Published online Aug 28, 2021. doi: 10.3748/wjg.v27.i32.5297
Peer-review started: February 18, 2021
First decision: May 1, 2021
Revised: May 3, 2021
Accepted: August 3, 2021
Article in press: August 3, 2021
Published online: August 28, 2021
Processing time: 187 Days and 14 Hours
Approximately 20% of cirrhotic patients with ascites develop umbilical herniation. These patients usually suffer from multisystemic complications of cirrhosis, have a significantly higher risk of infection, and require accurate surveillance– especially in the context of the coronavirus disease 2019 pandemic. The rupture of an umbilical hernia, is an uncommon, life-threatening complication of large-volume ascites and end-stage liver disease resulting in spontaneous paracentesis, also known as Flood syndrome. Flood syndrome remains a challenging condition for clinicians, as recommendations for its management are lacking, and the available evidence for the best treatment approach remains controversial. In this paper, four key questions are addressed regarding the management and prevention of Flood syndrome: (1) Which is the best treatment approach–conservative treatment or urgent surgery? (2) How can we establish the individual risk for herniation and possible hernia rupture in cirrhotic patients? (3) How can we prevent umbilical hernia ruptures? And (4) How can we manage these patients in the conditions created by the coronavirus disease 2019 pandemic?
Core Tip: Flood syndrome is a rare, life-threatening complication of large-volume ascites and end-stage liver disease resulting in a sudden umbilical hernia rupture and spontaneous paracentesis. It remains a challenge for clinicians, as recommendations for the management of this syndrome are lacking. The establishment of the individual risk for herniation and possible hernia rupture, timely prevention, and elective surgical treatment might reduce the risk of complications and the need for urgent surgery.
- Citation: Strainiene S, Peciulyte M, Strainys T, Stundiene I, Savlan I, Liakina V, Valantinas J. Management of Flood syndrome: What can we do better? World J Gastroenterol 2021; 27(32): 5297-5305
- URL: https://www.wjgnet.com/1007-9327/full/v27/i32/5297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i32.5297
Ascites is one of the most common complications of liver cirrhosis, manifesting to a variable extent in over 50% of cases. It is also one of the signs of decompensated cirrhosis that is associated with a poor prognosis[1,2]. The occurrence of ascites impairs patients’ working and social lives, leads to more frequent hospitalization, requires chronic treatment, and is a direct cause of further complications–such as spontaneous bacterial peritonitis, restrictive ventilatory dysfunction, and abdominal hernias[2]. The incidence of an umbilical hernia (UH) in cirrhotic patients with ascites is approximately 20%, which is 10 times higher than in the general population, and may be up to 40% in cases of large-volume ascites[3-6].
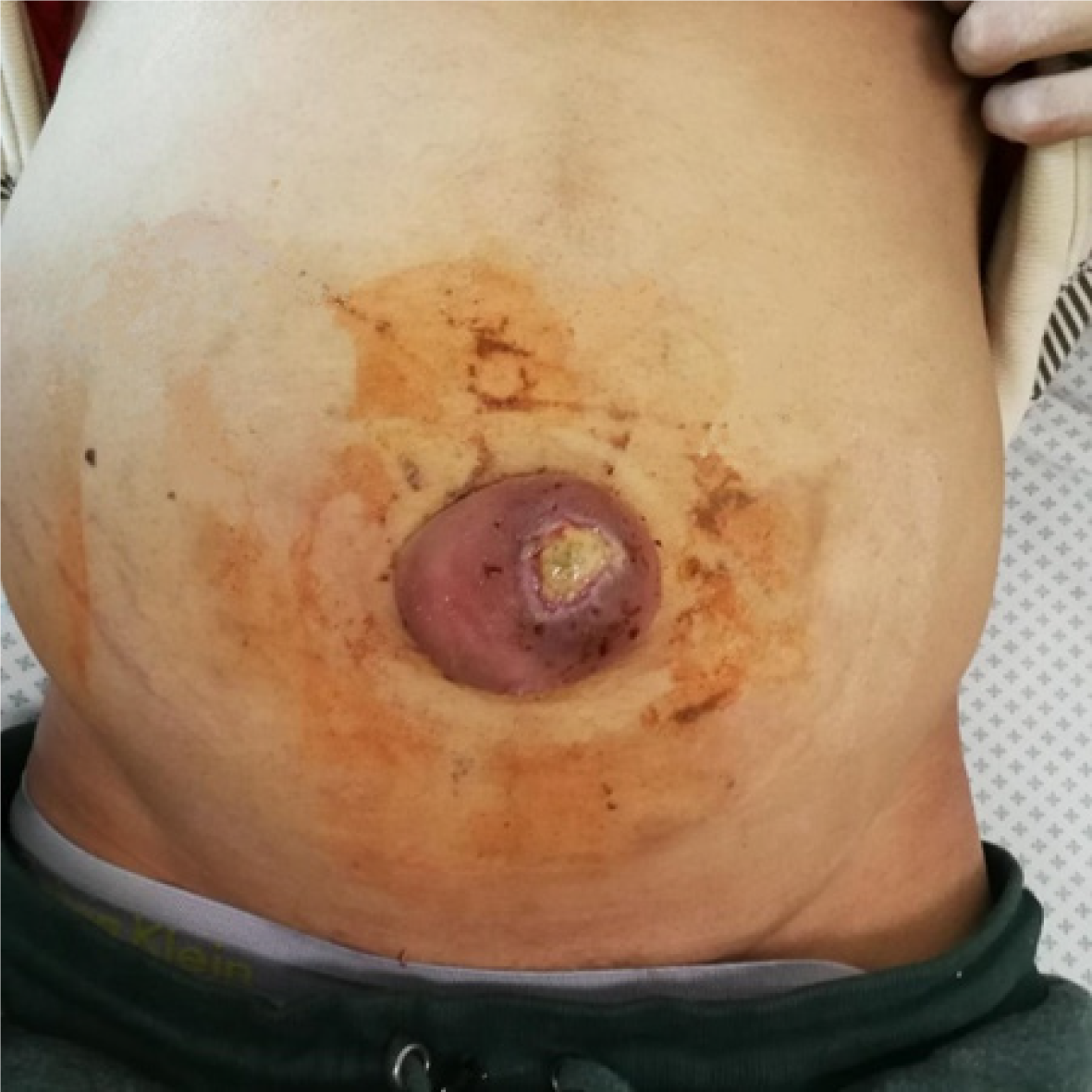
In rare cases, patients with large-volume ascites develop the severe complication of a ruptured UH, resulting in the leakage of ascitic fluid through a skin lesion (Figure 1). This is also known as Flood syndrome, and it was first described by Frank B. Flood in 1961[5,7,8]. This is a life-threatening complication with a significant morbidity and mortality rate of 30%. The rupture of the UH is contributed to by local trauma or a sudden rise in intraabdominal pressure caused by coughing, vomiting, straining, increasing ascites, or heavy lifting[9,10].
Possible complications of the ruptured UH are the evisceration of the small intestine, the incarceration of the bowel, cellulitis, peritonitis, sepsis, and hypotension caused by massive paracentesis[11,12]. The management of Flood syndrome is the subject of much debate due to the lack of high-quality prospective studies and the absence of clear recommendations.
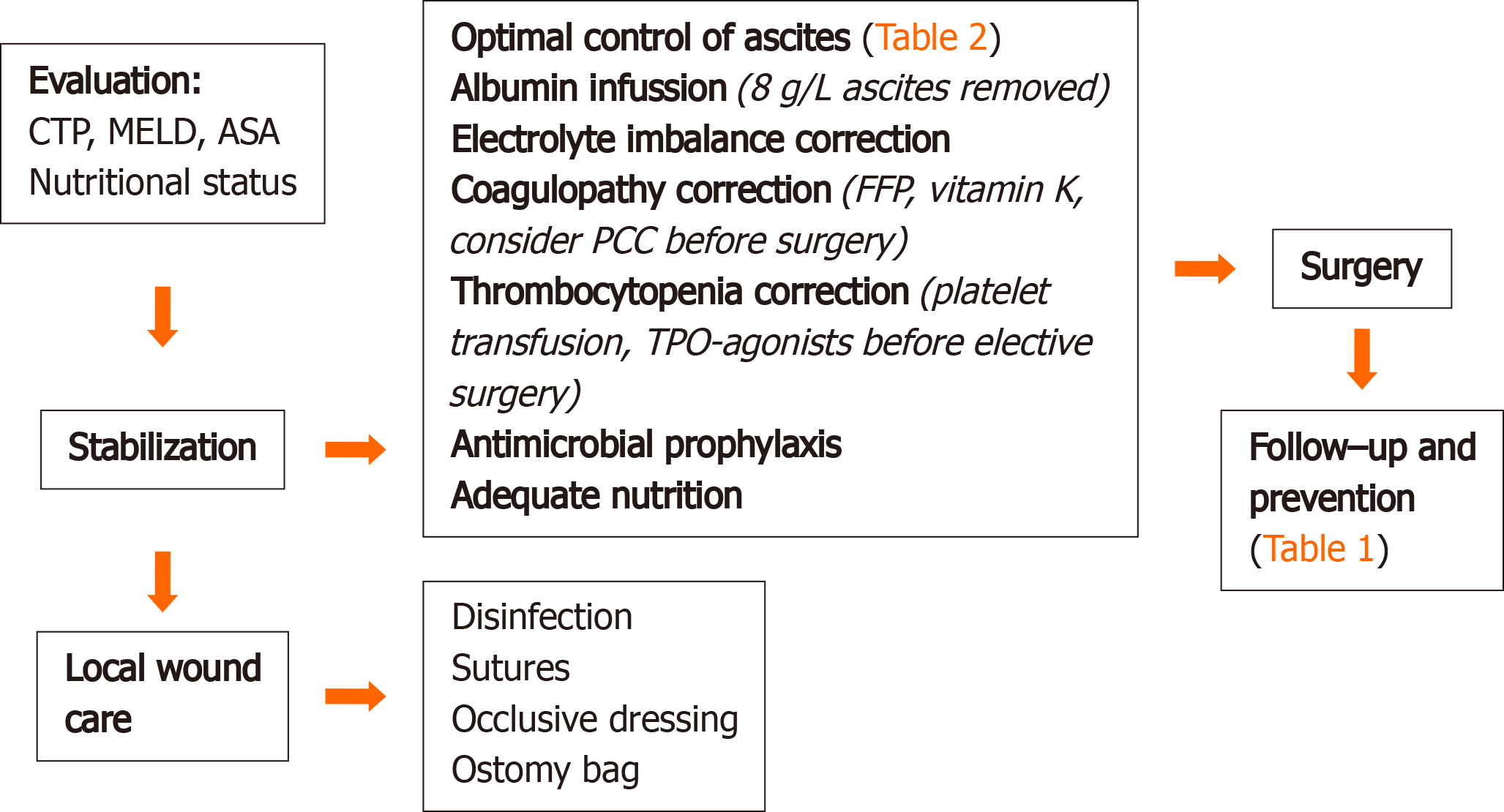
Mortality rates after urgent surgical repair of the hernia vary between 6%–20%, compared to 60%–80% after only supportive care[5,13-15]. The stabilization of the patient’s condition, local wound care, optimal control of ascites, correction of the electrolyte imbalance, coagulopathy and thrombocytopenia, and antimicrobial prophylaxis are the critical points for managing a ruptured UH[5,13,16]. The timing of the surgery is also contested, as there is no reliable data on the optimal duration of conservative treatment. The surgical procedure is often delayed due to the high incidence of postoperative complications, such as wound dehiscence and evisceration caused by the re-accumulation of the ascites, bleeding due to coagulopathy, and the inadequate synthesis of clotting factors[5]. The key points of the management of Flood syndrome are summarized in Figure 2.
Evaluation: Patients with liver disease are at a higher risk of surgical and anesthesia-related complications. This risk depends upon the type of liver disease and its severity, the surgical procedure, and the type of anesthesia[17]. Both the model for end-stage liver disease (MELD) score and the Child-Turcotte-Pugh (CTP) classification, combined with the American Society of Anesthesiologists Classification, have been suggested for the stratification of the risks of hernia surgery, but neither appear to be ubiquitously applicable[17]. The main predictor of operative risk in patients with cirrhosis is the CTP classification, but more recent studies suggest that the MELD score might be superior in this regard[18]. Patients with a CTP score > 10 or a MELD score > 20 pose a high risk of postoperative decompensation, including liver failure, worsening encephalopathy, bleeding, wound infection, renal failure, hypoxia, intractable ascites, UH recurrence, and prolonged ileus[5,17,19]. Therefore, it is important to stabilize the patient’s condition and to initiate conservative treatment prior to surgery.
Local wound care: Local wound care is one of the first steps in the management of Flood syndrome. Proper disinfection of the area around the skin lesion and control of the peritoneal fluid leakage are crucial in preventing infection and excessive ascites loss. Massive spontaneous paracentesis may lead to severe electrolyte disbalance and paracentesis-induced circulatory dysfunction. Macerations at the UH site might be addressed with two Z sutures and covered with an occlusive dressing. An ostomy bag is used to collect the remaining ascitic fluid.
Stabilization: The optimal control of ascites is one of the main goals in managing a ruptured UH and preventing herniation. Inadequately controlled ascites increase the risk of wound infection and the relapse of the hernia by 75%[5,14]. The main strategies of controlling ascites according to the European Association for the Study of the Liver guidelines are presented in Table 1.
| Risk establishment | Prevention |
| Questions to ask | |
| (1) Ascites control: (a) Changes in the abdomen volume; (b) Fluid balance; (c) Weight; (d) Use of prescribed treatment; (2) Nutrition (any signs of malnutrition?); (3) Alcohol intake; (4) Surgeries in the abdomen; (5) Pre-existing hernias; (6) Employment (heavy lifting activities); (7) Comorbidities; (8) Constipation; (9) Medicaments used; and (10) Changes in the abdomen, umbilical area visual appearance | (1) Education; (2) Risk establishment; and (3) Risk management: (a) Lifestyle modification; (b) Management of the underlying liver disease; (c) Management of ascites; (d) Doctor-patient communication; and (e) Communication between medical specialists |
| Patients with UH | |
| (1) All the above; (2) Avoid heavy lifting, rapid movement; (3)Abdominal surgeon consult; and (4) Elective surgery in stable patients |
Plasma volume expansion therapy with albumin infusion (8 g/L ascites removed) is recommended to prevent paracentesis-induced circulatory dysfunction in cases with a high volume (5 L of ascites) of spontaneous paracentesis[2]. There is also some promising data regarding cheaper alternative preventive measures, such as colloids, vasoconstrictors (midodrine, terlipressin), and lowering the standard doses of the albumin, but these approaches are not yet recommended by international guidelines[20-23].
It is also essential to evaluate the patient’s nutritional status, as these patients are often cachectic and malnourished. A protein-rich diet (2000 kcal/d, with 40–50 g of protein) is recommended to address the patient’s nutritional needs. The patient’s dietary needs might be covered orally with additional protein supplementation or via enteral feeding. Vitamin B group supplementation is also mandatory, especially in patients with alcohol-related cirrhosis[24].
Patients with liver disease undergoing surgery might be at risk of both thrombosis and bleeding, due to dysregulated coagulation and a diminished hemostatic reserve[25-27]. Therefore, cirrhotic patients need to be carefully assessed for the risk of bleeding before surgery. Prothrombin time, international normalized ratio (INR), and platelet count have been formally recommended for the purpose of determining coagulation status in clinical practice. However, the relationship between prothrombin time/INR and the risk of bleeding in cirrhosis is disputable. Isolated evaluations of bleeding or clotting time are also of little prognostic value in patients with liver diseases during pre-operative screening[28].
Fresh frozen plasma and vitamin K therapy (phytomenadione 10 mg/mL) are currently used to address coagulopathy as a standard of care to manage active bleeding or prophylaxis before invasive procedures[29,30]. In cases of urgent surgery, prothrombin complex concentrate (PCC) might be used to prevent bleeding in selected patients with severe coagulopathy. Drebes et al[26] recently reviewed PCC’s clinical use in patients with acute/chronic liver disease in a retrospective single-center study[26]. In their study, 20–25 IU/kg of PCC was administered to patients with INR 4, and 30 IU/kg for patients with INR 4. PCC therapy effectively improved the results of the coagulation test, with no evidence of an increased risk of thromboembolism. These findings highlighted the need to assess further PCC’s potential role as a form of hemostatic therapy in liver disease patients. The use of PCC before urgent surgery should therefore be considered.
Thrombocytopenia is another issue associated with liver cirrhosis. Prophylactical or periprocedural platelet transfusion, splenic artery embolization, transjugular intrahepatic portosystemic shunts, and a splenectomy are well documented methods of treating thrombocytopenia. Recently, thrombopoietin receptor agonists (avatrombopag and lusutrombopag) were approved by the United States Food and Drug Administration for the non-invasive treatment of thrombocytopenia in patients with chronic liver disease undergoing a surgical procedure. These new drugs are considered safe and effective alternatives to platelet transfusions[31,32].
Surgical approach: The importance of the surgical treatment of the UH rupture in patients with ascites was first described by Kirkpatrick and Schubert, who calculated that the mortality rate was 60% in conservatively treated patients, compared to 14% in those who underwent herniorrhaphy[15]. However, there is a much higher risk of complications and a higher morbidity rate after the urgent surgical repair of an UH[33,34].
In terms of the surgical method, most authors suggest that the best option seems to be a primary closure with non-absorbable sutures[5,9,14]. Synthetic meshes may not be an appropriate technique in patients with ascites due to a higher risk of infection and a possible ingrowth of the mesh[5,35]. However, a single randomized study of 80 patients demonstrated that the use of a synthetic mesh in cirrhotic patients with complicated UHs was related to a lower rate of hernia reoccurrence[16]. UHs reoccurred in 14.2% of patients who underwent suture repair, compared to 2.7% in the mesh repair group (P < 0.05). The incidence of infection at the surgical site was not significant in either group: 8.5% in the conventional fascial repair group and 16.2% in the mesh repair group (P > 0.05). However, the mean duration of hospital stay was significantly longer in the mesh repair group (P < 0.05)[16].
Finally, it is essential to treat these patients in a tertiary center with specialized surgical and intensive care units due to the increased risk of infection, bleeding, and postoperative decompensation of cirrhosis[36]. Smaller hospitals cannot provide appropriate care because of their lack of experience[7,37]. In such cases, several alternative methods are described, including the successful placement of a pig-tail drain with no recurrence of ascitic fluid leakage and the injection of fibrin glue into the defect to stem drainage[38-40]. These approaches allow natural wound healing, though they remain temporary solutions. Ulceration or necrosis over a UH should be considered a dangerous sign; a warning of an impending rupture, and such patients should be referred to urgent surgical treatment[11].
The main etiological factors for the development of UHs are ascites, weakened abdominal wall muscles, malnutrition, and the recanalization of pre-existing openings promoted by increased abdominal pressure[5,6,37].
A UH rupture is usually the result of multiple risk factors. Alcohol consumption is one of the major causes of decompensated cirrhosis with ascites. Cirrhosis alone causes malnutrition and sarcopenia, resulting in weakened abdominal wall muscles. Harmful alcohol consumption is also associated with nutritional deficiencies, including protein-calorie malnutrition and cachexia[24,41]. It is also necessary to consider comorbidities that can put limitations on the patient’s physical activity and nutrition, such as epilepsy, depression, and related medication (antiepileptics, antidepressants). Previous abdominal surgeries and pre-existing hernias are also severe risk factors for UHs due to the presence of a weakened abdominal wall. Therefore, careful anamnesis is essential (Table 1).
General measures for UH prevention consist of the continuous education of the patient and their family members, lifestyle modification, and the avoidance of alcohol, tobacco, constipation, and heavy lifting. Hernia trusses are controversial as they may worsen the hernia, and they should only be used temporarily, if at all[42].
The main methods of preventing UH occurrence and rupture specifically for cirrhotic patients are the optimal control of ascites using medical therapies or regular paracentesis as per general guidelines (Table 2)[2,5,17].
| Treatment | |
| Main measures | Moderate restriction of sodium intake, 80–120 mmoL/d (4.6-6.9 g of salt/d) |
| Adequate nutrition: Protein-rich diet (2000 kcal/d, protein–40-50 g/d), vitamin therapy | |
| Correction of electrolyte imbalance | |
| Adequate fluid intake: No restriction needed in patients with normal serum sodium concentration; in hyponatremic patients (< 130 mmoL/L), restrict fluid intake to 1.0-1.5 L/d | |
| Daily track of weight (or measure fluid intake and diuresis) | |
| The maximum recommended weight loss during diuretic therapy: (1) 0.5 kg/d in patients without edema; and (2) 1 kg/d in patients with edema | |
| Mild and moderate ascites (grade Iº-IIº) | Aldosterone antagonists: Spironolactone 50-100 mg/d (maximum of 400 mg/d) ± loop diuretics: Furosemide 20-40 mg/d (maximum of 160 mg/d) |
| Torasemide (10-40 mg/d) if no response to furosemide | |
| Distal diuretics: Amiloride 5-20 mg/d; triamterene 100 mg 2 k./d. (if aldosterone antagonists are not tolerated) | |
| Combined dosage of diuretics: Spironolactone 50-100-200-300-400 mg/d (in 100 mg steps) + furosemide 20-40-80-120-160 mg/d (in 40 mg steps) (or adequate doses of other diuretics) | |
| Large ascites (grade IIIº) | LVP |
| Albumin infusion (8 g/L of ascitic fluid removed) | |
| Minimal effective dose of diuretics to prevent the re-accumulation of ascites after LVP | |
| Refractory ascites | Repeated partial or large volume paracentesis + albumin infusion |
| Withdrawn diuretics | |
| Transjugular intrahepatic portosystemic shunt | |
| Alternative drugs: (1) α1 adrenergic agonists–midodrine 7.5 mg 3 times/d; (2) Vasopressin analog–terlipressin 1-2 mg/d intravenous; and (3) α2 adrenergic agonists–clonidine | |
| Alfapump system | |
| Liver transplantation |
The management of underlying liver disease improves the outlook for the reversible components of hepatic decompensation. In most patients with alcoholic cirrhosis, abstinence may reduce or even normalize portal pressure, resulting in the increased ease of treatment of ascites or their disappearance altogether[1]. Alcohol-induced liver cirrhosis is a double pathology, hence the management of both liver cirrhosis and dependence is essential. The main treatment methods are total abstinence, correction of malnutrition, vitamins, and microelements, and the primary and secondary prophylaxis of the complications of cirrhosis. Specific treatment with S–adenosyl–L–methionine, propyluracil, colchicine, and anabolic steroids is debated[41].
It is also essential to monitor carefully the patient with ascites and to consider elective UH repair as soon as herniation appears. This can help to prevent UH rupture and minimize the number of postoperative complications. The watch-and-wait policy was previously applied to patients with ascites and UHs, and surgery was performed only after the occurrence of complications[6]. However, in recent decades, some changes have occurred as novel studies have demonstrated that elective UH repair in patients with liver cirrhosis and ascites can be performed with less morbidity. Conservative management leads to a higher incidence of incarceration, with subsequent hernia repair in the emergency setting[34,43,44]. Marsman et al[43] compared the outcome of operative and conservative treatment in patients with liver cirrhosis and ascites complicated by UHs. Elective hernia repair was successful without complications or recurrence in around 70.5% of patients, while complications such as wound-related problems (superficial wound infection, necrosis, hematoma) were noted in 17.6% of cases. Initial conservative management was effective in only 23% of patients[41]. A single-center prospective study on patients listed for liver transplantation showed that elective UH repair is preferable to urgent repair in patients with cirrhosis and ascites[44].
Therefore, elective herniorrhaphy is now recommended in patients with cirrhosis and ascites after the patient is stabilized using optimal medical management, to prevent an emergent presentation of incarceration or UH rupture[17,33]. There are several contraindications for elective surgery in patients with liver disease, such as acute liver failure, acute renal failure, acute viral hepatitis, alcoholic hepatitis, cardiomyopathy, hypoxemia, severe coagulopathy, and thrombocytopenia (despite treatment)[17].
Efforts should be taken to avoid recurrent ascites in the postoperative period, as they lead to impaired wound healing and possible risks of dehiscence[17]. Measures for preventing the reoccurrence of the UH after surgery are the same as preventive methods against UH rupture (Table 1).
According to the literature, just 33% of patients with ascites receive only treatments that are supported by guidelines and high-quality evidence[43]. Furthermore, over one-third of patients hospitalized due to cirrhosis complications are readmitted within 1 mo of discharge[45,46]. Many patients do not seek proper care until decompensation, and some do not follow or cannot afford treatment. Clearly, the current management of cirrhosis leaves room for improvement, especially during the conditions of the coronavirus disease 2019 (COVID-19) pandemic.
Clinicians must be aware that the COVID-19 pandemic has negatively impacted the care of patients with chronic liver disease[47]. This might result in a higher incidence of decompensated cirrhosis when patients do not receive treatment correction on time. There is also an increased risk of a growing incidence of alcohol-induced and non-alcoholic fatty liver disease-related cirrhosis. Patient care is challenging; along with telemedicine and the near-impossibility of physical examination, clinicians must be more accurate and foresee potential future issues. Moreover, not every patient can or indeed wants to use telemedicine, as many patients represent vulnerable cohorts. However, there are some steps that can be taken to address these challenges.
First, we must make more effort than ever before to encourage and educate patients and their relatives. Visual methods can be used, such as written instructions for the patient (in a patient-friendly form), educational video seminars, and other materials. We must ask key questions (during teleconsultation) to establish the patient’s risks (Table 1), and we must cooperate. Cooperation between patient and doctor and communication between different medical specialists is essential in managing patients with liver cirrhosis and ascites. All clinicians must be aware of the possible complications of ascites and refer the patient to a specialist consultation in a timely manner.
The rupture of the UH and spontaneous paracentesis in patients with decompensated cirrhosis is a rare and dangerous complication that remains a challenge for gastroenterologists, surgeons, and anesthesiologists. The stabilization of the patient’s overall condition, adequate control of ascites, and local wound care are the main goals of therapy before the primary repair of the hernia. Gastroenterologists and general practitioners should establish the risk to the individual patient regarding herniation and possible hernia rupture via careful follow-up. Timely prevention of UH and elective surgical treatment can reduce the risk of complications and the need for urgent surgery, decrease overall hospitalization time, and improve the patient’s quality of life.
We would like to thank Vilnius University Hospital’s Santaros Clinic for their close collaboration.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Lithuanian Society of Gastroenterology; Lithuanian Society of Immunology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Lithuania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gallo P S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Biecker E. Diagnosis and therapy of ascites in liver cirrhosis. World J Gastroenterol. 2011;17:1237-1248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1818] [Article Influence: 259.7] [Reference Citation Analysis (2)] |
| 3. | Coelho JC, Claus CM, Campos AC, Costa MA, Blum C. Umbilical hernia in patients with liver cirrhosis: A surgical challenge. World J Gastrointest Surg. 2016;8:476-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (3)] |
| 4. | Dumas M, Breton JC, Pestre Alexandre M, Girard PL, Giordano C. [Current status of the therapy of human African trypanosomiasis]. Presse Med. 1985;14:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Chatzizacharias NA, Bradley JA, Harper S, Butler A, Jah A, Huguet E, Praseedom RK, Allison M, Gibbs P. Successful surgical management of ruptured umbilical hernias in cirrhotic patients. World J Gastroenterol. 2015;21:3109-3113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Salamone G, Licari L, Guercio G, Campanella S, Falco N, Scerrino G, Bonventre S, Geraci G, Cocorullo G, Gulotta G. The abdominal wall hernia in cirrhotic patients: a historical challenge. World J Emerg Surg. 2018;13:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Malespin M, Moore CM, Fialho A, de Melo SW Jr, Benyashvili T, Kothari AN, di Sabato D, Kallwitz ER, Cotler SJ, Lu AD. Case Series of 10 Patients with Cirrhosis Undergoing Emergent Repair of Ruptured Umbilical Hernias: Natural History and Predictors of Outcomes. Exp Clin Transplant. 2019;17:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | FLOOD FB. Spontaneous perforation of the umbilicus in Laennec's cirrhosis with massive ascites. N Engl J Med. 1961;264:72-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Good DW, Royds JE, Smith MJ, Neary PC, Eguare E. Umbilical hernia rupture with evisceration of omentum from massive ascites: a case report. J Med Case Rep. 2011;5:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Cobb WS, Burns JM, Kercher KW, Matthews BD, James Norton H, Todd Heniford B. Normal intraabdominal pressure in healthy adults. J Surg Res. 2005;129:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 311] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Choo EK, McElroy S. Spontaneous bowel evisceration in a patient with alcoholic cirrhosis and an umbilical hernia. J Emerg Med. 2008;34:41-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | DeLuca IJ, Grossman ME. Flood syndrome. JAAD Case Rep. 2015;1:5-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Fagan SP, Awad SS, Berger DH. Management of complicated umbilical hernias in patients with end-stage liver disease and refractory ascites. Surgery. 2004;135:679-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Telem DA, Schiano T, Divino CM. Complicated hernia presentation in patients with advanced cirrhosis and refractory ascites: management and outcome. Surgery. 2010;148:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 15. | Kirkpatrick S, Schubert T. Umbilical hernia rupture in cirrhotics with ascites. Dig Dis Sci. 1988;33:762-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Ammar SA. Management of complicated umbilical hernias in cirrhotic patients using permanent mesh: randomized clinical trial. Hernia. 2010;14:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Northup PG, Friedman LS, Kamath PS. AGA Clinical Practice Update on Surgical Risk Assessment and Perioperative Management in Cirrhosis: Expert Review. Clin Gastroenterol Hepatol. 2019;17:595-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 18. | O'Leary JG, Friedman LS. Predicting surgical risk in patients with cirrhosis: from art to science. Gastroenterology. 2007;132:1609-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Friedman LS. Surgery in the patient with liver disease. Trans Am Clin Climatol Assoc. 2010;121:192-204; discussion 205. [PubMed] |
| 20. | Moreau R, Asselah T, Condat B, de Kerguenec C, Pessione F, Bernard B, Poynard T, Binn M, Grangé JD, Valla D, Lebrec D. Comparison of the effect of terlipressin and albumin on arterial blood volume in patients with cirrhosis and tense ascites treated by paracentesis: a randomised pilot study. Gut. 2002;50:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Singh V, Kumar R, Nain CK, Singh B, Sharma AK. Terlipressin vs albumin in paracentesis-induced circulatory dysfunction in cirrhosis: a randomized study. J Gastroenterol Hepatol. 2006;21:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Tandon P, Tsuyuki RT, Mitchell L, Hoskinson M, Ma MM, Wong WW, Mason AL, Gutfreund K, Bain VG. The effect of 1 mo of therapy with midodrine, octreotide-LAR and albumin in refractory ascites: a pilot study. Liver Int. 2009;29:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Alsebaey A, Rewisha E, Waked I. Paracentesis-induced circulatory dysfunction: are there albumin alternatives? Egyp Liver J. 2020;10:39. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J; DGEM (German Society for Nutritional Medicine); Ferenci P, Holm E, Vom Dahl S, Müller MJ, Nolte W; ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006;25:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 405] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 25. | Monroe DM, Hoffman M. The coagulation cascade in cirrhosis. Clin Liver Dis. 2009;13:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Drebes A, de Vos M, Gill S, Fosbury E, Mallett S, Burroughs A, Agarwal B, Patch D, Chowdary P. Prothrombin Complex Concentrates for Coagulopathy in Liver Disease: Single-Center, Clinical Experience in 105 Patients. Hepatol Commun. 2019;3:513-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Agarwal B, Wright G, Gatt A, Riddell A, Vemala V, Mallett S, Chowdary P, Davenport A, Jalan R, Burroughs A. Evaluation of coagulation abnormalities in acute liver failure. J Hepatol. 2012;57:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Harrison MF. The Misunderstood Coagulopathy of Liver Disease: A Review for the Acute Setting. West J Emerg Med. 2018;19:863-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Shah NL, Intagliata NM, Northup PG, Argo CK, Caldwell SH. Procoagulant therapeutics in liver disease: a critique and clinical rationale. Nat Rev Gastroenterol Hepatol. 2014;11:675-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55:965-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 355] [Article Influence: 27.3] [Reference Citation Analysis (35)] |
| 31. | Saab S, Brown RS Jr. Management of Thrombocytopenia in Patients with Chronic Liver Disease. Dig Dis Sci. 2019;64:2757-2768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Khemichian S, Terrault NA. Thrombopoietin Receptor Agonists in Patients with Chronic Liver Disease. Semin Thromb Hemost. 2020;46:682-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Odom SR, Gupta A, Talmor D, Novack V, Sagy I, Evenson AR. Emergency hernia repair in cirrhotic patients with ascites. J Trauma Acute Care Surg. 2013;75:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Gray SH, Vick CC, Graham LA, Finan KR, Neumayer LA, Hawn MT. Umbilical herniorrhapy in cirrhosis: improved outcomes with elective repair. J Gastrointest Surg. 2008;12:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Smith R. Hernia Repair in Patients with Cirrhosis. In: Lim R, editor. Multidisciplinary Approaches to Common Surgical Problems. Springer, 2019: 267-281. [DOI] [Full Text] |
| 36. | Bhangui P, Laurent A, Amathieu R, Azoulay D. Assessment of risk for non-hepatic surgery in cirrhotic patients. J Hepatol. 2012;57:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Andraus W, Pinheiro RS, Lai Q, Haddad LBP, Nacif LS, D'Albuquerque LAC, Lerut J. Abdominal wall hernia in cirrhotic patients: emergency surgery results in higher morbidity and mortality. BMC Surg. 2015;15:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Sadik KW, Bonatti H, Schmitt T. Injection of fibrin glue for temporary treatment of an ascites leak from a ruptured umbilical hernia in a patient with liver cirrhosis. Surgery. 2008;143:574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Melcher ML, Lobato RL, Wren SM. A novel technique to treat ruptured umbilical hernias in patients with liver cirrhosis and severe ascites. J Laparoendosc Adv Surg Tech A. 2003;13:331-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Nguyen ET, Tudtud-Hans LA. Flood Syndrome: Spontaneous Umbilical Hernia Rupture Leaking Ascitic Fluid-A Case Report. Perm J. 2017;21:16-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018;113:175-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 560] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 42. | Kingsnorth A, LeBlanc K. Hernias: inguinal and incisional. Lancet. 2003;362:1561-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 628] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 43. | Marsman HA, Heisterkamp J, Halm JA, Tilanus HW, Metselaar HJ, Kazemier G. Management in patients with liver cirrhosis and an umbilical hernia. Surgery. 2007;142:372-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Eker HH, van Ramshorst GH, de Goede B, Tilanus HW, Metselaar HJ, de Man RA, Lange JF, Kazemier G. A prospective study on elective umbilical hernia repair in patients with liver cirrhosis and ascites. Surgery. 2011;150:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Kanwal F, Kramer JR, Buchanan P, Asch SM, Assioun Y, Bacon BR, Li J, El-Serag HB. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012;143:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (2)] |
| 46. | Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 47. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (1)] |