Published online Aug 14, 2021. doi: 10.3748/wjg.v27.i30.4963
Peer-review started: January 28, 2021
First decision: May 2, 2021
Revised: May 17, 2021
Accepted: July 20, 2021
Article in press: July 20, 2021
Published online: August 14, 2021
Processing time: 193 Days and 15.7 Hours
Pancreatic cancer is a dismal disease with high incidence and poor survival rates. With the aim to improve overall survival of pancreatic cancer patients, new therapeutic approaches are urgently needed. Protein kinases are key regulatory players in basically all stages of development, maintaining physiologic functions but also being involved in pathogenic processes. c-Jun N-terminal kinases (JNK) and p38 kinases, representatives of the mitogen-activated protein kinases, as well as the casein kinase 1 (CK1) family of protein kinases are important mediators of adequate response to cellular stress following inflammatory and metabolic stressors, DNA damage, and others. In their physiologic roles, they are responsible for the regulation of cell cycle progression, cell proliferation and differentiation, and apoptosis. Dysregulation of the underlying pathways consequently has been identified in various cancer types, including pancreatic cancer. Pharmacological targeting of those pathways has been the field of interest for several years. While success in earlier studies was limited due to lacking specificity and off-target effects, more recent improvements in small molecule inhibitor design against stress-activated protein kinases and their use in combination therapies have shown promising in vitro results. Consequently, targeting of JNK, p38, and CK1 protein kinase family members may actually be of particular interest in the field of precision medicine in patients with highly deregulated kinase pathways related to these kinases. However, further studies are warranted, especially involving in vivo investigation and clinical trials, in order to advance inhibition of stress-activated kinases to the field of translational medicine.
Core Tip: Since pancreatic cancer patients are generally confronted with poor prognosis, optimized therapeutic strategies are urgently needed. To establish new treatment options, efforts in drug development have increasingly focused on targeting protein kinases. In the cellular response to various stress signals, c-Jun N-terminal kinases (JNK) and p38 kinases as well as members of the casein kinase 1 (CK1) family are of special interest. Concentrating on pancreatic carcinoma in this review, we summarize the key roles of JNK, p38, and CK1 and provide an overview of recent achievements in the development of small molecule kinase inhibitors against these kinases.
- Citation: Traub B, Roth A, Kornmann M, Knippschild U, Bischof J. Stress-activated kinases as therapeutic targets in pancreatic cancer. World J Gastroenterol 2021; 27(30): 4963-4984
- URL: https://www.wjgnet.com/1007-9327/full/v27/i30/4963.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i30.4963
Pancreatic cancer is a severe disease, with overall 5-year survival rates less than 10% and only very little improvement over the last decades[1]. It is currently the fourth most common cause of cancer-related deaths, and it is expected by 2030 that pancreatic cancer will have surpassed colon and breast/prostate cancer to move up to second rank of cancer-related deaths[2]. Contributing to the immense challenge of treating pancreatic cancer, dysregulation of multiple signaling pathways can frequently be detected. With the genetic hallmark mutation of KRAS in over 90% of all pancreatic cancer patients, the high relevance of kinase-driven pathways is underlined[3].
So far, classic chemotherapeutic agents have only shown moderate success in prolonging overall survival of patients suffering from pancreatic ductal adenocarcinoma (PDAC). However, more and more personalized therapy concepts are becoming promising options, especially the use of small molecule inhibitors specifically targeting newly identified drug targets, such as deregulated protein kinases. Of special interest are kinases activated in cellular stress situations, like mitogen-activated protein kinases (MAPKs) and members of the casein kinase 1 (CK1) family, which phosphorylate signal integration molecules like p53 and β-catenin finally resulting in activation of processes leading to cell cycle arrest or apoptosis.
MAPKs are key players in transducing extracellular stimuli into intracellular signaling cascades and therefore represent interesting drug targets. Multiple isoforms have been identified, which can be clustered into six groups of MAPKs. The most prominent of those are the extracellular-regulated kinases 1 and 2 (ERK1/2), the c-Jun N-terminal kinases 1, 2, and 3 (JNK1/2/3), and the p38 kinases α, β, γ, and δ[4,5]. As a response to various stimuli such as growth factors, cytokines, and environmental stress, MAPK-triggered phosphorylation of their target transcription factors (TFs) marks the endpoint of an intracellular kinase cascade. This cascade consists of ligands binding to their cell membrane receptors, recruitment of GTPase (e.g., RAS) to the plasma membrane, and activation of MAPK kinase kinases (MKKKs or MAPKKKs, e.g., RAF) as well as MAPK kinases (MKKs or MAPKKs, e.g., MEK1/2)[5-7]. ERK1/2 belong to the best-studied kinases among MAPKs. Their relevance for pancreatic cancer has been well documented, especially as ERKs exert their functions downstream of mutant KRAS[8,9]. JNK and p38 can be grouped together as stress-activated protein kinases (SAPKs), as their pathways are regularly activated by environmental stressors, like nutrient deprivation, inflammatory cytokines, or ultraviolet irradiation[5,10].
A remarkable association with tumorigenesis and tumor progression has also been demonstrated for the CK1 family of protein kinases. Being among the first kinases described in history, involvement of CK1 isoforms in several essential signal transduction pathways has been reported within the last decades. As a key cascade in developmental processes, the (canonical) Wnt/β-catenin signaling pathway can also be involved in promoting cell proliferation through activation of oncogenes like c-myc and cyclin D1[11,12]. All human CK1 isoforms were identified to fulfill negative as well as positive regulatory functions in canonical Wnt signaling, thereby either acting as tumor suppressors or contributing to Wnt-induced oncogenic processes[13-15]. CK1δ and ε might furthermore promote canonical instead of non-canonical Wnt signaling, consequently resulting in reduced JNK-mediated Wnt signaling and apoptosis[16,17]. In addition, apoptosis mediated by Fas can also be down-regulated by CK1δ- and ε-mediated stabilization of Bid[18]. Apart from signaling associated with proliferation, differentiation, and apoptosis, CK1 is also involved in further mechanisms of the cellular stress response, including functions in immune response and inflammation, regulation of microtubule dynamic processes, autophagy, and DNA damage-related signal transduction[19-21]. Especially well documented is the regulatory function of CK1 isoforms in p53-mediated signal transduction with CK1δ even forming an autoregulatory feedback loop with p53[19].
Cancer itself already forms a stressful environment on the tumor cells, induced by hypoxia and nutrient deprivation as well as metabolic and replication stress. Additionally, cancer cells face genotoxic stress exerted by chemo- and radiotherapies. In this regard, pancreatic cancer is no exception since tumors of the pancreas are known for their dense stroma with impaired vasculature and the association with metabolic stressors, like diabetic conditions. This review aims to elucidate the role of the stress-activated kinases: JNK and p38 but also CK1 in the pathogenesis of pancreatic cancer and their potential as therapeutic targets.
The first description of JNK as a 54 kDa protein kinase activated upon peritoneal cycloheximide injection into rats dates back to 1990[22]. So far, three different JNK isoforms have been identified in the human genome, including JNK1 (MAPK8), JNK2 (MAPK9), and JNK3 (MAPK10), whereas expression of JNK3 is mainly restricted to brain, heart, and testes[23]. Alternative splicing results in generation of at least 10 different JNK isoforms with molecular weights ranging from 46 kDa to 55 kDa[24]. Activation of JNKs is dependent on phosphorylation of threonine and tyrosine residues by upstream kinases. For JNK1, phosphorylation of Thr180 and Tyr182 within its kinase subdomain VIII has been demonstrated to be essential[25]. In the activation process of JNK1, the upstream kinases MKK4 and MKK7 both fulfill non-redundant functions, with MKK4 preferably phosphorylating Tyr residues while MKK7 favors Thr residues. Phosphorylation of Thr180 is sufficient for JNK1 activation, however, dual phosphorylation by both kinases is required for full JNK activation[26,27]. Further upstream of the signaling cascade, a large variety of at least 14 MKKKs can activate MKK4 and MKK7[23,28].
Merging the influence of multiple upstream kinases into fewer effector kinases enables the cells to respond to a variety of stimuli, like growth factors and cytokines, reactive oxygen species, physical interactions with other cells and the extracellular matrix, as well as cellular stressors[29]. The variety of different stimuli requires multiple cell membrane receptors engaging into the JNK pathway. These include G-protein coupled receptors, Wnt-receptors, transforming growth factor-β receptors, and tumor necrosis factor-α (TNF-α) receptors[30]. Signal transfer within the SAPK pathway is generally orchestrated by docking motifs for upstream kinases and downstream substrates, as well as scaffold proteins. Those scaffold proteins express docking sites for MKKKs, MKKs, and MAPKs and play an important role in the correct stimulus response through the kinase cascade[29,31].
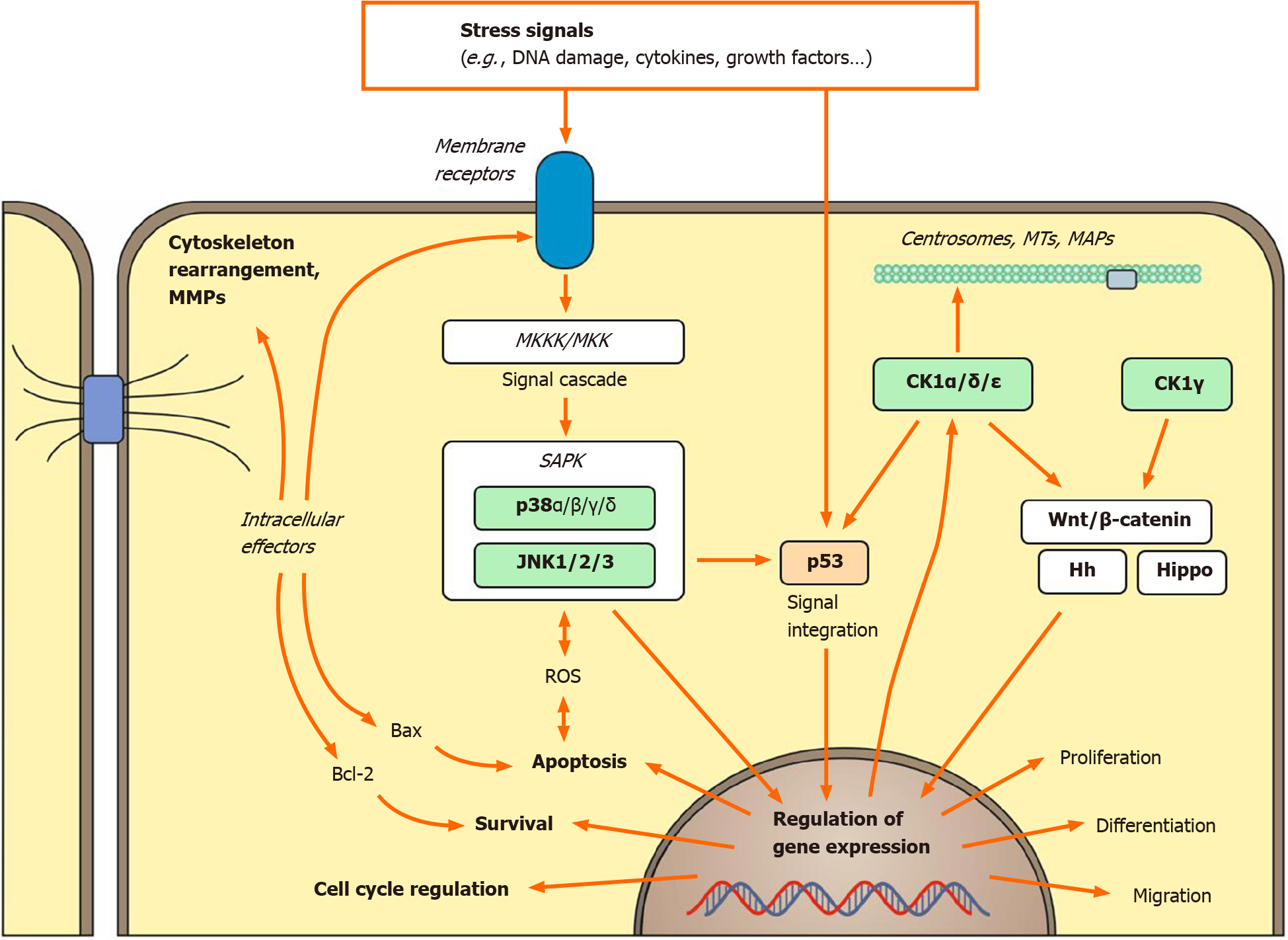
Even more diverse than the upstream mediators are the possible JNK substrates. As all MAPKs, JNK is a proline-targeted serine/threonine kinase, thus preferably phosphorylating Ser-Pro as well as Thr-Pro motifs[4,32]. So far, the list of JNK substrates includes more than 100 targets, among them TFs like c-Jun, p53, c-myc, and β-catenin, microtubule-associated proteins, components of focal-adhesion-complex and cell-to-cell-adhesion, as well as apoptosis-regulating proteins like Bcl-2 and Bax[30]. Figure 1 shows an overview of MAPK-related cellular functions.
The first p38 MAPK was discovered in 1994, and today four isoforms (p38α, β, γ, and δ, corresponding to MAPK14, 11, 12 and 13) are known[33,34]. While p38α is ubiquitously expressed, the other isoforms show differential tissue distribution, with p38β being mainly expressed in the brain, p38γ in skeletal muscle, and p38δ in endocrine glands[35]. Dual phosphorylation of Thr180 and Tyr182 in a Thr-Gly-Tyr motif are required for full p38 kinase activation[36]. MKK3 and MKK6 specifically activate p38, but while MKK6 can activate all isoforms, MKK3 is unable to phosphorylate p38β[37,38]. MKK4 can also contribute to p38 activation[39,40]. Multiple MKKKs contribute to the activation of MKK3 and MKK6, among which some are shared with the JNK pathway. By engaging specific MKKKs in response to defined stimuli, cells are enabled to elicit the correct stress response[35]. In T cells, a kinase cascade-independent pathway of p38α activation has also been described[41]. Similar to JNK, there are many p38-specific substrates, ranging from TFs to other protein kinases and apoptosis-regulating proteins[35].
The involvement of JNK and p38 in the pathogenesis of cancer has been studied extensively. Their role has been debated controversially since both can exhibit pro- as well as anti-tumorigenic functions[42,43]. For both kinases, the cellular effects provoked by JNK and p38 depend on the type, strength, and duration of the stimulus[44,45]. However, the influence of SAPKs on cancer development and progression is apparent as major cancer characteristics like cell proliferation, migration, and apoptosis are influenced by p38 and JNK. Pathways associated with these characteristics will now be discussed in general and specifically in pancreatic cancer.
A primary example of differential regulation in SAPK-related pathways is the interaction of JNKs and c-Jun. In non-stimulated cells, JNK2 activity leads to degradation of c-Jun, while after stimulation, JNK1 phosphorylates and stabilizes c-Jun. Consequently, knockdown of JNK1 decreased fibroblast proliferation through reduced activity of Activator protein-1 (AP-1), a TF of which phospho-c-Jun is a vital component[46,47]. Phosphorylation of c-Jun has also been identified as a critical step in RAS-induced tumorigenesis. Oncogenic RAS uses the same phosphorylation sites as JNK on c-Jun and promotes transformation of rat embryonic fibroblasts, while c-Junnullcells are resistant to RAS-induced transformation[48,49]. However, the role of JNK in this process is under dispute. In TP53-/-mouse embryonic fibroblasts, dual knockout of JNK1 and 2 reduced KRASG12D-induced transformation and suppressed in vivo growth. Furthermore, KRASG12D-induced lung tumor formation in mice was similarly reduced by JNK knockout[50]. This effect was in part attributed to JNK2, as only JNK2- but not JNK1-deprived mouse embryonic fibroblasts resisted RAS-induced transformation, although increased levels of AP-1 and phospho-c-Jun were observed[51]. However, not all transforming effects of RAS seem to be controlled by JNK, and in contrast to the before-mentioned studies, loss of JNK in RAS mutant cells may also contribute to enhanced tumorigenesis through apoptosis regulation[52]. A possible explanation for these findings is JNK-controlled cell cycle progression, as fibroblasts with knockdown of JNK2 show faster G1/S progression, while their JNK1-deprived counterparts show an opposing phenotype[46].
Besides proliferation, pro- and anti-apoptotic signals are also mediated by JNK in dependency of the stimulus. JNK regulates the expression of Bcl-2 family members and thereby influences apoptosis mediated via the mitochondrial pathway[44]. In TNF-mediated apoptosis, at early time-points, JNK activation triggers pro-survival pathways, while functioning JNK signaling is required for TNF-mediated apoptosis under persistent stimulation[53]. In murine cancer models, JNK1 was shown to promote chemically induced liver cancer, a finding that was also confirmed in human hepatocellular carcinoma[54,55]. On the other hand, knockdown of JNK1 rendered mice more susceptible to chemically induced skin tumors, while knockdown of JNK2 exerted an opposite effect[56].
The influence of p38 on oncogenesis is generally thought to be tumor suppressive, but protumorigenic functions like promotion of invasiveness has also been reported[44]. p38α controls proliferation through regulation of cell cycle progression at the G1/S and G2/M phases[57]. p38 can inhibit G1/S progression, e.g., through downregulation of cyclin D1[40,58] or by phosphorylation of p53 and retinoblastoma protein[59-61]. Alternatively, p38 can also promote cell cycle progression through the induction of cyclin A or interference with the retinoblastoma protein pathway[62,63]. p38-mediated phosphorylation of p53 also activates the G2/M checkpoint in response to DNA double-strand breaks. Ironically, this also offers a survival benefit for tumor cells and increases therapy resistance, as DNA damaging drugs become less effective with functional p38 signaling[64-66]. Other tumor-promoting roles include formation of a pro-invasive phenotype through induction of matrix metalloproteinases and tumor cell dormancy, enabling metastatic relapse[42].
The influence of the SAPK pathway on pancreatic cancer has been studied on patient samples as well as genetically engineered cell lines and mouse models, while patient cohorts are especially helpful to study population risk factors. Handra-Luca et al[67] offered an immunohistochemical analysis of the MAPK pathway in 99 surgically resected pancreatic cancer specimens. While high immunoreactivity for ERK1/2 was consistently associated with a worse prognosis, high expression levels of p38 could be associated with shorter recurrence-free survival in patients without adjuvant treatment. Strong staining for MKK4 was associated with increased proliferation[67]. Contrarily hereto, phosphorylated p38 with activated downstream TFs was identified as a favorable biomarker after surgical resection and associated with reduced number of lymph node metastases. Labeling of phospho-p38 showed no changes through the different cancer stages. Furthermore, pharmacologically inhibiting p38 in vitro and in vivo resulted in enhanced JNK signaling and enhanced cell growth[68]. Phospho-p38 was reported to increase during tumor progression, which is consistent with reports claiming that p38 has tumor suppressive functions during early carcinogenesis but switches towards a tumor promoting phenotype later on[69]. In our own previous work, we were able to dissect the isoform-specific functions of p38 in pancreatic cancer by genetically targeting p38 isoforms α and β. We confirmed an in vivo tumor suppressive phenotype of p38α but also showed a pro-invasive function. Additionally, we showed a tumor suppressive role of p38β, opposing p38α[70]. Interestingly, oncogenic KRAS induced activation of p38 and phenotypically increased invasion[71,72].
Increased phospho-JNK staining was observed in pancreatic cancer tissues compared to normal controls[73] and increased phospho-JNK1 staining was determined as an independent predictor of peritoneal spread[74]. Furthermore, serum auto-antibodies against JNK2 were identified as potential biomarker in pancreatic cancer patients[75]. By isoform-specific knockdown of JNK1 and 2 in MiaPaCa-2 and Panc-1 cells, a tumor promoting role could be attributed to JNK1, while JNK2 seems to exert suppressive functions in pancreatic cancer[76]. Upstream kinases of JNK have also been studied in pancreatic cancer, but their distinct role in tumor formation and progression remains elusive. MEKK1, as a representative of JNK-activating MKKKs, was shown to contribute to pancreatic cancer cell survival. However, unlike in other cancer cell lines, JNK signaling was not affected by knockout of MEKK1 in the PDAC cell line Panc-1[77]. MKK4, a direct upstream kinase of JNK, was expressed in the majority of resected specimens, while expression levels were reduced in matched metastatic samples. As further hints for a potential tumor suppressive role, patients with loss of MKK4 were associated with shorter survival, and pancreatic cancer cell lines frequently showed loss-of-heterozygosity for MKK4[78,79]. However, ectopic expression of MKK4 stimulated proliferation and migration of ASPC-1 and BxPC3 cells[80].
Both kinases also act in pancreatic cancer outside neoplastic cells. Ptf1aCre/+; KrasG12D/+; JNK1−/−mice showed significantly smaller tumors than their JNK1+/-counterparts. Tumors induced by transplantation of murine PDAC cells were larger in wild-type mice than in JNK1-/-mice lacking JNK signaling in stromal and immune cells. Interestingly, mice heterozygous for JNK1 showed less infiltrating CD8+ T cells, possibly due to JNK-mediated downregulation of chemokine secretion of tumor-associated fibroblasts[81]. On the other hand, alternative activation of p38 through the T cell receptor in CD4+ T cells resulted in more aggressive disease through secretion of pro-inflammatory cytokines like interleukin-17 and TNF-α[82].
Due to the involvement of SAPKs within a variety of cellular processes and diseases, multiple researchers and the pharmaceutical industry have focused on identifying pharmacological inhibitors.
Generally, JNK small molecule inhibitors can be grouped into adenosine tripho
| Inhibitor | IC50 (µmol/L) | Observed effects in cell culture and in vivo data | Ref. |
| JNK inhibitor II(SP600125) | 0.040 (JNK1); 0.040 (JNK2); 0.090 (JNK3) | Antitumor effects in cancer cell lines of thyroid, stomach, lung, colon, pancreas, and brain | [104,185-189] |
| JNK inhibitor XVI(JNK-IN-8) | 0.005 (JNK1); 0.019 (JNK2); 0.980 (JNK3) | Covalent binding to JNK inactivates kinase function; Sensitizes pancreatic cancer cells and triple negative breast cancer cells to 5-FU/FOLFOX and triple negative breast cancer cells to lapatinib treatment | [190-192] |
| Bentamapimod(AS602801) | 0.080 (JNK1); 0.090 (JNK2); 0.230 (JNK3) | Cytotoxic effects observed on cancer stem cells derived from pancreatic cancer, non-small cell lung cancer, ovarian cancer, and glioblastoma | [103,193] |
| SB203580 | 0.034 (p38) | Synergistic effects observed in combination with cisplatin in vitro and in vivo; Inhibition of gemcitabine-induced apoptosis in combination therapy (tested on PK-1 and PCI-43 PDAC cell lines); IC50(p38) = 0.08-0.20 µmol/L in vivo) | [194-198] |
| SB202190 | 0.050 (p38α); 0.100 (p38β2); 0.600 (CK1) | Inhibition of gemcitabine-induced apoptosis in combination therapy (tested on PK-1 and PCI-43 PDAC cell lines); Inhibits resistance of colon cancer cell lines towards irinotecan | [93,197,199,200] |
| SB239063 | 0.044 (p38α and β) | Dose-dependent growth inhibition observed in three pancreatic cancer cell lines | [68,201] |
Strikingly, most studies report an antitumor effect of pharmacological SAPK inhibition. However, it needs to be noted that all inhibitors were pan-JNK inhibitors and p38α or pan-p38 inhibitors, respectively. As mentioned above, we previously reported on marked isoform-specific differences. By using genetic pathway disruption, isoform-specific tumor suppressive functions of JNK2 and p38β were detected and consequently, targeting of these isoforms might increase the risk of failure in clinical studies[76]. This effect could not be observed in our study, as pharmacological inhibition of JNK also reduced cell growth in various cell lines. Up to now, clinical studies using p38 inhibitors failed or only showed moderate success. Only one JNK inhibitor (CC-401) has been clinically evaluated for the treatment of cancer (NCT00126893), but this study has been discontinued[88,89].
In an early study, Ding et al[90] showed that the p38 inhibitor SB203580 [half maximal inhibitory concentration (IC50) = 34 nmol/L] increases the number of Panc-1 cells in S phase as well as their proliferation. Decreased p38 activity was confirmed by detection of reduced levels of phospho-activating transcription factor. However, the same study also revealed increased phosphorylation levels of ERK1/2 and JNK[90]. Increased activation of ERK1/2, possibly as a compensation mechanism or off-target effect under SB203580 treatment, has also been shown in other reports[91,92]. Therefore, it remains unclear if the observed increased proliferation is a consequence of the loss of p38-dependent tumor suppressive actions or rather of increased ERK1/2 signaling. More recently, off-target effects of SB203580 and the closely related compound SB202190 were also described by Shanware and colleagues[93], reporting on cellular effects interestingly caused by off-target inhibition of CK1. Similar to the above mentioned studies, Zhong et al[68] reported on the growth enhancing effects not only for SB203580 but also for SB202190 and SB239063 on three different pancreatic cancer cell lines. Interestingly, while environmental stressors (hypoxia and reduced serum levels) led to reduced proliferation in PDAC cell lines, p38 inhibition abolished these effects. Again, increased phospho-JNK levels after p38 inhibition were reported, and JNK inhibition through SP600125 abolished the effects of p38 inhibition in vitro. The pan-JNK inhibitor SP600125 (IC50(JNK1) = 40 nmol/L; IC50(JNK2) = 40 nmol/L; IC50(JNK3) = 90 nmol/L) also reduced the in vivo growth of cell lines with high phospho-p38 Levels[68]. The crosstalk of p38 and JNK has been described in various contexts previously. Although there is evidence for a synergistic role of both SAPKs in activation of downstream targets[94], an opposing function of both has also been well documented[95-97]. Possible regulation mechanisms of JNK through p38 include upstream MKKKs (MLK3, TAK1) as well as nuclear factor-κB[44]. Consequently, targeting single MAPKs is highly challenging. An alternative approach to using inhibitors could be selectively activating pathways of interest. The small molecule triptonide was shown to selectively activate the MEKK4-MKK4-p38 pathway without significantly altering phosphorylation levels of JNK and ERK1/2. This resulted in dose-dependent growth reduction of six pancreatic cancer cell lines as well as in vivo xenografts by inducing G2/M arrest and reduced expression of cyclin-dependent kinase 3[98].
In contrast to those studies showing an overall growth restraining effect of p38 in pancreatic cancer, Yang et al[99] performed a screen of p38α expression in various cancer samples of The Cancer Genome Atlas database and identified an overexpression of p38α in PDAC samples. The same study also reported enhanced phospho-p38 labeling in PDAC tissues compared to adjacent normal tissue and mostly attributed phospho-p38 labeling to cancer cells. When treating Pan02 cells with SB203580 or the p38α- and β-specific inhibitor LY2228820, Yang et al[99] reported growth-restricting effects. However, it needs to be noted that the used inhibitor concentrations were relatively high and potential off-target effects cannot be excluded. Finally, in order to address the issue of lacking sensitivity, possible binding pockets in p38, enabling the design of more selective inhibitor compounds in future, were identified by in silico modeling[99].
Previous studies indirectly suggested a growth-promoting effect of JNKs on pancreatic cancer. Takahashi et al[73] observed growth inhibition in vitro and in vivo after treatment with SP600125. This was associated with G1 arrest and downregulation of cyclin D1 in vitro. In genetically-engineered mouse models (Ptf1acre/+, LSL-KrasG12D/+, and Tgfbr2flox/flox) SP600125 reduced neoangiogenesis and expression levels of CD44 in PDAC cells[73]. Together with CD133, CD44 is considered as a potential marker for cancer stem cells (CSCs) or CSC-like cells (CSCLCs)[100,101]. Increased levels of phospho-JNK was also shown in CSCLCs of pancreatic cancer and other human malignancies[102]. Inhibition of JNK by the pan-JNK inhibitors SP600125 or AS602801 as well as genetic targeting of JNK1 and 2 via small interfering RNA-mediated knockdown reduced levels of CD133+ cells in an isolated subpopulation of pancreatic cancer-derived CSCLCs and abolished their self-renewal capacity in vitro and in vivo[102,103]. Besides growth suppression and interference with CSCs, induction of cellular differentiation can be another mechanism of JNK inhibition-mediated tumor suppression[104].
In clinical practice, SAPK inhibition will rather be used for combination therapy approaches instead of single-agent therapy. Therefore, the interference of SAPK inhibitors with standard of care chemotherapeutics is highly relevant. In addition to single treatment of CSCLCs, the group of Suzuki et al[105] also investigated the effects of JNK inhibition in combination with gemcitabine and 5-fluorouracil (5-FU). While CSCLCs expectedly were more resistant to these agents, pretreatment with SP600125 had a synergistic effect in combination with gemcitabine and 5-FU in a reactive oxygen species-based way of action[105]. The observed synergistic effect can furthermore be explained by JNK-mediated effects on multidrug resistance. Multidrug resistance is not only a hallmark of CSCs but of cancer cells in general, and multidrug transporters like P-glycoprotein reduce intracellular drug levels[106]. In this context, high JNK levels were shown to decrease P-glycoprotein levels in pancreatic and gastric cancer, thereby increasing intracellular drug concentrations as well as drug sensitivity[107].
The interplay of the p38 pathway and gemcitabine treatment has been well studied in pancreatic cancer cells. Apoptosis mediated through gemcitabine was consistently associated with p38 activation as well as caspase-dependent cleavage of poly (ADP-ribose) polymerase and heat shock protein 27 phosphorylation. Inhibition of p38 by SB203580 reversed these effects. Similarly, inhibition of MAPK-activated protein kinase 2, a downstream target of p38, abolished gemcitabine-mediated apoptosis in pancreatic cancer. However, combination of p38 inhibitors with mitomycin C showed synergistic effects[108].
In 1954 for the first time, an enzyme was isolated from liver tissue, which was able to phosphorylate the milk protein casein[109]. Fifteen years later, two distinct protein kinases with the ability to phosphorylate casein (at least in vitro) were described and termed casein kinase 1 (CK1) and casein kinase 2 (CK2), meanwhile renamed protein kinases CK1 and CK2[110]. Despite their common nomenclature and the ability to phosphorylate casein, protein kinases CK1 and CK2 are highly different with respect to their classification and cellular functions. While CK2 belongs to the CMGC [containing cyclin-dependent kinase, MAPK, glycogen synthase kinase 3 (GSK3), and cdc2-like kinase families] group of the human kinome, CK1 forms an independent family of protein kinases[111].
In the human genome, six CK1 isoforms are encoded (α, γ1, γ2, γ3, δ, and ε), and several splice variants can originate from post-transcriptional processing. While all CK1 isoforms are highly conserved within the kinase domain, the sequences of the N- and C-terminal noncatalytic domains can be quite variable[20,112]. Although the protein kinases of the CK1 family are generally considered to be constitutively active, several regulatory mechanisms have been described. Expression and/or activity levels of CK1 isoforms can be enhanced by insulin or cellular stress executed by viral transformation, topoisomerase inhibitor treatment, or γ-irradiation. Regulation of enzymatic activity is also possible on the protein level, e.g., by modulation of subcellular localization, interaction with other proteins, or (auto-)phosphorylation in particular targeting the C-terminal regulatory domain but also the kinase domain[20,112].
Most CK1 isoforms are localized in the cytosol. Only the CK1αL variant possessing a second nuclear localization signal in the L-exon can be localized to the nucleus[113]. Due to C-terminal palmitoylation, CK1γ can be associated with the plasma membrane[114-116]. By modulation of subcellular localization, CK1 isoforms can be brought in proximity with different substrate pools. Substrate recognition motifs for CK1 can generally be found on most cellular proteins and, to date, more than 150 substrates being phosphorylated by CK1 isoforms at least in vitro have been reported[20]. Thereby, CK1 shows strong preferences for acidic or phospho-primed substrates presenting the canonical consensus sequence (phospho-Ser/phospho-Thr-X-X-(X)-Ser/Thr). In addition, several alternative noncanonical motifs targeted by CK1 have been described[117,118].
The broad range of substrates phosphorylated by CK1 gives a hint of the numerous cellular processes potentially regulated by CK1 family members. These processes involve cell proliferation and differentiation, DNA processing and repair, as well as cytoskeleton maintenance just to name few of them. In particular, essential signal transduction pathways involving CK1-mediated regulation include Wnt and Hedgehog (Hh) signaling as well as regulation of circadian rhythm[20]. Consequently, deregulation or dysfunction of CK1 isoforms involved in regulation of these signaling pathways can result in deregulated signal transduction and subsequent development of pathological states.
CK1 isoforms have been implicated in several signaling pathways such as the canonical and noncanonical Wnt as well as Hh and Hippo signaling pathways, which play an important role in tissue development, growth, and homeostasis[119-122]. Aberrant signaling as well as mutations of key regulator proteins of these pathways can lead to various cancer entities[123-127]. The connection between CK1 and cancer has been strengthened through the discovery of their targets such as β-catenin, p53, and mouse double minute homologue 2 and 4, which hold important roles as key regulators in signaling pathways and are generally thought to be involved in cancer development (Figure 1)[128,129]. Considering the reported CK1-mediated phos
As one of the best characterized CK1-regulated processes, the Wnt signaling pathway has an important regulatory role in cell proliferation, differentiation, and cell polarity[120,130-133]. Altered expression levels of key regulators within the pathway are associated to oncogenesis, both through increased expression of positive regulators and decreased expression of negative regulators[134-137]. Several studies showed that all CK1 isoforms are implicated in the Wnt signaling pathway and either exert positive or negative regulatory functions, respectively[14]. Acting as positive regulators of the canonical Wnt signaling pathway, CK1γ, δ, and ε were found to initiate the transcription of proto-oncogenes like cyclin D1 and c-myc resulting in increased cell proliferation and cell survival[13,138,139]. For instance, mutations within the C-terminal region of CK1δ were shown to alter its physiological role, increase the oncogenic potential, and promote colonic adenoma development[140]. Additionally, CK1 isoforms exhibit oncogenic characteristics associated to the inhibition of apoptotic processes. This assumption is supported by the findings that CK1δ and CK1ε contribute to the switching mechanism between the canonical and the non-canonical Wnt/Rac1/JNK pathway, where they may favor the canonical Wnt pathway to the detriment of JNK-mediated apoptosis[17,141]. In many Wnt-driven cancers, CK1α protein expression is suppressed, leading to an activation of proliferative processes via the Wnt pathway. In addition, the absence of CK1α leads to a critical involvement of p53 in controlling invasiveness, which was shown in a model for colon cancer[15].
The importance of CK1 isoforms within various signaling pathways is strengthened by reports linking CK1 to phosphorylation of components in Hh signaling pathway. Although the activity of the Hh signaling pathway is reduced in adulthood, it is critical for embryonic development, organogenesis, and maintenance of healthy adult cells[119]. In the adult organism, Hh signaling contributes to the regulation of epithelial maintenance and tissue regeneration; consequently mutations and dysregulation of components of this signaling pathway promote tumorigenesis and cancer development[142-145]. As seen in Wnt signaling, CK1 isoforms appear to have contrasting effects on Hh signaling. Acting as a negative regulator, CK1 promotes proteolysis of GLI TF and prevents target gene transcription[146-148]. In order to fulfill its positive function, CK1α and G-protein coupled receptor kinase 2 phosphorylate the positive Hh regulator Smoothened homologue precursor, thereby inducing its active conformation[149].
The major functions of the Hippo pathway have been defined to correct organ maturation through restriction of organ size by regulating cell proliferation and apoptosis[150]. As such, dysregulated Hippo signaling can trigger tumorigenesis and cancer. CK1 isoforms have been proposed to regulate Hippo signaling through phosphorylation of a phosphodegron signal in Yes-associated protein after receiving priming phosphorylation by large tumor suppressors 1 and 2. As a result, the phospho
In a recent study analyzing messenger RNA-based gene expression data of the International Cancer Genome Consortium Pancreatic Cancer Australia cohort, high expression levels of CK1δ detected in patients with pancreatic cancer were correlated with poor survival. Increased expression of CK1δ could be found in patients with metastatic pancreatic carcinoma, and CK1δ expression was furthermore strongly correlated with the tumor grade[152]. This observation is in line with previous studies reporting upregulation of CK1 isoforms in PDAC in general[153,154] and describing increased expression of CK1δ and CK1ε in a patient cohort with higher-graded PDAC[155]. Cell line-specific elevated expression levels of CK1δ and/or CK1ε were also detected in various tumor cell lines[152,155]. Independent of the detected protein levels, CK1δ- and CK1ε-specific kinase activities in extracts obtained from various pancreatic cancer cell lines (MiaPaCa-2, BxPC3, PancTu-1, and Colo357) significantly differed from each other by up to six orders of magnitude[156].
In general, due to the involvement of CK1 isoforms in various pathways related to tumorigenesis, altered expression and/or activity levels of CK1 isoforms can be associated with increased oncogenic potential. Using the breast cancer cell line MCF7, a regulatory function of CK1ε has been identified in the Akt pathway[157]. This is of particular interest because Akt is frequently upregulated in PDAC[158,159]. In detail, CK1ε is able to inhibit protein phosphatase 2B, consequently resulting in increased Akt phosphorylation levels and enhanced Akt kinase activity. Inhibition of CK1ε in MCF7 cells by the small molecule inhibitor IC261 has been demonstrated to reduce Akt phosphorylation as well as Akt-mediated phosphorylation of GSK3β[157]. Quite similar findings could be made using PDAC cells. Also in this case, phosphorylation of Akt was reduced in response to treatment with IC261[160]. However, these results were only based on observations made in preliminary experiments, and effects were obtained by using extremely high concentrations of the rather unspecific early-stage inhibitor IC261.
Apart from altered expression and/or activity levels, mutations in the coding sequence for CK1 isoforms can also be associated with increased oncogenic functions of the resulting CK1 mutant proteins. Several mutations in CSNK1D, the gene coding for human protein kinase CK1δ, identified in different types of cancer (e.g., colorectal carcinoma, lung squamous cell carcinoma, bladder urothelial carcinoma, and pancreatic carcinoma) were analyzed for their enzyme kinetic parameters and their sensitivity towards the treatment with several CK1-specific small molecule inhibitors. Among the tested mutants, hyperactive (e.g., R127L and R127Q) as well as nearly inactive (e.g., E247K and L252P) variants could be characterized. Especially, the hyperactive CK1δ mutant R127Q showed enhanced sensitivity towards the treatment with various CK1-specific inhibitors. The two tested CK1δ mutants exclusively detected in PDAC (Q399* and H414Y) only showed slightly reduced kinase activity when compared to wild-type CK1δ[161]. The online analysis tool cBioPortal for Cancer Genomics lists even more mutations detected in PDAC and affecting CK1 isoforms, among them frameshift deletions as well as nonsense and missense mutations[162-165]. However, these mutations have so far not been investigated for their oncogenic potential.
Unfortunately, no detailed information on the role of CK1 isoforms in formation of metastasis from primary tumors located in the pancreas has been available so far. In general, the zinc-finger TF Snail is phosphorylated by CK1ε and GSK3β in a hierarchical manner. Snail can promote epithelial-mesenchymal transition by repressing expression of E-cadherin but is degraded by the proteasome upon phosphorylation by CK1ε and GSK3β. Pharmacological inhibition (using the inhibitor IC261) or RNA interference-mediated downregulation of CK1δ inhibits phospho
Results obtained from numerous studies conducted within the last 10-15 years characterized the protein kinases of the CK1 family as well-established drug targets. While early-stage small molecule inhibitors (e.g., IC261[168]) only demonstrated low target selectivity, several recent-stage CK1-specific inhibitors with enhanced selectivity and improved potency in the nanomolar range are available to date (Table 2)[20,112,169]. So far, none of these inhibitors advanced to the stage of clinical trials, and the use of these compounds was limited to biochemical and cell culture-based testing or animal models.
| Inhibitor | IC50 CK1 (µmol/L) | Observed effects in cell culture and in vivo data | Ref. |
| IC261 | 1.000 ± 0.30 (CK1δ) | Reduced growth of ASPC-1, BxPC3, Capan-1, Colo357, MiaPaCa-2, Panc-1, Panc89, and PancTu-1 at 1.25 µmol/L concentration of IC261; Subcutaneous xenograft model using PancTu-2: reduced tumor size with IC261 or gemcitabine (no synergism with gemcitabine), downregulation of anti-apoptotic genes/upregulation of cell cycle- and cell death-associated regulators; Notable off target effects (affecting the cytoskeleton and ion channels) | [155,168,182-184,202] |
| compound 11b | 0.004 ± 0.001 (CK1δ); 0.025 ± 0.004 (CK1ε); 0.010 (p38α) | Cytotoxic effects observed on Colo357 (EC50 = 3.5 ± 0.3 µmol/L) and Panc89 (1.5 ± 0.4 µmol/L) | [174] |
| compound 3c | 1.600 (CK1δ/ε) | In a panel of cell lines only effective against Panc-1 (EC50 = 9.3 ± 0.0 µmol/L); Cytotoxic effects observed on A549 (lung carcinoma) and Hek293 (normal cells) significantly higher EC50 values | [175] |
| compound 2 | 0.070 ± 0.01 (CK1δkd); 0.520 ± 0.05 (CK1ε) | Cytotoxic effects observed on BxPC3 (EC50 = 0.11 ± 0.01 µmol/L), Colo357 (0.13 ± 0.02 µmol/L), MiaPaCa (0.26 ± 0.02 µmol/L), PancTu-1 (0.70 ± 0.02 µmol/L), and Panc-1 (0.35 ± 0.08 µmol/L); Cell line-specific effects observed in screening against a panel of 82 tumor cell lines | [178] |
| IWP-4 | 1.020 ± 0.13 (CK1δ); 7.070 ± 2.01 (CK1ε) | Cytotoxic effects observed on A818-6 (EC50 = 0.93 ± 0.07 µmol/L), MiaPaCa-2 (0.23 ± 0.01 µmol/L), Panc-1 (0.23 ± 0.02 µmol/L), Panc89 (0.58 ± 0.12 µmol/L), and Capan (0.23 ± 0.01 µmol/L); Inhibition of Wnt signaling (Wnt3A overexpression, autocrine/paracrine) with IC50 = 0.71 ± 0.38 µmol/L; Inhibition of Wnt signaling (Wnt3A-conditioned medium, autocrine/paracrine) with EC50 = 1.47 ± 0.55 µmol/L | |
| SR-3029 | 0.044 (CK1δ); 0.260 (CK1ε) | Cytotoxic effects observed on Panc-1 (EC50 = 0.023 µmol/L), MiaPaCa2 (0.370 µmol/L), and BxPC3 (0.131 µmol/L); Mouse pharmacokinetic studies with promising results for animal model use of SR-3029; Orthotopic xenograft model using Panc-1, reduced tumor size using SR-3029 and/or gemcitabine (synergism with gemcitabine due to upregulation of dCK) | [152,180] |
Quite recently, we characterized optimized 4,5-diarylimidazoles as highly effective ATP-competitive inhibitors of CK1δ. Substituted isoxazoles were originally designed as inhibitors of p38α MAPK, but they share the same pharmacophore moiety that is necessary to inhibit CK1δ[93,170-172]. Substituting the isoxazole scaffold with an imidazole scaffold resulted in the generation of highly potent dual-specific inhibitors of p38α MAPK and CK1δ[173]. By further optimizing these imidazole-based compounds, CK1 isoform-specific inhibitors with IC50 values in the low nanomolar range like compounds 11b [IC50(CK1δ) = 4 nmol/L], 12a (19 nmol/L), and 16b (8 nmol/L) could be developed, which represent the most potent CK1δ-specific inhibitors described so far. Because IC50 values determined for the highly related isoform CK1ε are increased by six to 12 orders of magnitude (with 25, 227, and 81 nmol/L for 11b, 12a, and 16b, respectively), these compounds can also be considered to be selective for CK1δ. Compound 11b even demonstrated superior selectivity towards CK1δ among a panel of more than 321 protein kinases. However, full selectivity with respect to side-effects on p38α MAPK could still not be achieved for this set of compounds, but IC50 values determined for p38α are three-fold higher compared to CK1δ. Finally, 11b demonstrated significant effects on pancreatic cancer cell lines, with half maximal effective concentration (EC50) values in the low micromolar range [EC50(Colo357) = 3.5 µmol/L, EC50(Panc89) = 1.5 µmol/L)[174].
Apart from isoxazole- and imidazole-derived molecules, the quinazoline-based inhibitors (N-(1H-pyrazol-3-yl)quinazolin-4-amines) 3c and 3d have been shown to inhibit CK1δ and ε (IC50(CK1δ/ε) = 1.6 and 1.4 µmol/L, respectively). In a panel of human cancer cell lines, compound 3c even demonstrated selective cytotoxicity against the PDAC cell line Panc-1, with an EC50 value of 9.3 µmol/L [for all others no EC50 value could be determined (> 100 µmol/L), except for A549 with 29.7 and HEK293 with 71.1 µmol/L]. Compound 3d also demonstrated effects on Panc-1 cells but only with an extremely high EC50 value of 69.4 µmol/L. However, the mechanism of selectivity of the tested quinazoline-based inhibitor remains to be determined[175].
Within the last decade, several benzimidazole-based inhibitors have demonstrated significant inhibition of CK1δ variants and superior isoform selectivity over CK1δ. The series of compounds described by Leban et al[176] originates from piperidinyl-thiazoles originally designed to inhibit nuclear factor-κB[176]. Following modification, these compounds also demonstrated significant inhibition of CK1 family members. Most significant inhibition of CK1δ kinase domain (CK1δkd) with superior isoform selectivity over CK1ε could be determined for compound 5 (IC50(CK1δkd) = 29 nmol/L, IC50(CK1ε) = 199 nmol/L). Compound 5 also induced apoptosis in various tumor cell lines with cell line-specific effects and only moderate levels of apoptosis in Colo357 pancreatic cancer cells (tested at 4 µmol/L concentration)[177]. As reported by Richter and colleagues[178], the highly related but structurally slightly different compound 1 showed three-fold stronger inhibition of CK1δkd (IC50 = 10 nmol/L). By further improving the physicochemical properties of this difluoro-dioxolo-benzoimidazole derivative, inhibitor potency in vitro could be maintained for modified compound 2 (IC50(CK1δkd) = 0.07 µmol/L, IC50(CK1ε) = 0.52 µmol/L) while significantly increasing the effects observed on a panel of cancer cell lines. In comparison to compound 1, the effects on cell viability were significantly increased for cell lines treated with compound 2, among them the pancreatic cancer cell lines BxPC3, Colo357, MiaPaCa, PancTu-1, and Panc-1 (see Table 2 for EC50 data)[178].
Being structurally related to benzimidazole-based inhibitors, compounds derived from inhibitors of Wnt production (IWP) have recently been described as CK1-specific inhibitors. IWP-2 and IWP-4 as well as the further optimized compound 19 displayed rather potent inhibition of CK1δkd in vitro (IC50(CK1δ) = 0.32, 1.02, and 0.09 µmol/L for IWP-2, IWP-4, and compound 19) and also demonstrated significant effects on the proliferation of pancreatic cancer cell lines as determined for IWP-4-treated A818-6 (EC50 = 0.93 µmol/L), MiaPaCa (0.23 µmol/L), Panc-1 (0.23 µmol/L), and Panc89 (0.58 µmol/L) cells[179].
As a benzimidazole-based inhibitor containing a purine scaffold compound SR-3029 has been described as highly potent and selective inhibitor of CK1δ (IC50(CK1δ) = 44 nmol/L, IC50(CK1ε) = 260 nmol/L)[180]. SR-3029 shows improved cellular activity on the human melanoma cell line A375 (EC50 = 86 nmol/L) and the triple-negative breast cancer cell line MDA-MB-231[181]. These results suggested favorable cell penetration for SR-3029, and mouse pharmacokinetic properties indicated that SR-3029 actually was sufficient for use in xenograft studies[180].
Recently, SR-3029 has been tested for its effects on the proliferation of PDAC cell lines Panc-1, MiaPaCa-2, and BxPC3, thereby obtaining EC50 values in the submicromolar range (23, 370, and 131 nmol/L, respectively). Furthermore, synergistic effects have been detected for the treatment of MiaPaCa-2 and Panc-1 cells with a combination of SR-3029 and gemcitabine, the standard of care used in treatment of locally advanced and metastatic PDAC. Same effects could be observed after silencing of CK1δ by small interfering RNA. The mechanism of synergy could be explained by upregulation of deoxycytidine kinase subsequent to inhibition of CK1δ by SR-3029, resulting in enhanced metabolism and anti-proliferative effects of gemcitabine. Anti-proliferative effects of SR-3029 and synergy with gemcitabine could also be observed in vivo by using an orthotopic xenotransplantation mouse model. Tumors obtained from injection of Panc-1 cells into the pancreas were significantly smaller after treatment with SR-3029 or gemcitabine, and tumor size was even more reduced after combination therapy[152].
In a previous xenotransplantation study, the early-stage CK1-specific inhibitor IC261 had already demonstrated therapeutic potential. Tumor cell growth of a panel of established pancreatic cancer cell lines (ASPC-1, BxPC3, Capan-1, Colo357, MiaPaCa-2, Panc-1, Panc89, and PancTu-1) was significantly reduced by treatment with 1.25 µmol/L IC261 in vitro, and the size of tumors obtained after subcutaneous injection of PancTu-2 cells was significantly smaller after treatment with IC261. In the tumor tissue, downregulation of several anti-apoptotic genes (e.g., Bcl-2 family members) and upregulation of cell cycle- and cell death-associated regulators (e.g., p21, ataxia-telangiectasia mutated kinase, checkpoint kinase 1) could be observed following treatment with IC261 or gemcitabine[155]. However, and in contrast to the above mentioned recent study by Vena and colleagues[152], IC261 failed to sensitize gemcitabine-resistant PancTu-1 cells to treatment with gemcitabine, and no synergistic or additive action in combination with gemcitabine could be demonstrated for IC261. This failure can be due to the unspecific effects meanwhile described for IC261. Apart from its specific action on CK1 family members, IC261 is able to bind tubulin with an affinity similar to the spindle poison colchicine. IC261 can therefore be considered as a microtubule polymerization inhibitor by directly exerting its effects on microtubules independent of CK1 blockage[182,183]. Moreover, within the concentration range necessary to block CK1 kinase activity, IC261 is also able to block voltage-gated sodium channels, and consequently, well-characterized recent-stage CK1-specific inhibitor compounds like SR-3029 should be used for targeting CK1 isoforms instead of using the unspecific early-stage inhibitor IC261[184].
In recent years, there has been a lot of evidence for the involvement of stress-activated kinases like JNK and p38 but also CK1 in the pathogenesis of pancreatic cancer. Furthermore, remarkable progress has been made in designing specific small molecule inhibitors to effectively target these kinases in vitro and in vivo and to reduce off-target effects. Interestingly, due to similarities in protein structure, some inhibitor compounds even demonstrate dual inhibition of p38 and CK1 isoforms. However, further mechanisms and benefits from dual kinase inhibition have not been studied in detail. Furthermore, conclusive results from using specific inhibitors in clinical trials remain to be obtained, and knowledge on the interplay of these inhibitors with standard of care chemotherapeutics needs to be acquired in future studies.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cherri S S-Editor: Ma YJ L-Editor: A P-Editor: Liu JH
| 1. | Hidalgo M, Cascinu S, Kleeff J, Labianca R, Löhr JM, Neoptolemos J, Real FX, Van Laethem JL, Heinemann V. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology. 2015;15:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 369] [Article Influence: 36.9] [Reference Citation Analysis (2)] |
| 2. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5128] [Article Influence: 466.2] [Reference Citation Analysis (0)] |
| 3. | Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 859] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 4. | Turjanski AG, Vaqué JP, Gutkind JS. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240-3253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 327] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 2004] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 6. | Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 1350] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 7. | Avruch J, Khokhlatchev A, Kyriakis JM, Luo Z, Tzivion G, Vavvas D, Zhang XF. Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade. Recent Prog Horm Res. 2001;56:127-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 285] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 8. | Collisson EA, Trejo CL, Silva JM, Gu S, Korkola JE, Heiser LM, Charles RP, Rabinovich BA, Hann B, Dankort D, Spellman PT, Phillips WA, Gray JW, McMahon M. A central role for RAF→MEK→ERK signaling in the genesis of pancreatic ductal adenocarcinoma. Cancer Discov. 2012;2:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 9. | Hayes TK, Neel NF, Hu C, Gautam P, Chenard M, Long B, Aziz M, Kassner M, Bryant KL, Pierobon M, Marayati R, Kher S, George SD, Xu M, Wang-Gillam A, Samatar AA, Maitra A, Wennerberg K, Petricoin EF 3rd, Yin HH, Nelkin B, Cox AD, Yeh JJ, Der CJ. Long-Term ERK Inhibition in KRAS-Mutant Pancreatic Cancer Is Associated with MYC Degradation and Senescence-like Growth Suppression. Cancer Cell. 2016;29:75-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 10. | Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279-3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1960] [Cited by in RCA: 2231] [Article Influence: 123.9] [Reference Citation Analysis (0)] |
| 11. | He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3533] [Cited by in RCA: 3602] [Article Influence: 133.4] [Reference Citation Analysis (0)] |
| 12. | Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2796] [Cited by in RCA: 2853] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 13. | McKay RM, Peters JM, Graff JM. The casein kinase I family in Wnt signaling. Dev Biol. 2001;235:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Price MA. CKI, there's more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 2006;20:399-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Elyada E, Pribluda A, Goldstein RE, Morgenstern Y, Brachya G, Cojocaru G, Snir-Alkalay I, Burstain I, Haffner-Krausz R, Jung S, Wiener Z, Alitalo K, Oren M, Pikarsky E, Ben-Neriah Y. CKIα ablation highlights a critical role for p53 in invasiveness control. Nature. 2011;470:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Qiu WJ, Liu DX, Neo SY, He X, Lin SC. Differential molecular assemblies underlie the dual function of Axin in modulating the WNT and JNK pathways. J Biol Chem. 2001;276:32152-32159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Cong F, Schweizer L, Varmus H. Casein kinase Iepsilon modulates the signaling specificities of dishevelled. Mol Cell Biol. 2004;24:2000-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B, Martinou JC. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 267] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Knippschild U, Milne DM, Campbell LE, DeMaggio AJ, Christenson E, Hoekstra MF, Meek DW. p53 is phosphorylated in vitro and in vivo by the delta and epsilon isoforms of casein kinase 1 and enhances the level of casein kinase 1 delta in response to topoisomerase-directed drugs. Oncogene. 1997;15:1727-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Xu P, Ianes C, Gärtner F, Liu C, Burster T, Bakulev V, Rachidi N, Knippschild U, Bischof J. Structure, regulation, and (patho-)physiological functions of the stress-induced protein kinase CK1 delta (CSNK1D). Gene. 2019;715:144005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Carrino M, Quotti Tubi L, Fregnani A, Canovas Nunes S, Barilà G, Trentin L, Zambello R, Semenzato G, Manni S, Piazza F. Prosurvival autophagy is regulated by protein kinase CK1 alpha in multiple myeloma. Cell Death Discov. 2019;5:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Kyriakis JM, Avruch J. pp54 microtubule-associated protein 2 kinase. A novel serine/threonine protein kinase regulated by phosphorylation and stimulated by poly-L-lysine. J Biol Chem. 1990;265:17355-17363. [PubMed] |
| 23. | Davis RJ. Signal transduction by the JNK group of MAP kinases. In: Letts LG, Morgan DW. Inflammatory Processes. Basel: Birkhäuser, 2000: 13-21. |
| 24. | Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Dérijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760-2770. [PubMed] |
| 25. | Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)--from inflammation to development. Curr Opin Cell Biol. 1998;10:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1195] [Cited by in RCA: 1223] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 26. | Dérijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2509] [Cited by in RCA: 2594] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 27. | Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 290] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Tournier C, Whitmarsh AJ, Cavanagh J, Barrett T, Davis RJ. The MKK7 gene encodes a group of c-Jun NH2-terminal kinase kinases. Mol Cell Biol. 1999;19:1569-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 146] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 378] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 30. | Zeke A, Misheva M, Reményi A, Bogoyevitch MA. JNK Signaling: Regulation and Functions Based on Complex Protein-Protein Partnerships. Microbiol Mol Biol Rev. 2016;80:793-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 385] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 31. | Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 601] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 32. | Sheridan DL, Kong Y, Parker SA, Dalby KN, Turk BE. Substrate discrimination among mitogen-activated protein kinases through distinct docking sequence motifs. J Biol Chem. 2008;283:19511-19520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15:369-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 504] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 34. | Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2031] [Cited by in RCA: 2049] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 35. | Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1289] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 36. | Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420-7426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1750] [Cited by in RCA: 1812] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 37. | Alonso G, Ambrosino C, Jones M, Nebreda AR. Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J Biol Chem. 2000;275:40641-40648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273:1741-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 447] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 39. | Dérijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1253] [Cited by in RCA: 1287] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 40. | Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, Kyuuma M, Takeshita T, Flavell RA, Davis RJ. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 392] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 41. | Salvador JM, Mittelstadt PR, Guszczynski T, Copeland TD, Yamaguchi H, Appella E, Fornace AJ Jr, Ashwell JD. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nat Immunol. 2005;6:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 42. | Igea A, Nebreda AR. The Stress Kinase p38α as a Target for Cancer Therapy. Cancer Res. 2015;75:3997-4002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 43. | Tournier C. The 2 Faces of JNK Signaling in Cancer. Genes Cancer. 2013;4:397-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 44. | Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1980] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 45. | Martínez-Limón A, Joaquin M, Caballero M, Posas F, de Nadal E. The p38 Pathway: From Biology to Cancer Therapy. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 46. | Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell. 2004;15:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 317] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 47. | Fuchs SY, Dolan L, Davis RJ, Ronai Z. Phosphorylation-dependent targeting of c-Jun ubiquitination by Jun N-kinase. Oncogene. 1996;13:1531-1535. [PubMed] |
| 48. | Johnson R, Spiegelman B, Hanahan D, Wisdom R. Cellular transformation and malignancy induced by ras require c-jun. Mol Cell Biol. 1996;16:4504-4511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 231] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Smeal T, Binetruy B, Mercola DA, Birrer M, Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991;354:494-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 642] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 50. | Cellurale C, Sabio G, Kennedy NJ, Das M, Barlow M, Sandy P, Jacks T, Davis RJ. Requirement of c-Jun NH(2)-terminal kinase for Ras-initiated tumor formation. Mol Cell Biol. 2011;31:1565-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | Nielsen C, Thastrup J, Bøttzauw T, Jäättelä M, Kallunki T. c-Jun NH2-terminal kinase 2 is required for Ras transformation independently of activator protein 1. Cancer Res. 2007;67:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Kennedy NJ, Sluss HK, Jones SN, Bar-Sagi D, Flavell RA, Davis RJ. Suppression of Ras-stimulated transformation by the JNK signal transduction pathway. Genes Dev. 2003;17:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Ventura JJ, Hübner A, Zhang C, Flavell RA, Shokat KM, Davis RJ. Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell. 2006;21:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 255] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 54. | Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest. 2008;118:3943-3953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 255] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 55. | Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA. 2006;103:10544-10551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 353] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 56. | She QB, Chen N, Bode AM, Flavell RA, Dong Z. Deficiency of c-Jun-NH(2)-terminal kinase-1 in mice enhances skin tumor development by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 2002;62:1343-1348. [PubMed] |
| 57. | Ambrosino C, Nebreda AR. Cell cycle regulation by p38 MAP kinases. Biol Cell. 2001;93:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Thoms HC, Dunlop MG, Stark LA. p38-mediated inactivation of cyclin D1/cyclin-dependent kinase 4 stimulates nucleolar translocation of RelA and apoptosis in colorectal cancer cells. Cancer Res. 2007;67:1660-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 59. | Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA, Ambrosino C, Sauter G, Nebreda AR, Anderson CW, Kallioniemi A, Fornace AJ Jr, Appella E. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet. 2002;31:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 344] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 60. | She QB, Bode AM, Ma WY, Chen NY, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 2001;61:1604-1610. [PubMed] |
| 61. | Gubern A, Joaquin M, Marquès M, Maseres P, Garcia-Garcia J, Amat R, González-Nuñez D, Oliva B, Real FX, de Nadal E, Posas F. The N-Terminal Phosphorylation of RB by p38 Bypasses Its Inactivation by CDKs and Prevents Proliferation in Cancer Cells. Mol Cell. 2016;64:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 62. | Philips A, Roux P, Coulon V, Bellanger JM, Vié A, Vignais ML, Blanchard JM. Differential effect of Rac and Cdc42 on p38 kinase activity and cell cycle progression of nonadherent primary mouse fibroblasts. J Biol Chem. 2000;275:5911-5917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Wang S, Nath N, Minden A, Chellappan S. Regulation of Rb and E2F by signal transduction cascades: divergent effects of JNK1 and p38 kinases. EMBO J. 1999;18:1559-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Thornton TM, Rincon M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci. 2009;5:44-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 348] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 65. | Lee ER, Kim JY, Kang YJ, Ahn JY, Kim JH, Kim BW, Choi HY, Jeong MY, Cho SG. Interplay between PI3K/Akt and MAPK signaling pathways in DNA-damaging drug-induced apoptosis. Biochim Biophys Acta. 2006;1763:958-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 66. | Hernández Losa J, Parada Cobo C, Guinea Viniegra J, Sánchez-Arevalo Lobo VJ, Ramón y Cajal S, Sánchez-Prieto R. Role of the p38 MAPK pathway in cisplatin-based therapy. Oncogene. 2003;22:3998-4006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Handra-Luca A, Lesty C, Hammel P, Sauvanet A, Rebours V, Martin A, Fagard R, Fléjou JF, Faivre S, Bédossa P, Ruszniewski P, Couvelard A. Biological and prognostic relevance of mitogen-activated protein kinases in pancreatic adenocarcinoma. Pancreas. 2012;41:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Zhong Y, Naito Y, Cope L, Naranjo-Suarez S, Saunders T, Hong SM, Goggins MG, Herman JM, Wolfgang CL, Iacobuzio-Donahue CA. Functional p38 MAPK identified by biomarker profiling of pancreatic cancer restrains growth through JNK inhibition and correlates with improved survival. Clin Cancer Res. 2014;20:6200-6211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Amsterdam A, Shpigner L, Raanan C, Schreiber L, Melzer E, Seger R. Dynamic distribution of ERK, p38 and JNK during the development of pancreatic ductal adenocarcinoma. Acta Histochem. 2014;116:1434-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Tian X, Traub B, Xie X, Zhou S, Henne-Bruns D, Knippschild U, Kornmann M. Opposing Oncogenic Functions of p38 Mitogen-activated Protein Kinase Alpha and Beta in Human Pancreatic Cancer Cells. Anticancer Res. 2020;40:5545-5556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Dreissigacker U, Mueller MS, Unger M, Siegert P, Genze F, Gierschik P, Giehl K. Oncogenic K-Ras down-regulates Rac1 and RhoA activity and enhances migration and invasion of pancreatic carcinoma cells through activation of p38. Cell Signal. 2006;18:1156-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Choi BH, Philips MR, Chen Y, Lu L, Dai W. K-Ras Lys-42 is crucial for its signaling, cell migration, and invasion. J Biol Chem. 2018;293:17574-17581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 73. | Takahashi R, Hirata Y, Sakitani K, Nakata W, Kinoshita H, Hayakawa Y, Nakagawa H, Sakamoto K, Hikiba Y, Ijichi H, Moses HL, Maeda S, Koike K. Therapeutic effect of c-Jun N-terminal kinase inhibition on pancreatic cancer. Cancer Sci. 2013;104:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Lu W, Wei W, de Bock GH, Zhou H, Li Q, Shen X. The roles of Wnt5a, JNK and paxillin in the occurrence of metastasis of pancreatic adenocarcinoma. Int J Clin Oncol. 2014;19:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Bracci PM, Zhou M, Young S, Wiemels J. Serum autoantibodies to pancreatic cancer antigens as biomarkers of pancreatic cancer in a San Francisco Bay Area case-control study. Cancer. 2012;118:5384-5394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 76. | Tian X, Traub B, Shi J, Huber N, Schreiner S, Chen G, Zhou S, Henne-Bruns D, Knippschild U, Kornmann M. c-Jun N-terminal kinase 2 suppresses pancreatic cancer growth and invasion and is opposed by c-Jun N-terminal kinase 1. Cancer Gene Ther. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 77. | Hirano T, Shino Y, Saito T, Komoda F, Okutomi Y, Takeda A, Ishihara T, Yamaguchi T, Saisho H, Shirasawa H. Dominant negative MEKK1 inhibits survival of pancreatic cancer cells. Oncogene. 2002;21:5923-5928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Teng DH, Perry WL 3rd, Hogan JK, Baumgard M, Bell R, Berry S, Davis T, Frank D, Frye C, Hattier T, Hu R, Jammulapati S, Janecki T, Leavitt A, Mitchell JT, Pero R, Sexton D, Schroeder M, Su PH, Swedlund B, Kyriakis JM, Avruch J, Bartel P, Wong AK, Tavtigian SV. Human mitogen-activated protein kinase kinase 4 as a candidate tumor suppressor. Cancer Res. 1997;57:4177-4182. [PubMed] |
| 79. | Xin W, Yun KJ, Ricci F, Zahurak M, Qiu W, Su GH, Yeo CJ, Hruban RH, Kern SE, Iacobuzio-Donahue CA. MAP2K4/MKK4 expression in pancreatic cancer: genetic validation of immunohistochemistry and relationship to disease course. Clin Cancer Res. 2004;10:8516-8520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Wang L, Pan Y, Dai JL. Evidence of MKK4 pro-oncogenic activity in breast and pancreatic tumors. Oncogene. 2004;23:5978-5985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 81. | Sato T, Shibata W, Hikiba Y, Kaneta Y, Suzuki N, Ihara S, Ishii Y, Sue S, Kameta E, Sugimori M, Yamada H, Kaneko H, Sasaki T, Ishii T, Tamura T, Kondo M, Maeda S. c-Jun N-terminal kinase in pancreatic tumor stroma augments tumor development in mice. Cancer Sci. 2017;108:2156-2165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Alam MS, Gaida MM, Bergmann F, Lasitschka F, Giese T, Giese NA, Hackert T, Hinz U, Hussain SP, Kozlov SV, Ashwell JD. Selective inhibition of the p38 alternative activation pathway in infiltrating T cells inhibits pancreatic cancer progression. Nat Med. 2015;21:1337-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 83. | Siddiqui MA, Reddy PA. Small molecule JNK (c-Jun N-terminal kinase) inhibitors. J Med Chem. 2010;53:3005-3012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 84. | Haller V, Nahidino P, Forster M, Laufer SA. An updated patent review of p38 MAP kinase inhibitors (2014-2019). Expert Opin Ther Pat. 2020;30:453-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 85. | Wang W, Ma C, Mao Z, Li M. JNK inhibition as a potential strategy in treating Parkinson's disease. Drug News Perspect. 2004;17:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | L. Clinical candidates of small molecule p38 MAPK inhibitors for inflammatory diseases. MAP Kinase. 2016;4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Lee JK, Kim NJ. Recent Advances in the Inhibition of p38 MAPK as a Potential Strategy for the Treatment of Alzheimer's Disease. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 88. | Celgene Corporation. Phase 1 Study to Determine the Optimal Biologic Dose of CC-401 in Subjects With High-Risk Myeloid Leukemia. [accessed 2021 Jan 22]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/study/NCT00126893 ClinicalTrials.gov Identifier: NCT00126893. |
| 89. | Messoussi A, Feneyrolles C, Bros A, Deroide A, Daydé-Cazals B, Chevé G, Van Hijfte N, Fauvel B, Bougrin K, Yasri A. Recent progress in the design, study, and development of c-Jun N-terminal kinase inhibitors as anticancer agents. Chem Biol. 2014;21:1433-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 90. | Ding XZ, Adrian TE. MEK/ERK-mediated proliferation is negatively regulated by P38 map kinase in the human pancreatic cancer cell line, PANC-1. Biochem Biophys Res Commun. 2001;282:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Henklova P, Vrzal R, Papouskova B, Bednar P, Jancova P, Anzenbacherova E, Ulrichova J, Maurel P, Pavek P, Dvorak Z. SB203580, a pharmacological inhibitor of p38 MAP kinase transduction pathway activates ERK and JNK MAP kinases in primary cultures of human hepatocytes. Eur J Pharmacol. 2008;593:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 92. | Birkenkamp KU, Tuyt LM, Lummen C, Wierenga AT, Kruijer W, Vellenga E. The p38 MAP kinase inhibitor SB203580 enhances nuclear factor-kappa B transcriptional activity by a non-specific effect upon the ERK pathway. Br J Pharmacol. 2000;131:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 93. | Shanware NP, Williams LM, Bowler MJ, Tibbetts RS. Non-specific in vivo inhibition of CK1 by the pyridinyl imidazole p38 inhibitors SB 203580 and SB 202190. BMB Rep. 2009;42:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Hazzalin CA, Cano E, Cuenda A, Barratt MJ, Cohen P, Mahadevan LC. p38/RK is essential for stress-induced nuclear responses: JNK/SAPKs and c-Jun/ATF-2 phosphorylation are insufficient. Curr Biol. 1996;6:1028-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 180] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 95. | Wada T, Stepniak E, Hui L, Leibbrandt A, Katada T, Nishina H, Wagner EF, Penninger JM. Antagonistic control of cell fates by JNK and p38-MAPK signaling. Cell Death Differ. 2008;15:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 96. | Stepniak E, Ricci R, Eferl R, Sumara G, Sumara I, Rath M, Hui L, Wagner EF. c-Jun/AP-1 controls liver regeneration by repressing p53/p21 and p38 MAPK activity. Genes Dev. 2006;20:2306-2314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 97. | Nemoto S, Sheng Z, Lin A. Opposing effects of Jun kinase and p38 mitogen-activated protein kinases on cardiomyocyte hypertrophy. Mol Cell Biol. 1998;18:3518-3526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 179] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 98. | Zhang B, Meng M, Xiang S, Cao Z, Xu X, Zhao Z, Zhang T, Chen B, Yang P, Li Y, Zhou Q. Selective activation of tumor-suppressive MAPKP signaling pathway by triptonide effectively inhibits pancreatic cancer cell tumorigenicity and tumor growth. Biochem Pharmacol. 2019;166:70-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Yang L, Sun X, Ye Y, Lu Y, Zuo J, Liu W, Elcock A, Zhu S. p38α Mitogen-Activated Protein Kinase Is a Druggable Target in Pancreatic Adenocarcinoma. Front Oncol. 2019;9:1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 100. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2427] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 101. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2137] [Article Influence: 118.7] [Reference Citation Analysis (0)] |
| 102. | Okada M, Shibuya K, Sato A, Seino S, Suzuki S, Seino M, Kitanaka C. Targeting the K-Ras--JNK axis eliminates cancer stem-like cells and prevents pancreatic tumor formation. Oncotarget. 2014;5:5100-5112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 103. | Okada M, Kuramoto K, Takeda H, Watarai H, Sakaki H, Seino S, Seino M, Suzuki S, Kitanaka C. The novel JNK inhibitor AS602801 inhibits cancer stem cells in vitro and in vivo. Oncotarget. 2016;7:27021-27032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 104. | Konno T, Ninomiya T, Kohno T, Kikuchi S, Sawada N, Kojima T. c-Jun N-terminal kinase inhibitor SP600125 enhances barrier function and elongation of human pancreatic cancer cell line HPAC in a Ca-switch model. Histochem Cell Biol. 2015;143:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Suzuki S, Okada M, Shibuya K, Seino M, Sato A, Takeda H, Seino S, Yoshioka T, Kitanaka C. JNK suppression of chemotherapeutic agents-induced ROS confers chemoresistance on pancreatic cancer stem cells. Oncotarget. 2015;6:458-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 106. | Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4043] [Cited by in RCA: 4146] [Article Influence: 180.3] [Reference Citation Analysis (0)] |
| 107. | Zhou J, Liu M, Aneja R, Chandra R, Lage H, Joshi HC. Reversal of P-glycoprotein-mediated multidrug resistance in cancer cells by the c-Jun NH2-terminal kinase. Cancer Res. 2006;66:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 108. | Li Y, Köpper F, Dobbelstein M. Inhibition of MAPKAPK2/MK2 facilitates DNA replication upon cancer cell treatment with gemcitabine but not cisplatin. Cancer Lett. 2018;428:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 109. | BURNETT G, KENNEDY EP. The enzymatic phosphorylation of proteins. J Biol Chem. 1954;211:969-980. [PubMed] |
| 110. | Pinna LA, Baggio B, Moret V, Siliprandi N. Isolation and properties of a protein kinase from rat liver microsomes. Biochim Biophys Acta. 1969;178:199-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 111. | Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5931] [Cited by in RCA: 5961] [Article Influence: 259.2] [Reference Citation Analysis (0)] |
| 112. | Knippschild U, Krüger M, Richter J, Xu P, García-Reyes B, Peifer C, Halekotte J, Bakulev V, Bischof J. The CK1 Family: Contribution to Cellular Stress Response and Its Role in Carcinogenesis. Front Oncol. 2014;4:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 113. | Fu Z, Chakraborti T, Morse S, Bennett GS, Shaw G. Four casein kinase I isoforms are differentially partitioned between nucleus and cytoplasm. Exp Cell Res. 2001;269:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 114. | Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 456] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 115. | Robinson LC, Menold MM, Garrett S, Culbertson MR. Casein kinase I-like protein kinases encoded by YCK1 and YCK2 are required for yeast morphogenesis. Mol Cell Biol. 1993;13:2870-2881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 116. | Vancura A, Sessler A, Leichus B, Kuret J. A prenylation motif is required for plasma membrane localization and biochemical function of casein kinase I in budding yeast. J Biol Chem. 1994;269:19271-19278. [PubMed] |
| 117. | Marin O, Bustos VH, Cesaro L, Meggio F, Pagano MA, Antonelli M, Allende CC, Pinna LA, Allende JE. A noncanonical sequence phosphorylated by casein kinase 1 in beta-catenin may play a role in casein kinase 1 targeting of important signaling proteins. Proc Natl Acad Sci USA. 2003;100:10193-10200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 118. | Kawakami F, Suzuki K, Ohtsuki K. A novel consensus phosphorylation motif in sulfatide- and cholesterol-3-sulfate-binding protein substrates for CK1 in vitro. Biol Pharm Bull. 2008;31:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059-3087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2300] [Cited by in RCA: 2336] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 120. | Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3831] [Cited by in RCA: 4052] [Article Influence: 202.6] [Reference Citation Analysis (0)] |
| 121. | Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 533] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 122. | Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 956] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 123. | Bao Y, Hata Y, Ikeda M, Withanage K. Mammalian Hippo pathway: from development to cancer and beyond. J Biochem. 2011;149:361-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 124. | Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4088] [Cited by in RCA: 4445] [Article Influence: 233.9] [Reference Citation Analysis (0)] |
| 125. | Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1213] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 126. | Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1948] [Cited by in RCA: 1881] [Article Influence: 125.4] [Reference Citation Analysis (0)] |
| 127. | Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 621] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 128. | Knippschild U, Gocht A, Wolff S, Huber N, Löhler J, Stöter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 442] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 129. | Knippschild U, Wolff S, Giamas G, Brockschmidt C, Wittau M, Würl PU, Eismann T, Stöter M. The role of the casein kinase 1 (CK1) family in different signaling pathways linked to cancer development. Onkologie. 2005;28:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 130. | Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286-3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 1971] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 131. | McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989;58:1075-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 394] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 132. | Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 480] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 133. | Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell. 1997;90:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 531] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 134. | Sinnberg T, Menzel M, Kaesler S, Biedermann T, Sauer B, Nahnsen S, Schwarz M, Garbe C, Schittek B. Suppression of casein kinase 1alpha in melanoma cells induces a switch in beta-catenin signaling to promote metastasis. Cancer Res. 2010;70:6999-7009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 135. | Xiang XJ, Liu YW, Chen DD, Yu S. Differential expression of Dickkopf-1 among non-small cell lung cancer cells. Mol Med Rep. 2015;12:1935-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 136. | Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1301] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 137. | Nagahata T, Shimada T, Harada A, Nagai H, Onda M, Yokoyama S, Shiba T, Jin E, Kawanami O, Emi M. Amplification, up-regulation and over-expression of DVL-1, the human counterpart of the Drosophila disheveled gene, in primary breast cancers. Cancer Sci. 2003;94:515-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 138. | Wong C, Chen C, Wu Q, Liu Y, Zheng P. A critical role for the regulated wnt-myc pathway in naive T cell survival. J Immunol. 2015;194:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 139. | Fusté NP, Fernández-Hernández R, Cemeli T, Mirantes C, Pedraza N, Rafel M, Torres-Rosell J, Colomina N, Ferrezuelo F, Dolcet X, Garí E. Cytoplasmic cyclin D1 regulates cell invasion and metastasis through the phosphorylation of paxillin. Nat Commun. 2016;7:11581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 140. | Tsai IC, Woolf M, Neklason DW, Branford WW, Yost HJ, Burt RW, Virshup DM. Disease-associated casein kinase I delta mutation may promote adenomatous polyps formation via a Wnt/beta-catenin independent mechanism. Int J Cancer. 2007;120:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 141. | Zhang Y, Qiu WJ, Chan SC, Han J, He X, Lin SC. Casein kinase I and casein kinase II differentially regulate axin function in Wnt and JNK pathways. J Biol Chem. 2002;277:17706-17712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 142. | Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 845] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 143. | Lindemann RK. Stroma-initiated hedgehog signaling takes center stage in B-cell lymphoma. Cancer Res. 2008;68:961-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 144. | Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 302] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 145. | Wicking C, Smyth I, Bale A. The hedgehog signalling pathway in tumorigenesis and development. Oncogene. 1999;18:7844-7851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 146. | Denef N, Neubüser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102:521-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 427] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 147. | Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 605] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 148. | Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 929] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 149. | Chen Y, Sasai N, Ma G, Yue T, Jia J, Briscoe J, Jiang J. Sonic Hedgehog dependent phosphorylation by CK1α and GRK2 is required for ciliary accumulation and activation of smoothened. PLoS Biol. 2011;9:e1001083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 150. | Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010;24:72-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 1144] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 151. | Xu F, Wang YL, Chang JJ, Du SC, Diao L, Jiang N, Wang HJ, Ma D, Zhang J. Mammalian sterile 20-like kinase 1/2 inhibits the Wnt/β-catenin signalling pathway by directly binding casein kinase 1ε. Biochem J. 2014;458:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 152. | Vena F, Bayle S, Nieto A, Quereda V, Aceti M, Frydman SM, Sansil SS, Grant W, Monastyrskyi A, McDonald P, Roush WR, Teng M, Duckett D. Targeting Casein Kinase 1 Delta Sensitizes Pancreatic and Bladder Cancer Cells to Gemcitabine Treatment by Upregulating Deoxycytidine Kinase. Mol Cancer Ther. 2020;19:1623-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 153. | Grützmann R, Boriss H, Ammerpohl O, Lüttges J, Kalthoff H, Schackert HK, Klöppel G, Saeger HD, Pilarsky C. Meta-analysis of microarray data on pancreatic cancer defines a set of commonly dysregulated genes. Oncogene. 2005;24:5079-5088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 154. | Grützmann R, Pilarsky C, Staub E, Schmitt AO, Foerder M, Specht T, Hinzmann B, Dahl E, Alldinger I, Rosenthal A, Ockert D, Saeger HD. Systematic isolation of genes differentially expressed in normal and cancerous tissue of the pancreas. Pancreatology. 2003;3:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 155. | Brockschmidt C, Hirner H, Huber N, Eismann T, Hillenbrand A, Giamas G, Radunsky B, Ammerpohl O, Bohm B, Henne-Bruns D, Kalthoff H, Leithäuser F, Trauzold A, Knippschild U. Anti-apoptotic and growth-stimulatory functions of CK1 delta and epsilon in ductal adenocarcinoma of the pancreas are inhibited by IC261 in vitro and in vivo. Gut. 2008;57:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 156. | Giamas G, Hirner H, Shoshiashvili L, Grothey A, Gessert S, Kühl M, Henne-Bruns D, Vorgias CE, Knippschild U. Phosphorylation of CK1delta: identification of Ser370 as the major phosphorylation site targeted by PKA in vitro and in vivo. Biochem J. 2007;406:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 157. | Modak C, Bryant P. Casein Kinase I epsilon positively regulates the Akt pathway in breast cancer cell lines. Biochem Biophys Res Commun. 2008;368:801-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 158. | Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. Inhibition of the phosphatidylinositol 3'-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2002;1:989-997. [PubMed] |
| 159. | Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, Umeshita K, Sakon M, Ishikawa O, Ohigashi H, Nakamori S, Monden M, Aozasa K. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:2846-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 160. | Modak C, Chai J. Potential of casein kinase I in digestive cancer screening. World J Gastrointest Oncol. 2009;1:26-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 161. | Liu C, Witt L, Ianes C, Bischof J, Bammert MT, Baier J, Kirschner S, Henne-Bruns D, Xu P, Kornmann M, Peifer C, Knippschild U. Newly Developed CK1-Specific Inhibitors Show Specifically Stronger Effects on CK1 Mutants and Colon Cancer Cell Lines. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 162. | Broad Institute TCGA Genome Data Analysis Center. Analysis Overview for Pancreatic Adenocarcinoma (Primary solid tumor cohort) - 28 January 2016; 2016 [cited 2021 Jan 22]. Database: cBioPortal [Internet]. Available from: https://www.cbioportal.org/study/summary?id=paad_tcga. |
| 163. | Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM; Australian Pancreatic Cancer Genome Initiative; Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 2548] [Article Influence: 283.1] [Reference Citation Analysis (0)] |
| 164. | Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9144] [Cited by in RCA: 12749] [Article Influence: 980.7] [Reference Citation Analysis (0)] |
| 165. | Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8187] [Cited by in RCA: 11220] [Article Influence: 935.0] [Reference Citation Analysis (0)] |
| 166. | Xu Y, Lee SH, Kim HS, Kim NH, Piao S, Park SH, Jung YS, Yook JI, Park BJ, Ha NC. Role of CK1 in GSK3beta-mediated phosphorylation and degradation of snail. Oncogene. 2010;29:3124-3133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 167. | Ye LC, Jiang C, Bai J, Jiang J, Hong HF, Qiu LS. Knockdown of casein kinase 1e inhibits cell proliferation and invasion of colorectal cancer cells via inhibition of the wnt/β-catenin signaling. J Biol Regul Homeost Agents. 2015;29:307-315. [PubMed] |
| 168. | Mashhoon N, DeMaggio AJ, Tereshko V, Bergmeier SC, Egli M, Hoekstra MF, Kuret J. Crystal structure of a conformation-selective casein kinase-1 inhibitor. J Biol Chem. 2000;275:20052-20060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 169. | Cozza G, Pinna LA. Casein kinases as potential therapeutic targets. Expert Opin Ther Targets. 2016;20:319-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 170. | Peifer C, Kinkel K, Abadleh M, Schollmeyer D, Laufer S. From five- to six-membered rings: 3,4-diarylquinolinone as lead for novel p38MAP kinase inhibitors. J Med Chem. 2007;50:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 171. | Peifer C, Urich R, Schattel V, Abadleh M, Röttig M, Kohlbacher O, Laufer S. Implications for selectivity of 3,4-diarylquinolinones as p38alphaMAP kinase inhibitors. Bioorg Med Chem Lett. 2008;18:1431-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 172. | Godl K, Wissing J, Kurtenbach A, Habenberger P, Blencke S, Gutbrod H, Salassidis K, Stein-Gerlach M, Missio A, Cotten M, Daub H. An efficient proteomics method to identify the cellular targets of protein kinase inhibitors. Proc Natl Acad Sci USA. 2003;100:15434-15439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 279] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 173. | Peifer C, Abadleh M, Bischof J, Hauser D, Schattel V, Hirner H, Knippschild U, Laufer S. 3,4-Diaryl-isoxazoles and -imidazoles as potent dual inhibitors of p38alpha mitogen activated protein kinase and casein kinase 1delta. J Med Chem. 2009;52:7618-7630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 174. | Halekotte J, Witt L, Ianes C, Krüger M, Bührmann M, Rauh D, Pichlo C, Brunstein E, Luxenburger A, Baumann U, Knippschild U, Bischof J, Peifer C. Optimized 4,5-Diarylimidazoles as Potent/Selective Inhibitors of Protein Kinase CK1δ and Their Structural Relation to p38α MAPK. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 175. | Karthikeyan C, Jharia P, Waiker DK, Nusbaum AC, Amawi H, Kirwen EM, Christman R, Arudra SKC, Meijer L, Tiwari AK, Trivedi P. N-(1H-Pyrazol-3-yl)quinazolin-4-amines as a novel class of casein kinase 1δ/ε inhibitors: Synthesis, biological evaluation and molecular modeling studies. Bioorg Med Chem Lett. 2017;27:2663-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 176. | Leban J, Baierl M, Mies J, Trentinaglia V, Rath S, Kronthaler K, Wolf K, Gotschlich A, Seifert MH. A novel class of potent NF-kappaB signaling inhibitors. Bioorg Med Chem Lett. 2007;17:5858-5862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 177. | Bischof J, Leban J, Zaja M, Grothey A, Radunsky B, Othersen O, Strobl S, Vitt D, Knippschild U. 2-Benzamido-N-(1H-benzo[d]imidazol-2-yl)thiazole-4-carboxamide derivatives as potent inhibitors of CK1δ/ε. Amino Acids. 2012;43:1577-1591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 178. | Richter J, Bischof J, Zaja M, Kohlhof H, Othersen O, Vitt D, Alscher V, Pospiech I, García-Reyes B, Berg S, Leban J, Knippschild U. Difluoro-dioxolo-benzoimidazol-benzamides as potent inhibitors of CK1δ and ε with nanomolar inhibitory activity on cancer cell proliferation. J Med Chem. 2014;57:7933-7946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 179. | García-Reyes B, Witt L, Jansen B, Karasu E, Gehring T, Leban J, Henne-Bruns D, Pichlo C, Brunstein E, Baumann U, Wesseler F, Rathmer B, Schade D, Peifer C, Knippschild U. Discovery of Inhibitor of Wnt Production 2 (IWP-2) and Related Compounds As Selective ATP-Competitive Inhibitors of Casein Kinase 1 (CK1) δ/ε. J Med Chem. 2018;61:4087-4102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 180. | Bibian M, Rahaim RJ, Choi JY, Noguchi Y, Schürer S, Chen W, Nakanishi S, Licht K, Rosenberg LH, Li L, Feng Y, Cameron MD, Duckett DR, Cleveland JL, Roush WR. Development of highly selective casein kinase 1δ/1ε (CK1δ/ε) inhibitors with potent antiproliferative properties. Bioorg Med Chem Lett. 2013;23:4374-4380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 181. | Rosenberg LH, Lafitte M, Quereda V, Grant W, Chen W, Bibian M, Noguchi Y, Fallahi M, Yang C, Chang JC, Roush WR, Cleveland JL, Duckett DR. Therapeutic targeting of casein kinase 1δ in breast cancer. Sci Transl Med. 2015;7:318ra202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 182. | Cheong JK, Nguyen TH, Wang H, Tan P, Voorhoeve PM, Lee SH, Virshup DM. IC261 induces cell cycle arrest and apoptosis of human cancer cells via CK1δ/ɛ and Wnt/β-catenin independent inhibition of mitotic spindle formation. Oncogene. 2011;30:2558-2569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 183. | Stöter M, Krüger M, Banting G, Henne-Bruns D, Knippschild U. Microtubules depolymerization caused by the CK1 inhibitor IC261 may be not mediated by CK1 blockage. PLoS One. 2014;9:e100090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 184. | Föhr KJ, Knippschild U, Herkommer A, Fauler M, Peifer C, Georgieff M, Adolph O. State-dependent block of voltage-gated sodium channels by the casein-kinase 1 inhibitor IC261. Invest New Drugs. 2017;35:277-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 185. | Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681-13686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2167] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 186. | Grassi ES, Vezzoli V, Negri I, Lábadi Á, Fugazzola L, Vitale G, Persani L. SP600125 has a remarkable anticancer potential against undifferentiated thyroid cancer through selective action on ROCK and p53 pathways. Oncotarget. 2015;6:36383-36399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 187. | Kim JH, Kim TH, Kang HS, Ro J, Kim HS, Yoon S. SP600125, an inhibitor of Jnk pathway, reduces viability of relatively resistant cancer cells to doxorubicin. Biochem Biophys Res Commun. 2009;387:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 188. | Li JY, Huang JY, Xing B, Ren KW, Li M, Wei D, Gu PY, Chen G, Gu B, Zhang GF, Hu WX. SP600125, a JNK inhibitor, suppresses growth of JNK-inactive glioblastoma cells through cell-cycle G2/M phase arrest. Pharmazie. 2012;67:942-946. [PubMed] |
| 189. | Lu YY, Chen TS, Wang XP, Qu JL, Chen M. The JNK inhibitor SP600125 enhances dihydroartemisinin-induced apoptosis by accelerating Bax translocation into mitochondria in human lung adenocarcinoma cells. FEBS Lett. 2010;584:4019-4026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 190. | Lipner MB, Peng XL, Jin C, Xu Y, Gao Y, East MP, Rashid NU, Moffitt RA, Herrera Loeza SG, Morrison AB, Golitz BT, Vaziri C, Graves LM, Johnson GL, Yeh JJ. Irreversible JNK1-JUN inhibition by JNK-IN-8 sensitizes pancreatic cancer to 5-FU/FOLFOX chemotherapy. JCI Insight. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 191. | Ebelt ND, Kaoud TS, Edupuganti R, Van Ravenstein S, Dalby KN, Van Den Berg CL. A c-Jun N-terminal kinase inhibitor, JNK-IN-8, sensitizes triple negative breast cancer cells to lapatinib. Oncotarget. 2017;8:104894-104912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 192. | Zhang T, Inesta-Vaquera F, Niepel M, Zhang J, Ficarro SB, Machleidt T, Xie T, Marto JA, Kim N, Sim T, Laughlin JD, Park H, LoGrasso PV, Patricelli M, Nomanbhoy TK, Sorger PK, Alessi DR, Gray NS. Discovery of potent and selective covalent inhibitors of JNK. Chem Biol. 2012;19:140-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 193. | Cicenas J, Zalyte E, Rimkus A, Dapkus D, Noreika R, Urbonavicius S. JNK, p38, ERK, and SGK1 Inhibitors in Cancer. Cancers (Basel). 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 194. | Kumar S, Jiang MS, Adams JL, Lee JC. Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem Biophys Res Commun. 1999;263:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 227] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 195. | Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, Strickler JE, McLaughlin MM, Siemens IR, Fisher SM, Livi GP, White JR, Adams JL, Young PR. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2614] [Cited by in RCA: 2664] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 196. | Habiro A, Tanno S, Koizumi K, Izawa T, Nakano Y, Osanai M, Mizukami Y, Okumura T, Kohgo Y. Involvement of p38 mitogen-activated protein kinase in gemcitabine-induced apoptosis in human pancreatic cancer cells. Biochem Biophys Res Commun. 2004;316:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 197. | Koizumi K, Tanno S, Nakano Y, Habiro A, Izawa T, Mizukami Y, Okumura T, Kohgo Y. Activation of p38 mitogen-activated protein kinase is necessary for gemcitabine-induced cytotoxicity in human pancreatic cancer cells. Anticancer Res. 2005;25:3347-3353. [PubMed] |
| 198. | Pereira L, Igea A, Canovas B, Dolado I, Nebreda AR. Inhibition of p38 MAPK sensitizes tumour cells to cisplatin-induced apoptosis mediated by reactive oxygen species and JNK. EMBO Mol Med. 2013;5:1759-1774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 199. | Paillas S, Boissière F, Bibeau F, Denouel A, Mollevi C, Causse A, Denis V, Vezzio-Vié N, Marzi L, Cortijo C, Ait-Arsa I, Askari N, Pourquier P, Martineau P, Del Rio M, Gongora C. Targeting the p38 MAPK pathway inhibits irinotecan resistance in colon adenocarcinoma. Cancer Res. 2011;71:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 200. | Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 2121] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 201. | Underwood DC, Osborn RR, Kotzer CJ, Adams JL, Lee JC, Webb EF, Carpenter DC, Bochnowicz S, Thomas HC, Hay DW, Griswold DE. SB 239063, a potent p38 MAP kinase inhibitor, reduces inflammatory cytokine production, airways eosinophil infiltration, and persistence. J Pharmacol Exp Ther. 2000;293:281-288. [PubMed] |
| 202. | Rena G, Bain J, Elliott M, Cohen P. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 2004;5:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |