Published online Aug 14, 2021. doi: 10.3748/wjg.v27.i30.5088
Peer-review started: April 7, 2021
First decision: May 27, 2021
Revised: May 28, 2021
Accepted: July 15, 2021
Article in press: July 15, 2021
Published online: August 14, 2021
Processing time: 124 Days and 15.9 Hours
As a country with a high burden of hepatitis B, China has about 86 million cases of hepatitis B virus infection, ranking the first in the world. Currently, there are about 390000 deaths due to hepatitis B-related complications such as liver cirrhosis and liver cancer every year. Consequently, how to control portal hypertension, improve liver functional reserve, and reduce the incidence of hepatic failure and liver cancer in such patients is the focus of current clinical attention. Previous clinical study in our center suggested that at 24 mo after transjugular intrahepatic portosystemic shunt (TIPS), the liver functional reserve of patients with hepatitis B cirrhosis was better than that of patients with alcohol-induced and immune cirrhosis, which may be related to the effective etiological treatment.
To investigate the clinical efficacy of three first-line antiviral drugs recommended by the guidelines of prevention and treatment for chronic hepatitis B in China (2019) in the treatment of patients with hepatitis B-related cirrhosis who had received a TIPS.
The clinical data of 137 patients with hepatitis B-related cirrhosis with portal hypertension after receiving TIPS at our centre between March 2016 and December 2020 were analysed retrospectively. According to different anti-viral drugs, the patients were divided into entecavir (ETV) (n = 70), tenofovir alafenamide fumarate (TAF) (n = 32), and tenofovir disoproxil fumarate (TDF) (n = 35) groups. The cumulative incidence of hepatic encephalopathy and hepatocellular carcinoma, survival, and changes in hepatic reserve function and glomerular filtration rate in patients treated with different antiviral drugs within 24 mo after surgery were investigated.
At 24 mo after surgery, the Child–Pugh score in the TAF group (6.97 ± 0.86) was lower than that in the TDF (7.49 ± 0.82; t = -2.52, P = 0.014) and ETV groups (7.64 ± 1.17; t = -2.92, P = 0.004). The model for end-stage liver disease score in the TAF group at 24 mo after surgery was 9.72 ± 1.5, which was lower than that in the TDF (10.74 ± 2.33; t = -2.09, P = 0.040) and ETV groups (10.97 ± 2.17; t = -2.93, P = 0.004). At 24 mo after surgery, the estimated glomerular filtration rate (eGFR) in the TAF group (104.41 ± 12.54) was higher than that in the TDF (93.54 ± 8.97) and ETV groups (89.96 ± 9.86) (F = 21.57, P < 0.001).
At 24 mo after surgery, compared with TDF and ETV, TAF has significant advantages in the improvement of liver functional reserve and eGFR.
Core Tip: As a country with a high burden of hepatitis B, China has about 86 million cases of hepatitis B virus (HBV) infection, ranking the first in the world. Hepatitis B cirrhosis complicated with portal hypertension is characterized by persistent HBV replication and aggravated liver inflammation and fibrosis. Considering the fact that there is currently no report on the clinical efficacy of antiviral therapy for patients with hepatitis B cirrhosis after transjugular intrahepatic portosystemic shunt, we believe that this study has appreciated clinical reference value for the selection of anti-HBV drugs in such patients.
- Citation: Yao X, Huang S, Zhou H, Tang SH, Qin JP. Clinical efficacy of antiviral therapy in patients with hepatitis B-related cirrhosis after transjugular intrahepatic portosystemic shunt. World J Gastroenterol 2021; 27(30): 5088-5099
- URL: https://www.wjgnet.com/1007-9327/full/v27/i30/5088.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i30.5088
Patients with chronic hepatitis B (CHB) cirrhosis complicated with severe portal hypertension-related complications rarely accept liver transplantation because of limited liver source and high medical costs. As a result, how to improve the liver functional reserve, reduce the incidence of liver cancer, and enhance the survival rate of such patients has become the focus of clinical research. Transjugular intrahepatic portosystemic shunt (TIPS) is an effective method to treat the complications secondary to liver cirrhosis complicated with portal hypertension. While portal hypertension-related complications in patients with CHB cirrhosis are effectively controlled, early initiation of antiviral therapy can significantly reduce long-term liver-related complications, such as hepatocellular carcinoma (HCC) and hepatic failure, and improve the survival rate. At present, tenofovir disoproxil fumarate (TDF), entecavir (ETV), and tenofovir alafenamide fumarate (TAF) are recommended as the first-line treatment options for CHB patients by major international and domestic guidelines for the diagnosis, treatment, and management of CHB. Moreover, previous clinical study[1] in our center suggested that the use of an 8-mm stent during TIPS could not only effectively shunt, but also relieve portal pressure. Twenty-four months after surgery, the liver functional reserve of patients with hepatitis B cirrhosis was better than that of patients with alcohol-induced and immune cirrhosis, which may be related to the effective etiological treatment. Based on the above studies, the clinical data of patients with hepatitis B cirrhosis after TIPS treated with different antiviral drugs were retrospectively analyzed in this study. However, TAF entered the Chinese market since November 2018. Current data on the efficacy and safety of TAF in Chinese people are rare, especially in patients with CHB cirrhosis complicated with portal hypertension whose portal vein pressure is relieved after TIPS. Therefore, we evaluated the clinical efficacy of the three antiviral drugs through this retrospective study, with an aim to provide reference for antiviral treatment of patients with CHB cirrhosis after TIPS.
The clinical data of 137 patients with CHB cirrhosis complicated with portal hypertension treated by TIPS with a Viator stent at our center from March 2016 to December 2020 were collected. The inclusion criteria included: (1) Adult patients with CHB cirrhosis complicated with portal hypertension treated by TIPS for the first time; (2) patients receiving TIPS with a shunt channel dilated by an 8-mm balloon and an 8-mm TIPS-specific stent (Viator stent) implanted; and (3) patients undergoing monotherapy (ETV/TDF/TAF) for hepatitis B virus (HBV) for more than one year, with complete virological response maintained 3, 6, and 12 mo after surgery, as evidenced by a low HBV DNA (the lower limit of detection of HBV DNA was 100 IU/mL). The exclusion criteria were as follows: (1) Patients with liver cancer confirmed by imaging examination before surgery, and a history of splenectomy or partial splenic artery embolization; (2) patients with stent dysfunction; (3) patients with preoperative Child-Pugh score for hepatic function > 13 and underlying nephropathy [estimated glomerular filtration rate (eGFR) < 90 mL/min]; (4) patients with cavernous transformation of the portal vein or portal thrombosis; (5) patients with preoperative hepatic encephalopathy (HE), hepatorenal syndrome, and hepatopulmonary syndrome; (6) patients with a history of major liver surgery such as hepatectomy, surgical shunt, and liver transplantation; (7) patients with severe coagulation disorders; (8) patients with severe right heart failure and pulmonary hypertension; (9) patients with uncontrolled intrahepatic infection [alanine aminotransferase (ALT) was more than 10 times higher than the upper limit of normal] and systemic infection; (10) pregnant and lactating patients or patients with pregnancy plan; and (11) patients with liver cirrhosis treated with warfarin and other anticoagulants after surgery. All the patients met the indications of the United States TIPS guidelines[2] and the American Association of the Study of Liver Diseases (AASLD) guidelines for the prevention and treatment of CHB[3]. This study was approved by the hospital ethics committee. The patients and their families were fully informed and signed an informed consent form.
Digital subtraction angiography (DSA) was carried out using the AXIOM-Artist DSA system (Siemens, Germany) and Mark V high-pressure syringes. Surgical materials included a RUPS-100 puncture kit, a straight-needle multi-side hole catheter, an Opta Pro balloon catheter, embolization coils, a Cobra catheter (Cook, United States), a stiffening exchange guide wire, and a Viatorr stent (Gore and Associates, United States). Additionally, TDF (300 mg/tablet, Beite Pharmaceutical Co., Ltd.), ETV tablets (0.5 mg/tablet, Sino-American Shanghai Squibb Pharmaceutical Co., Ltd.), and TAF tablets (25 mg/tablet, Gilead, United States) were used in this study.
Preoperative preparation: Preoperatively, routine examinations including routine blood test, liver and kidney function test, HBV-DNA quantitation, and prothrombin time test were performed. All the patients underwent enhanced computed tomography (CT) of the liver and three-dimensional (3D) reconstruction of the hepatic vein-portal vein. The anatomic relationship between the hepatic vein and the portal vein was analyzed to guide the puncture of the portal vein during surgery.
TIPS approach: All the patients received TIPS via the right jugular vein. Under the guidance of a guide wire, the puncture system passed through the superior vena cava and the right atrium to the right hepatic vein or the hepatic segment of the inferior vena cava. According to the images obtained from preoperative enhanced CT of the liver and 3D reconstruction of the hepatic vein-portal vein, the puncture of the portal vein branch was guided. After the safety of the puncture was evaluated, portal vein pressure was measured and the collateral circulation vessels causing esophagogastric varices were embolized. Then, a stent was inserted after balloon dilation puncturing the channel, followed by another portal vein angiography and measurement of portal vein pressure. The detailed operations have been reported in a previous study[4]. All operations were successfully completed by the same group of professionals using an 8-mm balloon and an 8-mm Viatorr stent, without severe operation-related complications.
Postoperative follow-up: The data on HBV-DNA quantitation, liver and kidney function, and coagulation were collected before surgery and 1, 3, 6, 12, and 24 mo after surgery. Ascites and HE were understood by ultrasonography and follow-up records. Child-Pugh grade and the model for end-stage liver disease (MELD) were obtained, respectively. The follow-up endpoints of follow-up was death at 24 mo after surgery, no response to follow-up for consecutive 2 times or more, liver transplantation, or study deadline (December, 2020). The evaluation methods of follow-up were referred to the references[5-7].
All measurement data are expressed as the mean ± SD or percentages. The portal pressure gradient before and after treatment was analyzed using the paired t-test or chi-square test. The incidence of HE and liver cancer and survival rate were calculated with the Kaplan-Meier curve and compared by the log-rank test. All the data were statistically analyzed using SPSS 22.0. P < 0.05 was considered significantly significant.
A total of 137 patients with liver cirrhosis were enrolled, including 75 males and 62 females, with an average age of 54.0 ± 9.1 years. According to different antiviral drugs, the patients were divided into an ETV group (n = 70), a TAF group (n = 32), and a TDF group (n = 35). Gender, age, preoperative and postoperative portal pressure gradient, preoperative and postoperative Child-Pugh grade, MELD score, the proportion of hepatitis B e antigen (HBeAg)-positive patients, median HBV DNA level, median time to TAF/TDF/ETV therapy, and kidney function index (eGFR or sCr) showed no statistically significant differences among the three groups (Table 1).
| Clinical characteristic | TAF (n = 32) | TDF (n = 35) | ETV (n = 70) | Statistic | P value | Total (n = 137) |
| Gender, n (%) | X2 = 1.033 | 0.597 | ||||
| Male | 20 | 18 | 37 | 75 | ||
| Female | 12 | 17 | 33 | 52 | ||
| Age (yr, mean ± SD) | 53.8 ± 8.5 | 54.4 ± 7.3 | 54.5 ± 10.3 | t = 0.234 | 0.815 | 54.3 ± 9.2 |
| Child-Pugh classification | X2 = 1.623 | 0.805 | ||||
| A | 9 | 14 | 21 | 44 | ||
| B | 18 | 17 | 37 | 72 | ||
| C | 5 | 4 | 12 | 21 | ||
| Child-Pugh score | 6.94 ± 1.29 | 7.46 ± 2.14 | 7.26 + 1.64 | F = 1.119 | 0.330 | 7.31 ± 1.75 |
| MELD score | 10.37 ± 2.94 | 9.69 ± 2.96 | 10.41 ± 2.93 | F = 0.775 | 0.463 | 10.22 ± 2.93 |
| Prothrombin time (s) | 14.40 ± 1.86 | 15.14 ± 2.81 | 14.95 ± 2.73 | F = 0.930 | 0.354 | 14.74 ± 2.59 |
| Albumin (g/L) | 35.27 ± 5.65 | 34.14 ± 5.01 | 34.43 ± 4.33 | F = 0.496 | 0.610 | 34.55 + 4.82 |
| eGFR (mL/min/1.73 m2) | 91.22 ± 10.67 | 90.37 ± 14.24 | 90.19 ± 12.05 | F = 0.078 | 0.925 | 90.47 ± 12.27 |
| Cr (μmol/L) | 75.04 ± 36.60 | 78.07 ± 39.72 | 71.56 ± 23.70 | F = 0.940 | 0.349 | 74.04 ± 31.51 |
| Total bilirubin (μmol/L) | 36.83 ± 14.74 | 38.62 ± 17.21 | 34.10 ± 15.69 | F = 0.868 | 0.387 | 35.89 ± 15.89 |
| PPG before TIPS (mmHg) | 25.41 ± 4.82 | 25.71 ± 5.49 | 25.84 ± 5.49 | F = 0.073 | 0.929 | 25.71 ± 5.31 |
| PPG after TIPS (mmHg) | 9.52 ± 2.28 | 9.97 ± 3.04 | 10.13 ± 2.81 | F = 0.530 | 0.590 | 9.95 ± 2.75 |
| Deaths in 24 mo, n (%) | 3 (9.4) | 4 (11.4) | 10 (14.3) | X2 = 0.517 | 0.772 | 17 (13.7) |
| HCC in 24 mo, n (%) | 2 (6.3) | 2 (5.7) | 7 (10) | X2 = 0.712 | 0.700 | 11 (9.3) |
| HE in 24 mo, n (%) | 6 (18.8) | 8 (22.9) | 18 (25.7) | X2 = 0.770 | 0.681 | 32 (25.4) |
TIPS via the right jugular vein was performed successfully in all the 137 patients, without severe intraoperative complications such as abdominal hemorrhage and bile duct hemorrhage. The collateral circulation causing esophagogastric varices was embolized and the stent was implanted successfully. The success rate of surgery was 100%, and the short-term hemostasis rate was 100%. The portal pressure gradient (mmHg) decreased from 25.71 ± 5.32 preoperatively to 9.95 ± 2.75 postoperatively (t = 37.32, P < 0.001).
