Published online Aug 14, 2021. doi: 10.3748/wjg.v27.i30.5112
Peer-review started: February 16, 2021
First decision: May 1, 2021
Revised: May 13, 2021
Accepted: July 9, 2021
Article in press: July 9, 2021
Published online: August 14, 2021
Processing time: 174 Days and 14.2 Hours
There is an increased risk of atherosclerosis in patients with chronic hepatitis C or human immunodeficiency virus, but there is scarce data on hepatitis B virus infection. The hypothesis of this study is that hepatitis B virus infection increases the risk of carotid plaques and subclinical atherosclerosis in naïve hepatitis B e antigen (HBeAg) negative subjects.
To assess the rate of carotid plaques and subclinical atherosclerosis in naïve HBeAg negative subjects in comparison with a cohort of healthy controls.
Prospective case-control collaborative study conducted in two tertiary hospitals. Four hundred and two subjects prospectively recruited at the outpatient clinic were included from May 2016 to April 2017: 201 naïve HBeAg-negative hepatitis B virus-infected [49 chronic hepatitis B (CHB) and 152 inactive carriers(ICs)] and 201 healthy controls. Anthropomorphic and metabolic measures, liver stiffness and carotid Doppler ultrasound were performed. Subclinical atherosclerosis was established on an intima-media thickness increase of ≥1.2 mm and/or the presence of carotid plaques. Normally distributed quantitative variables were compared with the Student t test and those with a non-normal distribution with the Mann-Whitney U test. Categorical variables were compared between groups using the χ2 or Fisher exact test.
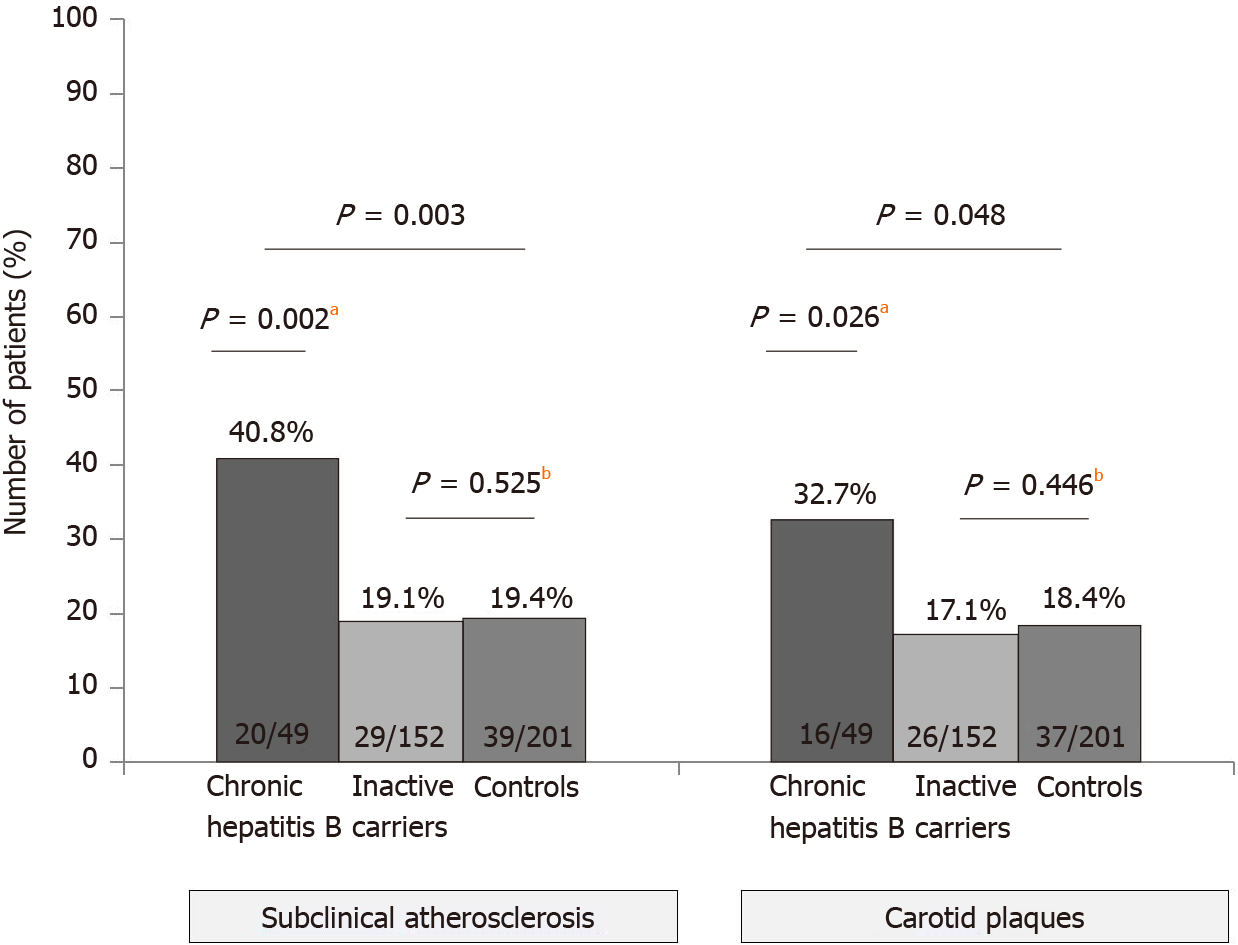
Carotid plaques were found more often in CHB (32.7%) than ICs (17.1%) or controls (18.4%) (P = 0.048). Subclinical atherosclerosis was also increased in CHB (40.8%) vsICs (19.1%) or controls (19.4%) (P = 0.003). No differences in the risk of atherosclerosis were observed between controls and ICs. The factors inde
Untreated HBeAg-negative CHB is an independent risk factor for carotid plaques and subclinical atherosclerosis, while ICs present a similar risk to controls.
Core Tip: This prospective case-control collaborative study aimed to assess whether chronic infection by hepatitis B was associated with risk of carotid plaques and subclinical atherosclerosis. Overall, 402 subjects were recruited, 201 naïve hepatitis B e antigen-negative hepatitis B virus-infected and 201 healthy controls Patients with hepatitis B e antigen-negative chronic hepatitis B presented a higher rate of carotid plaques than non-infected controls,but no differences were observed between controls and hepatitis B inactive carriers. These results suggest that hepatitis B infection may have a role as a cardiovascular risk factor in patients with chronic hepatitis B.
- Citation: Riveiro-Barciela M, Marcos-Fosch C, Martinez-Valle F, Bronte F, Orozco O, Sanz-Pérez I, Torres D, Salcedo MT, Petta S, Esteban R, Craxi A, Buti M. Naïve hepatitis B e antigen-negative chronic hepatitis B patients are at risk of carotid atherosclerosis: A prospective study. World J Gastroenterol 2021; 27(30): 5112-5125
- URL: https://www.wjgnet.com/1007-9327/full/v27/i30/5112.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i30.5112
More than 257 million people worldwide are infected with hepatitis B virus (HBV)[1], and more than 780000 die each year due to the infection[2]. Chronic HBV infection is a dynamic condition that passes through several phases, being the hepatitis B e antigen (HBeAg)-negative form the most common in Western countries[1]. Currently, patients are classified as HBeAg-negative chronic hepatitis B (CHB) when they have increased HBV DNA and alanine aminotransferase (ALT) levels and liver fibrosis and/or necroinflammation or as HBeAg-negative chronic infection/inactive carriers (ICs)when they have low HBV DNA and normal ALT levels and associated with absent or mild liver damage[3].
An increased risk of cardiovascular events has been associated with some viral infections like hepatitis C virus (HCV)[4] or human immunodeficiency virus (HIV)[5] as well as autoimmune diseases[6]. The cause of atherosclerosis in these patients is not fully explained by conventional risk factors, and endothelial dysfunction has been suggested as the underlying mechanism causing the early atherosclerotic process. This endothelial dysfunction is mainly associated with the persistent inflammatory state linked to these diseases (HCV, HIV and autoimmune diseases). In fact, eradication of HCV infection has shown a positive impact on carotid atherosclerosis[7]. Both the presence of carotid plaques or measurement of the intima-media thickness (IMT) are accepted and validated surrogate markers for early diagnosis of subclinical atherosclerosis leading to increased cardiovascular risk[8].
Chronic HBV infection has been associated with a propensity to mount proinflammatory immune reactions[9,10], including higher oxidative stress[11], that may predispose to a higher subclinical atherosclerosis.
The aim of this study was to assess whether the stage of HBeAg-negative chronic HBV infection impacts the presence of both carotid plaques and subclinical atherosclerosis. Another aim was to evaluate if the risk of both carotid plaques and subclinical atherosclerosis in HBeAg-negative patients differ to those of healthy controls.
Two hundred and one patients with chronic HBV infection and naïve to antiviral therapy were prospectively recruited at the outpatient clinics of two tertiary hospitals (Di.Bi.M.I.S., University of Palermo, Italy and Vall d’Hebron Hospital, Spain) from May 2016 to April 2017. Inclusion criteria were hepatitis B surface antigen (HBsAg) positive for more than 6 mo, HBeAg-negative and no prior exposure to antiviral therapy. Exclusion criteria were previous cardiovascular events (acute myocardial infarction or ischemic stroke), liver transplantation, HCV, hepatitis D or HIV coinfection, history of hepatocellular carcinoma or evidence of liver disease of mixed etiology (autoimmune hepatitis, Wilson’s disease, hemochromatosis, α1-antitriypsin deficiency). In addition, 201 healthy individuals matched for sex, age and body mass index were recruited as controls at the outpatient clinics from the same centers. In particular, no patient had a history of previous cardiovascular events, evidence of HBV infection (HBsAg and anti-HBc negative), HCV or HIV, or history of rheumatic or oncological disease. Importance of selection of naïve patients was crucial in view of the effect of antiviral therapy in both liver immunity and carotid plaques in subjects with HCV treated with direct-acting antivirals[7].
Naïve patients with HBV infection were classified into CHB and IC according to the recommendations of European Association for the Study of the Liver[3]: HBeAg-negative CHB was established on HBV DNA > 2000 IU/mL plus fluctuating or persistently elevated ALT levels and/or histological evidence of at least moderate fibrosis and/or necroinflammation; HBeAg-negative chronic infection or IC state was established on persistently normal ALT levels plus HBV DNA < 2000 IU/mL or HBV DNA 2000-20000 IU/mL plus evidence of mild or absent hepatic necroinflammation and fibrosis. Diagnosis of liver cirrhosis was established by liver biopsy (Ishak score 5 or 6) or transient elastography values > 13.1 kPa[12]. This study was conducted in accordance with the Declaration of Helsinki guidelines and the principles of Good Practice and was approved by the Ethics Committee of both hospitals (PR(AG)245/2015).
Data on demographics (sex, age and race), toxic exposure (alcohol, tobacco), cardio
Central obesity was defined as a waist circumference greater than 102 cm in men and 88 cm in women. Insulin resistance was determined with the homeostasis model assessment[13]. The nonalcoholic fatty liver disease (NAFLD) score was also cal
Carotid Doppler ultrasound study (Vivid I, General Electric, GE Healthcare, Horten, Norway, equipped with a 3.5-10 MHz linear transducer) was performed to determine the IMT. B-mode ultrasound with a semi-automatic edge-detection algorithm was used to measure the medium and maximum IMT on the far wall of both the right and left side of the common carotid artery at 1 cm before the bifurcation, measuring at least 250 mm of a straight arterial segment. The presence of an atheroma plaque was established based on the Manheim criteria, as a focal structure that encroached into the arterial lumen by at least 0.5 mm or 50% of the surrounding IMT value or demon
Normally distributed quantitative variables were compared with the Student t test and those with a non-normal distribution with the Mann-Whitney U test. Quantitative variables were expressed as the median and interquartile range or mean and standard deviation depending on the group size. Categorical variables were compared between groups using the χ2 or Fisher exact test, as appropriate.Variables with a P value < 0.10 in the univariate model were analyzed in a multivariate logistic regression model. Quantitative variables were also introduced as categorical (median or mean of the overall cohort) in order to increase the potency of the models. In the case of homeostasis model assessment, values from included patients were contrasted with the normal from general population[18]. Odds ratios (ORs) and 95% confidence intervals were calculated for the independent predictive factors of carotid plaques and subclinical atherosclerosis. Only patients with available data for all the variables considered in the analysis were included in the multivariate logistic regression models.
Because enrollment of patients with CHB was difficult due to the limitation to naïve subjects, the number of CHB and ICs differed. For this reason, a propensity score analysis matched by sex, age and main cardiovascular risk factors was carried out by using the package of R[19]. P values < 0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS, version 26.0 (SPSS Inc, Armonk, NY, United States).
In total, 402 individuals were enrolled: 201 chronic HBV-infected and 201 healthy controls. Overall, 218 (54.2%) were males, and the more common cardiovascular risk factors were active or former smokers (33.3%), alcohol intake (25.8%) and dyslipidemia (19.9%). Both alcohol intake and central obesity were more common in the control group. Table 1 shows the baseline characteristics of the included cohorts of patients.
| Controls | Chronic hepatitis B | Inactive carriers | |||
| n = 201 | n = 49 | n = 152 | P value1 | P value2 | |
| Age, yr | 48.1 ± 10.2 | 48.4 ± 12.0 | 46.5 ± 13.4 | 0.29 | 0.28 |
| Male sex (%) | 103 (51.2) | 31 (64.6) | 84 (54.9) | 0.13 | 0.16 |
| Race (%) | < 0.001 | 0.48 | |||
| Caucasian | 186 (92.5) | 35 (72.9) | 102 (66.7) | ||
| Asian | 2 (1.0) | 6 (12.5) | 11 (7.2) | ||
| African | 0 (0) | 6 (12.5) | 28 (18.3) | ||
| Hispanic | 13 (6.5) | 1 (2.1) | 12 (7.8) | ||
| Cardiovascular risk factors (%) | |||||
| Tobacco exposure | 74 (36.8) | 15 (31.3) | 45 (29.6) | 0.09 | 0.48 |
| Alcohol intake3 | 70 (34.8) | 11 (22.9) | 23 (15.4) | < 0.001 | 0.17 |
| Hypertension | 40 (19.9) | 11 (22.9) | 27 (17.8) | 0.46 | 0.28 |
| Diabetes | 4 (2.0) | 4 (8.3) | 6 (3.9) | 0.08 | 0.2 |
| Dyslipidemia | 46 (22.9) | 3 (6.3) | 31 (20.4) | 0.08 | 0.02 |
| Central obesity | 52 (25.9) | 10 (20.8) | 27 (17.9) | 0.04 | 0.4 |
| BMI, kg/m2 | 25.3 ± 3.6 | 26.0 ± 3.9 | 25.2 ± 4.0 | 0.76 | 0.22 |
| Liver cirrhosis (%) | 0 (0) | 12 (24.4) | 0 (0) | < 0.001 | < 0.001 |
| ALT, IU/mL | 22.6 ± 12.7 | 59.7 ± 48.6 | 25.6 ± 16.7 | < 0.001 | < 0.001 |
| GGT, IU/mL | 30.4 ± 30.9 | 60.7 ± 87.9 | 31.5 ± 63.3 | 0.24 | < 0.001 |
| LDL, mg/dL4 | 131.9 ± 38.3 | 116.9 ± 30.7 | 118.1 ± 32.6 | 0.002 | 0.82 |
| Triglycerides, mg/dL | 108.0 ± 56.7 | 96.4 ± 46.3 | 106.8 ± 59.1 | 0.54 | 0.26 |
| C-reactive protein, mg/dL5 | 0.29 ± 0.42 | 0.84 ± 1.90 | 1.00 ± 9.00 | 0.42 | 0.88 |
| HOMA index5 | 2.05 ± 1.84 | 4.20 ± 3.50 | 3.40 ± 3.90 | < 0.001 | 0.18 |
| HBsAg, logIU/mL | - | 3.6 ± 0.8 | 2.9 ± 1.2 | - | 0.001 |
| HBV DNA, logIU/mL | - | 4.4 ± 1.8 | 2.4 ± 1.1 | - | < 0.001 |
| Transient elastography, kPa | 4.5 ± 1.4 | 11.3 ± 10.9 | 5.5 ± 2.4 | < 0.001 | < 0.001 |
| CAP, dB/m | 246.5 ± 54.5 | 227.4 ± 55.0 | 227.2 ± 56.2 | 0.001 | 0.98 |
In the HBV-infected group, 152 (75.6%) were ICs and 49 (24.4%) CHB. In the latter, 12 (24.4%) patients had liver cirrhosis. Baseline characteristics according to the classification of HBV infection were summarized in Table 1. Most patients were Caucasian (68.2%), and the median age was 47 years. Demographical features did not differ between the two groups. Dyslipidemia was more common in ICs than in patients with CHB, whereas the prevalence of the remaining cardiovascular risk factors was similar. ALT, HBV DNA and HBsAg values as well as liver stiffness were significantly higher in patients with CHB.
No differences were observed between the HBV-infected group and the control group in terms of gender and age, although some cardiovascular risk factors such as central obesity and dyslipidemia were more common among non-HBV infected individuals (Table 1). In fact, although increased values of liver stiffness were observed in patients with HBV infection, CAP levels were higher in subjects within the control group.
Overall, patients with HBV infection presented higher rates of both carotid plaques (20.9% vs 18.4%) and subclinical atherosclerosis (24.4% vs 19.4%), though these differences did not reach statistical significance (P = 0.31 and P = 0.14, respectively). When the three groups were analyzed separately, taking into account the state of HBV infection, we learnt that patients with CHB had higher rates of carotid plaques (32.7%) and subclinical atherosclerosis (40.8%) than controls (18.4% and 19.4%, respectively), as shown in Figure 1. However, the rates were similar when only ICs and controls were compared (carotid plaques: 17.1% vs 18.4%, P = 0.446; subclinical atherosclerosis: 19.1% vs 19.4%, P = 0.525). Although the typical cardiovascular risk factors were linked with both carotid plaques and subclinical atherosclerosis on the univariate analysis, on the multivariate (Table 2) the only factors independently associated with the presence of subclinical atherosclerosis were older age (OR 1.45, P < 0.001), diagnosis of CHB (OR 1.23, P < 0.001) and diabetes (OR 1.13, P = 0.028). Similar results were observed regarding the carotid plaques, with age over 50 years (OR 1.43, P < 0.001) and CHB (OR 1.18, P = 0.004) as independent risk factors.
| Subclinical atherosclerosis | Carotid plaques | |||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |||
| Age, years | < 0.001 | < 0.001 | ||||
| Age > 50 years | < 0.001 | 1.45 (1.24-1.48) | < 0.001 | < 0.001 | 1.43 (1.21-1.44) | < 0.001 |
| Male sex | 0.004 | 0.336 | 0.003 | 0.212 | ||
| Central obesity | 0.008 | 0.073 | 0.007 | 0.141 | ||
| Tobacco exposure | 0.003 | 0.081 | 0.002 | 0.187 | ||
| Alcohol intake1 | 0.109 | - | 0.073 | 0.929 | ||
| Arterial hypertension | < 0.001 | 0.949 | < 0.001 | 0.690 | ||
| Diabetes mellitus | 0.004 | 1.13 (1.03-1.59) | 0.028 | 0.01 | 0.082 | |
| Dyslipidemia | 0.001 | < 0.001 | 0.095 | |||
| Chronic hepatitis B | 0.001 | 1.23 (1.11-1.41) | < 0.001 | 0.016 | 1.18 (1.06-1.34) | 0.004 |
| Transient elastography, kPa | 0.01 | 0.090 | 0.073 | 0.438 | ||
| Transient elastography > 5.7 kPa | 0.008 | 0.048 | ||||
| CAP, dB/m | < 0.001 | 0.989 | < 0.001 | 0.577 | ||
| CAP > 238 dB/m | < 0.001 | < 0.001 | ||||
| AST, IU/mL | 0.115 | - | 0.152 | - | ||
| AST > 27 IU/mL | 0.102 | 0.131 | ||||
| GGT, IU/mL | < 0.001 | 0.067 | 0.001 | 0.947 | ||
| GGT > 36 IU/mL | < 0.001 | 0.001 | ||||
| Triglycerides, mg/dL | 0.011 | 0.059 | 0.018 | 0.957 | ||
| Triglycerides > 106 mg/dL | 0.009 | 0.009 | ||||
| LDL, mg/dL | 0.651 | - | 0.180 | - | ||
| HOMA index | 0.038 | 0.073 | 0.278 | 0.105 | ||
| HOMA index > 1.2 | 0.150 | 0.489 | ||||
| HOMA index > 3 | 0.002 | 0.018 | ||||
Though this is a prospective study, due to the different number of HBV-infected subjects included in each group, a propensity score analysis including all patients with CHB (n = 49) and a cohort with the same number of IC and controls, balanced by age, sex and main cardiovascular risk factors, was carried out. The multivariate analysis of this propensity score revealed similar results as shown the analysis performed with the overall cohort, with older age (OR 1.30, P = 0.01) and CHB state (OR 1.26, P = 0.03) as independent risk factors associated with the presence of carotid plaques (Table 3).
| Groups of study | Univariate analysis | Multivariate analysis | ||||
| Chronic hepatitis B, n = 49 | Inactive carriers, n = 49 | Controls, n = 49 | P value | OR (95%CI) | P value | |
| Age, yr | 48.4 ± 12.0 | 48.7 ± 13.0 | 47.1 ± 11.3 | 0.78 | ||
| Age > 50 yr (%) | 25 (51.0) | 24 (49.0) | 23 (46.9) | 0.92 | 1.30 (1.12-1.50) | 0.01 |
| Male sex (%) | 31 (64.6) | 31 (63.3) | 31 (63.3) | 1 | 0.72 | |
| Caucasian race (%) | 35 (72.9) | 32 (65.3) | 38 (77.6) | 0.41 | - | |
| Cardiovascular risk factors (%) | ||||||
| Tobacco exposure | 15 (31.3) | 13 (26.5) | 16 (32.7) | 0.80 | 0.71 | |
| Hypertension | 11 (22.9) | 10 (20.4) | 11 (22.4) | 0.96 | 0.40 | |
| Diabetes | 4 (8.3) | 5 (10.2) | 3 (6.1) | 0.76 | 0.57 | |
| Dyslipidemia | 3 (6.3) | 3 (6.1) | 5 (10.2) | 0.68 | 0.54 | |
| Central obesity | 10 (20.8) | 10 (20.4) | 10 (20.4) | 1 | 0.06 | |
| BMI, kg/m2 | 26.0 ± 3.9 | 26.3 ± 4.9 | 26.4 ± 3.8 | 0.90 | 0.16 | |
| ALT, IU/mL | 48.6 ± 9.4 | 26.9 ± 9.4 | 25.2 ± 9.6 | < 0.001 | 0.08 | |
| GGT, IU/mL | 60.7 ± 87.9 | 42.7 ± 106.0 | 37.7 ± 50.9 | 0.45 | 0.07 | |
| LDL, mg/dL1 | 116.9 ± 30.7 | 120.3 ± 32.6 | 112.7 ± 35.3 | 0.72 | 0.87 | |
| Triglycerides, mg/dL | 96.4 ± 46.3 | 99.0 ± 57.5 | 115.3 ± 77.1 | 0.30 | 0.41 | |
| HOMA index2 | 4.2 ± 3.5 | 4.7 ± 5.8 | 2.6 ± 2.6 | 0.14 | 0.40 | |
| Transient elastography, kPa | 11.3 ± 10.9 | 5.7 ± 3.0 | 5.0 ± 7.9 | < 0.001 | 0.80 | |
| CAP, dB/m | 227.4 ± 55.0 | 232.1 ± 48.6 | 251.5 ± 62.8 | 0.095 | 0.49 | |
| Chronic hepatitis B state (%) | 49 (100) | 0 (0) | 0 (0) | < 0.001 | 1.26 (1.09-1.47) | 0.03 |
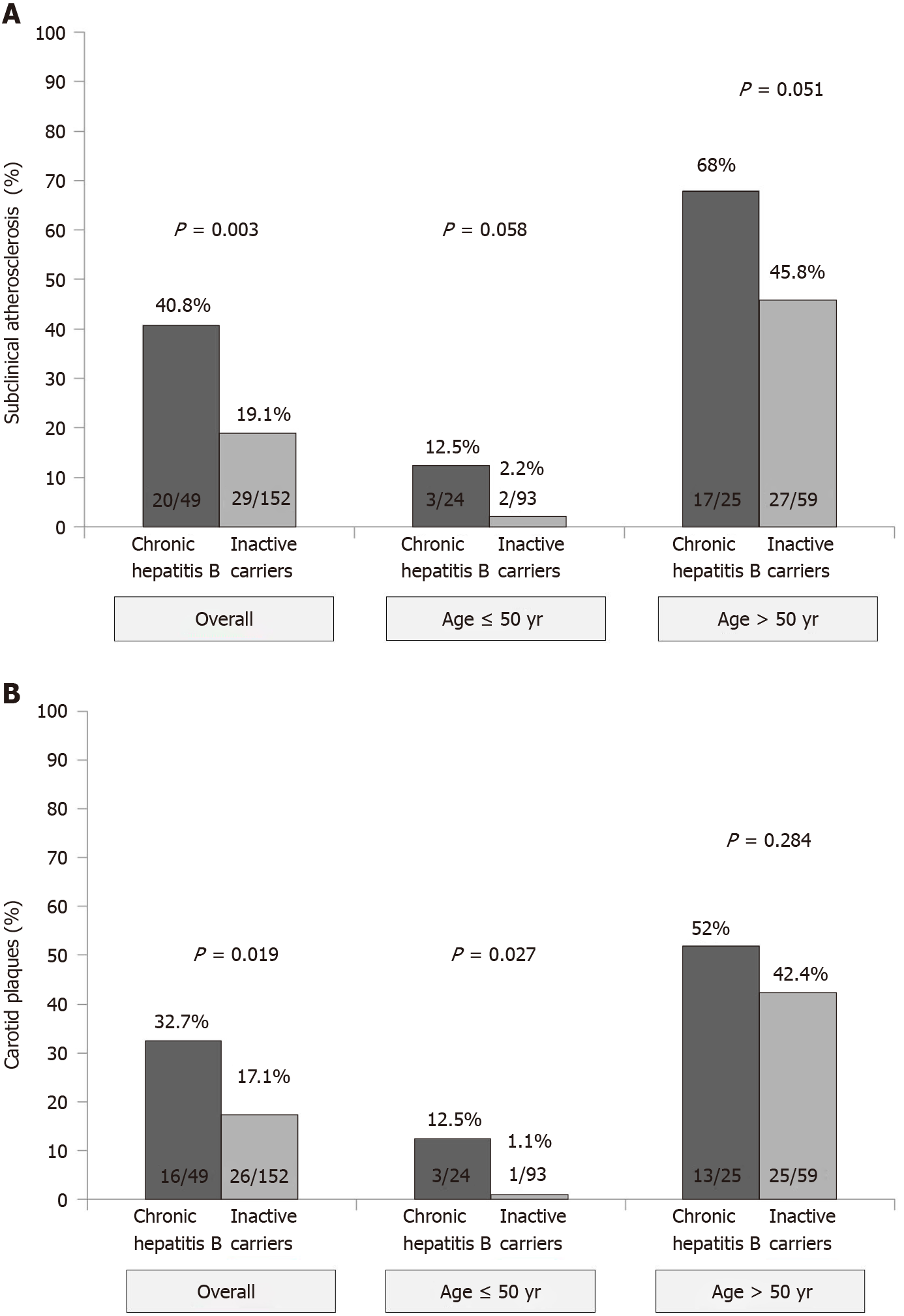
Overall, 49 (24.4%) patients had subclinical atherosclerosis, including 42 (20.9%) with carotid plaques, 19 (9.5%) with increased IMT (≥ 1.2 mm) and 12 (6%) with both findings. The prevalence of both subclinical atherosclerosis (P = 0.003) and carotid plaques (P = 0.019) was higher in patients with CHB than ICs (Figure 2). Liver cirrhosis was associated with an increased risk of subclinical atherosclerosis (42.0% vs 23.0%) although the difference did not reach statistical significance (P = 0.13). The impact of CHB on the presence of subclinical atherosclerosis remained when patients were stratified by age (Figure 2). In those ≤ 50 years, the prevalence of subclinical atherosclerosis was 12.5% in CHB patients and only 2.2% in ICs (P = 0.058). In patients aged over 50 years, those with CHB also had a higher prevalence of subclinical atherosclerosis (68.0% vs 45.8%, P = 0.051). Age was strongly associated with the presence of subclinical atherosclerosis (P < 0.001). On multivariate analysis, factors independently associated with the presence of subclinical atherosclerosis were older age (OR 1.11, P < 0.001), increased values of gamma-glutamyltransferase (OR 5.9, P = 0.007) and CHB (OR 3.35, P = 0.017). When age was introduced as a categorical variable (threshold of 50 years), both CHB and age remained as predictive factors of subclinical atherosclerosis (Table 4).
| Subclinical atherosclerosis | No subclinical atherosclerosis | Univariate analysis | Multivariate analysis | Adjusted multivariate analysis | |||
| n = 49 | n = 152 | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, yr | 57 (53.5-62.0) | 42 (33.0-52.0) | < 0.001 | 1.10 (1.06-1.16) | < 0.001 | 1.19 (1.12-1.25) | < 0.001 |
| Age > 50 yr (%) | 45 (91.8) | 46 (30.3) | < 0.001 | 21.9 (6.7-71.8) | < 0.001 | ||
| Male sex (%) | 31 (63.3) | 84 (55.3) | 0.207 | - | - | ||
| BMI, kg/m2 | 70.0 (64-77) | 69.5 (62-80) | 0.261 | - | - | ||
| Central obesity (%) | 13 (26.5) | 24 (16.0) | 0.079 | - | 0.298 | 0.356 | |
| Tobacco exposure (%) | 22 (44.9) | 38 (25.2) | 0.008 | - | 0.208 | 0.920 | |
| Alcohol intake1 (%) | 14 (28.5) | 20 (13.5) | 0.016 | - | 0.876 | 0.092 | |
| Arterial hypertension (%) | 18 (36.7) | 20 (13.2) | 0.001 | - | 0.789 | 0.419 | |
| Diabetes mellitus (%) | 5 (10.2) | 5 (3.3) | 0.067 | - | 0.994 | 0.457 | |
| Dyslipidemia (%) | 16 (32.6) | 18 (11.9) | 0.001 | - | 0.876 | 0.786 | |
| Chronic hepatitis B (%) | 20 (40.8) | 28 (18.4) | 0.002 | 3.35 (1.20-9.10) | 0.017 | 1.89 (1.75-2.04) | < 0.001 |
| Liver cirrhosis (%) | 5 (10.2) | 7 (4.6) | 0.138 | - | - | ||
| ALT, IU/mL | 29 (22.0-43.5) | 24 (17.0-34.0) | 0.310 | - | -- | ||
| ALT > ULN (%) | 10 (20.4) | 22 (14.5) | 0.220 | - | - | ||
| GGT, IU/mL | 31 (18.0-62.5) | 20 (16.0-28.0) | < 0.001 | - | 0.120 | ||
| GGT > ULN (%) | 16 (32.7) | 8 (5.3) | < 0.001 | 5.90 (1.60-21.40) | 0.007 | 1.27 (1.19-1.36) | < 0.001 |
| HbA1c, % | 5.5 (5.3-5.8) | 5.4 (5.1-5.6) | 0.004 | - | 0.78 | 0.551 | |
| HbA1c ≥ 6% (%) | 7 (14.6) | 6 (4.1) | 0.018 | - | - | ||
| HOMA index | 3.3 (2.2-6.0) | 2.4 (1.7-3.6) | 0.038 | - | 0.054 | ||
| HOMA index > 3 (%) | 22 (45.8) | 51 (34.2) | 0.102 | - | - | ||
| HBsAg, logIU/mL | 3.2 (2.5-3.6) | 3.3 (2.4-4.0) | 0.321 | - | - | ||
| HBV DNA, logIU/mL | 3.1(2.4-3.8) | 2.9 (2.3-3.5) | 0.533 | - | - | ||
| Transient elastography, kPa | 6.2(4.2-10.3) | 5.2 (4.2-6.9) | 0.059 | -- | 0.327 | 1.01 (1.00-1.01) | < 0.001 |
| CAP, dB/m | 246 (210.0-289.0) | 213 (185.0-261.5) | 0.004 | -- | 0.220 | 1.000 (1.000-1.001) | 0.006 |
| CAP > 227 dB/m (%) | 29 (67.4) | 51 (38.3) | 0.001 | -- | 0.172 | ||
In terms of carotid plaques, impact of CHB was especially important in patients aged ≤ 50 years (Figure 2). On the multivariate analysis, only age (age > 50 years, OR 1.45, 95%confidence interval 1.30-1.62, P < 0.001) and increased gamma-glutamyltransferase levels (gamma-glutamyltransferase > 36 IU/mL, OR 1.19, 95%confidence interval 1.04-1.37, P = 0.012) independently impacted the presence of carotid plaques.
Four patients with CHB and liver cirrhosis presented a NAFLD score > 0.675, suggesting significant fibrosis. Two of them had subclinical atherosclerosis, but none had a history of diabetes, and their body mass index was < 25 kg/m2 and CAP < 250 dB/m. Otherwise, they presented an HBV DNA > 2000 IU/mL, suggesting that fibrosis was likely related to CHB.
The results of this prospective collaborative study including well-characterized HBeAg negative chronic HBV infection show that CHB is independently associated with the presence of both carotid plaques and subclinical atherosclerosis. These results suggest that HBV infection may have a role as a cardiovascular risk factor in naïve patients with CHB.
There are few studies assessing the potential effect of HBV infection on develo
The suggested mechanisms to explain HBV-related atherosclerosis is direct vascular damage by the virus and particularly accelerated oxidative damage and the pro-inflammatory state of chronic HBsAg carriers[21]. Knowledge about the immune response in HBV-infected patients has increased considerably in recent years[9]. The production of proinflammatory cytokines (e.g., interleukin1b, tumor necrosis factoralpha) steadily increases during early life until it reaches the state of chronic low-grade systemic inflammation that occurs in elderly persons[22]. HBeAg-negative CHB[3] has been linked with a propensity to mount proinflammatory immune reactions[9]. In this population, liver inflammation is triggered by HBV-specific CD8 T cells, and it is associated with increased levels of chemokines and natural killer cell activation[23]. This proinflammatory state is independent of ALT levels and even HBV DNA levels, which usually fluctuate in this stage of the disease[24]. However, it has been clearly associated with progression of liver disease[9].
In our study, neither ALT levels nor HBV DNA were associated with an increased prevalence of subclinical atherosclerosis. This fact may be explained by the inclusion of patients with CHB with normal ALT but increased values of HBV DNA and liver damage at liver biopsy. On the other hand, some of the patients with liver cirrhosis presented relatively low HBV DNA levels. Older age and CHB status were independent factors associated with increased carotid plaques and subclinical atherosclerosis, in line with the proinflammatory state induced by older age and progression of liver damage.
Serum paraoxonase-1 and arylesterase activities, plasma free sulfhydryl groups and total antioxidant capacity, all factors associated with increased susceptibility to atherogenesis[24,25], are lower in HBV patients than in non-infected controls[26]. This finding can also contribute to the development of atherosclerosis in patients with HBV infection. Moreover, the association between fibrosis progression and exacerbated immune responses in patients with CHB is well established[9,10,27], so this dysfunctional immunological response might also bring an increase in cardiovascular risk.
Accordingly, HCV infection has been linked with increased prevalence of carotid plaques in those patients with evidence of advanced liver fibrosis[4]. In that study, Petta et al[4] showed that 73 of 174 HCV patients (42%) had carotid plaques, with older age and liver fibrosis as independent factors associated with carotid atherosclerosis, results in line with our findings because age and CHB were the two variables independently linked with increased risk of both carotid plaques and subclinical atherosclerosis. The role of liver damage is especially relevant in view of the lack of statistical differences when HBV ICs were compared with controls, suggesting that HBV infection may predispose to cardiovascular risk only when it is associated with a proinflammatory state, as described in patients with CHB[9,27].
This study has some limitations. First, the fact that only naïve patients were included turned out in a relatively low number of patients with HBeAg negative CHB and inferior to the cohort of HBV ICs. However, these patients were well characterized, and all met the European Association for the Study of the Liver criteria for CHB, including 24% with liver cirrhosis. Second, there were some differences among the groups. In order to minimize this potential bias, a propensity score was carried out, confirming the role of CHB status as cardiovascular risk factor. Moreover, data presented herein derived from a prospective, collaborative cohort of well-characterized patients, including different ethnicity and therefore HBV genotypes.
Interestingly, since reversion of liver fibrosis in patients with CHB is possible due to nucleos(t)ide analog therapy[28], it would be appealing to assess the potential impact of oral antiviral therapy on early atherosclerosis related to HBV infection, especially to view the effect of antiviral treatment for HCV in the overall cardiovascular risk and specifically in the carotid plaques[7].
In conclusion, in this prospective, case-control collaborative study, presence of subclinical atherosclerosis and carotid plaques were more frequent in patients with HBV infection than controls. The presence of liver damage was an independent factor associated with subclinical atherosclerosis and carotid plaques, regardless of the classical cardiovascular factors.
There is an increased risk of atherosclerosis in patients with chronic hepatitis C and also in individuals with human immunodeficiency virus infection.
There is scarce data on the potential role of hepatitis B virus infection as a cardio
To assess whether the stage of hepatitis B e antigen (HBeAg)-negative chronic hepatitis B virus infection impacts the presence of both carotid plaques and subclinical atherosclerosis and to evaluate if the risk of both carotid plaques and subclinical atherosclerosis in HBeAg-negative patients differ to those of healthy controls.
Prospective case-control study with 402 subjects prospectively recruited at the outpatient clinic. Anthropomorphic and metabolic measures, liver stiffness and carotid Doppler ultrasound were performed.
Patients with HBeAg-negative chronic hepatitis B presented a higher rate of carotid plaques than healthy controls (32.7% vs 18.4%, P = 0.002), but no differences were observed between controls and hepatitis B inactive carriers. HBeAg-negative chronic hepatitis B was an independent risk factor for carotid plaques as well as age, dyslipidemia and central obesity.
These results suggest that hepatitis B infection may have a role as a cardiovascular risk factor in patients with chronic hepatitis B.
Further studies should assess the potential impact of oral antiviral therapy on early atherosclerosis related to hepatitis B virus infection.
Writing support was provided by Cavallo C.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar R S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 557] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 2. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9574] [Article Influence: 736.5] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3790] [Article Influence: 473.8] [Reference Citation Analysis (1)] |
| 4. | Petta S, Torres D, Fazio G, Cammà C, Cabibi D, Di Marco V, Licata A, Marchesini G, Mazzola A, Parrinello G, Novo S, Licata G, Craxì A. Carotid atherosclerosis and chronic hepatitis C: a prospective study of risk associations. Hepatology. 2012;55:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Lipshultz SE, Fisher SD, Lai WW, Miller TL. Cardiovascular risk factors, monitoring, and therapy for HIV-infected patients. AIDS. 2003;17 Suppl 1:S96-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Lewandowski LB, Kaplan MJ. Update on cardiovascular disease in lupus. CurrOpinRheumatol. 2016;28:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Petta S, Adinolfi LE, Fracanzani AL, Rini F, Caldarella R, Calvaruso V, Cammà C, Ciaccio M, Di Marco V, Grimaudo S, Licata A, Marrone A, Nevola R, Pipitone RM, Pinto A, Rinaldi L, Torres D, Tuttolomondo A, Valenti L, Fargion S, Craxì A. Hepatitis C virus eradication by direct-acting antiviral agents improves carotid atherosclerosis in patients with severe liver fibrosis. J Hepatol. 2018;69:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 8. | Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen TP, Sander D, Plichart M, Catapano AL, Robertson CM, Kiechl S, Rundek T, Desvarieux M, Lind L, Schmid C, DasMahapatra P, Gao L, Ziegelbauer K, Bots ML, Thompson SG; PROG-IMT Study Group. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379:2053-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 452] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 9. | Bertoletti A, Hong M. Age-Dependent Immune Events during HBV Infection from Birth to Adulthood: An Alternative Interpretation. Front Immunol. 2014;5:441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Maini MK, Gehring AJ. The role of innate immunity in the immunopathology and treatment of HBV infection. J Hepatol. 2016;64:S60-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 11. | Ishizaka N, Ishizaka Y, Takahashi E, Toda Ei E, Hashimoto H, Ohno M, Nagai R, Yamakado M. Increased prevalence of carotid atherosclerosis in hepatitis B virus carriers. Circulation. 2002;105:1028-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Viganò M, Paggi S, Lampertico P, Fraquelli M, Massironi S, Ronchi G, Rigamonti C, Conte D, Colombo M. Dual cut-off transient elastography to assess liver fibrosis in chronic hepatitis B: a cohort study with internal validation. Aliment Pharmacol Ther. 2011;34:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24499] [Article Influence: 612.5] [Reference Citation Analysis (0)] |
| 14. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2282] [Article Influence: 126.8] [Reference Citation Analysis (1)] |
| 15. | Xu L, Lu W, Li P, Shen F, Mi YQ, Fan JG. A comparison of hepatic steatosis index, controlled attenuation parameter and ultrasound as noninvasive diagnostic tools for steatosis in chronic hepatitis B. Dig Liver Dis. 2017;49:910-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 1280] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 17. | Espeland MA, Craven TE, Riley WA, Corson J, Romont A, Furberg CD. Reliability of longitudinal ultrasonographic measurements of carotid intimal-medial thicknesses. Asymptomatic Carotid Artery Progression Study Research Group. Stroke. 1996;27:480-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Shashaj B, Luciano R, Contoli B, Morino GS, Spreghini MR, Rustico C, Sforza RW, Dallapiccola B, Manco M. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2016;53:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 19. | Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137:693-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 271] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Targher G, Bertolini L, Padovani R, Rodella S, Arcaro G, Day C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol. 2007;46:1126-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Purnak T, Efe C, Beyazit Y, Ozaslan E, Astan R, Milanloglu A, Ozbalkan Z, Rizzo M. Recent insights into the relationship between inflammatory liver diseases and atherosclerosis. J Investig Med. 2011;59:904-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. CurrOpin Immunol. 2010;22:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 450] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 23. | Tan AT, Koh S, Goh W, Zhe HY, Gehring AJ, Lim SG, Bertoletti A. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J Hepatol. 2010;52:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Stabenow D, Frings M, Trück C, Gärtner K, Förster I, Kurts C, Tüting T, Odenthal M, Dienes HP, Cederbrant K, Protzer U, Knolle PA. Bioluminescence imaging allows measuring CD8 T cell function in the liver. Hepatology. 2010;51:1430-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Rosenblat M, Karry R, Aviram M. Paraoxonase 1 (PON1) is a more potent antioxidant and stimulant of macrophage cholesterol efflux, when present in HDL than in lipoprotein-deficient serum: relevance to diabetes. Atherosclerosis. 2006;187:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Karsen H, Binici I, Sunnetcioglu M, Baran AI, Ceylan MR, Selek S, Celik H. Association of paraoxonase activity and atherosclerosis in patients with chronic hepatitis B. Afr Health Sci. 2012;12:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87:1439-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 293] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 28. | Abayli B, Abaylı C, Gencdal G. Histopathological evaluation of long-term tenofovir disoproxil fumarate treatment in patients with hepatitis be antigen-negative chronic hepatitis B. World J GastrointestPharmacolTher. 2021;12:32-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |