Published online Aug 28, 2021. doi: 10.3748/wjg.v27.i32.5438
Peer-review started: April 21, 2021
First decision: June 30, 2021
Revised: July 12, 2021
Accepted: July 20, 2021
Article in press: July 20, 2021
Published online: August 28, 2021
Processing time: 125 Days and 12.5 Hours
Intestinal lymphoma is a rare tumor. Contrast-enhanced ultrasound (CEUS) findings of intestinal lymphoma have not been reported previously, and the relationship between CEUS and clinicopathological features and prognostic factors is still unknown.
To describe the B-mode US and CEUS features of intestinal lymphoma and in
This was a single-center retrospective study. Eighteen patients with histologically confirmed intestinal lymphoma underwent B-mode US and CEUS examinations between October 2016 and November 2019. We summarized the features of B-mode US and CUES imaging of intestinal lymphoma and compared the frequency of tumor necrosis in intestinal lymphomas with reference to different pathological subtypes (aggressive or indolent) and clinical stage (early or advanced). The time–intensity curve parameters of CEUS were also compared between patients with normal and elevated serum lactate dehydrogenase.
In B-mode imaging, four patterns were observed in intestinal lymphoma: Mass type (12/18, 66.7%), infiltration type (1/18, 5.6%), mesentery type (4/18, 22.2%) and mixed type (1/18, 5.6%). All cases were hypoechoic and no cystic areas were detected. On CEUS, most cases (17/18, 94.4%) showed arterial hyperechoic en
B-mode US and CEUS findings of intestinal lymphoma are characteristic. We observed a high rate of tumor necrosis, which appeared more frequently in ag
Core Tip: This is a small pilot study that described the appearance and pattern of intestinal lymphoma on B-mode ultrasound (US) and contrast-enhanced US. We revealed an unexpected contrast-enhanced US enhancement pattern with a high rate of tumor necrosis, which was found more frequently in aggressive than in indolent subtypes. This might provide additional information for clinical diagnosis and treat
- Citation: Cui NY, Gong XT, Tian YT, Wang Y, Zhang R, Liu MJ, Han J, Wang B, Yang D. Contrast-enhanced ultrasound imaging for intestinal lymphoma. World J Gastroenterol 2021; 27(32): 5438-5447
- URL: https://www.wjgnet.com/1007-9327/full/v27/i32/5438.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i32.5438
Gastrointestinal tract is the most common site for extranodal lymphomas[1,2]. Approximately 20%–25% of primary extranodal lymphomas occur in the gastrointestinal tract[3,4]. Around 50%–70% of the gastrointestinal lymphomas arise in the stomach, 20%–30% in the small bowel and 5%–20% in the large bowel[5]. Primary intestinal lymphomas are less common than gastric lymphomas and differ in clinical features, pathology, treatment and prognosis[6-10]. There are few studies on the ultrasound (US) diagnosis of intestinal lymphoma, most of which are case reports. Intestinal tumors are usually studied through endoscopic or radiological imaging techniques, which may explain the lack of studies on US in intestinal lymphomas[11]. However, previous studies have already shown that US is an effective method for detecting gastrointestinal lesions, especially in experienced hands[12]. US-guided biopsy can also establish a definite pathological diagnosis, avoiding unnecessary surgery.
Contrast-enhanced US (CEUS) has emerged as a new imaging technique that can evaluate tumor vascularity[13]. CEUS is now widely accepted and recommended in inflammatory bowel disease in the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines. CEUS is suggested for assessment of the vascularity of the gastrointestinal wall and gastrointestinal tumors[14]. To the best of our knowledge, CEUS features of intestinal lymphoma have not previously been described. The aim of this study was to illustrate CEUS patterns of intestinal lym
This is a single-center retrospective study based on a search of our imaging system from October 2016 to November 2019. Patients were excluded if they had a history of any other cancer or if they had previously received treatment. Written informed consent was obtained from each patient before the CEUS examinations.
Eighteen patients with histologically confirmed intestinal lymphoma were included, 10 men and 8 women, with age range 34–85 years. Definitive diagnosis for all the lesions was obtained by US-guided biopsy in 15 (83.3%) cases or by surgical resection in 3 (16.7%) cases. The diagnosis of intestinal lymphoma was based on the World Health Organization classification[15]. According to the Ann Arbor staging system, patients were classified into early stage (I/II) and advanced stage (III/IV). Pathological types were classified into aggressive and indolent lymphoma based on the World Health Organization classification. Primary intestinal lymphoma was defined as predominant intestinal lesion without previous peripheral lymphadenopathy or any lymphoma whose clinical presentation was related to intestinal involvement[16].
All patients underwent B-mode US and CEUS with a 3.0–5.0 -MHz C5-1 probe for the iU22 sonography system (Philips Healthcare Ultrasound, Bothell, WA, United States) prior to surgery or biopsy. All patients were fasted for 8–12 h. CEUS was performed on all tumors under same protocols with the following parameters: Mechanical index 0.08–0.11, contrast gain 50%–60% and dynamic range 40 dB and focal zone was set at maximal depth. SonoVue (4.8 mL; Bracco, Milan, Italy) was administrated through a forearm vein in bolus within 1–2 s, followed by flush of 5 mL 0.9% saline. Images were acquired immediately after contrast agent was injected and lasted for 3 min and were recorded and stored as raw data on personal hard disks.
US and CEUS images were reviewed by two experienced radiologists, who were blinded to all clinical and pathological information. If there was disagreement between them, the final determination was made after discussion. The tumor size, internal echogenicity and presence of bowel wall thickening were recorded. Bowel wall thickening was defined as > 3 mm for small bowel and > 5 mm for large bowel. The enhancement pattern, enhancement distribution, tumor necrosis and presence of irregular and enlarged vessels (diameter > 5 mm) were recorded. The enhancement pattern was classified as homogeneous or heterogeneous. The enhancement distribution was clarified as diffuse, centripetal or centrifugal. Tumor necrosis was consi
According to the EFSUMB guidelines[14], the arterial phase is defined as the first 30 s after the contrast agent is injected, and the venous phase as the period between 30 and 120 s. By using Q lab software version 5 (Philips Medical Systems) on the workstation, we selected a region of interest (ROI) in the most enhanced area of the lesion, and the ROI area was set to 25 mm2. The time–intensity curve (TIC) was au
The Fisher’s exact test was used to compare rate of tumor necrosis on CEUS between patients with Ann Arbor stage I/II and III/IV intestinal lymphomas and between patients with indolent and aggressive subtypes of intestinal lymphoma. Student’s t test was used to compare the TIC parameters between normal and elevated serum lactate dehydrogenase (LDH) groups. Statistical significance was determined at P < 0.05 (two sided). Statistical analyses were performed using SPSS version 13.0 (SPSS Chicago, IL, United States).
This patient group comprised 18 patients with non-Hodgkin’s lymphoma (NHL) of various subtypes. Seventeen intestinal lymphomas were of B-cell origin; among which 11 were diffuse large B-cell lymphoma (DLBCL), 3 were follicular lymphoma, 2 were mucosa-associated lymphoid tissue lymphoma and 1 was mantle cell lymphoma. Only one case was of T-cell origin (enteropathy-associated T-cell lymphoma). No instances of intestinal Hodgkin’s lymphoma were encountered. Primary intestinal lymphoma was diagnosed in 16 cases, whereas secondary lymphoma infiltration of the bowel was observed in 2 cases. Eight cases were classified as stage II, 4 as stage III and 6 as stage IV. The clinical presentations included abdominal pain (n = 7), palpable abdominal mass (n = 5), gastrointestinal bleeding (n = 2), constipation (n = 2), weight loss (n = 1) and fever (n = 1).
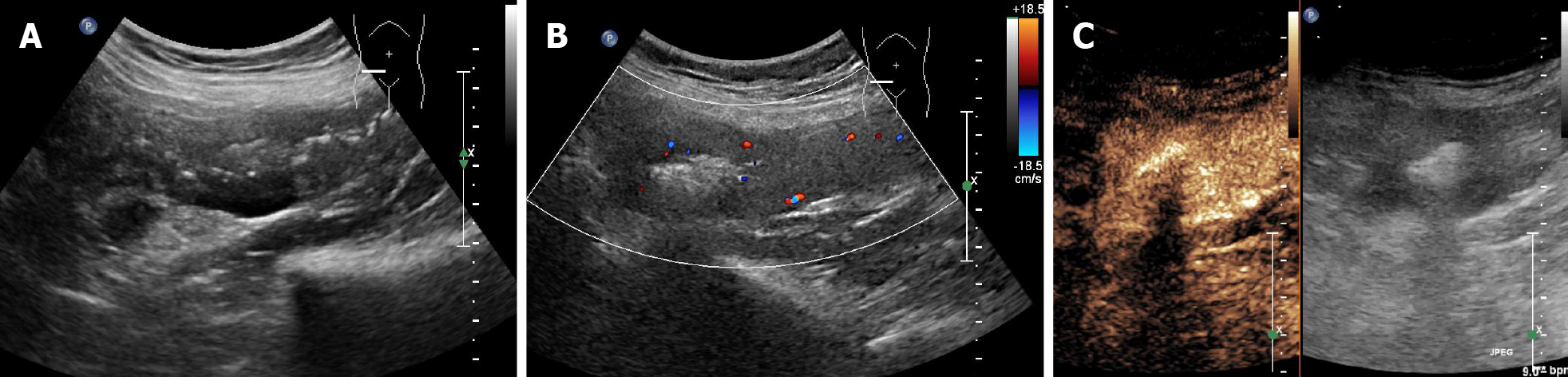
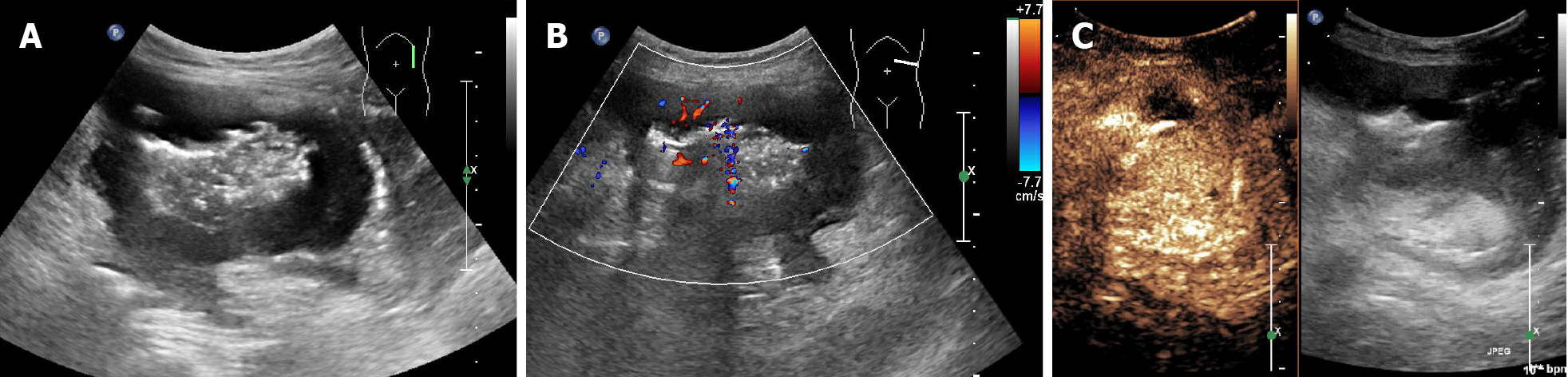
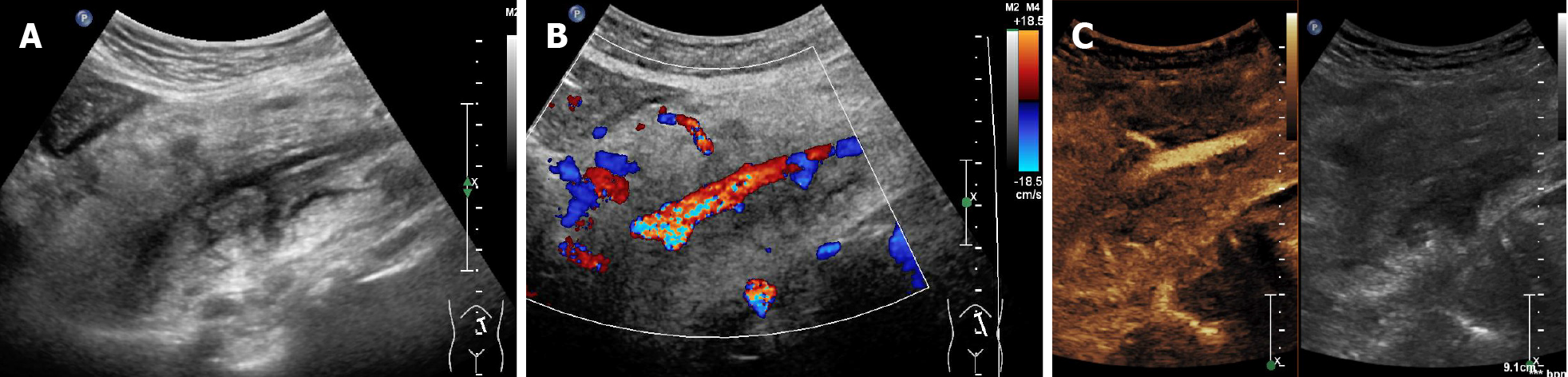
Four sonographic patterns of intestinal lymphoma were observed on B-mode imaging. The mass type (n = 12) presented as circumferential, marked bowel wall thickening forming masses, with loss of stratification, and hyperechoic lumen inside the mass (Figure 1A and B)[17,18]. The size of the masses ranged from 7.1 cm to 11.9 cm (mean 9.9 cm). The infiltration type presented as segmental bowel wall thickening without apparent mass formation (n = 1), with intestinal wall thickness of 2.6 cm (Figure 2A and B). The mesenteric type (n = 4) was characterized by multiple exophytic mesen
Rapid arterial enhancement followed by gradual washout in the venous phase was observed in all 18 patients (Figures 1C, 2C, and 3C). The peak enhancement was reached in the arterial phase or early venous phase. Seventeen cases presented with hyperechoic arterial enhancement, and only 1 case revealed hypoechoic arterial enhancement. Enhancement during the arterial phase was homogeneous (n = 7) or inhomogeneous (n = 11). Fifteen cases presented with diffuse enhancement, whereas three presented with centripetal enhancement. The visualization of irregular vessels was observed in 8 cases (44.4%). Tumor necrosis on CEUS was identified in 11 (61.1%) patients (Figure 1C), in which 1 case revealed extensive necrosis. Detailed information about B-mode US and CEUS findings of the 18 cases are shown in Table 1.
| No. | Age (yr) | Sex | Location | Pathological subtype | B-mode US pattern | CEUS enhancement pattern | Necrosis |
| 1 | 78 | M | Ileocecal region | DLBCL | Mass type | Diffuse, inhomogeneous, arterial hyperechoic uptake. gradually venous wash-out | Yes |
| 2 | 34 | M | Rectum | MATL | Mass type | Diffuse, inhomogeneous, arterial hyperechoic uptake. gradually venous wash-out | Yes |
| 3 | 43 | M | Mesentery | DLBCL | Mesenteric type | Diffuse, inhomogeneous, hyperechoic arterial uptake. gradually venous wash-out | Yes |
| 4 | 46 | M | Small intestine | FL | Mass type | Diffuse, homogeneous, hypoechoic, arterial uptake. gradually venous wash-out | No |
| 5 | 75 | F | Ascending colon | DLBCL | Infiltration type | Diffuse, homogeneous, hyperechoic arterial uptake. gradually venous wash-out | No |
| 6 | 78 | F | Jejunum, ileum | EATL | Mass type | Diffuse, inhomogeneous, hyperechoic arterial uptake. gradually venous wash-out | Yes |
| 7 | 52 | F | Jejunum | DLBCL | Mass type | Diffuse, inhomogeneous, hyperechoic arterial uptake. gradually venous wash-out | Yes |
| 8 | 74 | M | Small intestine | DLBCL | Mass type | Diffuse, inhomogeneous, hyperechoic arterial uptake. gradually venous wash-out | Yes |
| 9 | 52 | M | Jejunum, ileum | MATL | Mass type | Diffuse, homogeneous, hyperechoic arterial uptake. gradually venous wash-out | No |
| 10 | 61 | M | Mesentery | DLBCL | Mesenteric type | Diffuse, inhomogeneous, hyperechoic arterial uptake. gradually venous wash-out | Yes |
| 11 | 41 | F | Mesentery | DLBCL | Mesenteric type | Diffuse, inhomogeneous, hyperechoic arterial uptake. gradually venous wash-out | No |
| 12 | 46 | F | Small intestine | DLBCL | Mass type | Diffuse, inhomogeneous, hyperechoic arterial uptake. gradually venous wash-out | Yes |
| 13 | 64 | M | Ileocecum, ascending colon | DLBCL | Mass type | Diffuse, inhomogeneous, hyperechoic arterial uptake. gradually venous wash-out | Yes |
| 14 | 68 | F | Small intestine | DLBCL | Mixed type | Diffuse, inhomogeneous, hyperechoic arterial uptake. gradually venous wash-out | Yes |
| 15 | 61 | F | Small intestine | FL | Mass type | Diffuse, homogeneous, hyperechoic arterial uptake. gradually venous wash-out | No |
| 16 | 85 | M | Small intestine | MCL | Mass type | Diffuse, homogeneous, hyperechoic arterial uptake. gradually venous wash-out | No |
| 17 | 35 | M | Mesentery | FL | Mesenteric type | Diffuse, homogeneous, hyperechoic arterial uptake. gradually venous wash-out | No |
| 18 | 68 | F | Small intestine | DLBCL | Mass type | Diffuse, homogeneous, hyperechoic arterial uptake. gradually venous wash-out | Yes |
The mean diameter of the lesions with and without necrosis was 8.36 cm and 9.58 cm, respectively (P = 0.6). We compared the frequency of tumor necrosis in intestinal lymphomas with reference to different pathological subtypes or clinical stages. Tumor necrosis on CEUS was detected in 20.0% (1/5) of indolent intestinal lymphomas and in 76.9% (10/13) of aggressive subtypes (P = 0.047). Tumor necrosis was detected in 80% (8/10) of patients in III/IV stage and in 37.5% (3/8) patients in I/II stage (P = 0.145) (Table 2). Quantitative results of TIC parameters are shown in Table 3. The serum LDH value ranged from 130 to 968 U/L, with 10 patients’ values exceeding the upper limit of normal (248 U/L). There was no significant difference in TIC parameters between normal and elevated LDH groups (Table 4).
| Characteristic | With necrosis | Without necrosis |
| Tumor size (cm) | 8.36 | 9.58 |
| Stage | ||
| Early stage | 3 | 5 |
| Advanced stage | 8 | 2 |
| Subtypea | ||
| Indolent subtype | 1 | 4 |
| Aggressive subtype | 10 | 3 |
| TIC parameters | Range | mean ± SD |
| Rise time (s) | 2.90-27.62 | 13.74 ± 6.11 |
| Time to peak (s) | 7.64-36.00 | 24.41 ± 6.61 |
| Mean transit time (s) | 29.94-77.75 | 49.94 ± 13.98 |
| Time from peak to one half (s) | 36.38-127.85 | 77.88 ± 24.12 |
| Peak intensity (dB) | 7.8-29.28 | 17.57 ± 5.98 |
| WIS (dB/s) | 0.53-29.28 | 1.35 ± 1.09 |
| AUC | 590.99-3616.03 | 1772.05 ± 824.89 |
| TIC parameters | Normal LDH | Elevated LDH | P value |
| Rise time (s) | 14.34 | 12.78 | 0.693 |
| Time to peak (s) | 27.17 | 27.07 | 0.982 |
| Mean transit time (s) | 53.39 | 46.21 | 0.382 |
| Time from peak to one half (s) | 82.70 | 72.49 | 0.442 |
| Peak intensity (dB) | 16.50 | 16.68 | 0.955 |
| Wash in slope (dB/s) | 1.38 | 1.26 | 0.824 |
| Area under the curve (dBs) | 1699.47 | 1712.42 | 0.979 |
In this study, we reported 18 cases of intestinal lymphoma. The mean age of our patients was 58.94 ± 15.89 years with most cases between 50 and 70 years. There was a male predominance with a male to female ratio of 1.25: 1. Lymphomas were predominantly located in the small bowel, and the most common symptoms included palpable abdominal mass and abdominal pain, which is in agreement with the literature[20]. All the intestinal lymphomas were NHL. B-cell lymphomas were more frequent than T-cell lymphomas (17: 1), and the most common pathological subtype was DLBCL (11/18, 61.1%). These results correlated well with previous studies[3,21].
We confirmed some of the sonographic features reported earlier and provided new information regarding the CEUS findings in intestinal NHL. Our data showed that mass type was the most frequent form of intestinal NHL on B-mode imaging (12/18, 66.7%), which was in agreement with previous studies[22]. However, this finding was not specific because various intestinal abnormalities also can lead to bowel wall thickening. Nevertheless, bowel wall thickening in lymphoma is often significant (> 2 cm) compared to other malignancies and often located in the ileocecal region, with coexistence of perivisceral multiple lymph nodes. Diffuse or segmental bowel wall thickening is characteristic of intestinal lymphoma, especially when associated with bowel lumen dilation, but this US pattern only comprised 5.6% in our study.
In our study, rapid arterial enhancement followed by elimination of the contrast media in the venous phase was observed in all cases, which is similar with other malignancies. This finding is believed to be correlated with the presence of arteriovenous shunts and a well-represented circulatory bed. Thirteen cases exhibited diffuse enhancement, in accordance with previous studies[23]. Most (17/18, 94.4%) intestinal lymphomas displayed hyperechoic arterial enhancement, which is different from isoechoic or hypoechoic arterial enhancement observed in lymphoma infiltration of the spleen, lungs and kidneys[24-26]. The reason for this discrepancy is unclear and needs to be confirmed in larger populations.
It is notable that there was a high rate of necrosis (11/18, 61.1%) on CEUS, which is inconsistent with previous studies. Despite necrosis being prone to occur in large tumors, we found no correlation between the presence of tumor necrosis on CEUS and tumor size. Necrosis was not detected in 4 of the 9 cases with bulky lesions. Necrosis may indicate aggressive tumor growth, and thus be associated with poor prognosis. Some researchers have explored the prognostic implications of tumor necrosis at computed tomography (CT) or positron emission tomography in lymphoma. Saito et al[27] did not find an association between disease-free survival and presence of necrosis in lymph nodes in 60 patients with different NHL subtypes[27]. However, Adams et al[28] showed that patients with newly diagnosed DLBCL with tumor necrosis at CT had significantly worse progression-free survival and overall survival than patients without tumor necrosis at CT. The National Comprehensive Cancer Network International Prognostic Index score did not differ significantly between the two groups. Our study showed that the frequency of necrosis on CEUS was different between indolent and aggressive lymphoma subtypes, but it was not related to clinical stage. Since it as a retrospective study, patients were not followed up to assess prognosis.
Evaluation of prognosis by imaging is desirable but often not possible. It is known that tumor growth is accompanied by neoangiogenesis. The assessment of the bowel vasculature can provide functional information about tumor perfusion, particularly with the advent of the second generation of contrast agents for CEUS. The generation of TICs allows for objective measurement of blood flow parameters[29]. In our pre
US is not the method of choice for the diagnosis of intestinal lymphoma, because it may not detect small or deep lesions that can be obscured by bowel gas; it is often used as targeted US for evaluation of suspected region but has difficulty in thorough assessment and staging for advanced lymphoma. Intestinal lymphoma is conventionally diagnosed by CT, magnetic resonance imaging and positron emission to
This study had some limitations. Intestinal lymphoma is a rare entity and the sam
In this study, our findings on intestinal lymphoma contribute to knowledge of CEUS imaging. In this small group, intestinal lymphomas showed a characteristic enhan
Contrast-enhanced ultrasound (CEUS) is a new imaging technique that can evaluate tumor vascularity, and it has been proved to have value in the diagnosis and assess
There have been few studies on CEUS findings of intestinal lymphoma and their potential correlation with histopathological features.
Our present study aimed to describe the B-mode US and CEUS features of intestinal lymphoma and investigate the correlation of CEUS and histopathological features.
We summarized the features of B-mode US and CUES imaging of intestinal lymphoma of 18 patients and compared the frequency of tumor necrosis in intestinal lymphomas with reference to different pathological subtypes (aggressive or indolent) and clinical stage (early or advanced). Besides, the time–intensity curve parameters of CEUS were also compared between patients with normal and elevated serum lactate dehydrogenase (LDH).
In B-mode imaging, four patterns were observed in intestinal lymphoma: Mass type (12/18, 66.7%), infiltration type (1/18, 5.6%), mesentery type (4/18, 22.2%) and mixed type (1/18, 5.6%). All cases were hypoechoic and no cystic areas were detected. On CEUS, most cases (17/18, 94.4%) showed arterial hyperechoic enhancement. All cases showed arterial enhancement followed by venous wash out. A relatively high rate of tumor necrosis (11/18, 61.1%) was observed in this study. Tumor necrosis on CEUS was more frequent in aggressive subtypes (10/13, 76.9%) than in indolent subtypes (1/5, 20.0%) (P = 0.047). There were no correlations between tumor necrosis and lesion size and Ann Arbor stage. There was no significant difference in time–intensity curve parameters between normal and elevated LDH groups.
B-mode US and CEUS findings of intestinal lymphoma are characteristic. We observed a high rate of tumor necrosis, which appeared more frequently in aggressive patho
Based on the present results, we will expand the number of enrolled patients and explore the advantages of US compared with other imaging techniques in diagnosing intestinal lymphoma.
The authors like to thank Professor Hong-Da Chen, biostatistician at Office of Cancer Screening, National Cancer Center/National Clinical Research Center for Cancer/ Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Angeramo CA, Mannelli L, Okada M S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 2. | Levine MS, Rubesin SE, Pantongrag-Brown L, Buck JL, Herlinger H. Non-Hodgkin's lymphoma of the gastrointestinal tract: radiographic findings. AJR Am J Roentgenol. 1997;168:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Foukas PG, de Leval L. Recent advances in intestinal lymphomas. Histopathology. 2015;66:112-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17:697-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 245] [Cited by in RCA: 265] [Article Influence: 18.9] [Reference Citation Analysis (3)] |
| 5. | Nakamura S, Matsumoto T. Gastrointestinal lymphoma: recent advances in diagnosis and treatment. Digestion. 2013;87:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Dragosics B, Bauer P, Radaszkiewicz T. Primary gastrointestinal non-Hodgkin's lymphomas. A retrospective clinicopathologic study of 150 cases. Cancer. 1985;55:1060-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Koch P, del Valle F, Berdel WE, Willich NA, Reers B, Hiddemann W, Grothaus-Pinke B, Reinartz G, Brockmann J, Temmesfeld A, Schmitz R, Rübe C, Probst A, Jaenke G, Bodenstein H, Junker A, Pott C, Schultze J, Heinecke A, Parwaresch R, Tiemann M; German Multicenter Study Group. Primary gastrointestinal non-Hodgkin's lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001;19:3861-3873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 269] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Ruskoné-Fourmestraux A, Aegerter P, Delmer A, Brousse N, Galian A, Rambaud JC. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d'Etude des Lymphomes Digestifs. Gastroenterology. 1993;105:1662-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 109] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Fischbach W, Dragosics B, Kolve-Goebeler ME, Ohmann C, Greiner A, Yang Q, Böhm S, Verreet P, Horstmann O, Busch M, Dühmke E, Müller-Hermelink HK, Wilms K, Allinger S, Bauer P, Bauer S, Bender A, Brandstätter G, Chott A, Dittrich C, Erhart K, Eysselt D, Ellersdorfer H, Ferlitsch A, Fridrik MA, Gartner A, Hausmaninger M, Hinterberger W, Hügel K, Ilsinger P, Jonaus K, Judmaier G, Karner J, Kerstan E, Knoflach P, Lenz K, Kandutsch A, Lobmeyer M, Michlmeier H, Mach H, Marosi C, Ohlinger W, Oprean H, Pointer H, Pont J, Salabon H, Samec HJ, Ulsperger A, Wimmer A, Wewalka F. Primary gastric B-cell lymphoma: results of a prospective multicenter study. The German-Austrian Gastrointestinal Lymphoma Study Group. Gastroenterology. 2000;119:1191-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 134] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer. 2003;97:2462-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (9)] |
| 12. | Fukumoto A, Tanaka S, Imagawa H, Shishido T, Oka S, Yoshida S, Yamada H, Chayama K. Usefulness and limitations of transabdominal ultrasonography for detecting small-bowel tumors. Scand J Gastroenterol. 2009;44:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Malone CD, Fetzer DT, Monsky WL, Itani M, Mellnick VM, Velez PA, Middleton WD, Averkiou MA, Ramaswamy RS. Contrast-enhanced US for the Interventional Radiologist: Current and Emerging Applications. Radiographics. 2020;40:562-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O, Claudon M, Clevert DA, Correas JM, D'Onofrio M, Drudi FM, Eyding J, Giovannini M, Hocke M, Ignee A, Jung EM, Klauser AS, Lassau N, Leen E, Mathis G, Saftoiu A, Seidel G, Sidhu PS, ter Haar G, Timmerman D, Weskott HP. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 680] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 15. | Swerdlow SH, Resea IAF. WHO classification of tumours of haematopoietic and lymphoid tissues. International Agency for Research on Cancer, 2008. |
| 16. | Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract: a study of 117 cases presenting with gastrointestinal disease. Cancer. 1978;42:693-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Fakhry JR, Berk RN. The "target" pattern: characteristic sonographic feature of stomach and bowel abnormalities. AJR Am J Roentgenol. 1981;137:969-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Goerg C, Schwerk WB, Goerg K. Gastrointestinal lymphoma: sonographic findings in 54 patients. AJR Am J Roentgenol. 1990;155:795-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Lo Re G, Federica V, Midiri F, Picone D, La Tona G, Galia M, Lo Casto A, Lagalla R, Midiri M. Radiological Features of Gastrointestinal Lymphoma. Gastroenterol Res Pract. 2016;2016:2498143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Smith C, Kubicka RA, Thomas CR Jr. Non-Hodgkin lymphoma of the gastrointestinal tract. Radiographics. 1992;12:887-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Weindorf SC, Smith LB, Owens SR. Update on Gastrointestinal Lymphomas. Arch Pathol Lab Med. 2018;142:1347-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Sener RN, Alper H, Demirci A, Diren HB. A different sonographic "pseudokidney" appearance detected with intestinal lymphoma: "hydronephrotic-pseudokidney". J Clin Ultrasound. 1989;17:209-212. [PubMed] [DOI] [Full Text] |
| 23. | Rubaltelli L, Khadivi Y, Tregnaghi A, Stramare R, Ferro F, Borsato S, Fiocco U, Adami F, Rossi CR. Evaluation of lymph node perfusion using continuous mode harmonic ultrasonography with a second-generation contrast agent. J Ultrasound Med. 2004;23:829-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Trenker C, Neesse A, Görg C. Sonographic patterns of renal lymphoma in B-mode imaging and in contrast-enhanced ultrasound (CEUS)--a retrospective evaluation. Eur J Radiol. 2015;84:807-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Sutherland T, Temple F, Galvin A, Hennessy O. Contrast-enhanced ultrasound of the spleen: an introduction and pictorial essay. Insights Imaging. 2011;2:515-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Görg C. Transcutaneous contrast-enhanced sonography of pleural-based pulmonary lesions. Eur J Radiol. 2007;64:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Saito A, Takashima S, Takayama F, Kawakami S, Momose M, Matsushita T. Spontaneous extensive necrosis in non-Hodgkin lymphoma: prevalence and clinical significance. J Comput Assist Tomogr. 2001;25:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Adams HJA, de Klerk JMH, Fijnheer R, Dubois SV, Nievelstein RAJ, Kwee TC. Prognostic value of tumor necrosis at CT in diffuse large B-cell lymphoma. Eur J Radiol. 2015;84:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Greis C. Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS). Clin Hemorheol Microcirc. 2011;49:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Wang Y, Li L, Wang YX, Cui NY, Zou SM, Zhou CW, Jiang YX. Time-intensity curve parameters in rectal cancer measured using endorectal ultrasonography with sterile coupling gels filling the rectum: correlations with tumor angiogenesis and clinicopathological features. Biomed Res Int. 2014;2014:587806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Kim SJ, Hong JS, Chang MH, Kim JA, Kwak JY, Kim JS, Yoon DH, Lee WS, Do YR, Kang HJ, Eom HS, Park Y, Won JH, Mun YC, Kim HJ, Kwon JH, Kong JH, Oh SY, Lee S, Bae SH, Yang DH, Jun HJ, Kim YS, Yun HJ, Lee SI, Kim MK, Park EK, Kim WS, Suh C. Highly elevated serum lactate dehydrogenase is associated with central nervous system relapse in patients with diffuse large B-cell lymphoma: Results of a multicenter prospective cohort study. Oncotarget. 2016;7:72033-72043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |