient because we used anonymized data, we obtained informed consent using an opt-out option on our facility’s website (uniform resource locator below).
PECS was defined as localized abdominal pain and fever without apparent perforation after colorectal ESD. We used a visual analog scale (VAS) to evaluate localized abdominal pain. Nurses who were not research participants administered the VAS. The pain criteria were defined as a score ≥ 30 mm from the postoperative day (POD) 1 to discharge or increased by ≥ 20 mm from the hospital admission VAS score. Our fever criteria were body temperature ≥ 37.5 degrees or white blood cell count ≥ 10000 µ/L from POD 1 to discharge.
All patients underwent bowel preparation (polyethylene glycol, 2 L) before colorectal ESD. Then, the patients were sedated in the endoscopy room using intravenous injections of pentazocine (15-30 mg/kg body weight). We added midazolam (1-3 mg/session) if pentazocine was insufficient for sedation. Electrocardiogram moni
We used the electrosurgical-coagulation unit to detach the lesion’s colorectal mucosa. We changed the setting mode according to the procedure step and used dry-cut, swift-coagulation, and soft-coagulation modes. We calculated the power applied using the diagram in the manufacturer’s technical manual that shows the relationship between resistance (Ω) and power (W) in the swift-coagulation mode (Supplementary material). As human internal resistance is 1000-1600 Ω[24], the lesion resistance was defined as 1300 Ω. Thus, lesions are detached while the electrosurgical-coagulation unit automatically adjusts the power output. However, this automatic power adjustment is difficult to monitor.
The curve describing the relationship between power (vertical axis) and resistance (horizontal axis) in the manufacturer’s technical diagram (we used effect 4 in our study) showed that the power corresponding to 1300 Ω is 50 W (Supplementary material). Therefore, we obtained the formula: power consumption (J) = power (W) × time (s) from the relationship between power and power consumption. We used this formula to calculate the total Joule heat applied to the lesion.
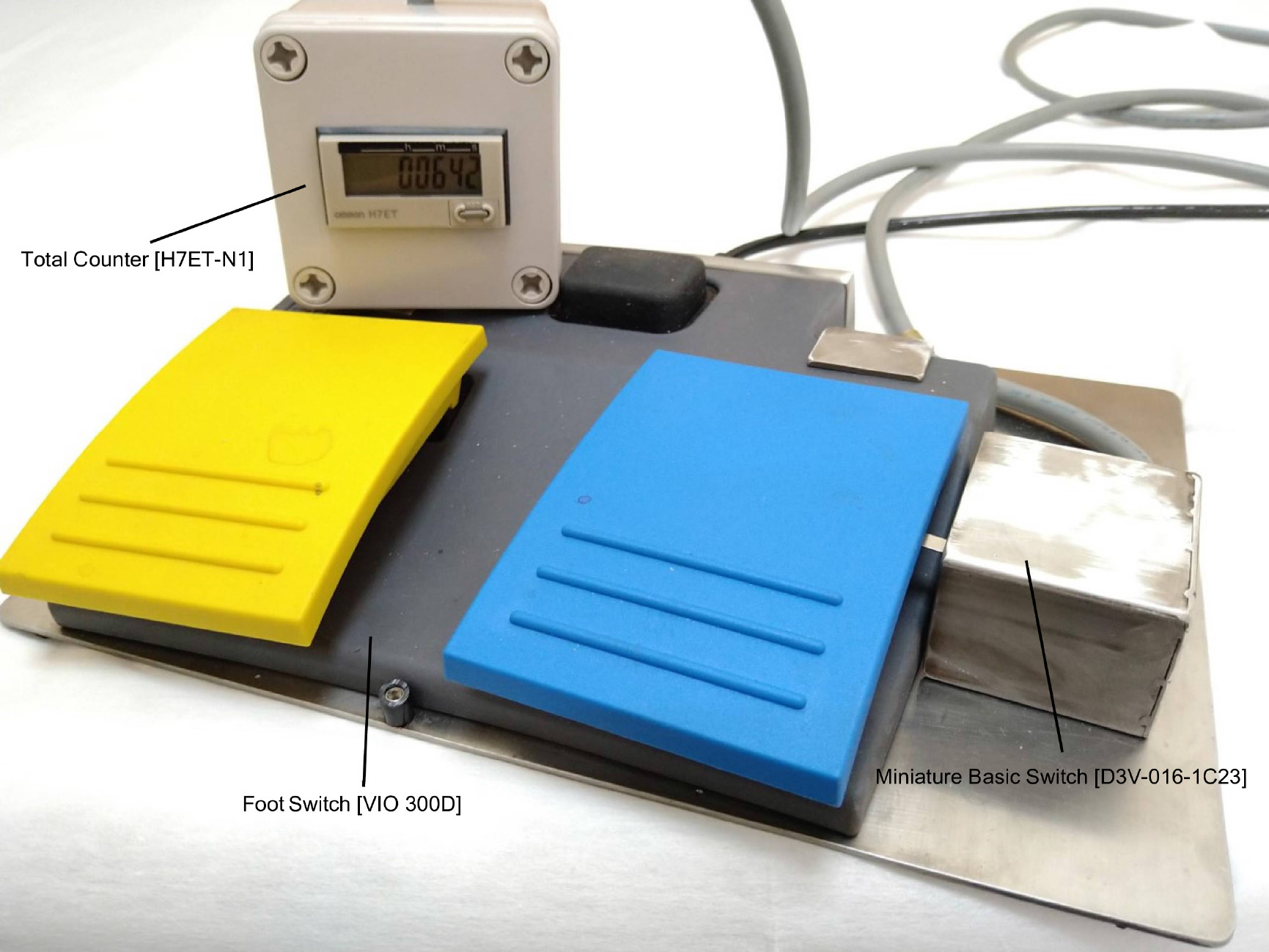
We developed a novel device to measure the swift coagulation time with a sensor [Miniature Basic Switch (D3V-016-1C23), OMRON Corporation, Kyoto, Japan] adjacent to the foot switch of the electrosurgical coagulation unit (Figure 1). This sensor was activated when the operator stepped down on the foot switch, and the swift-coagulation mode time was recorded by a counter [Total Counter (H7ET-N1), OMRON Corporation]. In cases where the swift-coagulation time was not measured, we retrospectively calculated the time using the data (swift-coagulation mode time/procedure time: Mean 3%) obtained from the measured cases. Patients exposed to more or less than the median Joule heat value (8460 J) were assigned to the high and low Joule heat groups, respectively.
In all patients, on POD 1, the vital signs and a blood sample were examined to check for bleeding, the pain VAS was administered, and radiography was performed to check for free air. We considered a fever, localized abdominal pain, or increased C-reactive protein suspicious for delayed perforation associated with PECS. If the pain or fever criteria were satisfied, we performed computed tomography. In the absence of adverse findings, patients resumed eating, starting with dinner on POD 1. If no fever, localized abdominal pain, or bleeding occurred after eating resumed, patients were discharged on POD 6.
We analyzed continuous and categorical variables using Mann-Whitney U and chi-square tests, respectively. In our multivariate analysis, we performed logistic re
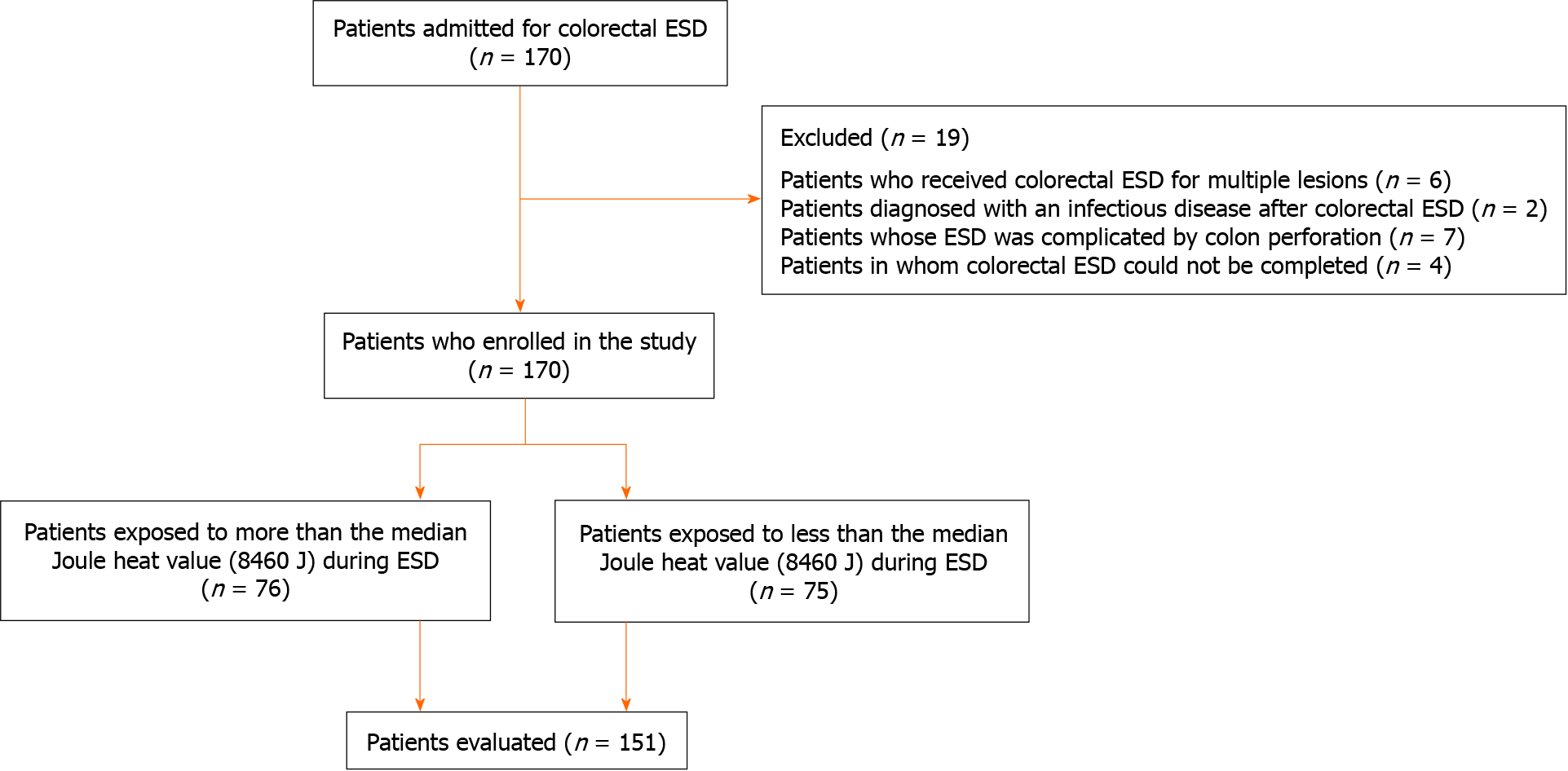
Of 170 patients admitted for colorectal ESD, 19 met the exclusion criteria (multiple lesions, n = 6; post-ESD infection, n = 2; perforation during ESD, n = 7; ESD discontinuation, n = 4) (Figure 2). We analyzed the remaining 151 patients [mean age, 67.3 years (range 39-92); male, 62.3%]. Tumors occurred in the right colon (n = 59, 39.1%), left colon (n = 41, 27.2%), and rectum (n = 51, 33.8%); R0 and en bloc resections were achieved in 115 patients (76.2%) and 135 patients (89.4%), respectively; and PECS occurred in 16 patients (10.6 %) (Table 1).
| Patient features | n (%) |
| Number of patients | 151 |
| Male | 94 (62.3) |
| Age (yr), mean ± SD | 67.3 ± 10.9 |
| BMI (kg/m2), mean ± SD | 22.9 ± 3.0 |
| Specimen size | |
| < 40 mm | 132 (87.4) |
| ≥ 40 mm | 19 (12.6) |
| Total Joule heat | |
| < 8460 J1 | 75 (49.7) |
| ≥ 8460 J | 76 (50.3) |
| Tumor location | |
| Right colon | 59 (39.1) |
| Left colon | 41 (27.1) |
| Rectum | 51 (33.8) |
| Tumor morphology | |
| 0-Is/Ip | 29 (19.2) |
| LST-G | 75 (49.7) |
| LST-NG (0-IIc) | 47 (31.1) |
| Depth of pathological invasion | |
| Tis (M) | 130 (86.1) |
| T1 (SM) | 21 (13.9) |
| Histological diagnosis | |
| Adenoma | 67 (44.4) |
| Adenocarcinoma | 80 (53.0) |
| Other | 4 (2.6) |
| ECOG performance status after ESD | |
| 0 | 144 (95.4) |
| ≥1 | 7 (4.6) |
| R0 resection | 115 (76.2) |
| ESD procedure performed by trainees | 52 (34.4) |
| Additional endoscopic therapy after ESD | 2 (1.3) |
| Additional surgery after ESD | 12 (7.9) |
| En bloc resection | 135 (89.4) |
| Intraoperative perforation or penetration | 7 (4.6) |
| Submucosal fibrosis | 45 (29.8) |
| PECS | 16 (10.6) |
The high (n = 76) and low (n = 75) Joule heat groups showed no statistically significant difference in sex; tumor morphology, location, or depth of pathological invasion; R0 resection rate; or incidence of SM fibrosis (Table 2). Univariate analyses revealed that patient age was significantly greater, and the PECS incidence was significantly higher in the high Joule group than in the low Joule group; the specimen size was also significantly larger in the high Joule group. Compared to the low Joule heat group, the high Joule heat group included more patients who had undergone ESD performed by a trainee, and the difference was significant. In addition, the R0 resection rate was significantly higher in the low Joule group. Multivariate analysis showed that the R0 resection rate [odds ratio (OR), 3.27; 95% confidence interval (CI): 1.26-8.45; P = 0.01] and PECS incidence (OR, 4.83; 95%CI: 1.08-21.50; P = 0.03) were significantly higher and the specimen size (OR, 1.07; 95%CI: 1.03-1.11; P < 0.01) and number of ESD procedures performed by a trainee (OR, 5.30; 95%CI: 2.32-12.10; P < 0.01) were significantly larger in the high Joule heat group than in the low Joule heat group (Table 3).
| High Joule heat | Low Joule heat | P value | |
| n = 76 | n = 75 | ||
| Sex | 0.54 | ||
| Male | 45 (59.2) | 49 (65.3) | |
| Female | 31 (40.8) | 26 (34.7) | |
| Age (yr), mean ± SD | 69 ± 11.5 | 66 ± 10.2 | < 0.01 |
| BMI (kg/m2), mean ± SD | 22.8 ± 2.3 | 23.0 ± 3.2 | 0.77 |
| Specimen size | < 0.01 | ||
| < 40 mm | 60 (78.9) | 73 (97.3) | |
| ≥ 40 mm | 16 (21.1) | 2 (2.7) | |
| Tumor location | 0.84 | ||
| Right colon | 28 (36.9) | 31 (41.3) | |
| Left colon | 21 (27.6) | 20 (26.7) | |
| Rectum | 27 (35.5) | 24 (32.0) | |
| Tumor morphology | 0.56 | ||
| 0-Is/Ip | 18 (23.7) | 21 (28.0) | |
| LST-G | 36 (47.4) | 29 (38.7) | |
| LST-NG (0-IIc) | 22 (28.9) | 25 (33.3) | |
| Depth of pathological invasion | 0.66 | ||
| Tis (M) | 64 (84.2) | 66 (88.0) | |
| T1 (SM) | 12 (15.8) | 9 (12.0) | |
| Histological diagnosis | 0.12 | ||
| Adenoma | 34 (44.7) | 33 (44.0) | |
| Adenocarcinoma | 42 (55.3) | 38 (50.7) | |
| Other | 0 (0.0) | 4 (5.3) | |
| Performance status after ESD | 0.45 | ||
| 0 | 71 (93.4) | 73 (97.3) | |
| ≥ 1 | 5 (6.6) | 2 (2.7) | |
| R0 resection | 50 (65.8) | 65 (86.7) | < 0.01 |
| ESD procedure performed by trainees | 37 (55.3) | 15 (13.3) | < 0.01 |
| Additional endoscopic therapy after ESD | 1 (1.3) | 1 (1.3) | 0.999 |
| Additional surgery after ESD | 8 (10.5) | 4 (5.3) | 0.38 |
| En bloc resection | 69 (90.8) | 73 (97.3) | 0.18 |
| Submucosal fibrosis | 27 (35.5) | 18 (24.0) | 0.17 |
| PECS | 13 (17.1) | 3 (4.0) | 0.02 |
| Odds ratio | 95%CI | P value | |
| Age | 1.03 | 0.99-1.06 | 0.17 |
| R0 resection | 3.27 | 1.26-8.45 | 0.01 |
| Specimen size | 1.07 | 1.03-1.11 | < 0.01 |
| ESD procedure performed by trainees | 5.30 | 2.32-12.10 | < 0.01 |
| PECS | 4.83 | 1.08-21.50 | 0.03 |
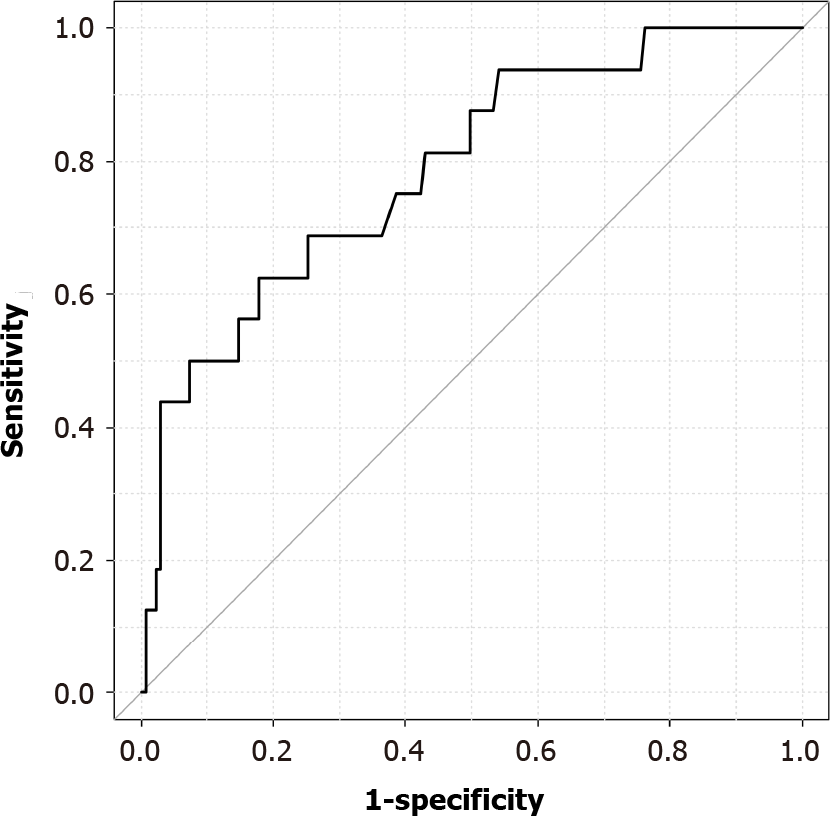
The area under the ROC curve showing the correlation between PECS and total Joule heat was 0.788 (95%CI: 0.666-0.909), and the PECS onset cutoff value was 15390 J (sensitivity, 0.625; specificity, 0.822) (Figure 3).
To our knowledge, this study, performed using our novel device, is the first to indicate high total Joule heat exposure during colorectal ESD as a PECS risk factor. Addi
PECS is a state of temporary inflammation resulting from transmission of electrocoagulation heat to the resection site muscle layer and serous membrane during endoscopic therapy[18,25,26]. Previously reported PECS risk factors include tumor location[11,13,20,27], SM fibrosis[27], long procedure time[20,21], and specimen dia
A study of lengthy procedures (mean, 90 min) demonstrated that long procedure time is a PECS risk factor[20]. However, several studies that did not include long procedure time as a PECS risk factor showed that the procedures were short (range, 52-67 min)[11,28,29]. Our study revealed that ESD procedures performed by trainees are associated with the onset of PECS. A previous study showed that ESD procedures performed by trainees are longer than those performed by experienced endoscopists[23]. Because the swift-coagulation mode time probably increases as the procedure time lengthens, high Joule heat accumulation is likely to occur during long ESDs, causing severe damage to the peeled mucosal surface, leading to PECS. Previous studies were limited in that they did not consider the impact of the ESD procedure time on the Joule heat delivered to the lesion. In this study, measurement of swift-coagulation mode time allowed us to investigate the association between PECS and total Joule heat. Our study revealed that the total Joule heat applied to the peeled surface is involved in the development of PECS. Furthermore, using the total Joule heat cutoff value of PECS occurrence could allow the introduction of PECS prevention measures, including online measurement of swift-coagulation mode time during colorectal ESD. For example, an online PECS alert system is promising.
First, because this was a retrospective cohort study, we could not rule out poor endoscope operability as a confounding factor. Second, as this was a single-center study, selection bias cannot be excluded. Nevertheless, we imposed strict inclusion and exclusion criteria to mitigate selection bias to the greatest extent possible. Last, the sample size was small. In the future, a prospective study should be performed to establish the validity of our results.
We found that Joule heat accumulation within the gastrointestinal wall is involved in the onset of PECS. ESD-related thermal damage to the mucosal peeled surface is probably a major component of the mechanism underlying PECS.
Few studies have examined the impact of endoscopic submucosal dissection (ESD)-related thermal damage on the onset of post-ESD electrocoagulation syndrome (PECS).
We hypothesized that electrocoagulation is associated with Joule heat capable of causing thermal damage to the gastrointestinal wall during long ESD operations.
We aimed to determine the association between high Joule heat and the onset of PECS.
We developed a novel device to measure the swift coagulation time with a sensor adjacent to the electrosurgical coagulation unit foot switch, which enabled us to calculate total Joule heat. PECS was defined as localized abdominal pain (visual analogue scale ≥ 30 mm during hospitalization or increased by ≥ 20 mm from the baseline) and fever (temperature ≥ 37.5 degrees or white blood cell count ≥ 10000 µ/L). Patients exposed to more or less than the median Joule heat value were assigned to the high and low Joule heat groups, respectively.
We evaluated 151 patients. The PECS incidence was 10.6% (16/151 cases), and all patients were followed conservatively and discharged without severe complications. In multivariate analysis, high Joule heat was an independent PECS risk factor. The area under the ROC curve showing the correlation between PECS and total Joule heat was high [0.788 (95% confidence interval: 0.666-0.909)].
Joule heat accumulation in the gastrointestinal wall is involved in the onset of PECS. ESD-related thermal damage of the peeled mucosal surface is probably a major component of the mechanism underlying PECS.
Future prospective studies should be performed to establish the validity of our results.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li YY S-Editor: Liu M L-Editor: A P-Editor: Yuan YY
| 1. | Puli SR, Kakugawa Y, Saito Y, Antillon D, Gotoda T, Antillon MR. Successful complete cure en-bloc resection of large nonpedunculated colonic polyps by endoscopic submucosal dissection: a meta-analysis and systematic review. Ann Surg Oncol. 2009;16:2147-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T, Fujii T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 3. | Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T, Yoshida S, Ikehara H, Otake Y, Nakajima T, Matsuda T, Saito D. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 593] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 4. | Kobayashi N, Yoshitake N, Hirahara Y, Konishi J, Saito Y, Matsuda T, Ishikawa T, Sekiguchi R, Fujimori T. Matched case-control study comparing endoscopic submucosal dissection and endoscopic mucosal resection for colorectal tumors. J Gastroenterol Hepatol. 2012;27:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisasbe T, Matsuda T, Ishikawa H, Sugihara K. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3262-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 6. | Wada Y, Kudo SE, Tanaka S, Saito Y, Iishii H, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisabe T, Tsuruta O, Kashida H, Ishikawa H, Sugihara K. Predictive factors for complications in endoscopic resection of large colorectal lesions: a multicenter prospective study. Surg Endosc. 2015;29:1216-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 275] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2009;41:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Watabe H, Yamaji Y, Okamoto M, Kondo S, Ohta M, Ikenoue T, Kato J, Togo G, Matsumura M, Yoshida H, Kawabe T, Omata M. Risk assessment for delayed hemorrhagic complication of colonic polypectomy: polyp-related factors and patient-related factors. Gastrointest Endosc. 2006;64:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Okamoto K, Watanabe T, Komeda Y, Kono T, Takashima K, Okamoto A, Kono M, Yamada M, Arizumi T, Kamata K, Minaga K, Yamao K, Nagai T, Asakuma Y, Takenaka M, Sakurai T, Matsui S, Nishida N, Chikugo T, Kashida H, Kudo M. Risk Factors for Postoperative Bleeding in Endoscopic Submucosal Dissection of Colorectal Tumors. Oncology. 2017;93 Suppl 1:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Jung D, Youn YH, Jahng J, Kim JH, Park H. Risk of electrocoagulation syndrome after endoscopic submucosal dissection in the colon and rectum. Endoscopy. 2013;45:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Hong MJ, Kim JH, Lee SY, Sung IK, Park HS, Shim CS. Prevalence and clinical features of coagulation syndrome after endoscopic submucosal dissection for colorectal neoplasms. Dig Dis Sci. 2015;60:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Yamashina T, Takeuchi Y, Uedo N, Hamada K, Aoi K, Yamasaki Y, Matsuura N, Kanesaka T, Akasaka T, Yamamoto S, Hanaoka N, Higashino K, Ishihara R, Iishi H. Features of electrocoagulation syndrome after endoscopic submucosal dissection for colorectal neoplasm. J Gastroenterol Hepatol. 2016;31:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Lee SP, Sung IK, Kim JH, Lee SY, Park HS, Shim CS, Ki HK. A randomized controlled trial of prophylactic antibiotics in the prevention of electrocoagulation syndrome after colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2017;86:349-357.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Lee SP, Kim JH, Sung IK, Lee SY, Park HS, Shim CS, Han HS. Effect of submucosal fibrosis on endoscopic submucosal dissection of colorectal tumors: pathologic review of 173 cases. J Gastroenterol Hepatol. 2015;30:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Hirasawa K, Sato C, Makazu M, Kaneko H, Kobayashi R, Kokawa A, Maeda S. Coagulation syndrome: Delayed perforation after colorectal endoscopic treatments. World J Gastrointest Endosc. 2015;7:1055-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Mori H, Kobara H, Rafiq K, Nishiyama N, Fujihara S, Oryu M, Masaki T. Effects of gastric irrigation on bacterial counts before endoscopic submucosal dissection: a randomized case control prospective study. PLoS One. 2013;8:e65377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Christie JP, Marrazzo J 3rd. "Mini-perforation" of the colon--not all postpolypectomy perforations require laparotomy. Dis Colon Rectum. 1991;34:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Nomura S, Shimura T, Katano T, Iwai T, Mizuno Y, Yamada T, Ebi M, Hirata Y, Nishie H, Mizushima T, Nojiri Y, Togawa S, Shibata S, Kataoka H. A multicenter, single-blind randomized controlled trial of endoscopic clipping closure for preventing coagulation syndrome after colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2020;91:859-867.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Arimoto J, Higurashi T, Kato S, Fuyuki A, Ohkubo H, Nonaka T, Yamaguchi Y, Ashikari K, Chiba H, Goto S, Taguri M, Sakaguchi T, Atsukawa K, Nakajima A. Risk factors for post-colorectal endoscopic submucosal dissection (ESD) coagulation syndrome: a multicenter, prospective, observational study. Endosc Int Open. 2018;6:E342-E349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Lee H, Cheoi KS, Chung H, Park JC, Shin SK, Lee SK, Lee YC. Clinical features and predictive factors of coagulation syndrome after endoscopic submucosal dissection for early gastric neoplasm. Gastric Cancer. 2012;15:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Saitoh Y, Tsuruta O, Sugihara KI, Igarashi M, Toyonaga T, Ajioka Y, Kusunoki M, Koike K, Fujimoto K, Tajiri H. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2020;32:219-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 271] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 23. | Hotta K, Oyama T, Shinohara T, Miyata Y, Takahashi A, Kitamura Y, Tomori A. Learning curve for endoscopic submucosal dissection of large colorectal tumors. Dig Endosc. 2010;22:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Freiberger H. Der elektrische widerstand des menschlichen körpers gegen technischen gleich- und wechselstrom. Berlin: Verlag von Julius Springer, 1934. |
| 25. | Waye JD, Lewis BS, Yessayan S. Colonoscopy: a prospective report of complications. J Clin Gastroenterol. 1992;15:347-351. [PubMed] |
| 26. | Cha JM, Lim KS, Lee SH, Joo YE, Hong SP, Kim TI, Kim HG, Park DI, Kim SE, Yang DH, Shin JE. Clinical outcomes and risk factors of post-polypectomy coagulation syndrome: a multicenter, retrospective, case-control study. Endoscopy. 2013;45:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Ito S, Hotta K, Imai K, Yamaguchi Y, Kishida Y, Takizawa K, Kakushima N, Tanaka M, Kawata N, Yoshida M, Ishiwatari H, Matsubayashi H, Ono H. Risk factors of post-endoscopic submucosal dissection electrocoagulation syndrome for colorectal neoplasm. J Gastroenterol Hepatol. 2018;33:2001-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Hanaoka N, Uedo N, Ishihara R, Higashino K, Takeuchi Y, Inoue T, Chatani R, Hanafusa M, Tsujii Y, Kanzaki H, Kawada N, Iishi H, Tatsuta M, Tomita Y, Miyashiro I, Yano M. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2010;42:1112-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Kobayashi R, Hirasawa K, Sato C, Makazu M, Kaneko H, Ikeda R, Fukuchi T, Sawada A, Ozeki Y, Taguri M, Takebayashi S, Maeda S. Utility of multi-detector computed tomography scans after colorectal endoscopic submucosal dissection: a prospective study. Gastrointest Endosc. 2018;87:818-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |