Published online Sep 14, 2021. doi: 10.3748/wjg.v27.i34.5775
Peer-review started: February 26, 2021
First decision: May 1, 2021
Revised: May 26, 2021
Accepted: August 18, 2021
Article in press: August 18, 2021
Published online: September 14, 2021
Processing time: 194 Days and 10.9 Hours
A progressive reduction in the secretion of pancreatic enzymes in patients with chronic pancreatitis (CP) results in malabsorption and ultimate malnutrition. However, the pathogenesis of malnutrition is multifactorial and other factors such as chronic inflammation, alcohol excess and poor dietary intake all contribute. Patients may restrict their dietary intake due to poor appetite or to avoid gastrointestinal symptoms and abdominal pain. Whilst up to half of patients with chronic pancreatitis are reportedly malnourished, the dietary intake of patients with CP is relatively understudied and has not been systematically reviewed to date.
To perform a systematic review and meta-analysis of the dietary intakes of patients with CP compared to healthy controls, and to compare the dietary intake of patients with alcohol-related CP and non-alcohol-related CP.
A systematic literature search was performed using EMBASE, MEDLINE, and Cochrane review on studies published between 1946 and August 30th, 2019. Adult subjects with a diagnosis of CP who had undergone dietary assessment were included in the systematic review (qualitative analysis). Studies on patients with other pancreatic diseases or who had undergone pancreatic surgery were not included. Studies comparing the dietary intake of patients with CP to that of healthy controls were included in the meta-analysis (quantitative analysis). Meta-analysis was performed using Review Manager 5.3. Newcastle Ottawa Scale (NOS) was used to assess quality of studies.
Of 6715 studies retrieved in the search, 23 were eligible for qualitative analysis while 12 were eligible for quantitative analysis. In the meta-analysis, the total energy (calorie) intake of patients with CP was similar to that of healthy controls [mean difference (MD): 171.3; 95% confidence interval (CI): -226.01, 568.5; P = 0.4], however patients with CP consumed significantly fewer non-alcohol calories than controls [MD: -694.1; 95%CI: -1256.1, (-132.1); P = 0.02]. CP patients consumed more protein, but carbohydrate and fat intakes did not differ significantly. Those with alcohol-related CP consumed more mean (standard deviation) calories than CP patients with a non-alcohol aetiology [2642 (1090) kcal and 1372 (394) kcal, respectively, P = 0.046], as well as more protein, fat, but not carbohydrate.
Although patients with CP had similar calorie intake to controls, studies that analysed the contribution of alcohol to energy intake showed that patients with CP consumed fewer non-alcohol calories than healthy controls. A high calorie intake, made up to a large degree by alcohol, may in part contribute to poor nutritional status in CP.
Core Tip: Patients with chronic pancreatitis appear to have a similar dietary intake to heathy controls, however, this diet may be made up of alcohol-derived calories to a considerable degree. This may account for the poor nutritional status frequently observed in this population.
- Citation: Ul Ain Q, Bashir Y, Kelleher L, Bourne DM, Egan SM, McMahon J, Keaskin L, Griffin OM, Conlon KC, Duggan SN. Dietary intake in patients with chronic pancreatitis: A systematic review and meta-analysis. World J Gastroenterol 2021; 27(34): 5775-5792
- URL: https://www.wjgnet.com/1007-9327/full/v27/i34/5775.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i34.5775
Chronic pancreatitis (CP) is a progressive, inflammatory disease of the pancreas, causing progressive fibrosis and eventual destruction of the gland[1]. Along with a gradual loss of pancreatic endocrine and exocrine function, a chronic hyper-inflammatory state increases the risk of undernutrition[2,3]. Almost 50% of CP patients are known to be undernourished[4], the reasons for which are multifactorial.
Maldigestion and malabsorption commonly co-exist with suboptimal dietary intake. Patients may self-restrict to ease symptoms of malabsorption, nausea, early satiety, and abdominal pain. Misleading dietary instructions or selective over-restriction of diet, for instance a fat-free diet to manage steatorrhea, may further cause suboptimal intake[5,6]. For some, chronic excess alcohol consumption may displace food resulting in reduced intake of dietary nutrients[7], while heavy smoking may further reduce appetite[8]. Further complications, including type 3c diabetes and chronic pain, and maldigestive symptoms including nausea, steatorrhoea, abdominal bloating and or discomfort may worsen already poor dietary intake in the latter stages of the disease[9].
In patients with CP, the prevalence of nutrient deficiencies and their consequences have been widely reported[10-14] however little is known regarding the dietary intake of patients with CP. One study on dietary intake in CP reported that 52% of the patients lost weight following diagnosis of CP, with those who lost weight consuming significantly fewer calories than those who did not[15]. Conversely in another study, patients with calcifying CP consumed higher amount of fats and proteins[16]. Therefore, despite being a digestive disease, no study has systematically reviewed dietary intake in CP.
To address this gap, we undertook a systematic review of published observational studies reporting dietary intake in patients with CP. Specifically, the primary objective was to compare energy (calorie) and macronutrient intake in CP patients with that of healthy controls. The secondary objectives were to determine if dietary intake in CP differed according to aetiology, and to determine and compare the micronutrient intakes of patients with CP with that of controls.
This study adhered to the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines[17]. No ethical approval was required due to the nature of the study.
Under the guidance of medical librarian (J.M), Ovid Medline (1946 to August 30th, 2019), Embase (1980 to September 30th, 2019) and Cochrane Review were searched. The search strategy was developed for MEDLINE and translated for use in EMBASE. Finally, we searched the Cochrane central register of control trials to assess for any available trials on our search. We searched for articles with a combination of exploded versions of Medical Subject Headings (MeSH) and keywords as follows: (“Pancreatitis, Chronic”, chronic pancreatitis) AND “diet”, “dietary intake”, “food intake”, “food quality”, “nutrient intake”, “nutritional assessment”, “nutrition intake”, “nutrition survey”, “nutritional survey”, ”nutritional surveys”, ”food”, “diet history”, “dietary history”, “food frequency questionnaire”, “food diary”, “food diaries”, “calorie intake”, energy intake”, “carbohydrates”, “dietary protein”, ”dietary fats”, “vitamins”, “minerals”, “macronutrient intake”, “micronutrient intake”, “eating”, “diet pattern”, “dietary pattern”, “diet records”, “dietary records”, “diet quality”, ”dietary quality”, “diet assessment”, “dietary assessment”.
Boolean operator “OR” was used to assess between each Mesh term and “AND” was used to combine the searches for chronic pancreatitis and dietary intake. Additionally, bibliographies of included studies, and abstracts of pertinent scientific conferences were also manually reviewed for other relevant articles. The search was not limited by language.
To be included, studies had to have quantified the dietary intake of patients with chronic pancreatitis. The PICO[18] model was used to determine the inclusion criteria as follows: patient population (adults of both sexes with a confirmed diagnosis of CP) with the data of interest (studies that measured dietary intake by any recognised means, including food-frequency questionnaires (FFQs), diet histories, food diaries, electronic (‘app’) measurement, or any other validated technique. Data on controls (healthy or non-pancreatitis) were recorded, if available. Publications had to be available in full text. Studies on patients with active acute pancreatitis, pancreatic carcinoma, cystic fibrosis, and total pancreatectomy were excluded. Data from children under the age of 18, those on enteral/parenteral nutrition, elemental diet or additional supplementation via parenteral routes were excluded. Duplicates, case-reports, reviews, editorial/comments, abstracts only, and animal studies were also excluded. Intervention studies where the diet was modified before assessment were excluded, unless baseline unmodified dietary assessment was available. However, data from patients that may have received dietary advice as part of routine clinical care were not excluded.
Two reviewers (QS and YB) independently reviewed abstracts and titles of the citations to determine suitability for final inclusion. Disagreement was resolved with discussion or if required, consultation with the third reviewer (SND). Once potentially relevant studies were identified, full publications were sought, and the selection criteria were applied.
Quality and risk of bias was assessed using the Newcastle-Ottawa scale (NOS)[19]. A total score of 9 stars can be awarded to each study based on: Up to four stars for the patient selection criteria, two stars for comparability of participants on the basis of design or analysis, and three stars for ascertainment of exposure. The study was deemed to be low quality (0-3 stars), moderate quality (4-6 stars), or high quality (7-9 stars) according to this scoring system.
For each eligible study, the following data were extracted: study details, patient characteristics (age, ethnicity, gender, smoking status, alcohol consumption, BMI), clinical details (disease status, diagnostic criteria, complications, duration of disease, controls if included), dietary intake (energy intake (calories), macronutrient intake (carbohydrate, fat, protein), vitamin intake (vitamins A, B, C, D, E and K), and mineral intake (iron and calcium). We attempted to contact study authors to clarify data where required.
Data were analysed using Review Manager (RevMan, version 5.3.5, The Copenhagen Nordic Cochrane Center, The Cochrane Collaboration, 2014). Data were presented as mean (g/d for macronutrients and kcal/d for caloric intake) and corresponding standard deviation (SD). Data were pooled using a random-effects model to give the most conservative estimates. Statistical heterogeneity was calculated as I2 (presented as 0%-100%). Values < 40% were considered as relatively unimportant, 40%-60% moderate heterogeneity and > 60% indicated substantial heterogeneity. P values < 0.05 were considered statistically significant. Publication bias was assessed using Egger’s test. Subgroup analysis for statistical associations were done using SPSS (version 20, IBM, United States).
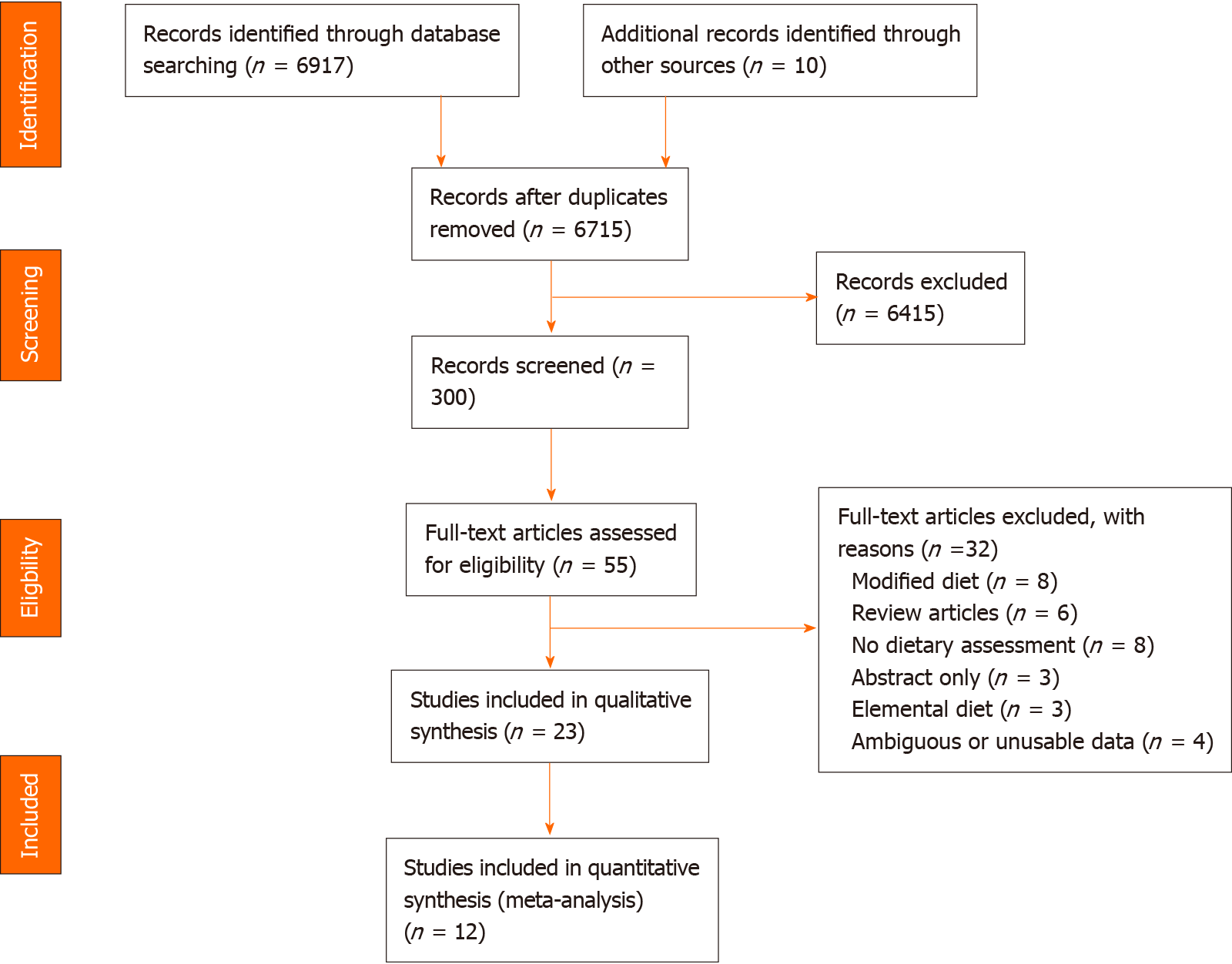
The PRISMA flow diagram is shown in Figure 1. Table 1 summarises the 23 articles retrieved in the search, representing 1,577 patients with confirmed CP (based on clinical symptoms and/or radiology). Of these, 12 studies comparing dietary intake of CP patients to that of controls were eligible for meta-analysis, representing 1048 patients with CP and 1,965 control subjects. Most case-controlled studies[6,7,15,20-29] recruited healthy controls, while five studies compared CP patients to patients with alcoholic cirrhosis[6,7,23,25,30], and one study[31] compared patients with pancreatic steatorrhea to those with intestinal steatorrhea. Nine studies originated from Asia (predominantly India), seven from Europe (predominantly France), five from the United States, two from South/Central America, and one each from Africa and Australasia. Most studies were done in the out-patient setting (22 studies)[6,7,15,20-30,32-34] while two studies[31,35] assessed hospitalised patients, and one study[36] assessed dietary intake by means of an international survey. All but three studies[26,29,36] were single centre.
| Ref. | Setting (subgroup) | Groups (n) | GenderM/F | Age (mean ± SD, yr) | BMI (mean ± SD, kg/m2) | Dietary assessment method (by whom) | NOS | ||||
| CP, n (type) | Control, n (type) | CP | Control | CP | Control | CP | Control | ||||
| Durbec et al[40] | Clinic (Marseille) | 193 (142 alcohol-related) | 490 (no PC, diabetes, liver disease) | 193/0 | 490/0 | 44.5 ± 10.5 | 46.6 ± 13.3 | 20.2 | 24.2 | Standardised questionnaire[39](dietitian) | 6 |
| Clinic (9 countries) | 247 | 521 (healthy) | 247/0 | 521/0 | 44.8 ± 13.4 | 46.7 ± 9.9 | 20.5 | 24.1 | As above (dietitian) | 6 | |
| Pitchumoni et al[30] | Clinic | 36 (36 alcohol-related) | 26 (cirrhosis) | NR | NR | NR | NR | NR | NR | NR (“detailed survey”, method not specified) (nutritionist) | 4 |
| Uscanga et al[35] | Hospital | 23 (23 alcohol-related) | 20 (healthy) | 21/2 | - | 41.2 ± 12.2 | - | - | - | NR (nutritionist) | 6 |
| Guarnier et al[27] | Clinic | 14 (8 alcohol-related) | - | 14/0 | - | 50 ± 11 | - | 21.8 (X) | - | Diet history and FFQ (dietitian) | 5 |
| Mezey et al[7] | Hospital | 42 (42 alcohol related) | 28 (healthy) | 25/17 | 19/9 | 40.7 ± 1.6 | 39.5 ± 1.8 | NR | NR | NR (dietitian) | 6 |
| Balakrishnan et al[36] | Clinic(India) | 28 | 23 (free of diabetes and digestive disease) | - | - | 32.4 | 24.7 | 16.2 | 18.4 | Questionnaire[40] (dietitian) | 7 |
| Balakrishnan et al[36] | Clinic(France) | 25 | 17 (free from diabetes and digestive disease) | - | - | 45.7 | 39.9 | 21.6 | 21.7 | FFQ (dietitian) | 7 |
| Dani et al[29] | Clinic(Belo Horizonte) | 28 (28 alcohol-related) | 28 (healthy) | - | - | - | - | - | - | NR (NR) | 5 |
| Dani et al[29] | Clinic(Sao Paulo) | 40 (40 alcohol-related) | 40 (healthy) | - | - | - | - | - | - | NR (NR) | 5 |
| Noel-Jorand et al[20] | Clinic | 31 (31 alcohol-related) | 34 (non-alcoholic, ‘normal’ controls) | 25/6 | 28/6 | 39.6 ± 6.0 | 44.8 ± 9.0 | 22 ± 2.7 | 22.9 ± 2.8 | Standardised questionnaire[41] (NR, ‘highly trained person’) | 6 |
| Laugier et al[26] | Clinic(African) | 34 (34 alcohol-related) | 40 (healthy) | 33/1 | 39/1 | 39.1 ± 1.3 | 35.2 ± 1.7 | NR | NR | Questionnaire[40] (NR) (dietitian) | 5 |
| Laugier et al[26] | Clinic (French) | 87 | 430 (healthy) | - | 41/6 | 40.6 ± 1.3 | 46.6 ± 0.8 | NR | NR | As above (NR) | 5 |
| Nakamura et al[6] | Clinic | 38 (29 alcohol-related; 8 idiopathic) | 35 (healthy) | 36/2 | - | 52.3 ± 11.3 | 51.6 ± 11.1 | 20.1 ± 2.1 | 23.6 ± 3.2 | 3-7-d food diary (dietitian) | 5 |
| Lévy et al[23] | Hospital or attending clinic | 56 (56 alcohol-related) | 50 (healthy) | n/a | - | 44.8 ± 8.4 | 43.1 ± 8.9 | NR | NR | NR “Detailed survey” (NR) | 5 |
