Published online Sep 21, 2021. doi: 10.3748/wjg.v27.i35.5890
Peer-review started: March 15, 2021
First decision: April 17, 2021
Revised: April 29, 2021
Accepted: August 10, 2021
Article in press: August 10, 2021
Published online: September 21, 2021
Processing time: 183 Days and 16.6 Hours
Zollinger-Ellison syndrome (ZES) associated with pancreatic or duodenal gastrinoma is characterized by gastric acid hypersecretion, which typically leads to gastroesophageal reflux disease, recurrent peptic ulcers, and chronic diarrhea. As symptoms of ZES are nonspecific and overlap with other gastrointestinal disorders, the diagnosis is often delayed with an average time between the onset of symptoms and final diagnosis longer than 5 years. The critical step for the diagnosis of ZES is represented by the initial clinical suspicion. Hypergastrinemia is the hallmark of ZES; however, hypergastrinemia might recognize several causes, which should be ruled out in order to make a final diagnosis. Gastrin levels > 1000 pg/mL and a gastric pH below 2 are considered to be diagnostic for gastrinoma; some specific tests, including esophageal pH-recording and secretin test, might be useful in selected cases, although they are not widely available. Endoscopic ultrasound is very useful for the diagnosis and the local staging of the primary tumor in patients with ZES, particularly in the setting of multiple endocrine neoplasia type 1. Some controversies about the management of these tumors also exist. For the localized stage, the combination of proton pump inhibitory therapy, which usually resolves symptoms, and surgery, whenever feasible, with curative intent represents the hallmark of gastrinoma treatment. The high expression of somatostatin receptors in gastrinomas makes them highly responsive to somatostatin analogs, supporting their use as anti-proliferative agents in patients not amenable to surgical cure. Other medical options for advanced disease are super-imposable to other neuroendocrine neoplasms, and studies specifically focused on gastrinomas only are scant and often limited to case reports or small retrospective series. The multidisciplinary approach remains the cornerstone for the proper management of this composite disease. Herein, we reviewed available literature about gastrinoma-associated ZES with a specific focus on differential diagnosis, providing potential diagnostic and therapeutic algorithms.
Core Tip: As symptoms of Zollinger-Ellison syndrome are nonspecific and overlap with other gastrointestinal disorders, most of these patients are usually referred to general gastroenterologists, leading to a diagnostic delay. A better disease awareness together with the maintenance of a high index of suspicion are necessary to make the final diagnosis. The proper management of Zollinger-Ellison syndrome due to a gastrinoma include both the medical treatment for symptom’s relief and surgery whenever feasible with curative intent; the multidisciplinary approach, with close cooperation between gastroenterologists and surgeons, and the referral to tertiary centers with great expertise in the neuroendocrine field are mandatory.
- Citation: Rossi RE, Elvevi A, Citterio D, Coppa J, Invernizzi P, Mazzaferro V, Massironi S. Gastrinoma and Zollinger Ellison syndrome: A roadmap for the management between new and old therapies. World J Gastroenterol 2021; 27(35): 5890-5907
- URL: https://www.wjgnet.com/1007-9327/full/v27/i35/5890.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i35.5890
Zollinger-Ellison syndrome (ZES) was firstly described in 1955 as associated with a neuroendocrine neoplasm (NEN) capable of ectopic gastrin secretion (namely gastrinoma)[1], resulting in gastric acid hypersecretion, which typically leads to gastroesophageal reflux disease (GERD), recurrent peptic ulcers, and chronic diarrhea. The terms gastrinoma and ZES have been frequently used as synonymous, although gastrinoma refers to the NEN secreting gastrin, whereas ZES refers to the clinical manifestations of the disease. ZES has an incidence of 1-1.5 cases/million per year[2]. Gastrinomas are NENs located in the duodenum (70%), pancreas (25%), and rarely (5%), in other sites, including stomach, liver, ovary, and lung. Gastrinoma is the most frequent functioning duodenal NEN and the second most frequently occurring functional pancreatic NEN (pNEN), following insulinoma; in turn, 15% of functioning pNENs is represented by gastrinoma. It may be sporadic, which is generally diagnosed between the ages of 50 and 70 years with a male to female ratio of 1.5-2:1[3], whilst 20%-30% of the patients develop ZES in the context of a genetic syndrome known as multiple endocrine neoplasia type 1 (MEN-1)[4].
The diagnosis of ZES is not always straightforward due to both non-specific symptoms and confounding factors including proton pump inhibitor (PPI) therapy, which might temporarily relieve symptoms. Furthermore, as these patients tend to be referred to gastroenterologists because of diarrhea and/or reflux disease disorder, despite a better awareness of the disease, the diagnosis might be challenging for those gastroenterologists with low experience in the neuroendocrine setting as well as for many oncologists who are less used to dealing with diarrhea and reflux disease. As a consequence, the average time between the onset of symptoms and the final diagnosis is often longer than 5 years[5,6], and nearly 25% of patients are metastatic at the first diagnosis and show a worse prognosis when compared to non-metastatic patients in whom the surgical management is associated with a promising 15-year survival rate of > 80%[7].
Furthermore, some controversies about the management of these tumors still exist, particularly regarding the exact role of surgery or medical treatment and the possible role of somatostatin analogs (SSAs)[3]. Given that gastrinoma and ZES need both a proper medical treatment for symptom relief and a surgical procedure whenever feasible, the multidisciplinary approach, with close cooperation between clinicians and surgeons, remains the cornerstone for proper management of this composite disease, which should be always referred to tertiary centers.
Herein, we review from a critical point of view current knowledge about gastrinoma-associated ZES, also providing potential diagnostic and therapeutic algorithms based on both evidence from literature and own personal experience.
Bibliographical searches were performed in PubMed using the following keywords: Gastrinoma; Zollinger Ellison syndrome; neuroendocrine neoplasms; pancreatic neuroendocrine neoplasm; duodenal neuroendocrine neoplasm; diagnosis; therapy; guidelines. We searched for all relevant articles published over the last 10 years. The reference lists from the studies returned by the electronic search were manually searched to identify further relevant reports. The reference lists from all available review articles, primary studies, and proceedings of major meetings were also considered. Articles published as abstracts were included, whereas non-English language papers were excluded.
ZES is characterized by gastric acid hypersecretion and consequent hyperchlorhydria resulting in severe acid-related peptic disease and diarrhea. The symptoms usually resolve when gastric acid secretion is controlled pharmacologically with PPIs[8,9]; of note, the disappearance of diarrhea following PPI treatment is typical of ZES and represents one of the factors contributing to the diagnostic delay. According to data from the literature, common symptoms include abdominal pain (75%), diarrhea (73%), heartburn (44%), and weight loss (17%)[6,8,10]. As these symptoms are both not specific and often less severe due to concomitant PPI treatment, the final diagnosis is often delayed and patients are diagnosed with irritable bowel syndrome or reflux disease by gastroenterologists with low or no knowledge of the disease[8,11].
The endoscopic features are also not specific and might include erosions and ulcers[12], however, ZES patients often present with multiple ulcers located at unusual sites, e.g., beyond the first or second portion of the duodenum[8,13]. Furthermore, enlarged gastric folds can be present in more than 90% of patients with ZES[11].
One should keep in mind that approximately 25% of gastrinomas occur in the context of MEN-1, which is characterized by the presence of parathyroid, pancreatic-duodenal, and pituitary tumors[14]; thus the occurrence of unexplained hypercalcemia might be a sign for possible MEN-1 syndrome-associated ZES[15,16], also taken into account that primary hyperparathyroidism is generally the presenting feature in the majority of cases of MEN-1 syndrome[8,16,17]. Of note, parathyroidectomy usually improves gastrin levels and basal acid output[16]. Finally, in ZES/MEN-1 patients, type 2 gastric NENs might occur[3].
Symptoms of ZES are nonspecific and overlap with other gastrointestinal (GI) disorders, which explains the frequent diagnostic delay.
As concerns chronic diarrhea in ZES, this is sustained by hyperchlorhydria and sodium and water malabsorption due to hypergastrinemia[18]. As afore-mentioned, diarrhea is one of the most frequent symptoms in ZES; up to 75% of patients manifest diarrhea[19], and this could be the sole presenting symptom in 3%-10% of the patients[20]. Moreover, chronic diarrhea is one of the most frequent symptoms requiring gastroenterologist referral; its diagnostic workup could be challenging because many different causes could cooperate to diarrhea development and recurrence (e.g., dietary habits, drugs). Recent British Society of Gastroenterology (BSG) guidelines for chronic diarrhea[21] tried to classify different causes of chronic diarrhea (Table 1) and to standardize a diagnostic work-up in these patients. Since hormone-secreting tumors are considered rare causes of chronic diarrhea, BSG guidelines suggest testing patients for these tumors only when other causes of diarrhea have been excluded. From a clinical point of view, the association between chronic diarrhea with both other ZES suggestive symptoms (e.g., chronic peptic ulcer disease) and clinical response to PPIs may be helpful in diagnosing this challenging syndrome, taking into account that the delay in diagnosis of ZES remains between 6 to 9 years from the first clinical presentation[9,19].
| Common | Infrequent | Rare |
| IBS-diarrhea | Small bowel bacterial overgrowth | Small bowel enteropathies (i.e. Whipple’s disease, tropical sprue, amyloid, etc.) |
| Bile acid diarrhea | Mesenteric ischemia | Hypoparathyroidism |
| Diet (artificial sweeteners, caffeine, FODMAP malabsorption, etc.) | Lymphoma | Addison’s disease |
| Colonic neoplasia | Surgical causes (small bowel resection, incontinence, etc.) | Hormone secreting tumors (i.e. VIPoma, gastrinoma, carcinoid) |
| IBD | Chronic pancreatitis | Autonomic neuropathy |
| Celiac disease | Radiation enteropathy | Factitious diarrhea |
| Drugs (antibiotics, NSAID, etc.) | Pancreatic carcinoma | Brainerd diarrhea |
| Overflow diarrhea | Hyperthyroidism | |
| Diabetes | ||
| Chronic infections (i.e. giardiasis) | ||
| Cystic fibrosis |
Abdominal pain and heartburn are frequently reported as symptoms of ZES[9]. As well as diarrhea, they are sustained by hyperchlorhydria, which directly damages GI mucosa, causing ulcers and erosions. Abdominal pain could be associated with peptic ulcers, which, differently from Helicobacter pylori or non-steroidal anti-inflammatory drug-related ulcers, are multiple, located at unusual locations (e.g., the third part of the duodenum, small bowel) and complicated by bleeding, penetration, perforation, or strictures[8,13,19].
Similar to peptic ulcer disease, chronic GERD is one of the most frequent manifestations of ZES[13]. Heartburn and regurgitation are the most typical symptoms, which are super-imposable to symptoms associated with typical GERD; differently from the typical syndrome, patients with ZES often present esophageal strictures due to over-exposition to acid reflux.
Again, the association between these symptoms and chronic diarrhea, after exclusion of other common GI etiologies, might raise the suspicion of ZES, which requires specific tests in order to get the final diagnosis.
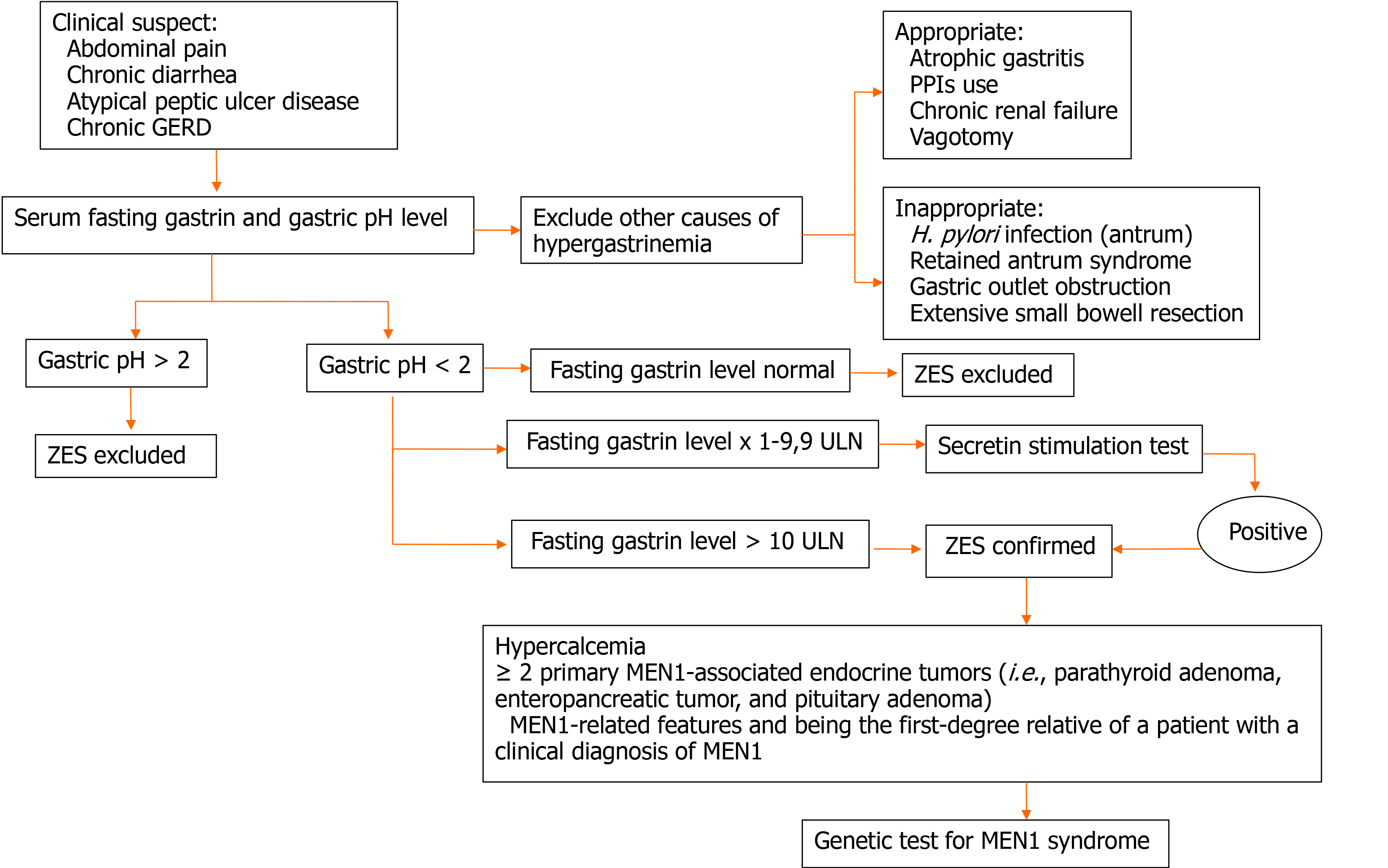
The diagnosis of ZES is quite challenging, also considered that the critical point is the initial suspicion of ZES. A suggested diagnostic algorithm is represented in Figure 1.
ZES is a clinical syndrome characterized by the following triad: (1) gastric acid hypersecretion, sustained by (2) fasting serum hypergastrinemia causing (3) peptic ulcer disease and diarrhea[1]. Hypergastrinemia is sustained by a gastrinoma, a rare NEN (located primarily in the duodenum or pancreas) that secretes gastrin.
Since ZES symptoms can be explained almost entirely by acid hypersecretion, PPIs, which significantly decrease acid secretion, can mitigate or resolve ZES symptoms, making ZES diagnosis even more challenging than in the past[9,22], but avoiding severe ZES complications.
Hypergastrinemia is the hallmark of ZES; however, hypergastrinemia might recognize several causes, which should be ruled out in order to make a final diagnosis of ZES[23]. In detail, it can be distinguished between (1) appropriate hypergastrinemia, due to atrophic gastritis (with or without pernicious anemia), anti-secretory therapy (PPIs or high-dose histamine H2-receptor antagonist, namely famotidine), chronic renal failure, Helicobacter pylori-related pan-gastritis, vagotomy, and (2) inappropriate hypergastrinemia that can be observed in ZES (sporadic or associated with MEN-1), antral-predominant Helicobacter pylori infection, retained-antrum syndrome, gastric-outlet obstruction, extensive small-bowel resection.
The diagnosis of ZES requires the demonstration of inappropriate gastrin secretion associated with gastric hyperchlorhydria, which corresponds to a gastric pH < 2[5]. Normal fasting gastrin levels are < 100 pg/mL; levels > 300 pg/mL are highly suspicious, and levels > 1000 pg/mL together with a gastric pH below 2 are considered to be diagnostic for gastrinoma[2,9,24]. Naso-gastric tube aspiration has classically been used to estimate gastric pH, but it can be uncomfortable for patients and can underestimate gastric acid output; alternatively, gastric pH can be measured during upper GI endoscopy, by aspiration of gastric juice for pH determination using either pH paper or a pH meter; while endoscopic sampling was shown to overestimate total acid volume, it provided more reproducible results and offered greater patient tolerance than nasogastric tube placement[5,23,25,26]. To avoid false-negative results, fasting serum gastrin levels and gastric pH should be measured after PPI withdrawal[2,6,27]. However, PPI withdrawal could be dangerous for ZES patients, because it could bring a dramatic increase in gastric acid secretion, hence causing severe peptic ulcer disease and its complications[23], thus the decision to stop the treatment should be tailored to every single patient. Then, it is usually suggested to start histamine H2-receptor antagonists (i.e. famotidine) as soon as PPIs are stopped in order to prevent complications due to gastric acid hypersecretion. Having a shorter duration of action compared to PPIs, H2-antagonists could be used until the evening before serum gastrin and gastric pH tests[23].
Localization of the primary tumor and its metastases is the first diagnostic step when ZES associated with gastrinoma is suspected.
Contrast-enhanced computed tomography (CT) scan is useful to identify primary tumor > 1 cm, pancreatic head tumors, and liver metastases, with a sensitivity between 59% and 78% and a specificity between 95% and 98%, respectively. Conversely, sensitivity decreases for tumor size < 1 cm and extra-pancreatic locations[28,29].
Contrast-enhanced magnetic resonance imaging (MRI) showed high specificity (namely 100%) in detecting small pancreatic tumors and liver metastases, whereas sensibility is sub-optimal varying from 25% to 85%. Of note, MRI showed a higher sensibility for liver metastases detection when compared to CT scan[28,30].
Somatostatin receptor scintigraphy (Octreoscan®) has been used to localize gastrinomas[8,31]. This test involves the administration of indium radio-labeled octreotide, which binds selectively to somatostatin receptors found on gastrinoma cells. It showed quite good sensitivity (between 77% and 78%) and a good specificity (93%-94%) for primary tumor detection and its metastases, although sensitivity decreases for small tumors (< 1 cm)[32]. Diagnostic accuracy of somatostatin receptor scintigraphy (Octreoscan®) can be improved by performing it in combination with single-photon emission CT (SRS-SPECT)[28]. Different studies showed higher sensitivity and specificity in primary tumor detection, 78%-88% and 97%, respectively, when compared to Octreoscan® alone[33-35].
In more recent years, somatostatin receptor positron emission tomography (PET) techniques have shown great promise for improving the localization of gastrinomas as well as other NENs[36-39] and for the detection of distant metastases, including bone lesions. The radioisotope 68Ga can be ligated to peptides that bind to somatostatin receptors found in abundance on the NEN surface[36]. This technique showed a higher sensibility and specificity (72%-100% and 83%-100%, respectively) when compared to the aforementioned diagnostic techniques in localizing the primary tumor, especially small size tumors[36,37,40]. Combining 68Ga-radiotracers with traditional CT scans (PET/CT) further enhances diagnostic accuracy compared to PET alone, showing a sensitivity of 93% and a specificity of 96% in primary tumor detection[41]. Gallium-68PET-scan should be always included in the diagnostic pathway of all NENs, including gastrinoma, in order to both identify the primary tumor and stage the disease.
Endoscopic ultrasound (EUS) has become an important diagnostic tool to localize gastrinomas, particularly small (i.e. < 2 cm) pancreatic lesions; its sensitivity and specificity are 75%-100% and 95%, respectively, for pancreatic tumors. Unfortunately, its sensitivity dramatically decreases in cases of duodenal localization, ranging from 38% to 63%[28,42]. A further advantage of this technique is the possibility of taking cytologic/histologic samples through a fine needle aspiration/biopsy (FNA/B) to confirm the diagnosis of NEN, even if false-negative results are possible mainly due to poor sampling adequacy. EUS-FNA/B is now considered the primary sampling technique for pancreatic tumors, with a sensitivity ranging between 80% and 90%, specificity at 96%[43], and a sampling adequacy rate of 83%-93%[44].
When used as a screening modality in asymptomatic patients with MEN-1, EUS has been reported to be more accurate than CT scan to detect smaller tumors[45]. Therefore, its diagnostic ability has led experts to recommend it as an annual screening modality for all patients with MEN-1, although recent evidence suggests that the growth rate of small pNENs (i.e. < 2 cm) is low and that EUS screening frequency can likely be extended[14,46].
Since one of the most common symptoms of ZES is GERD, it could be argued that esophageal pH-monitoring could be a useful tool to diagnose ZES. Recent BSG guidelines for esophageal manometry and esophageal pH monitoring[47] stated indications to perform esophageal pH-monitoring, also including as an indication GERD symptoms that did not respond to double dose of PPIs. This technique allows to diagnose an increased acid exposure, to evaluate the association between symptoms and acid or non-acid reflux, and to identify different phenotypes of upper symptoms (i.e. non-erosive reflux disease, hypersensitive esophagus, and functional heartburn).
ZES is not usually included in diagnosis performed by esophageal pH-monitoring, and, consequently, ZES reference standard for esophageal pH-monitoring is lacking. However, evidence of a high number of acidic reflux episodes (i.e. esophageal pH < 4), a high number of long (i.e. > 5 min) reflux episodes, a high percentage of time with esophageal pH < 4, both on a double dose of PPIs and off PPIs, could raise the suspicion of abnormal gastric acid secretion. This hypothesis should be confirmed by prospective studies; however, considering the rarity of this syndrome, it would be very difficult to obtain standard values to use in clinical practice; therefore, despite its potential utility, this test is not currently included in the standard diagnostic workup of gastrinoma.
Secretin provocative test in ZES diagnosis founds its application in controversial cases, that is patients with suspected ZES, gastric pH < 2 but fasting serum gastrin < × 10 upper limit of normal[9]. To perform a secretin stimulation test, fasting gastrin levels are obtained before intravenous (IV) administration of secretin and then 2, 5, and 10 min after infusion[25]. Patients with gastrinomas exhibit an inappropriate increase in gastrin production in response to secretin infusion[9]. This mechanism can be explained in part by the fact that secretin receptors are expressed directly on the gastrinoma cell surface[48]. Different cut-offs for positive tests have been proposed, including an absolute increase in gastrin concentration ≥ 110 pg/mL or ≥ 200 pg/mL or a 50% increase in gastrin concentration[49]. However, previous data suggested that a positive secretin-provocative test (≥ 120 pg/mL increase) has a sensitivity of 94% and specificity of 100%, respectively[50]. According to data from the literature, a false-negative response can occur in 6% to 20% of patients[51,52], whereas false-positive responses, ranging from 15% to 39% in different studies[52,53], are found in patients with pernicious anemia or chronic PPI use.
In order to reduce the risk of false-positive results, PPI treatment should be withdrawn, but, again, the decision should be discussed in a case-by-case manner to limit the risk of severe complications (e.g., perforation or bleeding). This might partially explain the reason why the secretin test can be difficult to be performed and should be reserved for strictly selected cases when the diagnosis is not straightforward.
MEN-1 is an autosomal dominant disorder, whose incidence has been estimated from random postmortem studies to be 0.25%, and to be 1%-18% in patients with primary hyperparathyroidism, 16%-38% in patients with gastrinomas, and less than 3% in patients with pituitary tumors[14]. From a clinical point of view, MEN-1 syndrome includes the occurrence of parathyroid adenoma (90%), entero-pancreatic tumor (30%-70%), being gastrinoma the most frequent (40%), and pituitary adenoma (30%-40%). Other tumors that might occur in MEN-1 patients are adrenal cortical tumor (40%), pheochromocytoma (< 1%), bronchopulmonary NEN (2%), thymic NEN (2%), gastric NEN (10%), lipomas, (30%), angiofibromas (85%), collagenomas (70%), and meningiomas (8%)[14].
In patients with an established diagnosis of gastrinoma-related ZES, MEN-1 syndrome might be present in approximately 25% of the cases. The presence of hypercalcemia due to hyperparathyroidism is one of the first signs. However, the diagnosis might be challenging in this specific setting as ZES does not usually develop in the absence of primary hyperparathyroidism, and hypergastrinemia has also been reported to be associated with hypercalcemia as a confounding factor[15]. Further
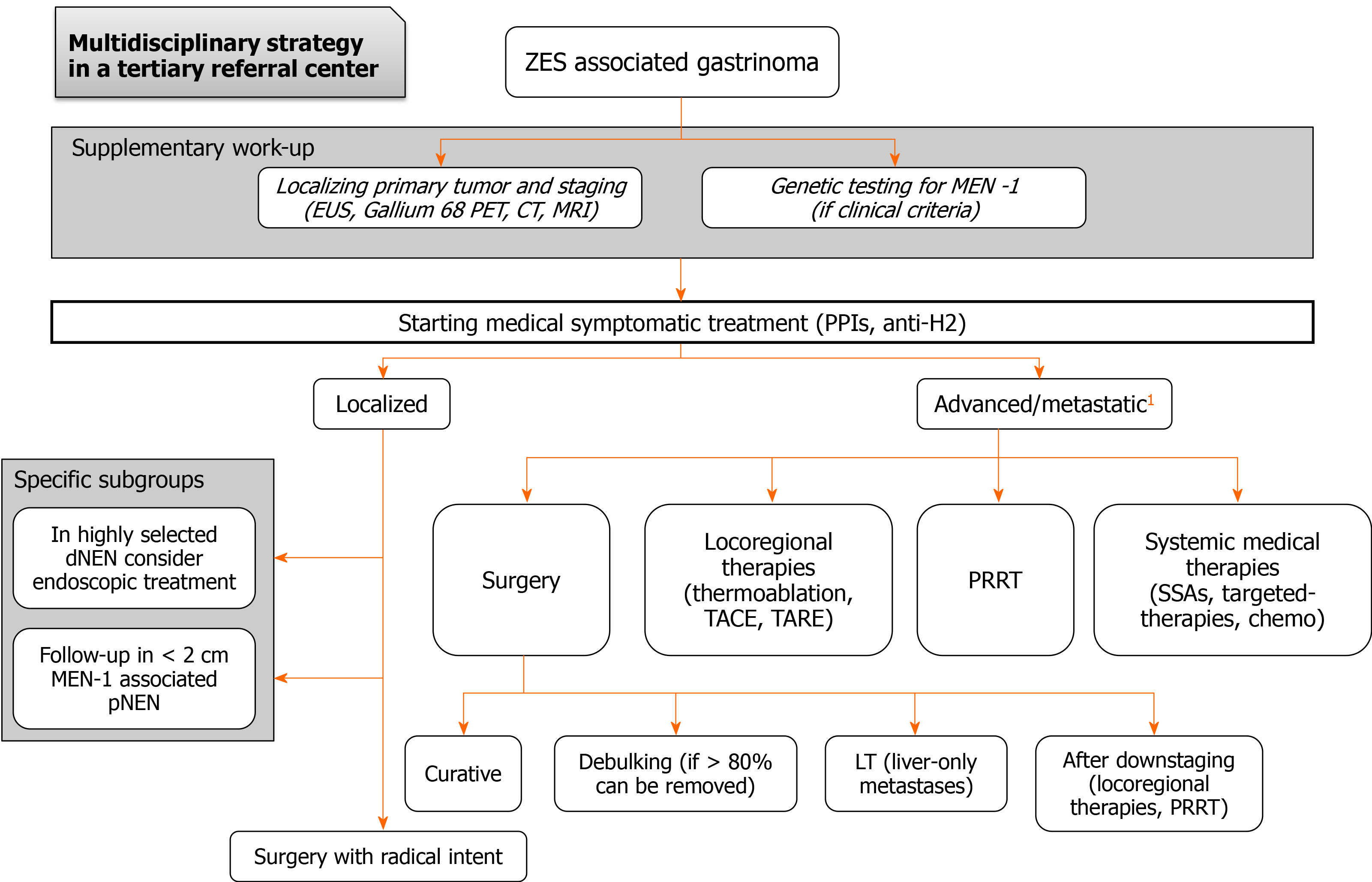
The management of gastrinoma and ZES includes both a proper medical treatment for symptom’s relief and surgery with curative intent whenever feasible. A proposed therapeutic algorithm is represented in Figure 2.
The role of surgery in the treatment of gastrinoma has changed completely from the introduction of PPIs in the 1980s. In fact, before the advent of an effective anti-secretory therapy, surgery was performed to control acid hypersecretion, mainly removing the target cells of gastrin through total gastrectomy. These operations were, by the way, affected by a high mortality rate due to acid-related complications in the postoperative course. With the use of PPIs, gastric hypersecretion was no longer a problem, and the main determinant of prognosis became the gastrinoma itself because of its malignant potential and surgical excision started to be proposed as a potentially curative therapy. From 1981, the National Institute of Health began a prospective study recruiting patients with ZES for surgical therapy, with a well-designed surgical protocol in order to capture the long-term results of the best available surgical approach. The study reported a 10-year overall survival (OS) and disease-free survival (DFS) of 94% and 34%, respectively[54]. Therefore, surgery has gradually changed its role and gastrinoma resection has started to be increasingly proposed to patients eligible for resection. Currently, across the most important guidelines, surgical excision is generally recommended either for sporadic gastrinoma or for MEN-1 associated gastrinoma if complete tumor removal is possible[2,55-58]. Subsequent studies reported a 20 year OS of 58%-71%, a 20-year disease-related survival of 73%-88%[59], and a 10-year DFS of 25%-50%[60]. Surgery of the primary tumor also demonstrated to reduce the occurrence of liver metastases[61-63], which are one of the main determinants of prognosis, and to improve DFS in comparison with non-surgical management[62].
The majority of gastrinomas (from 60% to 90% depending on the series)[42,60] occur in the duodenum, and, since these are often very small lesions (less than 1 cm) and located at the submucosal layer, tumor detection is not so straightforward. Therefore, the surgical technique should follow a stepwise approach to search for the tumor even in case of negative preoperative imaging. In this context, surgery has firstly a diagnostic purpose, which is quite uncommon in modern surgery and, given the peculiarity of this technique and the rarity of the disease, it should be performed by experienced surgeons in tertiary referral centers. After a complete abdominal exploration, the duodenum and the pancreatic head are mobilized (Kocher maneuver) and carefully palpated. Intra-operative ultrasound with a linear probe is then performed on the duodenum and pancreas looking for the primary tumor and on the liver in search for liver metastases. Intra-operative endoscopy is performed thereafter advancing the scope into the duodenum; duodenal gastrinomas may be found through trans-illumination of the bowel wall as non-trans-illuminated spots. If a lesion is identified, it should be marked with a suture and the duodenum opened around it for a full-thickness excision. If the described steps fail to reveal any lesion, a 3 cm longitudinal incision is made on the anterior aspect of the second portion of the duodenum, and the entire duodenal wall is palpated. Suspicious lesions are excised with a full-thickness rim of normal tissue and sent for pathology. The duodenum is then closed transversally, if possible, to minimize the risk of strictures[64,65]. In the hands of an experienced surgeon, lesions could be found in 98% of imaging-negative ZES patients, with a 50% curative rate[59], similar to that of imaging-positive patients. These findings suggest that surgery should be performed as soon as possible in sporadic ZES, despite negative imaging findings. Pancreatic gastrinomas should be enucleated if located 3 mm or farther from the main pancreatic duct. Conversely, lesions that are closer to the pancreatic duct require distal pancreatectomy with or without splenectomy if located in the body or tail of the gland and pancreaticoduodenectomy if located in the head/neck. Pancreaticoduodenectomy or distal pancreatectomy may be necessary also for local recurrence after enucleation[66].
Regional lymph nodes should always be removed because nodal metastases are present in almost half of the patients[54,67] and lymphadenectomy has been associated with increased DFS[68], as reported also for other pNENs[69-71]. The presence of primary gastrinoma located in a lymph node is controversial, however, several studies reported long disease-free survivors after resection of only a positive lymph node[72,73], and this supports the role of routine lymphadenectomy.
Since pancreaticoduodenectomy provides complete removal of the regional lymph nodes of the pancreatic head, the results in terms of DFS are better with respect to enucleation because of the higher chance of radicality[54,67]. However, given the high postoperative morbidity and the good prognosis also of patients with small residual disease, pancreaticoduodenectomy is not recommended as the standard operation for these patients[2,55-58]. Generally, the indication for surgery should always follow a thorough risk/benefit assessment within a multidisciplinary tumor board aiming at maximum radicality and minimum morbidity. This is particularly the case for MEN-1 patients; in these patients, who have generally an earlier age of onset, pNENs should be resected in low-risk patients, and surgery is generally recommended for tumors larger than 2 cm[14,58]. However, according to most authorities, as well as all guidelines, surgical resection for an attempted cure should be performed in ZES patients whenever possible[2,27,58]. This is particularly true for functioning duodenal NENs, including gastrinomas, which have been reported to express a high metastatic potential[74], thus a radical surgical approach should be the first choice in this specific setting. However, in highly selected cases (i.e. duodenal lesions ≤ 1 cm, limited to the submucosal layer and without lymph nodal involvement), endoscopic resection might also be considered, although the risk of undetected micro-metastases might represent an issue.
Another controversial issue is laparoscopic surgery; while it is widely adopted for pNENs, its role for gastrinomas is limited to patients in whom preoperative imaging gives an accurate definition of tumor location. Unfortunately, as already mentioned, extensive exploration is often needed for diagnostic purposes. In these cases, laparoscopy is inadequate, and laparotomy is mandatory.
The role of surgical resection in ZES patients with advanced metastatic disease or even with extensive invasive localized disease is not well-defined. In this setting, the possibility of surgical removal of all resectable tumors (cytoreductive surgery, debulking surgery) should be considered, and surgery is generally recommended if ≥ 80% of all disease can be removed (generally feasible in 5%-15% of all metastatic gastrinomas), although only a few reports containing primarily gastrinomas treated with this approach are currently available[10,72].
Finally, in highly selected metastatic gastrinomas, with liver-only metastases and fulfilling strict inclusion criteria, liver transplantation might be considered, even if its use remains controversial and the risk of tumor recurrence represents an issue[58].
Studies specifically focused on liver-directed therapies in the context of gastrinomas are scant; however, as for other NENs, the embolization approaches in the setting of gastrinoma are generally reserved for patients with metastatic unresectable hepatic metastases either limited to the liver or with a liver-predominant disease, particularly if locally symptomatic[10,58]. Of note, liver-directed therapies are used less frequently in ZES than in other metastatic NENs, because in ZES, the hormone excess-state can be well-controlled medically.
Among functioning NENs, gastrinoma is the most frequent type. There are two therapeutic goals in the management of patients with gastrinoma: The control of gastric acid hypersecretion and the treatment of the tumor itself.
The therapy for syndrome control is based on PPI (e.g., omeprazole, esomeprazole, lansoprazole, pantoprazole, etc.), which are highly effective drugs and considered the drugs of choice for suppressing acid secretion. PPIs effectively block gastric acid secretion by irreversibly binding to and inhibiting the hydrogen-potassium ATPase pump on the luminal surface of the parietal cell membrane. Theoretically, the choice and titration of anti-secretory therapy should be guided by the parameters of gastric acid secretions such as basal acid output (to reduce it below 10 mEq/h)[75], since using symptoms alone as a signal of efficacy might be misleading, even if in many centers these methods are not available. Therefore, in most cases, PPI therapy is started at an empirical maximized dosage. The recommended initial dose of omeprazole is 60 mg/daily or esomeprazole 120 mg/daily, lansoprazole 45 mg/daily, rabeprazole 60 mg/daily, pantoprazole 120 mg/daily, divided, twice-a-day[75-78]. The type of PPI used seems not to be of relevance and a systematic review of 12 randomized trials examining the relative effectiveness of different PPI doses and dosing regimens found no consistent differences in symptom resolution and esophagitis healing rates[79]. IV PPIs are indicated in patients with clinically significant upper GI bleeding from a suspected peptic ulcer. Omeprazole, pantoprazole, and esomeprazole are the only PPIs available as an IV formulation. The other patients can be treated with oral preparation.
As concerns efficacy, PPIs have significantly decreased the morbidity and mortality resulting from severe ulcer disease[80]. In 60% of patients, ulcer healing occurs within 2 wk; in 90%-100% of patients, healing occurs within 4 wk. PPIs are generally safe, even when used in high doses.
Once an effective clinical control of the peptic disease has been achieved, a gradual dose reduction is generally suggested[81,82]. In a study by Metz et al[83], 37 patients received high-dose omeprazole for almost 2 years, and nearly 50% were able to lower the dose down to 20 mg once daily, with 95% of patients experiencing safe long-term reductions in their medication dose. PPIs are generally well tolerated and can control hypergastrinemia in ZES for > 10 years (although some patients experience low vitamin B12 levels)[84].
No tachyphylaxis has been described. Therefore, the long duration of action, the fewer adverse effects, and the high potency make them superior to H2 blockers.
Regarding the use of H2-receptor antagonists in ZES, the dose usually is 4-8 times higher than the dose administered to patients with peptic ulcer disease. Although a good success rate exists, this treatment has been reported to fail in 50% of patients. Therefore, these drugs are never the first choice.
Only when PPIs are unable to control gastric acid secretion, SSAs can be considered, as they reduce gastrin secretion, even if they do not represent a first-line treatment at least for symptom control.
Even if this is not a treatment currently approved in localized gastrinoma, it is worth mentioning that in animals, the cholecystokinin-2 receptor antagonist YF476 has been shown to inhibit the development of enterochromaffin-like cell-tumors in susceptible animals with induced hypergastrinemia. Therefore, this drug could represent a potential option in ZES, not only to inhibit hypergastrinemia but also to prevent gastric NEN type 2 (e.g., associated with ZES/MEN-1). Furthermore, there continues to be interest in the development of cholecystokinin-2 receptor antagonists as anti-secretory agents[85]. However, strong evidence supporting the role of these molecules in this specific setting is lacking.
Approximately one-third of ZES patients present with metastatic disease to the liver[10,86]. There are several systemic therapeutic options for advanced gastrinoma, not substantially different from the ones for other NENs, however, studies evaluating specific response rates in gastrinomas alone are limited.
SSAs like octreotide and lanreotide are highly effective in controlling the symptoms associated with hormone hypersecretion in all functioning tumors[87,88]; furthermore, they can reduce gastrin levels and their anti-proliferative effect has been demonstrated in PROMID and CLARINET studies[89,90]. However, in these studies only a few cases of gastrinoma were included, and, even if different case reports and case series suggested the role of SSAs in controlling gastrin secretion and symptoms in ZES patients[91-94], to date only a few studies with a very low number of patients investigated specifically the role of SSAs in ZES[3].
The multitargeted tyrosine kinase inhibitor, sunitinib, has demonstrated an improved progression-free survival from 5.5 mo to 11.4 mo in metastatic pNENs[95]. Moreover, based on the results of two randomized, double-blind, prospective, placebo-controlled studies, the mammalian target of rapamycin-inhibitor everolimus has been approved in advanced both pancreatic[96] and extra-pNENs[96]. However, there are no specific studies on the effects of sunitinib/everolimus in the specific setting of gastrinomas.
Streptozocin, 5-fluorouracil, and doxorubicin have been used, with the response rate reported to be as high as 69%[97]. Despite these reported response rates, the true radiologic response rate is more probably between 10% and 40%[98,99]. More recently, anti-proliferative activity has also been shown for temozolomide. Data came from retrospective studies[100] as well as from a prospective randomized study comparing capecitabine plus temozolomide to temozolomide alone in pNENs that revealed a median progression-free survival longer in the combination arm (22.7 mo vs 14.4 mo, hazard ratio 0.58, P = 0.023), but satisfactory in both[101]. Moreover, a recent real-world analysis confirmed the combination of capecitabine and temozolomide as an active treatment for metastatic NENs[102]. Because of these studies, the use of capecitabine plus temozolomide has become routine for advanced pNENs, including gastrinomas.
Lastly, peptide receptor radionuclide therapy (PRRT) may be the most promising systemic therapy, and it has been repeatedly reported as particularly useful for symptom relief in functioning forms, even if this aspect might be less important in the setting of gastrinomas due to concomitant PPI treatment which is considered to be the first-line approach for symptoms’ control[10]. Two different isotopes have been used in most studies: 90Yttrium (90Y)- or 177Lutetium (177Lu)-labeled SSAs[103]. The approval of PRRT treatment comes from the promising results of a double-blinded, control phase 3 trial (NETTER-1)[104] in patients with advanced unresectable, midgut carcinoids and the results of treatment of 510 patients with advanced pNENs and other NENs[105,106]. According to data from the literature, gastrinomas are one of the malignant pNENs that were most responsive to PRRT; however, they also had one of the highest recurrence rates leading to a poorer prognosis[103,105]. In detail, in one study including 11 patients with metastatic ZES[107] treated with either 90Y-and/or 177Lu-labeled SSAs, the mean serum gastrin decreased by 81%, complete response occurred in 9%, partial tumor response in 45%, tumor stabilization in 45%, with a persistence of the antitumor effect for a median period of 14 mo in 64% of the cases. Another study[108] involving 30 gastrinoma patients treated with 90Y-labeled SSAs reported a partial response rate of 33% with a mean OS time of 40 mo.
As the diagnosis of ZES is challenging; the maintenance of a high index of suspicion is necessary to get the final diagnosis. Better disease awareness is useful to reduce the diagnostic delay, particularly due to the improper referral of patients to physicians with low or no expertise in the neuroendocrine field. The association between typical symptoms including chronic diarrhea, reflux disorder, and recurrent peptic disease particularly at unusual sites should raise the suspicion of ZES after exclusion of alternative and more common GI etiologies. The possibility of an underlying MEN-1 syndrome should be always considered, particularly in young patients with concomitant hypercalcemia suggestive of hyperparathyroidism and/or familiar history of MEN-1. A fasting gastrin level is generally the first step and confounding factors such as PPI use need to be considered. Gastric pH, esophageal pH-recording, and possibly a secretin stimulation test might be necessary as well, although the decision to perform them should be tailored to every single patient, considered both the need to withdraw PPI treatment and the limited availability of these tests in routine clinical practice. Tumor localization must be performed and EUS with the possibility of getting a sampling through FNA is considered to be a more accurate technique than conventional imaging for small lesions. Given the high expression of STTRs in gastrinomas, gallium-68PET-scan should be always included in the diagnostic pathway of all NENs, including gastrinoma, in order to both identify the primary tumor and to stage the disease.
Regarding the treatment of the localized disease, the two milestones are represented by PPIs for symptoms’ control and surgery with curative intent. The role of surgery in the treatment of gastrinoma has changed completely from the introduction of PPIs. In the past, total gastrectomy represented the sole effective treatment to treat ZES by removing the end-organ target of gastrin. With the use of PPIs, gastric hypersecretion was no longer considered a problem and surgical excision started to be proposed as a potentially curative therapy. Surgical removal of the primary tumor (and possibly its metastases) with curative intent should be, indeed, always performed. Unfortunately, the diagnosis is often made when the disease is too advanced for a surgical approach. The first step, again, is represented by syndrome control, based on PPIs, which are considered to be the drugs of choice for suppressing acid secretion. In order to achieve tumor growth control, SSAs constitute a viable option; studies specifically focused on advanced gastrinomas are scanty and often retrospective, however, according to data from the literature, treatments for the advanced disease are super-imposable to other NENs and include targeted therapies, chemotherapy, and PRRT. As there is a need for both a proper medical treatment for symptom’s relief and a surgical procedure whenever feasible with curative intent, the multidisciplinary approach, with close cooperation between clinicians and surgeons, remains the cornerstone for proper management of this composite disease. Due to the risk of overlapping ZES with other GI common disorders, referral to tertiary centers with great expertise in the neuroendocrine field is mandatory.
Pietro Invernizzi and Sara Massironi are members of the European Reference Network on Hepatological Diseases (ERN RARE LIVER), and they thank AMAF Monza ONLUS and AIRCS for their support.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Prisciandaro M S-Editor: Gao CC L-Editor: Filipodia P-Editor: Xing YX
| 1. | Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg. 1955;142:709-23; discussion, 724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 942] [Cited by in RCA: 696] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 2. | Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 985] [Article Influence: 109.4] [Reference Citation Analysis (1)] |
| 3. | Guarnotta V, Martini C, Davì MV, Pizza G, Colao A, Faggiano A; NIKE group. The Zollinger-Ellison syndrome: is there a role for somatostatin analogues in the treatment of the gastrinoma? Endocrine. 2018;60:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Ito T, Igarashi H, Jensen RT. Zollinger-Ellison syndrome: recent advances and controversies. Curr Opin Gastroenterol. 2013;29:650-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J Gastroenterol. 2012;18:5495-5503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Jensen RT, Niederle B, Mitry E, Ramage JK, Steinmuller T, Lewington V, Scarpa A, Sundin A, Perren A, Gross D, O'Connor JM, Pauwels S, Kloppel G; Frascati Consensus Conference; European Neuroendocrine Tumor Society. Gastrinoma (duodenal and pancreatic). Neuroendocrinology. 2006;84:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Keutgen XM, Nilubol N, Kebebew E. Malignant-functioning neuroendocrine tumors of the pancreas: A survival analysis. Surgery. 2016;159:1382-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Gibril F, Jensen RT. Zollinger-Ellison syndrome revisited: diagnosis, biologic markers, associated inherited disorders, and acid hypersecretion. Curr Gastroenterol Rep. 2004;6:454-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Mendelson AH, Donowitz M. Catching the Zebra: Clinical Pearls and Pitfalls for the Successful Diagnosis of Zollinger-Ellison Syndrome. Dig Dis Sci. 2017;62:2258-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Jensen RT, Ito T. Gastrinoma. 2020 Nov 21. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Grossman A, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. [PubMed] |
| 11. | Wilcox CM, Seay T, Arcury JT, Mohnen J, Hirschowitz BI. Zollinger-Ellison syndrome: presentation, response to therapy, and outcome. Dig Liver Dis. 2011;43:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Hatta W, Iijima K, Koike T, Kondo Y, Ara N, Asanuma K, Uno K, Asano N, Imatani A, Shimosegawa T. Endoscopic findings for predicting gastric acid secretion status. Dig Endosc. 2015;27:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Roy PK, Venzon DJ, Shojamanesh H, Abou-Saif A, Peghini P, Doppman JL, Gibril F, Jensen RT. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore). 2000;79:379-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 225] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML; Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97:2990-3011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 878] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 15. | Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore). 2004;83:43-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Norton JA, Venzon DJ, Berna MJ, Alexander HR, Fraker DL, Libutti SK, Marx SJ, Gibril F, Jensen RT. Prospective study of surgery for primary hyperparathyroidism (HPT) in multiple endocrine neoplasia-type 1 and Zollinger-Ellison syndrome: long-term outcome of a more virulent form of HPT. Ann Surg. 2008;247:501-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Davì MV, Boninsegna L, Dalle Carbonare L, Toaiari M, Capelli P, Scarpa A, Francia G, Falconi M. Presentation and outcome of pancreaticoduodenal endocrine tumors in multiple endocrine neoplasia type 1 syndrome. Neuroendocrinology. 2011;94:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 18. | Aamar A, Madhani K, Virk H, Butt Z. Zollinger-Ellison Syndrome: A Rare Case of Chronic Diarrhea. Gastroenterology Res. 2016;9:103-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Campana D, Piscitelli L, Mazzotta E, Bonora M, Serra C, Salomone L, Corinaldesi R, Tomassetti P. Zollinger-Ellison syndrome. Diagnosis and therapy. Minerva Med. 2005;96:187-206. [PubMed] |
| 20. | Simmons LH, Guimaraes AR, Zukerberg LR. Case records of the Massachusetts General Hospital. Case 6-2013. A 54-year-old man with recurrent diarrhea. N Engl J Med. 2013;368:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Arasaradnam RP, Brown S, Forbes A, Fox MR, Hungin P, Kelman L, Major G, O'Connor M, Sanders DS, Sinha R, Smith SC, Thomas P, Walters JRF. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd edition. Gut. 2018;67:1380-1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 201] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 22. | Corleto VD, Annibale B, Gibril F, Angeletti S, Serrano J, Venzon DJ, Delle Fave G, Jensen RT. Does the widespread use of proton pump inhibitors mask, complicate and/or delay the diagnosis of Zollinger-Ellison syndrome? Aliment Pharmacol Ther. 2001;15:1555-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Metz DC. Diagnosis of the Zollinger–Ellison syndrome. Clin Gastroenterol Hepatol. 2012;10:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist. 2014;19:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Phan J, Benhammou JN, Pisegna JR. Gastric Hypersecretory States: Investigation and Management. Curr Treat Options Gastroenterol. 2015;13:386-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Oh DS, Wang HS, Ohning GV, Pisegna JR. Validation of a new endoscopic technique to assess acid output in Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2006;4:1467-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, Marx SJ, Pasieka JL, Pommier RF, Yao JC, Jensen RT; North American Neuroendocrine Tumor Society (NANETS). NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 467] [Cited by in RCA: 397] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 28. | Krampitz GW, Norton JA. Current management of the Zollinger-Ellison syndrome. Adv Surg. 2013;47:59-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Reznek RH. CT/MRI of neuroendocrine tumours. Cancer Imaging. 2006;6:S163-S177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Thoeni RF, Mueller-Lisse UG, Chan R, Do NK, Shyn PB. Detection of small, functional islet cell tumors in the pancreas: selection of MR imaging sequences for optimal sensitivity. Radiology. 2000;214:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469-1492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 637] [Cited by in RCA: 542] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 32. | Stokkel MP, Rietbergen DD, Korse CM, Taal BG. Somatostatin receptor scintigraphy and chromogranin A assay in staging and follow-up of patients with well-differentiated neuroendocrine tumors. Nucl Med Commun. 2011;32:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Ruf J, von Wedel F, Furth C, Denecke T, Stelter L, Steffen IG, Schütte K, Arend J, Ulrich G, Klose S, Bornschein J, Apostolova I, Amthauer H. Significance of a Single-Time-Point Somatostatin Receptor SPECT/Multiphase CT Protocol in the Diagnostic Work-up of Gastroenteropancreatic Neuroendocrine Neoplasms. J Nucl Med. 2016;57:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Wong KK, Gandhi A, Viglianti BL, Fig LM, Rubello D, Gross MD. Endocrine radionuclide scintigraphy with fusion single photon emission computed tomography/computed tomography. World J Radiol. 2016;8:635-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 35. | Sainz-Esteban A, Olmos R, González-Sagrado M, González ML, Ruiz MÁ, García-Talavera P, Gamazo C, Villanueva JG, Cobo A, de Luis D. Contribution of ¹¹¹In-pentetreotide SPECT/CT imaging to conventional somatostatin receptor scintigraphy in the detection of neuroendocrine tumours. Nucl Med Commun. 2015;36:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10:2259-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 37. | Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, Kovacs P, Von Guggenberg E, Bale R, Virgolini IJ. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 688] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 38. | Skoura E, Michopoulou S, Mohmaduvesh M, Panagiotidis E, Al Harbi M, Toumpanakis C, Almukhailed O, Kayani I, Syed R, Navalkissoor S, Ell PJ, Caplin ME, Bomanji J. The Impact of 68Ga-DOTATATE PET/CT Imaging on Management of Patients with Neuroendocrine Tumors: Experience from a National Referral Center in the United Kingdom. J Nucl Med. 2016;57:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Sharma P, Arora S, Mukherjee A, Pal S, Sahni P, Garg P, Khadgawat R, Thulkar S, Bal C, Kumar R. Predictive value of 68Ga-DOTANOC PET/CT in patients with suspicion of neuroendocrine tumors: is its routine use justified? Clin Nucl Med. 2014;39:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Wild D, Bomanji JB, Benkert P, Maecke H, Ell PJ, Reubi JC, Caplin ME. Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2013;54:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 41. | Geijer H, Breimer LH. Somatostatin receptor PET/CT in neuroendocrine tumours: update on systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2013;40:1770-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 42. | Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004;240:757-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 43. | Zilli A, Arcidiacono PG, Conte D, Massironi S. Clinical impact of endoscopic ultrasonography on the management of neuroendocrine tumors: lights and shadows. Dig Liver Dis. 2018;50:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Atiq M, Bhutani MS, Bektas M, Lee JE, Gong Y, Tamm EP, Shah CP, Ross WA, Yao J, Raju GS, Wang X, Lee JH. EUS-FNA for pancreatic neuroendocrine tumors: a tertiary cancer center experience. Dig Dis Sci. 2012;57:791-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Thomas-Marques L, Murat A, Delemer B, Penfornis A, Cardot-Bauters C, Baudin E, Niccoli-Sire P, Levoir D, Choplin Hdu B, Chabre O, Jovenin N, Cadiot G; Groupe des Tumeurs Endocrines (GTE). Prospective endoscopic ultrasonographic evaluation of the frequency of nonfunctioning pancreaticoduodenal endocrine tumors in patients with multiple endocrine neoplasia type 1. Am J Gastroenterol. 2006;101:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Kappelle WF, Valk GD, Leenders M, Moons LM, Bogte A, Siersema PD, Vleggaar FP. Growth rate of small pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1: results from an endoscopic ultrasound based cohort study. Endoscopy. 2017;49:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Trudgill NJ, Sifrim D, Sweis R, Fullard M, Basu K, McCord M, Booth M, Hayman J, Boeckxstaens G, Johnston BT, Ager N, De Caestecker J. British Society of Gastroenterology guidelines for oesophageal manometry and oesophageal reflux monitoring. Gut. 2019;68:1731-1750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 48. | Long SH, Berna MJ, Thill M, Pace A, Pradhan TK, Hoffmann KM, Serrano J, Jensen RT. Secretin-receptor and secretin-receptor-variant expression in gastrinomas: correlation with clinical and tumoral features and secretin and calcium provocative test results. J Clin Endocrinol Metab. 2007;92:4394-4402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Metz DC, Buchanan M, Purich E, Fein S. A randomized controlled crossover study comparing synthetic porcine and human secretins with biologically derived porcine secretin to diagnose Zollinger-Ellison Syndrome. Aliment Pharmacol Ther. 2001;15:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Berna MJ, Hoffmann KM, Long SH, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore). 2006;85:331-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 51. | Poitras P, Gingras MH, Rehfeld JF. Secretin stimulation test for gastrin release in Zollinger-Ellison syndrome: to do or not to do? Pancreas. 2013;42:903-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Kuiper P, Biemond I, Masclee AA, Jansen JB, Verspaget HW, Lamers CB. Diagnostic efficacy of the secretin stimulation test for the Zollinger-Ellison syndrome: an intra-individual comparison using different dosages in patients and controls. Pancreatology. 2010;10:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Shah P, Singh MH, Yang YX, Metz DC. Hypochlorhydria and achlorhydria are associated with false-positive secretin stimulation testing for Zollinger-Ellison syndrome. Pancreas. 2013;42:932-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Norton JA, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, Goebel SU, Peghini PL, Roy PK, Gibril F, Jensen RT. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 312] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 55. | Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, Scoazec JY, Salazar R, Sauvanet A, Kianmanesh R; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 374] [Article Influence: 28.8] [Reference Citation Analysis (1)] |
| 56. | Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, Kulke MH, Liu EH, Metz DC, Phan AT, Sippel RS, Strosberg JR, Yao JC; North American Neuroendocrine Tumor Society. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 437] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 57. | Howe JR, Merchant NB, Conrad C, Keutgen XM, Hallet J, Drebin JA, Minter RM, Lairmore TC, Tseng JF, Zeh HJ, Libutti SK, Singh G, Lee JE, Hope TA, Kim MK, Menda Y, Halfdanarson TR, Chan JA, Pommier RF. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49:1-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 259] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 58. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 694] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 59. | Norton JA, Fraker DL, Alexander HR, Jensen RT. Value of surgery in patients with negative imaging and sporadic Zollinger-Ellison syndrome. Ann Surg. 2012;256:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases, or survival in patients with Zollinger-Ellison syndrome? Ann Surg. 2004;239:617-25; discussion 626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | Weber HC, Venzon DJ, Lin JT, Fishbein VA, Orbuch M, Strader DB, Gibril F, Metz DC, Fraker DL, Norton JA. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 302] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 62. | Norton JA, Fraker DL, Alexander HR, Gibril F, Liewehr DJ, Venzon DJ, Jensen RT. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006;244:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | Fraker DL, Norton JA, Alexander HR, Venzon DJ, Jensen RT. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg. 1994;220:320-8; discussion 328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 129] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Sugg SL, Norton JA, Fraker DL, Metz DC, Pisegna JR, Fishbeyn V, Benya RV, Shawker TH, Doppman JL, Jensen RT. A prospective study of intraoperative methods to diagnose and resect duodenal gastrinomas. Ann Surg. 1993;218:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome. Results of a 10-year prospective study. Ann Surg. 1992;215:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 142] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 66. | Norton JA, Krampitz GW, Poultsides GA, Visser BC, Fraker DL, Alexander HR, Jensen RT. Prospective Evaluation of Results of Reoperation in Zollinger-Ellison Syndrome. Ann Surg. 2018;267:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Giovinazzo F, Butturini G, Monsellato D, Malleo G, Marchegiani G, Bassi C. Lymph nodes metastasis and recurrences justify an aggressive treatment of gastrinoma. Updates Surg. 2013;65:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Bartsch DK, Waldmann J, Fendrich V, Boninsegna L, Lopez CL, Partelli S, Falconi M. Impact of lymphadenectomy on survival after surgery for sporadic gastrinoma. Br J Surg. 2012;99:1234-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Liu P, Zhang X, Shang Y, Lu L, Cao F, Sun M, Tang Z, Vollmar B, Gong P. Lymph node ratio, but not the total number of examined lymph nodes or lymph node metastasis, is a predictor of overall survival for pancreatic neuroendocrine neoplasms after surgical resection. Oncotarget. 2017;8:89245-89255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Conrad C, Kutlu OC, Dasari A, Chan JA, Vauthey JN, Adams DB, Kim M, Fleming JB, Katz MH, Lee JE. Prognostic Value of Lymph Node Status and Extent of Lymphadenectomy in Pancreatic Neuroendocrine Tumors Confined To and Extending Beyond the Pancreas. J Gastrointest Surg. 2016;20:1966-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 71. | Curran T, Pockaj BA, Gray RJ, Halfdanarson TR, Wasif N. Importance of lymph node involvement in pancreatic neuroendocrine tumors: impact on survival and implications for surgical resection. J Gastrointest Surg. 2015;19:152-60; discussion 160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 72. | Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Possible primary lymph node gastrinoma: occurrence, natural history, and predictive factors: a prospective study. Ann Surg. 2003;237:650-7; discussion 657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 73. | Chen Y, Deshpande V, Ferrone C, Blaszkowsky LS, Parangi S, Warshaw AL, Lillemoe KD, Fernandez-Del Castillo C. Primary lymph node gastrinoma: A single institution experience. Surgery. 2017;162:1088-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Massironi S, Campana D, Partelli S, Panzuto F, Rossi RE, Faggiano A, Brighi N, Falconi M, Rinzivillo M, Delle Fave G, Colao AM, Conte D. Heterogeneity of Duodenal Neuroendocrine Tumors: An Italian Multi-center Experience. Ann Surg Oncol. 2018;25:3200-3206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 75. | Jensen RT, Fraker DL. Zollinger-Ellison syndrome. Advances in treatment of gastric hypersecretion and the gastrinoma. JAMA. 1994;271:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Metz DC, Pisegna JR, Fishbeyn VA, Benya RV, Jensen RT. Control of gastric acid hypersecretion in the management of patients with Zollinger-Ellison syndrome. World J Surg. 1993;17:468-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Hirschowitz BI, Simmons J, Mohnen J. Clinical outcome using lansoprazole in acid hypersecretors with and without Zollinger-Ellison syndrome: a 13-year prospective study. Clin Gastroenterol Hepatol. 2005;3:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | Metz DC, Comer GM, Soffer E, Forsmark CE, Cryer B, Chey W, Pisegna JR. Three-year oral pantoprazole administration is effective for patients with Zollinger-Ellison syndrome and other hypersecretory conditions. Aliment Pharmacol Ther. 2006;23:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Ip S, Bonis P, Tatsioni A, Raman G, Chew P, Kupelnick B, Fu L, DeVine D, Lau J. Comparative Effectiveness of Management Strategies For Gastroesophageal Reflux Disease [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2005 Dec. Report No.: 06-EHC003-EF. [PubMed] |
| 80. | Quatrini M, Castoldi L, Rossi G, Cesana BM, Peracchi M, Bardella MT. A follow-up study of patients with Zollinger-Ellison syndrome in the period 1966-2002: effects of surgical and medical treatments on long-term survival. J Clin Gastroenterol. 2005;39:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Maton PN, Vinayek R, Frucht H, McArthur KA, Miller LS, Saeed ZA, Gardner JD, Jensen RT. Long-term efficacy and safety of omeprazole in patients with Zollinger-Ellison syndrome: a prospective study. Gastroenterology. 1989;97:827-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Poitras P, Gingras MH, Rehfeld JF. The Zollinger-Ellison syndrome: dangers and consequences of interrupting antisecretory treatment. Clin Gastroenterol Hepatol. 2012;10:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Metz DC, Pisegna JR, Fishbeyn VA, Benya RV, Feigenbaum KM, Koviack PD, Jensen RT. Currently used doses of omeprazole in Zollinger-Ellison syndrome are too high. Gastroenterology. 1992;103:1498-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Norton JA, Foster DS, Ito T, Jensen RT. Gastrinomas: Medical or Surgical Treatment. Endocrinol Metab Clin North Am. 2018;47:577-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 85. | Dockray GJ, Moore A, Varro A, Pritchard DM. Gastrin receptor pharmacology. Curr Gastroenterol Rep. 2012;14:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Ellison EC, Johnson JA. The Zollinger-Ellison syndrome: a comprehensive review of historical, scientific, and clinical considerations. Curr Probl Surg. 2009;46:13-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Tomassetti P, Migliori M, Gullo L. Slow-release lanreotide treatment in endocrine gastrointestinal tumors. Am J Gastroenterol. 1998;93:1468-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 88. | Eriksson B, Renstrup J, Imam H, Oberg K. High-dose treatment with lanreotide of patients with advanced neuroendocrine gastrointestinal tumors: clinical and biological effects. Ann Oncol. 1997;8:1041-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 89. | Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R; PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 1739] [Article Influence: 108.7] [Reference Citation Analysis (0)] |
| 90. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1288] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 91. | Saijo F, Naito H, Funayama Y, Fukushima K, Shibata C, Hashimoto A, Kitayama T, Nagao M, Matsuno S, Sasaki I. Octreotide in control of multiple liver metastases from gastrinoma. J Gastroenterol. 2003;38:905-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 92. | Granberg D, Jacobsson H, Oberg K, Gustavsson J, Lehtihet M. Regression of a large malignant gastrinoma on treatment with Sandostatin LAR: a case report. Digestion. 2008;77:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Yamaguchi M, Yamada Y, Hosokawa Y, Iwamoto R, Tamba S, Ihara A, Yamamoto K, Hoshida Y, Matsuzawa Y. Long-term suppressive effect of octreotide on progression of metastatic gastrinoma with multiple endocrine neoplasia type 1: seven-year follow up. Intern Med. 2010;49:1557-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 94. | Ruszniewski P, Laucournet H, Elouaer-Blanc L, Mignon M, Bonfils S. Long-acting somatostatin (SMS 201-995) in the management of Zollinger-Ellison syndrome: evidence for sustained efficacy. Pancreas. 1988;3:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1826] [Article Influence: 130.4] [Reference Citation Analysis (0)] |
| 96. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2113] [Article Influence: 150.9] [Reference Citation Analysis (0)] |
| 97. | Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1980;303:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 397] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 98. | Prakash L, Bhosale P, Cloyd J, Kim M, Parker N, Yao J, Dasari A, Halperin D, Aloia T, Lee JE, Vauthey JN, Fleming JB, Katz MH. Role of Fluorouracil, Doxorubicin, and Streptozocin Therapy in the Preoperative Treatment of Localized Pancreatic Neuroendocrine Tumors. J Gastrointest Surg. 2017;21:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 99. | Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, Yao JC. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762-4771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 409] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 100. | Cives M, Ghayouri M, Morse B, Brelsford M, Black M, Rizzo A, Meeker A, Strosberg J. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016;23:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 101. | Kunz PL, Catalano PJ, Nimeiri H, Fisher GA, Longacre TA, Suarez CJ, Yao JC, Kulke MH, Hendifar AE, Shanks JC, Shah MH, Zalupski M, Schmulbach EL, Reidy DL, Strosberg JR, O’Dwyer PJ, Benson AB. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). J Clin Oncol. 2018;36:4004-4004. [RCA] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 102. | Bongiovanni A, Liverani C, Foca F, Fausti V, Di Menna G, Mercatali L, De Vita A, Riva N, Calpona S, Miserocchi G, Spadazzi C, Cocchi C, Ibrahim T. Temozolomide Alone or Combined with Capecitabine for the Treatment of Metastatic Neuroendocrine Neoplasia: a "Real World" data analysis. Neuroendocrinology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 103. | Ito T, Igarashi H, Jensen RT. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): recent insights and advances. J Gastroenterol. 2012;47:941-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 104. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2228] [Article Influence: 278.5] [Reference Citation Analysis (0)] |
| 105. | Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, Feelders RA, van Aken MO, Krenning EP. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1076] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 106. | Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, van Eijck CH, Esser JP, Kam BL, Krenning EP. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23:2754-2762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 477] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 107. | Grozinsky-Glasberg S, Barak D, Fraenkel M, Walter MA, Müeller-Brand J, Eckstein J, Applebaum L, Shimon I, Gross DJ. Peptide receptor radioligand therapy is an effective treatment for the long-term stabilization of malignant gastrinomas. Cancer. 2011;117:1377-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 108. | Dumont RA, Seiler D, Marincek N, Brunner P, Radojewski P, Rochlitz C, Müller-Brand J, Maecke HR, Briel M, Walter MA. Survival after somatostatin based radiopeptide therapy with (90)Y-DOTATOC vs. (90)Y-DOTATOC plus (177)Lu-DOTATOC in metastasized gastrinoma. Am J Nucl Med Mol Imaging. 2015;5:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |