Published online Sep 21, 2021. doi: 10.3748/wjg.v27.i35.5932
Peer-review started: March 22, 2021
First decision: June 14, 2021
Revised: June 27, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: September 21, 2021
Processing time: 177 Days and 1.2 Hours
Primary gastric lymphomas (PGLs) are distinct lymphoproliferative neoplasms described as heterogeneous entities clinically and molecularly. Their main histological types are diffuse large B-cell lymphoma (DLBCL) or mucosa-associated lymphoma tissue. PGL has been one of the main fields of clinical research of our group in recent years. Although gastric DLBCLs are frequent, sufficient data to guide optimal care are scarce. Until today, a multidisciplinary approach has been applied, including chemotherapy, surgery, radiotherapy or a combination of these treatments. In this minireview article, we provide an overview of the clinical manifestations, diagnosis and staging of these diseases, along with their molecular pathogenesis and the most important related clinical published series. We then discuss the scientific gaps, perils and pitfalls that exist regarding the aforementioned studies, in parallel with the unmet need for future research and comment on the proper methodology for such retrospective studies. Aiming to fill this gap, we retrospectively evaluated the trends in clinical presentation, management and outcome among 165 patients with DLBCL PGL who were seen in our institutions in 1980-2014. The study cohort was divided into two subgroups, comparing the main 2 therapeutic options [cyclophosphamide doxorubicin vincristine prednisone (CHOP) vs rituximab-CHOP (R-CHOP)]. A better outcome with immunochemotherapy (R-CHOP) was observed. In the next 2 mo, we will present the update of our study with the same basic conclusion.
Core Tip: A few small, heterogeneous, retrospective studies have attempted to determine the optimal treatment for gastric diffuse large B-cell lymphoma, investigating the role of chemotherapy +/- rituximab, surgery and radiation in patient outcomes. Our retrospective research suggests that a better outcome is observed for these patients after the introduction of immunochemotherapy (rituximab-cyclophosphamide doxorubicin vincristine prednisone). Because statistical analysis might differ among various studies, it is crucial to correctly define the terms freedom from progression and lymphoma-specific survival. The latter provides information on whether the patients died from lymphoma or from other causes.
- Citation: Diamantidis MD, Papaioannou M, Hatjiharissi E. Primary gastric non-Hodgkin lymphomas: Recent advances regarding disease pathogenesis and treatment. World J Gastroenterol 2021; 27(35): 5932-5945
- URL: https://www.wjgnet.com/1007-9327/full/v27/i35/5932.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i35.5932
Primary gastric lymphomas (PGLs) are a diverse group of lymphoproliferative disorders that originate from the stomach and comprise many different histologic types. Either of diffuse large B-cell lymphoma (DLBCL) subtype or mucosa-associated lymphoma tissue (MALT) histology, PGL is the second most common gastric malignancy globally, following the adenocarcinoma of the stomach[1,2]. The latter is the most common form of gastric cancer and the fifth most common malignancy in the world[3]. Despite the fact that prevention and treatment of Helicobacter pylori infection (H. pylori I) has led to a decrease in its overall incidence, gastric cancer remains the 3rd most deadly cancer, with an estimated 783000 deaths in 2018 worldwide[4,5]. Therefore, accurately recognizing and diagnosing gastric cancer from gastric lymphomas is important, as these diseases are treated differently, and any confusion may result in inappropriate treatment management.
The gastrointestinal tract (GIT) is the most common site for the development of extranodal lymphomas. The incidence of these neoplasms has been increasing in recent years[2,6]. The stomach represents 30%-40% of all extranodal lymphomas and 55%-65% of all GI lymphomas. The incidence of PGL varies from 4% to 20% of extranodal non-Hodgkin lymphomas (NHLs) and reaches up to 5% of primary gastric neoplasms[2]. The incidence of PGL is estimated to be 1 per 100000 in Western countries[7]. B-cell lymphomas are more frequent in these countries than in Eastern countries[1].
To date, the term PGL was originally used to describe lymphomas that arise from the stomach. However, within the medical literature, controversy exists regarding the definition, staging and treatment of this entity. Most cases of PGLs are B-cell subtypes of NHLs. The majority of these subtypes have DLBCL histology and are classified as DLBCL of the stomach, not otherwise specified (NOS).
PGLs are histologically heterogeneous neoplasms. This contributes to a different biology, clinical presentation and prognosis and subsequently determines special therapeutic needs for each subtype[8,9]. For example, certain subtypes of PGLs, such as DLBCL, are more aggressive than others and require immediate therapy[8], whereas for patients with MALT histology, unique management is usually applied ranging from watch and wait to antibiotic-based treatment[9].
As stated above, PGLs are histologically, biologically and clinically heterogeneous neoplasms. Although gastric DLBCL is an extranodal high-grade lymphoma, it is considered less aggressive than its nodal counterpart and other extranodal DLBCL locations. Its appropriate treatment has not been satisfactorily determined, and treatment choices vary considerably. Human immunodeficiency virus, Epstein-Barr infection, hepatitis B virus, human T-cell lymphotropic virus 1, immunosuppression, celiac disease, inflammatory bowel disease, and H. pylori I have all been implicated in the factors predisposing patients to PGLs, increasing the risk of developing the disease[1,2,10]. PGL usually occurs in patients older than 50 years. There are many older patients over 80 years of age. Males are more prone to be diagnosed with PGL with a 2-3-fold higher risk than females[2].
This review mainly focuses on DLBCL gastric lymphoma, which is one of the main fields of our clinical research and comments briefly on MALT lymphoma. The molecular etiology and pathophysiology of DLBCL gastric lymphomas and the available clinical data for their optimal management will be discussed. In parallel, a brief review of the MALT subtype that represents almost 50% of PGLs will also be provided. This review aims to meet the therapeutic needs of those who are involved and/or interested in the treatment of GI-DLBCL lymphomas and extensively focuses on the role of rituximab, the first in class anti-CD20 monoclonal antibody (mAb), in the outcome of patients with PGL of the DLBCL subtype.
The stomach is the most common site for the development of extranodal lymphomas in the GI tract, accounting for 60% of cases, followed by the small bowel, ileum, cecum, colon and rectum[7]. Distinguishing PGL from secondary dissemination of the stomach due to primary nodal lymphoma can be difficult. No peripheral and mediastinal lymphadenopathy at the time of diagnosis, no spleen or liver infiltration and normal blood counts are in contrast to the presence of a secondary gastric lymphoma[11].
The diagnosis of PGL can be delayed for many years due to the presence of nonspecific symptoms, mimicking peptic ulcer disease, gastritis, functional gastric or even pancreatic disorder. The main symptoms include nausea, vomiting, anorexia, abdominal distention, fullness or pain, indigestion, dyspepsia and weight loss, whereas weakness, night sweats, fever, jaundice, hematemesis or melena are less common[2,7]. An obvious epigastric mass or perforation is rare as an initial pre
An appropriate endoscopic evaluation with generously sized tissue samples is the hallmark of diagnosis. The diagnostic accuracy of endoscopic biopsy is very high, reaching 90%. Endoscopic ultrasonography can improve this diagnostic accuracy. The diagnosis becomes difficult when there is deep infiltration and preservation of the mucosa. Computed tomography (CT), magnetic resonance imaging (MRI) and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) assist in the diagnosis and staging of PGL[1,7]. Sporadically, PGL might present as multifocal, clonally identical foci surrounded by macroscopically unaffected tissue. Thus, gastric mapping of unaffected mucosa is strongly recommended[13]. Bone marrow infiltration, B symptoms and elevated lactate dehydrogenase (LDH) are more frequently encountered in nodal lymphomas than in gastric lymphomas[13].
Different staging systems have been proposed for PGLs. The Ann Arbor staging system, which is widely used for primary nodal lymphomas, is considered unsatisfactory as PGLs originate from the lining of the stomach instead of the lymph nodes[7,13]. In recent years, a more specific Lugano staging system for PGLs was proposed and applied based on the Lugano score[14,15], which includes the following stages: Stage IE — Lymphoma is confined to the GIT (single lesion or multiple noncontiguous lesions): IE1 = mucosa, submucosa; IE2 = muscularis propria, serosa; Stage II — Lymphoma extends into the abdomen from the primary site within the GI tract: II1 = local nodal involvement; II2 = distant nodal involvement; Stage IIE — Penetration of serosa to involve adjacent organs or tissues; Stage IV — Disseminated extranodal involvement or concomitant supra diaphragmatic nodal involvement. Note: Stage III does not exist because gastric lymphoma is always below the diaphragm.
A complete staging work-up includes the following: Biochemical examinations, chest, abdomen and pelvis CT scan, bone marrow biopsy, thorough endoscopy including biopsies from the stomach, duodenum and gastroesophageal junction, endoscopic ultrasound, evaluation of the Waldeyer ring, investigation for H. pylori I, routine histology and immunohistochemistry. Cytogenetic studies and even fluorescence in situ hybridization (FISH) can all be used in biopsies to provide the appropriate information needed for optimal treatment. PET/CT scans have documented diagnostic and prognostic value only for DLBCL lymphomas, in contrast to MALT gastric lymphomas, which can be reported as false-negative because of the small tumor burden of the disease and their indolent behavior[13]. However, there is an unmet need regarding the use of PET scans in the clinical setting to guide treatment. Usually, this examination is performed before and after the end of treatment to guide therapeutic decisions as a standard of care. Because the stomach is an abdominal organ, it is unclear how PET scans can assist in the aforementioned necessary clinical decisions.
In general, the prognosis of extranodal lymphomas varies according to the affected organ; it is poor in the testis, central nervous system (CNS) and intestine, whereas it is quite good in the stomach, mediastinum and bone. Nevertheless, PGL is an aggressive malignancy characterized by rapid growth. However, the prognosis of DLBCL PGL is relatively good, with a 5-year overall survival (OS) higher than 80%[7].
The optimal treatment for DLBCL PGLs is not clear, because prospective clinical studies are missing. In the past, a spectrum of treatment approaches was applied, ranging from gastrectomy or radiotherapy alone to chemotherapy (cyclophosphamide doxorubicin vincristine prednisone, CHOP) or the combination of chemotherapy plus radiotherapy and surgery. Wang et al[16] compared surgery over conservative treatment in a retrospective study. Conservative treatment in this study included chemotherapy (CHOP) or radiotherapy alone, chemotherapy plus radiotherapy or H. pylori I eradication (HPE). The authors found superiority of surgery alone compared with conservative treatment in the DLBCL type regarding prognosis, but not in the MALT type[16]. Currently, the role of surgical resection has been minimized, even in cases of extreme intestinal obstruction, as immunochemotherapy can induce rapid and complete resolution of large obstructing tumor masses. Gastrectomy is restricted to the management of major complications, including perforation or hemorrhage of DLBCL PGLs.
In contrast, other studies demonstrated that DLBCL PGL is a potentially curable disease with rituximab-CHOP (R-CHOP)-like treatment, leading to long-term survival[17]. Investigators found that surgical treatment did not offer survival benefits when compared with chemotherapy for 5-year progression-free survival (PFS) and OS estimates and that no significant differences were noted in these endpoints for patients treated with R-CHOP or conventional chemotherapy[18].
Sohn et al[19] directly compared CHOP vs R-CHOP as a front-line approach in 93 patients with DLBCL PGL. With a median follow-up of 48 mo, no differences were noted among the 2 groups regarding OS, EFS and CR. High serum levels of β2-microglobulin were associated with worse OS and EFS in patients who received R-CHOP[19]. In a retrospective analysis of 95 Japanese patients, the clinical outcomes of gastric DLBCL were extremely favorable for localized-stage patients in the rituximab era. Conversely, these treatments were poor for advanced-stage patients[20]. Interestingly, an effective approach in treating deeply infiltrated DLBCL PGL patients by switching fractioned R-CHOP (rituximab d0, 50% dose of CHOP d1 and d5) to standard R-CHOP cycles guided by endoscopic ultrasonography has been proposed[21].
The following factors were identified as having a negative impact on survival: age above 65, Eastern Cooperative Oncology Group 2-3, B symptoms, bulky disease, IPI 3-4, more than 3 treatment lines, and absence of response to first-line treatment[17].
Conversely, other factors were considered negative for prognosis in the subsequent study: elevated LDH levels, chemotherapy or radiotherapy alone or the combination of chemotherapy plus radiotherapy[16]. The non-germinal center B-cell-like lymphoma (GCB) subtype has also been associated with shorter OS[18]. H. pylori I negativity, advanced Lugano stage and elevated LDH levels have been reported as adverse prognostic factors in gastric DLBCL[22].
Low serum albumin at diagnosis was the only risk factor for developing gastric complications, such as bleeding and stenosis, in patients with gastric DLBCL who received R-CHOP[23]. Furthermore, a low CD4:CD8 ratio at diagnosis is an inde
Some patients with DLBCL PGL also have a MALT component. The 5-year PFS and OS estimates were similar when de novo DLBCL patients were compared with DLBCL/MALT patients, suggesting that patients with a MALT component, along with DLBCL, might have the same biological type of lymphoma as de novo DLBCL patients[18]. In such DLBCL/MALT cases, an important deregulation of Bcl-2 and an upregulation of p53 protein of uncertain clinical significance have been observed[26]. A synopsis of the studies comparing R-CHOP vs CHOP for DLBCL PGLs is shown in Table 1. Indeed, there is a lack of a head-to-head comparison between CHOP and R-CHOP in PGLs.
| Ref. | Number of Pts | R-CHOP OS | CHOP OS | R-CHOP PFS | CHOP PFS | Comments | |
| Sohn et al[19], 2012 | Double-arm Retrospective Study (R-CHOP vs CHOP as 1st line treatment) | 93 (55 R-CHOP, 38 CHOP) | 3-yr 84.7% (P > 0.05) | 3-yr 94.7% (P > 0.05) | 3-yr 81.7% (EFS) (P > 0.05) | 3-yr 86% (EFS) (P > 0.05) | CR: (CHOP: 93.9%), (R-CHOP: 92.5%) |
| Liu et al[62], 2018 | Double-arm Retrospective Study (diagnosis: 1973-2000 era vs 2001-2014 era of immuno-CT) | SEER Database 7051 [(4186, 1973-2000), (2865, 2001-2014) | 5-yr 53% (P = 0.001) | 5-yr 47% (P = 0.001) | |||
| Tanaka et al[20], 2012 | Single-arm Retrospective Study (R-CHOP) | 95 | 3-yr 91% (localized disease); 3-yr 95% (localized disease); 3-yr 64% (localized disease) | 3-yr 91% (localized disease); 3-yr 92% (localized disease); 3-yr 43% (localized disease) | 6c. R-CHOP; 3-4 c. R-CHOP plus radiotherapy; R-CHOP ± radiotherapy | ||
| Couto et al[17], 2021 | Single-arm Retrospective Study (R-CHOP) | 101 | Not reached | Not reached | 80% CR (after 1st line); 54% CR (3 yrs FU) |
Regarding the role of radiotherapy, more data are available for patients with gastric MALT lymphoma or early-stage gastric lymphoma. When there is an unsatisfactory response to HPE, recurrence after HPE or in MALT cases negative for H. pylori I, gastric radiotherapy of the entire stomach plus irradiation of the pathological and perigastric lymph nodes (30-440 Gy, 15-20 fractions) has been proposed. However, it is less clear whether radiotherapy should be applied in cases of DLBCL PGLs. However, involved-field radiotherapy has a role, especially for patients with DLBCL PGL of advanced stage who achieve partial remission (PR) after immunochemotherapy (R-CHOP)[27]. R-CHOP plus additional local treatment for gastric lesions (e.g., consolidative radiotherapy or surgical resection) has also been recommended[28]. Alternatively, several studies have found that in the era of immunochemotherapy (R-CHOP), radiotherapy does not improve OS[29-31]. The side effects of radiotherapy should always be taken into account in clinical decision making[27].
Despite the presence of several clinical series involving primary gastric DLBCL lymphomas mainly addressing the issue of selecting the optimal treatment, there are sporadic single cases in the literature[22,32-34]. Some very rare, more aggressive cases of DLBCL lymphoma originating from the stomach and infiltrating the adrenals bilaterally have been reported[32,34]. The first patient presented with nausea, vomiting, abdominal pain and hypotension, was treated with glucocorticoids and died after developing respiratory failure, severe hypotension refractory to vasopressors and severe metabolic acidosis[34]. The second case was a DLBCL, PGL of the non-germinal center (non-GC) type. This patient received 8 cycles of rituximab therapy, 6 cycles of CHOP and 3 cycles of prophylactic intrathecal chemotherapy. The patient maintained a CR for approximately 14 mo after the completion of the aforementioned treatment. The latter is in favor of the hypothesis that DLBCL lymphomas of the stomach have a better prognosis than other DLBCL nodal and extranodal lymphomas. In contrast to the very dismal prognosis of primary adrenal lymphomas (PALs)[35], this patient survived, likely because the primary neoplasm was gastric DLBCL, which has better biological and clinical behavior for unknown molecular reasons (even though it is considered an aggressive neoplasm, being DLBCL).
Regarding the role of HPE, Nakamura et al[36] studied 420 patients with gastric MALT lymphoma and found a significant responsiveness to HPE therapy (77%), with treatment failure (relapse or progressive disease) occurring in only 9% of the patients. However, this primary refractory disease was not associated with a dismal outcome, as the subsequent therapy still yielded a 90% OS rate after 10 years[36].
Nevertheless, even though HPE has already been established as an optimal strategy for the management of gastric MALT lymphoma, there are conflicting results, either in favor of or against HPE for patients with DLBCL PGLs. Thus, HPE has been reported to be a suitable strategy for patients with DLBCL PGLs[37,38]. The concept of a less aggressive biological behavior for H. pylori I-dependent gastric DLBCL has been proposed with the suggestion to apply HPE in such cases[39]. However, it is not clear how accurately these lymphomas can be distinguished. Alternatively, high-grade gastric lymphomas can rapidly progress if they do not respond to HPE. The loss of H. pylori I dependency and the possible high-grade lymphomatic evolution/ transformation are separate and distinct events in the natural history of PGL[38,40]. The description of defined molecular markers linked to H. pylori I dependency of PGLs is beyond the scope of this article.
Moreover, a substantial portion of early-stage H. pylori I-positive gastric de novo DLBCLs remain H. pylori I-dependent and respond to antibiotic treatment (HPE). Prospective studies to validate these findings are needed[41]. Our personal opinion is that HPE should not be applied as monotherapy, even in the early stage of H. pylori I-positive DLBCL PGLs.
Extranodal lymphomas are distinct types of lymphomas that show a predilection for anatomical sites harboring extranodal lymphoid tissue, such as the CNS, testis, mediastinum, bone and GIT, in contrast to the typical pattern of the nodal counterpart in the lymph nodes for nodal lymphomas[42]. Extranodal lymphomas can even appear in immune-privileged (sanctuary) sites (CNS, testis) or arise in sites of chronic inflammation, effusions or other closed spaces within the body. The complex mechanisms of local immune evasion leading to extranodal lymphoproliferations have not been fully elucidated[43]. The capacity of mature lymphocytes to recirculate between blood and lymphoid tissue and to migrate to extranodal anatomical sites is crucial for the pathogenesis of the disease. During this process, lymphocytes interact with endothelial venules, mediated by receptor molecules (integrins and lymphocytes)[44].
The role of specific B-cell receptor (BCR) antigens has been proposed in the process of lymphomagenesis. Oncogenic translocations during BCR development and generation (VDJ rearrangement), the activation of mature B-cells and the germinal center reaction, the mechanisms of loss of immunological self-tolerance, and the role of infectious agents and autoantigens are all hallmarks and basic elements of lymphomagenesis, a complex multifactorial process, in both aggressive and indolent lymphomas[45]. Gastric DLBCL is a high-grade lymphoma compared to low-grade MALT lymphomas. Whether DLBCL transforms from low-grade MALT lymphoma or whether it arises de novo in the stomach is unknown. DLBCL gastric lymphoma has been associated with a lower CR and shorter survival than MALT lymphoma[2,46]. Nevertheless, transformed DLBCLs from MALTs are CD10- and Bcl-2-negative, while de novo DLBCLs are CD10- and Bcl-2-positive[31,46].
The oncogene Bcl-6 is located on chromosome 3q27 and is frequently present in the majority of extranodal high-grade lymphomas. Conversely, Bcl-2 oncogene expression was significantly lower in gastric lymphomas than in other primary extranodal high-grade B-cell lymphomas (HGBCLs). p53 protein expression did not differ significantly between these 2 groups[2].
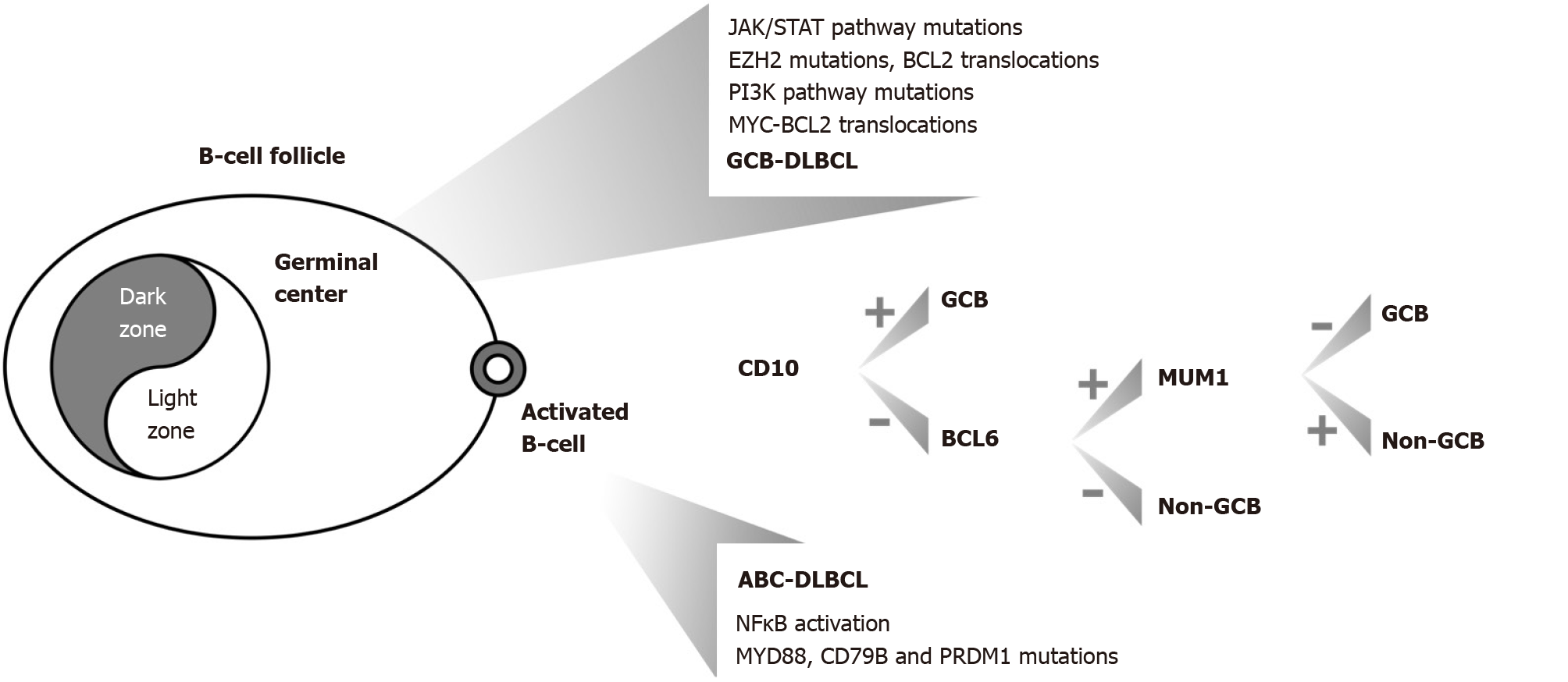
DLBCL is described by diffuse proliferation of large, atypical cells, with vesicular nuclei, prominent nucleoli, and basophilic cytoplasm. These cells typically express CD19, CD20, CD22 and CD79a (pan-B-cell markers). Bcl-6 is expressed in 60% of cases. FISH can identify poor prognostic subtypes of DLBCL, such as double-hit (DH) or triple-hit (TH) lymphomas (high-grade, B-cells), characterized by translocations of MYC and Bcl-2 and/or Bcl-6[47,48]. DH or TH lymphomas are defined by their genetic aberrations, irrespective of their morphology. Genetic variability has been documented for DLBCL PGL[47]. Gene expression profiling distinguishes DLBCL into GCB and non-GCB or activated B-cell-like (ABC) subtypes based on the cell of origin profile. ABC lymphomas show a worse prognosis than GCB lymphomas[49]. In routine diagnostic practice, this screening is conducted by immunohistochemistry based on the assessment of three markers (CD10, bcl-6 and MUM1)[50] (Figure 1).
More analytically, ABC DLBCLs are characterized by nuclear factor kappa beta (NF-κB) activation, showing a higher frequency of Bcl-2 amplifications, Bcl-6 rear
Interestingly, 2 HGBCLs were included in the recent revised WHO classification of lymphoid neoplasms. These entities are clinically and biologically distinct from DLBCL NOS and Burkitt lymphoma (BL). The HGBCL, NOS entity includes cases previously termed ‘unclassifiable, with features intermediate between DLBCL and BL’, or showing blastoid morphology but lacking DH/TH translocations[49].
High levels of Bcl-6 expression were found in GCB gastric lymphomas, whereas in the non-GCB cases, a high Bcl-6 expression level correlated importantly with mutations producing Bcl-6 deregulation, even if in the latter cases no correlation was found between survival rates[2].
Clinical studies addressing the role of programmed cell death 1 (PD-1) and its ligand (PD-L1) have shown promising results. PD-1 blockade in patients with PD-L1 expression on tumor cells has been linked with clinical responses. Investigators from Japan evaluated the role of PD-L1 expression on neoplastic and non neoplastic immune cells in the microenvironment (miPD-L1) in a retrospective study of patients with GI DLBCL lymphoma. They found that elevated miPD-L1 expression had a favorable impact on the outcome of these DLBCL patients, regardless of the anatomical site of the disease[52].
MALT lymphoma is a low-grade B-cell NHL, and the majority of cases (approximately 90%) are directly related to H. pylori I. However, 10% of gastric MALT lymphomas are H. pylori I negative[53]. Chronic H. pylori I of the gastric mucosa and the accompanying inflammation have been strongly linked to MALT lymphomagenesis. Moreover, abnormalities in the expression of various miRNAs contribute to the neoplastic gastric phenotype[54,55].
H. pylori I expresses proteins related to the corresponding genes, contributing to the related lymphomagenesis from the bacterium. These are cytotoxin-associated gene A (CagA), vacuolization cytotoxin A (VacA) and heat shock proteins (Hsps). The Cag pathogenicity island (a common gene sequence considered responsible for the pathophysiology of the infection) contains over 40 genes, which mainly code for a complex type IV secretion system. This pathogenicity island is usually absent from H. pylori I strains isolated from asymptomatic human carriers. The CagA protein is frequently co-expressed with the vacuolating cytotoxin VacA[56].
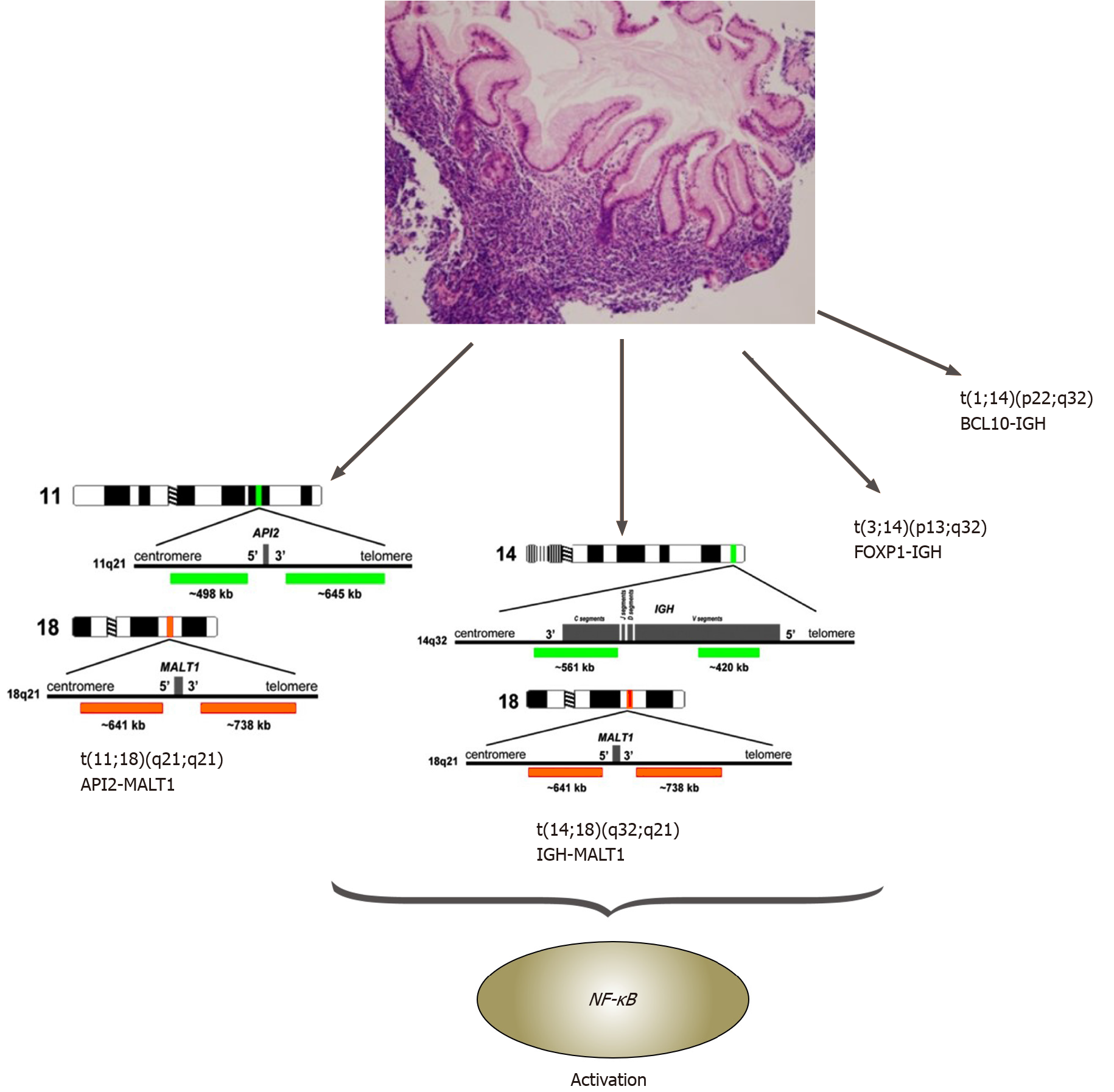
Hamoudi et al[57] established the connection between abnormal NF-κB signaling due to the chromosomal translocations, t(11;18)(q21;q21)/API2-MALT1, t(1;14) (p22;q32)/BCL10-IGH, t(14;18) (q32;q21)/IGH-MALT1 and t(3;14) (p13;q32)/FOXP1-IGH,in gastric MALT lymphomas[57,58] (Figure 2).
MALT1 and BCL10 proteins are involved in surface immune receptor-mediated activation of the NF-κB transcription factor; chromosomal translocations involving these genes are believed to exert their oncogenic activities through constitutive activation of the NF-κB pathway, leading to the expression of numerous genes important for cell survival and proliferation[40,55,57,58] (Figure 2).
In gastric MALT lymphoma, t(11;18)/API2-MALT1 is the most frequent translocation, detected in 20% of cases. This translocation fuses the N-terminal region of API2 to the C-terminal region of MALT1 and generates a functional chimeric fusion, which can activate the NF-κB pathway. Clinically, t(11;18) is more frequently associated with the absence of H. pylori I, and the majority of translocation-positive cases do not respond to HPE therapy. Interestingly, t(11;18)-positive cases rarely transform to DLBCL[55,58].
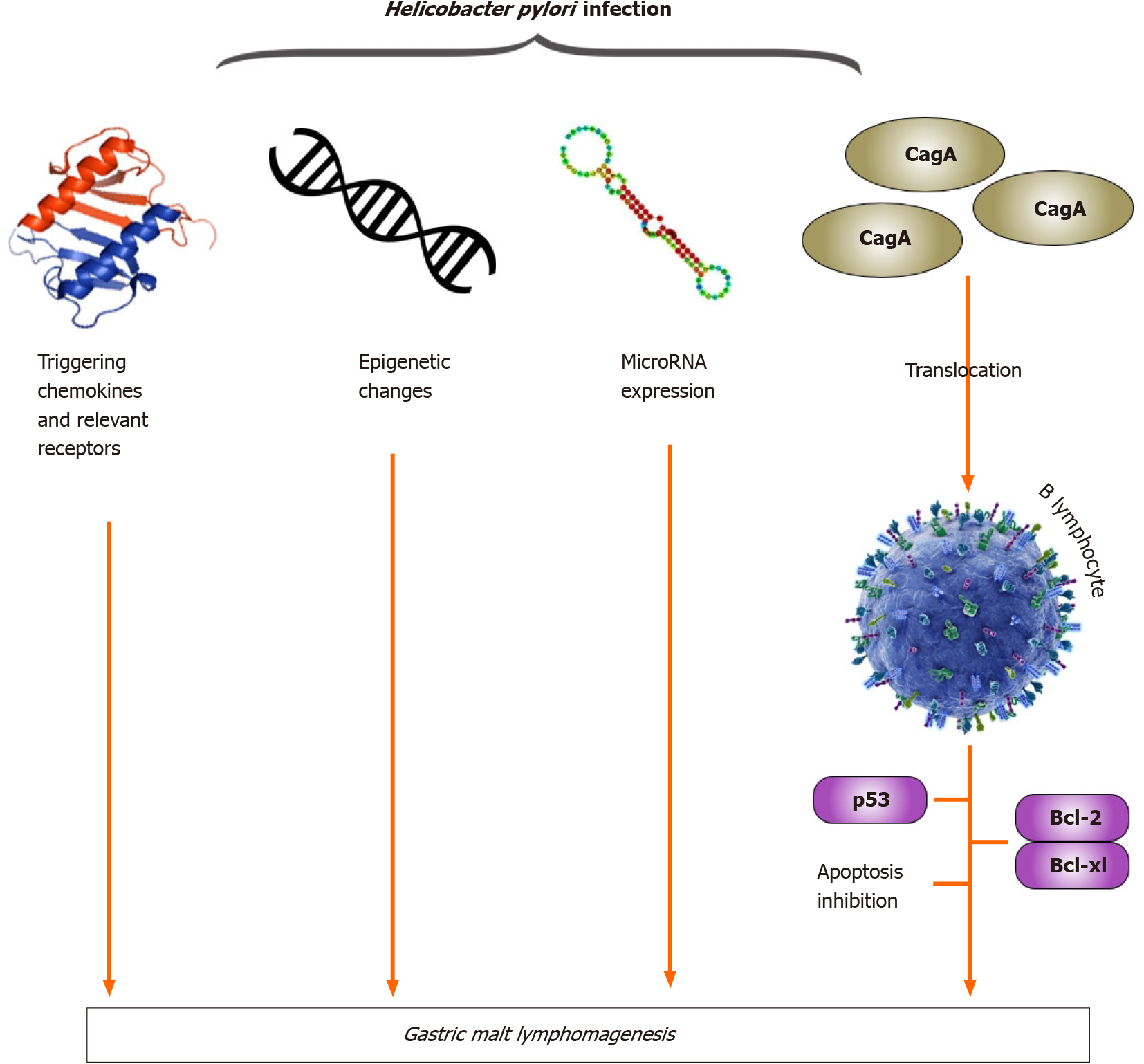
Gastric MALT lymphoma is indirectly influenced by H. pylori I through T-cell stimulation, and recent studies have shown that H. pylori-triggering chemokines and their receptors, H. pylori-associated epigenetic changes, H. pylori-regulated miRNA expression and tumor infiltration by CD4+ CD25+ regulatory T cells contribute to lymphomagenesis of gastric MALT lymphoma (Figure 3). Recent studies have also demonstrated that the translocation of CagA into B lymphocytes inhibits apoptosis through p53 accumulation, BAD phosphorylation and the upregulation of Bcl-2 and Bcl-XL expression (Figure 3). In gastric MALT lymphoma, CagA may stimulate lymphomagenesis directly through the regulation of signal transduction, and intracellular CagA is associated with H. pylori I dependence. These findings represent a substantial paradigm shift compared with the classical theory of H. pylori-reactive T cells contributing indirectly to the development of MALT lymphoma[40].
Other cytogenetic aberrations, often associated with one of the four main chro
While gastric DLBCLs are frequent, sufficient data to guide optimal care are still limited. In the past, gastrectomy was the treatment of choice for these patients. Nevertheless, due to the observed high morbidity rates linked with this procedure, novel therapeutic approaches have emerged, such as radiation and combination chemotherapy. Hence, until today, a multidisciplinary approach has been applied, including chemotherapy, surgery, radiotherapy or a combination of these modalities.
Today, immunochemotherapy with R-CHOP is the most acceptable option for treating gastric DLBCL, as for nodal DLBCL. R-CHOP was established as a standard approach for DLBCL patients; in the study of patients aged 60-80 years, the rate of complete response (CR) was significantly higher in the group that received R-CHOP vs CHOP[60]. Since then, a few small, heterogeneous, retrospective studies have attempted to determine the optimal management of gastric DLBCL, investigating the role of immunochemotherapy, surgery and radiation in patient outcomes[16-21,23,61-63].
Significant advances in diagnosis, treatment and response assessment options over the last years have been made in the field of high-grade lymphomas. Molecular characterization of DLBCL has also described 3 major lymphoma subgroups that correlate with distinct biological and clinical behavior (ABCs, GCBs, double hit lymphomas), supporting the rationale for distinct therapeutic options[48]. However, these advances were extracted from nodal DLBCL, while the intrinsic pathogenesis of primary gastric DLBCL is unclear, and similar studies on this particular type of lymphoma are lacking.
The heterogeneity of the various clinical retrospective studies investigating the outcomes of patients with DLBCL PGLs is impressive. For example, these studies differ in the number of patients, in the time intervals when each therapeutic approach was applied, or in the type of therapeutic approaches compared. Other studies calculate surgery alone and other surgeries with chemotherapy and/or radiotherapy, without separating treatment subgroups of patients. Some researchers place all DLBCL patients together into the statistical analysis, regardless of the anatomical site (stomach, intestine). Hence, comparisons are difficult and not head-to-head. Thus, evidence-based conclusions cannot be drawn, and these results should be regarded with caution.
Finally, the use of various staging systems combined with the variability in the applied procedures for staging make the application of meaningful comparisons among the published series difficult.
We retrospectively evaluated the clinical profile and the patterns of outcome among patients who were treated after the diagnosis of aggressive, B-cell, primary endocrine lymphoma (another type of extranodal lymphoma). The patients were diagnosed with either primary testicular lymphoma, primary thyroid lymphoma (PTHL), or PAL. Better outcomes were observed in patients with PTHL for whom the median OS had not been reached until the end date of the study, whereas the PAL group had the worst prognosis[35].
To better understand the nature and outcome of extranodal DLBCL PGL, we described patients’ and disease characteristics and assessed trends in treatment options, management and outcome among 159 newly diagnosed patients with primary gastric DLBCL who were seen in our institutions in the years 1971-2017.
Previously, we retrospectively evaluated the trends in clinical presentation, management and outcome among 165 consecutive patients with biopsy-proven primary gastric DLBCL who were seen in 1980-2014. The study cohort was divided into two subgroups based on the era of treatment (CHOP vs R-CHOP, before and after the initiation of rituximab). A better outcome after immunochemotherapy (R-CHOP) was observed comparatively[64].
Our novel manuscript and update of the same cohort of patients will be sent for peer review within the next 2 mo (under preparation). We have been preparing and analyzing it for years, focusing on the proper methodology and aiming to correct the perils and pitfalls seen in other relevant studies in the past. We will still have the same conclusion that a better outcome has been noted for the R-CHOP patient cohort, as in the past[64]. However, there are individual variations of the results regarding the OS and freedom from progression (FFP) time intervals, which will be analyzed accordingly, now that a longer follow-up of the patients has been achieved.
The term FFP is based on the strict scientific definition for this type of lymphoma and is preferable to define the aforementioned important endpoint for retrospective clinical studies. PFS has disadvantages in nonrandomized studies because in such studies, there is a lack of specific or concrete criteria for the comparison between time intervals (fixed check points), necessary for the re-evaluation of the disease and the definition of relapse in a similar way (for example, with CT or MRI). However, the term PFS is more widely used in the literature in an equivalent meaning for these lymphomas without being absolutely accurate or to the point in a strict scientific sense. We especially focused on defining FFP accurately, as this is crucial for this novel study. FFP for our novel update will be measured from the initiation of the first treatment until relapse or until death or until the last day of the study for the non relapsed patients or until the day of the last follow-up for the censored patients (lost to follow-up).
Per-protocol analysis will be used in our clinical research compared to intention-to-treat analysis. The latter is considered a better marker of treatment efficacy for prospective, randomized studies and not for retrospective studies.
Finally, lymphoma-specific survival, another important endpoint, will be measured from diagnosis until the time of death from lymphoma. The number of patients who died from causes other than lymphoma was not calculated at this endpoint. As the long-year follow-up continued, we noted a proportion of our patients dying from lymphoma but also other patients dying from causes other than lymphoma. This analysis is important because it attributes the specific hazard ratio to DLBCL gastric lymphoma (death risk) and separates causes of death other than lymphoma for patients who have survived longer. Importantly, when a patient died from another cause in addition to lymphoma, there was no relapse because the patient was in follow-up. Thus, the possible drug might have protected the patient from relapse, and these patients contributed to the studied time-to-event analysis.
In conclusion, retrospective studies, despite their limitations, if conducted with the correct methodology, can provide useful clinical information for treating patients. Our research in recent years has shown that immunochemotherapy (R-CHOP) is the optimal treatment for patients with DLBCL PGLs, as it is associated with a better outcome.
The authors would like to thank Tsangalas E for the preparation of the figures of this work.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ahmed M, Li G, Saito M S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | Peng JC, Zhong L, Ran ZH. Primary lymphomas in the gastrointestinal tract. J Dig Dis. 2015;16:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Juárez-Salcedo LM, Sokol L, Chavez JC, Dalia S. Primary Gastric Lymphoma, Epidemiology, Clinical Diagnosis, and Treatment. Cancer Control. 2018;25:1073274818778256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Milano AF. 20-Year Comparative Survival and Mortality of Cancer of the Stomach by Age, Sex, Race, Stage, Grade, Cohort Entry Time-Period, Disease Duration & Selected ICD-O-3 Oncologic Phenotypes: A Systematic Review of 157,258 Cases for Diagnosis Years 1973-2014: (SEER*Stat 8.3.4). J Insur Med. 2019;48:5-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 723] [Article Influence: 103.3] [Reference Citation Analysis (1)] |
| 5. | Zhang X, Yang J, Huang Q, Lyu J. Prognostic factors in patients with gastric adenocarcinoma using competing-risk analysis: a study of cases in the SEER database. Scand J Gastroenterol. 2019;54:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Violeta Filip P, Cuciureanu D, Sorina Diaconu L, Maria Vladareanu A, Silvia Pop C. MALT lymphoma: epidemiology, clinical diagnosis and treatment. J Med Life. 2018;11:187-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Ferreri AJ, Montalbán C. Primary diffuse large B-cell lymphoma of the stomach. Crit Rev Oncol Hematol. 2007;63:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Ikoma N, Badgwell BD, Mansfield PF. Multimodality Treatment of Gastric Lymphoma. Surg Clin North Am. 2017;97:405-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Fischbach W. Long-term follow-up of gastric lymphoma after stomach conserving treatment. Best Pract Res Clin Gastroenterol. 2010;24:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Shirwaikar Thomas A, Schwartz M, Quigley E. Gastrointestinal lymphoma: the new mimic. BMJ Open Gastroenterol. 2019;6:e000320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961;49:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 468] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Malipatel R, Patil M, Pritilata Rout P, Correa M, Devarbhavi H. Primary Gastric Lymphoma: Clinicopathological Profile. Euroasian J Hepatogastroenterol. 2018;8:6-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Psyrri A, Papageorgiou S, Economopoulos T. Primary extranodal lymphomas of stomach: clinical presentation, diagnostic pitfalls and management. Ann Oncol. 2008;19:1992-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin's Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2850] [Cited by in RCA: 3855] [Article Influence: 350.5] [Reference Citation Analysis (0)] |
| 15. | Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, Hoekstra OS, Hicks RJ, O'Doherty MJ, Hustinx R, Biggi A, Cheson BD. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048-3058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 1148] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 16. | Wang YG, Zhao LY, Liu CQ, Pan SC, Chen XL, Liu K, Zhang WH, Yang K, Chen XZ, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Clinical characteristics and prognostic factors of primary gastric lymphoma: A retrospective study with 165 cases. Medicine (Baltimore). 2016;95:e4250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Couto ME, Oliveira I, Domingues N, Viterbo L, Martins Â, Moreira I, Espírito-Santo A, Chacim S, Moreira C, Pereira D, Henrique R, Mariz J. Gastric Diffuse Large B-Cell Lymphoma: A Single-Center 9-Year Experience. Indian J Hematol Blood Transfus. 2021;1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Li X, Xia B, Guo S, Zhan Z, Zhang L, Zhao D, Wu X, Zhang Y. A retrospective analysis of primary gastric diffuse large B-cell lymphoma with or without concomitant mucosa-associated lymphoid tissue (MALT) lymphoma components. Ann Hematol. 2013;92:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Sohn BS, Kim SM, Yoon DH, Kim S, Lee DH, Kim JH, Lee SW, Huh J, Suh C. The comparison between CHOP and R-CHOP in primary gastric diffuse large B cell lymphoma. Ann Hematol. 2012;91:1731-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Tanaka T, Shimada K, Yamamoto K, Hirooka Y, Niwa Y, Sugiura I, Kitamura K, Kosugi H, Kinoshita T, Goto H, Nakamura S. Retrospective analysis of primary gastric diffuse large B cell lymphoma in the rituximab era: a multicenter study of 95 patients in Japan. Ann Hematol. 2012;91:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Liu Y, Liu Y, Zhao P, Zhang Q, Liu X, Lv F, Hong X, Cao J, Xue K. Switching Fractioned R-CHOP Cycles to Standard R-CHOP Cycles Guided by Endoscopic Ultrasonography in Treating Patients with Primary Gastric Diffuse Large B-Cell Lymphoma. Cancer Manag Res. 2020;12:5041-5048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Goto A, Nishikawa J, Ito S, Hideura E, Ogawa R, Hashimoto S, Okamoto T, Sakaida I. A Rapidly Developing Diffuse Large B cell Lymphoma of the Stomach. J Gastrointest Cancer. 2019;50:657-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Kadota T, Seo S, Fuse H, Ishii G, Itoh K, Yano T, Kaneko K, Tsukasaki K. Complications and outcomes in diffuse large B-cell lymphoma with gastric lesions treated with R-CHOP. Cancer Med. 2019;8:982-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Bai Z, Li Z, Guan T, Wang L, Wang J, Wu S, Su L. Primary Gastric Diffuse Large B-Cell Lymphoma: Prognostic Factors in the Immuno-Oncology Therapeutics Era. Turk J Haematol. 2020;37:193-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Wang X, Kan Y, Chen L, Ge P, Ding T, Zhai Q, Yu Y, Wang X, Zhao Z, Yang H, Liu X, Li L, Qiu L, Qian Z, Zhang H, Wang Y, Zhao H. miR-150 is a negative independent prognostic biomarker for primary gastrointestinal diffuse large B-cell lymphoma. Oncol Lett. 2020;19:3487-3494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Yonezumi M, Suzuki R, Suzuki H, Yoshino T, Oshima K, Hosokawa Y, Asaka M, Morishima Y, Nakamura S, Seto M. Detection of AP12-MALT1 chimaeric gene in extranodal and nodal marginal zone B-cell lymphoma by reverse transcription polymerase chain reaction (PCR) and genomic long and accurate PCR analyses. Br J Haematol. 2001;115:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Aleman BM, Haas RL, van der Maazen RW. Role of radiotherapy in the treatment of lymphomas of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010;24:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Kang HJ, Lee HH, Jung SE, Park KS, O JH, Jeon YW, Choi BO, Cho SG. Pattern of failure and optimal treatment strategy for primary gastric diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. PLoS One. 2020;15:e0238807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Avilés A, Nambo MJ, Neri N, Huerta-Guzmán J, Cuadra I, Alvarado I, Castañeda C, Fernández R, González M. The role of surgery in primary gastric lymphoma: results of a controlled clinical trial. Ann Surg. 2004;240:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Cuccurullo R, Govi S, Ferreri AJ. De-escalating therapy in gastric aggressive lymphoma. World J Gastroenterol. 2014;20:8993-8997. [PubMed] |
| 31. | Olszewska-Szopa M, Wróbel T. Gastrointestinal non-Hodgkin lymphomas. Adv Clin Exp Med. 2019;28:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Wakabayashi M, Sekiguchi Y, Shimada A, Ichikawa K, Sugimoto K, Tomita S, Izumi H, Nakamura N, Sawada T, Ohta Y, Komatsu N, Noguchi M. Diffuse large B-cell lymphoma solely involving bilateral adrenal glands and stomach: report of an extremely rare case with review of the literature. Int J Clin Exp Pathol. 2014;7:8190-8197. [PubMed] |
| 33. | Ceniceros-Cabrales AP, Sánchez-Fernández P. Perforated gastric diffuse large B-cell lymphoma: A case report and literature review. Rev Gastroenterol Mex (Engl Ed). 2019;84:412-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Hassan M, Mandal AK, Sidhu JS, Cardenas LM. Gastric diffuse large B-cell lymphoma with bilateral adrenal metastasis. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Hatjiharissi E, Diamantidis MD, Papaioannou M, Dimou T, Chrisoulidou A, Patakiouta F, Constantinou N, Pazaitou-Panayiotou K. Long-term outcome of primary endocrine non-Hodgkin lymphomas: does the site make the difference? QJM. 2013;106:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Nakamura S, Sugiyama T, Matsumoto T, Iijima K, Ono S, Tajika M, Tari A, Kitadai Y, Matsumoto H, Nagaya T, Kamoshida T, Watanabe N, Chiba T, Origasa H, Asaka M; JAPAN GAST Study Group. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 37. | Ferreri AJ, Govi S, Ponzoni M. The role of Helicobacter pylori eradication in the treatment of diffuse large B-cell and marginal zone lymphomas of the stomach. Curr Opin Oncol. 2013;25:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Paydas S. Helicobacter pylori eradication in gastric diffuse large B cell lymphoma. World J Gastroenterol. 2015;21:3773-3776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Kuo SH, Yeh KH, Chen LT, Lin CW, Hsu PN, Hsu C, Wu MS, Tzeng YS, Tsai HJ, Wang HP, Cheng AL. Helicobacter pylori-related diffuse large B-cell lymphoma of the stomach: a distinct entity with lower aggressiveness and higher chemosensitivity. Blood Cancer J. 2014;4:e220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Kuo SH, Cheng AL. Helicobacter pylori and mucosa-associated lymphoid tissue: what's new. Hematology Am Soc Hematol Educ Program. 2013;2013:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Kuo SH, Yeh KH, Wu MS, Lin CW, Hsu PN, Wang HP, Chen LT, Cheng AL. Helicobacter pylori eradication therapy is effective in the treatment of early-stage H pylori-positive gastric diffuse large B-cell lymphomas. Blood. 2012;119:4838-44; quiz 5057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 42. | Ollila TA, Olszewski AJ. Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence. Curr Treat Options Oncol. 2018;19:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 43. | King RL, Goodlad JR, Calaminici M, Dotlic S, Montes-Moreno S, Oschlies I, Ponzoni M, Traverse-Glehen A, Ott G, Ferry JA. Lymphomas arising in immune-privileged sites: insights into biology, diagnosis, and pathogenesis. Virchows Arch. 2020;476:647-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 44. | Taal BG, Burgers JM. Primary non-Hodgkin's lymphoma of the stomach: endoscopic diagnosis and the role of surgery. Scand J Gastroenterol Suppl. 1991;188:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Thurner L, Hartmann S, Neumann F, Hoth M, Stilgenbauer S, Küppers R, Preuss KD, Bewarder M. Role of Specific B-Cell Receptor Antigens in Lymphomagenesis. Front Oncol. 2020;10:604685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Bautista-Quach MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J Gastrointest Oncol. 2012;3:209-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 47. | Lin P, Medeiros LJ. The impact of MYC rearrangements and "double hit" abnormalities in diffuse large B-cell lymphoma. Curr Hematol Malig Rep. 2013;8:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Abramson JS. Hitting back at lymphoma: How do modern diagnostics identify high-risk diffuse large B-cell lymphoma subsets and alter treatment? Cancer. 2019;125:3111-3120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Foukas PG, Bisig B, de Leval L. Recent advances upper gastrointestinal lymphomas: molecular updates and diagnostic implications. Histopathology. 2021;78:187-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3156] [Article Influence: 143.5] [Reference Citation Analysis (1)] |
| 51. | Liu Y, Yu K, Li M, Zeng K, Wei J, Li X, Liu Y, Zhao D, Fan L, Yu Z, Wang Y, Li Z, Zhang W, Bai Q, Yan Q, Guo Y, Wang Z, Guo S. EZH2 overexpression in primary gastrointestinal diffuse large B-cell lymphoma and its association with the clinicopathological features. Hum Pathol. 2017;64:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Ishikawa E, Nakamura M, Shimada K, Tanaka T, Satou A, Kohno K, Sakakibara A, Furukawa K, Yamamura T, Miyahara R, Nakamura S, Kato S, Fujishiro M. Prognostic impact of PD-L1 expression in primary gastric and intestinal diffuse large B-cell lymphoma. J Gastroenterol. 2020;55:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Asano N, Iijima K, Koike T, Imatani A, Shimosegawa T. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphomas: A review. World J Gastroenterol. 2015;21:8014-8020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 54. | Vasilatou D, Sioulas AD, Pappa V, Papanikolaou IS, Triantafyllou K, Dimitriadis GD, Papageorgiou SG. The role of miRNAs and epigenetic mechanisms in primary gastric mucosa-associated lymphoid tissue lymphoma. Future Oncol. 2016;12:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Troppan K, Wenzl K, Neumeister P, Deutsch A. Molecular Pathogenesis of MALT Lymphoma. Gastroenterol Res Pract. 2015;2015:102656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, Tasca E, Azzurri A, D'Elios MM, Del Prete G, de Bernard M. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 57. | Hamoudi RA, Appert A, Ye H, Ruskone-Fourmestraux A, Streubel B, Chott A, Raderer M, Gong L, Wlodarska I, De Wolf-Peeters C, MacLennan KA, de Leval L, Isaacson PG, Du MQ. Differential expression of NF-kappaB target genes in MALT lymphoma with and without chromosome translocation: insights into molecular mechanism. Leukemia. 2010;24:1487-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Nakamura S, Matsumoto T. Helicobacter pylori and gastric mucosa-associated lymphoid tissue lymphoma: recent progress in pathogenesis and management. World J Gastroenterol. 2013;19:8181-8187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Deutsch AJ, Steinbauer E, Hofmann NA, Strunk D, Gerlza T, Beham-Schmid C, Schaider H, Neumeister P. Chemokine receptors in gastric MALT lymphoma: loss of CXCR4 and upregulation of CXCR7 is associated with progression to diffuse large B-cell lymphoma. Mod Pathol. 2013;26:182-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3975] [Cited by in RCA: 4037] [Article Influence: 175.5] [Reference Citation Analysis (0)] |
| 61. | Ge Z, Liu Z, Hu X. Anatomic distribution, clinical features, and survival data of 87 cases primary gastrointestinal lymphoma. World J Surg Oncol. 2016;14:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Liu PP, Xia Y, Bi XW, Wang Y, Sun P, Yang H, Li ZM, Jiang WQ. Trends in Survival of Patients with Primary Gastric Diffuse Large B-Cell Lymphoma: An Analysis of 7051 Cases in the SEER Database. Dis Markers. 2018;2018:7473935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Taal BG, Burgers JM, van Heerde P, Hart AA, Somers R. The clinical spectrum and treatment of primary non-Hodgkin's lymphoma of the stomach. Ann Oncol. 1993;4:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Hatjiharissi E, Diamantidis M, Papadopoulou A, Chatzileontiadou S, Gerofotis A, Pouptsis A, Karabatzakis N, Pentidou K, Patakiouta F, Konstantinou N, Papaioannou M. Long-term follow-up of patients with non-Hodgkin primary diffuse large B-cell lymphoma of the stomach: Better outcome after immunochemotherapy. Hematol Oncol. 2017;35:377. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |