Published online Aug 14, 2021. doi: 10.3748/wjg.v27.i30.5060
Peer-review started: March 4, 2021
First decision: May 5, 2021
Revised: May 19, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: August 14, 2021
Processing time: 159 Days and 1 Hours
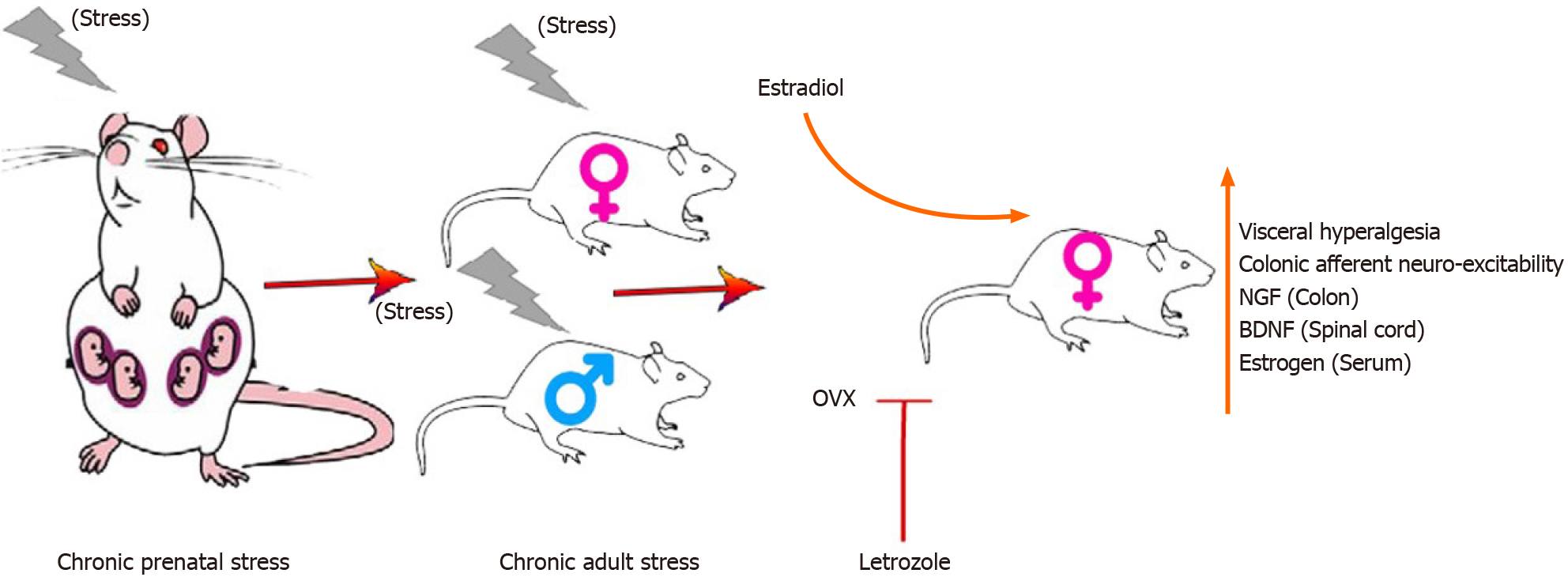
Chronic stress during pregnancy may increase visceral hyperalgesia of offspring in a sex-dependent way. Combining adult stress in offspring will increase this sensitivity. Based on the evidence implicating estrogen in exacerbating visceral hypersensitivity in female rodents in preclinical models, we predicted that chronic prenatal stress (CPS) + chronic adult stress (CAS) will maximize visceral hyperalgesia; and that estrogen plays an important role in colonic hyperalgesia.
The aim was to illuminate the role of estrogen in colonic hyperalgesia and its underlying mechanisms.
We established a CPS plus CAS rodent model in which the balloon was used to distend the colorectum. The single-fiber recording in vivo and patch clamp experiments in vitro were used to monitor the colonic neuron’s activity. The reverse transcription-polymerase chain reaction, western blot, and immunofluorescence were used to study the effects of CPS and CAS on colon primary afferent sensitivity. We used ovariectomy and letrozole to reduce estrogen levels of female rats respectively in order to assess the role of estrogen in female-specific enhanced primary afferent sensitization.
Spontaneous activity and single fiber activity were significantly greater in females than in males. The enhanced sensitization in female rats mainly came from low-threshold neurons. CPS significantly increased single-unit afferent fiber activity in L6-S2 dorsal roots in response. Activity was further enhanced by CAS. In addition, the excitability of colon-projecting dorsal root ganglion (DRG) neurons increased in CPS + CAS rats and was associated with a decrease in transient A-type K+ currents. Compared with ovariectomy, treatment with the aromatase inhibitor letrozole significantly reduced estrogen levels in female rats, confirming the gender difference. Moreover, mice treated with letrozole had decreased colonic DRG neuron excitability. The intrathecal infusion of estrogen increased brain-derived neurotrophic factor (BDNF) protein levels and contributed to the response to visceral pain. Western blotting showed that nerve growth factor protein was upregulated in CPS + CAS mice.
This study adds to the evidence that estrogen-dependent sensitization of primary afferent colon neurons is involved in the development of chronic stress-induced visceral hypersensitivity in female rats.
Core Tip: We investigated whether estrogen re-enhanced visceral hyperalgesia in chronic prenatal stress plus chronic adult stress models. After using physical ovariectomy or chemical inhibition with letrozole to reduce estrogen levels, we found that visceral hyperalgesia, colonic afferent neuronal excitability, nerve growth factor and brain-derived neurotrophic factor, and estrogen were all increased. The findings indicate that chronic stress-induced visceral hypersensitivity was estrogen dependent and that the hypersensitivity was mediated by estrogen-dependent sensitization of primary afferent colon neurons. The preclinical models provide key scientific evidence in support of developing gender-based visceral pain management.
- Citation: Chen JH, Sun Y, Ju PJ, Wei JB, Li QJ, Winston JH. Estrogen augmented visceral pain and colonic neuron modulation in a double-hit model of prenatal and adult stress. World J Gastroenterol 2021; 27(30): 5060-5075
- URL: https://www.wjgnet.com/1007-9327/full/v27/i30/5060.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i30.5060
Visceral pain of colonic origin is the most prominent symptom in irritable bowel syndrome (IBS) patients[1]. Female IBS patients report more severe pain that occurs more frequently and with longer episodes than in male patients[1,2]. The ratio of female to male IBS is about 2:1 among patients seen in medical clinics[3]. Moreover, females have a higher prevalence of IBS co-morbidities such as anxiety and depression[4,5] and are more vulnerable to stress-induced exacerbation of IBS symptoms compared with males[3,6,7].
Clinical studies show that early life adverse experiences are risk factors for the development of IBS symptoms, including visceral pain and ongoing chronic stress, especially abdominal pain[8-10]. These factors contribute to the development of visceral hypersensitivity, a key component of the IBS symptom complex and one that may be responsible for symptoms of pain[11,12]. Our previous research found that the female offspring of mothers subjected to chronic prenatal stress (CPS) had a markedly greater visceral sensitivity than their male littermates following challenge by another chronic adult stress (CAS) protocol. A critical molecular event in the development of this female-enhanced visceral hypersensitivity is upregulation of brain-derived neurotrophic factor (BDNF) expression in the lumbar-sacral spinal cord of female CPS + CAS rats[13]. However, the neurophysiological changes underlying the enhanced female-specific visceral hypersensitivity and the role of hormone in the development of stress-induced visceral hypersensitivity are not well understood.
Visceral hypersensitivity in IBS involves abnormal changes in neurophysiology throughout the brain-gut axis. In IBS, there is evidence for sensitization of primary afferents to jejunal distention and electrical stimulation[14], and there is evidence for increased sensitivity of lumbar splanchnic afferents[15,16]. In animal models of either early life adverse events or adult stress-induced visceral hypersensitivity[17], there is evidence of colon primary afferent sensitization. However, the studies were performed in male rodents. Therefore, in this study, we established a CPS and CAS rodent model to analyze the impact on female colon afferent neuron function and the role of estrogen. Our hypothesis was that female CPS offspring subjected to chronic stress as adults would exhibit greater colonic dorsal root ganglion (DRG) neuron sensitization compared with their male littermates, and that the enhanced visceral sensitization and primary afferent sensitization in females was estrogen dependent.
The Institutional Animal Care and Use Committee of the University of Texas Medical Branch at Galveston, TX approved all animal procedures. Experiments were performed on pregnant Sprague Dawley rats and their 8-wk-old to 16-wk-old male and female offspring. Rats were housed individual cages with access to food and water in a room with controlled conditions (22 ± 2 °C, relative humidity of 50% ± 5%), and a 12 h light/12 h dark cycle.
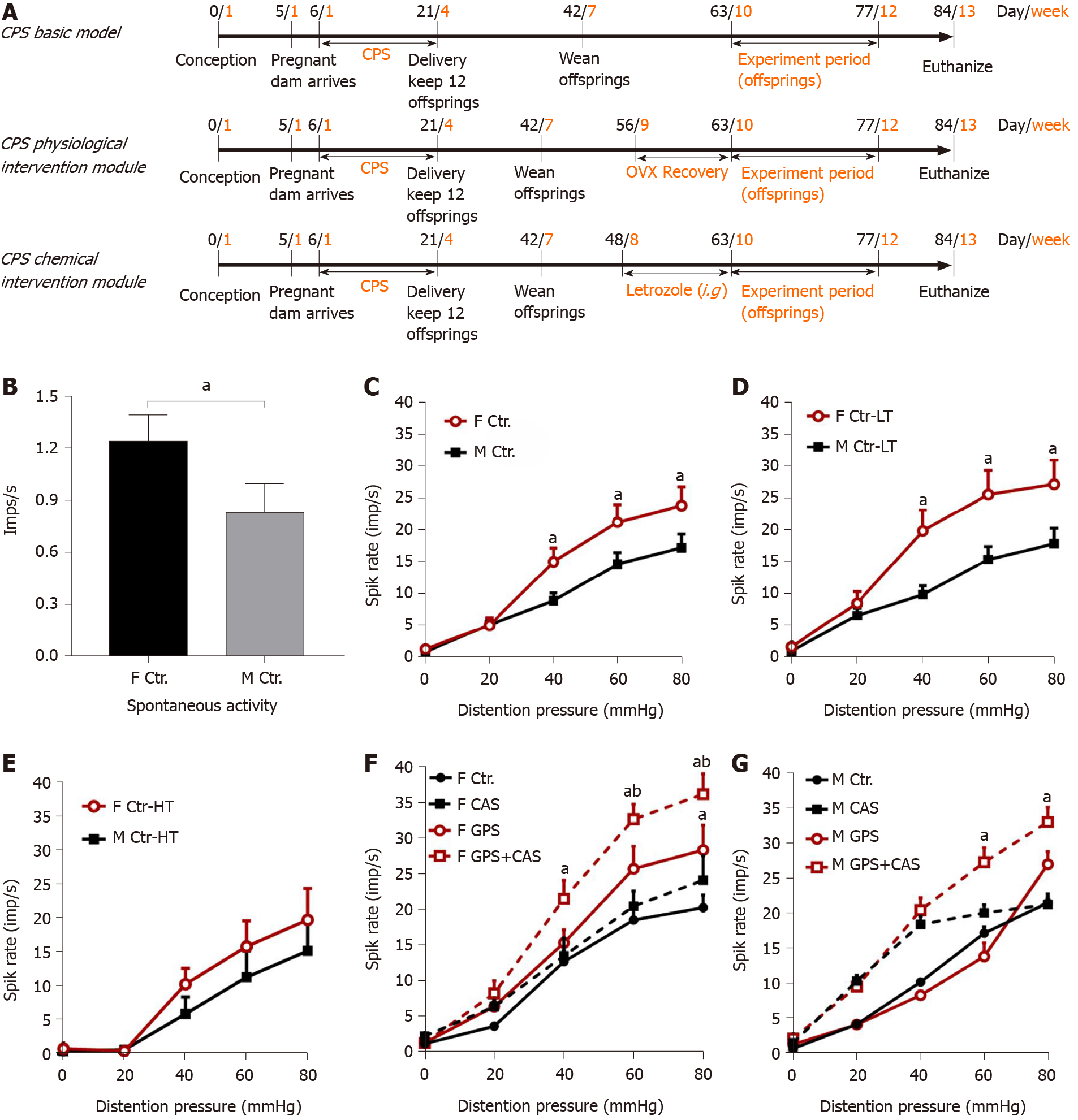
Pregnant dams were subjected to a CPS protocol that consisted of a random sequence of twice-daily applications of one of three stress sessions, a 1-h water-avoidance, 45-min cold-restraint, or a 20-min forced swim starting on day 6 and continuing until delivery on day 21. Male and female offspring of the stressed dams were designated CPS rats. Control dams received sham stress and their offspring were designated control rats. As adults at 8-16 wk of age, control and prenatally stressed offspring were challenged by the same CAS protocol for 9 d. Ovariectomy (OVX) or sham surgery was performed on female prenatal-stress offspring on day 56. Daily letrozole treatment was initiated on day 49, 2 wk prior to initiation of adult stress. Treatment was continued through the stress protocol. A schematic diagram of the study procedures is shown in Figure 1A.
Before OVX or letrozole treatment, vaginal smears were used to identify the estrus cycle phase. OVX or sham surgery was performed on female prenatal-stress offspring on day 56. The aromatase inhibitor letrozole [4,4’-(1H-1,2,4-triazol-l-yl-methylene)-bis-benzonitrile], (Novartis) 1.0 mg/kg was orally administered in the experimental group and vehicle (hydroxypropyl cellulose 0.3% in water) was given in the control group once daily for 14 d. Direct transcutaneous intrathecal injections of estrogen and letrozole were performed as described by Mestre et al[18].
Multiunit afferent discharges were recorded from the distal ends of L6-S2 dorsal rootlets decentralized close to their entry into the spinal cord. A bundle of multiunit fibers was distinguished into 2-6 single units off-line using wave mark template matching in Spike 2 software that differentiates spikes by shape and amplitude. Colonic afferent fibers were identified by their response to graded colorectal distention (CRD). A balloon was used to distend the colorectum. Isoflurane, 2.5%, followed by 50 mg/kg intraperitoneal sodium pentobarbital induced general anesthesia that was maintained by infusing a mixture of pentobarbital sodium + pancuronium bromide + saline by intravenous infusion through the tail vein. The adequacy of anesthesia was confirmed by the absence of corneal and pupillary reflexes and stability of the end-tidal CO2 level. A tracheotomy tube connected to a ventilator system provided a mixture of room air and oxygen. Expired CO2 was monitored and maintained at 3.5%. Body temperature was monitored and maintained at 37 °C by a servo-controlled heating blanket. A laminectomy from T12 to S2 exposed the spinal cord. The head was stabilized in a stereotaxic frame.
Retrograde fluorescence label injections: Labeling of colon-projecting DRG neurons was performed as previously described[13]. Under general 2% isoflurane anesthesia, the lipid soluble fluorescent dye, 1,1’-dioleyl-3,3,3’,3’-tetramethylindocarbocyanine methane-sulfonate (9-DiI, Invitrogen, Carlsbad, CA) was injected (50 mg/mL) into the muscularis externa on the exposed distal colon in 8 to 10 sites (2 μL each site). To prevent leakage, the needle was kept in place for 1 min following each injection.
Dissociation and culture of DRG neurons: Rats were deeply anesthetized with isoflurane followed by decapitation. Lumbosacral (L6–S2) DRGs were collected in ice cold and oxygenated dissecting solution, containing (in mM) 130 NaCl, 5 KCl, 2 KH2PO4, 1.5 CaCl2, 6 MgSO4, 10 glucose, and 10 HEPES, pH 7.2 (305 mOsm). After removal of the connective tissue, the ganglia were transferred to a 5 mL dissecting solution containing collagenase D (1.8 mg/mL; Roche) and trypsin (1.0 mg/mL; Sigma, St Louis, MO), and incubated for 1.5 h at 34.5 °C. DRGs were then taken from the enzyme solution, washed, and put in 0.5-2 mL of the dissecting solution containing DNase (0.5 mg/mL; Sigma). Cells were subsequently dissociated by gentle trituration 10 to 15 times with fire-polished glass pipettes and placed on acid-cleaned glass coverslips. The dissociated DRG neurons were kept in 1 mL DMEM (with 10% FBS) in an incubator (95% O2/5% CO2) at 37 °C overnight.
Whole-cell patch clamp recordings from dissociated DRG neurons: Before each experiment, a glass coverslip with DRG neurons was transferred to a recording chamber perfused (1.5 mL/min) with external solution containing (10 mM): 130 NaCl, 5 KCl, 2 KH2PO4, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH adjusted to pH 7.4 with NaOH (300 mOsm) at room temperature. Recording pipettes, pulled from borosilicate glass tubing, with resistance of 1-5 MΩ, were filled with solution containing (in mM): 100 KMeSO3, 40 KCl, and 10 HEPES, pH 7.25 adjusted with KOH (290 mOsm). DiI-labeled neurons were identified by fluorescence microscopy. Whole-cell currents and voltage were recorded from DiI-labeled neurons using a Dagan 3911 patch clamp amplifier. Data were acquired and analyzed by pCLAMP 9.2 (Molecular Devices, Sunnyvale, CA). The currents were filtered at 2–5 kHz and sampled at 50 or 100 s per point. While still under voltage clamp, the Clampex Membrane Test program (Molecular Devices) was used to determine membrane capacitance (Cm) and membrane resistance (Rm), during a 10 ms, 5 mV depolarizing pulse form a holding potential of −60 mV. The configuration was then switched to current clamp (0 pA) to determine other electrophysiological properties. After stabilizing for 2–3 min, the resting membrane potential was measured. The minimum acceptable resting membrane potential was −40 mV. Spontaneous activity (SA) was then recorded over two 30 s periods separated by 60 s without recording, as described by Bedi et al[19].
Transient A-type K+ current (IA) recording method in patch studies: To record voltage-gated K+ current (Kv), Na+ in control external solution was replaced with equimolar choline and the Ca2+ concentration was reduced to 0.03 mM to suppress Ca2+ currents and to prevent Ca2+ channels becoming Na+ conducting. The reduced external Ca2+ would also be expected to suppress Ca2+-activated K+ currents. The current traces of Kv in DRG neurons were measured at different holding potentials. The membrane potential was held at −100 mV and voltage steps were from −40 to +30 mV to record the total Kv. The membrane potential was held at −50 mV to record the sustained Kv. The IA currents were calculated by subtracting the sustained current from the total current. The current density (in pA/pF) was calculated by dividing the current amplitude by cell membrane capacitance.
Total RNA was extracted using RNeasy Mini Kits (QIAGEN, Valencia, CA). One microgram of total RNA was reverse-transcribed using the SuperScriptTM III First-Strand Synthesis System. PCR assays were performed on a StepOnePlus thermal cycler with 18 s as the normalizer using Applied Biosystems primer/probe set Rn02531967_s1 directed against the translated exon IX. Fold-change relative to control was calculated using the ΔΔCt method (Applied Biosystems).
Samples were lysed in RIPA buffer containing protease inhibitor cocktail and phenylmethanesulfonyl fluoride. Lysates were incubated for 30 min on ice and then centrifuged at 10 000 × g for 10 min at 4 °C. The protein concentration in the super
Frozen sections of colon tissue from control, CAS, CPS and CPS + CAS female rats were mounted on glass slides, and rehydrated in phosphate buffered saline at room temperature. The slides were treated for antigen retrieval and blocked with 10% normal goat serum diluted in 0.3% phosphate buffered saline-Triton for 1 h, and then incubated with NGF primary antibody in antibody diluent (Renoir Red, Biocare Medical, Concord, CA) at 4 °C overnight. The slides were exposed to fluorescent dye-conjugated secondary antibody for 2 h at room temperature, counterstained with 4',6-diamidino-2-phenylindole and coverslipped. Images were taken in fluorescence mode on an Olympus laser scanning confocal microscope and the average signal intensity was calculated by the bundled software.
Serum estradiol, adrenocorticotropic hormone (ACTH), and norepinephrine levels were measured using specific enzyme-linked immunosorbent assay kits for each analyte (CSB-E05110r, CSB-E06875r, CSB-E07022, Cusabio Bioteck CO., United States) following the manufacturer’s instructions.
Single fiber responses (impulses/second) to CRD were calculated by subtracting SA from the mean 30 s maximal activity during distension. Fibers were considered responsive if CRD increased their activity to 30% greater than the baseline value. Mechanosensitive single units were classified as high threshold (> 20 mmHg) or low threshold (≤ 20 mmHg) on the basis of their response threshold and profile during CRD. Single fiber activity data were analyzed by analysis of variance with repeated measures; CRD intensity was the repeated factor and the experimental group was the between-group factor. If significant main effects were present, the individual means were compared using the Fisher post-hoc test. All authors had access to the study data and reviewed and approved the final manuscript.
The basal activity of a spinal afferent fiber was defined as the average number of action potentials per second (impulses/sec) in the 60 s period before the onset of a distention stimulus. In male controls, 66% of the afferent fibers under study displayed SA and SA was significantly higher in female controls than in male controls (0.71 ± 0.21 vs 1.24 ± 0.20 imp/sec; Figure 1B). The average single fiber activity in response to CRD was significantly higher in female control rats compared with male controls (Figure 1C). We found that the enhanced sensitization in female rats mainly came from the low-threshold fibers (Figure 1D and E).
To assess the effects of CPS + CAS on colon afferent fiber activities, we compared average single colon afferent fiber activities projecting from dorsal roots S1-L6 in response to CRD in male and female control, CPS, control + CAS and CPS + CAS rats recorded approximately 24 h after the last stressor. In females, CPS significantly increased single-unit afferent activity in response to CRD vs control female rats (Figure 1F). CAS alone enhanced single-unit activity compared with control. The increase in average afferent responses after CAS in prenatally stressed female rats (44.0%) was significantly greater than the increase in female control rats (39.3%). In males, CPS had no significant effect on primary afferent responses (Figure 1G). When we compared males to females within each experimental group, we found that the average single fiber activity was significantly higher in female compared with male CPS + CAS rats (Figure 1F, G). The increased activity may contribute to the enhanced female visceral hypersensitivity previously reported in this model. Average single-fiber activities were significantly greater in control and CPS and CAS females than in their corresponding male experimental groups (Figure 1F and G). Both CAS and CPS + CAS rats had significantly increased primary afferent responses compared with control and CPS rats. Thus, our CPS and CAS protocols sensitized colon-projecting primary afferent fibers, with the greatest effects produced by the combination of CPS + CAS in both males and females.
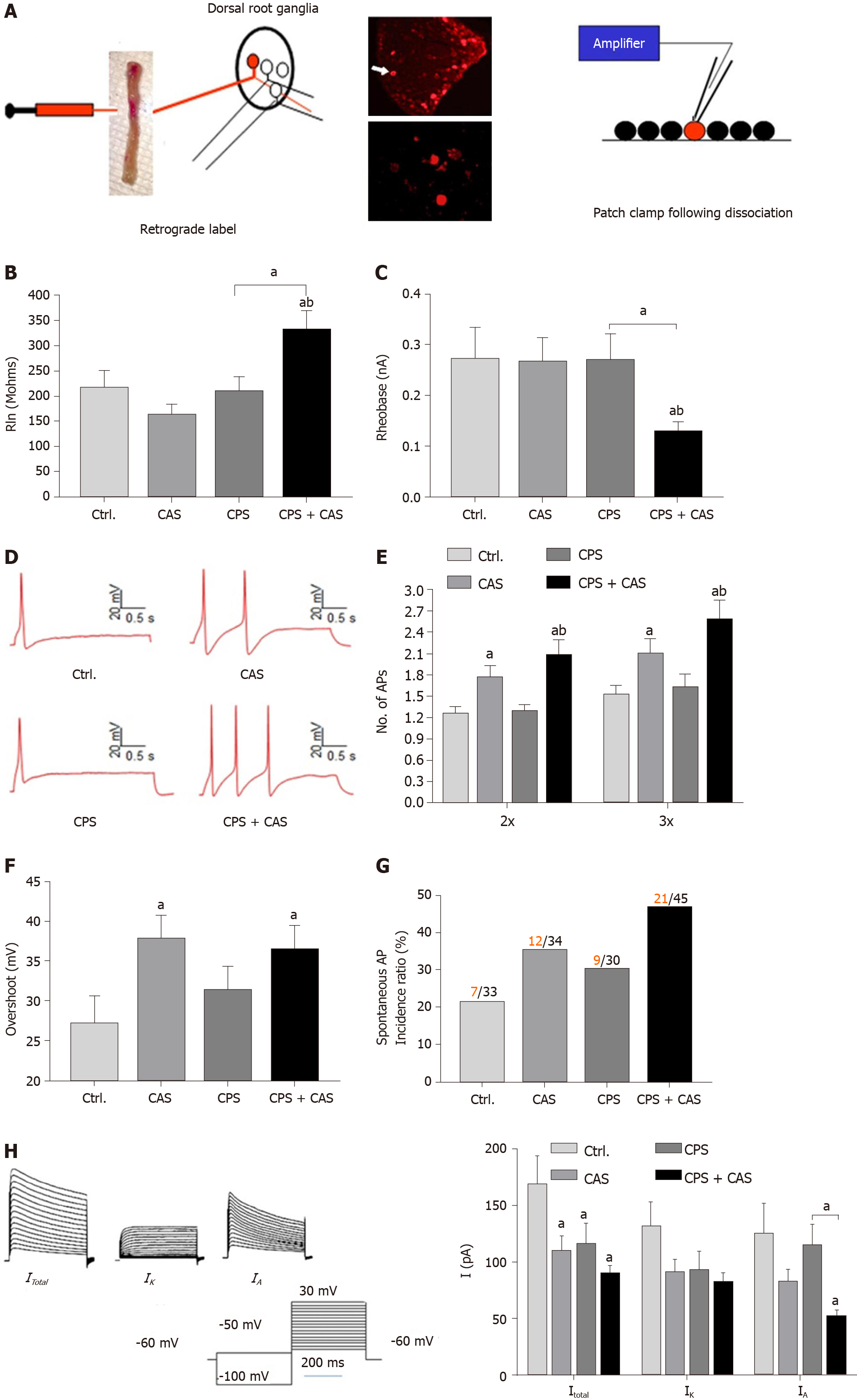
To elucidate the electrophysiological basis of enhanced stress-induced primary afferent activity in female rats, we performed patch clamp studies on acutely dissociated retrograde-labeled colon-projecting neurons from the L6-S2 DRGs in control, prenatal stress, adult stress only, and CPS + CAS female rats isolated 24 h after the last adult stressor (Figure 2A). Input resistance (Figure 2B) and rheobase (Figure 2C) were significantly decreased in neurons from CPS + CAS rats compared with the other three groups. The number of action potentials elicited at either 2 × or 3 × the rheobase were significantly greater in adult stress and CPS + CAS neurons compared with control and to CPS neurons (Figure 2D, E). CAS significantly increased action potential overshoot with or without CPS (Figure 2F), but it did not significantly alter other electrophysiological characteristics, such as number of spontaneous spikes, membrane capacitance (pF), resting membrane potential, cell diameter, time constant, and DRG neuron action potential amplitude and duration (Table 1).
| Classification | Ctr., n = 36 | CAS, n = 34 | CPS, n = 29 | CAS + CPS, n = 45 |
| Spontaneous spike number | 7.4 ± 4.0 | 16.9 ± 6.6 | 11.7 ± 4.8 | 44.8 ± 14.6c |
| Membrane capacitance (pF) | 72.1 ± 4.9 | 95.4 ± 5.8 | 85.1 ± 6.2 | 93.8 ± 8.9a |
| Action potential threshold (mV) | -27.1 ± 2.5 | -29.2 ± 1.8 | -34.6 ± 1.6 | -38.6 ± 1.4c |
| Resting membrane potential (mV) | -60.1 ± 1.7 | -50.1 ± 1.3 | -53.8 ± 1.5 | -56.4 ± 1.3 |
| Cell diameter (μm) | 32 ± 0.9 | 29 ± 0.6 | 31 ± 0.8 | 31 ± 0.6 |
| Time constant (μm) | 545.5 ± 51.1 | 737.7 ± 70.4 | 595.9 ± 54.1 | 535.3 ± 42.5 |
| Action potential amplitude (mV) | 79.0 ± 4.7 | 80.2 ± 3.9 | 77.4 ± 4.2 | 85.6 ± 3.7 |
| Duration (ms) | 8.38 ± 0.97 | 11.2 ± 0.95 | 12.3 ± 1.83 | 8.89 ± 0.60 |
The percentage of neurons with SA in was significantly greater in CPS + CAS rats than in control or CPS only rats (Figure 2G). Under voltage clamp conditions (Figure 2H), neurons from female CPS + CAS, CAS, CPS and control groups had IA and sustained outward rectifier K+ currents (IK). Compared with the other three groups, DRG neurons from CPS + CAS rats had significantly reduced average IA (P < 0.05). The average IK density was decreased but the change was not significant.
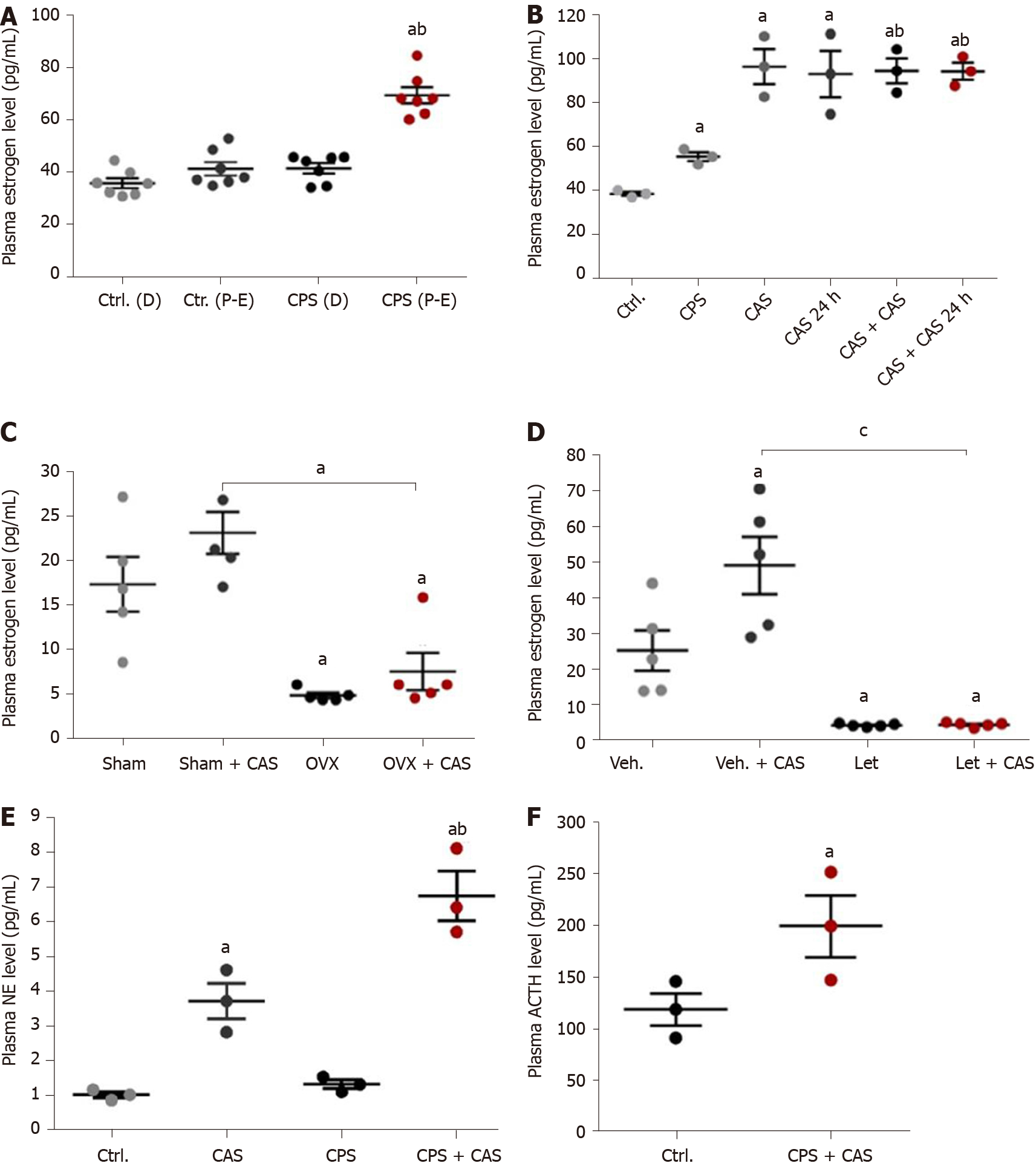
We did a vaginal smear test to identify the estrus cycle phases by identifying the vaginal cytological cell types. Estrogen concentration was significantly higher in the CPS proestrus/estrus phase compared with control diestrus, control proestrus/estrus, and CPS diestrus proestrus (P < 0.05; Figure 3A). Comparison of the plasma estrogen concentrations in control, CAS, CPS, CPS + CAS showed that CPS significantly increased plasma estrogen levels compared with the control rats and that CAS increased plasma estrogen level compared with the control and CPS rats (Figure 3B).
To determine whether estrogen contributed to stress-induced visceral hypersensitivity in prenatal stressed females, we reduced plasma estrogen levels by either OVX or letrozole treatment. OVX significantly lowered serum estradiol levels before and after CAS (Figure 3C). Treatment was continued throughout CAS. After treatment with letrozole, serum estradiol levels were significantly reduced (Figure 3D). To study the effects of gender and stress on norepinephrine and ACTH levels, we measured plasma norepinephrine levels in female rats from all four experimental groups. CAS alone significantly increased plasma norepinephrine levels compared with both the controls and with CPS alone (Figure 3E) Plasma norepinephrine levels were significantly increased in CPS + CAS rats compared with CAS alone as well as with controls and CPS. Plasma ACTH levels were significantly increased in CPS + CAS rats compared with controls. (Figure 3F).
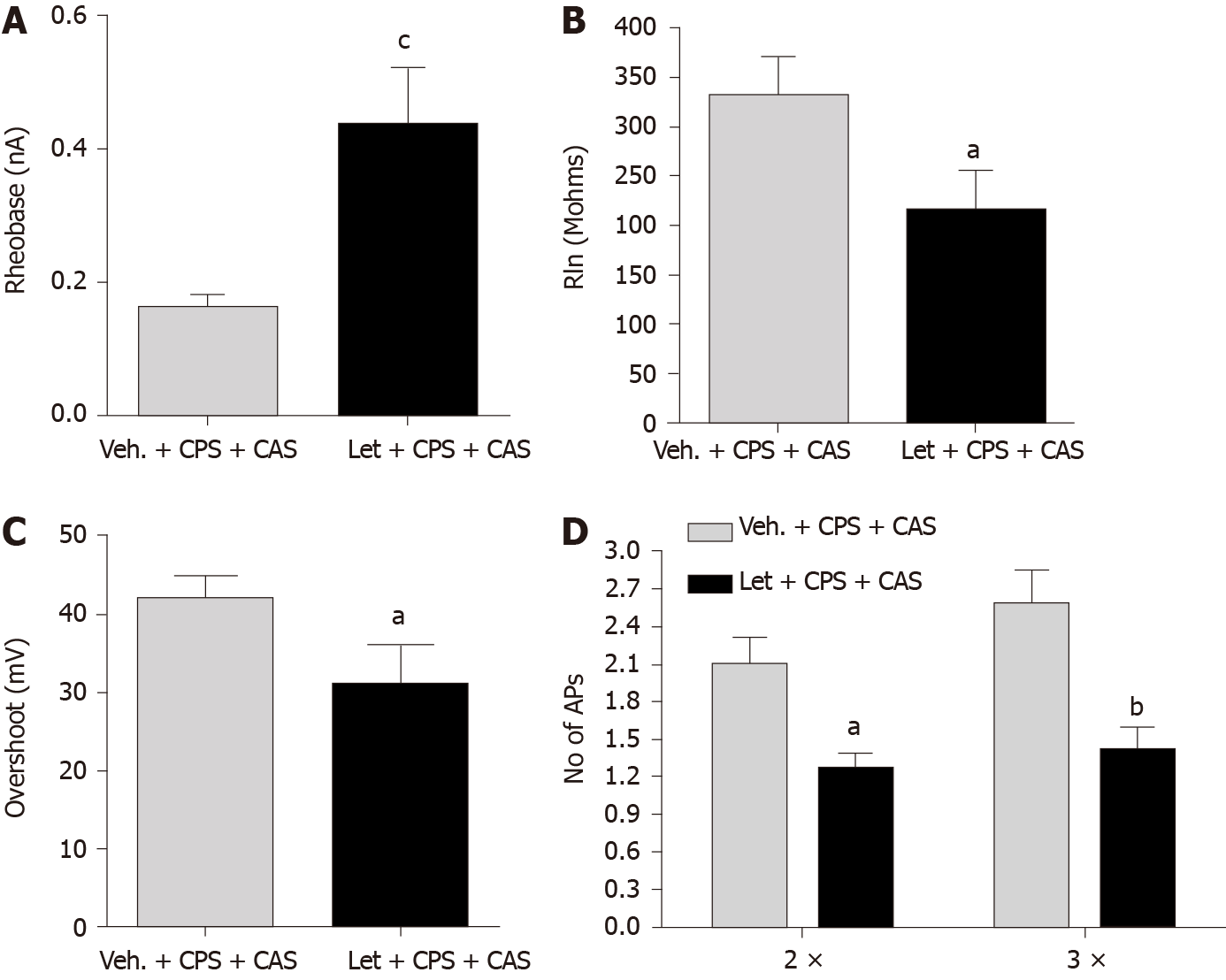
We performed patch clamp experiments on acutely isolated retrograde-labeled DRG neurons from CPS + CAS females with or without letrozole treatment 24 h after the last adult stressor. Letrozole treatment significantly increased rheobase (Figure 4A), and significantly reduced input resistance (Figure 4B). Action potential overshoot (Figure 4C) and the number of action potentials elicited by a current injection at either 2 × or 3 × rheobase were significantly reduced by letrozole treatment (Figure 4D). Other electrophysiological properties were not significantly altered (Table 2). We also recorded electromyographic activity to determine whether the reduction in visceral sensitivity in female CPS + CAS rats caused by OVX or systemic letrozole treatment reduced visceromotor responses. The findings demonstrated a significant decrease in excitability of colon-projecting L6-S2 neurons.
| Classification | Veh. + CAS + CPS, n = 60 | Let + CAS + CPS, n = 27 |
| Spontaneous spike number | 34.6 ± 11.3 | 8.04 ± 4.62c |
| Membrane capacitance (pF) | 86.8 ± 7.1 | 83.7 ± 6.7 |
| Action potential threshold (mV) | -34.6 ± 1.6 | -27.6 ± 2.1a |
| Resting membrane potential (mV) | -54.9 ± 1.3 | -54.6 ± 2.2 |
| Cell diameter (μm) | 31 ± 0.5 | 27 ± 1.6 |
| Time constant (um) | 502.7 ± 34.5 | 454.0 ± 36.6 |
| Action potential amplitude (mV) | 90.9 ± 3.7 | 89.8 ± 7.2 |
| Duration (ms) | 8.67 ± 0.63 | 10.70 ± 2.22 |
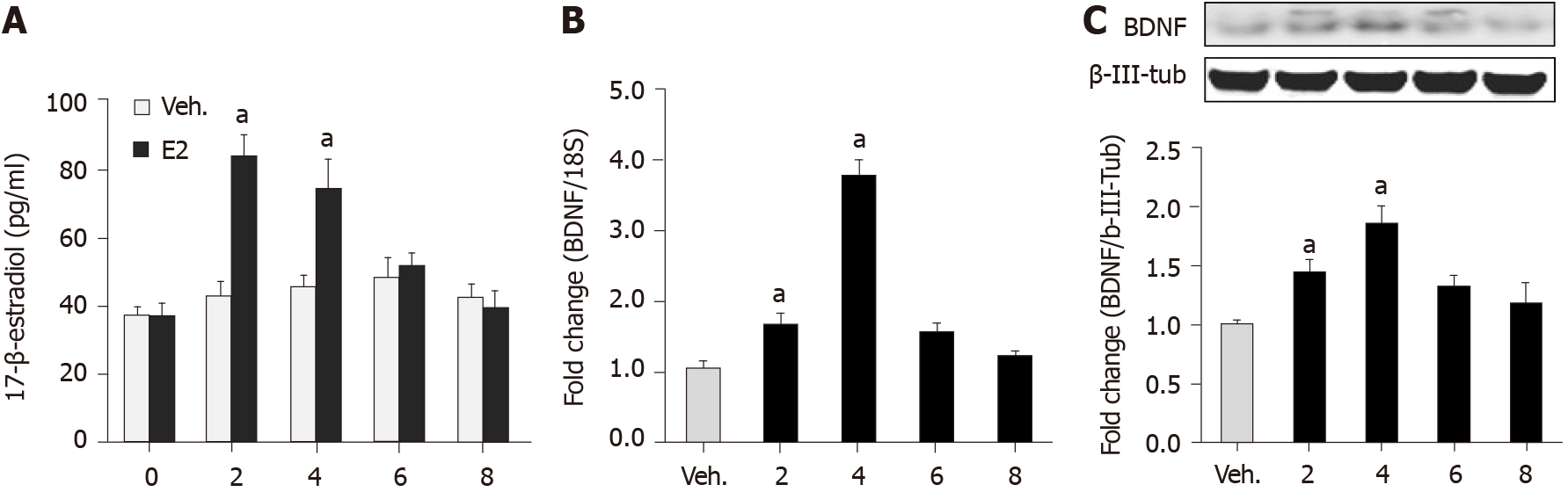
To investigate the effect of estrogen on BDNF expression, we measured BDNF mRNA and protein levels in the lumbar-sacral spinal cords of OVX and Sham CPS + CAS female rats. Systemic estradiol administration to naïve cycling females produced significant increases in plasma estrogen (Figure 5A), lumbar-sacral spinal cord BDNF mRNA (Figure 5B), and protein (Figure 5C). We also measured BDNF mRNA and protein levels in the lumbar-sacral spinal cords of OVX and Sham CPS + CAS female rats. BDNF mRNA and protein expression were significantly suppressed by OVX compared with sham rats. Another experiment showed that intrathecal infusion of estrogen into naïve female rats significantly increased BDNF protein levels, which proved that estrogen reversed the experimental results and contributed to the response to visceral pain[13].
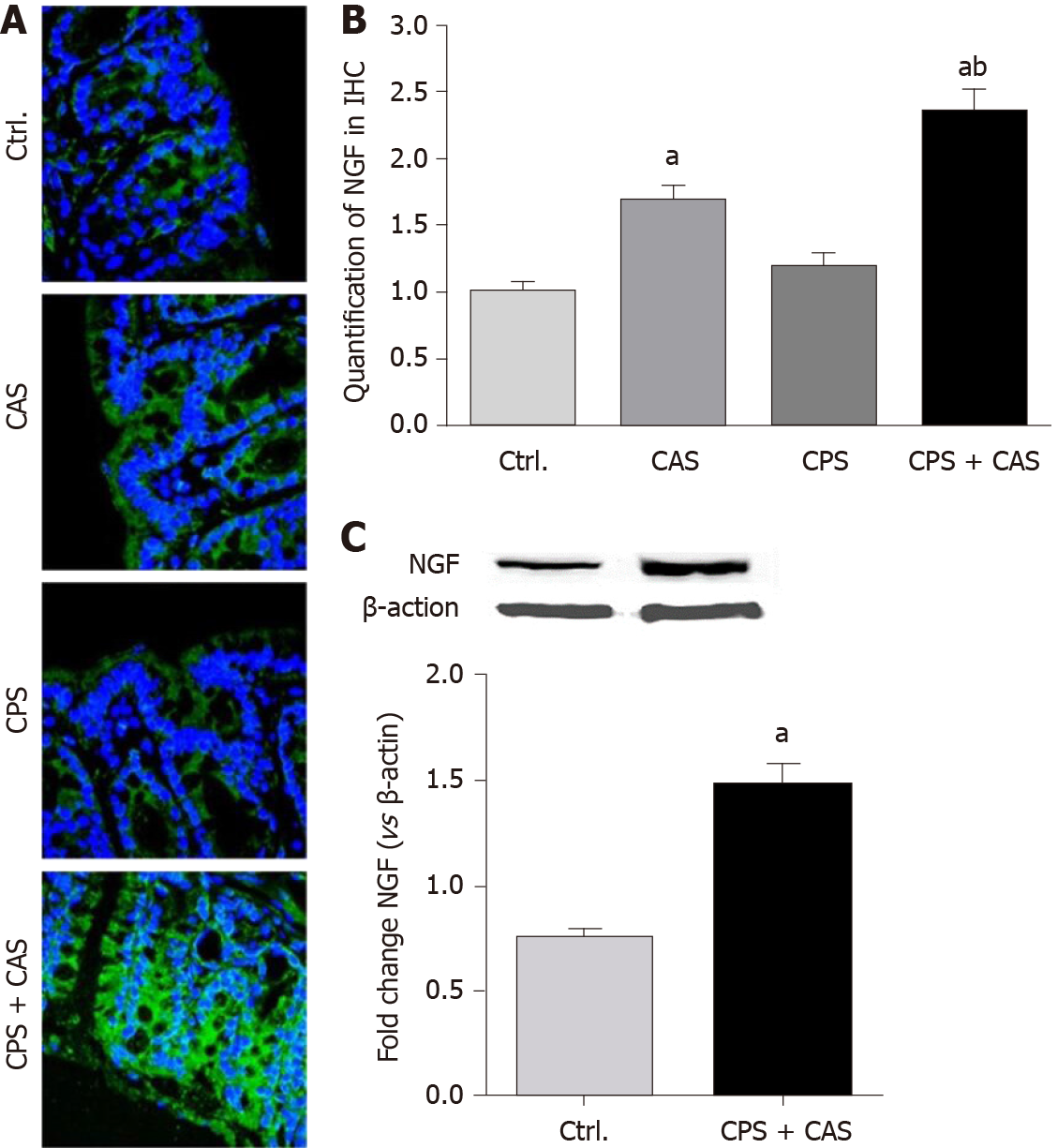
We examined NGF expression in the colons of females from all four experimental groups by immunohistochemistry (Figure 6A). Morphometric analysis showed that CAS and CPS + CAS significantly increased NGF levels in the colon wall, with the increase in CPS + CAS significantly greater that of CAS alone (Figure 6B). Western blotting showed that NGF protein was significantly upregulated in CPS + CAS rats compared with controls (Figure 6C).
Enhanced CPS-induced visceral hypersensitivity in female rats was associated with an increase in the responses of lumbosacral nerve fibers to CRD in both male and female offspring. These findings are further supported by data showing increased excitability of colon-projecting DRG neurons from females in patch clamp studies. The magnitude of the sensitization was the greatest in female CPS + CAS rats, suggesting that it made a major contribution to the observed enhanced female visceral hypersensitivity in our model.
Chronic stress is known to increase the excitability of colon-projecting DRG neurons in rats and mice. In adult male Sprague Dawley rats, colon DRG neuron sensitization was shown to be driven by increases in NGF expression in the colon muscularis externa[13]. In our model, we also observed a significant increase in colon NGF, but its potential role in primary afferent sensitization and visceral hypersensitivity was not investigated.. Other studies in male mice showed that stress, in the form of water avoidance, significantly increased the excitability of colon-projecting DRG neurons and that the combined activity of the stress mediators corticosterone and nore
When we tested on lumbar-sacral afferent fibers and dissociated neurons in patch clamp studies, we found significant decreases in transient potassium IA currents in neurons isolated from CPS + CAS females compared with the other three experimental groups. Declines in A-type Kv currents in DRG neurons have been associated with persistent pain in multiple chronic pain models[22]. Whether the decline was caused by changes in channel properties or expression was not investigated in this study. However, another study demonstrated that estrogen significantly shifted the activation curve of IA currents in the hyperpolarizing direction and that estrogen inhibited Kv (+) channels in mouse DRG neurons through a membrane ER-activated nongenomic pathway[23].
Our results showed that the excitability of colon-projecting neurons in CPS + CAS females was significantly reduced by systemic letrozole treatment, suggesting that estrogen contributed to the sensitization process. Previous studies show that estrogen receptors expressed on primary afferent neurons contributed to enhanced sensitivity in various pain models[24-26]. One study found no decline in the responses of colon-projecting nerve fibers to CRD following OVX and found no detectable estrogen receptor alpha immunoreactivity in colon-projecting DRG neurons[27]. The reasons for the differing results are not clear, but local production of estrogen in DRG neurons could be sufficient to sustain sensitization.
NGF and its receptors play important roles in the mechanism of visceral pain and hyperalgesia in women. For example, endometriosis is estrogen dependent and is commonly diagnosed. The main symptoms are various types of pelvic pain that have a serious effect on physical and mental health, but the mechanisms of abdominal pain are still unclear. Studies have shown NGF to be an inflammatory mediator and modulator of pain in adulthood[28].
In this study, we examined the sex differences and effects of estrogen on the acquisition of enhanced visceral hypersensitivity in the offspring of rats in a model of prenatal and adult stress as shown in Figure 7. Our study shows that estrogen acted in the spinal cord and the primary afferent neurons to enhance visceral nociception. Acute blockade of the endogenous synthesis of estrogens in rat spinal cord significantly reduced visceral hypersensitivity, suggesting that locally produced estrogen in the central nervous system can regulate nociceptive neurons to modulate visceral hypersensitivity. The chronic stress-estrogen-BDNF axis sensitized visceral hypersensitivity in the offspring of females subjected to CPS. The development of chronic stress-induced visceral hypersensitivity in female rats was estrogen dependent. A key component of this hypersensitivity was estrogen-dependent sensitization of primary afferent colon neurons. Our findings provide key scientific evidence in a preclinical model in support of developing gender-based treatment for abdominal pain in IBS.
Chronic stress during pregnancy may increase visceral hyperalgesia in the offspring. Combining adult stress in offspring will increase this sensitivity. Therefore, based on the evidence implicating estrogen exacerbates visceral hypersensitivity in female rodents in preclinical models, we predicted that chronic prenatal stress (CPS) + chronic adult stress (CAS) would maximize visceral hyperalgesia and that estrogen has an important role in colonic hyperalgesia.
The mechanisms of visceral hypersensitivity are not well defined. Understanding the neurophysiological mechanisms driving visceral hypersensitivity will spur the development of female pain-specific therapies.
The objective was to identify the enhancement of visceral hypersensitivity in a CPS + CAS model and explain the role of estrogen in that process.
A CPS + CAS rodent model was established. Single fiber recording in vivo and patch clamp experiments in vitro were used to monitor the activity of colonic neurons. Reverse transcription-polymerase chain reaction (RT-PCR), western blots, and immunofluorescence were used to study the effects of CPS and CAS on colon primary afferent sensitivity. We used ovariectomy (OVX) and letrozole to reduce estrogen levels in female rats in order to assess the role of estrogen in female-specific enhanced primary afferent sensitization.
A CPS + CAS rodent model was established. Single fiber recording in vivo and patch clamp experiments were used to monitor the colonic neuron activity in vitro. RT-PCR, western blots, and immunofluorescence were used to study the effects of CPS and CAS on colon primary afferent sensitivity. We used OVX and letrozole to reduce estrogen levels of female rats in order to assess the role of estrogen in female-specific enhanced primary afferent sensitization.
Spontaneous activity and single fiber activity were significantly greater in females than in males. The enhanced sensitization in female rats mainly came from the low-threshold neurons. CPS significantly increased single-unit afferent fiber activity in the L6-S2 dorsal roots in response. Activity was further enhanced by CAS. In addition, the increased excitability of colon-projecting DRG neurons in CPS + CAS rats was associated with a decrease in transient A-type K+ currents. Compared with OVX, letrozole treatment further reduced the estrogen levels of female rats, which confirmed the gender difference. Moreover, rats treated with letrozole had decreased colonic DRG neuron excitability. The intrathecal infusion of estrogen increased BDNF protein levels and contributed to the response to visceral pain. Western blots showed that nerve growth factor protein was upregulated in CPS + CAS rats.
This study adds to the evidence of the development of chronic stress-induced visceral hypersensitivity in females, and that it involves estrogen-dependent sensitization of primary afferent colon neurons.
This study demonstrated that CAS + CPS induced visceral hypersensitivity and that estrogen played a role in the process. Understanding the molecular and neuro
The authors would like to acknowledge the essential intellectual contributions of our recently deceased mentor, Dr. Sushil K Sarna. His guidance was essential to the successful completion of these studies. Thanks to Dr. Usman Ali for assistance in preparing and modifying the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bansal C, Sulkowski S S-Editor: Fan JR L-Editor: Filipodia P-Editor: Liu JH
| 1. | Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 950] [Article Influence: 41.3] [Reference Citation Analysis (1)] |
| 2. | Taub E, Cuevas JL, Cook EW 3rd, Crowell M, Whitehead WE. Irritable bowel syndrome defined by factor analysis. Gender and race comparisons. Dig Dis Sci. 1995;40:2647-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 223] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Cain KC, Jarrett ME, Burr RL, Rosen S, Hertig VL, Heitkemper MM. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Dig Dis Sci. 2009;54:1542-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Li X, Tian X, Lv L, Hei G, Huang X, Fan X, Zhang J, Pang L, Song X. Microglia activation in the offspring of prenatal Poly I: C exposed rats: a PET imaging and immunohistochemistry study. Gen Psychiatr. 2018;31:e000006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 344] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 7. | Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 665] [Article Influence: 73.9] [Reference Citation Analysis (1)] |
| 8. | Leserman J, Drossman DA. Relationship of abuse history to functional gastrointestinal disorders and symptoms: some possible mediating mechanisms. Trauma Violence Abuse. 2007;8:331-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, Chang L. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 10. | Mou L, Lei W, Chen J, Zhang R, Liu K, Liang X. Mediating effect of interpersonal relations on negative emotions and dysmenorrhea in female adolescents. Gen Psychiatr. 2019;32:e100008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Farzaei MH, Bahramsoltani R, Abdollahi M, Rahimi R. The Role of Visceral Hypersensitivity in Irritable Bowel Syndrome: Pharmacological Targets and Novel Treatments. J Neurogastroenterol Motil. 2016;22:558-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 12. | Simrén M, Törnblom H, Palsson OS, van Tilburg MAL, Van Oudenhove L, Tack J, Whitehead WE. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2018;67:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 13. | Chen J, Li Q, Saliuk G, Bazhanov S, Winston JH. Estrogen and serotonin enhance stress-induced visceral hypersensitivity in female rats by up-regulating brain-derived neurotrophic factor in spinal cord. Neurogastroenterol Motil. 2021;e14117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Accarino AM, Azpiroz F, Malagelada JR. Selective dysfunction of mechanosensitive intestinal afferents in irritable bowel syndrome. Gastroenterology. 1995;108:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 187] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Munakata J, Naliboff B, Harraf F, Kodner A, Lembo T, Chang L, Silverman DH, Mayer EA. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 253] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Yang B, Wei J, Ju P, Chen J. Effects of regulating intestinal microbiota on anxiety symptoms: A systematic review. Gen Psychiatr. 2019;32:e100056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 435] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, Grill RJ, Carlton SM, Walters ET. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci. 2010;30:14870-14882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Ibeakanma C, Ochoa-Cortes F, Miranda-Morales M, McDonald T, Spreadbury I, Cenac N, Cattaruzza F, Hurlbut D, Vanner S, Bunnett N, Vergnolle N. Brain-gut interactions increase peripheral nociceptive signaling in mice with postinfectious irritable bowel syndrome. Gastroenterology. 2011;141:2098-2108.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Ochoa-Cortes F, Guerrero-Alba R, Valdez-Morales EE, Spreadbury I, Barajas-Lopez C, Castro M, Bertrand J, Cenac N, Vergnolle N, Vanner SJ. Chronic stress mediators act synergistically on colonic nociceptive mouse dorsal root ganglia neurons to increase excitability. Neurogastroenterol Motil. 2014;26:334-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Zemel BM, Ritter DM, Covarrubias M, Muqeem T. A-Type KV Channels in Dorsal Root Ganglion Neurons: Diversity, Function, and Dysfunction. Front Mol Neurosci. 2018;11:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Du J, Wang Q, Hu F, Wang J, Ding H, Gao R, Xiao H, Wang L. Effects of estradiol on voltage-gated potassium channels in mouse dorsal root ganglion neurons. J Membr Biol. 2014;247:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Ferrari LF, Khomula EV, Araldi D, Levine JD. Marked Sexual Dimorphism in the Role of the Ryanodine Receptor in a Model of Pain Chronification in the Rat. Sci Rep. 2016;6:31221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Khomula EV, Ferrari LF, Araldi D, Levine JD. Sexual Dimorphism in a Reciprocal Interaction of Ryanodine and IP3 Receptors in the Induction of Hyperalgesic Priming. J Neurosci. 2017;37:2032-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Zhang H, Ding L, Shen T, Peng D. HMGB1 involved in stress-induced depression and its neuroinflammatory priming role: a systematic review. Gen Psychiatr. 2019;32:e100084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Ji Y, Tang B, Traub RJ. Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain. 2011;152:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Dawes JM, Andersson DA, Bennett DLH, Bevan S, McMahon SB. Inflammatory mediaters and modulators of pain. 6th ed. Philadelphia, Elsevier, 2013: 48-67. |