Published online Aug 7, 2021. doi: 10.3748/wjg.v27.i29.4862
Peer-review started: March 7, 2021
First decision: April 17, 2021
Revised: April 19, 2021
Accepted: July 19, 2021
Article in press: July 19, 2021
Published online: August 7, 2021
Processing time: 150 Days and 0.7 Hours
Sarcopenia is becoming a well-established player in evaluating patients with chronic liver disease. Data regarding its clinical significance and consequences in the course of liver disease have been growing; many of the data support the idea that it impacts decompensation event frequency, prolonged hospitalization, and mortality, as well as providing the possibility to better prioritize patients on lists awaiting liver transplantation. When assessing the whole clinical scope of the field, which includes malnutrition and frailty, as well as the complete spectrum of muscle mass, strength, and function, it becomes clear that a well-founded app
Core Tip: Knowledge regarding the influence of sarcopenia in the course of chronic liver disease has greatly expanded in the past ten years, especially with respect to cirrhosis. Data show that it has a great influence on disease decompensation and patient mortality, providing clues for the development of newer evaluation modalities and sarcopenia indices. Nonetheless, data regarding the therapeutic consequences and interventions remain scarce. This article attempts to summarize the current state of knowledge of this important clinical topic with a critical evaluation of some related groundbreaking studies.
- Citation: Hari A. Muscular abnormalities in liver cirrhosis. World J Gastroenterol 2021; 27(29): 4862-4878
- URL: https://www.wjgnet.com/1007-9327/full/v27/i29/4862.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i29.4862
Sarcopenia is an important part of the medical evaluation and treatment for chronic liver disease, especially in the advanced stage of the disease (i.e., cirrhosis), not only due to the very frequent coexistence of both medical entities but also because of its impact on the related clinical outcomes. This article focuses on the clinical aspects and consequences of sarcopenia in liver cirrhosis, common diagnostic procedures, and suggestions for clinical therapy. A review of the presented field in the scientific literature is provided, with the intentional omission of pathophysiology, for better transparency, clinical applicability, and an attempt to critically evaluate the most recent study findings.
To facilitate the understanding of common medical terms, a short paragraph summarizing their definitions is presented (see also Figure 1), followed by a brief description of the pathophysiological processes involved. For a more detailed review of the pathophysiology of sarcopenia in liver cirrhosis-especially regarding the po
Malnutrition is defined as a measurable change in physical and mental functions secondary to altered body composition and cell mass, resulting in an impaired quality of life and poor clinical outcomes. It is the consequence of insufficient protein and energy supplies.
It is well admitted that malnutrition participates in the onset of sarcopenia but the link between these two nutritional concepts remains confusing[5].
Sarcopenia is defined as the loss of muscle mass, muscle strength, and reduced physical function.
Frailty is a condition of increased vulnerability to endogenous and/or exogenous stressors associated with physiological decline.
Myosteatosis is pathological fat accumulation in muscles.
Sarcopenia is often equated as a state of advanced malnutrition. The condition is associated with complications such as poor mobility and quality of life and increased mortality. Muscle loss in sarcopenia is not solely at the expense of muscle atrophy, it also involves replacing muscle cells with fat and connective tissue. Protein supply in a sarcopenic patient is low due to chronic muscle degradation which is most clearly manifested during metabolic stress when muscle proteins should be mobilized very quickly to provide the body with amino acids for the liver, gut and immune system function. Sarcopenia in patients with liver cirrhosis is connected to age-related decline in muscle mass and to malnutrition. Studies show that malnutrition develops due to negative energy balance, loss of appetite, rapid satiety and poor food absorption. Concurrent illnesses or addictions (bacterial growth in the gut, pancreatic exocrine insufficiency, alcoholism) exacerbate the condition. Iatrogenic losses include ascites paracentesis and use of laxatives and diuretics. Frequent endoscopic imaging and laboratory examinations also affect diet and weight loss as well as sarcopenia. Therefore, liver cirrhosis is a state of accelerated starvation. In liver cirrhosis, cytokines that stimulate catabolic processes in the body are released. Due to the low glycogen stores, energy production is directed towards the breakdown of fatty acids. Gluconeogenesis is mainly pursued through amino acids breakdown, which worsens protein loss. Advancement of the state is also exacerbated by growth hormone and testoste
To facilitate the clinical application and evaluation of sarcopenia tests, The European Working Group on Sarcopenia and Older People provided consensus criteria for the diagnosis of sarcopenia using muscle mass, muscle strength, and muscle function as a practical clinical definition. In 2019, a revised definition was published. Pre-sarcopenia is defined as the presence of low muscle strength without its impact on muscle mass/quality or muscle function. Sarcopenia is defined as a low muscle strength with additional low muscle mass or quality. For severe sarcopenia, muscle mass, muscle strength, and muscle function needed to be impaired. As can be seen from the definition, muscle mass does not condition muscle function or strength. The end result of the sarcopenia process is a decline in muscle functional abilities assessed by frailty[6].
To assess frailty, the 5 Fried’s phenotypes of frailty as cited by Sinclair[7] are used. While patients with 3 or more phenotypes are defined as frail, patients with 1 or 2 phenotypes are defined as prefrail, and those with no phenotype as robust[7]. The concomitant presence of sarcopenia and obesity is significantly associated with prefrailty and frailty, especially in women with cirrhosis[8]. According to a well-defined description by Buchard et al[5], “sarcopenia [is] not identical to frailty but there is a major overlap between definitions and diagnosis criteria of the two pheno
In a well-designed study, a group of authors led by Traub et al[8] evaluated the applicability of the latest definition of sarcopenia in patients with cirrhosis. Computed tomography (CT) examination was used to assess muscle mass, hand grip test to assess muscle strength, and 4 m gait speed test to assess muscle function. Compared to the 2019 definition, they observed that the 2010 definition identified more sarcopenia cases in male patients. In patients with cirrhosis, muscle strength seems to be preserved longer, while muscle mass is already reduced, leading to a significant difference in sarcopenia diagnosis rates when using the 2019 definition. However, the gender imbalance seen in the 2010 definition seemed to be less pronounced with the 2019 definition. They objected to the lack of data whether the 2010 or 2019 criteria is better to predict clinical complications, poorer prognoses, and the effect of specific inter
Sarcopenia is present in 30% to 70% of patients with cirrhosis, probably less frequently in the population of patients with metabolic liver disease. The prevalence increases with the disease stage and increases sharply before liver transplantation[7,9]. Based on the Child–Pugh score, the annual rate of decrease in skeletal muscle mass is 1.3% in Child–Pugh A patients, 3.5% in Child–Pugh B patients, and 6.1% in Child–Pugh C patients[10]. The risk of developing sarcopenia is associated with male gender, ascites, and the degree of renal and hepatic dysfunction[9]. Overweight and obesity are as frequent as in general population, ranging from 20% to 40% and aggravating prognosis both in compensated and in decompensated cirrhosis[11]. Sarcopenic obesity (coexistence of sarcopenia and obesity) is present in one-fifth to one-third of patients with cirrhosis. It is determined by using an assessment of sarcopenia to which an assessment of obesity using a body mass index (BMI ≥ 25 or ≥30 kg/m2) is added. BMI corrected for ascites is probably the most practical to use (see below under the Anthropometry section), although it may shift patients to a lower BMI grade[12]. Myosteatosis occurs in up to 50% of patients with cirrhosis and can be identified in both sarcopenic and non-sarcopenic patients with or without obesity. It meditates inflammatory responses and has been associated with lower muscle fun
The main clinical consequences of sarcopenia are increased mortality per se, increased mortality from systemic bacterial infection, the occurrence of HE after transjugular intrahepatic portosystemic shunt (TIPSS) insertion, and the more frequent occurrence of acute on chronic liver failure. Regardless of the underlying disease, it has a significant effect on mortality in patients with HCC[9]. Ebadi et al[3] point out that it would be important to define strategies maintaining muscle mass in candidates prior to the TIPSS insertion. A study by Al-Azzawi et al[14] confirmed that the exis
In the group of patients awaiting liver transplantation, sarcopenia can affect re
Given the above-mentioned situation, it is not surprising that there are calls for the inclusion of sarcopenia among the factors of transplantation priority [Model for End-Stage Liver Disease (MELD) – score vs sarcopenia-MELD score]. A thorough study in this area evaluated patients on a transplant waiting list. To define sarcopenia, CT measurements with proposed cut-off values were used (see below). In this cohort of European patients, they demonstrated the sarcopenia impact on the increased morta
From a clinical point of view, it is important whether sarcopenia is worth assessing in the group of patients with cirrhosis and HCC. Sarcopenia probably affects the recurrence or progression of HCC in patients who are candidates for liver transplan
In recent years, it has been pointed out that sarcopenia is a systemic disease. Cardiac sarcopenia likely manifests as a heart failure with preserved ejection fraction[1]. Relationships between heart failure and depleted lean muscle mass are indisputable and go both ways. Increased mortality in sarcopenic patients could be partly explained by this bilateral effect, especially in the posttransplant period. Cardiac ultrasound (US) is suggested to assess cardiac function in a sarcopenic patient with cirrhosis[22]. Involvement of the diaphragm leads to reduced peak cough flow in the elderly, increased rate of respiratory infections through impaired airway clearance and diffi
Questionnaires take precedence over other tests because of their relatively short evaluation time and their possibility to monitor the condition in a dynamic timely manner[7]. Across the investigative options to assess malnutrition, Subjective Global Assessment (SGA) is the most frequently mentioned. Features of the SGA include a physical exam component that evaluates the loss of subcutaneous fat, peripheral or sacral edema, and muscle wasting. The quantity of muscle and subcutaneous tissue is graded subjectively by the examiner who then categorizes it as normal, mildly, moderately, or severely decreased. Multiple components on patient history are also evaluated. The first component is the amount of weight loss in the previous 6 mo. Supplementary historical features of the SGA include patient’s dietary intake and the presence of gastrointestinal symptoms experienced daily for at least 2 wk. Once the history and physical examination sections are completed, patients are classified as well nourished (SGA grade A), moderately malnourished or suspected of being malnou
Nutritional Risk Screening 2002 (NRS-2002), Liver Disease Undernutrition Scree
The NRS-2002 is a nutrition screening tool recommended by the ESPEN guidelines[17]. It includes three components―the nutritional score (BMI, weight loss, and dietary intake included), the disease severity score, and the age score (age > 70 years)[17,23]. Patients are classified as having no or low risk.
The LDUST assesses 6 factors that were identified as having the strongest associations with malnutrition in patients with chronic liver disease (nutrient intake, weight loss, loss of subcutaneous fat, loss of muscle mass, fluid accumulation, and a decline in functional status). The three potential patient responses are labeled and indicated as no signs of malnutrition, “mild to moderate” malnutrition, and “moderate to severe” malnutrition[24].
The RFH-NPT is a nutrition screening tool developed in the United Kingdom. It includes three major steps: (1) Patients who have alcoholic hepatitis or are undergoing tube feeding are immediately evaluated as high risk without proceeding to the next step; (2) Patients who do not have alcoholic hepatitis and are not undergoing tube feeding are assessed for fluid overload and its impact on food intake and weight loss; and (3) Patients who do not have fluid overload are assessed for nutritional status (BMI, unplanned weight loss, and daily dietary intake). Patients are stratified as being at low, moderate, or high risk[2,25].
The MUST includes three categories: Current BMI, unintentional weight loss, and the presence of any acute disease that could compromise nutritional intake for more than 5 d[25].
A large study assessed the importance of RFH-NPT in the Asian population with predominantly viral liver cirrhosis. The questionnaire proved to be useful in the group of patients with a low MELD score and for assessing the prognosis of the disease. The disadvantage of the study is that different questionnaires are only compared with each other, and with only basic laboratory and anthropometric parameters[25]. In a recent publication, Buchard et al[5] noted that RFH-NPT and LDUST, despite recommendations for their use in patients with liver cirrhosis, have not yet been associated with clinical issues such as survival and complications appearance. Moreover, LDUST is based on the patient’s statement and lacks objective data.
Myostatin is a natural muscle growth inhibitor. As a marker, it depends on gender and inflammatory processes in the body. In men with liver cirrhosis, it can be used to assess muscle mass, prognosis of decompensation events, and fit for surgery status. In contrast to Oshida et al[27] who came to different conclusions in the group of patients with compensated disease, the cited study used CT sarcopenia indices in a large group of patients with decompensated advanced chronic liver disease (dACLD). The limitation of the test is the decrease in the level of serum myostatin at a very low muscle mass stage which presents a major problem when defining possible cut-off values[26].
Another potential biomarker is irisin, a myokine, mainly expressed and secreted by skeletal muscles as well as functioning as an adipokine. A significant lower irisin level is proved to be a marker for muscle weakness and atrophy. In the group of patients with dACLD, the cited study demonstrated higher irisin levels in women without evidence of an association with irisin levels and the degree of hepatic impairment according to CHILD/MELD score, or the presence of ascites. Sarcopenia was assessed using a hand grip test and CT assessment of muscle mass[2]. In a related publication, a group led by Zhao et al[10] studied irisin levels in a group of patients with liver cirrhosis where a hand grip test and CT-assessed muscle mass were also used for the evaluation of sarcopenia. The difference between the studies was in the CT index used, as the latter used the proposed gold standard (see below). Lower irisin levels were demonstrated in the group of patients with a higher CHILD score. They also defined that it is not entirely clear whether this is the cause or the consequence of sarcopenia. Deficiency of the article are poorly defined criteria for the diagnosis of liver cirrhosis[10].
If muscles are damaged by diseases or vigorous exercise, titin is decomposed by proteolytic enzymes, and various titin fragments are detected in serum and urine. One of the isolated fragments is titin-N. The cited study defined its urinary excretion in a group of patients with metabolic-induced fatty liver disease. The study had a well-defined control group, but poorly defined comparative indices (CT indices? muscle and liver elastography? US parameters of skeletal muscle assessment?) in the article itself. According to their observations, titin-N was negatively associated with the amount of muscle mass (higher level in urine correlated with lower skeletal muscle mass) and positively associated with the occurrence of muscle myosteatosis (higher level in urine correlated with higher levels of US-assessed skeletal muscle fat). They also identified an association of the biomarker with the degree of muscle fibrosis progression which may be associated with functional muscle decline. For the latter, a knee extension test and a hand grip test were used as a comparative test[27].
According to EASL guidelines, two simple criteria can be used in everyday clinical practice to stratify patients at high risk of malnutrition: being underweight (BMI < 18.5 kg/m2) and having advanced decompensated cirrhosis (Child C patients)[28]. In pa
Of the measurements that can be performed with an ordinary tape measure, the most common are mid-arm muscle circumference (MAMC), mid-arm muscle area, and triceps skinfold (TSF), all of which are simple and rapid to perform low-cost tests that are not affected by the presence of fluid retention[7,28]. Some Asian studies mention calf circumference which showed a good association to the frailty[29] in their study population and for the assessment of sarcopenia in the group of patients with compensated ACLD (cACLD), or in patients without ascites[30]. The disadvantage of both studies is the assessment of muscle mass with tests that are less useful in the overweight or hypervolemic patients’ population.
This group of tests can be performed at the bedside or during outpatient consultations.
The Short Physical Performance Battery consisting of three methods - balance test, gait speed test, and five chair stand test - can be used to assess the patient’s functional ability[2,7].
Handgrip strength test (HS) is the most commonly studied test in this group using a calibrated dynamometer. The test depends on the patient’s age and BMI. An interesting study has shown that HS in combination with the MELD score in men awaiting liver transplantation can be superior to CT modality if mortality was observed as a clinical outcome. Study results could be explained due to the early decline in muscle function even before the decline in muscle composition and mass occurs[31].
To assess muscle function, a test cited by Buchard et al[5] is commonly mentioned - the Liver Frailty Index (LFI). Its role is to evaluate frailty by combining HS, chair stands, and balance tests. Using a provided cut-off, LFI was associated with mortality independently of the presence of HE and ascites[5,7].
The next frequently used test is a six-minute walk test which has a sensitivity of 90% for identifying patients with increased risk for pre-LT mortality when performed at less than 250 m[1,7].
A walking speed/gait test is offered as a third option[2,32]. In a study by Nishikawa et al[32], a 6 m walking test was performed to measure muscle function in a group of patients with liver cirrhosis, with walking speed (WS) and gait speed (GS) defined, respectively. As a reference to define sarcopenia, bioelectrical impedance analysis (BIA) was used and therefore only patients without ascites were included. They observed that improvement in WS requires quick movement, whereas the impro
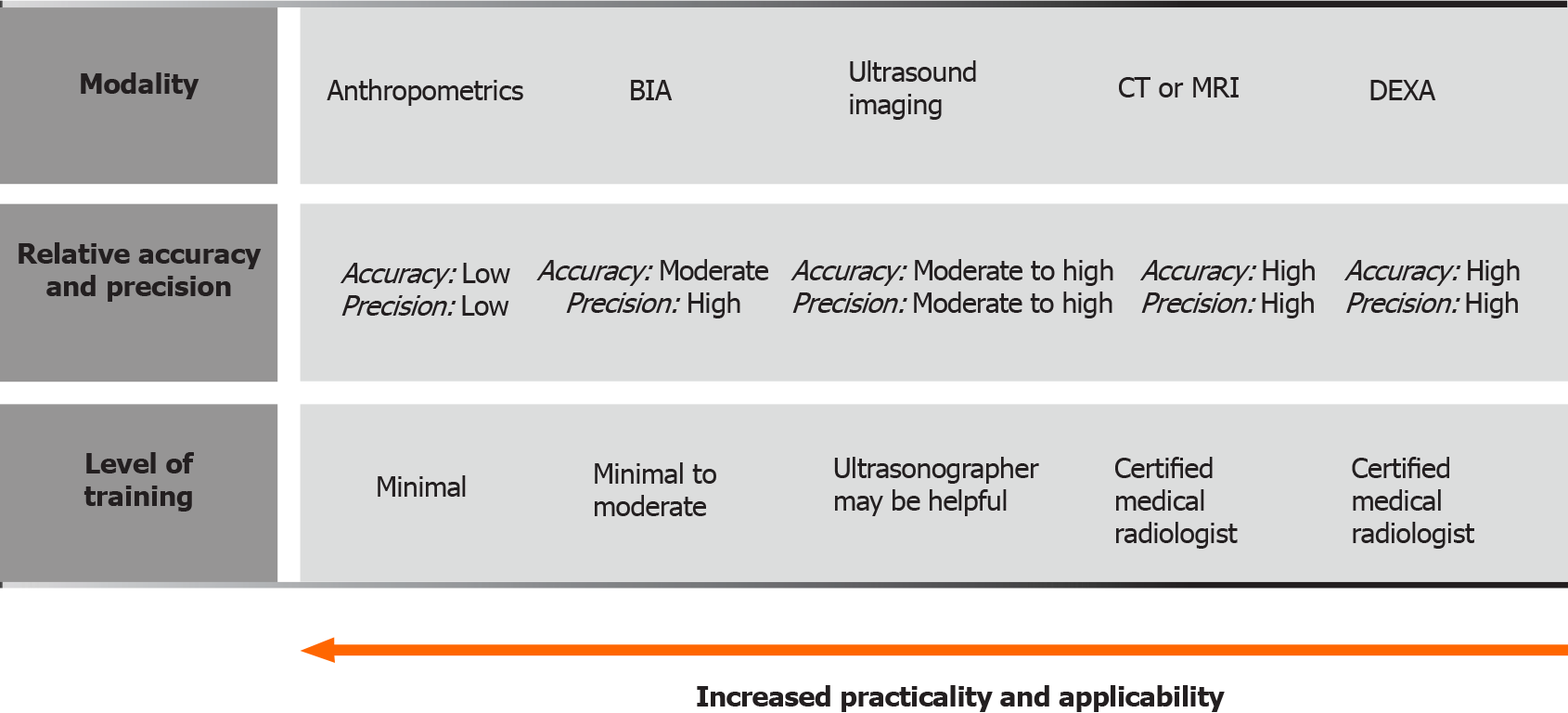
These diagnostic methods represent the foundation of modern clinical body compo
BIA: A fixed, low-voltage, high-frequency alternating current is introduced into the human body to assess body electrical conductivity together with resistance (impe
There are several parameters that can be measured by means of BIA: Body cell mass, total body water, extracellular water, extracellular mass, and body fat. Multifrequency BIA analysis has been proposed lately because it is less influenced by overhydration. It measures the above-mentioned parameters by passing a series of different electrical currents and electrical frequencies through the body. Segmental BIA can also be used to overcome the fluid retention bias[33].
PA was observed to be less affected by overhydration while being a reliable in
Dual energy x-ray absorptiometry: Dual energy x-ray absorptiometry (DEXA) uses low-dose x-rays to provide a comprehensive 3-dimensional analysis of the entire body, thus automatically breaking down each body compartment into bone mass, fat mass, and fat-free (or lean) mass. It is safe, inexpensive, readily available, and reproducible. In comparison to the CT scan, it uses less radiation. As with BIA, the problem of analysis may be water accumulation in muscle and fibrous tissue, especially in the elderly. Also, it is difficult to access in some medical centers[13,31,33].
In the group of patients with liver cirrhosis, it shows an association between muscle mass assessment and mortality[35]. Appendicular lean mass (APLM) has been pro
On the other hand, some studies report only a weak concordance between DEXA and CT when identifying sarcopenia in cirrhosis[24]. The rest state that DEXA indices show a sex-related distribution of body compartments. In cirrhotic women, more reduction in fat stores is observed with the maintenance of lean tissue. In men, the loss of lean tissue is the most featured early phenomenon. A described pattern is reflected by a weak association between muscle strength and muscle mass in cirrhotic women[33].
The following study in this area states the possibility of using proposed DEXA limb muscle mass indices which showed a good correlation compared to CT indices. The study was conducted in a small cohort of patients awaiting liver transplantation. They observed that fewer women were identified sarcopenic with DEXA than expected. They also cite the well-known DEXA deficiency as not being able to offer muscle structure quality determination compared to the CT examination[37].
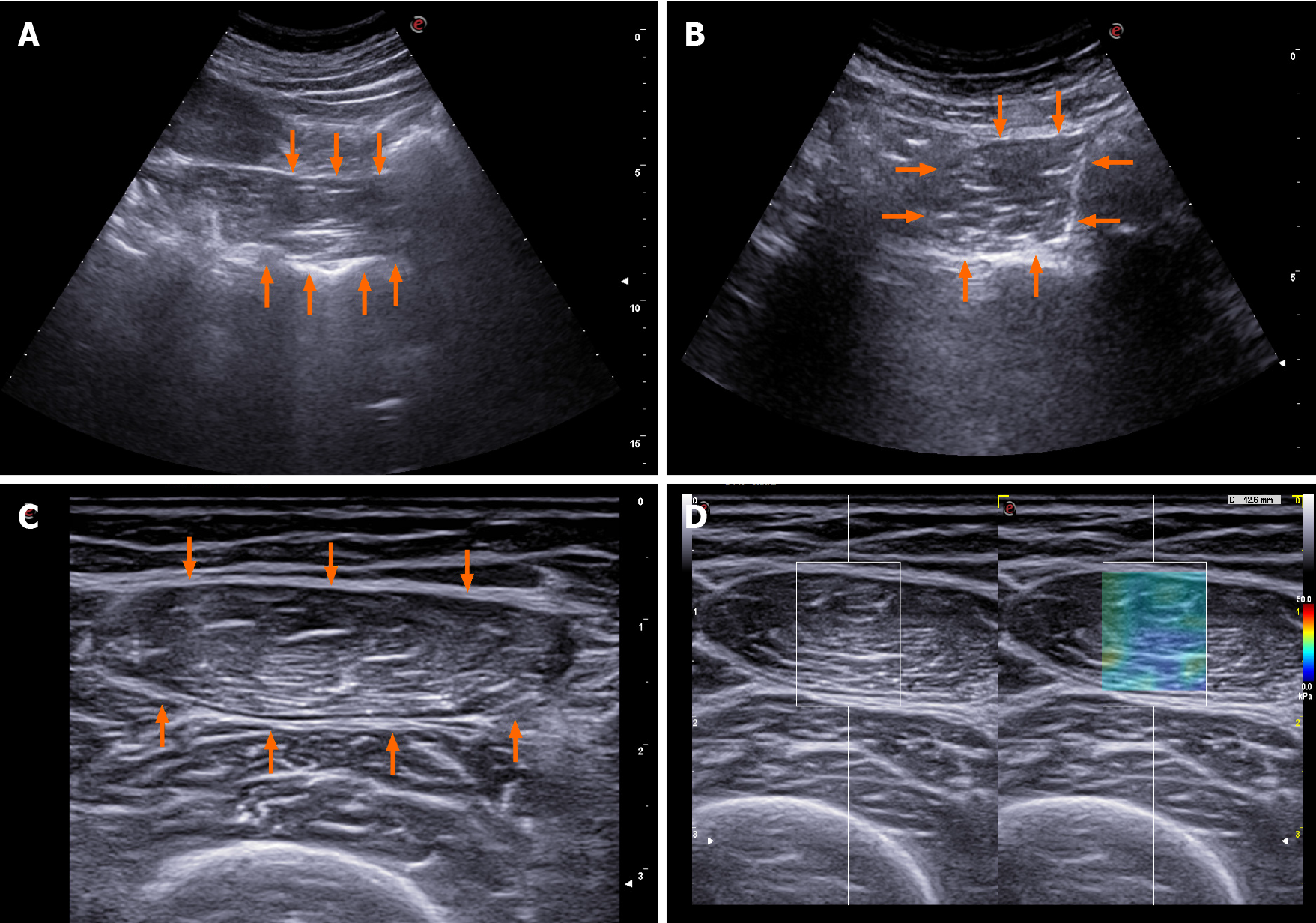
US: US can be used to evaluate echogenicity, diameter, cross-sectional area, and muscle volume. Performing three consecutive measurements and using their mean value as a final result is proposed. The patient should rest for at least 5 min before the measurement and not exercise less than 30 min before the examination. To assess the muscle contraction potential, muscle thickness can be measured before and after the movement, especially in muscles that have a significant change in diameter during contraction. The possibility of assessing microvascularization with contrast enhanced US and the assessment of pennation angle are mentioned. A special expert group for this field lists 39 muscle groups that can be evaluated using US examination. They propose standardizations of the measurement site and explore main problems of the investigative methodology that should be solved. The inability of assessing some muscle components with US is the most common problem. Other problems mentioned are visibility, dependency of the operator’s experience as well as the patient’s general condition impact on the measurement value, and its dependency on the equipment quality[38].
In this area, the quadriceps muscle evaluation, especially its thickness and quality, is most often cited in the literature. Pita et al[39] estimated a daily decline in muscle mass in patients with cirrhosis who were waiting for a liver transplant in the intensive care unit. In a relatively small cohort, CT-estimated muscle mass was used for comparison demonstrating the ability of the US to monitor muscle mass decline with daily measurements of rectus femoris muscle diameter, as well as the association between this result and mortality in the studied patients. Another frequently cited study from this area performed quadriceps diameter measurement and proposed a model that included BMI. It states that the US of quadriceps muscle is a low-cost, reliable, reproducible, and accurate estimate of muscle mass that can be completed at the bedside or in an outpatient clinical setting as well as repeated without concern of radiation exposure. Gender-specific nomograms that correlated well with CT control were suggested. The cohort of patients was small, but the comparability between the two operators was good[40].
Measurements of the tongue muscle thickness in the group of patients with liver cirrhosis showed an association with the CHILD and MELD score, and a distinctive difference between the group of healthy control group, but with no proven CT control correlation. The definition of liver cirrhosis in the article was relatively loose[41].
In a population of Japanese cirrhosis patients, Kobayashi et al[42] measured the area of the psoas muscle in the right groin area and balanced it by the square of body height. The examination was quick and performed at bedside in a large cohort of patients. The study showed a significant correlation between US and CT measure
In recent years, the use of elastography has been suggested to assess muscle stiffness which could indirectly point to the loss of muscle function. A well-structured study in a small cohort of patients showed an association between femoral muscle elastography results and frailty[44].
The US imaging of some of the above mentioned methods is showed in Figure 2.
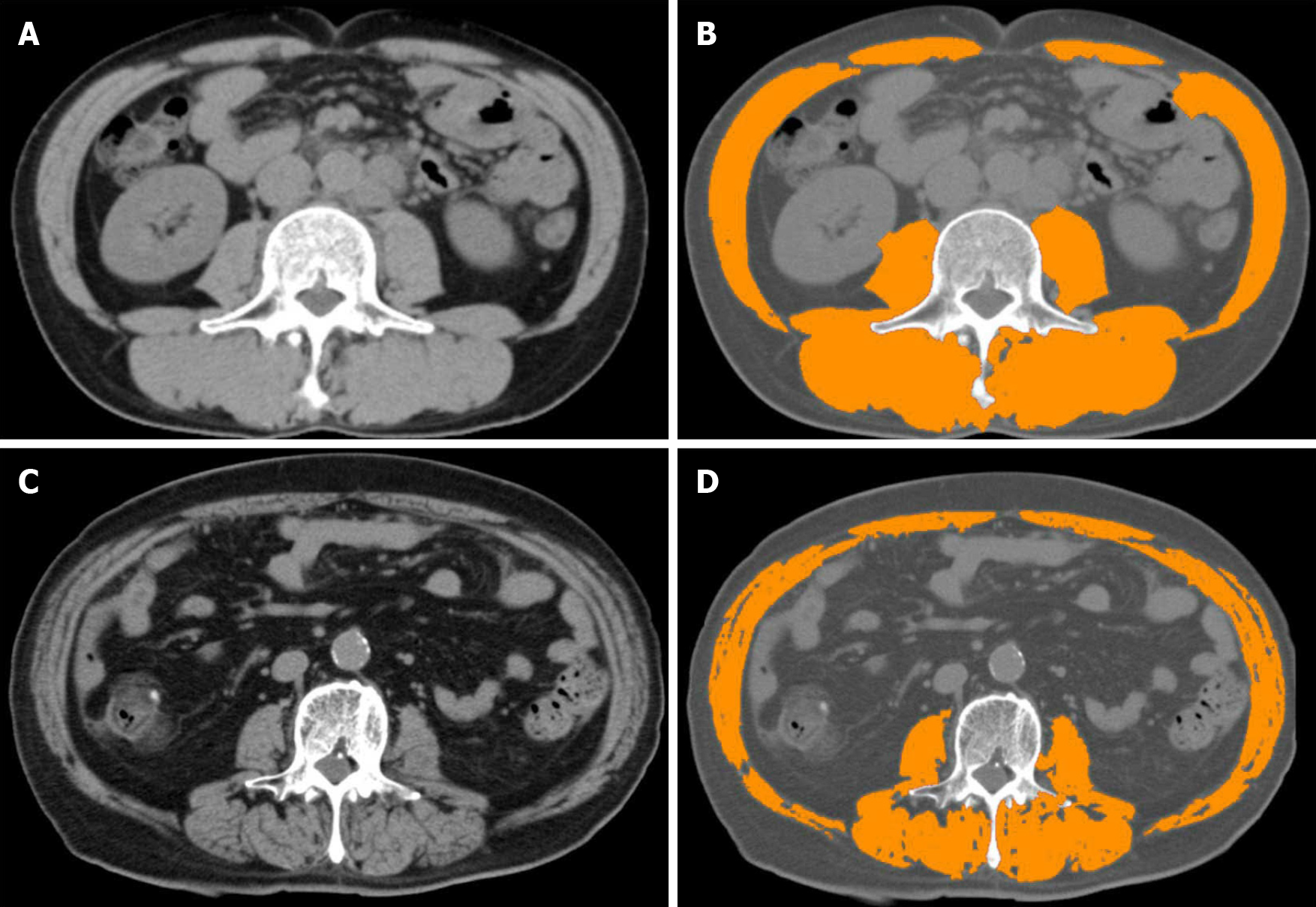
Cross-sectional imaging: CT: CT allows an accurate cross section assessment of muscle mass area and an estimate of tissue density using Hounsfield units (HU)[13]. It is recommended to use tissue that shows HU estimated density from -29 to +150 on two consecutive CT slices at L3 level. To make it more clinically applicable, it is suggested to use various computer programs which automatically and very reliably evaluate the muscle area (e.g., SliceOmatic, ImageJ, FatSeg, OsiriX)[7]. All these programs require manual image analysis by a radiologist. In this regard, some studies have tested the concept of a methodology to measure psoas muscle features on incidental CT scans using an automated, deep convolutional neural network model, a technology routinely used for facial recognition software. Automated measurement in the cirrhosis patient population proved to be comparable to that where the measu
The most common shortcomings of CT sarcopenia analysis are unclear possibility of recurrent/dynamic evaluation due to radiation exposure and the impact of the result on clinical decisions; unclear protocol of the examination method; radiation impact; examination costs; and the need for additional software to analyze images. In addition to the ability to differentiate three main body compartments, i.e., muscle, visceral, and subcutaneous adipose tissue, the ability to identify muscle radiodensity to determine ectopic fat accumulation in muscles, and the fact that it is probably not affected by the presence of ascites or edema, the pros are the relative ease, speed, accuracy, and accessibility of the examination in a hospital setting. There is a strong possibility of price and radiation exposure reduction to only 2.6 mSv by a single slice CT, and CT shows a very good reproducibility between different performers[7,19,31].
The test of choice is the L3 skeletal muscle index (SMI), a muscle area on a CT scan at the level of the L3 vertebrae corrected for height (Figure 3)[7]. Carey et al[24] recently defined sarcopenia as SMI < 50 cm2/m2 in males and 39 cm2/m2 in females in a cohort of cirrhotic patients in the North American region awaiting liver transplan
SMI seems to be a more complete and robust measurement than individual mea
The second common cited choice is the index first proposed by Durand et al[35].They demonstrated that the ratio between the transverse diameter of the psoas muscle measured at the L3 level (umbilicus is suggested as a reference point) and balanced for the patient’s height [psoas to height ratio (PTHR); unit mm/m] is an objective indicator of muscle loss and a predictor of mortality in patients with cirrhosis, independent of MELD or Na-MELD score. In their retrospective study, they also demonstrated the effect of the PTHR on mortality in patients with refractory ascites[35]. A study by Paternostro et al[47] tested the value of a similar index. They demon
To determine myosteatosis, CT should be performed without the use of a contrast agent. Muscle density is assessed by HU values[13]. To define myosteatosis, the proposed cut-off values are > 41 HU in patients with BMI < 24.9, and > 33 HU in those with a BMI > 25. In a group of patients with liver cirrhosis, Bhanji et al[50] demon
An interesting option offered by CT is the already mentioned possibility to assess the concomitant presence of sarcopenia and myosteatosis. In a small cohort of patients with cirrhosis, Nardelli et al[51] assessed the presence of sarcopenia and myosteatosis with suggested CT SMI and HU values. Minimal HE defined by psychomotor tests was more common in the group of patients with both factors, as was elevated blood ammonia.
Magnetic resonance imaging: Magnetic resonance imaging (MRI) is considered as an appealing test for the diagnosis of muscle wasting due to the lack of radiation exposure and high-quality images, including information on muscle quality as evident by fat infiltration[7]. When comparing results from CT and MRI images in Traub et al[8] study cohort, there was no difference in the detection rate of reduced muscle mass. However, the study did not compare CT and MRI images in the same patients since they used only imaging studies that were routinely performed. The study by Beer et al[52] included patients with cirrhosis who had clinical or imaging cirrhosis parameters. Additionally, the FIB-4 score was used to assess the degree of liver fibrosis. Portal hypertension was defined invasively, or its signs were evaluated by gastroscopy. MRI-assessed sarcopenia using PTHR index demonstrated an association between sarco
MRI offers another imaging technique, namely diffusion weighted imaging (DWI). DWI offers additional information regarding the composition and architecture of investigated tissue. DWI quantified by apparent diffusion coefficient (ADC) can reflect different pathological changes, such as cell density, extracellular matrix, nucleic areas, and membrane permeability, and may have a role in the diagnosis of different muscle disorders. In the cited study, ADC maps were thus created by the implemented software and manually drawn regions of interest on the ADC maps along the contours of the iliopsoas and paravertebral muscles to avoid fat areas and vessels. They confirmed that ADC can reflect pathological muscle changes since myositis and myopathy had statistically significantly higher ADC values in comparison to unaffec
The treatment of sarcopenia is focused on drug treatment possibilities, exercise and dietary measures, and the treatment of decompensated liver cirrhosis. There are currently no studies that prove the benefit of one or the other in terms of reducing mortality. There is a general benefit of the interventions in terms of reducing the feeling of fatigue; increasing vital capacity; improving muscle mass and ability to exercise; and last but not least, improving quality of life[7].
The general objectives of drug treatment are to focus on lowering blood ammonia and improving the action of growth hormone and testosterone in certain parts of the population with liver cirrhosis. Special emphasis should be placed on the elimination of poor appetite and complete absence of physical activity[1,2,7]. Studies suggest a possible beneficial effect of testosterone on improving muscle mass and function without significant side effects of treatment, but the evidence is yet not enough to recommend replacement therapy[2,7]. Extensive study data on the use of growth hormone in this area are not available. The importance of regulating thyroid hormone and blood sugar levels is also mentioned[24].
General nutrition advice concerns sufficient energy and adequate protein intake while avoiding prolonged fasting periods (> 6 h). The patient should eat three to five meals a day while the target caloric intake varies according to the patient’s BMI. As per EASL guidelines, at least 35 kcal/kg of actual body weight per day is recom
In the field of exercise, moderate intensity exercise is recommended for at least 30 min per day, 3-5 times per week. Physical exercise should start with a short-term warm-up and end with a stretching/cool-down phase. Although a combination of resistance and aerobic exercise is recommended, resistance exercise is more effective in reversing sarcopenia[9,13]. Avoidance of sedentary behavior should be recommended even in patients not willing to undergo a formal exercising program to increase daily physical activity within the context of NEAT (nonexercise activity thermogenesis). As opposed to scheduled exercise, NEAT encourages patients to take opportunities to increase their activity within their day-to-day activities[11]. Exercise brings improve
In the general complications of liver cirrhosis treatment area, there is evidence of the beneficial effect of TIPSS insertion on sarcopenia[1,7]. Thus, an uncontrolled study found that the insertion led to an increase in muscle mass and an improvement in overall prognosis. It also showed an improvement in psoas muscle area in 70% of patients with an increase in the mean muscle area after TIPSS insertion[2]. As previously stated, an already present sarcopenia could be considered a risk factor for mortality in patients who undergo TIPS placement[3], data, confirmed with another retrospective study in patients with cirrhosis who undergo TIPS placement for refractory ascites[54]. The therapeutic consequences of this findings are yet to be provided. The final treatment option for many patients with advanced liver cirrhosis is, of course, liver transplantation, which has been shown to have a beneficial effect on reversibility of muscle function after liver transplantation[9].
Dilemmas remain regarding the timeline of the proposed measures. It is hypothesized that an earlier intervention at a time when anabolic potential exists may be more effective than an intervention at a refractory stage of muscle wasting. Studies in this area are rare and have mostly been performed in very small populations in the field of cirrhosis[3]. To date, there is no evidence that exercise or dietary supplements affect sarcopenia through dysbiosis[4]. There is also no clear guidance on the group of patients with HCC and liver cirrhosis where interventions regarding exercise and dietary substitutions before and after treatment would probably be beneficial[21].
From their repeatability, comparability, accessibility, and realistic range for the correct clinical assessment of an individual patient, the main qualitative problem of sarco
The definition of CT-SMI as the gold standard for muscle mass assessment and the efforts of several study groups to define valid cirrhosis related sarcopenia thresholds are an important foundation for the future study and clinical applicability of this field. Indeed, some data suggest that even small changes in the cut-off values could mean very relevant shifts in the detection and study evaluation of sarcopenia in this population[8].
Study-malnourished areas in the field of sarcopenia and liver cirrhosis are the area of sarcopenia-CSPH interdependence, the area of vulnerable groups (HCC patients, alcoholism, morbid obesity), and the influence of sarcopenia on clinical outcomes in addition to mortality, especially on various forms of disease decompensation. A small number of studies pays attention to the field of sarcopenia treatment through the therapeutic interventions. Such studies are, of course, necessary for the long-term clinical applicability of the field but difficult to perform in larger cohorts.
According to certain clues, sarcopenia could have an important decision regarding the clinical choice for liver transplantation, both in terms of the patient’s higher priority and in terms of the patient’s ability to be fit for a major surgery. In countries that allow such graft allocation, this could lead to the selection of poorer transplants or living donor transplants, or to the rejection of the transplant process in the event of an estimated poor yield.
At the very end, an issue remains that we as clinical professionals find difficult to face. Does the current definition of sarcopenia by the above-mentioned tests have any relevant clinical implications regarding patients’ survival? Although most of the answers point to the affirmative, we are still quite a long way from the objective long-term goals of successful treatment in this area.
Nevertheless, we can certainly be justifiably pleased and proud to look at the huge leap of the last decade in terms of the knowledge gained in this clinically most relevant field. New possibilities for the use of existing and more modern indices of declining muscle mass and function in a patient with cirrhosis are coming to the fore. The tests mentioned in the article are increasingly striving for repeatability and simplicity, both in enabling dynamic sarcopenia monitoring and in reducing investigator dependent errors. A realistic desire for a study breakthrough in the upcoming years remains to define sarcopenia tests and indices that would ensure further ease of affordability and mass clinical applicability for a patient with liver cirrhosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang HC S-Editor: Liu M L-Editor: A P-Editor: Yuan YY
| 1. | Bhanji RA, Montano-Loza AJ, Watt KD. Sarcopenia in Cirrhosis: Looking Beyond the Skeletal Muscle Loss to See the Systemic Disease. Hepatology. 2019;70:2193-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Kukla M, Skladany L, Menżyk T, Derra A, Stygar D, Skonieczna M, Hudy D, Nabrdalik K, Gumprecht J, Marlicz W, Koulaouzidis A, Koller T. Irisin in Liver Cirrhosis. J Clin Med. 2020;9:3158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54:845-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 4. | Nishikawa H, Enomoto H, Nishiguchi S, Iijima H. Liver Cirrhosis and Sarcopenia from the Viewpoint of Dysbiosis. Int J Mol Sci. 2020;21:5254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Buchard B, Boirie Y, Cassagnes L, Lamblin G, Coilly A, Abergel A. Assessment of Malnutrition, Sarcopenia and Frailty in Patients with Cirrhosis: Which Tools Should We Use in Clinical Practice? Nutrients. 2020;12:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2); and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 599] [Cited by in RCA: 1499] [Article Influence: 249.8] [Reference Citation Analysis (0)] |
| 7. | Sinclair M. Controversies in Diagnosing Sarcopenia in Cirrhosis-Moving from Research to Clinical Practice. Nutrients. 2019;11:2454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Traub J, Bergheim I, Eibisberger M, Stadlbauer V. Sarcopenia and Liver Cirrhosis-Comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019. Nutrients. 2020;12:547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Hsu CS, Kao JH. Sarcopenia and chronic liver diseases. Expert Rev Gastroenterol Hepatol. 2018;12:1229-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Zhao M, Zhou X, Yuan C, Li R, Ma Y, Tang X. Association between serum irisin concentrations and sarcopenia in patients with liver cirrhosis: a cross-sectional study. Sci Rep. 2020;10:16093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Tandon P, Berzigotti A. Management of Lifestyle Factors in Individuals with Cirrhosis: A Pragmatic Review. Semin Liver Dis. 2020;40:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018;38:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 13. | Ebadi M, Montano-Loza AJ. Clinical relevance of skeletal muscle abnormalities in patients with cirrhosis. Dig Liver Dis. 2019;51:1493-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Al-Azzawi Y, Albo B, Fasullo M, Coukos J, Watts GJ, Tai R, Radcliffe D, Kroll-Desrosiers A, Devuni D, Szabo G. Sarcopenia is associated with longer hospital stay and multiorgan dysfunction in alcoholic hepatitis. Eur J Gastroenterol Hepatol. 2020;32:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Rodrigues SG, Brabandt B, Stirnimann G, Maurer MH, Berzigotti A. Adipopenia correlates with higher portal pressure in patients with cirrhosis. Liver Int. 2019;39:1672-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Paternostro R, Bardach C, Hofer BS, Scheiner B, Schwabl P, Asenbaum U, Ba-Ssalamah A, Scharitzer M, Bucscis T, Simbrunner B, Bauer D, Trauner M, Mandorfer M, Reiberger T, Lampichler K. Prognostic impact of sarcopenia in cirrhotic patients stratified by different severity of portal hypertension. Liver Int. 2021;41:799-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 403] [Article Influence: 67.2] [Reference Citation Analysis (3)] |
| 18. | van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Darwish Murad S, Feshtali S, van Ooijen PMA, Polak WG, Porte RJ, van Hoek B, van den Berg AP, Metselaar HJ, IJzermans JNM. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: A competing risk analysis in a national cohort. J Hepatol. 2018;68:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 19. | Stirnimann G, Ebadi M, Tandon P, Montano-Loza AJ. Should Sarcopenia Increase Priority for Transplant or Is It a Contraindication? Curr Gastroenterol Rep. 2018;20:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, Beaumont C, Esfandiari N, Myers RP. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol. 2015;6:e102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 21. | Marasco G, Serenari M, Renzulli M, Alemanni LV, Rossini B, Pettinari I, Dajti E, Ravaioli F, Golfieri R, Cescon M, Festi D, Colecchia A. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J Gastroenterol. 2020;55:927-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20:8061-8071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 171] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 23. | Anand AC. Nutrition and Muscle in Cirrhosis. J Clin Exp Hepatol. 2017;7:340-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 24. | Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, Dunn MA, Tsien C, Kallwitz ER, Ng V, Dasarathy S, Kappus M, Bashir MR, Montano-Loza AJ. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology. 2019;70:1816-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 25. | Wu Y, Zhu Y, Feng Y, Wang R, Yao N, Zhang M, Liu X, Liu H, Shi L, Zhu L, Yang N, Chen H, Liu J, Zhao Y, Yang Y. Royal Free Hospital-Nutritional Prioritizing Tool improves the prediction of malnutrition risk outcomes in liver cirrhosis patients compared with Nutritional Risk Screening 2002. Br J Nutr. 2020;124:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Skladany L, Koller T, Molcan P, Vnencakova J, Zilincan M, Jancekova D, Kukla M. Prognostic usefulness of serum myostatin in advanced chronic liver disease: its relation to gender and correlation with inflammatory status. J Physiol Pharmacol. 2019;70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 27. | Oshida N, Shida T, Oh S, Kim T, Isobe T, Okamoto Y, Kamimaki T, Okada K, Suzuki H, Ariizumi SI, Yamamoto M, Shoda J. Urinary Levels of Titin-N Fragment, a Skeletal Muscle Damage Marker, are Increased in Subjects with Nonalcoholic Fatty Liver Disease. Sci Rep. 2019;9:19498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 672] [Article Influence: 112.0] [Reference Citation Analysis (2)] |
| 29. | Nishikawa H, Yoh K, Enomoto H, Ikeda N, Aizawa N, Koriyama T, Nishimura T, Nishiguchi S, Iijima H. Anthropometric Measurements and Frailty in Patients with Liver Diseases. Diagnostics (Basel). 2020;10:433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Nishikawa H, Yoh K, Enomoto H, Iwata Y, Sakai Y, Kishino K, Shimono Y, Ikeda N, Takashima T, Aizawa N, Takata R, Hasegawa K, Koriyama T, Yuri Y, Nishimura T, Nishiguchi S, Iijima H. Calf Circumference as a Useful Predictor of Sarcopenia in Patients With Liver Diseases. In Vivo. 2020;34:2561-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Sinclair M, Chapman B, Hoermann R, Angus PW, Testro A, Scodellaro T, Gow PJ. Handgrip Strength Adds More Prognostic Value to the Model for End-Stage Liver Disease Score Than Imaging-Based Measures of Muscle Mass in Men With Cirrhosis. Liver Transpl. 2019;25:1480-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 32. | Nishikawa H, Enomoto H, Yoh K, Iwata Y, Sakai Y, Kishino K, Ikeda N, Takashima T, Aizawa N, Takata R, Hasegawa K, Ishii N, Yuri Y, Nishimura T, Iijima H, Nishiguchi S. Walking Speed: Japanese Data in Chronic Liver Diseases. J Clin Med. 2020;9:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Cichoż-Lach H, Michalak A. A Comprehensive Review of Bioelectrical Impedance Analysis and Other Methods in the Assessment of Nutritional Status in Patients with Liver Cirrhosis. Gastroenterol Res Pract. 2017;2017:6765856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Moriwaki EI, Enomoto H, Saito M, Hara N, Nishikawa H, Nishimura T, Iwata Y, Iijima H, Nishiguchi S. The Anthropometric Assessment With the Bioimpedance Method Is Associated With the Prognosis of Cirrhotic Patients. In Vivo. 2020;34:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Durand F, Buyse S, Francoz C, Laouénan C, Bruno O, Belghiti J, Moreau R, Vilgrain V, Valla D. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 287] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 36. | Georgiou A, Papatheodoridis GV, Alexopoulou A, Deutsch M, Vlachogiannakos I, Ioannidou P, Papageorgiou MV, Papadopoulos N, Yannakoulia M, Kontogianni MD. Validation of cutoffs for skeletal muscle mass index based on computed tomography analysis against dual energy X-ray absorptiometry in patients with cirrhosis: the KIRRHOS study. Ann Gastroenterol. 2020;33:80-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Lindqvist C, Brismar TB, Majeed A, Wahlin S. Assessment of muscle mass depletion in chronic liver disease: Dual-energy x-ray absorptiometry compared with computed tomography. Nutrition. 2019;61:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Perkisas S, Bastijns S, Baudry S, Bauer J, Beaudart C, Beckwée D, Cruz-Jentoft A, Gasowski J, Hobbelen H, Jager-Wittenaar H, Kasiukiewicz A, Landi F, Małek M, Marco E, Martone AM, de Miguel AM, Piotrowicz K, Sanchez E, Sanchez-Rodriguez D, Scafoglieri A, Vandewoude M, Verhoeven V, Wojszel ZB, De Cock AM. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur Geriatr Med. 2021;12:45-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 39. | Pita A, Ziogas IA, Ye F, Chen Y, Rauf MA, Matsuoka LK, Kaur N, Whang G, Zielsdorf SM, Bastas G, Izzy M, Alexopoulos SP. Feasibility of Serial Ultrasound Measurements of the Rectus Femoris Muscle Area to Assess Muscle Loss in Patients Awaiting Liver Transplantation in the Intensive Care Unit. Transplant Direct. 2020;6:e618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Tandon P, Low G, Mourtzakis M, Zenith L, Myers RP, Abraldes JG, Shaheen AA, Qamar H, Mansoor N, Carbonneau M, Ismond K, Mann S, Alaboudy A, Ma M. A Model to Identify Sarcopenia in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1473-1480.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 41. | Tandon M, Singh H, Singla N, Jain P, Pandey CK. Tongue thickness in health vs cirrhosis of the liver: Prospective observational study. World J Gastrointest Pharmacol Ther. 2020;11:59-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 42. | Kobayashi K, Maruyama H, Kiyono S, Ogasawara S, Suzuki E, Ooka Y, Chiba T, Kato N, Yamaguchi T. Application of transcutaneous ultrasonography for the diagnosis of muscle mass loss in patients with liver cirrhosis. J Gastroenterol. 2018;53:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Hari A, Berzigotti A, Štabuc B, Caglevič N. Muscle psoas indices measured by ultrasound in cirrhosis - Preliminary evaluation of sarcopenia assessment and prediction of liver decompensation and mortality. Dig Liver Dis. 2019;51:1502-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Becchetti C, Germani G, Burra P, Dufour JF, Berzigotti A. Bidimensional shear wave elastography of the rectus femoris muscle in patients with cirrhosis. J Hepatol. 2020;73:S696. [DOI] [Full Text] |
| 45. | Wang NC, Zhang P, Tapper EB, Saini S, Wang SC, Su GL. Automated Measurements of Muscle Mass Using Deep Learning Can Predict Clinical Outcomes in Patients With Liver Disease. Am J Gastroenterol. 2020;115:1210-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Marasco G, Sadalla S, Vara G, Golfieri R, Festi D, Colecchia A, Renzulli M. Imaging Software-Based Sarcopenia Assessment in Gastroenterology: Evolution and Clinical Meaning. Can J Gastroenterol Hepatol. 2021;2021:6669480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Paternostro R, Lampichler K, Bardach C, Asenbaum U, Landler C, Bauer D, Mandorfer M, Schwarzer R, Trauner M, Reiberger T, Ferlitsch A. The value of different CT-based methods for diagnosing low muscle mass and predicting mortality in patients with cirrhosis. Liver Int. 2019;39:2374-2385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 48. | Ebadi M, Wang CW, Lai JC, Dasarathy S, Kappus MR, Dunn MA, Carey EJ, Montano-Loza AJ; From the Fitness; Life Enhancement; and Exercise in Liver Transplantation (FLEXIT) Consortium. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopenia Muscle. 2018;9:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 49. | Ebadi M, Bhanji RA, Dunichand-Hoedl AR, Mazurak VC, Baracos VE, Montano-Loza AJ. Sarcopenia Severity Based on Computed Tomography Image Analysis in Patients with Cirrhosis. Nutrients. 2020;12:3463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 50. | Bhanji RA, Moctezuma-Velazquez C, Duarte-Rojo A, Ebadi M, Ghosh S, Rose C, Montano-Loza AJ. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int. 2018;12:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 51. | Nardelli S, Gioia S, Faccioli J, Riggio O, Ridola L. Sarcopenia and cognitive impairment in liver cirrhosis: A viewpoint on the clinical impact of minimal hepatic encephalopathy. World J Gastroenterol. 2019;25:5257-5265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Beer L, Bastati N, Ba-Ssalamah A, Pötter-Lang S, Lampichler K, Bican Y, Lauber D, Hodge J, Binter T, Pomej K, Simbrunner B, Semmler G, Trauner M, Mandorfer M, Reiberger T. MRI-defined sarcopenia predicts mortality in patients with chronic liver disease. Liver Int. 2020;40:2797-2807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 53. | Surov A, Paul L, Meyer HJ, Schob S, Engelmann C, Wienke A. Apparent Diffusion Coefficient Is a Novel Imaging Biomarker of Myopathic Changes in Liver Cirrhosis. J Clin Med. 2018;7:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Petridis I, Miraglia R, Maruzzelli L, Wan T, Berzigotti A, Bosch J, Volpes R. Sarcopenia predicts mortality after transjugular intrahepatic portosystemic shunt creation in patients with refractory ascites. J Hepatology. 2020;73:S717-S718. [DOI] [Full Text] |
| 55. | Tandon P, Raman M, Mourtzakis M, Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. 2017;65:1044-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (1)] |