Published online Aug 7, 2021. doi: 10.3748/wjg.v27.i29.4890
Peer-review started: January 29, 2021
First decision: March 29, 2021
Revised: April 3, 2021
Accepted: April 22, 2021
Article in press: April 22, 2021
Published online: August 7, 2021
Processing time: 187 Days and 7.4 Hours
Primary biliary cholangitis (PBC) is a serious disease that causes significant morbidity. PBC is confirmed with liver biopsy but autoantibodies are frequently used as proxies for diagnosis. The performance of autoantibodies for the diagnosis of PBC seems to vary widely across populations.
To assess the diagnostic performance of several autoantibodies for the diagnosis of PBC in Latin American individuals.
We studied 85 female adult Colombians, 43 cases with biopsy-confirmed PBC and 42 controls in whom a liver biopsy ruled out PBC. Plasma anti-mitochondrial antibodies (AMAs), anti-smooth muscle antibodies (ASMAs) and anti-nuclear antibodies (ANAs), as well as total immunoglobulin (Ig) M and IgG were determined using immunofluorescence or enzyme-linked immunosorbent assay in all study participants within 1 year of the biopsy. For all variables, values analyzed were those closest to the date of the biopsy. Patients with viral or alcoholic hepatitis were excluded.
Mean age at diagnosis was 58.7 years for cases and 56.9 years for controls, and the body mass index was lower among cases. Most cases received ursodeoxycholic acid, while most controls received vitamin E. Sjögren syndrome and Hashimoto’s thyroiditis were the most frequent autoimmune comorbidities of PBC. The prevalence of AMA positivity among PBC cases was unexpectedly low. The sensitivity and specificity values were respectively 44.2% and 76.2% for AMA, 74.4% and 38.1% for ANA, 14.0% and 73.8% for ASMA, 26.7% and 80.0% for IgG, and 57.1% and 85.7% for IgM. The combination of positive AMA plus positive IgM had 91% positive predictive value for PBC. Among AMA-negative cases, the most prevalent antibodies were ANA (87.5%). In all, 62% of AMA-positive and 84.6% of IgM-positive individuals had fibrosis in their biopsy.
AMA positivity was very low among female Latin American patients with PBC. The performance of all antibodies was quite limited. These results highlight the urgent need for better PBC biomarkers.
Core Tip: Primary biliary cholangitis (PBC) is confirmed by liver biopsy, but autoantibodies are frequently employed as an indirect diagnostic method. We studied 85 female adult Latin American patients, 43 with biopsy-confirmed PBC and 42 in whom liver biopsy ruled out PBC. The prevalence of anti-mitochondrial antibodies among PBC cases was only 44.2%. Anti-nuclear antibodies and anti-smooth muscle antibodies had similarly low diagnostic performance. Eighty-two percent of immunoglobulin M-positive individuals had fibrosis in their biopsy. Among female Latin American patients with PBC, the performance of any individual antibody for PBC diagnosis was quite limited.
- Citation: Guatibonza-García V, Gaete PV, Pérez-Londoño A, Puerto-Baracaldo DK, Gutiérrez-Romero SA, Mendivil CO, Tapias M. Poor performance of anti-mitochondrial antibodies for the diagnosis of primary biliary cholangitis in female Colombian patients: A single-center study. World J Gastroenterol 2021; 27(29): 4890-4899
- URL: https://www.wjgnet.com/1007-9327/full/v27/i29/4890.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i29.4890
Primary biliary cholangitis (PBC) is a chronic autoimmune liver disease characterized by cholestasis, autoantibodies, and chronic small bile duct injury[1]. In PBC, immune-mediated portal inflammation leads to a group of histological hallmarks: biliary epithelial damage, ductopenia, and fibrosis[2]. The disease was previously known as “primary biliary cirrhosis,” a term coined in 1950 but no longer in use because in fact most individuals are not cirrhotic at the time of diagnosis[3]. The population incidence of PBC is approximately 1 case per 100000 individuals per year, but there is significant geographic variation[4]. PBC is 10 times more common among women than men, and is frequently diagnosed between 40 and 50 years of age[5]. Patients with PBC may be asymptomatic, or may present with jaundice, fatigue, or pruritus[6].
The gold standard for PBC diagnosis is liver biopsy, but measurement of plasma autoantibodies is considered a key step of the initial approach[1]. There is a large repertoire of serologic tests with diagnostic potential for PBC including anti-mitochondrial antibodies (AMAs), anti-nuclear antibodies (ANAs), antibodies against smooth muscle (ASMA), titers of immunoglobulin (Ig) G and IgM, and more than 60 other immunoassays[7,8].
However, the performance of these tests varies widely across populations[4], and there is a critical lack of information about their diagnostic value in Hispanic patients. Within this context, the primary aim of our study was to evaluate various antibodies and antibody combinations for the diagnosis of PBC in Latin American individuals from Colombia.
We retrospectively collected information from 86 participants, 44 patients with a biopsy confirmed diagnosis of PBC and 42 controls in whom the results of liver biopsy ruled out PBC. Inclusion criteria were age over 18 years, having a liver biopsy performed between 2009 and 2019 and availability of a measurement of circulating AMA, ASMA and ANA by immunofluorescence or enzyme-linked immunosorbent assay (ELISA), within 1 year of the index liver biopsy. Exclusion criteria were alcohol consumption equal to or greater than two drinks a day on average, or viral hepatitis (serology for hepatitis B and hepatitis C viruses were available at the time of biopsy for all participants). All PBC diagnoses were confirmed or ruled out by a qualified pathologist with experience in liver diseases. Controls had to have biopsies reported as normal, non-alcoholic fatty liver disease or other, non-cholestatic liver diseases.
The definitive sample consisted of 85 participants, after we excluded the only male PBC case in order to have a more homogeneous study group. We extracted from their medical history data on demographics, blood chemistry, liver function tests, clinical presentation, concomitant medications, and autoimmune comorbidities. The laboratory values considered were those closest to the date of the biopsy. All clinical records were anonymized prior to data extraction and analysis. The study database was secured and only two researchers had access to it (Gaete PV and Puerto-Baracaldo DK).
We calculated sample size using the expression for diagnostic tests studies in which the expected prevalence of the condition of interest is known[9]. Concerning the results of AMA for diagnosis of PBC, if FP is the number of false positives, TN is the number of true negatives, Z is the value of the Z statistic for the 95th quantile of the standard normal distribution, SP is the minimum tolerable specificity (90%), W is the confidence interval width for the estimation of specificity obtained from the study (10%) and P is the expected prevalence of PBC among the included individuals (50%), the sample size for specificity (Nsp) is given by the following expression:
FP + TN = Z2 × SP × (1 – SP)/W2
Nsp = (FP + TN)/(1 – P)
This gave us a minimum sample size of 69 participants. Estimating an additional 20%, 86 participants were included.
Baseline characteristics were compared between cases and controls using t-tests for continuous variables and chi-squared tests for categorical variables. The sensitivity, specificity, and predictive values for PBC diagnosis were calculated for each antibody and their relevant combinations, considering liver biopsy as the gold standard. Additionally, we plotted receiver-operating characteristic (ROC) curves for titers of AMA, ASMA, and ANA. The association between antibody positivity and the presence of fibrosis was assessed using odds ratio (OR) calculated with univariate logistic regression. All statistical analyses were two-tailed and performed at a 5% significance level (alpha = 0.05) using IBM SPSS, version 20.0.
We studied 85 female adults, 43 with PBC and 42 controls. Mean age at diagnosis was 58.7 years for cases and 56.9 years for controls. Mean body mass index was lower among cases (P = 0.001), perhaps because a suspicion of nonalcoholic steatohepatitis was the indication for liver biopsy in many controls (Table 1). As expected, alkaline phosphatase was significantly higher among cases, but markers of hepatocellular damage were similar in both groups. Pruritus was the most common symptom in participants with PBC. Fibrosis in stages II-IV of the Batts-Ludwig classification was much more common in cases (39.5% vs 16.7%). In both groups, the most frequent pattern of ANA was centromeric. Most cases received ursodeoxycholic acid (UDCA) while most controls received vitamin E. Sjögren syndrome and Hashimoto’s thyroi
| Controls | Cases | P value | |
| Age at diagnosis | 58.7 ± 11.4 | 56.9 ± 10.2 | 0.44 |
| Body mass index | 26.8 ± 3.4 | 23.8 ± 2.4 | 0.001 |
| Total cholesterol (mg/dL) | 221 ± 52.5 | 221 ± 45.3 | 0.98 |
| LDL cholesterol (mg/dL) | 125 ± 40.2 | 124.8 ± 41.9 | 0.99 |
| HDL cholesterol (mg/dL) | 54.1 ± 28.7 | 58.5 ± 10.6 | 0.48 |
| Triglycerides (mg/dL) | 157 ± 64.6 | 134 ± 54.8 | 0.16 |
| Fold increment of ALKP above ULN | 1.82 ± 1.55 | 2.84 ± 2.06 | 0.013 |
| Fold increment of ALT above ULN | 2.27 ± 2.94 | 2.23 ± 1.97 | 0.94 |
| Fold increment of AST above ULN | 2.08 ± 3.37 | 2.06 ± 1.6 | 0.97 |
| Fold increment of GGT above ULN | 4.43 ± 3.57 | 6.74 ± 6.78 | 0.087 |
| Bilirubin (mg/dL) | 0.68 ± 0.42 | 1.5 ± 1.9 | 0.007 |
| Albumin (g/L) | 4.09 ± 0.32 | 4.05 ± 0.36 | 0.75 |
| International normalized ratio | 0.99 ± 0.09 | 1.0 ± 0.13 | 0.56 |
| Ascites, n (%) | 2 (4.8) | 3 (7) | 0.66 |
| Pruritus, n (%) | 12 (28.6) | 20 (46.5) | 0.08 |
| Degree of fibrosis, n (%)1 | |||
| 0 | 21 (50) | 18 (41.9) | 0.031 |
| 1 | 14 (33.3) | 8 (18.6) | |
| 2 | 1 (2.4) | 11 (25.6) | |
| 3 | 4 (9.5) | 5 (11.6) | |
| 4 | 2 (4.8) | 1 (2.3) | |
| Pattern of anti-nuclear antibodies, n (%) | |||
| Peripheral | 0 (0) | 1 (2.3) | 0.37 |
| Homogeneous | 0 (0) | 0 (0) | |
| Gross speckled | 0 (0) | 0 (0) | |
| Fine speckled | 0 (0) | 2 (4.7) | |
| Centromeric | 9 (21.4) | 12 (27.9) | |
| Nucleolar | 2 (4.8) | 2 (4.7) | |
| Laminar | 0 (0) | 0 (0) | |
| Cytoplasmic | 6 (14.3) | 2 (4.7) | |
| Medications at diagnosis, n (%) | |||
| Ursodeoxycholic acid | 16 (38.1) | 37 (86.0) | < 0.001 |
| Vitamin E | 15 (35.7) | 3 (7.0) | 0.001 |
| Statins | 6 (14.3) | 2 (4.7) | 0.13 |
| Antidiabetics | 5 (11.9) | 1 (2.3) | 0.085 |
| Immunosuppressors | 11 (26.2) | 13 (30.2) | 0.68 |
| Levothyroxine | 15 (35.7) | 15 (34.9) | 0.94 |
| Autoimmune comorbidities, n (%) | |||
| Sjögren syndrome | 4 (9.5) | 9 (20.9) | 0.14 |
| Limited systemic sclerosis | 2 (4.8) | 4 (9.3) | 0.41 |
| Diffuse systemic sclerosis | 4 (9.5) | 2 (4.7) | 0.381 |
| Hashimoto´s thyroiditis | 5 (11.9) | 6 (14) | 0.78 |
| Graves´ disease | 2 (4.8) | 0 (0) | 0.15 |
| Systemic lupus erythematosus | 1 (2.4) | 0 (0) | 0.31 |
| Inflammatory bowel disease | 0 (0) | 0 (0) | - |
| Rheumatoid arthritis | 1 (2.4) | 4 (9.3) | 0.178 |
| Hemolytic autoimmune anemia | 1 (2.4) | 0 (0) | 0.31 |
| Autoimmune thrombocytopenic purpura | 1 (2.4) | 0 (0) | 0.31 |
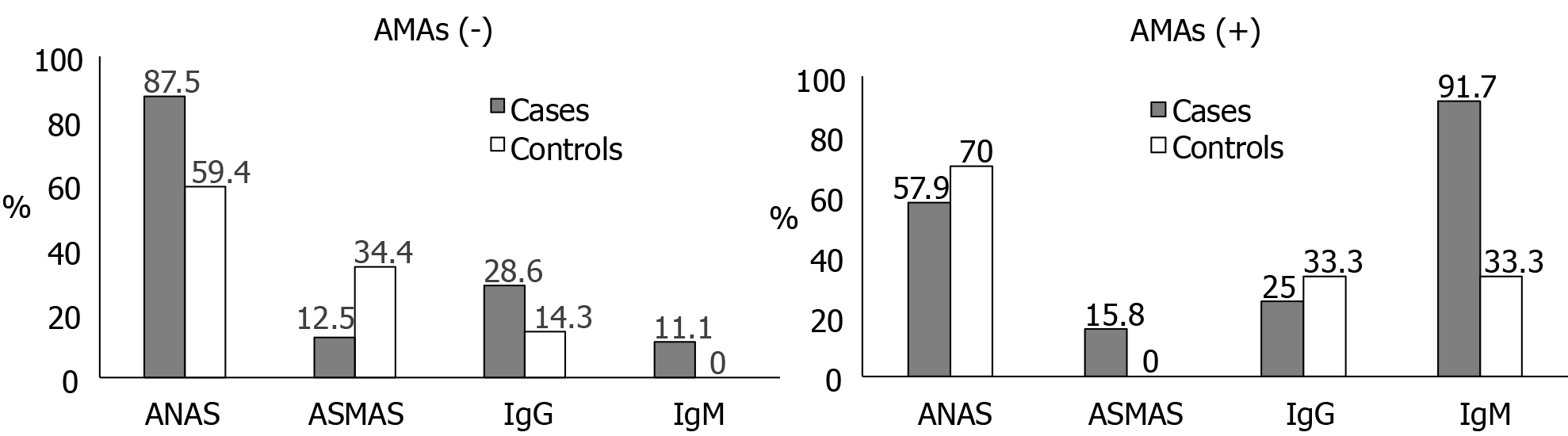
The prevalence of positivity for AMA in cases was surprisingly low, only 44.2%. Among participants with positive AMA, the most prevalent antibody was IgM, present in 91.7% of cases and 33.3% of controls. Meanwhile, among participants with negative AMA, the most prevalent antibodies were ANA, present in 87.5% of cases and 59.4% of controls. None of the AMA-negative controls had a positive IgM, while 11.1% of the AMA-negative cases did (Figure 1).
The diagnostic performance of AMA in our sample was lower than expected, with a sensitivity of 44.2% and a specificity of 76.2%. ANA showed better sensitivity (74.4%) but low specificity (38.1%). IgM had an acceptable balance between sensitivity (57.1%) and specificity (85.7%). Of note, the combination of positive AMA and positive ASMA had 100% specificity for confirmation of PBC. The combination of negative AMA and positive IgM exhibited 100% specificity and positive predictive value (Table 2).
| Antibody or antibody combination | Sensitivity | Specificity | PPV | NPV |
| AMA (+) | 44.2 | 76.2 | 65.5 | 57.1 |
| ANA (+) | 74.4 | 38.1 | 55.2 | 59.3 |
| ASMA (+) | 14.0 | 73.8 | 35.3 | 45.6 |
| IgG (+) | 26.7 | 80.0 | 66.7 | 42.1 |
| IgM (+) | 57.1 | 85.7 | 92.3 | 40.0 |
| AMA (+), ANA (+) | 25.6 | 83.3 | 61.1 | 52.2 |
| AMA (+), ANA (-) | 18.6 | 92.9 | 72.7 | 52.7 |
| AMA (-), ANA (+) | 48.8 | 54.8 | 52.5 | 51.1 |
| AMA (+), ASMA (+) | 7.0 | 100 | 100 | 51.2 |
| AMA (+), ASMA (-) | 37.2 | 76.2 | 61.5 | 54.2 |
| AMA (-), ASMA (+) | 7.0 | 73.8 | 21.4 | 43.7 |
| ANA (+), ASMA (+) | 9.3 | 83.3 | 36.4 | 47.3 |
| ANA (+) , ASMA (-) | 65.1 | 54.8 | 59.6 | 60.5 |
| ANA (-), ASMA (+) | 4.7 | 90.5 | 33.3 | 48.1 |
| AMA (+), IgG (+) | 13.3 | 90.0 | 66.7 | 40.9 |
| AMA (+), IgG (-) | 46.7 | 70.0 | 70.0 | 46.7 |
| AMA (-), IgG (+) | 13.3 | 90.0 | 66.7 | 40.9 |
| AMA (+), IgM (+) | 52.4 | 85.7 | 91.7 | 37.5 |
| AMA (+), IgM (-) | 4.8 | 71.4 | 33.3 | 20.0 |
| AMA (-), IgM (+) | 4.8 | 100 | 100 | 25.9 |
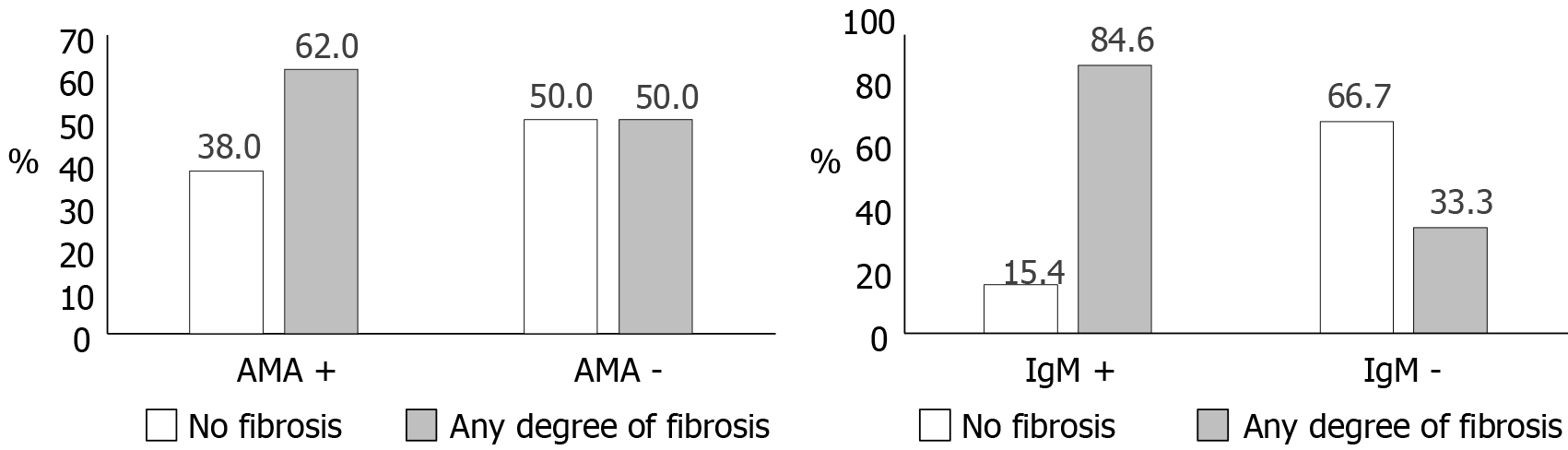
Fibrosis was more frequent among participants with positive AMA or IgM (Figure 2): Sixty-two percent of AMA-positive and 84.6% of IgM-positive individuals had any degree of fibrosis in biopsy. There was an equal proportion of persons with and without fibrosis among AMA-negative participants, while fibrosis was unusual (33.3%) when IgM was negative. Among cases only, the proportion with fibrosis was 63% for AMA-positive and 54% for AMA-negative individuals. The OR for the association between positive AMA and any fibrosis was not significant [OR:1.63, 95% confidence interval (CI): 0.67-4.09], while the association between positive IgM and fibrosis was strongly positive (OR:11.0, 95%CI: 1.73-69.9). Fibrosis was also differently associated with the predominant pattern in liver biopsy. The proportion of individuals with fibrosis was 80% for patients with lymphocytic infiltrate, 70.6% for patients with destructive non-suppurative cholangitis, 66.6% for patients with granulomatous cholangitis or florid lesions and 65% for patients with interphase hepatitis.
In separate exploratory ROC analyses of the plain reported titers of AMA and ANA, the best cutoff for AMA titers was 1:50, showing a sensitivity of 45.2%, a specificity of 81.1% and an area under the curve (C-statistic) of 66%. Meanwhile, the best cutoff for ANA titers was 1:120, with a sensitivity of 71.8%, a specificity of 52.5% and a C-statistic of 64.1%.
We performed a sensitivity analysis in which PBC status was defined as having a pathology diagnosis of PBC plus elevation of alkaline phosphatase. The results were very similar to those obtained with PBC status defined only by biopsy results, except for a slightly better negative predictive value for AMA (63.6 vs 57.1%) and slightly better sensitivity for IgM (63.2% vs 57.1%).
In this cross-sectional study, we assessed the performance of different autoimmunity markers for the diagnosis of biopsy confirmed PBC in the context of female Latin American patients. We were surprised to find that the majority of PBC cases were AMA-negative, and that no individual antibody or antibody pair provided a distinctly superior combination of sensitivity and specificity. Our results also suggested that IgM positivity can be a tool for the diagnosis of AMA-negative PBC and that IgM may serve as a marker of liver fibrosis. The results also show that simultaneous positivity for AMA and ASMA was a relevant confirmatory finding for PBC.
The clinical characteristics of our Hispanic patients regarding age, sex, symptoms, and liver function tests were in accordance with prior reports of PBC patients worldwide and in other Latin American countries[10-15]. Sjögren syndrome and Hashimoto´s thyroiditis were the most prevalent autoimmune comorbidities in our sample, as has been reported in Mexican[13] and Italian[16] PBC cohorts. The frequent coexistence of PBC and Sjögren syndrome could be explained by the exposure of self-antigens in biliary and salivary epithelial cells, as a result of apoptosis (secondary to infections or xenobiotics), leading to autoimmune epithelitis[17]. Among PBC patients, Hashimoto's thyroiditis is the most frequently reported thyroid disorder, followed by Graves´ disease[16]. However, a study comparing two European cohorts found that thyroid disease does not influence the rate of complications nor the natural history of PBC[18]. In general, the coexistence of thyroid and liver disease is usual, as thyroid disturbances are also frequent in primary sclerosing cholangitis and non-alcoholic fatty liver disease[19]. These findings highlight the need for autoimmune disease screening in Latin American patients with PBC.
The performance of AMA, ANA, ASMA, IgM, and IgG has been evaluated before, albeit seldom in Hispanics or Latin Americans. In a Mexican cohort of 78 PBC patients, 94.8% had positive AMA, 70.5% positive ANA and 8% positive ASMA[13]. This is in stark contrast to the rate of AMA positivity of cases in our study, only 44.2%. A meta-analysis of 24 studies (none of them from Latin America), reported 84.5% sensitivity and 97.8% specificity for AMA, also quite different from our results[20]. A meta-analysis of 11 studies from Asia, Europe, and North America reported 27% sensitivity and 98% specificity for ANA in AMA-negative patients. We, in turn, found a high rate of ANA positivity among AMA-negative cases (87%)[21]. In a group of AMA-negative PBC patients from the Mayo Clinic, there was a 21% rate of ASMA positivity[22], similar to the 12.5% we found. These findings suggest the presence of factors in our population that influence the diagnostic performance of the classic autoantibodies for histologically confirmed PBC diagnosis. One of such factors may be polymorphisms in genes for proteins involved in antigen presentation at the bile ducts. It has been demonstrated that cholangiocytes express not only MHC class I molecules, but also surface markers found on antigen presenting cells including MHC class II and co-stimulatory molecules (CD80, CD86, CD40)[23]. Since the distribution of HLA alleles differs substantially across Latin America[24], it is conceivable that individuals from certain populations are better presenters of ductal self-antigens, and elicit a stronger humoral self-immunity in the context of PBC. This may constitute a potential explanation for the stark contrast between our results and those from the prior Mexican study.
The previously mentioned Mexican study also described Sjögren syndrome as the most common autoimmune comorbidity of PBC. In contrast to our results, they reported a high prevalence of AMA positivity among PBC cases, evidencing variability even within Latino populations. A cross-sectional study in the US found that Hispanic patients with PBC had a particular clinical picture, characterized by a reduced clinical response to UDCA[25].
Even though AMA are the antibodies classically related to PBC, there is evidence that most patients with positive AMA do not have PBC findings in liver biopsy[26,27]. More than half of PBC cases in our sample were AMA-negative, much higher than in prior reports (5%-18%)[28]. Evidence that clinical characteristics or response to treatment is different in AMA-negative patients has been inconsistent[29]. Nonetheless, it is clear that diagnosis of these patients tends to be delayed, treatment is usually initiated later and complications like fibrosis or liver transplantation are more frequent[22,30,31]. A study found that seven of nine patients with AMA-negative PBC had the same immunohistochemistry findings as AMA-positive patients, supporting the idea that these subgroups have indeed the same disease[32]. One potential limitation of the use of AMAs in regular clinical practice is the availability of different techniques for their measurement, depending on the context (immunofluorescence vs ELISA), which may yield different diagnostic performance.
The utility of IgM in the study of PBC is being explored intensively, especially in the context of AMA-negative patients[33]. In our sample, the combination of negative AMA and positive IgM was highly specific for PBC. We also found that > 90% of AMA-positive cases had positive IgM. All things considered, IgM may be understood not only as a consequence of cholestasis, but also as a sign of alarm to look for fibrosis in PBC patients, even when AMA are negative. The mechanism underlying the high frequency of IgM positivity in AMA-positive PBC patients may involve polymor-phisms in the gene for toll-like receptor 9[34].
Strengths of the study include a homogeneous sample of well-defined patients and controls of the same sex, the availability of relevant information on comorbidities and current treatments, the fact that PBC status was confirmed by biopsy and the uniqueness of the patient population ethnicity. This study is a valuable source of demographic and diagnostic information for Hispanic/Latino patients with suspected PBC.
The main limitations are the cross-sectional nature of the study, which does not allow drawing definitive conclusions about causal associations, and its relatively modest sample size, although it was appropriate for the diagnostic evaluation of the tests analyzed. Future studies are needed to describe the time-course and severity of the disease, in order to better approach PBC diagnosis and treatment in these populations.
In summary, this study showed that a large proportion of PBC cases in Latin American patients can be AMA-negative, and that IgM can be a useful marker of fibrosis, in addition to cholestasis. For all these reasons, it is urgent to find a good marker for AMA-negative PBC, especially for Hispanic/Latino populations.
Primary biliary cholangitis (PBC) is a rare but serious and severely limiting autoimmune liver disease. Diagnosis of PBC is complicated by the different diagnostic performance of different autoantibodies in different populations.
There are limited data on the performance of autoantibodies for PBC diagnosis among Hispanic/Latino populations.
To assess the diagnostic performance of anti-mitochondrial antibodies (AMAs), anti-nuclear antibodies (ANAs), anti-smooth muscle antibodies (ASMAs), plasma immunoglobulin (Ig) M and plasma IgG for the diagnosis of histologically-confirmed PBC among female Colombian patients.
We studied 43 PBC cases and 42 controls in whom PBC was ruled out. All antibodies were measured using immunofluorescence or enzyme-linked immunosorbent assay within 1 year of the index biopsy. A sensitivity analysis was performed using pathology + elevated alkaline phosphatase to define case-control status. Patients with viral or alcoholic hepatitis were excluded.
The sensitivity of ANA was only 44.2%, specificity was 76.2%. No individual antibody or antibody combination displayed an acceptable combination of sensitivity and specificity, but the combination of positive AMA and positive ASMA had a very high specificity. IgM had particularly high specificity and positive predictive value.
We found a remarkably high prevalence of AMA-negative PBC among female Colombian patients. IgM served not only as a marker of PBC, but also as a marker of liver fibrosis.
It is urgent to find a good marker for AMA-negative PBC, especially for Hispanic/Latino populations.
We want to thank the Office of the Vice Provost for research (Vicerrectoría de Investigaciones) at Universidad de los Andes, their continued support throughout the development of this project.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Colombia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kato T S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LL
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 908] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 2. | Pinzani M, Luong TV. Pathogenesis of biliary fibrosis. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1279-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Heathcote EJ. Primary biliary cirrhosis: historical perspective. Clin Liver Dis. 2003;7:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Jepsen P, Grønbæk L, Vilstrup H. Worldwide Incidence of Autoimmune Liver Disease. Dig Dis. 2015;33 Suppl 2:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Lee CW, Ronnekleiv-Kelly S. Autoimmune Diseases of the Biliary Tract: A Review. Surg Clin North Am. 2019;99:185-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 922] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 7. | Sebode M, Weiler-Normann C, Liwinski T, Schramm C. Autoantibodies in Autoimmune Liver Disease-Clinical and Diagnostic Relevance. Front Immunol. 2018;9:609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Yamagiwa S, Kamimura H, Takamura M, Aoyagi Y. Autoantibodies in primary biliary cirrhosis: recent progress in research on the pathogenetic and clinical significance. World J Gastroenterol. 2014;20:2606-2612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Jones SR, Carley S, Harrison M. An introduction to power and sample size estimation. Emerg Med J. 2003;20:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 415] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 10. | Marzioni M, Bassanelli C, Ripellino C, Urbinati D, Alvaro D. Epidemiology of primary biliary cholangitis in Italy: Evidence from a real-world database. Dig Liver Dis. 2019;51:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Cheung KS, Seto WK, Fung J, Lai CL, Yuen MF. Epidemiology and Natural History of Primary Biliary Cholangitis in the Chinese: A Territory-Based Study in Hong Kong between 2000 and 2015. Clin Transl Gastroenterol. 2017;8:e116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Marschall HU, Henriksson I, Lindberg S, Söderdahl F, Thuresson M, Wahlin S, Ludvigsson JF. Incidence, prevalence, and outcome of primary biliary cholangitis in a nationwide Swedish population-based cohort. Sci Rep. 2019;9:11525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | González-Huezo MS, Delgado-Ayala LY, Osorio-Núñez AL, Meléndez-Mercado C. Autoimmune associations in a Mexican cohort with primary biliary cholangitis. Rev Gastroenterol Mex. 2019;84:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Melchor-Mendoza YK, Martínez-Benítez B, Mina-Hawat A, Rodríguez-Leal G, Duque X, Moran-Villota S. Ursodeoxycholic Acid Therapy in Patients with Primary Biliary Cholangitis with Limited Liver Transplantation Availability. Ann Hepatol. 2017;16:430-435. [PubMed] |
| 15. | Valera M JM, Smok S G, Poniachik T J, Oksenberg R D, Silva P G, Ferrario B M, Buckel G E, Brahm B J. [Primary biliary cirrhosis: a thirteen years experience]. Rev Med Chil. 2006;134:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Floreani A, Franceschet I, Cazzagon N, Spinazzè A, Buja A, Furlan P, Baldo V, Gershwin ME. Extrahepatic autoimmune conditions associated with primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 17. | Selmi C, Meroni PL, Gershwin ME. Primary biliary cirrhosis and Sjögren's syndrome: autoimmune epithelitis. J Autoimmun. 2012;39:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Floreani A, Mangini C, Reig A, Franceschet I, Cazzagon N, Perini L, Caballería L, Cocchio S, Baldo V, Parés A. Thyroid Dysfunction in Primary Biliary Cholangitis: A Comparative Study at Two European Centers. Am J Gastroenterol. 2017;112:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Silveira MG, Mendes FD, Diehl NN, Enders FT, Lindor KD. Thyroid dysfunction in primary biliary cirrhosis, primary sclerosing cholangitis and non-alcoholic fatty liver disease. Liver Int. 2009;29:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Hu S, Zhao F, Wang Q, Chen WX. The accuracy of the anti-mitochondrial antibody and the M2 subtype test for diagnosis of primary biliary cirrhosis: a meta-analysis. Clin Chem Lab Med. 2014;52:1533-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Zhang Q, Liu Z, Wu S, Duan W, Chen S, Ou X, You H, Kong Y, Jia J. Meta-Analysis of Antinuclear Antibodies in the Diagnosis of Antimitochondrial Antibody-Negative Primary Biliary Cholangitis. Gastroenterol Res Pract. 2019;2019:8959103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Juliusson G, Imam M, Björnsson ES, Talwalkar JA, Lindor KD. Long-term outcomes in antimitochondrial antibody negative primary biliary cirrhosis. Scand J Gastroenterol. 2016;51:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Chuang YH, Lan RY, Gershwin ME. The immunopathology of human biliary cell epithelium. Semin Immunopathol. 2009;31:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Arrieta-Bolaños E, Madrigal JA, Shaw BE. Human leukocyte antigen profiles of latin american populations: differential admixture and its potential impact on hematopoietic stem cell transplantation. Bone Marrow Res. 2012;2012:136087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Levy C, Naik J, Giordano C, Mandalia A, O'Brien C, Bhamidimarri KR, Schiff ER, Martin P. Hispanics with primary biliary cirrhosis are more likely to have features of autoimmune hepatitis and reduced response to ursodeoxycholic acid than non-Hispanics. Clin Gastroenterol Hepatol. 2014;12:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Tan S, Ayutyanont N, Bhattarai B, Movahedi Z, Jayaram L, Gish R, Nadir A. Clinical characteristics of antimitochondrial antibody-positive patients at a safety net health care system in Arizona. BMJ Open Gastroenterol. 2017;4:e000158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 27. | Berdichevski T, Cohen-Ezra O, Pappo O, Ben-Ari Z. Positive antimitochondrial antibody but normal serum alkaline phosphatase levels: Could it be primary biliary cholangitis? Hepatol Res. 2017;47:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Chascsa DM, Lindor KD. Antimitochondrial Antibody-Negative Primary Biliary Cholangitis: Is It Really the Same Disease? Clin Liver Dis. 2018;22:589-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Tan XY, Miao Q, Chen XY. [Clinicopathological analysis of anti-mitochondrial antibody negative primary biliary cholangitis-autoimmune hepatitis overlap syndrome]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Liu B, Shi XH, Zhang FC, Zhang W, Gao LX. Antimitochondrial antibody-negative primary biliary cirrhosis: a subset of primary biliary cirrhosis. Liver Int. 2008;28:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Sakauchi F, Mori M, Zeniya M, Toda G. Antimitochondrial antibody negative primary biliary cirrhosis in Japan: utilization of clinical data when patients applied to receive public financial aid. J Epidemiol. 2006;16:30-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Tsuneyama K, Van De Water J, Van Thiel D, Coppel R, Ruebner B, Nakanuma Y, Dickson ER, Gershwin ME. Abnormal expression of PDC-E2 on the apical surface of biliary epithelial cells in patients with antimitochondrial antibody-negative primary biliary cirrhosis. Hepatology. 1995;22:1440-1446. [PubMed] |
| 33. | Hirschfield GM, Dyson JK, Alexander GJM, Chapman MH, Collier J, Hübscher S, Patanwala I, Pereira SP, Thain C, Thorburn D, Tiniakos D, Walmsley M, Webster G, Jones DEJ. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018;67:1568-1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 222] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 34. | Kikuchi K, Lian ZX, Kimura Y, Selmi C, Yang GX, Gordon SC, Invernizzi P, Podda M, Coppel RL, Ansari AA, Ikehara S, Miyakawa H, Gershwin ME. Genetic polymorphisms of toll-like receptor 9 influence the immune response to CpG and contribute to hyper-IgM in primary biliary cirrhosis. J Autoimmun. 2005;24:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |