Published online Aug 14, 2021. doi: 10.3748/wjg.v27.i30.4999
Peer-review started: February 10, 2021
First decision: April 18, 2021
Revised: April 29, 2021
Accepted: July 13, 2021
Article in press: July 13, 2021
Published online: August 14, 2021
Processing time: 180 Days and 11 Hours
Metabolic associated fatty liver disease (MAFLD), formerly named “nonalcoholic fatty liver disease” occurs in about one-third of the general population of developed countries worldwide and behaves as a major morbidity and mortality risk factor for major causes of death, such as cardio
Core Tip: From studies using tissue-targeted animal models, it emerges that neither insulin resistance per se induces hepatic steatosis, nor steatosis induces whole-body insulin resistance. However, it is evident that reducing inflammation has several beneficial effects both at the hepatic and whole-body level. In fact, either hepatic or systemic inflammation act as major throttle of progressive liver and systemic diseases.
- Citation: Rebelos E, Iozzo P, Guzzardi MA, Brunetto MR, Bonino F. Brain-gut-liver interactions across the spectrum of insulin resistance in metabolic fatty liver disease. World J Gastroenterol 2021; 27(30): 4999-5018
- URL: https://www.wjgnet.com/1007-9327/full/v27/i30/4999.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i30.4999
In recent years, nonalcoholic fatty liver disease (NAFLD) has become the leading cause of chronic liver disease worldwide, and the endpoint complication of nonalcoholic steatohepatitis (NASH), a major indication for liver transplantation[1]. The magnitude of the problem is highlighted by a recent model that estimated a 178% increase in deaths caused by liver disease related to NASH by 2030[2]. Among noncirrhotic NAFLD patients, the leading cause of death is cardiovascular disease[3]. Fatty liver associates with the metabolic syndrome and predisposes to all diseases (cardio
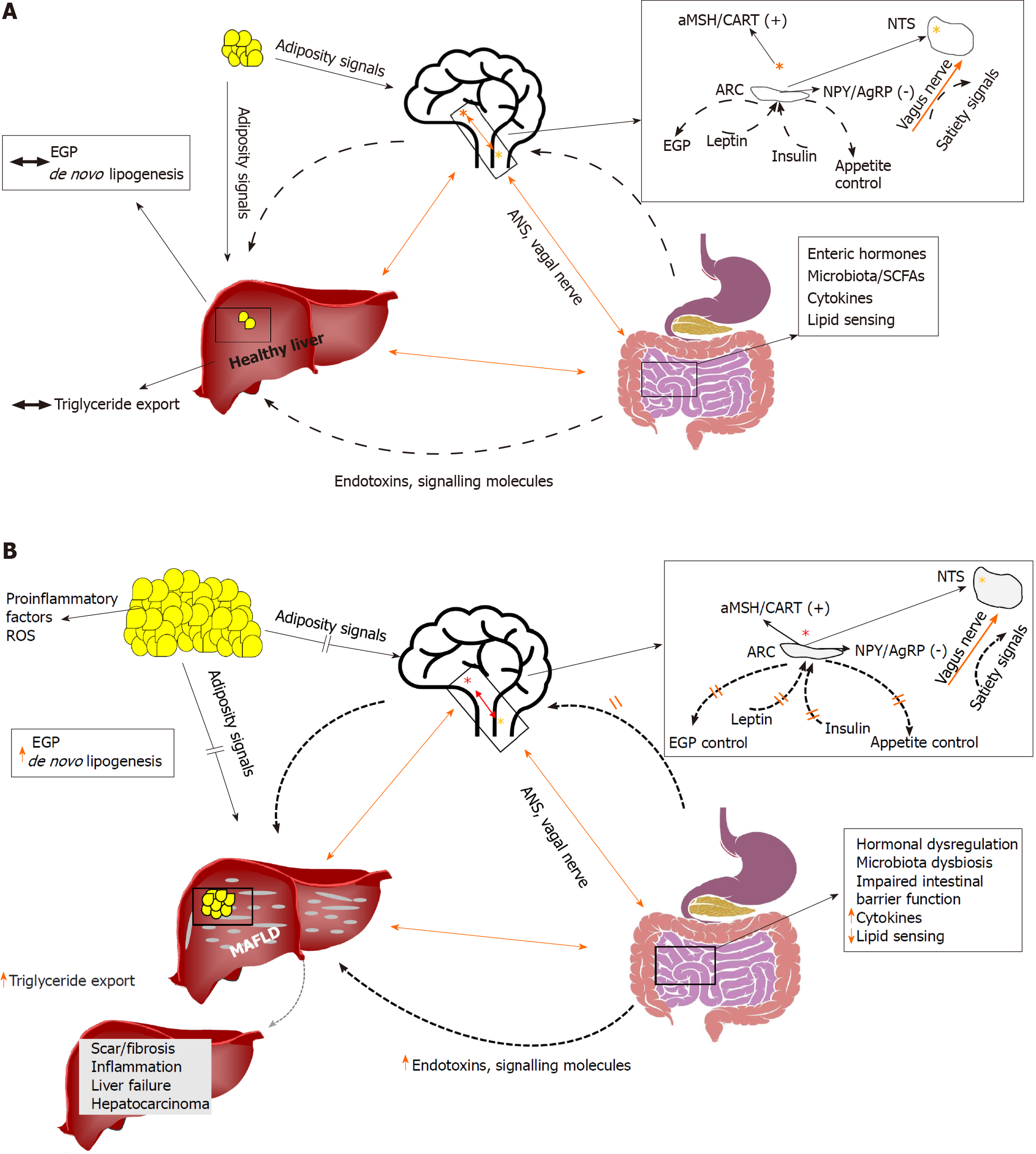
There are many complex physio-pathologic connections within the brain, gut, and liver (BGL) axis (Figure 1). While MAFLD per se contributes to an increased risk of neurodegeneration[7,8], one well known alteration of this axis is linked to hepatic encephalopathy (HE) a debilitating neuropsychiatric condition often associated with acute liver failure and/or cirrhosis[9]. However, the pathophysiologic mechanisms and treatment options involved in HE are much different from those involved with insulin resistance in MAFLD. Consistently, a recent study demonstrated that the nonabsorbable antibiotic rifaximin, a standard of care for HE had no effect on impro
Our review is focused on the early pathophysiology of BGL in the context of insulin resistance and specifically addresses two pillars of hepatic insulin resistance, namely dysregulated endogenous glucose production and MAFLD. Of note, highly selected patients with MAFLD without any features of the metabolic syndrome, have altered endogenous glucose production[11]. Specific emphasis will be given to novel findings from imaging studies, since imaging provides noninvasive in vivo “snapshots” of the tissues of interest. We and others have highlighted that there is an imperative need of noninvasive techniques, including imaging to identify effective biomarkers and early prognostic patterns of MAFLD[12,13]. Finally, we discuss new insights that can be gained from targeted animal models in which interventions such as the knockout (KO) of the insulin receptor or the GLUT4 glucose transporter in different tissues, or the primary upregulation of lipid synthesis help to elucidate the effect of insulin resistance on hepatic steatosis and vice versa.
Whereas the association between MAFLD and insulin resistance is well established, there is debate on their cause-effect relationships. Thus, it is not clear whether systemic insulin resistance induces the accumulation of lipids in the liver[11] or if hepatic steatosis is a major determinant of systemic insulin resistance[14]. In any case, once hepatic steatosis is established, other typical characteristics are observed such as insulin resistance, insufficient suppression of endogenous glucose production (EGP), increased insulin secretion, decreased whole-body glucose disposal, increased lipolysis with consequent enhanced lipid oxidation[11], decreased insulin clearance[15], and chronic oxidative stress[16]. Both insulin resistance and MAFLD are characterized by elevated circulating inflammatory markers[17,18]. The interplay between insulin resistance, ectopic fat accumulation in the liver, and inflammation is characterized by mutual positive regulation, i.e. a vicious circle[19]. On one hand, ectopic fat accumulation in the liver leads to lipotoxicity, low-grade inflammation and insulin resistance in the liver[19]. On the other hand, insulin resistance enhances lipotoxicity through unsuppressed lipolysis[19]. Finally, proinflammatory markers such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, which are typically increased in conditions of insulin resistance may further aggravate both insulin resistance and MAFLD[19].
In MAFLD patients, intrahepatic inflammation is the most important prognostic determinant of liver disease progression and systemic inflammatory markers correlate with hepatic inflammation[20]. A plausible hypothesis holds that in the context of MAFLD, inflammation (hepatic and/or systemic) acts as major disease accelerator[13].
Preclinical studies have shown that insulin acting directly on the brain may affect EGP. More specifically, Obici et al[21] have shown that an intracerebroventricular (ICV) injection of insulin suppresses EGP in rats. Human evidence is slowly accumulating, and recent clinical studies have confirmed the presence of a “brain-liver axis”. More specifically, intranasal insulin (INI) administration during the euglycemic hyper-insulinemic clamp was shown to suppress EGP in lean, but not in overweight, individuals[22]. Under the same euglycemic hyper-insulinemic conditions, brain imaging with 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) has shown that brain glucose uptake (BGU) correlates positively with EGP in morbidly obese individuals, but not in healthy lean individuals[23]. On the contrary, when INI was given during fasting conditions EGP was not affected, and similarly no correlation was found between BGU and EGP in the post-absorptive state. Taken together, the data suggest that under conditions of high systemic insulin levels like those typically seen in the postprandial state, the brain may directly control (i.e. suppress) EGP, but the control is lost with increased adiposity.
Other lines of research also suggest that the brain may control EGP. Intrac
Lipid delivery into the upper intestine has also been shown to suppress EGP through central action. More specifically, lipid delivery leads to long chain fatty acyl-CoA production that suppresses EGP, and the effect is abolished either after coadministration of the anesthetic tetracaine, by gut vagal deafferentation, or by hepatic vagotomy[27]. Wang and colleagues further demonstrated that in rats with insulin resistance induced by a high-fat diet (HFD), upper intestine lipid delivery failed to suppress EGP, suggesting a potential mechanism for dysregulated EGP in the context of insulin resistance. Along the same line, cholecystokinin (CCK) has also been reported to trigger gut-brain-liver axis control of HGP, and that HFD impaired CCK-induced afferent vagal signals to suppress HGP[28].
The gastrointestinal tract is a major producer of hormones, among which GLP-1, ghrelin, cholecystokinin are particularly involved in the BGL axis. GLP-1, an incretin hormone secreted from the L cells of the intestine in response to a meal, exhibits high fasting levels in subjects with insulin resistance and in whom GLP-1 does not increase sufficiently in response to a meal[29]. GLP-1-receptor mRNA has been found both in the hepatic portal region and in neurons[30]. Preclinical studies have suggested that GLP-1 may act on the liver through the nerve endings of the intestinal wall. Insulin clamp studies in GLP-1 receptor knockout mice showed a defective suppression of EGP[31], and the intraportal GLP-1 injection in rats increased the firing rate of the hepatic afferent of the vagus nerve[32]. In healthy individuals under conditions of pancreatic clamping (i.e. with stable insulin and glucagon concentrations), GLP-1 inhibits EGP, and the effect is mediated either through direct GLP-1 action on the liver or through neuron-mediated inhibition[33]. GLP-1 also contributes to suppression of appetite[34]. Thus, GLP-1 may play an important role in the pathophysiology of MAFLD; and in preliminary clinical studies, exenatide, a GLP-1 receptor agonist, was shown to decrease hepatic fat content and liver enzymes[35], and to improve liver histology[36]. Similarly, liraglutide was shown to improve NASH[37].
Ghrelin is a hormone that is mainly derived from the stomach and duodenum, and its main function is to control food intake by inducing appetite. Ghrelin has recently been shown to participate in the BGL axis, as under conditions of pancreatic clamping, intraduodenal ghrelin infusion resulted in increased HGP through neural-mediated action, as administration of ghrelin, while inhibiting the vagal afferent neurotransmission, abolished the ghrelin-induced increase of HGP[38]. Similarly, vagotomy or use of N-Methyl-D-aspartate blockers abolished the ghrelin effects on HGP. Ghrelin has also been shown to block the action of cholecystokinin. The ghrelin nutrient-sensitive effects on the gut may thus be attributed to its inhibition of cholecystokinin.
CCK is released from intestinal endocrine cells during feeding, and it binds to CCK-A receptors on gut vagal fibers that project the signal to the brainstem, causing termination of the meal[39]. Insulin and CCK have complimentary actions in inducing satiety, as ICV administration of insulin enhances the satiety effect of CCK[40], whereas fasting decreases it[41]. Similar complementary effects with CCK have been proposed for leptin, as ob/ob mice and Zucker rats have been shown to be relatively insensitive to the satiety effects of CCK[42].
Leptin is an adipokine, or adipocytokine with structural similarities with the cytokines of the type I cytokine family. Circulating leptin levels are directly related to expanded fat mass, but obesity is characterized by leptin resistance, and leptin resistance consists at least partially in a decreased capacity for leptin transport into the brain[43]. Even though secreted by adipose tissue, the main site of action of leptin is the central nervous system (CNS), and particularly in the hypothalamic nuclei. Leptin and insulin central actions are largely interconnected; both act on the arcuate nucleus to suppress the expression of the orexigenic peptides neuropeptide Y and agouti-related protein. Their action on other neurons is different and more complex, as leptin stimulates while insulin inhibits proopiomelanocortin neurons[44]. Apart from controlling EGP, appetite (and thus body weight), leptin is also implicated in MAFLD as it has been demonstrated that leptin deficient ob/ob mice have marked steatosis[45]. A recent preclinical study has shown that CNS-leptin signaling promotes hepatic triglyceride export and decreases de novo lipogenesis[46]; the authors propose intranasal leptin administration as potential new treatment of MAFLD.
As already highlighted, the tenth cranial, or vagus nerve, plays a pivotal role in the BGL axis communications, which are summarized in Figure 1. The enteric nervous system, also named the “second brain” or “brain in the gut” is considered as one of the autonomic nervous system divisions and consists of approximately 500 million neurons which produce a variety of neurotransmitters including acetylcholine, adrenaline, VIP and serotonin (5-HT). It has been shown that 5-HT promotes lipid accumulation in hepatocytes in vitro[47]. In line with that, short-term treatment with tryptophan inhibitors prevented the formation of 5-HT, which is a metabolite of tryptophan, and inhibited the development of hepatic steatosis in mice fed with a high carbohydrate diet without increasing the energy expenditure in adipose tissues[48]. In addition, the same study showed that inhibition of gut-derived serotonin ameliorated hepatic steatosis. Taken together, the data suggest that gut-derived serotonin is a regulator of hepatic lipid metabolism through a gut-liver axis. On the other hand, the gut microbiota regulates both the 5-HT synthesis and its release from the enteroendocrine cells, and 5-HT plays its role on the CNS as one of the most important central neurotransmitters in the regulation of mood, sleep, and pain[49]. Consistently, modification of central 5-HT levels was shown by Pagoto et al[50] to affect food preferences. Following acute tryptophan depletion (transient decrease of both peripheral and central 5-HT levels), overweight individuals increased their sweet calorie intake and preferred sweet foods. Of significant note is that most tryptophan was converted to kynurenine rather than to 5-HT, and under conditions of inflammation, the rate-limiting enzyme for this transformation could be upregulated. As kynurenine and 5-HT compete to cross the blood-brain barrier through the same transporter, it follows that inflammation-associated changes in kynurenine levels could impact on central 5-HT concentrations[51,52]. Thus, this is another pathway through which inflammation could decrease central 5-HT levels to prompt affected patients to increase their intake of sweets.
An altered gut microbiota composition is associated with obesity[53], and in obese humans specific microbiota compositions may be associated with impaired glucose control[54]. It is well established that a diet rich in fibers is healthy, with improvement of insulin sensitivity and glucose tolerance. The beneficial effects of increased fiber consumption are hypothesized to be mediated by the production of the short-chain fatty acids (SCFAs) acetate, propionate, and butyrate after fermentation of the fibers by the gut microbiota. SCFAs do not act just as substrate for colonocytes and enterocytes[55], but also as signaling molecules. For instance, SCFAs can stimulate the secretion of GLP-1 and peptide YY, and decrease the secretion of ghrelin[56-58]. Propionate and butyrate have been shown to activate intestinal gluconeogenesis (IGN), and interestingly, increased IGN was shown to have beneficial effects on glucose home
Decreased levels of the SCFA butyrate have also been associated with tight-junction abnormalities and increased intestinal permeability[61], which have been implicated in MAFLD pathogenesis and progression. Other microbiota-derived molecules might play also an important role. Deficiency of the macronutrient choline, which is implicated in the prevention of liver steatosis by promoting the assembly and excretion of very-low-density lipoprotein[62], has been observed in NAFLD patients and was associated with abundance of specific bacteria (Erysipelotrichia taxa), which are able to metabolize choline to trimethylamine (TMA) and its oxidized form TMAO, with the net effect of reducing choline bioavailability and increasing that of steatogenic TMAO[63].
Amino acid metabolism is also important. The branched-chain amino acids (BCAAs) valine, isoleucine and leucine, contribute to insulin resistance and hepatic steatosis, and can be synthesized and metabolized by specific gut bacteria. An intervention study in rats showed that the dietary administration of BCAA reduced the accumulation of liver fat through the modification of gut microbiota[64]. The effect occurred through the gut-brain axis, accompanied by microbiota-mediated production of the SCFA acetate, which activated the parasympathetic nervous system[65]. Other amino acids, such as tryptophan, phenylalanine, and tyrosine can be metabolized by gut bacteria that produce derivates with effects on metabolism and inflammation. For example, the essential amino acid tryptophan is the precursor of serotonin and can be converted into its indole intermediate, which in turn can reduce hepatic lipogenesis and inflammation[66].
Furthermore, the bacterial-derived endotoxin lipopolysaccharide (LPS) might contribute to local and systemic inflammation by the activation of the toll-like receptor 4 pathway. Increased abundance of endotoxin-producing bacterial strains has been found in the gut of obese patients compared with controls[67], suggesting its potential implication in the development of MAFLD and its progression to NASH, with the involvement of CNS dysfunction and inflammation.
Dietary patterns are able to modulate brain lipid composition and function[68] as well as hepatic lipid content[69]. It has been shown that unhealthy diets rich in saturated or monounsaturated fatty acids have unfavorable effects on gut microbiota composition[70], promoting an increase of LPS-producing bacteria and reduction of SCFAs leading to a systemic proinflammatory state occurring through the BGL axis[71].
Finally, gut microbiota-dependent regulation of neurotransmitters interacts with vagal afferent pathways to affect liver metabolism through the gut-brain axis. Gut bacteria can modify serotonin release[72], which has a brain-dependent effect on gastrointestinal motility and secretion, and energy expenditure. It promotes liver steatosis by local endocrine mechanisms[73], and modulates the production of several other molecules, such as gamma-aminobutyric acid, acetylcholine, histamine, norepinephrine, dopamine, and endocannabinoids that affect glucose and lipid metabolism and inflammation, as deeply reviewed elsewhere[74].
A useful experimental model for studying the effects of specific microbiota on host metabolism is provided by germ-free mice, which are resistant to high-fat diet-induced obesity[75] and protected from MAFLD. In a seminal study Le Roy et al[76] showed that germ-free mice that received fecal transplantation from C57BL/6J mice with HFD-induced hyperglycemia and increased plasma concentrations of proinflammatory cytokines, developed hepatic steatosis. On the contrary, germ-free mice that received stool from C57BL/6J mice that were non-responders to HFD and without hyper
Bile acids (BAs) are synthesized in the liver from cholesterol, stored in the gallbladder, and secreted after gallbladder emptying into the intestinal lumen upon food ingestion. As BAs move along the intestinal lumen, they contribute to the absorption of lipids and lipophilic vitamins. The majority of BAs (~95%) are re-absorbed by enterocytes and then transferred back to the liver where they are reused (i.e. enterohepatic circulation). BA are transformed to secondary BAs by the gut microbiota[78] and only in a small amount reach the systemic circulation and increases of plasma BA levels were reported after meals, suggesting that BAs could be a postprandial systemic signal[79].
In the last decades, important new insights have been gained, proposing BAs as important determinants of glucose homeostasis. Early studies showed that KO of the BA farnesoid X receptor (FXR) induced insulin resistance, whereas administration of BA agonists enhanced insulin sensitivity[80]. BA receptors are also present in the CNS, and it is now believed that the BA signal reaches the brain through three different pathways, one direct and two indirect[81]. The direct pathway consists in activating central FXR and Takeda G protein-coupled receptor (TGR5R) signaling after crossing the blood-brain barrier. Indirect activation of intestinal FXR and TGR5R results in the release of FGF19 and GLP-1, both of which can signal to the CNS. The pathways have been extensively reviewed by Mertens et al[81]. Even though major pathways of communication of the BGL axis through BAs were identified, their importance in pathophysiology warrants further investigation.
In Western societies, increased consumption of fructose began in the 1970s after the introduction of high-fructose corn syrup as a sweetener in soft drinks. Since then, the prevalence of obesity and T2D have substantially increased and a link between high fructose consumption and MAFLD has been established[82]. Fructose is a 5-carbon carbohydrate with peculiar characteristics, as upon entry in the cell it is phospho
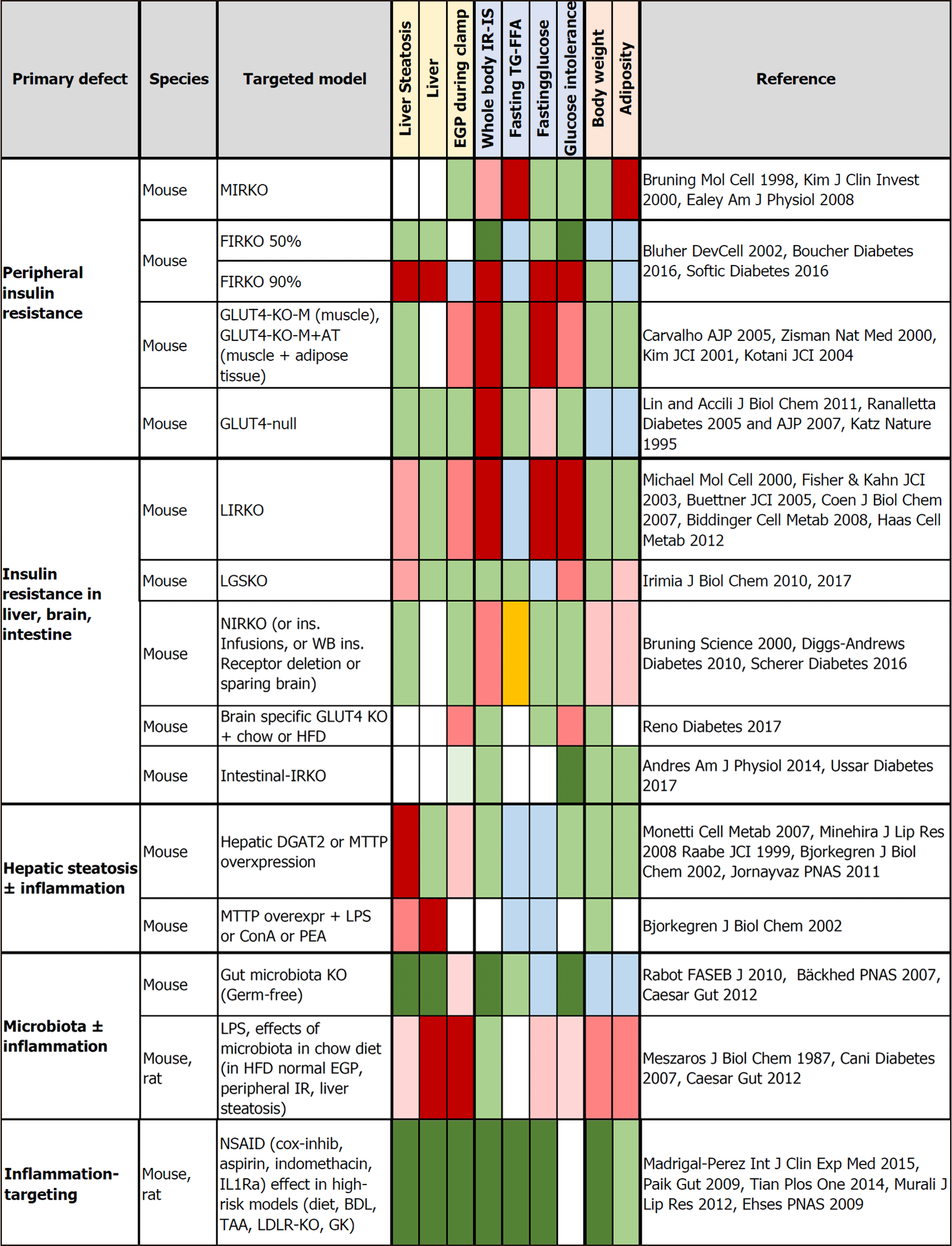
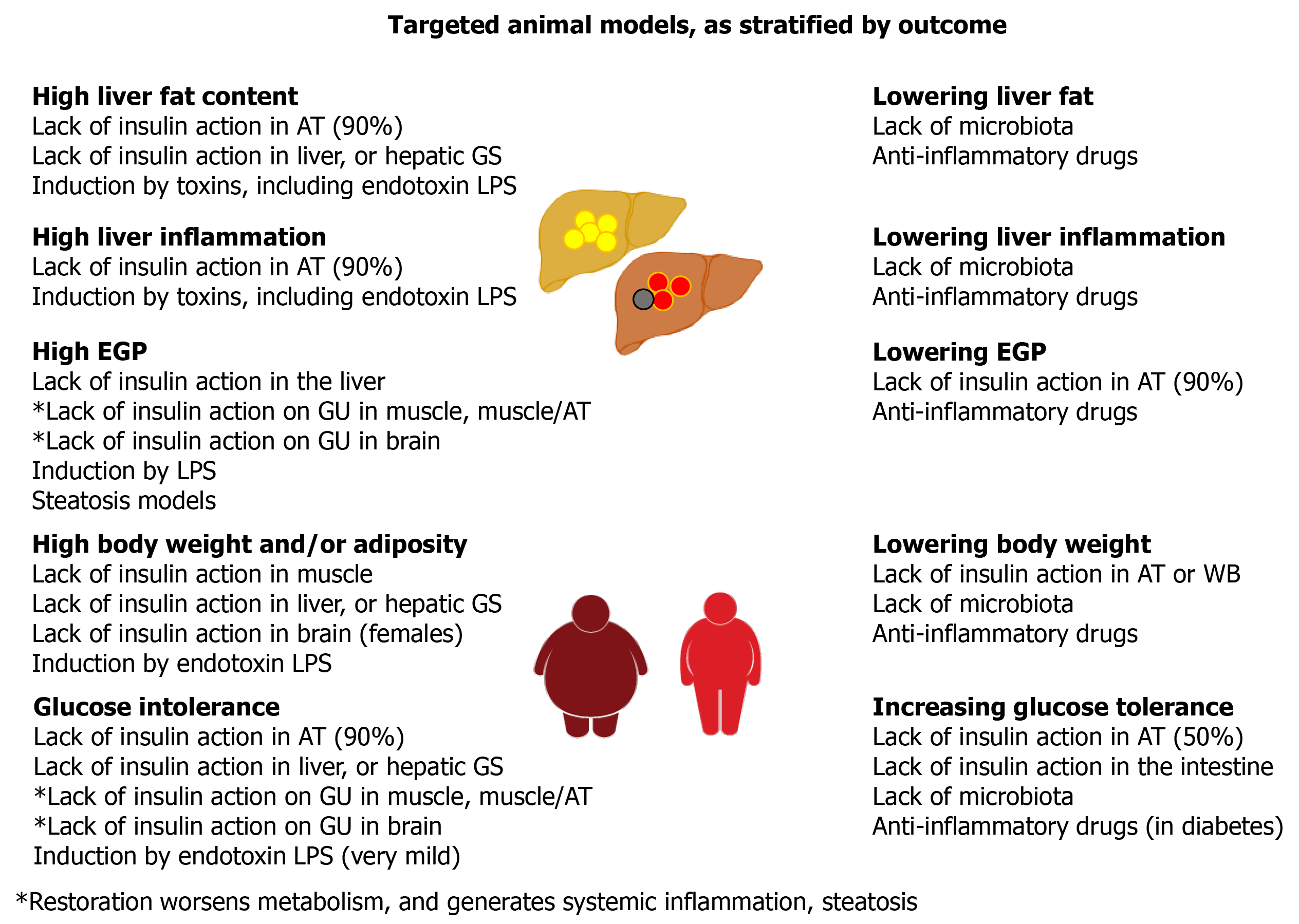
Based on the frequent coexistence of fatty liver, steatohepatitis, obesity, T2D, dysbiosis, insulin resistance, and low-grade inflammation, there is general acceptance of their possible causal interactions. However, their exact nature and implication in different subgroups of patients remains to be elucidated. In order to identify more specific causal relationships, we reviewed studies in tissue-targeted animal models in which the known primary event was either insulin resistance or hepatic steatosis or gut microbiota depletion or the induction or reduction of inflammation (Figures 2 and 3; Figure 2 is given more extensively in Supplementary Table 1). All the studies indicate that unless extreme lipodystrophy occurs (FIRKO-90%), severe insulin resistance in the whole body or skeletal muscle and/or adipose tissue does not cause hepatic steatosis and liver insulin resistance or inflammation[87-89]. GLUT4-null mice do not have hepatic steatosis or glucose intolerance and have normal EGP-related enzyme expression in their liver[90-92]. In the absence of insulin receptors or GLUT4 in both muscle and adipose tissue, glucose tolerance is normal or is less affected than expected from the degree of insulin resistance[93-95]. The key compensatory organs appeared to be adipose tissue (with upregulation in glucose uptake, increase in small adipocyte number), and the liver (with upregulated glucose uptake, balanced increase in lipid synthesis vs export). A chronic lack of insulin action in only the liver leads to unsuppressed EGP, resulting in severe glucose intolerance from early life onward, low insulin clearance, glycogen depletion, low expression of lipogenesis pathways, and resistance to high-fat diet-induced steatosis[96-99]. The effects of a short-term lack of insulin action are controversial[98,100]. With a normal diet in the chronic model, moderate liver steatosis occurs in older animals, with an elevation in liver enzymes, focal dysplasia, no fibrosis, and low circulating triglycerides, which may depend on blunted triglyceride export because of chronic brain exposure to hyperinsulinemia. In fact, acute vs chronic central insulin infusions have shown a transition from stimulation to suppression of hepatic triglyceride export[101], modulating liver fat content. Lack of insulin action in the brain also causes a moderate degree of hyperphagia in females (either with or without overweight), insulin resistance without hyperglycemia, or glucose intolerance and high or normal triglyceride levels[101-103]. Instead, the selective KO of brain GLUT4 results in normal peripheral insulin sensitivity, but unsuppressed EGP leading to glucose intolerance[104]. Although the intestine is assumed to contribute little to EGP, it was noted that the absence of insulin action in enterocytes ameliorated glucose tolerance, via reduced intestinal glucose absorption and downregulation of intestinal EGP enzyme expression[105,106]. From these studies, EGP (dys)regulation resulting from the action of insulin on the liver, brain, and gut seems to be the most prominent determinant of glucose (in)tolerance.
On the other hand, the primary induction of liver steatosis does not cause whole-body insulin resistance, glucose intolerance, or hepatic inflammation, but provokes an increase in hepatic ceramide and diacylglycerol content, and the enrichment of liver triglycerides with polyunsaturated fatty acids, which may increase susceptibility to inflammatory damage[107-111]. The exposure to toxins (including LPS) caused liver and lipid inflammation and reduced fasting glucose, insulin and triglyceride levels[108-111]. Consistently, older studies have shown that an injection of LPS leads to a major increase in fasting glucose consumption by the whole body, with a several-fold elevation in the liver and spleen glucose uptake lasting 48 h, possibly because of the content of macrophages[112]. More recent studies on chronic LPS infusion have revealed that, while inducing overweight and inflammation in the liver and in adipose tissue and muscle in chow and HFD fed mice, LPS caused steatosis and extra-hepatic insulin resistance only in HFD mice or hepatic insulin resistance (EGP) only in chow-fed mice, with a small impact on glucose tolerance[113]. This is in agreement with observations in germ-free mice lacking LPS showing lower liver fat, better glucose tolerance, higher insulin sensitivity, and normal circulating triglyceride and free fatty acid levels compared with colonized mice[114-116]. However, the selective inoculation of bacteria producing or not producing LPS in germ-free mice showed a direct effect only on adipose tissue inflammation and without hepatic or systemic impact[116]. All the evidence suggests that both the metabolic and hepatic effects of LPS require other microbial or dietary components and support a role for liver inflammation, but not steatosis per se, in the regulation of peripheral metabolism. In line with this, anti-inflammatory drugs have been shown to ameliorate liver function, steatosis, inflammation, and insulin resistance, with glucose and lipid lowering effects observed only in diabetic animals[117-121].
Liver biopsy is the current gold standard in both the diagnosis and follow-up of liver disease. The presence of ballooning degeneration of hepatocytes being the hallmark of steatohepatitis, but biopsy is invasive and unsuitable for frequent monitoring[122]. Liver function tests are useful, but not diagnostic or predictive of NASH and/or fibrosis in individual patient. Imaging tools can capture and measure liver steatosis. Among them, magnetic resonance imaging (MRI)-magnetic resonance spectroscopy (MRS) has the highest sensitivity, but it is complex and not always accessible. Recently mono- and multiparametric scores obtained by the AI-processing of common ultrasound images have been proposed for repeated follow-up of liver fat and are validated against spectroscopic magnetic resonance technology[123]. Vibration-controlled transient elastography and magnetic resonance elastography provide useful measures of the combined inflammation-fibrosis index, but a reliable distinction between them remains to be achieved in the diagnostic field[12].
MRI can measure the proton density fat fraction (PDFF) and has been shown to be an objective, accurate, and reproducible quantitative indicator of hepatic fat content across the entire liver. MRI-PDFF has been validated against liver histology, and shown to be more sensitive in detecting changes in hepatic fat content and treatment response in clinical trials[124-126].
Finally, multiparametric MRI makes it possible to establish scores for assessment and quantification of liver fibrosis and inflammation, with accurate prediction of clinical outcomes in patients with chronic liver disease of mixed etiologies and/or steatosis[127]. The animal studies discussed above showed that LPS-induced liver inflammation is characterized by very high hepatic glucose uptake, possibly because of macrophages. The notion that activated macrophages and lymphocytes have high glucose-avidity has supported the use studies with PET imaging of the glucose analogue (18F)-FDG in inflammatory conditions, such as osteomyelitis, sarcoidosis, vasculitis, or vulnerable atherosclerosis plaques[128,129]. Some studies, although lacking liver biopsies, have explored the relationship between computed tomography-determined steatosis and fasting (18F)-FDG-PET imaging[130-132], yielding controversial results. Two recent reports that used biopsy-proven liver diagnosis and compartmental modeling of (18F)-FDG in the liver, found an inverse relationship between hepatic inflammation grades and liver blood flow, i.e. the K1 rate constant representing the flow-dependent delivery of (18F)-FDG to the liver[133,134]. Other rate constants (e.g., fractional extraction) did not correlate with histology grades. Unfortunately, these human studies addressed relative indices and not the absolute rate of hepatic glucose uptake (HGU), which is given by the product of (18F)-FDG fractional uptake × circulating glucose levels, and they did not quantify EGP.
We have previously validated a method to simultaneously estimate EGP [by (18F)-FDG plasma clearance] together with HGU (by imaging) during (18F)-FDG-PET, addressing their relationship with liver steatosis (by MRI-MRS) in type 2 diabetic or morbidly obese patients. The studies indicate that hepatic insulin resistance and steatosis are, to some extent, proportional and improve after weight loss by bariatric surgery in morbidly obese individuals[135]. However, very-low-calorie diets in less severe obesity had effects on glucose tolerance, EGP, and liver fat, but not on HGU[136], whereas glucose lowering by SGTL2 inhibitors in diabetic patients had a significant effect on glucose control and liver fat, but not on EGP or HGU[137]. Taken together, the studies suggest that liver fat is not a cause of hepatic dysmetabolism, but rather a consequence of glucose intolerance. It is also important to keep in mind that the euglycemic insulin clamp that was used in the studies, did not reflect the daily metabolic physiology of patients, in which glucose and insulin levels increase and decrease together after meals or under fasting conditions. HGU and EGP are dependent on the changing insulin and glucose levels, and chronic hyperglycemia and hyperinsulinemia are commonly present. For example, PET imaging studies in minipigs underscore the relevance of circulating glucose by showing that hyperglycemic- compared with euglycemic-hyperinsulinemia enhanced HGU, hepatic triglyceride content and triglyceride release in proportion to glycemia[138]. The euglycemic clamp thus provides relevant information on the sole action of insulin on tissue metabolism, being insufficient to characterize the more complex relationship between glucose and lipid metabolism occurring in the liver in real life.
By using a fatty acid PET tracer, we demonstrated that overweight was characterized by an elevation in fasting hepatic fatty acid oxidation, with normal rates of triglyceride incorporation[139]. Liver steatosis occurred in obese subjects, in whom weight loss was able to reduce hepatic fatty acid uptake and liver steatosis in a proportional manner, and EGP[140]. Notably, a chronic, i.e. 1-wk treatment with acipimox, suppressing fatty acid levels and liver fatty acid uptake provoked a significant improvement in systemic and liver insulin sensitivity and decreased circulating triglycerides and liver enzymes, but did not change liver fat content, as measured with MRS in healthy individuals[140]. Thus, liver and systemic insulin sensitivity were improved, together with liver function and independent of hepatic triglyceride accumulation. Again, in spite of cross-sectional correlations and consensual changes after weight loss, intervention studies disconnect liver steatosis per se from other adverse metabolic consequences, at least in healthy subjects.
In spite of the new light on pathophysiology shed by the above studies, two major needs remain unmet. Firstly, none of the above PET imaging studies included sufficient histologic information to address the progression of liver steatosis into steatohepatitis and/or fibrosis, thus the specific factors of disease progression are not yet identified. However, sufficient knowledge exists to design targeted studies for a more effective demonstration of the potential of PET-CT as diagnostic tools. Secondly, the relevance of other organs in compensating or aggravating liver disease needs a better understanding in order to address appropriate treatment strategies and targets, and intervention-time windows. We have just started to examine the brain-liver-gut axis in humans by PET imaging. Our studies during euglycemic clamp revealed a positive relationship between BGU and EGP and the predictive value of BGU of glucose homeostasis in diabetic subjects following bariatric surgery[23]. We also detected a high fasting-uptake of fatty acids in the brain in obese and morbidly obese individuals[141]. A greater elevation of BGU was also observed in reward-related but not in behavior-controlling regions in response to sensory stimulation by chocolate stimuli in overweight women with high food-addiction scores, compared with women with lower scores, independent of peripheral substrate and hormone levels, which were shown to be similar. Only in the former group was BGU reduced after a low-calorie diet, independent of similar peripheral changes[142]. Thus, high or unbalanced BGU is associated with a variety of high-risk behavioral and metabolic aspects. More important, the study underscores that the same phenotype can result from different mechanisms, and that mechanistic or intervention studies pooling patients based on a similar phenotype (e.g., obesity or T2D) may be misleading both on detection of cause and on the evaluation of treatment efficacy. Evaluating the BGL axis by PET imaging with double-tracer oral glucose loading, we showed that the administration of exenatide (a GLP-1R agonist) in subjects with impaired glucose tolerance decreased EGP and HGU. A decrease in the intestinal absorption of oral glucose resulted in lower insulin levels, with an increased proportion of orally ingested glucose that was retained by the liver and increased BGU in most brain regions[143]. That underlines the importance of integrating intestinal metabolism and absorptive effects under real life circumstances in the study of the BGL axis. The quantification of intestinal glucose uptake by PET imaging has been recently validated, showing that intestinal insulin resistance in the jejunum was improved by bariatric surgery in obese subjects and in the large and small intestine by metformin and mildly improved in the small intestine of diabetic patients by rosiglitazone. Intestinal fatty acid uptake was elevated and further increased in obese subjects after bariatric surgery. Interestingly, parallel animal model observations showed that the human body could release glucose and fatty acids from the circulation into the gut lumen[144], which suggests that the gut can be a way to actively eliminate excess substrate and that the body feeds substrates to the gut microbiota, potentially modulating its composition and function. EGP was either decreased, unchanged or increased in the studies, again indicating a possible confounding effect of morbid obesity or a disconnect between insulin action in gut and liver. These studies, primarily planned to address insulin sensitivity, have set the stage for the design of gut-targeted and BGL-targeted imaging approaches under metabolic conditions that are relevant to this interaction, including the study of microbiomics.
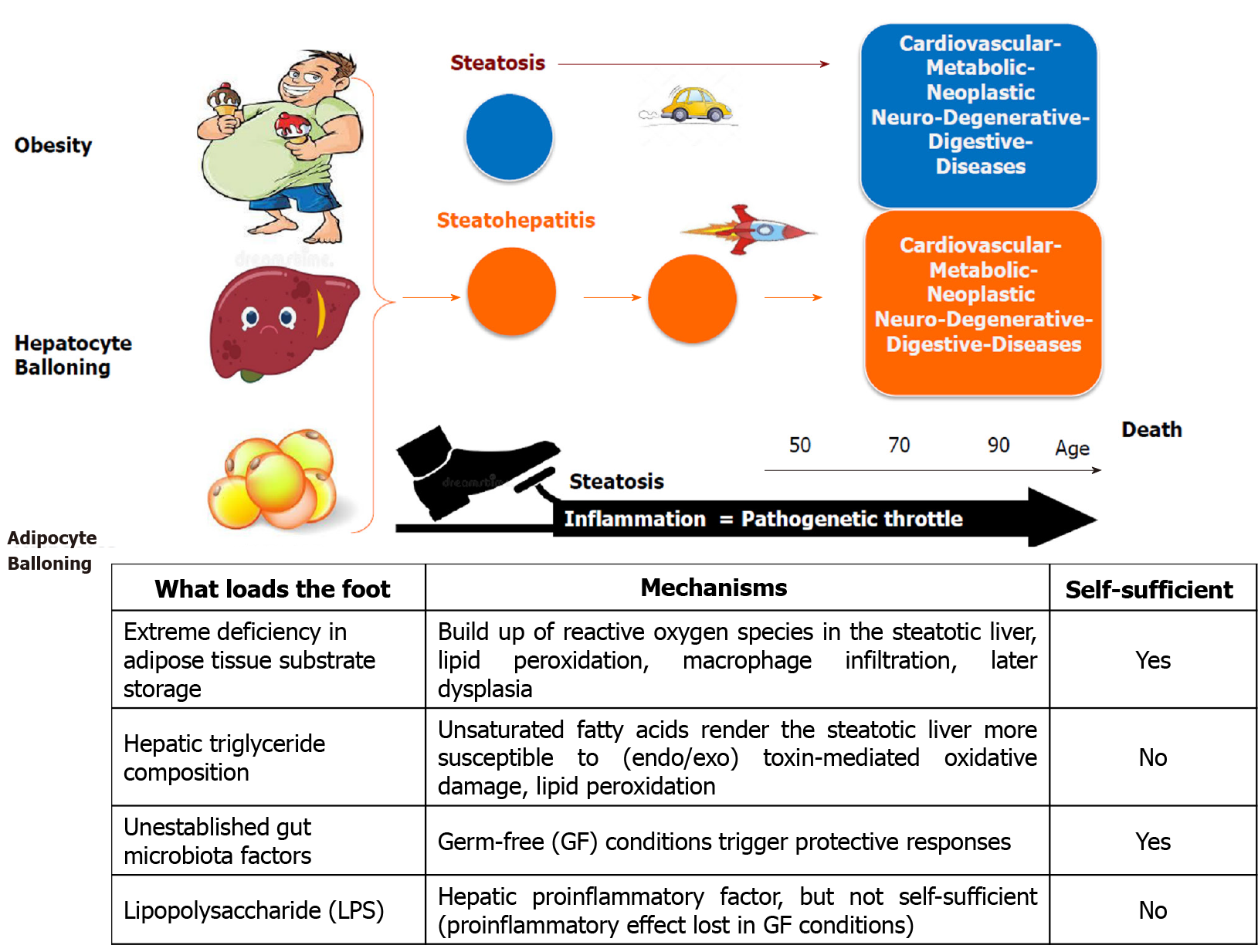
We reviewed the most recent knowledge of the complex interplay among the organs of the BGL axis in the pathophysiology of insulin resistance and MAFLD, presenting the best established interconnections between brain, gut and liver in the context of insulin resistance and hepatic steatosis. From studies using tissue-targeted animal models it emerges that insulin resistance per se does not induce hepatic steatosis, nor does steatosis induce whole-body insulin resistance. However, it is evident that reducing inflammation has several beneficial effects both at the hepatic and whole-body level. In fact, inflammation (either hepatic or systemic) acts as major throttle of progressive liver and systemic diseases. This paradigm is illustrated in Figure 4 together with the important diagnostic and prognostic role of the three hallmark characteristics of progressive fatty liver disease, namely visceral obesity (abdominal ballooning) and/or ballooning degeneration of hepatocytes and adipocytes (thus predominant adipocyte hypertrophy rather than hyperplasia).
There is currently no approved treatment for MAFLD, which is a multifaceted syndrome caused by pathogenetic mechanisms that, in animal studies, consistently appear to be diverse. Understanding and being able to identify and measure different factors that trigger and/or accelerate the pathogenesis of steatosis and its progression is a key issue for successful risk-stratification, prevention, and drug development.
With several drugs being potentially beneficial in the treatment of MAFLD, future clinical investigations should address carefully the most appropriate stratification of MAFLD patients to study their specific effects on liver and systemic inflammation, liver fat, and insulin resistance. On the other hand, the approach of both system biology and medicine has to be applied to address the unmet needs in the understanding of the pathophysiology of insulin resistance in different subsets of patients with MAFLD. The pooling of MAFLD patients just on the basis of their common phenotypic characteristic in clinical studies and trials can be highly misleading for both mechanistic understanding and for new drug development as the same phenotype can result from different and time/stage-evolving mechanisms, each requiring a very targeted approach (i.e. personalized, timely, and adequate for the disease-stage). Studies targeting the BGL axis might unveil new underlying mechanisms and fill the existing knowledge gaps in the causal links between insulin resistance and MAFLD pathophysiology, paving the way for the development of innovative diagnostic and therapeutic approaches.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ma J S-Editor: Zhang H L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, Eguchi Y, Wong VW, Negro F, Yilmaz Y, Romero-Gomez M, George J, Ahmed A, Wong R, Younossi I, Ziayee M, Afendy A; Global Nonalcoholic Steatohepatitis Council. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2019;17:748-755.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 577] [Article Influence: 96.2] [Reference Citation Analysis (0)] |
| 2. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1714] [Article Influence: 244.9] [Reference Citation Analysis (0)] |
| 3. | Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1415] [Cited by in RCA: 1319] [Article Influence: 219.8] [Reference Citation Analysis (0)] |
| 4. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2212] [Article Influence: 442.4] [Reference Citation Analysis (1)] |
| 5. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2841] [Article Influence: 568.2] [Reference Citation Analysis (1)] |
| 6. | Tameez-Ud-Din A, Alam F, Tameez-Ud-Din A, Chaudhary FMD. Auto-Brewery Syndrome: A Clinical Dilemma. Cureus.. 2020;16 ;12(10):e10983 [PMID: 33209539; PMCID: PMC7667719 DOI: 10.7759/cureus.10983. |
| 7. | Solas M, Milagro FI, Ramírez MJ, Martínez JA. Inflammation and gut-brain axis link obesity to cognitive dysfunction: plausible pharmacological interventions. Curr Opin Pharmacol. 2017;37:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Seo SW, Gottesman RF, Clark JM, Hernaez R, Chang Y, Kim C, Ha KH, Guallar E, Lazo M. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology. 2016;86:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 9. | Mancini A, Campagna F, Amodio P, Tuohy KM. Gut : liver : brain axis: the microbial challenge in the hepatic encephalopathy. Food Funct. 2018;9:1373-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Finlin BS, Zhu B, Boyechko T, Westgate PM, Chia CW, Egan JM, Kern PA. Effect of Rifaximin Treatment on Endotoxemia and Insulin Sensitivity in Humans. J Endocr Soc. 2019;3:1641-1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 540] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 12. | Mancini M, Summers P, Faita F, Brunetto MR, Callea F, De Nicola A, Di Lascio N, Farinati F, Gastaldelli A, Gridelli B, Mirabelli P, Neri E, Salvadori PA, Rebelos E, Tiribelli C, Valenti L, Salvatore M, Bonino F. Digital liver biopsy: Bio-imaging of fatty liver for translational and clinical research. World J Hepatol. 2018;10:231-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 13. | Tilg H, Effenberger M. From NAFLD to MAFLD: when pathophysiology succeeds. Nat Rev Gastroenterol Hepatol. 2020;17:387-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 14. | Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, Halavaara J, Yki-Järvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023-3028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 644] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 15. | Bril F, Lomonaco R, Orsak B, Ortiz-Lopez C, Webb A, Tio F, Hecht J, Cusi K. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:2178-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Videla LA, Rodrigo R, Araya J, Poniachik J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic Biol Med. 2004;37:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5683] [Cited by in RCA: 6395] [Article Influence: 355.3] [Reference Citation Analysis (1)] |
| 18. | Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjøro K, Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 460] [Article Influence: 24.2] [Reference Citation Analysis (2)] |
| 19. | Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 20. | Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 444] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 21. | Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 645] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 22. | Heni M, Wagner R, Kullmann S, Gancheva S, Roden M, Peter A, Stefan N, Preissl H, Häring HU, Fritsche A. Hypothalamic and Striatal Insulin Action Suppresses Endogenous Glucose Production and May Stimulate Glucose Uptake During Hyperinsulinemia in Lean but Not in Overweight Men. Diabetes. 2017;66:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Rebelos E, Immonen H, Bucci M, Hannukainen JC, Nummenmaa L, Honka MJ, Soinio M, Salminen P, Ferrannini E, Iozzo P, Nuutila P. Brain glucose uptake is associated with endogenous glucose production in obese patients before and after bariatric surgery and predicts metabolic outcome at follow-up. Diabetes Obes Metab. 2019;21:218-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Meek TH, Wisse BE, Thaler JP, Guyenet SJ, Matsen ME, Fischer JD, Taborsky GJ Jr, Schwartz MW, Morton GJ. BDNF action in the brain attenuates diabetic hyperglycemia via insulin-independent inhibition of hepatic glucose production. Diabetes. 2013;62:1512-1518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Liao GY, An JJ, Gharami K, Waterhouse EG, Vanevski F, Jones KR, Xu B. Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nat Med. 2012;18:564-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599-609, 609.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1135] [Cited by in RCA: 1216] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 27. | Wang PY, Caspi L, Lam CK, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TK. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Rebelos E, Astiarraga B, Bizzotto R, Mari A, Manca ML, Gonzalez A, Mendez A, Martinez CA, Hurwitz BE, Ferrannini E. GLP-1 response to sequential mixed meals: influence of insulin resistance. Clin Sci (Lond). 2017;131:2901-2910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965-4973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 266] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 31. | Ayala JE, Bracy DP, James FD, Julien BM, Wasserman DH, Drucker DJ. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology. 2009;150:1155-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol. 1996;271:E808-E813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Seghieri M, Rebelos E, Gastaldelli A, Astiarraga BD, Casolaro A, Barsotti E, Pocai A, Nauck M, Muscelli E, Ferrannini E. Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia. 2013;56:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 1063] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 35. | Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Wintle ME, Maggs DG. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 406] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 36. | Kenny PR, Brady DE, Torres DM, Ragozzino L, Chalasani N, Harrison SA. Exenatide in the treatment of diabetic patients with non-alcoholic steatohepatitis: a case series. Am J Gastroenterol. 2010;105:2707-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team; Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1471] [Article Influence: 163.4] [Reference Citation Analysis (1)] |
| 38. | Lin Y, Liang Z, He L, Yang M, Liu D, Gu HF, Liu H, Zhu Z, Zheng H, Li L, Yang G. Gut ghrelin regulates hepatic glucose production and insulin signaling via a gut-brain-liver pathway. Cell Commun Signal. 2019;17:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Smith GP, Gibbs J. The satiating effect of cholecystokinin. Curr Concepts Nutr. 1988;16:35-40. [PubMed] |
| 40. | Figlewicz DP, Sipols AJ, Seeley RJ, Chavez M, Woods SC, Porte D Jr. Intraventricular insulin enhances the meal-suppressive efficacy of intraventricular cholecystokinin octapeptide in the baboon. Behav Neurosci. 1995;109:567-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 41. | Stein LJ, Porte D Jr, Figlewicz DP, Woods SC. Effect of fasting interval on CCK-8 suppression of food intake in the baboon. Am J Physiol. 1986;250:R851-R855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | McLaughlin CL, Baile CA, Della-Fera MA. Changes in brain CCK concentrations with peripheral CCK injections in Zucker rats. Physiol Behav. 1986;36:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20:1341-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 234] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 44. | Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91:389-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 45. | Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 589] [Cited by in RCA: 579] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 46. | Hackl MT, Fürnsinn C, Schuh CM, Krssak M, Carli F, Guerra S, Freudenthaler A, Baumgartner-Parzer S, Helbich TH, Luger A, Zeyda M, Gastaldelli A, Buettner C, Scherer T. Brain leptin reduces liver lipids by increasing hepatic triglyceride secretion and lowering lipogenesis. Nat Commun. 2019;10:2717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 47. | Osawa Y, Kanamori H, Seki E, Hoshi M, Ohtaki H, Yasuda Y, Ito H, Suetsugu A, Nagaki M, Moriwaki H, Saito K, Seishima M. L-tryptophan-mediated enhancement of susceptibility to nonalcoholic fatty liver disease is dependent on the mammalian target of rapamycin. J Biol Chem. 2011;286:34800-34808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 48. | Namkung J, Shong KE, Kim H, Oh CM, Park S. Inhibition of Serotonin Synthesis Induces Negative Hepatic Lipid Balance. Diabetes Metab J. 2018;42:233-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 938] [Cited by in RCA: 1286] [Article Influence: 116.9] [Reference Citation Analysis (1)] |
| 50. | Pagoto SL, Spring B, McChargue D, Hitsman B, Smith M, Appelhans B, Hedeker D. Acute tryptophan depletion and sweet food consumption by overweight adults. Eat Behav. 2009;10:36-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Gupta A, Osadchiy V, Mayer EA. Brain-gut-microbiome interactions in obesity and food addiction. Nat Rev Gastroenterol Hepatol. 2020;17:655-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 52. | Stavrum AK, Heiland I, Schuster S, Puntervoll P, Ziegler M. Model of tryptophan metabolism, readily scalable using tissue-specific gene expression data. J Biol Chem. 2013;288:34555-34566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1408] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 54. | Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1766] [Cited by in RCA: 2148] [Article Influence: 179.0] [Reference Citation Analysis (0)] |
| 55. | Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1507] [Cited by in RCA: 1355] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 56. | Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1524] [Cited by in RCA: 1635] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 57. | Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 513] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 58. | Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009;89:1751-1759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 508] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 59. | Delaere F, Duchampt A, Mounien L, Seyer P, Duraffourd C, Zitoun C, Thorens B, Mithieux G. The role of sodium-coupled glucose co-transporter 3 in the satiety effect of portal glucose sensing. Mol Metab. 2012;2:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1618] [Article Influence: 147.1] [Reference Citation Analysis (0)] |
| 61. | Chen P, Torralba M, Tan J, Embree M, Zengler K, Stärkel P, van Pijkeren JP, DePew J, Loomba R, Ho SB, Bajaj JS, Mutlu EA, Keshavarzian A, Tsukamoto H, Nelson KE, Fouts DE, Schnabl B. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology. 2015;148:203-214.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 238] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 62. | Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4469] [Cited by in RCA: 4061] [Article Influence: 290.1] [Reference Citation Analysis (0)] |
| 63. | Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 545] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 64. | Iwao M, Gotoh K, Arakawa M, Endo M, Honda K, Seike M, Murakami K, Shibata H. Supplementation of branched-chain amino acids decreases fat accumulation in the liver through intestinal microbiota-mediated production of acetic acid. Sci Rep. 2020;10:18768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 65. | Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 951] [Article Influence: 105.7] [Reference Citation Analysis (1)] |
| 66. | Ji Y, Gao Y, Chen H, Yin Y, Zhang W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 67. | Fei N, Bruneau A, Zhang X, Wang R, Wang J, Rabot S, Gérard P, Zhao L. Endotoxin Producers Overgrowing in Human Gut Microbiota as the Causative Agents for Nonalcoholic Fatty Liver Disease. mBio. 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 68. | Bascoul-Colombo C, Guschina IA, Maskrey BH, Good M, O'Donnell VB, Harwood JL. Dietary DHA supplementation causes selective changes in phospholipids from different brain regions in both wild type mice and the Tg2576 mouse model of Alzheimer's disease. Biochim Biophys Acta. 2016;1861:524-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 69. | Pinçon A, De Montgolfier O, Akkoyunlu N, Daneault C, Pouliot P, Villeneuve L, Lesage F, Levy BI, Thorin-Trescases N, Thorin É, Ruiz M. Non-Alcoholic Fatty Liver Disease, and the Underlying Altered Fatty Acid Metabolism, Reveals Brain Hypoperfusion and Contributes to the Cognitive Decline in APP/PS1 Mice. Metabolites. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 70. | Wolters M, Ahrens J, Romaní-Pérez M, Watkins C, Sanz Y, Benítez-Páez A, Stanton C, Günther K. Dietary fat, the gut microbiota, and metabolic health - A systematic review conducted within the MyNewGut project. Clin Nutr. 2019;38:2504-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 71. | Higarza SG, Arboleya S, Gueimonde M, Gómez-Lázaro E, Arias JL, Arias N. Neurobehavioral dysfunction in non-alcoholic steatohepatitis is associated with hyperammonemia, gut dysbiosis, and metabolic and functional brain regional deficits. PLoS One. 2019;14:e0223019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 72. | Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O'Donnell TA, Brierley SM, Ingraham HA, Julius D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell. 2017;170:185-198.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 605] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 73. | Choi W, Namkung J, Hwang I, Kim H, Lim A, Park HJ, Lee HW, Han KH, Park S, Jeong JS, Bang G, Kim YH, Yadav VK, Karsenty G, Ju YS, Choi C, Suh JM, Park JY. Serotonin signals through a gut-liver axis to regulate hepatic steatosis. Nat Commun. 2018;9:4824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 74. | Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 885] [Article Influence: 147.5] [Reference Citation Analysis (1)] |
| 75. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718-15723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4530] [Cited by in RCA: 4417] [Article Influence: 210.3] [Reference Citation Analysis (4)] |
| 76. | Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, Perlemuter G, Cassard-Doulcier AM, Gérard P. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 715] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 77. | Gangarapu V, Ince AT, Baysal B, Kayar Y, Kılıç U, Gök Ö, Uysal Ö, Şenturk H. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 78. | Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1372] [Cited by in RCA: 1491] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 79. | Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1044] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 80. | Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 697] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 81. | Mertens KL, Kalsbeek A, Soeters MR, Eggink HM. Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front Neurosci. 2017;11:617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 82. | Ter Horst KW, Serlie MJ. Fructose Consumption, Lipogenesis, and Non-Alcoholic Fatty Liver Disease. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 215] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 83. | Rorabaugh JM, Stratford JM, Zahniser NR. A relationship between reduced nucleus accumbens shell and enhanced lateral hypothalamic orexin neuronal activation in long-term fructose bingeing behavior. PLoS One. 2014;9:e95019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1370-R1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 85. | Luo S, Monterosso JR, Sarpelleh K, Page KA. Differential effects of fructose versus glucose on brain and appetitive responses to food cues and decisions for food rewards. Proc Natl Acad Sci USA. 2015;112:6509-6514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 86. | Spruss A, Kanuri G, Stahl C, Bischoff SC, Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab Invest. 2012;92:1020-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 87. | Blüher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 579] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 88. | Boucher J, Softic S, El Ouaamari A, Krumpoch MT, Kleinridders A, Kulkarni RN, O'Neill BT, Kahn CR. Differential Roles of Insulin and IGF-1 Receptors in Adipose Tissue Development and Function. Diabetes. 2016;65:2201-2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 89. | Softic S, Boucher J, Solheim MH, Fujisaka S, Haering MF, Homan EP, Winnay J, Perez-Atayde AR, Kahn CR. Lipodystrophy Due to Adipose Tissue-Specific Insulin Receptor Knockout Results in Progressive NAFLD. Diabetes. 2016;65:2187-2200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 90. | Lin HV, Accili D. Reconstitution of insulin action in muscle, white adipose tissue, and brain of insulin receptor knock-out mice fails to rescue diabetes. J Biol Chem. 2011;286:9797-9804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 91. | Ranalletta M, Jiang H, Li J, Tsao TS, Stenbit AE, Yokoyama M, Katz EB, Charron MJ. Altered hepatic and muscle substrate utilization provoked by GLUT4 ablation. Diabetes. 2005;54:935-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Ranalletta M, Du XQ, Seki Y, Glenn AS, Kruse M, Fiallo A, Estrada I, Tsao TS, Stenbit AE, Katz EB, Charron MJ. Hepatic response to restoration of GLUT4 in skeletal muscle of GLUT4 null mice. Am J Physiol Endocrinol Metab. 2007;293:E1178-E1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 93. | Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, Wojtaszewski JF, Hirshman MF, Virkamaki A, Goodyear LJ, Kahn CR, Kahn BB. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med. 2000;6:924-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 575] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 94. | Kim JK, Zisman A, Fillmore JJ, Peroni OD, Kotani K, Perret P, Zong H, Dong J, Kahn CR, Kahn BB, Shulman GI. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J Clin Invest. 2001;108:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 95. | Kotani K, Peroni OD, Minokoshi Y, Boss O, Kahn BB. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J Clin Invest. 2004;114:1666-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87-97. [PubMed] |
| 97. | Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Alemán JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 363] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 98. | Haas JT, Miao J, Chanda D, Wang Y, Zhao E, Haas ME, Hirschey M, Vaitheesvaran B, Farese RV Jr, Kurland IJ, Graham M, Crooke R, Foufelle F, Biddinger SB. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab. 2012;15:873-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 99. | Fisher SJ, Kahn CR. Insulin signaling is required for insulin's direct and indirect action on hepatic glucose production. J Clin Invest. 2003;111:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 100. | Buettner C, Patel R, Muse ED, Bhanot S, Monia BP, McKay R, Obici S, Rossetti L. Severe impairment in liver insulin signaling fails to alter hepatic insulin action in conscious mice. J Clin Invest. 2005;115:1306-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 101. | Scherer T, Lindtner C, O'Hare J, Hackl M, Zielinski E, Freudenthaler A, Baumgartner-Parzer S, Tödter K, Heeren J, Krššák M, Scheja L, Fürnsinn C, Buettner C. Insulin Regulates Hepatic Triglyceride Secretion and Lipid Content via Signaling in the Brain. Diabetes. 2016;65:1511-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 102. | Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1616] [Cited by in RCA: 1531] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 103. | Diggs-Andrews KA, Zhang X, Song Z, Daphna-Iken D, Routh VH, Fisher SJ. Brain insulin action regulates hypothalamic glucose sensing and the counterregulatory response to hypoglycemia. Diabetes. 2010;59:2271-2280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 104. | Reno CM, Puente EC, Sheng Z, Daphna-Iken D, Bree AJ, Routh VH, Kahn BB, Fisher SJ. Brain GLUT4 Knockout Mice Have Impaired Glucose Tolerance, Decreased Insulin Sensitivity, and Impaired Hypoglycemic Counterregulation. Diabetes. 2017;66:587-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 105. | Andres SF, Santoro MA, Mah AT, Keku JA, Bortvedt AE, Blue RE, Lund PK. Deletion of intestinal epithelial insulin receptor attenuates high-fat diet-induced elevations in cholesterol and stem, enteroendocrine, and Paneth cell mRNAs. Am J Physiol Gastrointest Liver Physiol. 2015;308:G100-G111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 106. | Ussar S, Haering MF, Fujisaka S, Lutter D, Lee KY, Li N, Gerber GK, Bry L, Kahn CR. Regulation of Glucose Uptake and Enteroendocrine Function by the Intestinal Epithelial Insulin Receptor. Diabetes. 2017;66:886-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 107. | Minehira K, Young SG, Villanueva CJ, Yetukuri L, Oresic M, Hellerstein MK, Farese RV Jr, Horton JD, Preitner F, Thorens B, Tappy L. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J Lipid Res. 2008;49:2038-2044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 108. | Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV Sr, Hevener AL, Farese RV Jr. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 422] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 109. | Raabe M, Véniant MM, Sullivan MA, Zlot CH, Björkegren J, Nielsen LB, Wong JS, Hamilton RL, Young SG. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999;103:1287-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 351] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 110. | Björkegren J, Beigneux A, Bergo MO, Maher JJ, Young SG. Blocking the secretion of hepatic very low density lipoproteins renders the liver more susceptible to toxin-induced injury. J Biol Chem. 2002;277:5476-5483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Jornayvaz FR, Birkenfeld AL, Jurczak MJ, Kanda S, Guigni BA, Jiang DC, Zhang D, Lee HY, Samuel VT, Shulman GI. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc Natl Acad Sci USA. 2011;108:5748-5752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 112. | Mészáros K, Lang CH, Bagby GJ, Spitzer JJ. Contribution of different organs to increased glucose consumption after endotoxin administration. J Biol Chem. 1987;262:10965-10970. [PubMed] |
| 113. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4564] [Article Influence: 253.6] [Reference Citation Analysis (1)] |
| 114. | Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, Raymond F, Mansourian R, Chou CJ. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948-4959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 382] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 115. | Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2086] [Cited by in RCA: 1877] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 116. | Caesar R, Reigstad CS, Bäckhed HK, Reinhardt C, Ketonen M, Lundén GÖ, Cani PD, Bäckhed F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut. 2012;61:1701-1707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 117. | Madrigal-Perez VM, García-Rivera A, Rodriguez-Hernandez A, Ceja-Espiritu G, Briseño-Gomez XG, Galvan-Salazar HR, Soriano-Hernandez AD, Guzman-Esquivel J, Martinez-Fierro ML, Newton-Sanchez OA, Buenrostro BA, Rodriguez-Sanchez IP, López-Lemus UA, Lara-Esqueda A, Delgado-Enciso I. Preclinical analysis of nonsteroidal anti-inflammatory drug usefulness for the simultaneous prevention of steatohepatitis, atherosclerosis and hyperlipidemia. Int J Clin Exp Med. 2015;8:22477-22483. [PubMed] |
| 118. | Paik YH, Kim JK, Lee JI, Kang SH, Kim DY, An SH, Lee SJ, Lee DK, Han KH, Chon CY, Lee SI, Lee KS, Brenner DA. Celecoxib induces hepatic stellate cell apoptosis through inhibition of Akt activation and suppresses hepatic fibrosis in rats. Gut. 2009;58:1517-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 119. | Qiao H, Bai J, Chen Y, Tian J. Modeling the excretion of FDG in human kidneys using dynamic PET. Comput Biol Med. 2008;38:1171-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 120. | Murali G, Milne GL, Webb CD, Stewart AB, McMillan RP, Lyle BC, Hulver MW, Saraswathi V. Fish oil and indomethacin in combination potently reduce dyslipidemia and hepatic steatosis in LDLR(-/-) mice. J Lipid Res. 2012;53:2186-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 121. | Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, Irminger JC, Kergoat M, Portha B, Homo-Delarche F, Donath MY. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci USA. 2009;106:13998-14003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 122. | Salvati A, Faita F, Cavallone D, Gabriele R, Colombatto P, Coco B, Romagnoli V, Oliveri F, Bonino F, Brunetto MR. Steatosis/steatohepatitis: how sustainable is the non-invasive instrumental differential diagnosis in clinical practice. Hepatoma Res. 2021;7:14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 123. | Di Lascio N, Avigo C, Salvati A, Martini N, Ragucci M, Monti S, Prinster A, Chiappino D, Mancini M, D'Elia D, Ghiadoni L, Bonino F, Brunetto MR, Faita F. Steato-Score: Non-Invasive Quantitative Assessment of Liver Fat by Ultrasound Imaging. Ultrasound Med Biol. 2018;44:1585-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 124. | Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, Hassanein T, Patton HM, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 2009;251:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 125. | Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, Middleton M, Brunt EM, Loomba R, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 422] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 126. | Kang GH, Cruite I, Shiehmorteza M, Wolfson T, Gamst AC, Hamilton G, Bydder M, Middleton MS, Sirlin CB. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging. 2011;34:928-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 127. | Pavlides M, Banerjee R, Sellwood J, Kelly CJ, Robson MD, Booth JC, Collier J, Neubauer S, Barnes E. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol. 2016;64:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 128. | Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infection and inflammation. Radiographics. 2005;25:1357-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 329] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 129. | Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, Rafique A, Hargeaves R, Farkouh M, Fuster V, Fayad ZA. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 346] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 130. | Abikhzer G, Alabed YZ, Azoulay L, Assayag J, Rush C. Altered hepatic metabolic activity in patients with hepatic steatosis on FDG PET/CT. AJR Am J Roentgenol. 2011;196:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 131. | Lin CY, Lin WY, Lin CC, Shih CM, Jeng LB, Kao CH. The negative impact of fatty liver on maximum standard uptake value of liver on FDG PET. Clin Imaging. 2011;35:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 132. | Keramida G, Potts J, Bush J, Dizdarevic S, Peters AM. Hepatic steatosis is associated with increased hepatic FDG uptake. Eur J Radiol. 2014;83:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 133. | Wang G, Corwin MT, Olson KA, Badawi RD, Sarkar S. Dynamic PET of human liver inflammation: impact of kinetic modeling with optimization-derived dual-blood input function. Phys Med Biol. 2018;63:155004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 134. | Sarkar S, Corwin MT, Olson KA, Stewart SL, Liu CH, Badawi RD, Wang G. Pilot Study to Diagnose Nonalcoholic Steatohepatitis With Dynamic 18F-FDG PET. AJR Am J Roentgenol. 2019;212:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 135. | Immonen H, Hannukainen JC, Iozzo P, Soinio M, Salminen P, Saunavaara V, Borra R, Parkkola R, Mari A, Lehtimäki T, Pham T, Laine J, Kärjä V, Pihlajamäki J, Nelimarkka L, Nuutila P. Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non-diabetic patients. J Hepatol. 2014;60:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 136. | Viljanen AP, Iozzo P, Borra R, Kankaanpää M, Karmi A, Lautamäki R, Järvisalo M, Parkkola R, Rönnemaa T, Guiducci L, Lehtimäki T, Raitakari OT, Mari A, Nuutila P. Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J Clin Endocrinol Metab. 2009;94:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 137. | Latva-Rasku A, Honka MJ, Kullberg J, Mononen N, Lehtimäki T, Saltevo J, Kirjavainen AK, Saunavaara V, Iozzo P, Johansson L, Oscarsson J, Hannukainen JC, Nuutila P. The SGLT2 Inhibitor Dapagliflozin Reduces Liver Fat but Does Not Affect Tissue Insulin Sensitivity: A Randomized, Double-Blind, Placebo-Controlled Study With 8-Week Treatment in Type 2 Diabetes Patients. Diabetes Care. 2019;42:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 138. | Guiducci L, Lionetti V, Burchielli S, Simi C, Masi S, Liistro T, Pardini S, Porciello C, Di Cecco P, Vettor R, Calcagno A, Ciociaro D, Recchia FA, Salvadori PA, Iozzo P. A dose-response elevation in hepatic glucose uptake is paralleled by liver triglyceride synthesis and release. Endocr Res. 2011;36:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 139. | Iozzo P, Bucci M, Roivainen A, Någren K, Järvisalo MJ, Kiss J, Guiducci L, Fielding B, Naum AG, Borra R, Virtanen K, Savunen T, Salvadori PA, Ferrannini E, Knuuti J, Nuutila P. Fatty acid metabolism in the liver, measured by positron emission tomography, is increased in obese individuals. Gastroenterology. 2010;139:846-856, 856.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 140. | Rigazio S, Lehto HR, Tuunanen H, Någren K, Kankaanpää M, Simi C, Borra R, Naum AG, Parkkola R, Knuuti J, Nuutila P, Iozzo P. The lowering of hepatic fatty acid uptake improves liver function and insulin sensitivity without affecting hepatic fat content in humans. Am J Physiol Endocrinol Metab. 2008;295:E413-E419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 141. | Rebelos E, Hirvonen J, Bucci M, Pekkarinen L, Nyman M, Hannukainen JC, Iozzo P, Salminen P, Nummenmaa L, Ferrannini E, Nuutila P. Brain free fatty acid uptake is elevated in morbid obesity, and is irreversible 6 months after bariatric surgery: A positron emission tomography study. Diabetes Obes Metab. 2020;22:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 142. | Guzzardi MA, Garelli S, Agostini A, Filidei E, Fanelli F, Giorgetti A, Mezzullo M, Fucci S, Mazza R, Vicennati V, Iozzo P, Pagotto U. Food addiction distinguishes an overweight phenotype that can be reversed by low calorie diet. Eur Eat Disord Rev. 2018;26:657-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 143. | Daniele G, Iozzo P, Molina-Carrion M, Lancaster J, Ciociaro D, Cersosimo E, Tripathy D, Triplitt C, Fox P, Musi N, DeFronzo R, Gastaldelli A. Exenatide Regulates Cerebral Glucose Metabolism in Brain Areas Associated With Glucose Homeostasis and Reward System. Diabetes. 2015;64:3406-3412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 144. | Koffert JP, Mikkola K, Virtanen KA, Andersson AD, Faxius L, Hällsten K, Heglind M, Guiducci L, Pham T, Silvola JMU, Virta J, Eriksson O, Kauhanen SP, Saraste A, Enerbäck S, Iozzo P, Parkkola R, Gomez MF, Nuutila P. Metformin treatment significantly enhances intestinal glucose uptake in patients with type 2 diabetes: Results from a randomized clinical trial. Diabetes Res Clin Pract. 2017;131:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |