Published online Aug 28, 2021. doi: 10.3748/wjg.v27.i32.5424
Peer-review started: April 9, 2021
First decision: May 27, 2021
Revised: June 22, 2021
Accepted: August 10, 2021
Article in press: August 10, 2021
Published online: August 28, 2021
Processing time: 137 Days and 20.5 Hours
Sorafenib is an oral drug that prolongs overall survival (OS) in patients with hepatocellular carcinoma. Adverse events, including hand-foot skin reaction (HFSR), lead to permanent sorafenib discontinuation.
To clarify the association between interventions for adverse events and patient prognosis.
We performed a retrospective, multicenter study of patients treated with sorafenib monotherapy between May 2009 and March 2018. We developed a mutual cooperation system that was initiated at the start of sorafenib treatment to effectively manage adverse events. The mutual cooperation system entailed patients receiving consultations during which pharmacists provided accurate information about sorafenib to alleviate the fear and anxiety related to adverse events. We stratified the patients into three groups: Group A, patients without HFSR but with pharmacist intervention; Group B, patients with HFSR and phar
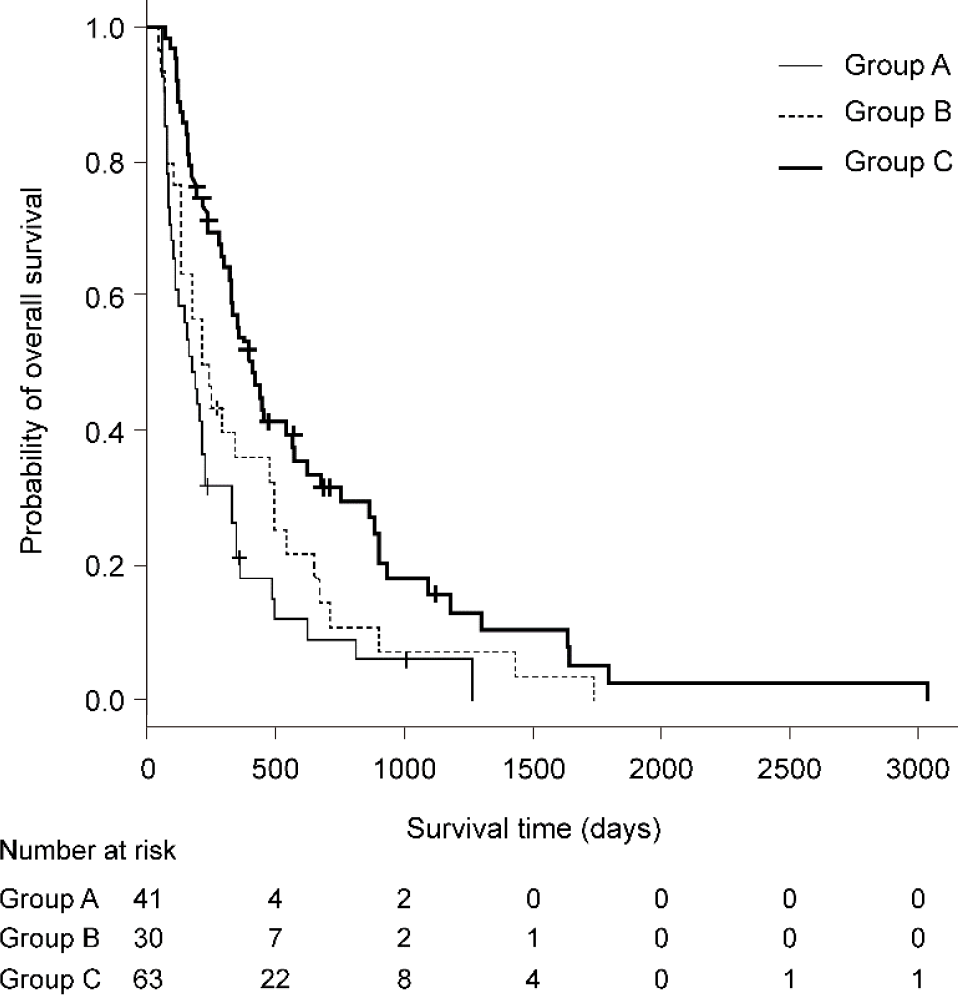
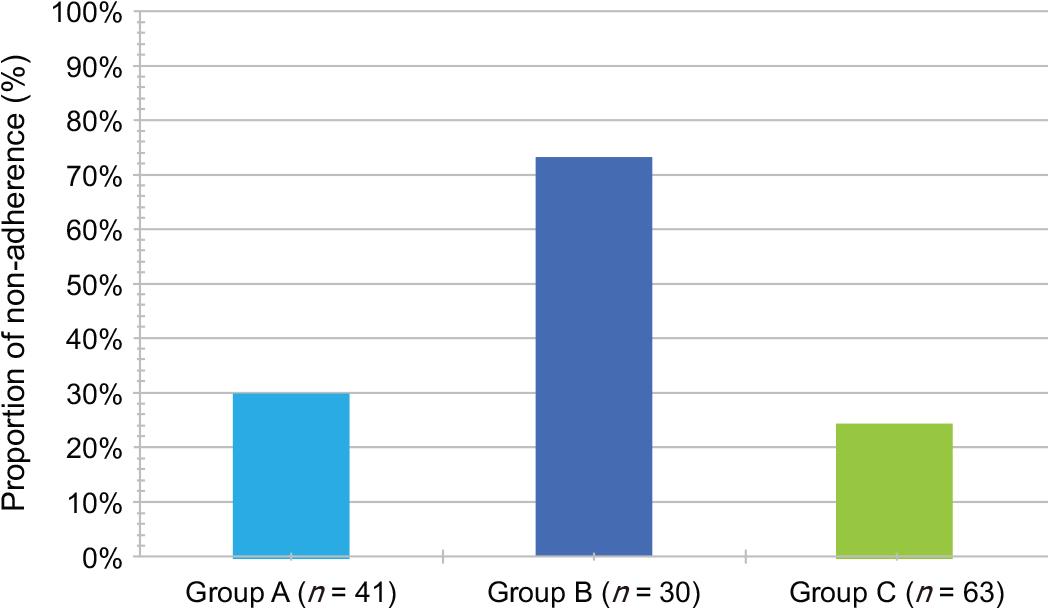
We enrolled 134 patients (Group A, n = 41; Group B, n = 30; Group C, n = 63). The median OS was significantly different between Groups A and C (6.2 vs 13.9 mo, p < 0.01) but not between Groups A and B (6.2 vs 7.7 mo, P = 0.62). Group A vs Group C was an independent OS predictor (HR, 0.41; 95%CI: 0.25-0.66; P < 0.01). In Group B alone, TTF was significantly lower and the nonadherence rate was higher (P < 0.01). In addition, the Spearman’s rank correlation coefficients bet
The mutual cooperation system increased treatment duration and improved prognosis in patients with HFSR. Future prospective studies (e.g., randomized controlled trials) and improved adherence could help prevent OS underestima
Core Tip: We investigated the effect of cooperation between oncologists and pharma
- Citation: Ochi M, Kamoshida T, Araki M, Ikegami T. Prolonged survival in patients with hand-foot skin reaction secondary to cooperative sorafenib treatment. World J Gastroenterol 2021; 27(32): 5424-5437
- URL: https://www.wjgnet.com/1007-9327/full/v27/i32/5424.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i32.5424
Sorafenib is a multikinase inhibitor used to treat advanced hepatocellular carcinoma (HCC)[1,2]. Although sorafenib prolongs overall survival (OS) in patients with HCC, it is associated with various adverse events (AEs) that may lead to permanent discontinuation[3].
Previous studies found that hand-foot skin reaction (HFSR) was a prognostic marker of longer survival[4-6]. While HFSR is an important predictor of survival outcomes in clinical practice and clinical sorafenib trials, AE management could influence the efficacy of HFSR as a prognostic factor. A recent study showed that increased clinician experience with AEs reduced the potential for discontinuing sorafenib therapy, resulting in a longer OS in patients with HCC[7]. Nevertheless, it takes a long time for clinicians to develop the necessary experience for the mana
As sorafenib is administered orally, its successful use for HCC treatment relies on patient medication adherence. However, many studies indicate that patients with cancer are sometimes nonadherent when prescribed oral drugs[8,9], and AEs are the main cause of poor adherence[10]. Poor adherence can lead to poor outcomes, and clinicians may wrongly conclude that a drug is ineffective because the response to treatment is insufficient[11].
It is important for patients to actively participate in making treatment decisions and then receive treatment according to their decisions to improve adherence[12]. We introduced behavior change techniques (patient education, medication regimen ma
Effective AE management that improves medication adherence has a considerable impact on survival outcomes. Previous single-center studies suggest that healthcare provider interventions improve adherence, and the onset of HFSR was a favorable prognostic factor of OS in patients with HCC[15,16]. However, little is known about the association between prognosis and medication adherence in patients with HCC, and multicenter studies on this relationship are lacking. Therefore, we aimed to compare the impact of different AE interventions on patient prognosis.
We retrospectively evaluated patients with advanced HCC treated with sorafenib monotherapy and no subsequent chemotherapeutic agent between May 2009 and March 2018 using the medical records of the following core hospitals in Japan: Hitachi General Hospital, Ibaraki Prefectural Central Hospital, Ibaraki Cancer Center, and Tokyo Medical University Ibaraki Medical Center. These hospitals are core hospitals that were designated by the government to provide specialized cancer medical care. The patients were separated into three groups: Group A, patients without HFSR but with pharmacist intervention (this intervention was performed by pharmacists who did not share interview information with the oncologist; it is called nonmutual co
We included patients with stage B or C HCC according to the Barcelona Clinic Liver Cancer (BCLC) staging system. The indication criteria for sorafenib administration were as follows: Child-Pugh grade A or B; Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; alanine aminotransferase < 5-fold the upper limit of the normal range; total bilirubin level < 2.0 mg/dL; neutrophil count > 1500/µL; hemoglobin level ≥ 8.5 g/dL; platelet count > 75000/µL; and no dialysis requirement. The study exclusion criteria were as follows: Patients with a history of thrombosis or ischemic heart disease, pregnant women and those who could become pregnant, and patients with brain metastases. Our study protocol was approved by the ethics com
We collected patient data from the start of sorafenib, including age, sex, etiology of underlying liver disease, Child-Pugh score, history of present illness, medical history, tumor marker level [alpha-fetoprotein (AFP)], ECOG performance status, and relevant laboratory tests, including total bilirubin, albumin, and international normalized ratio (INR). Laboratory tests and tumor marker levels were obtained every 8–10 wk until permanent sorafenib discontinuation.
Sorafenib response evaluations on computed tomography (CT) were scheduled for 8 wk after the first treatment, and subsequent evaluations were planned every 8 wk. Thoracic, abdominal, and pelvic CT scans were performed with intravenous iodinated contrast media. CT evaluations were conducted based on the modified Response Eva
Pharmacists with special expertise provided medical care at the pharmacist’s out
We developed a mutual cooperation system that was initiated at the start of sorafenib treatment to manage AEs effectively. Although patients in Groups B and C received medical advice from oncologists and pharmacists, the systems differed. Group C received 20- to 30-min consultations during which pharmacists provided accurate information about sorafenib to alleviate fear and anxiety related to AEs. After each visit, the pharmacist summarized the consultation results in a report and discussed their findings with an oncologist. Group B patients received a 5- to 10-min session during which pharmacists provided the same information about sorafenib that Group C had received. These pharmacist consultations were brief because a thorough medical examination by an oncologist had already been completed. Furthermore, the phar
Sorafenib was administered at a dose of 400 or 800 mg/d. The initial dose of sorafenib was determined by an oncologist. At the start of sorafenib treatment, all patients received information about AEs from a pharmacist and an oncologist. Patients who developed an AE could confer with their consulting pharmacist or prescribing oncologist. Pharmacists collected and recorded patient data, including the AE grade (according to NCI-CTCAE version 5.0), AE time of onset, and emotional response of the patient to the AE. Oncologists performed dose modifications throughout treat
Sorafenib was permanently discontinued when any of the following events occurred: (1) Tumor progression, defined as either radiologic (by the mRECIST criteria) or clinically progressive disease (e.g., ECOG performance status decline or onset of severe symptoms with no connection to liver failure); (2) Unacceptable AEs, defined as moderate to severe AEs (e.g., grades 2–4) that persisted after dose reduction or temporary treatment interruption; or (3) Liver decompensation, defined as gastroin
Categorical variables were assessed using the chi-square test and are presented as frequencies or percentages. Continuous variables were analyzed using the Mann-Whitney U test and are expressed as the mean ± SD. OS and TTF were evaluated using the Kaplan-Meier method. A landmark analysis[20] was performed to consider HFSR cases that might have occurred if a patient with HCC had not died as a guarantee-time bias. The analysis was performed using the time when the highest-grade HFSR occurred in 50% or more of the patients as a landmark (here, 30 d). The log-rank test was used to estimate differences in survival curves. Additionally, we used Cox regression analyses to evaluate the relationship between the time to the occurrence of an event and explanatory variables. Logistic regression analyses were used to evaluate the relationship between nonadherence and explanatory variables.
We included the following baseline characteristics as variables in our univariate analysis: age, sex, etiology of liver disease, bilirubin level, albumin level, INR, BCLC stage, ECOG performance status, macrovascular invasion, extrahepatic spread, serum AFP level, and number of previous transarterial chemoembolization (TACE) proce
We included 134 patients [median age, 69 years (range, 41–89 years); male, n = 99; female, n = 35] with advanced HCC who received sorafenib monotherapy without posttreatment (Group A, n = 41; Group B, n = 30; Group C, n = 63). The main etiological factor was hepatitis C virus (HCV) (77/134 patients, 57.5%), followed by hepatitis B virus (HBV) (30/134 patients, 22.4%).
All patients had cirrhosis [Child-Pugh A, n = 117 (87.3%); Child-Pugh B, n = 17 (12.7%)]. HCC was BCLC stage B in 55 patients (41.0%) and BCLC stage C in 79 patients (59.0%). None of the patients had a second primary cancer. Portal vein thrombosis was present in 35 patients (26.1%), and extrahepatic metastases were found in 67 patients (50.0%) (Table 1).
| Group A (n = 41) | Group B (n = 30) | Group C (n = 63) | P value | |
| Age (yr) | 70 (43–89) | 67 (41–87) | 69 (48–87) | 0.233 |
| Sex | ||||
| Male, n (%) | 33 (80.5) | 22 (73.3) | 44 (69.8) | 0.481 |
| Child-Pugh class, n (%) | 0.288 | |||
| A | 33 (80.5) | 27 (90.0) | 57 (90.5) | |
| B | 8 (19.5) | 3 (10.0) | 6 (9.5) | |
| Etiology, n (%) | ||||
| HCV | 22 (53.7) | 11 (36.7) | 44 (69.8) | 0.235 |
| HBV | 9 (21.9) | 10 (33.3) | 11 (17.5) | 0.417 |
| Other | 10 (24.4) | 9 (30.0) | 8 (12.7) | 0.109 |
| Portal vein thrombosis | 14 (34.1) | 10 (33.3) | 11 (17.5) | 0.099 |
| Extrahepatic spread, n (%) | 20 (48.8) | 19 (63.3) | 28 (44.4) | 0.230 |
| AFP (ng/mL), n (%) | ||||
| > 400 | 17 (41.5) | 17 (56.7) | 29 (46.0) | 0.437 |
| BCLC staging, n (%) | 0.333 | |||
| Stage B | 15 (36.6) | 10 (33.3) | 30 (47.6) | |
| Stage C | 26 (63.4) | 20 (66.7%) | 33 (52.4) | |
| ECOG performance status, n (%) | 0.955 | |||
| 0 | 30 (75.6) | 22 (73.3) | 46 (73.0) | |
| 1 | 10 (24.4) | 8 (26.7) | 17 (27.0) | |
| Total bilirubin (mg/dL) | 0.99 ± 0.36 | 0.91 ± 0.37 | 0.83 ± 0.33 | 0.052 |
| Albumin (g/L) | 3.61 ± 0.50 | 3.73 ± 0.54 | 3.82 ± 0.49 | 0.301 |
| INR | 1.14 ± 0.11 | 1.15 ± 0.16 | 1.14 ± 0.19 | 0.481 |
| Pre-sorafenib TACE procedures, n (%) | 0.531 | |||
| 0 | 13 (31.7) | 9 (30.0) | 20 (31.8) | |
| 1 | 3 (7.3) | 6 (20.0) | 13 (20.6) | |
| 2 | 6 (14.6) | 1 (3.3) | 4 (6.4) | |
| 3 | 4 (9.8) | 2 (6.7) | 8 (12.7) | |
| 4 | 5 (12.2) | 3 (10.0) | 12 (19.0) | |
| > 5 | 10 (24.4) | 9 (30.0) | 6 (9.5) |
An AE of at least grade 1 was observed in all patients after sorafenib administration. However, none of the patients experienced any grade 4 AEs. The main AEs in all groups were fatigue (30.6%), diarrhea (39.6%), hypertension (31.3%), anorexia (29.9%), and thrombocytopenia (38.8%) (Table 2). Many patients required temporary sorafenib interruption because of AEs (Group A, 19.5%; Group B, 6.7%; Group C, 41.3%). Of patients who temporarily stopped taking sorafenib, the rate of those who resumed treatment at a reduced dose was the highest in Group C (Group A vs Group B, P = 0.70; Group A vs Group C, P = 0.11; Group B vs Group C, P < 0.01) (Table 3).
| Group A (n = 41) | Group B (n = 30) | Group C (n = 63) | ||||||||||
| All grades | Grade 1 | Grade 2 | Grade 3 | All grades | Grade 1 | Grade 2 | Grade 3 | All grades | Grade 1 | Grade 2 | Grade 3 | |
| Any adverse event | 37 (90.2) | 30 (73.2) | 18 (43.9) | 5 (12.2) | 30 (100) | 28 (93.3) | 24 (80.0) | 14 (46.6) | 63 (100) | 58 (92.1) | 36 (57.1) | 14 (22.2) |
| Hand-foot skin reaction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 30 (100) | 15 (50.0) | 10 (33.3) | 5 (16.7) | 63 (100) | 39 (61.9) | 19 (30.2) | 5 (7.9) |
| Anemia | 17 (41.5) | 8 (19.5) | 8 (19.5) | 1 (2.4) | 20 (66.6) | 13 (43.3) | 4 (13.3) | 3 (10.0) | 23 (36.5) | 14 (22.2) | 7 (11.1) | 2 (3.2) |
| Diarrhea | 15 (36.6) | 12 (29.3) | 3 (7.3) | 0 (0) | 11 (36.6) | 9 (30.0) | 1 (3.3) | 1 (3.3) | 27 (42.8) | 21 (33.3) | 2 (3.2) | 4 (6.3) |
| Fatigue | 14 (34.1) | 10 (26.8) | 3 (7.3) | 0 (0) | 11 (36.6) | 7 (23.3) | 4 (13.3) | 0 (0) | 16 (25.4) | 10 (15.9) | 6 (9.5) | 0 (0) |
| Anorexia | 14 (34.1) | 10 (24.4) | 3 (7.3) | 1 (2.4) | 7 (23.3) | 5 (16.7) | 1 (3.3) | 1 (3.3) | 19 (30.1) | 15 (23.8) | 4 (6.3) | 0 (0) |
| Hypertension | 12 (29.3) | 10 (24.4) | 2 (4.9) | 0 (0) | 7 (23.3) | 2 (6.6) | 5 (16.7) | 0 (0) | 23 (36.5) | 16 (25.4) | 4 (6.3) | 3 (4.8) |
| Thrombocytopenia | 9 (22.0) | 2 (4.9) | 4 (9.7) | 3 (7.3) | 23 (76.7) | 10 (33.3) | 8 (26.7) | 5 (16.7) | 20 (31.7) | 10 (15.9) | 5 (7.9) | 5 (7.9) |
| Alopecia | 6 (14.6) | 4 (9.7) | 2 (4.9) | 0 (0) | 2 (6.6) | 2 (6.6) | 0 (0) | 0 (0) | 20 (31.7) | 19 (30.2) | 1 (1.6) | 0 (0) |
| Hepatic encephalopathy | 1 (2.4) | 0 (0) | 1 (2.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Group A (n = 41) | Group B (n = 30) | Group C (n = 63) | Group A vs Group B, P value | Group A vs Group C, P value | Group B vs Group C, P value | |
| Dose reduction to initial dose of sorafenib | 8/41 (19.5%) | 2/30 (6.7%) | 26/63 (41.3%) | 0.700 | 0.108 | 0.005 |
| Re-escalation to initial dose of sorafenib | 2/8 (25.0%) | 2/2 (100.0%) | 2/26 (7.7%) | NA | NA | NA |
CT examinations performed every 2 mo showed that the disease control rate (DCR) gradually decreased in all groups. The response rate (RR) and DCR 8 mo after the start of sorafenib treatment were the highest in Group C (RR, 9.5%; DCR, 65.1%) (Table 4).
| Group A (n = 41) | Group B (n = 30) | Group C (n = 63) | |
| Complete response | 0 | 1 | 1 |
| Partial response | 1 | 1 | 5 |
| Stable disease | 10 | 11 | 35 |
| Progressive disease | 30 | 17 | 22 |
| Response rate | 2.4% | 6.7% | 9.5% |
| Disease control rate | 26.8% | 43.3% | 65.1% |
The main causes of permanent drug discontinuation were HCC progression and sorafenib-related AE intolerance. Permanent discontinuation due to AE intolerance occurred most frequently in Group B (Group A (17.1%) vs Group B (60.0%), P < 0.01; Group A (17.1%) vs Group C (20.6%), P = 1.00; Group B (60.0%) vs Group C (20.6%), P < 0.05) (Table 5).
| Group A (n = 41) | Group B (n = 30) | Group C (n = 63) | Group A vs Group B, P value | Group A vs Group C, P value | Group B vs Group C, P value | |
| Progression | 26 (63.4) | 7 (23.3) | 33 (52.4) | 0.006 | 1.000 | 0.046 |
| Intolerance | 7 (17.1) | 18 (60.0) | 13 (20.6) | 0.002 | 1.000 | 0.001 |
| Liver failure | 6 (14.6) | 3 (10.0) | 8 (12.7) | 1.000 | 1.000 | 1.000 |
| Other | 2 (4.9) | 2 (6.7) | 9 (14.3) | 1.000 | 0.690 | 1.000 |
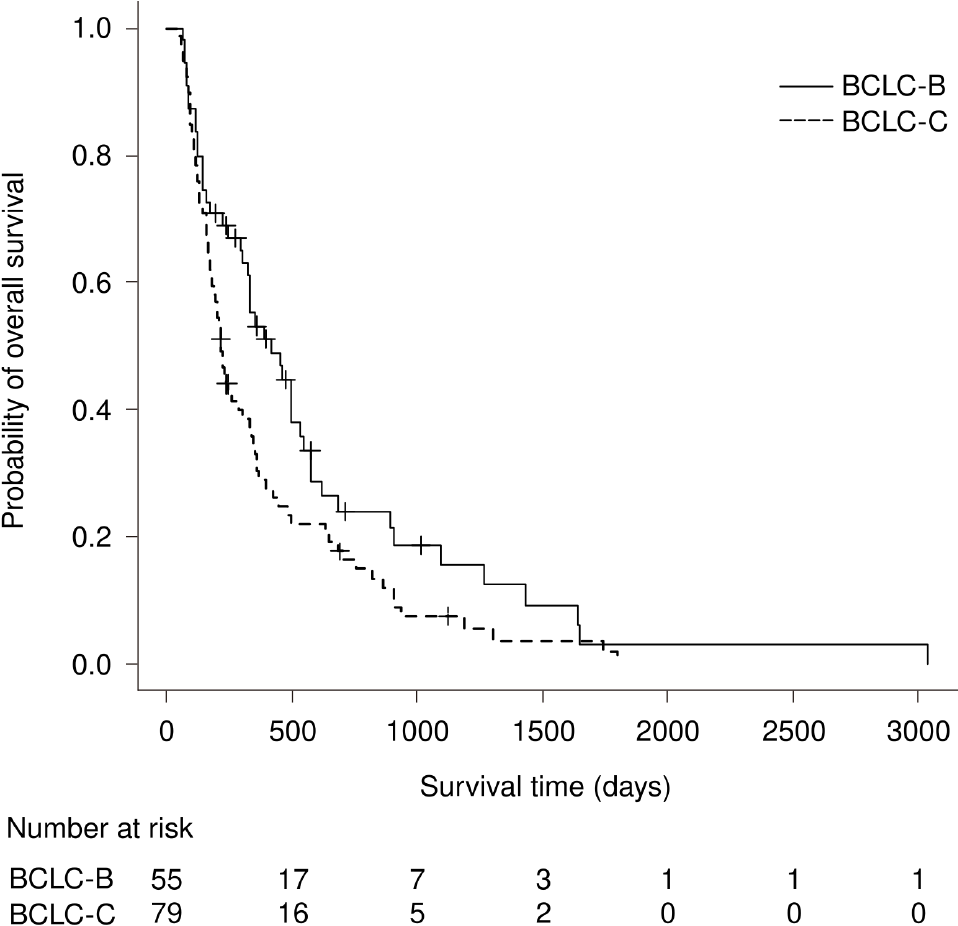
The median OS was 6.2 mo in Group A, 7.7 mo in Group B, and 13.9 mo in Group C. The difference in the median OS was significant between Groups A and C (P < 0.01). In multivariate analysis, Group A vs Group C (HR, 0.41; 95%CI: 0.25–0.66; P < 0.01) and BCLC-B (HR, 0.60, 95%CI: 0.41–0.89; P = 0.01) were independent predictors of survival (Figures 1 and 2, Table 6).
| Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (within 70 yr) | 0.867 | 0.603–1.246 | 0.440 | - | - | - |
| Male | 1.216 | 0.802–1.842 | 0.357 | - | - | - |
| Etiology (HBV) | 1.313 | 0.809–2.133 | 0.271 | - | - | |
| BCLC stage B | 0.667 | 0.459–0.969 | 0.033 | 0.601 | 0.405–0.891 | 0.011 |
| Portal vein thrombosis | 1.677 | 1.092–2.575 | 0.018 | 1.133 | 0.674–1.903 | 0.638 |
| Extrahepatic spread | 0.740 | 0.509–1.074 | 0.113 | 0.671 | 0.419–1.076 | 0.098 |
| AFP (> 400 ng/mL) | 1.282 | 0.893–1.839 | 0.178 | 1.370 | 0.936–2.006 | 0.105 |
| ECOG Performance status 1 | 0.752 | 0.488–1.158 | 0.196 | 1.042 | 0.636–1.708 | 0.869 |
| Group A vs Group B | 0.703 | 0.431–1.147 | 0.159 | 0.658 | 0.398–1.088 | 0.103 |
| Group A vs Group C | 0.431 | 0.281–0.663 | < 0.001 | 0.407 | 0.253–0.654 | < 0.001 |
| Sorafenib administration period (second half vs first half) | 1.205 | 0.840–1.728 | 0.311 | - | - | - |
The median TTF in Group C was 5.0 mo (95%CI: 3.8–6.5), which was the highest of all the groups [Group C (5.0 mo) vs Group A (2.1 mo), P < 0.01; Group C (5.0 mo) vs Group B (0.5 mo), P < 0.01). In multivariable Cox regression analysis, Group A vs Group B (HR, 1.69, 95%CI, 1.04–2.75; P = 0.03) and Group A vs Group C (HR, 0.53; 95%CI: 0.35–0.81; P < 0.01) were significant predictors of TTF (Table 7). The pro
| Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (within 70 yr) | 1.059 | 0.747–1.501 | 0.747 | - | - | - |
| Male | 0.926 | 0.625–1.372 | 0.703 | - | - | - |
| Etiology (HBV) | 1.208 | 0.768–1.901 | 0.413 | - | - | - |
| BCLC stage C | 1.311 | 0.921–1.866 | 0.132 | - | - | - |
| Portal vein thrombosis | 1.379 | 0.925–2.056 | 0.115 | 1.011 | 0.662–1.545 | 0.958 |
| Diarrhea | 0.675 | 0.473–0.965 | 0.031 | 0.654 | 0.449–0.952 | 0.027 |
| Hypertension | 1.070 | 0.735–1.556 | 0.725 | - | - | - |
| ECOG Performance status 1 | 0.687 | 0.446–1.058 | 0.089 | 0.725 | 0.463–1.135 | 0.159 |
| Group B vs Group A | 1.670 | 1.034–2.698 | 0.036 | 1.694 | 1.044–2.748 | 0.033 |
| Group C vs Group A | 0.495 | 0.328–0.747 | < 0.001 | 0.529 | 0.346–0.811 | 0.003 |
| Sorafenib administration period (second half vs first half) | 0.980 | 0.694–1.384 | 0.908 | - | - | - |
| Adjusted analyses | |||
| OR | 95%CI | P value | |
| Male | 0.352 | 0.141–0.877 | 0.025 |
| Child-Pugh stage B | 3.830 | 1.180–12.400 | 0.025 |
| Diarrhea | 0.472 | 0.198–1.120 | 0.089 |
| Group B vs Group A | 0.113 | 0.036–0.356 | < 0.001 |
| Group B vs Group C | 0.091 | 0.031–0.266 | < 0.001 |
The Spearman’s rank correlation coefficients between OS and TTF in each group were 0.41 (Group A; P < 0.01), 0.13 (Group B; P = 0.51), and 0.58 (Group C; P <0.01). There was a highly significant correlation between OS and TTF in Group C. However, there was no correlation between OS and TTF in Group B.
We investigated the effect of cooperation between oncologists and pharmacists on the prognosis of patients with advanced HCC treated with sorafenib monotherapy. In the present study, the occurrence of HFSR was associated with improved patient prog
Several studies have indicated that the emergence of HFSR is associated with prolonged survival in patients with advanced HCC treated with sorafenib[5,6,21]. However, these studies did not evaluate the correlation between medication adherence and survival after the appearance of an AE, including HFSR. Targeted therapies, including sorafenib, can result in unexpected AEs that do not occur after the administration of earlier chemotherapy drugs[22]. Oncologists must recognize these novel AEs at an early stage and provide appropriate treatment to the extent possible. However, previous studies revealed that optimal AE management requires considerable ex
The use of sorafenib is associated with various AEs, including gastrointestinal, constitutional, or dermatologic events[1,2], and their management may require dose reduction or temporary discontinuation to avoid sorafenib treatment cessation. For example, an appropriate sorafenib dose reduction yielded a decreased rate of per
In our study, only the mutual cooperation system promoted dose reduction after an AE and extended the TTF. In the mutual cooperation system, pharmacists were responsible for collecting data on AE grades, educating patients, and managing any leftover medicine. Furthermore, they documented their findings in a report for the oncologist. On the other hand, in the nonmutual cooperation system, an oncologist examined the patient before pharmacists were involved in patient management. Oncologists were required to evaluate AE grading data, educate patients, and determine the appropriate sorafenib dose. Only after the medical examination did a pharmacist provide additional patient management, and their findings were not reported to the oncologist. In the nonmutual cooperation system, the oncologist had to obtain a substantial amount of information to maintain or revise the sorafenib treatment regimen within 5 to 10 min.
Given these differences between the systems, our results suggest that the mutual cooperation system led to appropriate dose reductions, as reflected by the extended TTF. However, we do not recommend starting sorafenib at half the standard dose (800 mg/d). Dose reductions were guided by the results obtained by the mutual coope
A previous study showed that despite dramatic improvements in adjuvant hormo
Hurdles to medication adherence are complex and include patient-, clinician-, and healthcare system-related factors. Patient-related factors, such as limited involvement in the treatment decision-making process, poor health literacy, doubts about medication effectiveness, and previous adverse effects, influence adherence. Clinician-related factors include failure to recognize nonadherence, poor patient communi
Surprisingly, this study revealed that the prognostic value of HFSR was enhanced by appropriate medication adherence. On the other hand, BCLC-B HCC was an independent predictor of improved OS[29]. BCLC stage did not affect the difference in OS between Groups A and C, as there was no significant between-group difference in the baseline stage distribution.
We have reasonable evidence to confirm the validity of our results. First, variables such as age, sex, etiology, ECOG performance status, liver function, comorbidities, and TACE procedure count were not significantly different between the groups. Second, we verified that all patients had received sorafenib monotherapy and no subsequent chemotherapy; therefore, neither our patients’ prognoses nor the prolonged OS we observed was affected by other chemotherapeutic agents.
Nevertheless, our study has a few limitations. First, our study design was based on the mutual cooperation system. After a patient was first checked by a specialized pharmacist, the oncologist determined whether to prescribe sorafenib based on the pharmacist’s report. However, patients were not required to participate in the mutual cooperation system, and involvement was subject to the patient’s wish. After the patients underwent a medical examination by an oncologist, a specialized pharmacist could also provide patient guidance about sorafenib. While patients who were un
The mutual cooperation system increased treatment duration and improved prognosis in patients with HFSR secondary to sorafenib treatment. Additionally, the mutual cooperation system allowed us to promptly initiate sorafenib treatment. Our study clearly demonstrates the clinical and research benefits of this system. The mutual cooperation system for sorafenib treatment management described in this study could be applied to the management of patients treated with other multikinase inhibitors to extend OS. The increased OS resulting from the mutual cooperation system could have a substantial impact on the design of clinical studies in which sorafenib is used as the control drug. Additionally, nonadherence may have adversely affected OS in previous studies, leading researchers to underestimate drug efficacy. We propose that future clinical investigations designed to improve medication adherence could eliminate OS underestimation.
Although sorafenib prolongs overall survival (OS) in patients with hepatocellular carcinoma (HCC), the drug is associated with various adverse events (AEs) that may lead to permanent discontinuation.
The authors postulated that mutual cooperative intervention for AEs could improve OS in patients with HCC.
The aim of this study is to clarify the association between AE interventions and patient prognosis.
The authors developed a mutual cooperation system that was initiated at the start of sorafenib treatment to manage AEs effectively. The system entailed pharmacist con
The authors enrolled 134 patients (Group A, n = 41; Group B, n = 30; Group C, n = 63). The median OS significantly differed between Groups A and C (6.2 vs 13.9 mo, P < 0.01) but not between Groups A and B (6.2 vs 7.7 mo, P = 0.62). Group A vs Group C was an independent OS predictor (HR, 0.41; 95%CI: 0.25–0.66; P < 0.01). In Group B alone, the time to treatment failure (TTF) was significantly shorter, while the no
The mutual cooperation system increased the treatment duration and improved the prognosis of patients with HFSR.
Future prospective studies (e.g., randomized controlled trials) and improved adheren
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yu F S-Editor: Ma YJ L-Editor: A P-Editor: Yuan YY
| 1. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10266] [Article Influence: 603.9] [Reference Citation Analysis (2)] |
| 2. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4649] [Article Influence: 273.5] [Reference Citation Analysis (0)] |
| 3. | Iavarone M, Cabibbo G, Biolato M, Della Corte C, Maida M, Barbara M, Basso M, Vavassori S, Craxì A, Grieco A, Cammà C, Colombo M. Predictors of survival in patients with advanced hepatocellular carcinoma who permanently discontinued sorafenib. Hepatology. 2015;62:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Vincenzi B, Santini D, Russo A, Addeo R, Giuliani F, Montella L, Rizzo S, Venditti O, Frezza AM, Caraglia M, Colucci G, Del Prete S, Tonini G. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist. 2010;15:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Reig M, Torres F, Rodriguez-Lope C, Forner A, LLarch N, Rimola J, Darnell A, Ríos J, Ayuso C, Bruix J. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 6. | Díaz-González Á, Sanduzzi-Zamparelli M, Sapena V, Torres F, LLarch N, Iserte G, Forner A, da Fonseca L, Ríos J, Bruix J, Reig M. Systematic review with meta-analysis: the critical role of dermatological events in patients with hepatocellular carcinoma treated with sorafenib. Aliment Pharmacol Ther. 2019;49:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Tovoli F, Ielasi L, Casadei-Gardini A, Granito A, Foschi FG, Rovesti G, Negrini G, Orsi G, Renzulli M, Piscaglia F. Management of adverse events with tailored sorafenib dosing prolongs survival of hepatocellular carcinoma patients. J Hepatol. 2019;71:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, Apperley JF, Szydlo R, Desai R, Kozlowski K, Paliompeis C, Latham V, Foroni L, Molimard M, Reid A, Rezvani K, de Lavallade H, Guallar C, Goldman J, Khorashad JS. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 695] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 9. | Partridge AH, Archer L, Kornblith AB, Gralow J, Grenier D, Perez E, Wolff AC, Wang X, Kastrissios H, Berry D, Hudis C, Winer E, Muss H. Adherence and persistence with oral adjuvant chemotherapy in older women with early-stage breast cancer in CALGB 49907: adherence companion study 60104. J Clin Oncol. 2010;28:2418-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Greer JA, Amoyal N, Nisotel L, Fishbein JN, MacDonald J, Stagl J, Lennes I, Temel JS, Safren SA, Pirl WF. A Systematic Review of Adherence to Oral Antineoplastic Therapies. Oncologist. 2016;21:354-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 11. | Ibrahim AR, Eliasson L, Apperley JF, Milojkovic D, Bua M, Szydlo R, Mahon FX, Kozlowski K, Paliompeis C, Foroni L, Khorashad JS, Bazeos A, Molimard M, Reid A, Rezvani K, Gerrard G, Goldman J, Marin D. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117:3733-3736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2:323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 355] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 13. | Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008;27:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 1606] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 14. | Viswanathan M, Golin CE, Jones CD, Ashok M, Blalock SJ, Wines RC, Coker-Schwimmer EJ, Rosen DL, Sista P, Lohr KN. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157:785-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 539] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 15. | Ochi M, Kamoshida T, Ohkawara A, Ohkawara H, Kakinoki N, Hirai S, Yanaka A. Multikinase inhibitor-associated hand-foot skin reaction as a predictor of outcomes in patients with hepatocellular carcinoma treated with sorafenib. World J Gastroenterol. 2018;24:3155-3162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Shomura M, Kagawa T, Shiraishi K, Hirose S, Arase Y, Koizumi J, Mine T. Skin toxicity predicts efficacy to sorafenib in patients with advanced hepatocellular carcinoma. World J Hepatol. 2014;6:670-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3302] [Article Influence: 220.1] [Reference Citation Analysis (36)] |
| 18. | Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 853] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 19. | Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 544] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 20. | Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 940] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Howell J, Pinato DJ, Ramaswami R, Bettinger D, Arizumi T, Ferrari C, Yen C, Gibbin A, Burlone ME, Guaschino G, Sellers L, Black J, Pirisi M, Kudo M, Thimme R, Park JW, Sharma R. On-target sorafenib toxicity predicts improved survival in hepatocellular carcinoma: a multi-centre, prospective study. Aliment Pharmacol Ther. 2017;45:1146-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014;11:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Raoul JL, Adhoute X, Penaranda G, Perrier H, Castellani P, Oules V, Bourlière M. Sorafenib: Experience and Better Manage-ment of Side Effects Improve Overall Survival in Hepatocellular Carcinoma Patients: A Real-Life Retrospective Analysis. Liver Cancer. 2019;8:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 24. | Forner A, Da Fonseca LG, Díaz-González Á, Sanduzzi-Zamparelli M, Reig M, Bruix J. Controversies in the management of hepatocellular carcinoma. JHEP Rep. 2019;1:17-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Hershman DL. Sticking to It: Improving Outcomes by Increasing Adherence. J Clin Oncol. 2016;34:2440-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | McCowan C, Shearer J, Donnan PT, Dewar JA, Crilly M, Thompson AM, Fahey TP. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 281] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 27. | Kini V, Ho PM. Interventions to Improve Medication Adherence: A Review. JAMA. 2018;320:2461-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 346] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 28. | Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 29. | Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. 2017;67:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 453] [Article Influence: 56.6] [Reference Citation Analysis (0)] |