INTRODUCTION
Chronic viral hepatitis B and C infections remain a significant burden on global health. Modern directly acting antivirals (DAAs) have changed the landscape of hepatitis C virus (HCV) treatment with their high rates of sustained virological response and superior tolerability. HCV has a significant global impact with the World Health Organisation estimating that 71 million people worldwide are chronically infected[1]. Between 15%-30% of patients with chronic HCV infection will develop cirrhosis and its related complications within 20 years. On the other hand, despite the development and wide availability of the hepatitis B vaccine, WHO estimated that the global incidence of chronic hepatitis B virus (HBV) infection was 257 million people in 2015[2].
Both of these chronic diseases result in substantial economic burden. The mean annual all-cause medical cost for healthcare in an American cohort with HCV was $43891 as compared to $17989 in patients without HCV[3]. A retrospective study which conducted an analysis on the United States Veteran population with chronic HBV, calculated the total cost per patient per year to be $39240 for those treated with nucleoside analogues[4]. Treatment of HCV, particularly in the early stages of fibrosis, significantly mitigates the economic burden from HCV and its resultant hepatic complications[3]. The cost of treatment of HBV has decreased significantly over the decades as Entecavir and Tenofovir disproxil fumurate are now both off-patent. On the international market, the median price of WHO-prequalified generic Tenofovir has been reduced from US$208 per year to US$32 per year in 2016[2]. Cost of HCV treatment with DAAs is significant, ranging from $40000 to $90000 depending on the drug and treatment duration. Thankfully, many countries such as Australia and Canada have heavily subsidised treatment, allowing the widespread use of DAAs.
The advent of modern DAA therapies has revolutionised the treatment of HCV. More patients are willing to start treatment with the promise of high cure rates, shorter durations of treatment and limited adverse effects. Early access to effective curative treatment will have significant long-term impact on the outcomes of HCV related liver disease. Advances in HCV therapy have led to a commitment by the member states of WHO to try to eliminate viral hepatitis by 2030. For HBV therapy, nucleos(t)ide analogues (NA) are potent antiviral agents which are becoming more widely available in low to middle economic countries. NAs suppress viral replication and therefore result in improved long-term clinical outcomes in chronic liver disease by reducing the progression of liver injury to advanced fibrosis, cirrhosis, liver failure and hepatocellular carcinoma (HCC). As a direct consequence, the requirements for liver transplantation are also reduced[5,6].
The aim of this review article is to summarise the current literature regarding modern antiviral treatment of chronic HBV and HCV infection and the effects of therapy on clinical outcomes of chronic liver disease.
EFFECTS OF CHRONIC HEPATITIS B TREATMENT ON CLINICAL OUTCOMES
Prevalence
Globally, chronic HBV is one of the most common infectious diseases[7]. In 2015, the World Health Organisation (WHO) estimated that 257 million people, equivalent to approximately 3.5% of the world’s population, were living with chronic HBV[7]. In addition, 2.7 million people were coinfected with HBV and HIV[7]. Chronic HBV is associated with development of complications including cirrhosis, liver failure and hepatocellular carcinoma. HBV is a leading cause of liver cancer worldwide[8] and up to one in four people with chronic HBV will die from liver cancer or liver failure if their HBV is not monitored or treated appropriately[7]. The prevalence of HBV varies widely depending on country and is predominantly determined by age of exposure; approximately 90% of infected infants progress to develop chronic HBV, as opposed to a rate of 5% following exposure of immunocompetent adults to HBV[9]. In 2015, 65% of all infected persons with HBV were located in African and Western Pacific regions[7].
Chronic HBV infection is preventable using a readily accessible vaccine. In 2017 there were 4762 confirmed HBV cases reported in the United Kingdom and of these, 324 were acute cases of HBV[10]. The prevalence of HBV has been reduced through the incorporation of routine vaccination in the immunisation schedule in Australia and United Kingdom. In 2017, there was a 73% self-reported uptake of at least one dose of the HBV vaccine in England[11]. In Australia, there were an estimated 226566 people (0.9% of the population) living with chronic HBV[12]. Additionally, it is estimated that of those living with chronic HBV, only 68% have been diagnosed[12]. In 2020, only 22% people with chronic HBV were engaged with regular care and receiving regular monitoring or antiviral therapy[12]. Furthermore, only 9.3% were receiving antiviral therapy[13]. The national target in Australia is to have 50% of chronic HBV patients engaged with care and 20% receiving antiviral therapy by 2022[12].
Decompensation and mortality
Currently, chronic infection with HBV remains incurable. The goals of modern antiviral therapy for chronic HBV are to (1) improve survival and quality of life by preventing disease progression to cirrhosis, decompensation and HCC; (2) induce virological seroconversion of e-antigen and surface antigen; (3) induce long-term suppression of HBV DNA levels; and (4) induce normalisation of serum alanine aminotransferase (ALT) levels which reflect a biochemical response to therapy[14].
Chronic HBV infection can progress to advanced hepatic fibrosis and eventually, cirrhosis. Hepatic decompensation can occur in the context of cirrhosis or acute flare as a result of HBV reactivation. Decompensation presents clinically with the development of ascites, jaundice, encephalopathy or variceal bleeding. In HBV infection, hepatic decompensation occurs with a high HBV-DNA viral load and is more common in patients who are e-Ag positive[15].
Hui et al[16] conducted a study of 96 chronic HBV patients over three years and reported 29% of patients developed decompensation. The most common clinical manifestation of decompensation was ascites (70%) followed by variceal bleeding (34%), jaundice (26%) and hepatic encephalopathy (5%). Almost a third of subjects had more than one feature of decompensation. Das et al[17] performed a retrospective cohort study involving 253 HBV-related decompensated cirrhotic patients. They reported a mean 5-year survival rate of 19%; the commonest causes of death included hepatorenal syndrome (32%), HCC (28%), variceal bleeding (23%), liver failure (9%) and hepatic encephalopathy (9%).
Acute-on-chronic liver failure is associated with a high mortality rate and occurs in patients with chronic HBV who have experienced an acute liver decompensation event which results in jaundice, ascites, coagulopathy and/or encephalopathy[15]. Acute liver insults may result from recrudescence of HBV replication, viral superinfection (with hepatitis D, HCV, or hepatitis E virus), acute variceal bleeding, infection and/or alcoholic hepatitis[15]. Studies assessing acute-on-chronic liver failure in patients with chronic HBV infection report that antiviral therapy (including Entecavir and Tenofovir) reduces all-cause mortality[15,18,19].
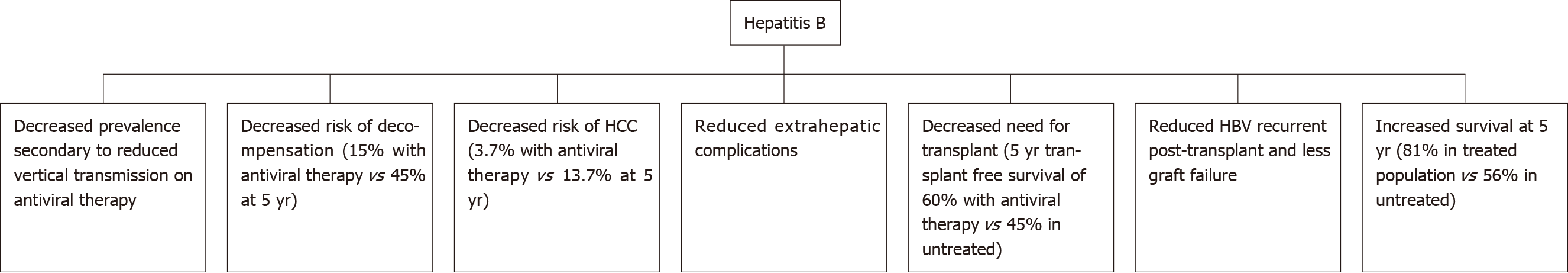
Antiviral therapy suppresses viral replication and reduces the likelihood of loss of hepatic function. The literature shows that antiviral therapy reduces the probability of decompensation and progression of liver cirrhosis[15] (Figure 1). The use of antiviral therapy in HBV-related liver decompensation results in improvement of liver function, as illustrated by improved MELD scores and patients being delisted for liver transplantation[15]. Therefore, guidelines recommend commencement of antiviral therapy in all cirrhotic patients with HBV, irrespective of viral load level. A Korean study by Kim et al[20] demonstrated a 15% probability of developing hepatic decompensation 5-years after commencement of antiviral therapy which was less compared to those who remained untreated and had a 45% probability of decompensation after 5 years. Overall, treatment with antivirals results in significantly improved prognosis in HBV-related cirrhosis as evidenced by 5 year survival rates of 81% in those treated with NAs compared to 56% in the untreated individuals[20].
Figure 1 Outcomes of hepatitis B after introduction of antiviral therapy.
HCC: Hepatocellular carcinoma; HBV: Hepatitis B virus.
Nucleos(t)ide analogues such as Entecavir and Tenofovir disoproxil fumurate (TDF) are considered first line agents due to their excellent safety profile, high efficacy and minimal to no risk of resistance developing in treatment-naïve patients[15]. Entecavir induces undetectable viral load in up to 89% of patients with decompensated liver disease after 12 mo[21,22]. Additionally, commencement of antiviral therapy is associated with improvement in prognosis, with a substantial reduction in Model of End stage Liver Disease (MELD) and Child-Pugh (CP) scores after twelve months of therapy[15]. Several studies have reported a mean improvement of both MELD and CP scores by up to 2 points (compared to baseline) at twelve months in patients who respond to treatment with Entecavir or TDF[21,23-26].
Pegylated-interferon alpha could be considered as an initial treatment option for patients with compensated cirrhosis, with a treatment duration of 48 wk. It is contraindicated in patients with hepatic decompensation. Observational studies which followed up patients for a mean of 84 months and compared treatment with pegylated-interferon alpha vs no treatment reported a significant decrease in the risk of HCC, with a cumulative incidence rate of 14% at 5 years in those treated vs 23% in untreated patients. There was no difference in all-cause mortality or decompensated liver disease[17,20,27-29].
Several studies have shown that antiviral therapy improves mortality in liver cirrhosis. Suppression of viral replication prevents progression of liver disease and therefore reduces the risk of liver decompensation and HCC, resulting in improved overall survival. The 5-year survival rate in untreated patients with HBV with compensated cirrhosis is 80%-85% vs 14%-35% in decompensated cirrhosis[15]. Additionally, a Korean cohort study of 204 patients demonstrated the positive prognostic effect of antiviral therapy in cirrhotic patients with HBV[20]. This study reported the 5-year survival rate in untreated cirrhotic patients was 56%; the survival breakdown based on CP score was 74% for CP-A, 34% for CP-B and 10% for CP-C[20]. For those who were treated with antiviral therapy, the overall 5-year survival rates were 81%; 91% for CP-A, 63% for CP-B and 62% for CP-C[20].
A large study by Jang et al[26] which followed 707 patients with HBV-related decompensation for seven years illustrated the long-term benefits of viral suppression in decompensated cirrhotic patients and a trend to benefit in regard to transplant-free survival with antiviral therapy. This study reported a 5-year transplant-free survival of nearly 60% with antiviral therapy compared with 46% in the untreated group. Usually, patients with chronic HBV-related decompensated cirrhosis who are listed for transplantation have a much lower 5-year survival compared with those who undergo transplantation with fulminant HBV infection[26]. In addition, up to 60% of those patients listed for transplantation were able to either delay or negate the need for liver transplantation[26]. However, of the patients with severe hepatic dysfunction in this study, 13% of patients died within first six months of initiating antiviral treatment[26]. Hence, patients with severe decompensation should be considered for liver transplantation because it may take several months of antiviral therapy for liver function to recover.
HCC
Recent studies have reported that the incidence of annual HCC in patients with chronic HBV ranges from 0.9%–1.4% in non-cirrhotic patients and up to 5.4% in those with cirrhosis[30]. The majority of the published literature demonstrates that antiviral therapy with nucleos(t)ide analogues (NAs) reduces the risk of HCC in patients with chronic HBV, after adjusting for known background risk factors including older age, male gender, cirrhosis, family history, HBeAg positive status, elevated HBV DNA and serum ALT levels[13,31,32]. Asian studies that compared data with or without NA treatment reported significant treatment-related reductions in risk of HCC risk by up to 30% in cirrhotic patients and 80% in non-cirrhotic patients[30].
Hosaka et al[29] compared 472 Entecavir-treated patients with 1143 untreated patients and found that the incidence rate of HCC at 5 years was 3.7% in the Entecavir group and 13.7% in the control group, after elimination of baseline differences by propensity score matching. Additionally, after adjustment for a number of known HCC risk factors, Entecavir treatment was independently associated with reduced HCC risk[29]. A European multicentre study reported that the HCC risk began to decrease after 5 years of antiviral therapy[33]. The reduction in HCC incidence with treatment was further supported by a Taiwanese nationwide study which assessed propensity score-matched patients on antiviral therapy; those receiving NA therapy had a significantly lower 7 year HCC incidence of 7% compared to 23% in the untreated cohort[13]. The benefit of antiviral treatment was observed in both non-cirrhotic and cirrhotic patients[13].
A large study conducted by Lee et al[5] which consisted of a post-hoc analysis of two prospective cohorts from Hong Kong and Korea (n = 818 patients), reported the risk of HCC did not decrease after 5 years of antiviral therapy. This study finding suggests that cirrhotic patients may require longer therapy before a decline in HCC risk eventuates[5]. The higher rates of HCC may also reflect improved mortality rates related to current clinical practice, which translates to more time for HCC to develop[34].
The mechanisms by which antiviral therapy decrease HCC risk may include reductions in the hepatic inflammation and nuclear signaling pathways that lead to neoplastic transformation on a cellular level[35,36]. NAs also reverse fibrosis and the wound-healing response known to be associated with the pathogenesis of HCC[37]. Antiviral therapy may reduce the expression of hepatitis B x-protein to levels that are insufficient for HCC development, or act at a genomic level by preventing integration of HBV DNA into host chromosomes and thus affect its malignant potential[38].
Thus, HCC risk is not completely eliminated with modern antiviral therapy, and there remains a risk of developing HCC even in the context of negative hepatitis B e antigen (HBeAg) and/or negative hepatitis B surface antigen (HBsAg) status[39-42]. Seroconversion of HBsAg is associated with better clinical outcomes and lower rates of HCC incidence[32]. Even so, several studies have illustrated that the risk of HCC remains elevated in treated HBeAg-negative patients for at least 5 years after initiation of NA treatment. Therefore, HCC surveillance should be continued long term, even after seroconversion of HBsAg[31,40,43].
The association between virological suppression and HCC development remains controversial due to conflicting results. In Asian studies, virological suppression is associated with lower rates of HCC – especially in cirrhotics[30,32]. On the other hand, European studies have not consistently reported this association. This difference may be related to variation in patient characteristics such as genotype distribution[6].
A Greek cohort with 321 HBeAg-negative chronic HBV patients treated with Entecavir, reported an HCC incidence of 1.2% at a median follow-up of 1.5 years[40]. The 5-year cumulative HCC incidence rate in Entecavir-treated patients was 9% in cirrhotic patients and less than 1% in non-cirrhotic patients[40]. This result was similar to two other Asian studies[29,44]. Old age was identified to be an independent risk factor associated with increased risk of HCC[40]. A Korean retrospective analysis of 829 patients who had achieved seroconversion of HBsAg and followed up over 3464 patient-years found that the annual rate of HCC was 0.6%; the estimated annual incidence of HCC was 3% in cirrhotic patients and 0.3% in non-cirrhotic patients[39]. Independent risk factors associated with the development of HCC included liver cirrhosis, male gender and age[3] 50 years at time of HBsAg seroconversion[39]. Therefore, HCC surveillance should be recommended to continue in patients who have undergone HBsAg seroconversion if they are cirrhotic or over the age of 50 years, regardless of cirrhotic status[39].
In patients who have undergone resection of HCC, an elevated viral load and absence of antiviral therapy are independent risk factors for recurrence of HCC[45]. Choi et al[34] conducted a historical cohort study of 1695 patients treated with Entecavir (n = 813) or TDF (n = 882) after curative intent hepatectomy for early-stage HCC (BCLC Stage 0/A) between 2010 and 2018. During a median follow-up duration of 38 months with continuous antiviral therapy, 33% of patients developed HCC recurrence. A comparison between Entecavir and TDF therapy revealed that TDF was associated with a significantly higher recurrence-free survival, higher overall survival rates and lower rates of HCC recurrence. The 3-year recurrence free survival rates were 73% and 64% in the TDF and Entecavir groups, respectively. TDF was also significantly associated with a lower risk of both early (i.e., within 2-years of resection) (adjusted hazard ratio 0.79, 95%CI, 0.64-0.97, P = 0.03) and late HCC recurrence (adjusted hazard ratio, 0.68; 95%CI, 0.47-0.97; P = 0.03) compared to Entecavir. Whilst the results of this study imply better clinical outcomes associated with TDF in regard to rates of recurrence of HCC, this was an observational study which may be subject to bias and confounding. Additionally, other limitations of the study include missing data, heterogeneity in duration of antiviral therapy treatment (i.e., before or after surgery), and TDF being approved as standard-of-care therapy in Korea in December 2020 which falls within the study period.
Liver transplantation
NAs have been associated with reduced rates of liver transplantation for patients with chronic hepatitis B. A retrospective study included 5374 patients with chronic HBV, 2000 patients were treated with NA (Entecavir) and 3374 patients were treated with Lamivudine in a tertiary centre in South Korea[46]. The study demonstrated that Entecavir was associated with a significantly lower risk of death or transplantation (hazard ratio 0.49, 95%CI 0.38-0.64)[46]. A further analysis of 860 paired cirrhotic patients replicated the finding (hazard ratio 0.42, 95%CI 0.31– 0.57)[46].
Recurrence of HBV post-liver transplantation occurs when circulating HBV virions from extrahepatic reservoirs engraft the new liver. HBV re-infection has been associated with early acute cellular rejection within the first year of liver transplantation[47]. Risk factors associated with HBV recurrence include high level of viraemia at time of transplantation, HBeAg-positive status, or history of drug resistance to oral HBV antiviral therapy[47,48]. Post-transplant immunosuppression can also induce viral replication and subsequent HBV infection in patients who have received donor liver graft with hepatitis B core antibody positivity[49]. Administration of hepatitis B immunoglobulin (HBIG) to transplant recipients has been associated with lower HBV recurrence and improved survival of 80%, compared to 80% HBV recurrence and 50% survival if no HBIG was given[47,48]. The addition of NA therapy to HBIG in liver transplant recipients has resulted in a further reduction in HBV recurrence and improved prognosis[47]. A study conducted by Cholongitas et al[50] which had a median follow up period of 30 mo, reported HBV recurrence was much lower in patients who received HBIG plus NA (Entecavir/Tenofovir) – 1%, compared to those who received HBIG plus Lamivudine – 6% (P = 0.0004).
Extrahepatic manifestations
Extrahepatic manifestations may be observed with acute or chronic HBV infection. It is postulated that circulating immune complexes and viral antigens activate the complement cascade resulting in organ injury, most commonly to the kidneys, vessel wall, skin and joints[51]. A multicentre retrospective cross-sectional study of HBV patients and found that 16% exhibited clinical extrahepatic manifestations - sensory-motor deficiency, sicca syndrome, myalgia, glomerulonephritis and arthralgia arthritis [52]. Other associations reported in the literature include a serum sickness-like syndrome, polyarteritis nodosa, cryoglobulinaemia, lichen planus, Gianotti-Crosti syndrome and Guillain-Barre syndrome. Awareness and recognition of these clinical manifestations are important to facilitate early diagnosis, monitoring and treatment of HBV infection and the associated organ injury.
Infection with HBV is associated with a variety of renal diseases including membranous nephropathy, membranoproliferative glomerulonephritis and rarely IgA nephropathy. A prospective French study by Mallet et al[53] monitored the renal function of patients with compensated chronic HBV following treatment with NAs. Treatment with TDF or Entecavir, especially in patients with an initial HBV DNA titre of 100 000IU/mL or greater, resulted in an increased estimated Glomerular filtration rate (eGFR) throughout the follow up period (median of 2.7 years)[53]. Similarly, a recent meta-analysis by Fu et al[54] assessed the efficacy and safety of NA monotherapy for HBV-related glomerulonephritis in 7 trials involving 182 participants. They found that antiviral therapy induced remission of proteinuria and increased HBeAg clearance.
Polyarteritis nodosa (PAN) is a multisystem necrotizing vasculitis typically affecting muscular medium-sized and small arteries. Hepatitis B has been observed in 17%-35% of patients diagnosed with PAN, although the frequency is likely decreasing due to HBV vaccination[55]. The American College of Rheumatology (ACR) has established ten criteria for the classification of PAN in patients with a vasculitis and recognises the evidence of HBV infection as one of the diagnostic criteria[56]. Other systemic features of PAN may include cutaneous livedo reticularis, testicular pain, mononeuropathy or polyneuropathy, renal artery aneurysm and unexpected weight loss. In patients in whom PAN is associated with HBV (HBV-PAN), the major focus of therapy is the use of antiviral agents for the treatment of the underlying viral disorder. Immunosuppressive therapy in the form of glucocorticoids may also be used in the short term for control of the systemic inflammation[55]. In several studies, clinical remission of PAN was observed in patients who were treated with antiviral agents and in very few patients who instead received only immunosuppressive treatment[55,57].
Mixed cryoglobulinaemia (types II and III) is caused by immunocomplexes composed of polyclonal IgG in association with either monoclonal or polyclonal IgM. These immunoglobulins precipitate with cold temperature, resulting in purpura, renal disease, arthralgia or arthritis. Cryoglobulinaemic vasculitis can develop in 1-4% of HBV patients. Treatment with NAs has been found to provide significant clinical response, preventing extrahepatic organ complications[58].
EFFECTS OF CHRONIC HEPATITIS C TREATMENT ON CLINICAL OUTCOMES
Prevalence
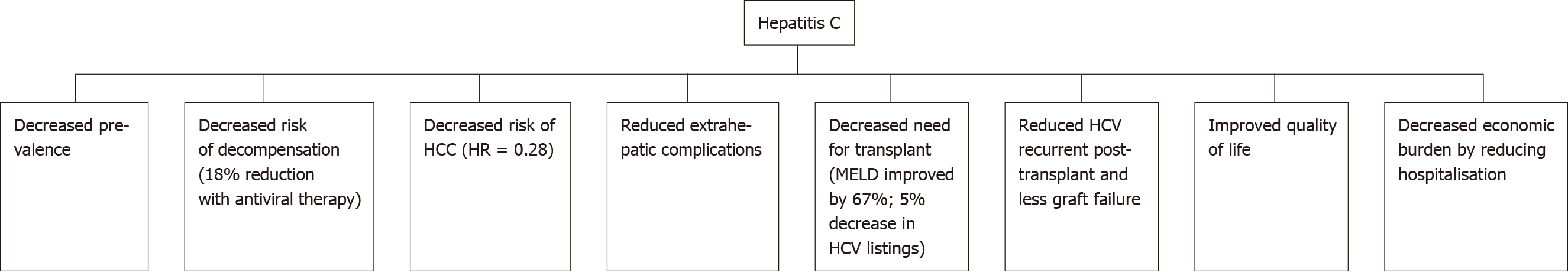
The WHO estimates that there are up to 71 million people worldwide who are chronically infected with HCV[1]. In Australia, mandatory reporting of HCV diagnosis has been required since the 1990s. This allows for more accurate assessment of HCV numbers. As of 2017, it was estimated that up to 182,144 people in Australia had HCV. The incidence of new HCV infections has steadily declined since 2000. Government subsidised DAA treatment was made generally available in 2016. At the end of 2018, an estimated 130089 Australians had HCV, a decrease of approximately 50000 patients with the use of antiviral therapy (Figure 2).
Figure 2 Outcomes of hepatitis C after introduction of antiviral therapy.
HCC: Hepatocellular carcinoma; MELD: Model of End stage Liver Disease; HCV: Hepatitis C virus.
In the United Kingdom, the prevalence is estimated to have decreased in recent years by one-third due to DAA treatment. Approximately 118000 people had HCV in 2019 as compared to 174000 in 2015[59]. It is expected that the prevalence will continue to steadily decrease with a corresponding increase in the number of patients who have been successfully treated.
Decompensation and mortality
It is well recognised that chronic HCV places considerable financial burden on the healthcare system, primarily related to presentations with decompensated liver disease. These individuals have prolonged stays in hospital and frequent readmissions. A United Kingdom study demonstrated that 21.1 d was the mean length of stay in decompensated HCV patients without HCC. This translates to a mean cost of 9120 pounds per year. Within Europe, annual hospitalisation costs are estimated at 8000-20000 euros for decompensated patients in France and up to 28000 euros in the Netherlands. The inpatient hospital burden appears to be greatest within the first year after the first decompensation event[60]. With the aging population, there are increasing numbers of patients presenting to Emergency Departments with HCV-related issues which result in hospitalisation[61]. In the Scottish population, between 1996 and 2013, the total number of HCV-related inpatient admissions with decompensation increased 16-fold[60].
In the United Kingdom-based Extended Access Program cohort, achieving a sustained viral response (SVR) in HCV cirrhotics was associated with a reduction in decompensation rates of 18% within the first 6 mo[62]. Lower decompensation rates result in decreased hospitalisation rates, reduced length of stay and most importantly, mortality reduction[63]. In the Veterans Affairs cohort, achieving SVR in patients with advanced liver disease (pre-treatment FIB-4 scores of > 3.25) resulted in a 74% reduction in mortality risk, after adjusting for baseline variables[64].
A rise in the hepatic venous pressure gradient (HVPG) is the main driver behind cirrhosis-related complications such as variceal bleeding and ascites. HCV eradication is associated with a reduction in HVPG which translates to decreased complications. This was demonstrated in a prospective study by Mandorfer et al[65] who measured HVPG pre- and post-treatment of HCV. In patients with subclinical portal hypertension (HVPG 6-9 mmHg), portal hypertension resolved in 63% of patients. In the group of patients with HVPG 10-15 mmHg, portal hypertension resolved in 43% of the cohort. The highest risk group with HVPG > 16 mmHg showed a reduction in HVPG in 35% of patients but portal hypertension did not resolve in any of the patients[65]. This indicates the importance of treating HCV early to achieve SVR and reversibility of portal hypertension prior to development of end-stage irreversible portal hypertension and its related complications.
Quality of life
Chronic HCV infection has clearly defined effects on hepatic fibrosis, cirrhosis and subsequently, increased risk of HCC. There have been many studies looking into the clinical effects of hepatitis C-related liver disease. Patient related outcomes (PROs) or impact on quality of life are equally important measures but harder to quantify. Previous studies have demonstrated that health-related quality of life (HRQOL) is impacted by HCV and HCV causes a more severe reduction in HRQOL than certain chronic diseases such as diabetes[66,67].
Cacoub et al[68] investigated the impact of HCV treatment on patient-related outcomes in a large study. Data were derived from 11 multicentre phase 3 clinical trials across a range of countries. These included trials based on interferon therapy, ribavirin-based and DAA therapies. PROs were measured using a combination of four different validated questionnaires to encompass different domains including physical, emotional and social impact as well as work productivity, fatigue and functional well-being. Patients were asked to fill in the questionnaires at baseline, during treatment and post-treatment while being blinded to their HCV RNA levels.
This study demonstrated that modern antiviral therapy was associated with improvement in PROs as early as four weeks after initiation of treatment[68]. By week 4, average PRO scores had improved by 6%, with the greatest improvements in the domain of emotional wellbeing and worry. This pattern continued throughout treatment course (increased by 8%) and was sustained up to SVR-24 (increased by 10%), becoming more pronounced with time. PROs were significantly better in the non-interferon, non-ribavirin group[68]. This provides additional support for the superiority of modern DAAs over older treatment regimes.
A systematic review by Younossi et al[69] also demonstrated that HCV infection negatively impacted patients’ HRQOL. In particular, scores were lower in the mental health domains as compared to physical health. The prevalence of depression in HCV patients was estimated to be 24% compared to 17% in the non-HCV control group. Based on this data, the risk of developing depression in patients with HCV is substantially increased (RR = 2.30)[69]. In addition to the physical benefits derived by HCV clearance, the significant mental health improvements are also an important reason to treat HCV patients as early as possible.
HCC
HCC is the second leading cause of cancer-related death worldwide. Early on in the post-DAA treatment era, there was initial concern about an unexpected increase in the incidence of HCC after achieving viral clearance. These results were later disputed by other studies which had longer follow-up periods and larger cohorts with control groups. A cohort study by Kanwal et al[70] demonstrated that SVR was associated with a significantly lower risk of HCC as compared to patients who remained infected (0.90 vs 3.45 HCC per 100-person years; adjusted HR = 0.28). Two large cohort studies by Ioannou et al[71] and Singer et al[72] also showed a significantly reduced risk of HCC following SVR with adjusted HR = 0.84 and 0.29, respectively. Cheung et al[62] carried out a prospective study involving 406 patients with decompensated cirrhosis and demonstrated that the incidence of HCC was 4% within the first 6 months of DAA treatment. This was equivalent to the matched control group with untreated patients. The evidence now overwhelmingly favours the absence of any increased risk of HCC following SVR[73,74].
Post-treatment studies from the interferon era have shown that regression of fibrosis is a gradual process that can take years. As fibrosis assessment is mostly performed using non-invasive markers now, there is limited data assessing fibrosis regression post SVR with DAAs using the gold standard of liver biopsy. One small study showed that cirrhosis resolved in 7 out of 14 patients following DAA treatment[75]. A large retrospective Brazilian study of 400 patients reported that liver stiffness measurement (measured with Fibroscan) decreased significantly post-SVR (13.6 kPa vs 10.2 kPa, P < 0.001) with 42% of the treated cohort experiencing a reduction of LSM by at least 30%[76]. Thus, early viral clearance offers advantages of prevention and regression of hepatic fibrosis.
Although treatment with DAAs does not confer additional risk of HCC development, it is still important to note that patients remain at risk of HCC even following SVR. Multivariate analyses from several studies have identified risk factors for HCC occurrence post DAA treatment. These include advanced liver fibrosis (F3 or F4), older age and male sex[77,78]. Therefore, it is imperative that patients with these risk factors should continue to undergo HCC surveillance even after successfully achieving viral clearance.
Liver transplantation
HCV is one of the leading reasons for liver transplantation in the Western world. Twenty-four percent of liver transplants performed in Europe between 1999 and 2009 were HCV-related[79]. The recurrence of HCV post-transplant was also an issue leading to accelerated graft dysfunction and eventual failure. DAA treatment can reduce the need for transplantation by reducing decompensation rates. A pooled analysis of 800 decompensated cirrhotic patients demonstrated that up to 60% of patients had an improvement of their MELD score after treatment[62]. In the United Kingdom, the introduction of treatment with DAAs has resulted in a decreased number of HCV-related cirrhosis patients being listed for transplant. The rate in 2013 of new registrations with HCV-related cirrhosis was 10% and this reduced to 5% in 2016 (P < 0.001) In this cohort, the median UKELD for HCV remained stable at 55 in the pre and post-DAA era[62,80,81]. There has been concern that treatment of HCV patients on the transplant waitlist will decrease their MELD score, reducing their chances of receiving a transplant. Despite the reduction in their MELD score, some of these patients still require a transplant as they are experiencing symptoms of decompensation. Ironically, the lower MELD score makes it less likely for them to receive a transplant, thus leaving this group of patients in “purgatory”. This data indicates that the threshold which physicians were using for listing patients had not changed over time and that the concept of entering “MELD purgatory” was not occurring in the United Kingdom[81].
Other centres in Europe have reported an improvement in liver function of HCV patients on the transplant waitlist following DAA treatment to the point where up to 1 in 4 patients can be removed from the waitlist or delisted. This decline in listing numbers is similar to that noted a decade ago after the introduction of antiviral therapy for hepatitis B. In an era where there is a chronic shortage of donor organs and an increasing need, early treatment with DAAs is beneficial in reducing the number of patients needing to progress to transplant[82,83].
HCV recurrence in the graft was a common problem post-transplant in patients with HCV. This contributed to accelerated fibrosis progression due to an immune incompetent state, resulting in graft dysfunction and eventual failure. With the introduction of DAAs, treatment of HCV recurrence in the post-transplant setting now prevents graft loss and the need for re-transplantation in this cohort[82,83].
Extrahepatic manifestations
HCV infection has an impact not only on the liver, but also systemically. A meta-analysis of 22 studies demonstrated that HCV infection increases the risk of cardiovascular mortality, with a two-fold higher risk of subclinical carotid plaques amongst HCV patients. HCV patients also have a higher risk of peripheral artery disease, cardiovascular events and cardiovascular mortality[84]. These changes are theorized to be due to systemic inflammation and release of inflammatory cytokines within the body caused by the virus. Based on this theory, treatment of HCV should result in a reduction in risk of extrahepatic manifestations.
A prospective Italian study by Nevola et al[85] evaluated HCV patients before and 24 wk after treatment with DAAs. After achieving SVR, patients demonstrated an improvement in fasting blood glucose levels and reduced insulin resistance, regardless of the fibrosis state. In patients with impaired fasting glucose pre-treatment, 32% had normal blood glucose levels following SVR. Forty-five percent of diabetic patients experienced an improvement in glycaemic control, with some patients managing to reduce or cease their diabetic medications[85]. This indicates that HCV clearance is associated with a significant change in glucose metabolism, independent of baseline BMI. This has also been confirmed by other studies[86-90].
Changes in lipid profiles also occur following SVR, commonly manifest with an increase in LDL levels. The effect on HDL and triglycerides is less consistent across studies[91-95]. Lipid disturbances require closer monitoring in patients who have achieved viral clearance. Although lipid profiles may deteriorate following SVR, renal function improves. Patients in the Nevola cohort experienced an improvement in eGFR by 10% compared to baseline, regardless of the fibrosis status[85].
Depression is co-morbid with HCV infection in up to one-third of cases. Patients are often marginalized and experience psychosocial stigma due to their positive HCV status. From a physiological standpoint, it is thought that the systemic inflammation caused by the virus and the invasion of HCV into the nervous system results in disruptions of neurotransmission pathways. Moez et al[96] used the Beck Depression inventory scale to prospectively assess change post HCV treatment in their cohort of Egyptian patients and found a significant decrease in scores 1 month and 3 months post-SVR which correlated with an improvement in mood symptoms.