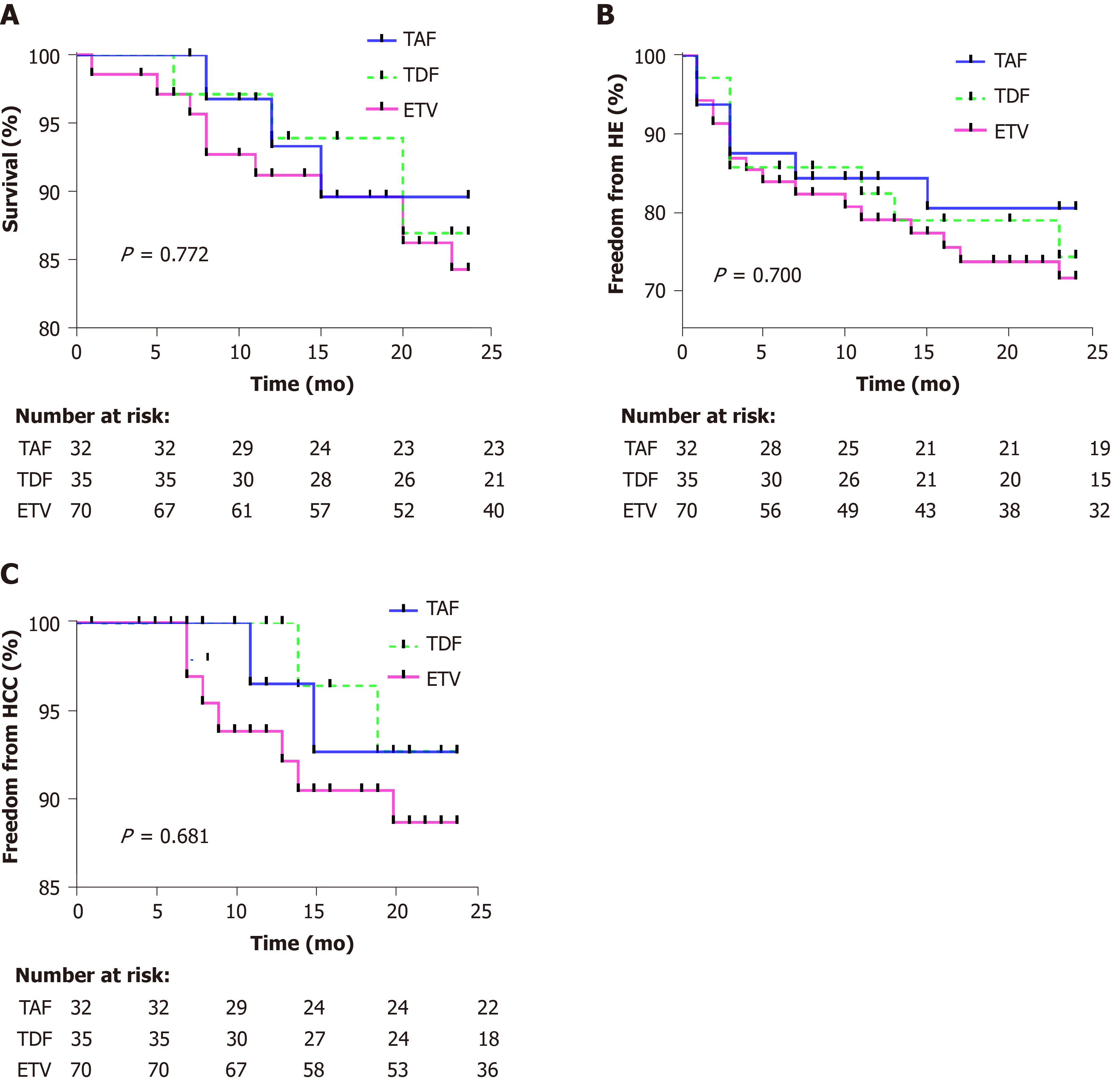
The median follow-up time was 24 (20, 24) mo. The survival rates at 12 and 24 mo after surgery was 93.3% and 89.6% in the TAF group, 93.9% and 86.9% in the TDF group, and 91.2% and 84.3% in the ETV group, respectively (log rank χ2 = 0.517, P = 0.772) (Figure 1A). During the follow-up, a total of 17 patients died, with a median time to death of 12 (7, 20) mo, including three cases in the TAF group, four in the TDF group, and ten in the ETV group. The causes of death included primary liver cancer in 11 (2/2/7) patients, sepsis/infection in 4 (1/1/2), and hepatorenal syndrome in 2 (0/1/1).
One, three, six, twelve, and twenty-four months after surgery, the incidence of HE was 6.2%, 12.5%, 12.5%, 15.6%, and 19.5% in the TAF group, 2.9%, 14.3%, 14.3%, 17.6%, and 25.7% in the TDF group, and 5.7%, 13.1%, 16.1%, 20.9%, and 28.3% in the ETV group (log rank χ2 = 0.712, P = 0.700) (Figure 1B). During the follow-up, HE occurred in 32 patients, with a median time to occurrence of 3 mo. There were six cases of HE in the TAF group (HE grade I/II/III-IV, 4/2/0), eight in the TDF group (HE grade I/II/III-IV, 6/1/1), and 18 in the ETV group (HE grade I/II/III-IV, 14/2/2).
Twelve and twenty-four months after surgery, the incidence of liver cancer was 3.4% and 7.3% in the TAF group, 3.6% and 7.3% in the TDF group, and 6.1% and 11.3% in the ETV group, respectively (log rank χ2 = 0.77, P = 0.681) (Figure 1C). During the follow-up, liver cancer occurred in 11 patients, with a median time to occurrence of 11 (8, 14) mo (TAF vs TDF vs ETV, 2/2/7).
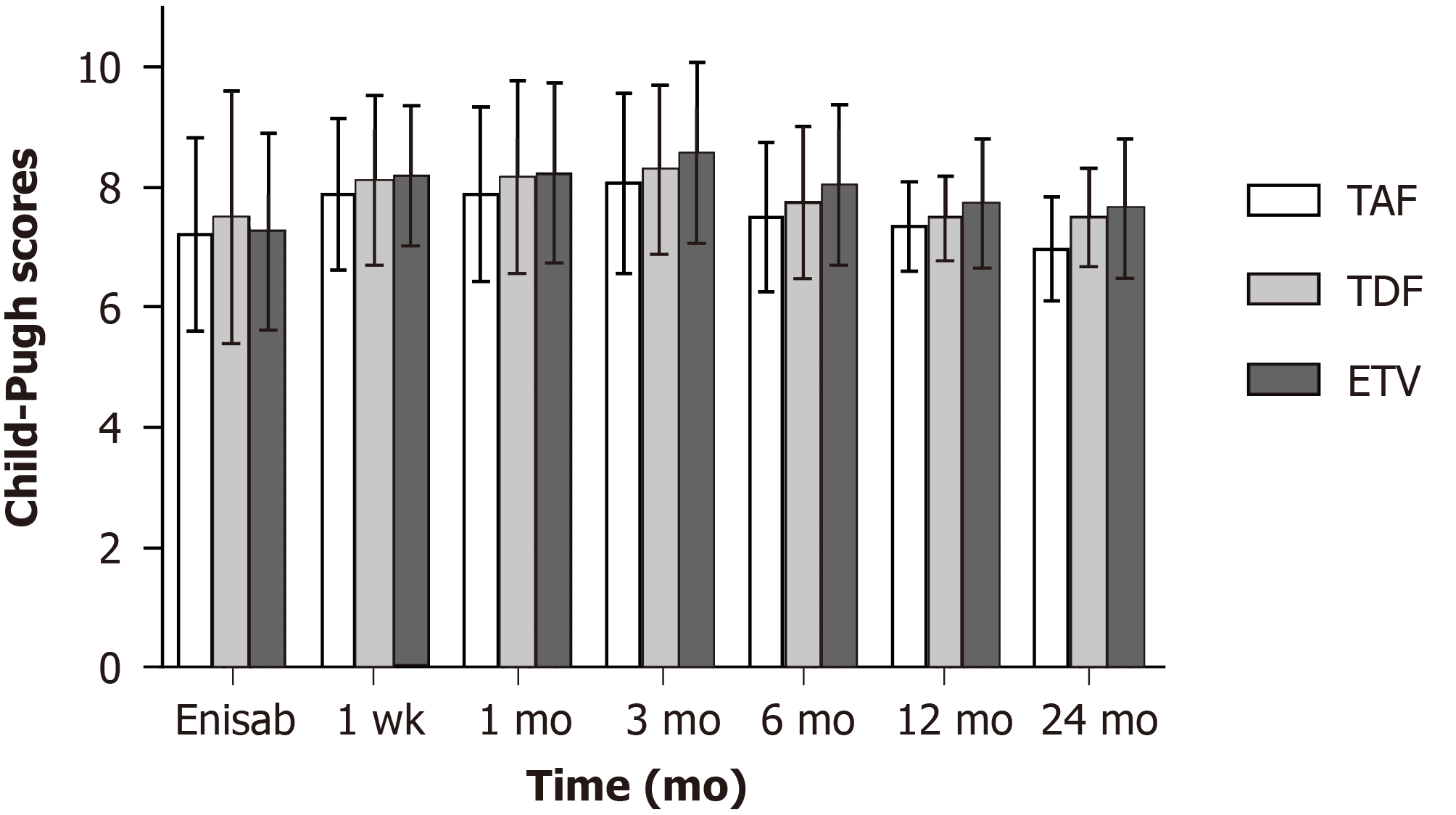
Child-Pugh score showed no significant differences in the three groups before and 12 mo after surgery (P > 0.05). Twenty-four months after surgery, Child-Pugh score in the TAF group was 6.97 ± 0.86, which was lower than those in the TDF group (7.49 ± 0.82; t = -2.52, P = 0.014) and ETV group (7.64 ± 1.17; t = -2.92, P = 0.004) (Figure 2).
Twenty-four months after surgery, the constituent ratio of Child-Pugh stage A/B/C (60.8/34.8/4.4) in the TAF group was improved compared with that (28.1/56.3/15.6) before surgery (χ2 = 6.47, P = 0.039), but the constituent ratio of Child-Pugh stage A/B/C in the TDF/ETV group presented no difference from that before surgery (P > 0.05) (Table 2).
| Baseline | 1 mo | 3 mo | 6 mo | 12 mo | 24 mo | |
| TAF, n (%) | 9/18/5 (32) | 4/23/5 (32) | 3/23/6 (32) | 6/22/4 (32) | 7/18/2 (27) | 14/8/1 (23) |
| Child-Pugh A/B/C (%) | 28.1/56.3/15.6 | 12.5/71.9/12.7 | 12.7/74.6/15.6 | 18.8/68.8/12.5 | 25.9/66.7/7.4 | 60.9/34.8/4.3 |
| TDF(n) | 14/17/4 (35) | 4/25/6 (35) | 4/23/8 (35) | 7/21/5 (33) | 6/20/3 (29) | 7/11/3 (21) |
| Child-Pugh A/B/C (%) | 40.0/48.6/11.4 | 11.4/71.4/12.0 | 11.4/65.8/22.8 | 21.2/63.6/15.2 | 20.7/68.9/10.4 | 33.3/52.4/14.3 |
| ETV, n (%) | 21/37/12 (70) | 9/47/13 (69) | 6/47/16 (69) | 11/44/11 (66) | 11/42/6 (59) | 11/24/5 (40) |
| Child-Pugh A/B/C (%) | 30.3/52.9/17.1 | 13.1/68.1/18.8 | 8.7/68.1/23.2 | 16.7/66.6/16.7 | 18.6/71.2/10.2 | 27.5/60.0/12.5 |
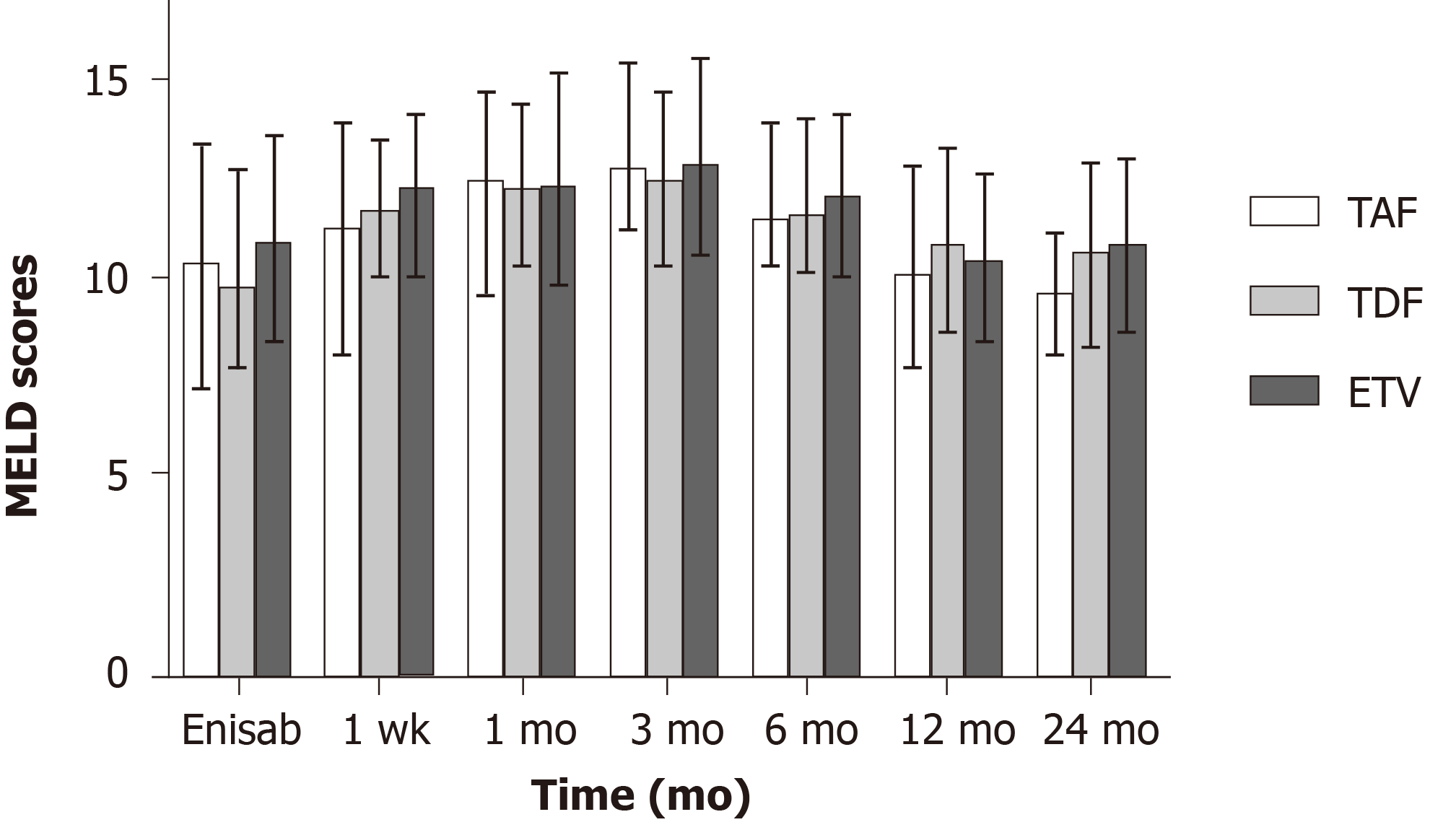
No statistically significant differences were found in MELD score before and 12 mo after surgery in the three groups (P > 0.05). Twenty-four months after surgery, MELD score in the TAF group was 9.72 ± 1.5, which was lower than those in the TDF group (10.74 ± 2.33; t = -2.09, P = 0.040) and ETV group (10.97 ± 2.17; t = -2.93, P = 0.004) (Figure 3).
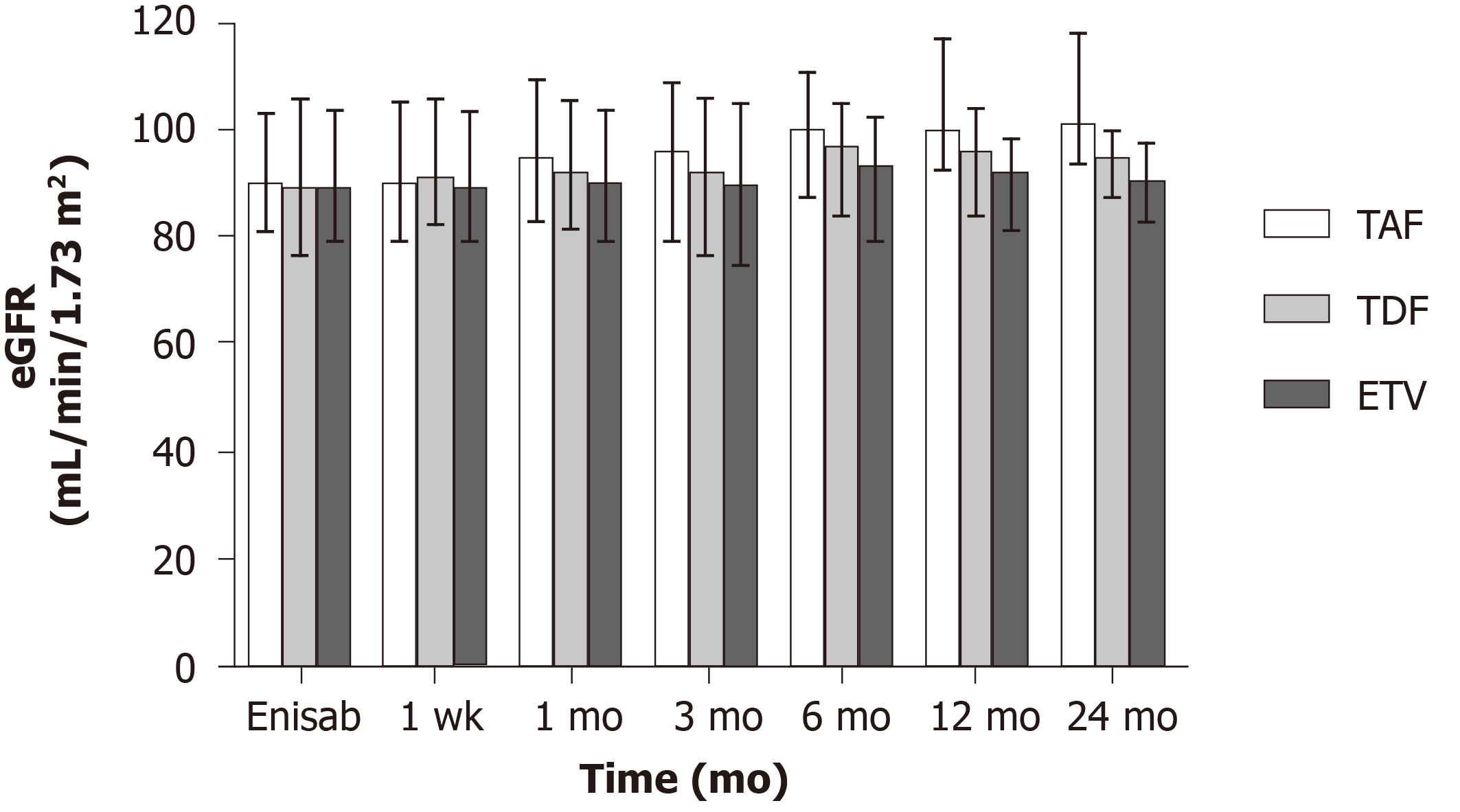
In the three groups, eGFR change showed no significant differences 3 mo after surgery (P > 0.05). Six months after surgery, eGFR level in the TAF group was 99.72 ± 11.52, which was higher than that in the ETV group (91.24 ± 12.60; t = 3.24, P = 0.002), but not significantly different from that in the TDF group (94.97 ± 12.52; t = 1.61, P = 0.112). Twelve months after surgery, eGFR level in the TAF group was 103.44 ± 13.02, which was higher than those in the TDF group (94.11 ± 12.34; t = 3.08, P = 0.004) and ETV group (90.03 ± 11.04; t = 5.37, P < 0.001). Twenty-four months after surgery, eGFR level in the TAF group was 104.41 ± 12.54, which was higher than those (93.54 ± 8.97) in the TDF (93.54 ± 8.97) and ETV (89.96 ± 9.86) groups (F = 21.57, P < 0.001) (Figure 4).
As a country with a high burden of hepatitis B, China has about 86 million cases of HBV infection, ranking the first in the world. Currently, there are about 30 million CHB patients, and 390000 deaths due to hepatitis B-related complications such as liver cirrhosis and liver cancer every year[8,9]. Hepatitis B cirrhosis complicated with portal hypertension is characterized by persistent HBV replication and aggravated liver inflammation and fibrosis. Without timely control, it may progress to severe post-cirrhosis complications such as esophagogastric variceal bleeding, HE, intractable hydrothorax and ascites, and even hepatic failure and liver cancer, leading to death. Consequently, how to control portal hypertension, improve liver functional reserve, and reduce the incidence of hepatic failure and liver cancer in such patients is the focus of current clinical attention. TIPS is an effective minimally invasive interventional method for the treatment of portal hypertension-related complications. It is worthy of further clinical exploration on the management of patients with hepatitis B cirrhosis using individualized antiviral therapy after surgery. Considering the fact that there is no report on the clinical efficacy of antiviral therapy for patients with hepatitis B cirrhosis after TIPS, we believe that this study has appreciated clinical reference value for the selection of anti-HBV drugs in such patients.
CHB is one of the main causes of HCC, cirrhosis-related complications, and liver-related death all over the world[10]. Chronic HBV infection is mainly treated by maximizing the long-term inhibition of HBV replication, relieving the inflammation and necrosis of hepatocytes and fibrous hyperplasia of the liver, delaying and reducing the occurrence of hepatic failure, decompensated cirrhosis, HCC, and other complications, improving the quality of life of the patients, and prolonging their survival time[11-13]. Antiviral therapy is a long-term or even lifelong process. The efficacy and safety of drugs are important factors that clinicians and patients need to consider. Previous studies have shown that in CHB patients with initial treatment, the incidence of drug resistance 5 years after ETV therapy is about 1.2%[14,15], while the clinical drug resistance of TDF and TAF has not been definitely reported[16,17]. Moreover, these three drugs are the first choice of oral antiviral drugs recommended by international and domestic guidelines, characterized by high barrier to resistance, strong effect, and high safety. In this study, all the included patients were those with hepatitis B cirrhosis after TIPS, and treated with the three antiviral drugs. During follow-up, the percentage of complete virological response of HBV DNA at 3, 6, and 12 mo after surgery was 100%, without breakthrough in virology. We believe that after continuous antiviral therapy, complete virological response can be achieved, inflammatory activity of the liver can be reduced or even become static, liver fibrosis can be reversed, and the regeneration ability of residual hepatocytes can be enhanced, which can further improve the liver functional reserve of the patients. In this study, TAF showed a better effect in improving the liver functional reserve of patients with hepatitis B cirrhosis at 24 mo after TIPS than TDF and ETV, the potential mechanism of which may be attributed to the stronger antiviral effect and ALT normalization ability of TAF. Previous global phase III clinical studies 108/110 on TAF[18,19] showed that compared with TDF, CHB patients treated with TAF could achieve a higher proportion of ALT normalization at both the 48th and 96th weeks. Moreover, an increasing number of real-world studies[20-22] demonstrated that about 30% of CHB patients receiving long-term ETV therapy failed to achieve complete virological response (the lower limit of detection of HBV DNA was 20 IU/mL), while most patients could achieve complete virological response after switching to TAF therapy. All these studies suggest that TAF has more prominent advantages in antiviral ability and/or ALT normalization. In our center, the lower limit of detection of HBV DNA was 100 IU/mL, which could not be used to accurately identify the CHB population with a low level of HBV DNA replication. However, the above findings may still have certain reference value for antiviral therapy in patients with hepatitis B cirrhosis complicated with portal hypertension after TIPS.
In fact, for patients with cirrhosis, especially for those with decompensated cirrhosis, liver transplantation is the only way to remove the lesion and radically cure cirrhosis. However, transplantation is rarely conducted in Asia. Especially in China, fewer patients can receive liver transplantation because of the limitation in funds and liver sources. For patients with hepatitis B cirrhosis, effective etiological treatment combined with control of portal hypertension and improvement of liver functional reserve is the key to long-term survival of patients with decompensated hepatitis B cirrhosis who cannot accept liver transplantation. TIPS is the only minimally invasive method for relieving portal hypertension. Previous clinical study[1] in our center suggested that the use of an 8-mm stent during TIPS could not only effectively shunt, but also relieve portal pressure. Postoperatively, the liver functional reserve was affected by shunt in a short time, but the special stent with an inner diameter of 8 mm had limited shunt. The liver functional reserve could be restored to the preoperative level 12 mo after surgery. Twenty-four months after surgery, the liver functional reserve of patients with hepatitis B cirrhosis was better than that of patients with alcohol-induced and immune cirrhosis, which may be related to the effective etiological treatment. Based on the above studies, the clinical data of patients with hepatitis B cirrhosis after TIPS treated with different antiviral drugs were retrospectively analyzed in this study. Before this study, many studies[23-27] in the past 2 years have explored the effects of different antiviral drugs on the long-term occurrence of HCC in patients with CHB, most of which are retrospective cohort studies, and some were systematic reviews and meta-analyses, with non-unified conclusions. However, it can be concluded that nucleotide drugs (represented by TFV) are equal or superior to nucleoside analogues (represented by ETV) in the long-term risk of HCC, especially in some patients with high-risk factors for HCC, such as compensated cirrhosis or decompensated cirrhosis. In this study, it was found that during the follow-up, the incidence of HE and liver cancer as well as survival rate had no statistical significance among the three groups. We believe that it was related to the small number of patients enrolled in this study and short follow-up time. Because TAF has not been clinically applied in China for a long time, the long-term clinical outcomes of the three different antiviral drugs in the treatment of patients with hepatitis B cirrhosis after TIPS need to be further clarified by expanding the sample size and prolonging the observation time.
According to the Chinese guidelines for TIPS operation in 2019[28], TIPS can effectively improve glomerular filtration rate, increase renal blood flow, and reduce serum creatinine and aldosterone levels. The eGFR of all the patients included in this study was improved at varying degrees 6 mo after surgery. We believe that the short-term changes in renal function are related to the changes in systemic hemodynamics after TIPS, and effective control of portal pressure can improve renal blood supply and glomerular filtration rate. The safety of TAF for the bone and kidney has been proved to be higher than that of TDF in previous global phase III clinical studies 108/110[18,19]. Additionally, the 2018 AASLD HBV guidelines[11] suggest no advantages or disadvantages between ETV and TDF for the risk of long-term renal and skeletal complications. Compared with TDF, TAF is associated with a lower proportion of patients with skeletal and renal abnormalities. Moreover, in the 2017 EASL guidelines[12], TAF or ETV is superior to TDF in CHB patients > 60 years old with bone diseases and renal changes. With the same baseline, eGFR of the TAF group was better than the other two groups 12-24 mo after surgery in this study. We believe that in the limited follow-up time, there were differences among the three drugs in the treatment of patients with hepatitis B cirrhosis after TIPS. TAF may have a smaller effect on the renal function of patients with decompensated hepatitis B cirrhosis than the other two drugs, which further indicates that TAF may have a smaller effect on the renal function of patients with CHB cirrhosis. Through clinical practice, we believe that patients with hepatitis B cirrhosis after TIPS can benefit from long-term treatment by active antiviral therapy and choosing TAF, which has a smaller effect on renal function and can potently inhibit viruses.
This study has several limitations. First, this is a retrospective cohort study. Although most indexes in different treatment groups were consistent at the baseline in this study, there were still potential unpredictable biases. Second, in this study, HBeAg status, HBsAg titer level, and ALT that may affect the long-term prognosis were not recorded and analyzed. Finally, the time from the approval of TAF in China to its clinical application is still short, and the number of patients receiving TAF therapy is relatively small. Therefore, the sample size of this study is small, and we need to further expand the sample size, and use propensity score matching statistical method to avoid excessive biases and baseline mismatch as much as possible. This study is continuing to determine whether the beneficial effect of TAF will last for a long time, and we will also conduct a longer-term clinical observation on the antiviral therapy in patients with hepatitis B cirrhosis after TIPS.
In our center, the operation and clinical management were performed by the same group of doctors. Through clinical practice, we believe that improving the long-term survival rate of patients with hepatitis B cirrhosis after TIPS involves a complex situation, which is determined by a large number of non-hemodynamic factors. Age, degree of renal failure, chronic inflammation, urease-producing intestinal bacteria, bacterial translocation and malnutrition/atrophy are other very important factors in the regulation of the treatment. Early initiation of antiviral therapy and optimization of antiviral therapy are also important factors. In conclusion, compared with TDF and ETV, TAF has significant advantages in the improvement of liver functional reserve and eGFR. The difference in the long-term effect of TAF on HCC occurrence needs further observation and clarification.
At 24 mo after surgery, compared with TDF and ETV, TAF has significant advantages in the improvement of liver functional reserve and eGFR. The difference in the long-term effect of TAF on HCC occurrence needs further observation and clarification.
Hepatitis B cirrhosis complicated with portal hypertension is characterized by persistent hepatitis B virus (HBV) replication and aggravated liver inflammation and fibrosis. Without timely control, it may progress to severe post-cirrhosis complications such as esophagogastric variceal bleeding, hepatic encephalopathy (HE), intractable hydrothorax and ascites, and even hepatic failure and liver cancer, leading to death. Consequently, how to control portal hypertension, improve liver functional reserve, and reduce the incidences of hepatic failure and liver cancer in such patients is the focus of current clinical attention. Previous clinical study in our center suggested that the use of an 8-mm stent during transjugular intrahepatic portosystemic shunt (TIPS) could not only effectively shunt, but also relieve portal pressure. Twenty-four months after surgery, the liver functional reserve of patients with hepatitis B cirrhosis was better than that of patients with alcohol-induced and immune cirrhosis. Based on the above studies, the clinical data of patients with hepatitis B cirrhosis after TIPS treated with different antiviral drugs were retrospectively analyzed in this study.
TIPS is an effective minimally invasive interventional method for the treatment of portal hypertension-related complications. It is worthy of further clinical exploration on the management of patients with hepatitis B cirrhosis using individualized antiviral therapy after surgery. Considering the fact that there is no report on the clinical efficacy of antiviral therapy for patients with hepatitis B cirrhosis after TIPS, we believe that this study has appreciated clinical reference value for the selection of anti-HBV drugs in such patients.
To explore the clinical efficacy of the three antiviral drugs through this retrospective study, so as to provide reference for antiviral treatment of patients with chronic hepatitis B (CHB) cirrhosis after TIPS.
The clinical data of 137 patients with hepatitis B-related cirrhosis with portal hypertension after receiving TIPS at our center between March 2016 and December 2020 were analysed retrospectively. According to different anti-viral drugs, the patients were divided into entecavir (ETV) (n = 70), tenofovir alafenamide fumarate (TAF) (n = 32), and tenofovir disoproxil fumarate (TDF) (n = 35) groups. The cumulative incidence of HE and hepatocellular carcinoma (HCC), survival, and changes in hepatic reserve function and glomerular filtration rate in patients treated with different antiviral drugs within 24 mo after surgery were investigated.
At 24 mo after surgery, compared with TDF and ETV, TAF had significant advantages in the improvement of liver functional reserve and estimated glomerular filtration rate (eGFR).
Through clinical practice, compared with TDF and ETV, TAF has significant advantages in the improvement of liver functional reserve and eGFR. The difference in the long-term effect of TAF on HCC occurrence needs further observation and clarification. The author’s team believes that improving the long-term survival rate of patients with hepatitis B cirrhosis after TIPS involves a complex situation, which is determined by a large number of non-hemodynamic factors. Early initiation of antiviral therapy and optimization of antiviral therapy are important factors. Age, degree of renal failure, chronic inflammation, urease-producing intestinal bacteria, bacterial translocation, and malnutrition/atrophy are other very important factors in the regulation of the treatment.
The difference in the long-term effect of TAF on HCC occurrence needs further observation and clarification.
Thanks to Director Jian-Ping Qin and Dr. Shan-Hong Tang for their contributions to this manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alkarkoushi RR, Senyuz O S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Yao X, Zhou H, Huang S, Tang SH, Qin JP. Effects of transjugular intrahepatic portosystemic shunt using the Viatorr stent on hepatic reserve function in patients with cirrhosis. World J Clin Cases. 2021;9:1532-1542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 405] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 3. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1588] [Article Influence: 176.4] [Reference Citation Analysis (2)] |
| 4. | Qin JP, Tang SH, Jiang MD, He QW, Chen HB, Yao X, Zeng WZ, Gu M. Contrast enhanced computed tomography and reconstruction of hepatic vascular system for transjugular intrahepatic portal systemic shunt puncture path planning. World J Gastroenterol. 2015;21:9623-9629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M, Christie JM; Clinical Services and Standards Committee of the British Society of Gastroenterology. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 416] [Article Influence: 41.6] [Reference Citation Analysis (2)] |
| 6. | Chinese Society of Hepatology; Chinese Medical Association. Xu X, Duan Z, Ding H, Li W, Jia J, Wei L, Linghu E, Zhuang H. Chinese guidelines on the management of ascites and its related complications in cirrhosis. Hepatol Int. 2019;13:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Bercu ZL, Fischman AM, Kim E, Nowakowski FS, Patel RS, Schiano TD, Chang CY, Lookstein RA. TIPS for refractory ascites: a 6-year single-center experience with expanded polytetrafluoroethylene-covered stent-grafts. AJR Am J Roentgenol. 2015;204:654-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 2001] [Article Influence: 200.1] [Reference Citation Analysis (4)] |
| 9. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64718] [Article Influence: 16179.5] [Reference Citation Analysis (177)] |
| 10. | Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 408] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 11. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2847] [Article Influence: 406.7] [Reference Citation Analysis (0)] |
| 12. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3804] [Article Influence: 475.5] [Reference Citation Analysis (1)] |
| 13. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1961] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 14. | Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N, Zhang H, Tenney DJ, Tamez R, Iloeje U. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 466] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 15. | Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392:2313-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 369] [Article Influence: 52.7] [Reference Citation Analysis (2)] |
| 16. | Liu Y, Corsa AC, Buti M, Cathcart AL, Flaherty JF, Miller MD, Kitrinos KM, Marcellin P, Gane EJ. No detectable resistance to tenofovir disoproxil fumarate in HBeAg+ and HBeAg- patients with chronic hepatitis B after 8 years of treatment. J Viral Hepat. 2017;24:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | Park ES, Lee AR, Kim DH, Lee JH, Yoo JJ, Ahn SH, Sim H, Park S, Kang HS, Won J, Ha YN, Shin GC, Kwon SY, Park YK, Choi BS, Lee YB, Jeong N, An Y, Ju YS, Yu SJ, Chae HB, Yu KS, Kim YJ, Yoon JH, Zoulim F, Kim KH. Identification of a quadruple mutation that confers tenofovir resistance in chronic hepatitis B patients. J Hepatol. 2019;70:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, Ahn SH, Izumi N, Chuang WL, Bae H, Sharma M, Janssen HLA, Pan CQ, Çelen MK, Furusyo N, Shalimar D, Yoon KT, Trinh H, Flaherty JF, Gaggar A, Lau AH, Cathcart AL, Lin L, Bhardwaj N, Suri V, Mani Subramanian G, Gane EJ, Buti M, Chan HLY; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018;68:672-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 19. | Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, Hui AJ, Janssen HL, Chowdhury A, Tsang TY, Mehta R, Gane E, Flaherty JF, Massetto B, Gaggar A, Kitrinos KM, Lin L, Subramanian GM, McHutchison JG, Lim YS, Acharya SK, Agarwal K; GS-US-320-0110 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 352] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 20. | Sun Y, Wu X, Zhou J, Meng T, Wang B, Chen S, Liu H, Wang T, Zhao X, Wu S, Kong Y, Ou X, Wee A, Theise ND, Qiu C, Zhang W, Lu F, Jia J, You H. Persistent Low Level of Hepatitis B Virus Promotes Fibrosis Progression During Therapy. Clin Gastroenterol Hepatol. 2020;18:2582-2591.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 21. | Ogawa E, Nomura H, Nakamuta M, Furusyo N, Koyanagi T, Dohmen K, Ooho A, Satoh T, Kawano A, Kajiwara E, Takahashi K, Azuma K, Kato M, Shimoda S, Hayashi J; Kyushu University Liver Disease Study (KULDS) Group. Tenofovir alafenamide after switching from entecavir or nucleos(t)ide combination therapy for patients with chronic hepatitis B. Liver Int. 2020;40:1578-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Li ZB, Li L, Niu XX, Chen SH, Fu YM, Wang CY, Liu Y, Shao Q, Chen G, Ji D. Switching from entecavir to tenofovir alafenamide for chronic hepatitis B patients with low-level viraemia. Liver Int. 2021;41:1254-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir Is Associated With Lower Risk of Hepatocellular Carcinoma Than Entecavir in Patients With Chronic HBV Infection in China. Gastroenterology. 2020;158:215-225.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 24. | Gu L, Yao Q, Shen Z, He Y, Ng DM, Yang T, Chen B, Chen P, Mao F, Yu Q. Comparison of tenofovir versus entecavir on reducing incidence of hepatocellular carcinoma in chronic hepatitis B patients: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2020;35:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Choi WM, Choi J, Lim YS. Effects of Tenofovir vs Entecavir on Risk of Hepatocellular Carcinoma in Patients With Chronic HBV Infection: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2021;19:246-258.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 26. | Li M, Lv T, Wu S, Wei W, Wu X, Ou X, Ma H, Chow SC, Kong Y, You H, Jia J. Tenofovir versus entecavir in lowering the risk of hepatocellular carcinoma development in patients with chronic hepatitis B: a critical systematic review and meta-analysis. Hepatol Int. 2020;14:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Chang TS, Yang YH, Chen WM, Shen CH, Tung SY, Yen CW, Hsieh YY, Lee CP, Tsai ML, Hung CH, Lu SN. Long-term risk of primary liver cancers in entecavir versus tenofovir treatment for chronic hepatitis B. Sci Rep. 2021;11:1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Chinese College of Interventionalists. [CCI clinical practice guidelines: management of TIPS for portal hypertension (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:582-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |