Published online Aug 28, 2021. doi: 10.3748/wjg.v27.i32.5351
Peer-review started: January 28, 2021
First decision: May 2, 2021
Revised: May 15, 2021
Accepted: July 19, 2021
Article in press: July 19, 2021
Published online: August 28, 2021
Processing time: 208 Days and 2.8 Hours
The close relationship of medicine with technology and the particular interest in this symbiosis in recent years has led to the development of several computed artificial intelligence (AI) systems aimed at various areas of medicine. A number of studies have demonstrated that those systems allow accurate diagnoses with histological precision, thus facilitating decision-making by clinicians in real time. In the field of gastroenterology, AI has been applied in the diagnosis of pathologies of the entire digestive tract and their attached glands, and are increasingly accepted for the detection of colorectal polyps and confirming their histological classification. Studies have shown high accuracy, sensitivity, and specificity in relation to expert endoscopists, and mainly in relation to those with less experience. Other applications that are increasingly studied and with very promising results are the investigation of dysplasia in patients with Barrett's esophagus and the endoscopic and histological assessment of colon inflammation in patients with ulcerative colitis. In some cases AI is thus better than or at least equal to human abilities. However, additional studies are needed to reinforce the existing data, and mainly to determine the applicability of this technology in other indications. This review summarizes the state of the art of AI in gastroenterological pathology.
Core Tip: The uses of artificial intelligence (AI) in gastroenterology are growing from day to day. Many published studies have demonstrated the potential benefits of using this technology in clinical practice. We review here the most recent studies related to the major hot topics of AI in gastroenterology, namely colorectal polyps, dysplasia in Barrett's esophagus, and inflammation in ulcerative colitis. The machine seems to cooperate with the human, helping him to exceed his abilities and making the future of this symbiosis very promising.
- Citation: Correia FP, Lourenço LC. Artificial intelligence application in diagnostic gastrointestinal endoscopy - Deus ex machina? World J Gastroenterol 2021; 27(32): 5351-5361
- URL: https://www.wjgnet.com/1007-9327/full/v27/i32/5351.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i32.5351
Artificial intelligence (AI) is a concept that involves the fields of science and engineering, and was defined by one of its founders, Alan Turing, as the ability of a computer to achieve human performance in cognitive tasks[1]. Machine Learning (ML) is an AI subfield that allows the identification of patterns that can be used to analyze a particular situation[2]. ML can "learn" and improve with experience gained from provided data sets and then apply that information to similar scenarios in the future[2]. Deep learning (DL) is an AI subfield that uses artificial neural networks that are similar to the human brain, and which the capacity to learn and make decisions on its own[2]. Since 1970, AI has been used in medicine for various purposes, including improving the effectiveness of diagnosis, facilitating therapeutic and disease monitoring, and improving overall patient outcomes[2,3].
Gastroenterology is a medical specialty with great potential for integrating AI technology, as the diagnosis of gastrointestinal conditions relies much on image-based investigations (endoscopy and radiology)[4]. Numerous recent studies have investigated the applicability of AI in such diverse areas of gastroenterology, as in the investigation of dysplasia in Barrett's esophagus (BE), diagnosis of gastroesophageal reflux disease, differentiation of acute and chronic pancreatitis, detection and classification of colorectal polyps, characterization of colonic inflammatory activity in patients with inflammatory bowel disease (IBD), among others[2].
In this review, we aimed to describe the latest uses of AI in gastroenterology and the evidence that allows understanding how far these developments can supplant human abilities. We conducted a search of several platforms (PubMed, MEDLINE and EMBASE), with no time limit for articles that compared the performance of these systems and endoscopists.
The epidemiology of esophageal cancer has changed over the years. The increasing incidence of esophageal adenocarcinoma since the 1970s, has made it the most common type of esophageal cancer, mainly in the western countries[5,6]. BE is considered a premalignant condition[5,6] with a risk of progression to esophageal adenocarcinoma of up to 0.3% per year[7]. Endoscopic surveillance programs are based on the fact that progression tends to occur gradually from low-grade to high-grade dysplasia and esophageal adenocarcinoma. The aim is to find premalignant and malignant lesions at an early stage[8].
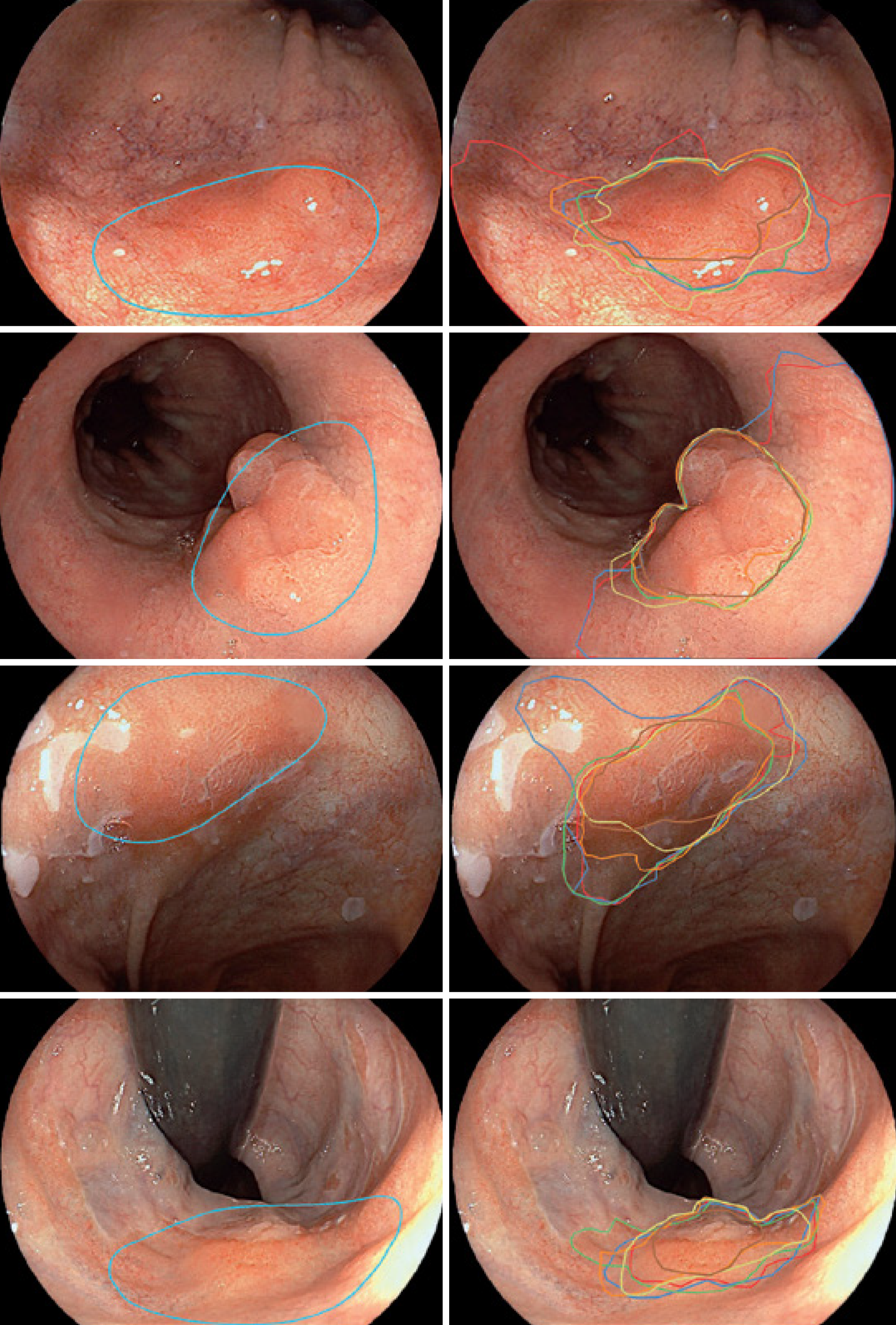
Because of the possible impact on the survival of the patients, there has been a growing interest in the application of AI and ML in patients with BE[9]. Several studies have reported high rates of sensitivity, specificity and accuracy with computer-aided detection (CAD) systems in detecting and delineating dysplastic lesions in patients with BE[10-13]. In comparison with expert endoscopists, the CAD system had an accuracy of 88% vs 73%, sensitivity of 93% vs 72%, and specificity of 83% vs 74%[10]. In addition, the area of delineation of the lesion by the CAD system overlapped that of expert endoscopists (Figure 1)[10].
Despite the promising results and the data showing that the detection speed for dysplastic lesions was compatible with the real-time use of endoscopic surveillance, this application has only recently been tried in real time. Thus, Ebigbo et al[14] used the CAD system in 14 patients simultaneously with endoscopic examination by BE-expert endoscopists. The performance of the AI system was similar to that of the experienced endoscopists, with a sensitivity of 83.7%, a specificity of 100%, and an accuracy of 89.9%, suggesting its potential to improve endoscopic surveillance of BE, mainly by nonexpert endoscopists.
Currently, there is no evidence that advanced imaging techniques, such as chromoendoscopy, autofluorescence endoscopy, or confocal laser endomicroscopy, offer advantages over high-definition white-light endoscopy (WLE)[7]. Volumetric laser endomicroscopy (VLE) is a recent endoscopic imaging technology that uses optical coherence tomography to produce high-resolution scans of 6-cm segments of the esophagus, with surface and subsurface image depth greater that 3 mm[15,16]. In this way, the real-time diagnosis of surface and subsurface lesions, as well as guiding their endoscopic treatment is possible[15,16]. VLE scans comprise a large amount of visual information with numerous gray-shade images that make interpretation complex and time consuming even by experts[17-19]. CAD systems have been developed that are better at identifying early BE neoplasia in ex vivo VLE images than VLE experts are[17], mainly when multiframe analysis is used[18]. However, further studies are needed to validate in vivo data. Trindade et al[19] also created AI image enhancement software called intelligent real-time image segmentation, that identifies three VLE features previously associated with histologic dysplasia (i.e. hyper-reflective surface, hyporeflective structures and lack of a layered architecture) and displays them as different colors superimposed over the VLE image to facilitate interpretation. Studies are underway to assess the effectiveness of this AI system.
It is now known that colorectal cancer (CRC) surveillance with colonoscopy of both the right and left colon is associated with a reduction in CRC mortality[20-22]. The importance of a meticulous high-quality colonoscopy is a result of the inverse relationship that exists between the adenoma detection rate and the risk of interval CRC or advanced-stage CRC[23]. The high rate (up to 30%) of missed adenomas during screening colonoscopy led to the development of computer-aided detection (CADe) systems, which are programs based on DL, in an attempt to mitigate this problem[24]. The first study[25] on the use of CADe in the detection of colon polyps was published in 2003, and it demonstrated good results. However, only static images were used. Since then, several systems have been developed and used with real-time endoscopy. In recent systematic review and meta-analysis, Hassan et al[26] assessed the relationship between the increased detection of polyps and the main features of the detected lesions. The review included five randomized clinical trials with a total of 4354 patients (2163 in the CADe and 2191 in the control group). The highlight of the results was that the adenoma detection rate in the CADe group was significantly higher (36.6%) than that in the control group (25.2%) regardless of adenoma size, location, and morphology of the polyps. The rates of detection of advanced adenomas were not significantly different. The authors concluded that AI could benefit colorectal cancer surveillance, as the factors that affect the detection of lesions by human observers, such as size and morphology, do not interfere with detection by the AI system. In addition, the study shows that the AI system did not affect the efficiency of colonoscopy, maintaining similar withdrawal time in both groups. The results are supported by those in prospective study by Liu et al[27] in which the adenoma detection rate was higher in the CADe group than in the control group (29.01% vs 20.91%) and a meta-analysis by Barua et al[28] that found an absolute increase of 10.3% in the detection rate of adenomas. The increase was mainly the result of increased detection of nonadvanced diminutive adenomas, serrated adenomas, and hyperplastic polyps[26,27]. In addition to the smaller size, other characteristics of the polyps first detected by the CADe system were isochromia, flat shape, unclear boundary, partly behind colon folds, and at the edge of the visual field[27]. Thus, the features of the polyps seem to contribute to a higher probability of missed lesions, however Lui et al[29] found that 79% of missed lesions can be detected with AI support during the first examination and considered that the main cause of missed lesions was human error, such as less endoscopist experience or the presence of multiple polyps that can increase endoscopist distraction or fatigue. We did not find consistent evidence for the use of advanced imaging techniques in the detection of polyps by CAD systems.
One of the problems of CRC surveillance is the high cost of polypectomy and pathological examination, in particular given the high prevalence of diminutive polyps ≤ 5 mm[30]. It has been proven that a resect and discard strategy for diminutive polyps has an important economic impact, without losing efficacy in CRC surveillance[30]. An optical biopsy of diminutive polyps supported by image-enhanced endoscopic may be appropriate. The Preservation and Incorporation of Valuable endoscopic Innovations (PIVI) group of the American Society for Gastrointestinal Endoscopy (ASGE)[31] recommends that for a “diagnose-and-leave” strategy, endoscopic diagnosis should provide a 90% or higher negative predictive value for adenomatous histology when used with high confidence. For a “resect and discard” strategy for adenomas ≤ 5 mm, it is recommended that an optical biopsy with high confidence, when combined with a histologic assessment of polyps larger than 5 mm, should provide ≥ 90% agreement in the assignment of post polypectomy surveillance intervals compared with decisions based on pathological assessment of all identified polyps.
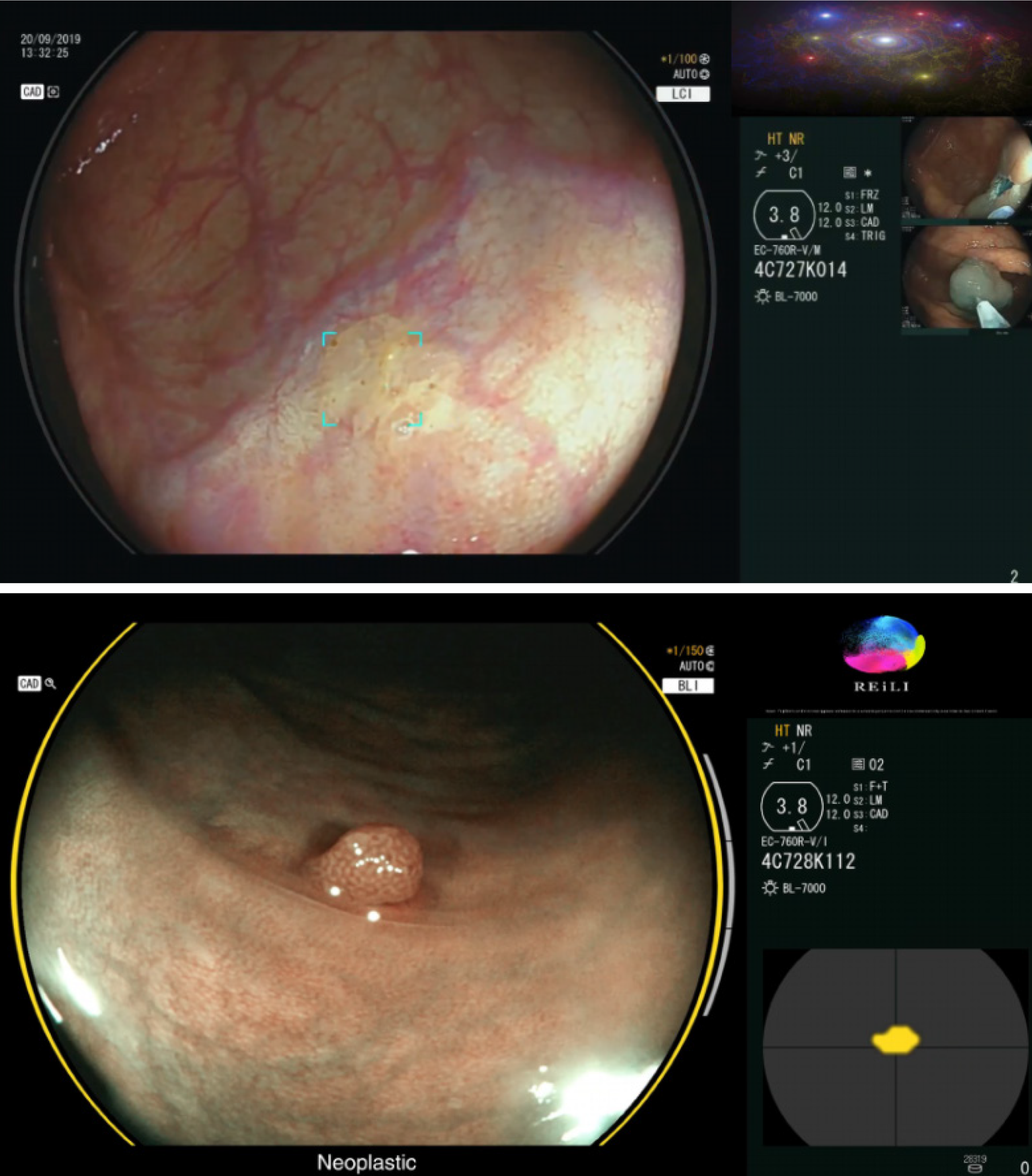
The optical diagnosis of colorectal polyps has not yet been widely implemented because of the lack of endoscopist experience and the considerable learning curve of advanced imaging modalities[32,33]. To overcome this problem, AI systems such as, computer-aided diagnostic (CADx) that help to predict the histology (i.e. neoplastic or non-neoplastic) of detected polyps (Figure 2) have been developed with the aim of avoiding unnecessary endoscopic biopsies or resection[33-35]. Yang et al[35] created DL models that classify colorectal lesions histologically using white-light colonoscopy images. They found an accuracy of 79.5% in distinguishing neoplastic and non-neoplastic lesions and 87.1% in distinguishing advanced and nonadvanced colorectal lesions. When comparing the performance of endoscopists and CADx for the classification of polyps, they found that CADx performed better than less experienced endoscopists, but not better than experts.
Combinations of advanced imaging modalities, such as narrow-band imaging (NBI) or endocytoscopy, and CAD systems improved the performance of AI-assisted optical biopsy[34]. Several studies have shown the potential of combining NBI with CADx in the classification of polyps[36-38]. Song et al[37] developed a DL model to predict colorectal polyp histology based on NBI near-focus images. Their study showed a good diagnostic accuracy (81.3%-82.4%) that was comparable to expert endoscopists and was significantly more accurate than trainee endoscopists. CADx performance was consistent regardless of polyp size, location, or morphology, with an average histological assessment time of 0.02-0.04 s. They concluded that an AI CAD system can help inexperienced endoscopists in the histological assessment of colorectal polyps and increase the confidence of experts regarding their assessments.
A recent systematic review and meta-analysis that included 18 studies (15 retrospective and three prospective) and 7680 images of colorectal polyps was performed to evaluate the prediction of histology[39]. AI had an accuracy of 0.96 (AUC), with a sensitivity of 92.3% and a specificity of 89.8%. Comparing studies that used NBI with those that used non-NBI techniques, they found a significantly better accuracy (AUC 0.98 vs 0.84) in the NBI studies. When they evaluated performance in the histological characterization of diminutive polyps, they found that the accuracy overlapped and that the pooled negative predictive value was 91.3%-95.1%, which complies with the threshold of the "resect and discard" strategy of the ASGE. The difference in the performance of AI and endoscopists was not significant except in studies that included nonexpert endoscopists, in which the performance AI was significantly better than that of nonexpert endoscopists.
Endocytoscopy (EC) is an advanced endoscopic technique in which a contact light microscopy system is integrated into the distal tip of a conventional colonoscope[40]. This technique has performed well in differentiating between diminutive neoplastic and non-neoplastic colorectal polyps both alone[41] and in combination with NBI (EC-NBI)[42]. The combined use of EC and CAD (EC-CAD) to classify the histology of colorectal polyps has been studied. According to Mori et al[40], this technology achieved a sensitivity of 92%, a specificity of 79.5%, and an accuracy of 89.2%. The performance was comparable to EC image evaluation by experts, but significantly better than evaluation by trainees for identifying neoplastic polyps.
Ulcerative colitis (UC) is a chronic inflammatory disease, and the therapeutic goals have changed over the last decades, from the treatment of symptoms toward mucosal healing, with the aim of modifying the natural history of disease[43-45]. Mucosal healing can be defined by endoscopic evaluation because the inflammation is limited to the mucosal layer[44]. However, it is currently known that histological evidence of inflammation, even in patients with mucosal healing, is a prognostic marker of a two-to-three-fold increase in the risk of colitis relapse and colon cancer[44,45]. Conse
Several studies have used AI for the macroscopic and histological characterization of colic mucosa in UC. Takenaka et al[47] developed a deep neural network for evaluation of UC with the aim of predicting endoscopic remission, a UC endoscopic index of severity score of 0; and histologic remission, a Geboes score of ≤ 3 points using endoscopic images. In the training set, they used 40,758 images of colonoscopies and the histologic results of 6885 biopsies from the same patients. They validated the results in a prospective study of 875 patients with 4187 endoscopic images and 4104 biopsy specimens. In the validation phase, the AI system was able to identify patients with endoscopic remission with 90.1% of accuracy and histologic remission with 92.9% of accuracy using only endoscopic images. The results suggest that computer-aided diagnosis can reliably assist endoscopic and histologic disease activity in UC without the need for mucosal biopsies. It should be noted that the study used only white-light images (WLIs). Chromoendoscopy was not used after WLI evaluation.
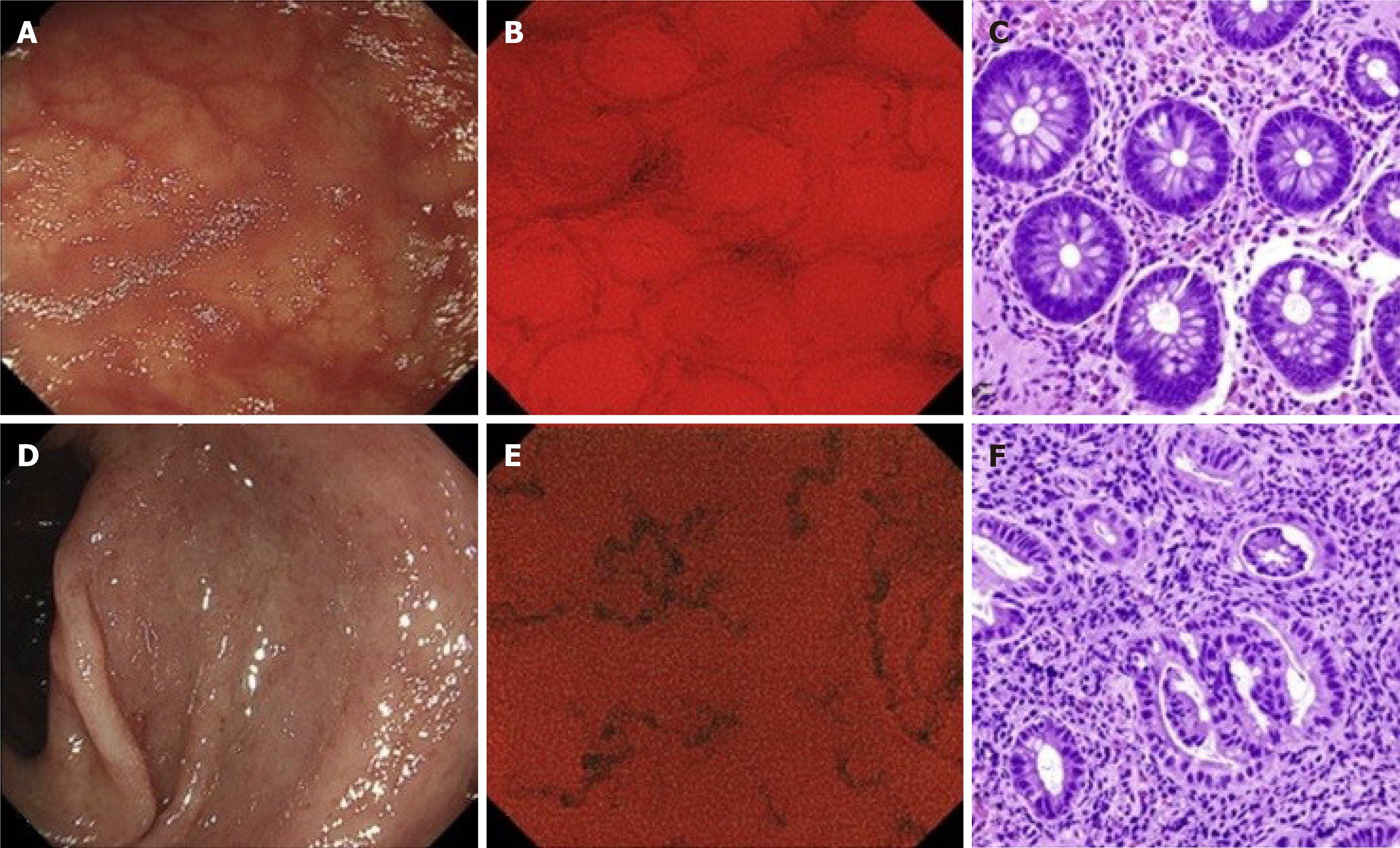
However, there are doubts that conventional WLI can reliably identify persistent histologic inflammation. Alternatives such as NBI, confocal laser endomicroscopy, or EC have emerged[48]. Despite their excellent diagnostic performance, these techniques are highly dependent on experienced endoscopists[48]. Maeda et al[48] developed a CAD system that used AI and EC to predict persistent histologic inflammation (Figure 3). It was used to retrospectively evaluate 12900 EC images for ML and 9935 EC images for validation, in addition assessment of biopsy samples by experienced pathologists blinded to the endoscopy results. The algorithm had 74% sensitivity, 97% specificity, and 91% accuracy in identifying histologic inflammation, and perfect reproducibility. The results are promising, and the authors concluded that the symbiosis achieved with the CAD system and EC has the potential to support immediate intervention and ultimately reduce the number of required biopsy samples.
There is a lack of published studies of the potential benefit of AI in assessment of disease activity in Crohn’s disease. Evidence of the applicability of AI to the surveillance of dysplasia in patients with IBD is still lacking. Maeda et al[49] described a case in which an AI system assisted in the detection of dysplastic lesions in a patient with UC. They concluded that AI may have potential in this context, especially for nonexpert endoscopists.
The evidence for a role of AI in diagnostic endoscopy is growing, with increasing supporting evidence in dysplasia detection in BE, identification and assessment of colorectal polyps, and characterization of colonic mucosa in UC. A number of possible applications are still under evaluation (Table 1)[50-58]. Deus ex machina is a Latin expression meaning “God from the machine”. The term was coined from the conventions of ancient Greek theatre, where actors who were playing gods were brought onto stage using a machine[59]. Later it was used in the story plots of theatre plays and movies to describe un unexpected and sudden solution to resolve main issues or tragedies. AI is currently being developed for use in endoscopy daily practice. It seems to be a quick solution of the long-standing problems of the interobserver agreement and the subjective “feeling” and accuracy of the endoscopist in diagnostic exams. Apart from the complexity of improving or overcoming human evaluation, there are also availability issues that will limit the access to this new technology.
| Disease | Detection | Ref. |
| Esophageal squamous cell carcinoma (ESCC) | Detection of early ESCC | Yang et al[50] |
| Kumagai et al[51] | ||
| Helicobacter pylori infection | Diagnosis of Helicobacter pylori infection | Mohan et al[52] |
| Gastric cancer (GC) | Detection of preneoplastic lesions and early gastric cancerPrediction the invasion depth of GC | Anta et al[53] |
| Nagao et al[54] | ||
| Celiac disease | Early detection of celiac disease | Sana et al[55] |
| Gastrointestinal bleeding | Detection of small bowel bleeding with wireless capsule endoscopic | Aoki et al[56] |
| Tsuboi et al[57] | ||
| Pancreatic lesions | Differentiation of pancreatic cancer from chronic pancreatitis and normal pancreas | Tonozuka et al[58] |
In the meantime, the added value of electronic systems to human practice must not be underrated as they would definitely contribute to the standardization of diagnostic endoscopy worldwide. Apart from that, savings in the time, effort, economic, and human resources needed for diagnostic exams, would make room for improvements in endoscopic therapeutic procedures, which are growing in complexity over time.
In conclusion, AI is currently used to optimize diagnostic endoscopy procedures, with growing indications and evidence over time. Although it may not be ready for prime-time use worldwide, the future of AI is near. It will depend on us, human endoscopists, to define how far these machines can help us with (and solve?) our naturally imperfect issues.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Portugal
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gora MJ S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Li JH
| 1. | Ramesh AN, Kambhampati C, Monson JR, Drew PJ. Artificial intelligence in medicine. Ann R Coll Surg Engl. 2004;86:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 2. | Kaul V, Enslin S, Gross SA. History of artificial intelligence in medicine. Gastrointest Endosc. 2020;92:807-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 315] [Article Influence: 63.0] [Reference Citation Analysis (1)] |
| 3. | Liu R, Rong Yan, Peng Z. A review of medical artificial intelligence. Global Health J. 2020;4:42-45. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Sung JJ, Poon NC. Artificial intelligence in gastroenterology: where are we heading? Front Med. 2020;14:511-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1059] [Article Influence: 117.7] [Reference Citation Analysis (0)] |
| 6. | Maitra I, Date RS, Martin FL. Towards screening Barrett's oesophagus: current guidelines, imaging modalities and future developments. Clin J Gastroenterol. 2020;13:635-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Weusten B, Bisschops R, Coron E, Dinis-Ribeiro M, Dumonceau JM, Esteban JM, Hassan C, Pech O, Repici A, Bergman J, di Pietro M. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 372] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 8. | Sharma P, Shaheen NJ, Katzka D, Bergman JJGHM. AGA Clinical Practice Update on Endoscopic Treatment of Barrett's Esophagus With Dysplasia and/or Early Cancer: Expert Review. Gastroenterology. 2020;158:760-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 9. | de Souza LA Jr, Palm C, Mendel R, Hook C, Ebigbo A, Probst A, Messmann H, Weber S, Papa JP. A survey on Barrett's esophagus analysis using machine learning. Comput Biol Med. 2018;96:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | de Groof AJ, Struyvenberg MR, van der Putten J, van der Sommen F, Fockens KN, Curvers WL, Zinger S, Pouw RE, Coron E, Baldaque-Silva F, Pech O, Weusten B, Meining A, Neuhaus H, Bisschops R, Dent J, Schoon EJ, de With PH, Bergman JJ. Deep-Learning System Detects Neoplasia in Patients With Barrett's Esophagus With Higher Accuracy Than Endoscopists in a Multistep Training and Validation Study With Benchmarking. Gastroenterology. 2020;158:915-929.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 11. | Hashimoto R, Requa J, Dao T, Ninh A, Tran E, Mai D, Lugo M, El-Hage Chehade N, Chang KJ, Karnes WE, Samarasena JB. Artificial intelligence using convolutional neural networks for real-time detection of early esophageal neoplasia in Barrett's esophagus (with video). Gastrointest Endosc. 2020;91:1264-1271.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 12. | Lui TKL, Tsui VWM, Leung WK. Accuracy of artificial intelligence-assisted detection of upper GI lesions: a systematic review and meta-analysis. Gastrointest Endosc. 2020;92:821-830.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | de Groof J, van der Sommen F, van der Putten J, Struyvenberg MR, Zinger S, Curvers WL, Pech O, Meining A, Neuhaus H, Bisschops R, Schoon EJ, de With PH, Bergman JJ. The Argos project: The development of a computer-aided detection system to improve detection of Barrett's neoplasia on white light endoscopy. United European Gastroenterol J. 2019;7:538-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Ebigbo A, Mendel R, Probst A, Manzeneder J, Prinz F, de Souza LA Jr, Papa J, Palm C, Messmann H. Real-time use of artificial intelligence in the evaluation of cancer in Barrett's oesophagus. Gut. 2020;69:615-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 15. | Wolfsen HC. Volumetric Laser Endomicroscopy in Patients With Barrett Esophagus. Gastroenterol Hepatol (N Y). 2016;12:719-722. [PubMed] |
| 16. | Wolfsen HC, Sharma P, Wallace MB, Leggett C, Tearney G, Wang KK. Safety and feasibility of volumetric laser endomicroscopy in patients with Barrett's esophagus (with videos). Gastrointest Endosc. 2015;82:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Swager AF, van der Sommen F, Klomp SR, Zinger S, Meijer SL, Schoon EJ, Bergman JJGHM, de With PH, Curvers WL. Computer-aided detection of early Barrett's neoplasia using volumetric laser endomicroscopy. Gastrointest Endosc. 2017;86:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 18. | Struyvenberg MR, van der Sommen F, Swager AF, de Groof AJ, Rikos A, Schoon EJ, Bergman JJ, de With PHN, Curvers WL. Improved Barrett's neoplasia detection using computer-assisted multiframe analysis of volumetric laser endomicroscopy. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Trindade AJ, McKinley MJ, Fan C, Leggett CL, Kahn A, Pleskow DK. Endoscopic Surveillance of Barrett's Esophagus Using Volumetric Laser Endomicroscopy With Artificial Intelligence Image Enhancement. Gastroenterology. 2019;157:303-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Pan J, Xin L, Ma YF, Hu LH, Li ZS. Colonoscopy Reduces Colorectal Cancer Incidence and Mortality in Patients With Non-Malignant Findings: A Meta-Analysis. Am J Gastroenterol. 2016;111:355-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Niikura R, Hirata Y, Suzuki N, Yamada A, Hayakawa Y, Suzuki H, Yamamoto S, Nakata R, Komatsu J, Okamoto M, Kodaira M, Shinozaki T, Fujishiro M, Watanabe T, Koike K. Colonoscopy reduces colorectal cancer mortality: A multicenter, long-term, colonoscopy-based cohort study. PLoS One. 2017;12:e0185294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Doubeni CA, Corley DA, Quinn VP, Jensen CD, Zauber AG, Goodman M, Johnson JR, Mehta SJ, Becerra TA, Zhao WK, Schottinger J, Doria-Rose VP, Levin TR, Weiss NS, Fletcher RH. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut. 2018;67:291-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 23. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1561] [Article Influence: 141.9] [Reference Citation Analysis (0)] |
| 24. | Wang P, Liu P, Glissen Brown JR, Berzin TM, Zhou G, Lei S, Liu X, Li L, Xiao X. Lower Adenoma Miss Rate of Computer-Aided Detection-Assisted Colonoscopy vs Routine White-Light Colonoscopy in a Prospective Tandem Study. Gastroenterology. 2020;159:1252-1261.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 25. | Karkanis SA, Iakovidis DK, Maroulis DE, Karras DA, Tzivras M. Computer-aided tumor detection in endoscopic video using color wavelet features. IEEE Trans Inf Technol Biomed. 2003;7:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Hassan C, Spadaccini M, Iannone A, Maselli R, Jovani M, Chandrasekar VT, Antonelli G, Yu H, Areia M, Dinis-Ribeiro M, Bhandari P, Sharma P, Rex DK, Rösch T, Wallace M, Repici A. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93:77-85.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 308] [Article Influence: 77.0] [Reference Citation Analysis (1)] |
| 27. | Liu P, Wang P, Glissen Brown JR, Berzin TM, Zhou G, Liu W, Xiao X, Chen Z, Zhang Z, Zhou C, Lei L, Xiong F, Li L, Liu X. The single-monitor trial: an embedded CADe system increased adenoma detection during colonoscopy: a prospective randomized study. Therap Adv Gastroenterol. 2020;13:1756284820979165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 28. | Barua I, Vinsard DG, Jodal HC, Løberg M, Kalager M, Holme Ø, Misawa M, Bretthauer M, Mori Y. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy. 2021;53:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (1)] |
| 29. | Lui TKL, Hui CKY, Tsui VWM, Cheung KS, Ko MKL, Foo DCC, Mak LY, Yeung CK, Lui TH, Wong SY, Leung WK. New insights on missed colonic lesions during colonoscopy through artificial intelligence-assisted real-time detection (with video). Gastrointest Endosc. 2021;93:193-200.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. 2010;8:865-869, 869.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 31. | ASGE Technology Committee, Abu Dayyeh BK, Thosani N, Konda V, Wallace MB, Rex DK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, Maple JT, Murad FM, Siddiqui UD, Banerjee S. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2015;81:502.e1-502.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 248] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 32. | Castela J, Mão de Ferro S, Rosa I, Lage P, Ferreira S, Pereira Silva J, Cortez Pinto J, Vale Rodrigues R, Moleiro J, Claro I, Esteves S, Dias Pereira A. Real-Time Optical Diagnosis of Colorectal Polyps in the Routine Clinical Practice Using the NICE and WASP Classifications in a Nonacademic Setting. GE Port J Gastroenterol. 2019;26:314-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Mori Y, Neumann H, Misawa M, Kudo SE, Bretthauer M. Artificial intelligence in colonoscopy - Now on the market. What's next? J Gastroenterol Hepatol. 2021;36:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 34. | Mori Y, Kudo SE, Berzin TM, Misawa M, Takeda K. Computer-aided diagnosis for colonoscopy. Endoscopy. 2017;49:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 35. | Yang YJ, Cho BJ, Lee MJ, Kim JH, Lim H, Bang CS, Jeong HM, Hong JT, Baik GH. Automated Classification of Colorectal Neoplasms in White-Light Colonoscopy Images via Deep Learning. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Byrne MF, Chapados N, Soudan F, Oertel C, Linares Pérez M, Kelly R, Iqbal N, Chandelier F, Rex DK. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut. 2019;68:94-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 412] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 37. | Song EM, Park B, Ha CA, Hwang SW, Park SH, Yang DH, Ye BD, Myung SJ, Yang SK, Kim N, Byeon JS. Endoscopic diagnosis and treatment planning for colorectal polyps using a deep-learning model. Sci Rep. 2020;10:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 38. | Rodriguez-Diaz E, Baffy G, Lo WK, Mashimo H, Vidyarthi G, Mohapatra SS, Singh SK. Real-time artificial intelligence-based histologic classification of colorectal polyps with augmented visualization. Gastrointest Endosc. 2021;93:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 39. | Lui TKL, Guo CG, Leung WK. Accuracy of artificial intelligence on histology prediction and detection of colorectal polyps: a systematic review and meta-analysis. Gastrointest Endosc. 2020;92:11-22.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 40. | Mori Y, Kudo SE, Wakamura K, Misawa M, Ogawa Y, Kutsukawa M, Kudo T, Hayashi T, Miyachi H, Ishida F, Inoue H. Novel computer-aided diagnostic system for colorectal lesions by using endocytoscopy (with videos). Gastrointest Endosc. 2015;81:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 41. | Utsumi T, Sano Y, Iwatate M, Sunakawa H, Teramoto A, Hirata D, Hattori S, Sano W, Hasuike N, Ichikawa K, Fujimori T. Prospective real-time evaluation of diagnostic performance using endocytoscopy in differentiating neoplasia from non-neoplasia for colorectal diminutive polyps (≤ 5 mm). World J Gastrointest Oncol. 2018;10:96-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Kataoka S, Kudo SE, Misawa M, Nakamura H, Takeda K, Toyoshima N, Mori Y, Ogata N, Kudo T, Hisayuki T, Hayashi T, Wakamura K, Baba T, Ishida F. Endocytoscopy with NBI has the potential to correctly diagnose diminutive colorectal polyps that are difficult to diagnose using conventional NBI. Endosc Int Open. 2020;8:E360-E367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Danese S, Roda G, Peyrin-Biroulet L. Evolving therapeutic goals in ulcerative colitis: towards disease clearance. Nat Rev Gastroenterol Hepatol. 2020;17:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 44. | Chateau T, Feakins R, Marchal-Bressenot A, Magro F, Danese S, Peyrin-Biroulet L. Histological Remission in Ulcerative Colitis: Under the Microscope Is the Cure. Am J Gastroenterol. 2020;115:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 45. | Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol. 2014;12:929-34.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 46. | Holmer AK, Dulai PS. Using Artificial Intelligence to Identify Patients With Ulcerative Colitis in Endoscopic and Histologic Remission. Gastroenterology. 2020;158:2045-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Takenaka K, Ohtsuka K, Fujii T, Negi M, Suzuki K, Shimizu H, Oshima S, Akiyama S, Motobayashi M, Nagahori M, Saito E, Matsuoka K, Watanabe M. Development and Validation of a Deep Neural Network for Accurate Evaluation of Endoscopic Images From Patients With Ulcerative Colitis. Gastroenterology. 2020;158:2150-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 48. | Maeda Y, Kudo SE, Mori Y, Misawa M, Ogata N, Sasanuma S, Wakamura K, Oda M, Mori K, Ohtsuka K. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest Endosc. 2019;89:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 49. | Maeda Y, Kudo SE, Ogata N, Misawa M, Mori Y, Mori K, Ohtsuka K. Can artificial intelligence help to detect dysplasia in patients with ulcerative colitis? Endoscopy. 2021;53:E273-E274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 50. | Yang XX, Li Z, Shao XJ, Ji R, Qu JY, Zheng MQ, Sun YN, Zhou RC, You H, Li LX, Feng J, Yang XY, Li YQ, Zuo XL. Real-time artificial intelligence for endoscopic diagnosis of early esophageal squamous cell cancer (with video). Dig Endosc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 51. | Kumagai Y, Takubo K, Kawada K, Aoyama K, Endo Y, Ozawa T, Hirasawa T, Yoshio T, Ishihara S, Fujishiro M, Tamaru JI, Mochiki E, Ishida H, Tada T. Diagnosis using deep-learning artificial intelligence based on the endocytoscopic observation of the esophagus. Esophagus. 2019;16:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 52. | Mohan BP, Khan SR, Kassab LL, Ponnada S, Mohy-Ud-Din N, Chandan S, Dulai PS, Kochhar GS. Convolutional neural networks in the computer-aided diagnosis of Helicobacter pylori infection and non-causal comparison to physician endoscopists: a systematic review with meta-analysis. Ann Gastroenterol. 2021;34:20-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Arribas Anta J, Dinis-Ribeiro M. Early gastric cancer and Artificial Intelligence: Is it time for population screening? Best Pract Res Clin Gastroenterol. 2021;52-53:101710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Nagao S, Tsuji Y, Sakaguchi Y, Takahashi Y, Minatsuki C, Niimi K, Yamashita H, Yamamichi N, Seto Y, Tada T, Koike K. Highly accurate artificial intelligence systems to predict the invasion depth of gastric cancer: efficacy of conventional white-light imaging, nonmagnifying narrow-band imaging, and indigo-carmine dye contrast imaging. Gastrointest Endosc. 2020;92:866-873.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 55. | Sana MK, Hussain ZM, Shah PA, Maqsood MH. Artificial intelligence in celiac disease. Comput Biol Med. 2020;125:103996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Aoki T, Yamada A, Aoyama K, Saito H, Tsuboi A, Nakada A, Niikura R, Fujishiro M, Oka S, Ishihara S, Matsuda T, Tanaka S, Koike K, Tada T. Automatic detection of erosions and ulcerations in wireless capsule endoscopy images based on a deep convolutional neural network. Gastrointest Endosc. 2019;89:357-363.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 57. | Tsuboi A, Oka S, Aoyama K, Saito H, Aoki T, Yamada A, Matsuda T, Fujishiro M, Ishihara S, Nakahori M, Koike K, Tanaka S, Tada T. Artificial intelligence using a convolutional neural network for automatic detection of small-bowel angioectasia in capsule endoscopy images. Dig Endosc. 2020;32:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 58. | Tonozuka R, Itoi T, Nagata N, Kojima H, Sofuni A, Tsuchiya T, Ishii K, Tanaka R, Nagakawa Y, Mukai S. Deep learning analysis for the detection of pancreatic cancer on endosonographic images: a pilot study. J Hepatobiliary Pancreat Sci. 2021;28:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 59. | Chondros T, Milidonis K, Vitzilaios G, Vaitsis J. “Deus-Ex-Machina” reconstruction in the Athens theater of Dionysus. Mech Mach Theory. 2013;67:172-191. [DOI] [Full Text] |