| Nakamura et al[31] | Hospital | 15 (15 pancreatic steatorrhoea) | 7 (7 intestinal steatorrhoea) | 15/0 | 5/2 | 54.3 ± 7.5 | 52.4 ± 22.2 | 18.4 ± 2.6 | 19.4 ± 1.0 | 3-7 d food diary (NR) | 4 |
| Vaona et al[21] | Clinic | 40 | 75 (healthy) | 40/0 | 75/0 | 50 ± 10.0 | 50 ± 10.0 | 21.9 ± 3.1 | 26.1 ± 3.1 | 7-d food diary and 24-h recall (dietitian) | 6 |
| Singh et al[33]1 | Clinic | 76 (60% tropical PC; 40% alcohol-related) | - | 63/13 | - | 32 (NR) | - | NR | - | FFQFor n = 20 subgroup, 24-h dietary recall (dietitian) | 5 |
| Hamberg et al[38] | Clinic | 6 (6 alcohol-related) | - | 4/2 | - | 50.3 ± 8.6 | - | NR | - | 3-d food diary and diet history (dietitian) | 5 |
| Midha et al[22] | Clinic | 120 (idiopathic CP) | 120 (healthy subjects attending gastroenterology OPD) | 74/46 | NR (sex-matched) | 29.6 ± 10.5 | NR (age-matched) | 19.4 ± 3.4 | 21.3 ± 3.7 | 24 dietary recall and FFQ (dietitian) | 5 |
| Singh et al[39] | Clinic (Group 1 in RCT) | 29 | - | 24/5 | - | 32 ± 10.0 | - | 17.2 ± 1.2 | - | FFQ and 24-h recall (dietitian) | 5 |
| Singh et al[39] | Clinic (Group 2 in RCT) | 31 | - | 26/5 | - | 28 ± 10.0 | - | 16.7 ± 1.6 | - | ||
| Tinju et al[37]1 | Clinic | 94 (23% alcohol-related, 44% tropical, 28% idiopathic) | - | 61/31 | n/a | 40.2 ± 131 | - | NR | NR | FFQ and 24-h recall (dietitian) | 5 |
| Sathiaraj et al[15] | Clinic | 89 (89 tropical PC diagnosed within 1 yr) | 101 | 60/29 | - | 32.1 ± 14 | NR | NR | NR | FFQ (dietitian) | 6 |
| Quilliot et al[28] | Clinic | 80 (80 alcohol-related) | 20 (healthy) | 75/5 | NR | 57.6 ± 9.1 | 44 ± 11 | 25.7 ± 4.5 | 25 ± 2 | 7-d dietary recall and FFQ (NR) | 5 |
| Sobral-Oliveira et al[25] | Clinic | 20 (20 alcohol-related, current non-drinkers) | 16 (healthy never drinkers) | 20/0 | 16/0 | 54.1 ± 11.4 | 54.1 ± 18.1 | 23.7 ± 4.6 | 26 ± 3.7 | 7-d diet history (dietitian) | 6 |
| Turner et al[32] | Clinic | 83 | - | 61/22 | - | 43.4 (NR) | - | 23.8 (NR) | - | 24-h dietary recall (NR) | 6 |
| Roberts et al[24] | Clinic | 52 (40% alcohol-related, 23% idiopathic, 12 autoimmune) | 48(healthy) | 35/17 | 19/29 | 50 ± 15.0 | 54 ± 13.0 | 24 ± 6.0 | 31 ± 8.0 | FFQ (Vioscreen software) (NR) | 6 |
Regarding dietary assessment, a variety of methods were used (some studies used combined methods to estimate intake). Four studies used food diaries (between 3 and 7 d diaries), 9 studies used food frequency questionnaires (FFQ, of which one used a software technology VioScreen), six studies used a 24-h recall method, one study used a 7-d recall method, three studies employed a diet history method, four studies (all conducted in the 1970s or 1980s) referenced a particular dietary assessment technique, and attempts to obtain the original references were not successful, and five studies did not report the exact dietary assessment method employed. Twenty-two out of the 23 included studies used an a posteriori or data- driven approach to assess diet quality, while one study used a priori measure of diet quality (diet indices)[24], specifically the healthy eating index (HEI) and Mediterranean diet score (MED). Dietary assessment was performed by a dietitian (n = 15) or nutritionist (n = 2) in most cases, and 6 studies did not characterise the individual conducting the assessment. Only six of the 23 studies were published in the last 10 years (2010 or later). Seven studies had been published more than 30 years ago (earlier than 1990).
Patients with CP had a pooled average age of 43 years (range 29-57.6 years), while healthy controls had a pooled average age of 45 years (range 24.7-54 years). 19 studies reported gender distribution, 1152 (86.8%) of CP patients were male while 175 (13.2%) were female. Thirteen studies reported the BMI of CP patients (pooled mean BMI 21.5 kg/m2, range 16.2-25.7 kg/m2), while the reported mean BMI of controls was 24.8 kg/m2 (range 18.4-27.3 kg/m2). The average BMI of CP patients from studies conducted on subjects from Eastern/African countries was lower than those conducted on subjects from Western countries (US, Europe) (BMI 18.2 and 22.5 kg/m2 respectively, P = 0.042). When comparing control subjects from studies from Eastern and Western countries the BMIs did not differ (20.5 and 25 kg/m2 respectively, P = 0.066).
According to the NOS for quality assessment, one study[36] was deemed to be “high-quality” while no studies were deemed to be “low-quality”, with the remainder being medium-quality.
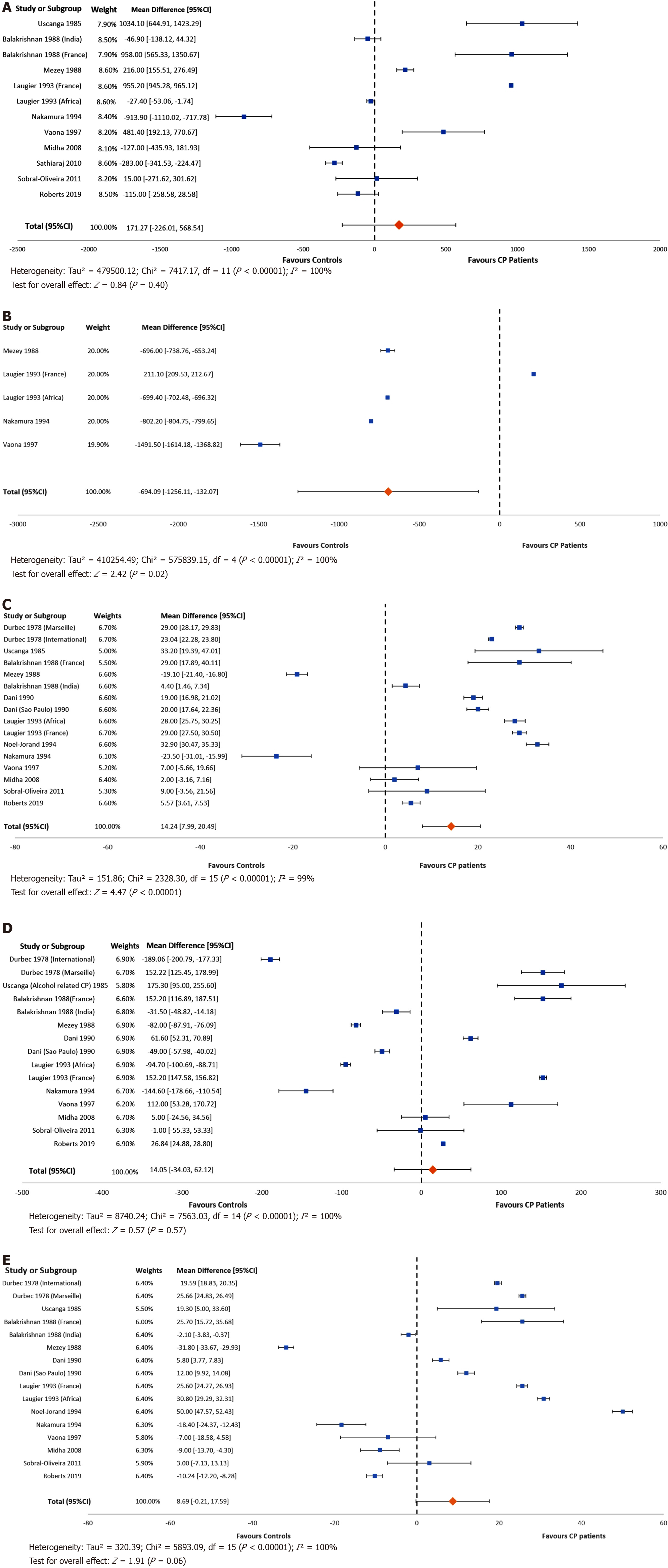
Meta-analysis was used to combine study results. Ten studies reported energy (calorie) intakes for both patients with CP and healthy controls, and two studies reported data for geographic groups separately, which were reported independently in the forest plot. There was no difference between the energy intakes of patients with CP and healthy controls (MD: 171.3; 95%CI: -226.01, 568.5; P = 0.4) (Figure 2A), however when analysing data from four studies reporting the intake of non-alcohol calories (five separate study groups), patients with CP consumed significantly fewer non-alcohol calories than healthy controls [MD: -694.1; 95%CI: -1256.1, (-132.1); P = 0.02] (Figure 2B). From 13 studies (16 separate study groups), patients with CP had a higher intake of protein than healthy controls (MD: 14.2; 95%CI: 7.99, 20.49; P < 0.001, Figure 2C). From 11 studies (15 separate study groups), the consumption of carbohydrate (MD: 14.05; 95%CI: -34.03, 62.12; P = 0.57, Figure 2D) did not differ between groups. Similarly, from 12 studies (16 separate study groups) the intake of dietary fat (MD: 8.69; 95%CI: -0.21, 17.59; P = 0.06, Figure 2E) did not differ between the groups. Heterogeneity was considerably high for all outcome measures.
Energy intake and macronutrient composition: Macro- and micronutrient intakes of patients and controls in all studies included in the systematic review are summarised in Table 2. For patients with CP, energy (calorie) intake ranged from 842 to 4178 kcal/d, while the energy intake of controls ranged from 810 to 4178 kcal/d. Where there was higher energy intake among patients with CP, the reasons varied between studies. High calorie consumption was due to higher fat and protein intake in one study[23], higher carbohydrate intake in one study[21], and due to a high intake of all macronutrients in another[35]. In one study[7], the calorie intake was high due to excessive alcohol consumption, with alcohol comprising 50% of total caloric intake. The percentage of energy intake consumed as carbohydrate ranged from 22.1% to 73%, the percentage of energy consumed as protein ranged from 9.1% to 21.3%, and the percentage of energy consumed as fat ranged from 16 to 35%. For controls, the corresponding percentages were 27.5% to 61% (carbohydrate), 10.5% to 16.8% (protein) and 19.8% to 36.6% (fat). In the majority of studies reporting lower energy intake among patients with CP, those with poor energy intake showed some form of malnutrition (defined by lower BMI/loss of weight) after onset of disease when compared to controls. In one study that compared subtypes of CP[37], patients with tropical CP had the lowest intake of energy, attributable to a low intake of dietary fat.
| Ref. | Number of subjects | Energy intake kcal/d (SD) | Carbohydrate intake g/d (SD) | Protein intake g/d (SD) | Fats intake g/d (SD) | Alcohol intake g/d (SD) | ||||||
| CP | Control | CP | Control | CP | Control | CP | Control | CP | Control | CP | Control | |
| Durbec et al[40] | 193 (Marseille) | 490 | NR | NR | 452.5 (178.1) | 300.4 (104.4) | 123.8 (56.4) | 94.8 (29.5) | 122.6 (50.4) | 96.97 (28.8) | 144 (78.7) | 43.6 (56.6) |
| 247 (International) | 521 | NR | NR | 389 (174.3) | 305 (111.8) | 119.2 (61) | 96.1 (33.9) | 115.7 (54.1) | 97.8 (32.9) | 149.9 (89.1) | 37.2 (55.1) | |
| Pitchumoni et al[30] | 36 | 26 | 10501 (523) | 8101 (330) | NR | NR | 67 (22) | 56 (23) | 59 (33) | 40 (22) | > 200 | > 200 |
| Uscanga et al[35] | 23 | 20 | 3367 (748.6) | 2332 (548.9) | 532.6 (157.7) | 357.3 (109.3) | 117.6 (26.2) | 84.4 (19.9) | 83.7 (29.4) | 64.4 (17.7) | 203.9 (100.2) | NR |
| Guarnier et al[27] | 14 | UC | 1893 (296) | UC | 258.5 (44.3) | UC | 66.5 (10.3) | UC | 66.5 (14.8) | UC | NR | UC |
| Mezey et al[7]2 | 42 | 28 | 2,514 (142) | 2298 (115) | 139.2 (10.0) | 221 (13.7) | 57.1 (4.8) | 76.2 (4.8) | 53.8 (4.2) | 85.6 (3.7) | 177.8 (14.5) | 47.7 (11.6) |
| Balakrishnan et al[36]2 | 28 (India) | 23 | 1966 (154.8) | 2012 (173.6) | 354 (22.4) | 385.7 (37.2) | 55.7 (5.8) | 51.3 (4.9) | 23.4 (2.8) | 25.5 (3.4) | NR | NR |
| 105 (France) | 476 | 3411 (NR) | 2453 (NR) | 452.5 (178) | 300.3 (104.4) | 123.8 (56.4) | 94.8 (29.5) | 122.6 (50.4) | 96.9 (28.8) | NR | NR | |
| Dani et al[29] | 28 (Belo Horizonte) | 28 | NR | NR | 282(21.9) | 220(12.2) | 64(3.9) | 45(3.8) | 40(4.3) | 34(3.4) | 396.6(286.0) | NR |
| 40 (Sao Paulo) | 40 | NR | NR | 270 (21.6) | 319 (19.3) | 100 (5.9) | 80 (4.8) | 97 (4.8) | 85 (4.7) | 295.3 (171.3) | NR | |
| Noel-Jorand et al[20] | 31 | 34 | NR | 1919 (NR) | NR | 133 (NR) | 119 (NR) | 86 (NR) | 120 (NR) | 70 (NR) | 232 (NR) | < 20 (NR) |
| Laugier et al[26]2 | 34 (Africa) | 40 | 2339 (56.8) | 2366 (55.3) | 301 (15.2) | 395 (10.1) | 145 (5.9) | 117 (3.4) | 66 (3.8) | 35 (2.6) | 130 (12.7) | 34 (5.5) |
| 87 (France) | 430 | 3409 (45.4) | 2454 (55.3) | 452 (21.8) | 300 (6.5) | 124 (7.1) | 95 (1.8) | 123 (6.3) | 97 (1.7) | 150 (11.4) | 43 (3.8) | |
| Nakamura et al[6] | 38 | 35 | 1650 (366) | 2564 (477) | 237 (63.9) | 382 (82.5) | 73 (15) | 96 (17.5) | 38 (12.3) | 56 (13.6) | 58 (130.6) | 78 (110.4) |
| Lévy et al[23] | 56 | 50 | 4178 (1906-13345) | 4178 (1433-6418) | 295 (108.8-541) | 290 (797-772) | 117 (34.6-162.7) | 109 (22.6-181.6) | 171 (59.7-447.7) | 145 (42.2-332.7) | 17.53 (0.9-64.5) | 17.83 (2.3-50.4) |
| Nakamura et al[31] | 15 | 7 | 1674 (188.2) | 1985 (226.8) | NR | NR | NR | NR | 45(8) | 54(7.9) | NR | NR |
| Vaona et al[21] | 40 | 75 | 25844 (850.2) | 21034 (528.5) | 362 (168) | 250 (120) | 95 (33) | 88 (33) | 76 (25) | 83 (38) | 37 (44) | 32 (26) |
| Singh et al[33] | 20 | UC | 1760 (375) | UC | 274 (55) | UC | 48 (13) | UC | 53 (23) | UC | NR | UC |
| Hamberg et al[38]2 | 7 | UC | NR | UC | NR | UC | 36.8 (8.46) | UC | NR | UC | NR | UC |
| Midha et al[22] | 120 | 120 | 1650 (699) | 1663 (499) | 262 (125) | 257 (108) | 56 (24) | 54 (16) | 38 (20) | 47 (17) | NR | NR |
| Singh et al[39] | 29 (Group 1)5 | UC | 2188 (672) | UC | 346 (140) | UC | 73 (24) | UC | 52 (24) | UC | NR | UC |
| 31 (Group 2)5 | UC | 2053 (689) | UC | 324 (126) | UC | 69 (22) | UC | 48 (18) | UC | NR | UC | |
| Tinju et al[37]6 | 100 | UC | 1398 (515.9) | UC | 231 (98.8) | UC | 47 (18.8) | UC | 37 (18.7) | UC | NR | UC |
| Sathiaraj et al[15] | 89 | 101 | 1093 (257) | NR | 195 (56) | UC | 31 (10) | NR | 19 (7) | NR | NR | NR |
| Quilliot et al[28] | 80 | 20 | 2512 (644) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sobral-Oliveira et al[25] | 20 | 16 | 1637 (546) | 1622 (542) | 224 (87) | 225 (79) | 71 (24) | 70 (21) | 51 (17) | 49 (23) | 253 (229) | NR |
| Turner et al[32] | 83 | 54 | 842 (NR) | 1143 (NR) | 89 (NR) | 116 (NR) | 46 (NR) | 60 (NR) | 25 (NR) | 48 (NR) | 80 (NR) | 30 (NR) |
| Roberts et al[24] | 52 | 48 | 1549 (NR) | 1664 (NR) | 208 (NR) | 181 (NR) | 66 (NR) | 60 (NR) | 52 (NR) | 62 (NR) | NR | NR |
Protein intake: Twenty studies[6,7,15,20-27,29,30,32,33,35-40] determined the protein intake of patients with CP, 15 of which were controlled[6,7,20-26,29,30,32,35-37,40]. Of those, 13 studies[6,7,20-26,29,35-37,40] compared the protein intake of CP patients to that of healthy controls, while one study compared to patients with alcoholic cirrhosis[30] and another to patients with acute pancreatitis[32]. The protein intake of patients with CP ranged from 35 to 145 g/d, compared to 45 to 117g/d among healthy controls. Five studies[20,26,29,35,40] reported that patients with CP consumed a higher intake of protein than healthy controls. In one of these studies[40], the relative risk of developing CP increased as a function of the quantity of protein (and alcohol) consumed. Another study on African patients[26] reported that high protein intake was mostly derived from high bean consumption. Eight studies[6,7,15,27,32,33,36,37] reported the opposite: that patients with CP consumed less protein than controls, with protein intake being 20% lower than the recommended daily allowance in two studies[33,37]. Five of these eight studies were conducted in Eastern countries, specifically India and Japan. One study[36] compared protein intake in patients with India with that of patients in France, finding that intake was lower in the former. Three studies considered the source of dietary protein[6,20,23]. Of these, two studies[6,23] reported that patients with CP consumed more animal-based protein than controls.
Carbohydrate intake: Eighteen studies[6,7,15,21-27,29,32,33,35-37,39,40] analysed the carbohydrate intake of patients with CP, of which 13 studies[6,7,21-26,29,32,35,36,40] were controlled. In the 13 controlled studies, there were 17 separate groups studied. Among controlled studies, carbohydrate intake ranged from 89 to 533 g for patients with CP, and 116 to 386 g for controls. Of the controlled studies, three[21,26,35] reported that patients with CP had a higher intake of carbohydrates than controls (in one of those studies[26], only one of the study arms reported that patients with CP consumed significantly more carbohydrate than controls). Four studies reported the opposite[6,7,26,32], stating that patients with CP consumed lower amounts of carbohydrates than controls (in one of those studies[26], only the African group reported that patients with CP consumed significantly less carbohydrate than controls). The other controlled studies reported no significant difference in carbohydrate intake between patients with CP and controls.
Fat intake: Seven studies[18,21,23,24,33,34,38] reported a higher intake of dietary fat in patients with CP than in controls. In several studies, a higher intake of fat was associated with the development of CP[18,21,24,33,38] and earlier onset of disease[18,38]. Three studies provided details on the source of dietary fat[6,20,23]. In one study[6] conducted in Japan, CP patients had statistically lower fat intake from both animal and plant sources, but significantly higher intake of marine fat (fish oil). Two studies analysed patients’ intake of saturated fat, monounsaturated and polyunsaturated fat[20,23], reporting that patients with alcohol-associated CP consumed significantly more dietary fat than both control patients with cirrhosis[20,23] and healthy controls[20].
Alcohol intake: Noel-Jorand and colleagues[20] noted that patients with CP had reached the same threshold of alcohol consumption as patients with alcoholic liver cirrhosis prior to diagnosis of their respective diseases (204-232 g/d per subject), however those who developed CP consumed alcohol over a shorter time-frame than those who developed cirrhosis. Lévy et al[23] similarly reported that patients with CP had a lower mean duration than those with alcoholic cirrhosis. Durbec et al[40] reported that the total quantity of alcohol consumed, as well as the duration of consumption, prior to diagnosis was lower in women than in men. This was mirrored in a study from the United States in which women with CP had a lower duration of alcohol consumption than men with CP[7]. Three studies compared patients with alcohol-related CP to those with CP of other causes. Patients with alcohol-related CP consumed more calories[35,37], carbohydrate[35] and protein[29]. Table 3 compares intake between those with alcohol-related CP and CP of other causes. Those with alcohol-related CP had a higher overall energy intake than those with non-alcohol-related CP, along with a higher protein and fat intake, but not carbohydrate intake.
Geographical differences: Studies reporting a higher consumption of energy were mostly conducted in Western countries (United States, Europe, Mexico), and the majority were published over 20 years ago. Studies reporting a lower consumption of energy tended to arise from Eastern countries (mainly India and Japan), and the majority had been published more recently. The consumption of carbohydrate and protein appeared to be similar in Western and Eastern countries, while the average intake of fat was lower in eastern/African countries (mean 35.8 g/d) compared to Western countries (mean 75 g/d).
Micronutrient intake: Only four studies measured micronutrient intake (Table 4), of which two studies were controlled (both recruited healthy controls). Vitamin A was expressed in various forms and it was not clear which specific form was presented in some cases. In a controlled study from Japan[6], controls consumed more vitamin A, vitamin B1, vitamin B2, calcium and iron, but not vitamin C.
| Ref. | Vitamin A (SD), IU | Vitamin B1 (SD) mg | Vitamin B2 (SD) mg | Vitamin C (mg) | Vitamin D (mcg) | Vitamin E (IU/mg) | Vitamin K (mg) | Calcium (SD), mg | Iron (SD), mg | |
| Nakamura et al[6] | CP | 2337.9 (3018.9) | 0.9 (0.3) | 1.28 (0.4) | 98.5 (38.5) | NM | NM | NM | 501.5 (145.4) | 9.9 (2.7) |
| Control1 | 4016.3 (4902.7) | 1.21 (0.3) | 1.61 (0.5) | 85.0 (48.2) | NM | NM | NM | 624.3 (227.2) | 12.1 (2.8) | |
| Tinju et al[37] | CP | M: 3659.7 (6546.8); F: 3888.4 (3936.1) | NM | NM | M: 54.0 (70.5); F: 50.0 (68.7) | NM | NM | NM | M: 496.3 (307.9); F: 485.8 (272) | M: 11.4 (6.7); F: 7.1 (2.8) |
| Control | UC | UC | UC | UC | UC | UC | UC | UC | UC | |
| Turner et al[32]2 | CP | 278 (μg/d) | NM | NM | 25.3 | NM | NM | NM | NM | NM |
| Control | UC | UC | UC | UC | UC | UC | UC | UC | UC | |
| Roberts et al[24]3 | CP | 744.4 (RAE) | NM | NM | NM | 5.88 | 11.77 | 64 | NM | NM |
| Control2 | 651.3 (RAE) | NM | NM | NM | 4.03 | 11.54 | 99.5 | NM | NM |
This is the first systematic review to quantify the nutritional intake of patients with CP. While total calorie consumption was not different between patients with CP and healthy controls, patients with CP consumed significantly fewer non-alcohol calories than controls. Those with CP also consumed significantly more protein than healthy controls, however the intake of carbohydrate and fat did not differ. When comparing patients with alcohol-related CP to those with a non-alcohol aetiology, patients with alcohol-related CP consumed more calories, protein and fat than controls, but consumed a similar amount of carbohydrates. This review has highlighted the dearth of research on this topic in general. Only five of the studies retrieved in the search were published in the ten years preceding this paper.
The contribution of alcohol to overall energy intake is an important finding. Five studies were meta-analysed to compare non-alcohol caloric intake to that of healthy controls. In one study from the US on alcohol-related CP, as much as half of all calories consumed were derived from alcohol[7]. In another study conducted both in France and Africa, percentage calories from alcohol were as high as 31% and 39% respectively[24]. The contribution of alcohol to weight is not straightforward. Each gram of alcohol provides 7.1 (29.7 kJ) kcal whereas each gram of carbohydrate approximately provides 4 kcal/g[41], therefore, those consuming large amounts of alcohol should eventually gain excess weight. However, in a Brazilian study comparing asymptomatic alcohol-aetiology CP patients to non-pancreatitis patients with excessive alcohol consumption and healthy controls, patients with CP had lower BMI, body fat and lean body mass, despite a higher alcohol-derived caloric consumption. They also had magnesium and vitamin deficiencies, as well dyslipidaemia and increased risk of cardiovascular disease[25]. Calories derived from alcohol have no nutritive value. Where the same amount of calories was consumed by two groups of non-pancreatic healthy subjects (one receiving calories from carbohydrates and the other receiving the same calories from alcohol), those obtaining energy from alcohol did not gain weight, even when alcohol-derived calorie consumption was increased[42]. Malnutrition secondary to alcohol consumption is multi-factorial and not just due to non-nutritive calorie provision. One study reported that patients consuming high levels of alcohol (23% of total calories) tended to displace carbohydrate intake. However, where alcohol intake was higher than 30% of total calories, the intake of proteins, fat and vitamins declined considerably[43].
As well as being responsible for the development of CP and the progression of disease, and aside from the effects of nutrient displacement, a high intake of alcohol is known to directly contribute to malnutrition. Alcohol has direct toxic effects on the gastrointestinal tract and liver, leading to impaired digestion, reduced absorption of nutrients into the blood, and impaired utilisation or increased degradation of those nutrients. As well as causing malabsorption, alcohol also increases systemic inflammation increasing energy requirement, and leading to weight loss[44]. All these effects occur independently and parallel to the decreased secretion of pancreatic enzymes and chronic inflammation that occur as a consequence of CP, hence, cumulative damage occurs in CP patients with excessive alcohol consumption. Patients with CP may reduce their intake of alcohol following diagnosis[24], however most studies show that they continue to consume high quantities of alcohol[45].
Patients with CP may not be classically underweight, however they may be sarcopenic, defined as low skeletal muscle mass, low muscle strength, and low muscle function leading to impaired physical performance[46,47]. A systematic review[47] reported that sarcopenia is prevalent in 17%-64% of patients with CP, and negatively affects outcomes such as quality of life, hospitalisation, mortality and may be associated with a lower islet yield following total pancreatectomy with islet autotransplantation. Sarcopenic obesity describes the state where excess body weight and reduced muscle mass or strength occur simultaneously. In the 12 studies meta-analysed for total calorie intake, only seven reported BMI. Of those, all reported a mean BMI in the underweight or normal BMI range for patients with CP. Only two of the five studies meta-analysed for non-alcohol calorie intake reported BMI, with both reporting BMI to be in the normal range. Crucially, BMI alone is a poor indicator of individual nutritional status and body composition, with sarcopenia being present independent of BMI[47-49]. In addition, impaired measures of functional capacity (such as hand-grip strength) have been reported in patients with CP, independent of weight or BMI[12,50]. Contrary to the ‘textbook’ description of an underweight patient, those with CP are frequently overweight or obese[12]. In the present review, whilst none of the studies measured sarcopenia or sarcopenic obesity, patients with CP appeared to have an adequate or high caloric consumption. However, a high percentage of calories as alcohol might explain the nutritional deficits reported in some.
The issue of the macronutrient composition of the diet is more complex. In our meta-analysis of 12 studies (investigating 16 different study populations), we showed that patients with CP consumed significantly more dietary protein compared to healthy controls. Recently-published consensus guidelines state that patients with CP do not need to follow specific restrictions on protein, fat or carbohydrate intake, but instead should follow a “well-balanced” diet, except for those who are malnourished, where a high protein, high calorie dietary pattern is warranted[9,51]. It is not known if patients included in the meta-analysis had received dietary counselling at any time preceding their inclusion in the studies. Several studies have examined the role of protein intake in the development of CP. In a paper published in 1973, Sarles et al[16] reported the results of an international study of dietary intake in CP in 16 countries across 4 continents. The study, which did not meet the criteria for inclusion into the systematic review, reported that subjects with alcoholism who developed chronic calcific pancreatitis had higher protein and fat intakes than controls. Conversely in a subgroup from India, chronic calcific pancreatitis was found in ‘occasional alcoholics’ who were deficient in protein. In a study published in the same decade by the same author[40], two study groups were assessed, including a group from France, and a second group of Caucasian patients from 9 international centres. This study also recruited controls and was eligible for inclusion in the systematic review and meta-analysis. It found that there was a small increase in the relative risk of developing chronic pancreatitis with increased protein intake, although the study counselled against a “protein-poor” diet due to the risk of malabsorption and protein deficiency. Most studies reporting a high protein intake for patients with CP had recruited patients with an alcohol aetiology[7,21,23,24,28]. Studies on patients with non-alcohol aetiology appeared to report a lower protein intake[20].
Regarding fat intake, the study by Durbec and Sarles[40] reported that both a low-fat and high-fat diet increased the relative risk of developing chronic pancreatitis, suggesting consumption of an “average fat” diet (85-110 g fat per day). Perhaps unexpectedly, our meta-analysis showed that there was no difference in fat intake between patients with CP and healthy controls. The forest plot (Figure 2E) shows that most studies reported that patients had a higher intake than controls, and the p-value was just above the arbitrary 0.05. Patients were traditionally advised to follow a low-fat (or even fat-free) diet in an effort to reduce the symptom burden associated with pancreatic exocrine insufficiency. The most recent guidelines[9,51] recommend against restricting fat intake for all but the most severe cases of intractable malabsorption. This is in part because protein and carbohydrate are similarly malabsorbed, resulting in adverse symptoms, and simple fat restriction does not address this fact. Furthermore, achieving protein requirement is also more difficult with a restrictive diet. Treatment with optimal dosage of PERT treats malabsorption of all macronutrients and allows for a more “normal” dietary intake. The studies described above, suggesting that higher dietary intakes of protein and fat might be related to the development of CP, were published during the 1970s, before the importance of adequate treatment with PERT was appreciated. The relationship between the development of CP, the macronutrient composition of the diet, along with the interplay between nutritional status and alcohol intake are therefore not well understood.
There is a clear gap in the literature on the dietary intake of micronutrients in CP. Only four studies[6,24,32,37] have investigated this, one of which was uncontrolled[32]. Only one study measured the dietary intake of fat-soluble vitamins D, E and K[24]. However, this is a critical issue for patients with CP, who are known to have micronutrient deficiency. Again, a high intake of calories with little nutritive value is certain to exacerbate nutritional deficiencies[12,52-58].
This study was also the first to systematically review the methodology of dietary assessment techniques for this patient group, finding considerable heterogeneity, which presented a clear challenge for the interpretation of data, and thereby limits the generalisability of results. A notable number of studies did not report the methods used, while others utilised retrospective techniques and some used prospective methods such as food diaries. Each method carries its own risk of bias. For example, retrospective techniques such as FFQs and dietary recall may be affected by recall bias, as well with problems with subject memory, cognition and motivation. The reported nutrient intake in some studies appeared to be considerably high compared to contemporaneous national surveys. For example, Lévy et al[23] reported the dietary intake of newly diagnosed alcoholic CP patients from 1995 and reported an intake for both groups in excess of 4,000 kcal and 100 g protein per day. This is considerably higher than values reported by other national surveys around that time, including the French national dietary study from 1993-94 [mean energy intake in males of 2,390 kcal of which 16.6% came from protein (approximately 99g)[59]]. Similarly, the Irish North-South Food Consumption Survey 1997-99 found that men aged 18-64 years had a mean energy intake of 2,632kcal and 100g protein[60]. FFQs, used in the French study, have been shown to overestimate energy intake[59]. However, this study was not eligible for inclusion in the meta-analysis due to incompatible data.
This systematic review was limited by the heterogenous nature of the included studies. More importantly, the method of dietary assessment differed considerably between studies, which may account for the wide range of intakes reported and may have resulted in both under- and over-estimation of actual intake. However, the meta-analysis was designed to report the differences in intake between patients with CP and controls rather than absolute values (and the methodology for patients and controls was the same within each individual study), which should minimise this error. In addition, the meta-analysis compared patients with CP to healthy controls only, and excluded studies with other types of controls. Hence, despite inter-group heterogeneity, intra-group diversity was minimal. Furthermore, as heterogeneity is high in large scale studies, this may have further added to the heterogeneity observed, as most of our included studies recruited large patient numbers. Only 12 of the 23 studies retrieved were deemed eligible for meta-analysis, and fewer were suitable for some of the analyses, such as non-alcohol calories. This was in part due to the inclusion of studies that recruited healthy controls only.
Only six studies were published within the last ten years. Nonetheless, we believe that the results are applicable to present-day patients. A potential variable was the inclusion of a wide range of ethnic groups. The differences in nutrient intake reported may have been a consequence of geographic location rather than the effect of CP. However, the inclusion of healthy controls from the same geographic region as the patients with CP would have minimised this limitation. In addition, most patients with CP recruited to the various studies were male, but the same restriction wasn’t applied for controls. Therefore, the gender differences might explain higher caloric intake by CP patients in some studies. No study had compared dietary intake by gender. As with all systematic reviews, this study was inherently limited by the quality of the studies included, although only one study was deemed to be of low quality. In some cases, different units and methods of reporting were used, necessitating extrapolation or recalculation.
This systematic review and meta-analysis supports the notion that while patients consume apparently adequate energy, the quality of the dietary intake is poor, in part due to the contribution of alcohol to overall energy intake. This review has also highlighted the critical lack of recent studies on this topic. Further studies should aim to characterise the dietary intake of patients with CP, with robust study designs and adequate sample size. Longitudinal research would help to determine the effects of various dietary patterns and alcohol abuse on diet. Intervention studies of therapeutic dietary strategies as well as early dietetic consultation in patients with CP are sorely needed.
Patients with chronic pancreatitis (CP) are a high risk of developing malnutrition due to progressive exocrine insufficiency, malabsorption, and adverse gastro-intestinal symptoms. Poor dietary intake and excess alcohol consumption may exacerbate malnutrition.
Although CP is a digestive disease, little is known regarding the dietary intake of patients with this condition, and no systematic reviews or meta-analyses have been undertaken to date.
The purpose of this systematic review and meta-analysis was to compare the macronutrient and micronutrient intake of patients with CP to that of healthy subjects, and to determine if there was a difference in dietary intake according to aetiology.
With the guidance of a medical librarian, we conducted a search of multiple databases including Ovid Medline, EMBASE and Cochrane Review.
Twenty-three studies were retrieved in the search and included in the systematic review (representing 1577 patients with CP), of which 12 were eligible for meta-analysis (representing 1048 patients with CP and 1965 healthy controls). There was no statistical difference in the energy (calorie) intakes of patients with CP and controls. However, when analysing studies reporting non-alcohol calorie consumption, patients with CP consumed significantly fewer calories than health control subjects. Those with CP consumed more protein than healthy controls, but there was no difference in the consumption of carbohydrate and fat. Heterogeneity was considerably high for all outcome measures. A broad variety of methods was used in the various studies to estimate dietary intake, and most had been published more than ten years prior to this review.
This was the first meta-analysis to evaluate the dietary intake of patients with CP. Although the calorie intake of patients with CP did not differ from that of healthy controls, studies that analysed the contribution of alcohol to energy intake showed that those with CP consumed fewer non-alcohol calories than controls. This may contribute to the poor nutritional status of patients with CP.
The considerable heterogeneity made it difficult to make general conclusions, and the broad range of dietary assessment methods used means that under-or over-estimation of intake may have occurred. There were few data for micronutrient intake, a considerable research gap considering the common occurrence of biochemical deficiency in this patient group. There were few recently-published studies. Future studies should aim to characterise the dietary macronutrient and micronutrient intakes of patients with CP with robust methodology and study design.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Irish Nutrition and Diet Institute (INDI); Health and Social Care Professionals Regulator, Ireland (CORU); Pancreatic Society of Great Britain and Ireland (PSGBI); American Pancreatic Association.
Specialty type: Surgery
Country/Territory of origin: Ireland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lei C S-Editor: Ma YJ L-Editor: A P-Editor: Liu JH
| 1. | Sarles H. Etiopathogenesis and definition of chronic pancreatitis. Dig Dis Sci. 1986;31:91S-107S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Rasch S, Valantiene I, Mickevicius A, Beer S, Rosendahl J, Charnley RM, Robinson SM. Chronic pancreatitis: Do serum biomarkers provide an association with an inflammageing phenotype? Pancreatology. 2016;16:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Hébuterne X, Hastier P, Péroux JL, Zeboudj N, Delmont JP, Rampal P. Resting energy expenditure in patients with alcoholic chronic pancreatitis. Dig Dis Sci. 1996;41:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Armbrecht U. [Chronic pancreatitis: weight loss and poor physical performance - experience from a specialized rehabilitation centre]. Rehabilitation (Stuttg). 2001;40:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 5. | Goebell H, Hotz J, Hoffmeister H. Hypercaloric nutrition as aetiological factor in chronic pancreatitis. Z Gastroenterol. 1980;18:94-97. [PubMed] |
| 6. | Nakamura T, Arai Y, Terada A, Kudoh K, Imamura K, Machida K, Kikuchi H, Takebe K. Dietary analysis of Japanese patients with chronic pancreatitis in stable conditions. J Gastroenterol. 1994;29:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Mezey E, Kolman CJ, Diehl AM, Mitchell MC, Herlong HF. Alcohol and dietary intake in the development of chronic pancreatitis and liver disease in alcoholism. Am J Clin Nutr. 1988;48:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 382] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 9. | Arvanitakis M, Ockenga J, Bezmarevic M, Gianotti L, Krznarić Ž, Lobo DN, Löser C, Madl C, Meier R, Phillips M, Rasmussen HH, Van Hooft JE, Bischoff SC. ESPEN guideline on clinical nutrition in acute and chronic pancreatitis. Clin Nutr. 2020;39:612-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 10. | Duggan SN, O'Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas. 2012;41:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Duggan SN, Smyth ND, Murphy A, Macnaughton D, O'Keefe SJ, Conlon KC. High prevalence of osteoporosis in patients with chronic pancreatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 12. | Duggan SN, Smyth ND, O'Sullivan M, Feehan S, Ridgway PF, Conlon KC. The prevalence of malnutrition and fat-soluble vitamin deficiencies in chronic pancreatitis. Nutr Clin Pract. 2014;29:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Martínez-Moneo E, Stigliano S, Hedström A, Kaczka A, Malvik M, Waldthaler A, Maisonneuve P, Simon P, Capurso G. Deficiency of fat-soluble vitamins in chronic pancreatitis: A systematic review and meta-analysis. Pancreatology. 2016;16:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Hoogenboom SA, Lekkerkerker SJ, Fockens P, Boermeester MA, van Hooft JE. Systematic review and meta-analysis on the prevalence of vitamin D deficiency in patients with chronic pancreatitis. Pancreatology. 2016;16:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Sathiaraj E, Gupta S, Chutke M, Mahurkar S, Mansard MJ, Rao GV, Reddy DN. Malnutrition is not an etiological factor in the development of tropical pancreatitis--a case-control study of southern Indian patients. Trop Gastroenterol. 2010;31:169-174. [PubMed] |
| 16. | Sarles H. An international survey on nutrition and pancreatitis. Digestion. 1973;9:389-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 55] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9207] [Cited by in RCA: 8050] [Article Influence: 536.7] [Reference Citation Analysis (2)] |
| 18. | Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. 2018;106:420-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 540] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 19. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford; 2000. |
| 20. | Noel-Jorand MC, Bras J. A comparison of nutritional profiles of patients with alcohol-related pancreatitis and cirrhosis. Alcohol Alcohol. 1994;29:65-74. [PubMed] |
| 21. | Vaona B, Armellini F, Bovo P, Rigo L, Zamboni M, Brunori MP, Dall'O E, Filippini M, Talamini G, Di Francesco V, Frulloni L, Micciolo R, Cavallini G. Food intake of patients with chronic pancreatitis after onset of the disease. Am J Clin Nutr. 1997;65:851-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Midha S, Singh N, Sachdev V, Tandon RK, Joshi YK, Garg PK. Cause and effect relationship of malnutrition with idiopathic chronic pancreatitis: prospective case-control study. J Gastroenterol Hepatol. 2008;23:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Lévy P, Mathurin P, Roqueplo A, Rueff B, Bernades P. A multidimensional case-control study of dietary, alcohol, and tobacco habits in alcoholic men with chronic pancreatitis. Pancreas. 1995;10:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Roberts KM, Golian P, Nahikian-Nelms M, Hinton A, Madril P, Basch K, Conwell D, Hart PA. Does the Healthy Eating Index and Mediterranean Diet Score Identify the Nutritional Adequacy of Dietary Patterns in Chronic Pancreatitis? Dig Dis Sci. 2019;64:2318-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Sobral-Oliveira MB, Faintuch J, Guarita DR, Oliveira CP, Carrilho FJ. Nutritional profile of asymptomatic alcoholic patients. Arq Gastroenterol. 2011;48:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Laugier R, Bernard JP, Laroche R, Kadende P, N'Dabaneze E, Saunière JF, Dupuy P. Exocrine pancreatic secretion in normal controls and chronic calcifying pancreatitis patients from Burundi: possible dietary influences. Digestion. 1993;54:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Guarnieri G, Toigo G, Situlin R, Crapesi L, Del Bianco MA, Zanettovich A, Mandero E, Resetta G. Muscle biopsy studies on protein-energy malnutrition in patients with chronic relapsing pancreatitis. Infusionsther Klin Ernahr. 1986;13:166, 168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Quilliot D, Forbes A, Dubois F, Gueant JL, Ziegler O. Carotenoid deficiency in chronic pancreatitis: the effect of an increase in tomato consumption. Eur J Clin Nutr. 2011;65:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Dani R, Mott CB, Guarita DR, Nogueira CE. Epidemiology and etiology of chronic pancreatitis in Brazil: a tale of two cities. Pancreas. 1990;5:474-478. [PubMed] |
| 30. | Pitchumoni CS, Sonnenshein M, Candido FM, Panchacharam P, Cooperman JM. Nutrition in the pathogenesis of alcoholic pancreatitis. Am J Clin Nutr. 1980;33:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Nakamura T, Takebe K, Tando Y, Arai Y, Yamada N, Ishii M, Kikuchi H, Imamura K. Faecal triglycerides and fatty acids in the differential diagnosis of pancreatic insufficiency and intestinal malabsorption in patients with low fat intakes. J Int Med Res. 1995;23:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Turner RC, Brazionis LB, McDermott R. Intake patterns of food nutrients and other substances associated with chronic pancreatitis. Pancreatology. 2013;13:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Singh N, Joshi YK, Saraya A, Tandon RK. Nutritional profile of patients with chronic pancreatitis. Asia Pac J Clin Nutr. 1999;8:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Lin Y, Tamakoshi A, Hayakawa T, Ogawa M, Ohno Y; Research Committee on Intractable Pancreatic Diseases. Associations of alcohol drinking and nutrient intake with chronic pancreatitis: findings from a case-control study in Japan. Am J Gastroenterol. 2001;96:2622-2627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Uscanga L, Robles-Díaz G, Sarles H. Nutritional data and etiology of chronic pancreatitis in Mexico. Dig Dis Sci. 1985;30:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Balakrishnan V, Sauniere JF, Hariharan M, Sarles H. Diet, pancreatic function, and chronic pancreatitis in south India and France. Pancreas. 1988;3:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Tinju J, Reshmi S, Rajesh G, Balakrishnan V. Anthropometric, biochemical, clinical and dietary assessment for malnutrition in south Indian patients with chronic pancreatitis. Trop Gastroenterol. 2010;31:285-290. [PubMed] |
| 38. | Hamberg O, Andersen V, Sonne J, Larsen S, Vilstrup H. Urea synthesis in patients with chronic pancreatitis: relation to glucagon secretion and dietary protein intake. Clin Nutr. 2001;20:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Singh S, Midha S, Singh N, Joshi YK, Garg PK. Dietary counseling versus dietary supplements for malnutrition in chronic pancreatitis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2008;6:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Durbec JP, Sarles H. Multicenter survey of the etiology of pancreatic diseases. Relationship between the relative risk of developing chronic pancreaitis and alcohol, protein and lipid consumption. Digestion. 1978;18:337-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 156] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Westerfeld WW, Schulman MP. Metabolism and caloric value of alcohol. J Am Med Assoc. 1959;170:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Pirola RC, Lieber CS. The energy cost of the metabolism of drugs, including ethanol. Pharmacology. 1972;7:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 118] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Gruchow HW, Sobocinski KA, Barboriak JJ. Alcohol, nutrient intake, and hypertension in US adults. JAMA. 1985;253:1567-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 162] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 45. | Coté GA, Yadav D, Slivka A, Hawes RH, Anderson MA, Burton FR, Brand RE, Banks PA, Lewis MD, Disario JA, Gardner TB, Gelrud A, Amann ST, Baillie J, Money ME, O'Connell M, Whitcomb DC, Sherman S; North American Pancreatitis Study Group. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:266-73; quiz e27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 46. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 7793] [Article Influence: 1298.8] [Reference Citation Analysis (1)] |
| 47. | Kuan LL, Dennison AR, Garcea G. Prevalence and Impact of Sarcopenia in Chronic Pancreatitis: A Review of the Literature. World J Surg. 2021;45:590-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | OConnor D, Kok T, Purcell C, Duggan S, Conlon K. Investigating the prevalence of sarcopenia in chronic pancreatitis in an irish cohort: A CT-scan based pilot study. Pancreatology. 2014;3:S74. [DOI] [Full Text] |
| 49. | Olesen SS, Büyükuslu A, Køhler M, Rasmussen HH, Drewes AM. Sarcopenia associates with increased hospitalization rates and reduced survival in patients with chronic pancreatitis. Pancreatology. 2019;19:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 50. | Verhaegh BP, Reijven PL, Prins MH, Brouns JH, Masclee AA, Keulemans YC. Nutritional status in patients with chronic pancreatitis. Eur J Clin Nutr. 2013;67:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Löhr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, Haas S, Akisik F, Kartalis N, Iglesias-Garcia J, Keller J, Boermeester M, Werner J, Dumonceau JM, Fockens P, Drewes A, Ceyhan G, Lindkvist B, Drenth J, Ewald N, Hardt P, de Madaria E, Witt H, Schneider A, Manfredi R, Brøndum FJ, Rudolf S, Bollen T, Bruno M; HaPanEU/UEG Working Group. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J. 2017;5:153-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 425] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 52. | de Rijk FE, Kempeneers MA, Bruno MJ, Besselink MG, van Goor H, Boermeester MA, van Geenen EJ, van Hooft JE, van Santvoort HC, Verdonk RC; Dutch Pancreatitis Study Group. Suboptimal care for chronic pancreatitis patients revealed by moderate to low adherence to the United European Gastroenterology evidence-based guidelines (HaPanEU): A Netherlands nationwide analysis. United European Gastroenterol J. 2020;8:764-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Sikkens EC, Cahen DL, Koch AD, Braat H, Poley JW, Kuipers EJ, Bruno MJ. The prevalence of fat-soluble vitamin deficiencies and a decreased bone mass in patients with chronic pancreatitis. Pancreatology. 2013;13:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 54. | Duggan SN, Purcell C, Kilbane M, O'Keane M, McKenna M, Gaffney P, Ridgway PF, Boran G, Conlon KC. An association between abnormal bone turnover, systemic inflammation, and osteoporosis in patients with chronic pancreatitis: a case-matched study. Am J Gastroenterol. 2015;110:336-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 55. | Lindkvist B, Phillips ME, Domínguez-Muñoz JE. Clinical, anthropometric and laboratory nutritional markers of pancreatic exocrine insufficiency: Prevalence and diagnostic use. Pancreatology. 2015;15:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 56. | Quilliot D, Dousset B, Guerci B, Dubois F, Drouin P, Ziegler O. Evidence that diabetes mellitus favors impaired metabolism of zinc, copper, and selenium in chronic pancreatitis. Pancreas. 2001;22:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Papazachariou IM, Martinez-Isla A, Efthimiou E, Williamson RC, Girgis SI. Magnesium deficiency in patients with chronic pancreatitis identified by an intravenous loading test. Clin Chim Acta. 2000;302:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Vujasinovic M, Hedström A, Maisonneuve P, Valente R, von Horn H, Löhr JM, Haas SL. Zinc deficiency in patients with chronic pancreatitis. World J Gastroenterol. 2019;25:600-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Volatier JL, Verger P. Recent national French food and nutrient intake data. Br J Nutr. 1999;81 Suppl 2:S57-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Harrington KE, McGowan MJ, Kiely M, Robson PJ, Livingstone MB, Morrissey PA, Gibney MJ. Macronutrient intakes and food sources in Irish adults: findings of the North/South Ireland Food Consumption Survey. Public Health Nutr. 2001;4:1051-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |