Published online Aug 7, 2021. doi: 10.3748/wjg.v27.i29.4746
Peer-review started: January 28, 2021
First decision: June 14, 2021
Revised: June 23, 2021
Accepted: July 22, 2021
Article in press: July 22, 2021
Published online: August 7, 2021
Processing time: 188 Days and 2.9 Hours
Ischemic bowel disease (ISBODI) includes colon ischemia, acute mesenteric ischemia (AMI) and chronic mesenteric ischemia (CMI). Epidemiologically, colon ischemia is the most common type followed by AMI and CMI. There are various risk factors for the development of ISBODI. Abdominal pain is the common presenting symptom of each type. High clinical suspicion is essential in ordering appropriate tests. Imaging studies and colonoscopy with biopsy are the main diagnostic tests. Treatment varies from conservative measures to surgical resection and revascularization. Involvement of multidisciplinary team is essential in managing ISBODI. Although open surgery with revascularization plays an important role, recently there is an increasing interest in percutaneous endovas
Core Tip: Ischemic bowel disease (ISBODI) includes a spectrum of diseases due to inadequate blood supply to the bowel wall. In this review, we will be discussing the epidemiology, risk factors, pathophysiology, clinical aspects, investigations, current management protocols and prognosis of each type of ISBODI.
- Citation: Ahmed M. Ischemic bowel disease in 2021. World J Gastroenterol 2021; 27(29): 4746-4762
- URL: https://www.wjgnet.com/1007-9327/full/v27/i29/4746.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i29.4746
Ischemic bowel disease (ISBODI) is the most common vascular disorder of the gastrointestinal tract. ISBODI results from inadequate supply of oxygenated blood to any part of the bowel wall. ISBODI is a heterogenous group of disorders that can be divided into small intestinal ischemia or mesenteric ischemia, and large intestinal ischemia or colonic ischemia (CI) or ischemic colitis (IC). Clinically mesenteric ischemia is further classified as acute mesenteric ischemia (AMI) and chronic mesenteric ischemia (CMI). Although ISBODI is not that common in clinical practice, the morbidity and mortality can be high if this entity is not recognized and treated emergently.
CI is the most common entity of ISBODI. The exact epidemiology of ISBODI is not known. One study showed that the overall age-adjusted and sex-adjusted incidence of CI was about 6.1 cases per 100000 person-years in 1976-1980 and it increased by about 4-fold to 22.9/100000 person-years over the course of few decades (2005-2009)[1]. The prevalence of AMI is 1 in 1000 hospital admissions, whereas the prevalence of CMI is 1 in 100000 individuals[2]. The incidence of CMI is probably only 2-3 per 100000 persons per year[3]. AMI represents an uncommon cause of acute abdominal pain as the overall incidence of AMI is 0.09% to 0.2% of all acute surgical admissions[4].
ISBODI is commonly seen in the elderly population with mesenteric vascular atherosclerosis[5]. Other risk factors include diabetes mellitus, hypertension, coronary artery disease, peripheral artery disease, atrial fibrillation, congestive heart failure, recent myocardial infarction, shock, chronic renal failure requiring hemodialysis, severe dehydration, chronic obstructive lung disease, irritable bowel syndrome, sickle cell crisis with microvascular occlusion, rheumatic autoimmune diseases, substance abuse like amphetamine abuse or cocaine abuse, long distance running, hereditary and acquired thrombophilia[6]. Iatrogenic intestinal ischemia can occur post-surgically (abdominal aortic aneurysm surgery, colectomy, bypass surgery), due to dislodgement of intravascular devices, and secondary to use of certain medications–estrogen, digoxin, danazol, alosetron, pseudo ephedrine, vasopressin, Gold, psychotropic drugs, sumatriptan, serotoninergic agonists and anatagonists immunomodulators, laxatives and non-steroidal anti-inflammatory drugs[7,8]. Enterocolonic ischemia may also be due to systemic or infectious vasculitis, fibromuscular dysplasia, radiation and amyloidosis[9]. Colonic obstruction due to neoplasm, diverticular disease, adhesion, volvulus, intussusception, internal hernia with obstruction or fecal impaction may also contribute to CI although in most cases the precipitating cause remains unidentified. The CI usually occurs proximal to the obstruction[10].
There are several physiological factors which can make the colon more susceptible to ischemia. There is less blood flow to the colon (per 100 g of tissue) as compared to other parts of the gastrointestinal tract. Functional colonic motor activity further decreases colonic blood flow. The colon also depends on collateral circulation frequently. During hypotensive episodes, the colon cannot autoregulate well compared with the rest of the gastrointestinal tract[11].
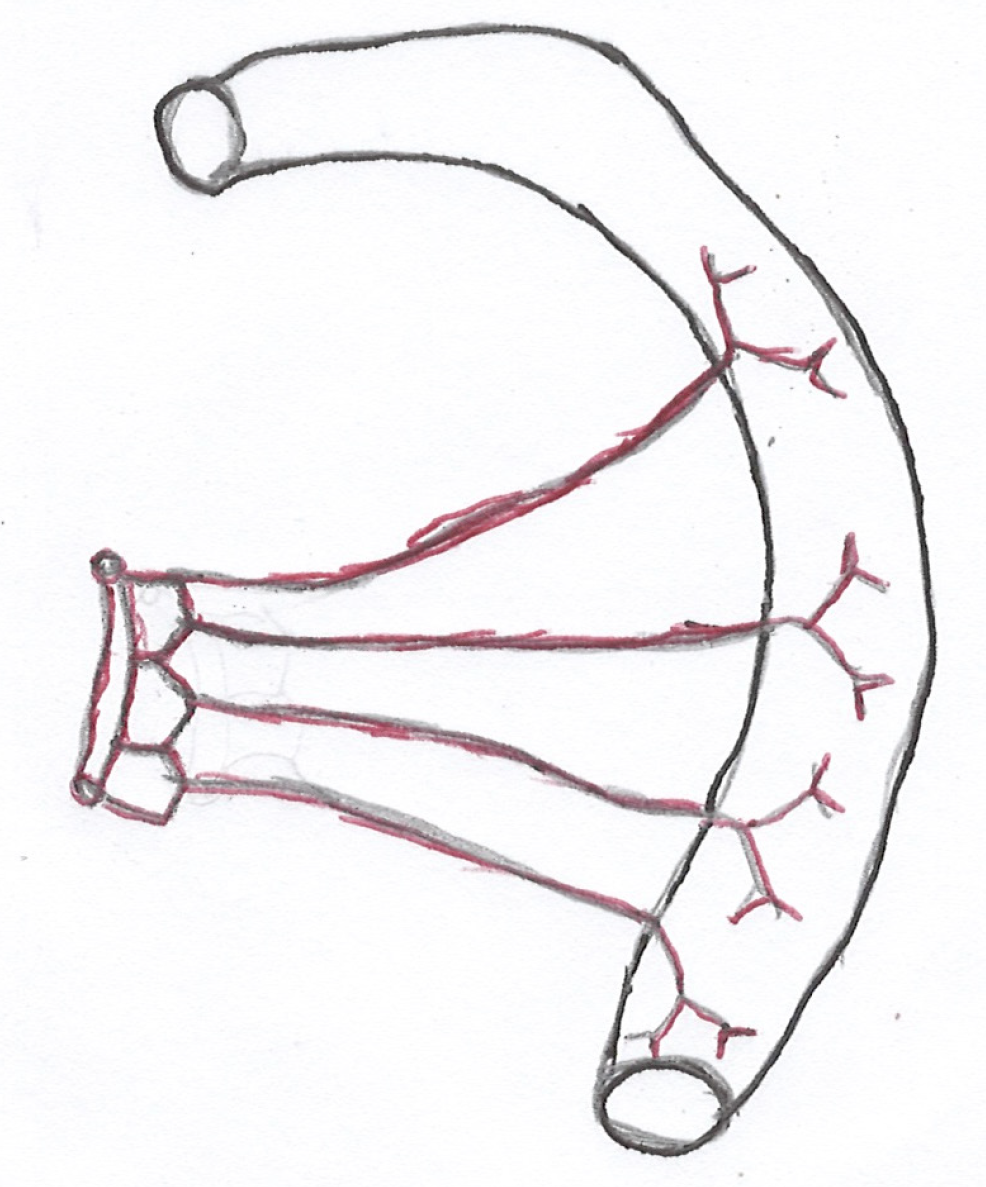
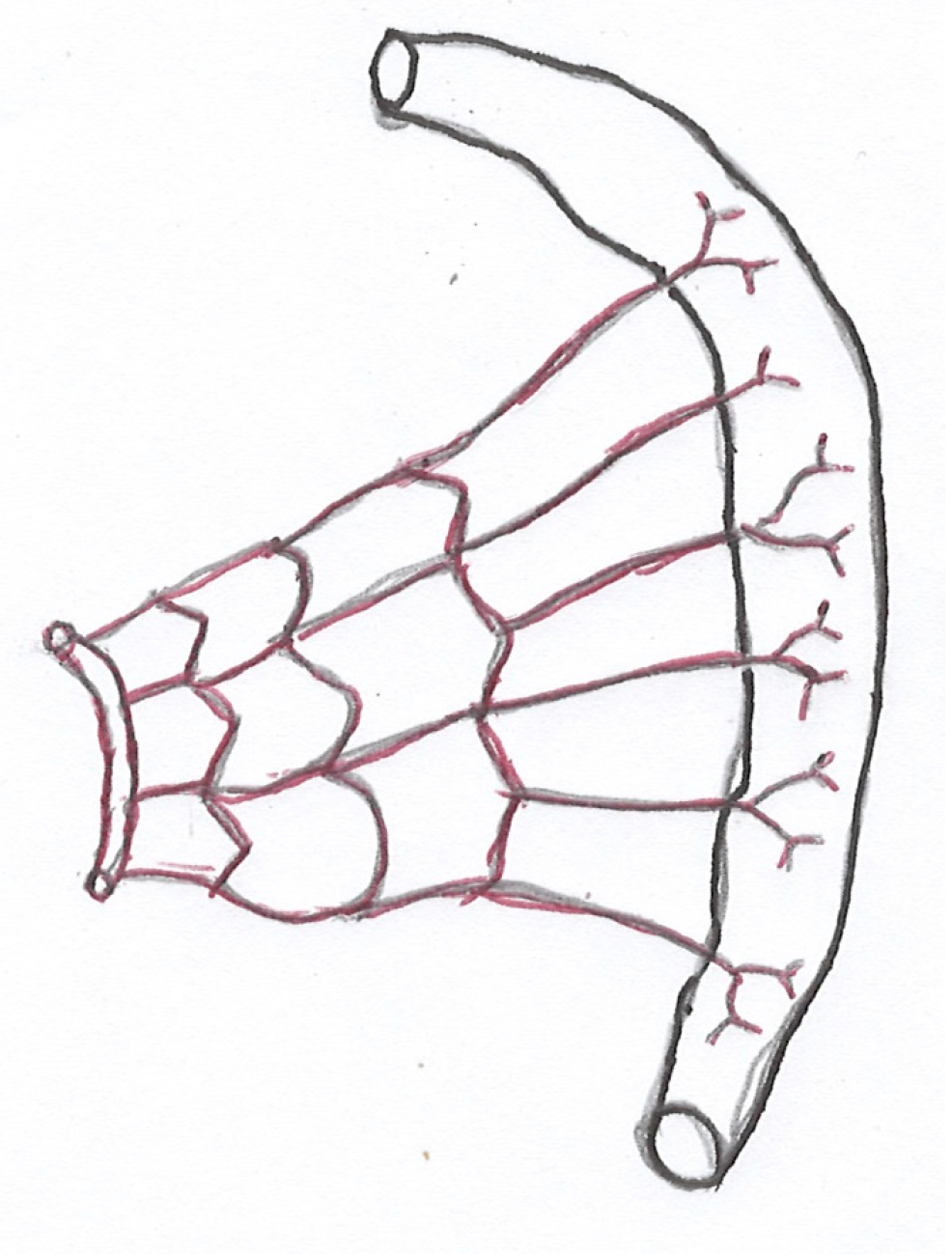
It is important to have a clear knowledge of the vascular supply and venous drainage of the intestine to understand the pathophysiology of ISBODI. Small intestine and large intestine derive their blood supply mainly from the superior mesenteric artery (SMA) and the inferior mesenteric artery (IMA). Proximal part of the duodenum (up to the level of ampulla of Vater) receives its blood supply from the celiac artery (CA) via gastroduodenal artery (GDA) (a branch of hepatic artery) and superior pancreaticoduodenal artery (a branch of GDA)[12]. Rest of the small bowel and right two-third of the colon (up to splenic flexure) are supplied by SMA. Numerous branches arise from the SMA to supply the jeunum and ileum. These branches pass between the layers of the mesentery and form anastomotic arcades from which straight arteries called vasa recta arise to supply jejunal and ileal walls. Jejunal arterial arcades are less in number but they have long vasa recta. Ileal arterial arcades are more in number but they have short vasa recta (Figures 1 and 2)[13]. As a result, jejunum is more vulnerable to ischemic injury than ileum.
Left one-third of the colon and upper two-third of the rectum are supplied by IMA (most diminutive of 3 mesenteric arteries). Lower one third of the rectum is supplied by middle rectal artery (a branch of anterior division of internal iliac artery) and inferior rectal artery (a branch of internal pudendal artery). The transverse colon is mostly supplied by the middle colic artery which is a branch of the SMA. It may also receive blood supply from the marginal artery which is the anastomotic arcade between the left colic and right colic arteries. There are 3 watershed zones in the colon that receives blood supply from the distal branches of two large arteries. As they have the fewest vascular collaterals, they are prone to develop non-occlusive IC at the watershed zones when blood flow decreases. The watershed zones include the splenic flexure or Griffith’s point, rectosigmoid region or Sudeck’s point and the ileocecal region[14]. The small bowel mucosa is more sensitive to ischemia as compared to colonic mucosa but it has tremendous regenerative potential. On the other hand, colonic mucosa is less easily damaged by ischemia but once destroyed, slow recovery occurs[15].
Of 25% of resting cardiac output and 35% of post-prandial cardiac output go to the mesenteric circulation[16]. Mucosa and submucosa of the intestine receive 70% of mesenteric blood while muscularis propria and serosa receive the remainder (30%) of mesenteric blood. Mesenteric ischemia does not occur until the blood supply is reduced by more than 50%[17] or patient’s mean arterial pressure does not go below 45 mmHg[18]. Significant injury to the intestine may not occur even if a 75% reduction in blood flow occurs for up to 12 h but complete occlusion of blood flow will cause bowel wall infarction within 6 h.
The venous drainage follows the arterial supply forming venae rectae and venous arcades so that duodenal venous drainage occurs via the splenic vein or superior mesenteric vein (SMV); jejunal, ileal and proximal (two-third) colonic venous drainage occurs via SMV, and distal (one-third) colonic venous drainage occurs via inferior mesenteric vein. Ultimately, these veins drain into the portal vein[19]. Atherosclerosis, thromboembolism, hypoperfusion (as a result of arrhythmia, heart failure, shock), hypercoagulable state, vasculitis, venous occlusion or any mechanical obstruction to the mesenteric blood vessels may to lead to ISBODI[20]. In most cases of SMA embolism, duodenum, proximal jejunum and transverse colon get spared as emboli lodge 6 to 8 cm beyond its origin, often beyond the origin of middle colic artery. Emboli lodge into the SMA more easily possibly because of its large diameter and its origin from the aorta at an acute angle[21]. On the other hand, SMA thrombosis generally occurs at the origin of SMA at the site of severe atherosclerotic narrowing[22]. Proximal colon may become ischemic because of involvement of ileocolic artery. Mesenteric venous thrombosis (MVT) generally involves the SMV but inferior mesenteric veinous (IMV) thrombosis can occur in up to 11% of all cases of MVT[23]. Venous thrombosis leads to intestinal edema and increased vascular resistance leading to bowel ischemia. Non-occlusive mesenteric ischemia (NOMI) results from mesenteric vasoconstriction associated systemic hypotension and low flow state in the splanchnic circulation. NOMI can cause small bowel as well as proximal CI and necrosis because of involvement of ileocolic artery.
AMI occurs due to sudden decrease in blood flow to varying portion of the small intestine and/or colon. The ischemic process can be severe enough to cause instant bowel wall infarction. The main mechanism of ischemic injury is hypoxia and cellular acidosis due to local accumulation of lactic acid and other cellular metabolic by-products secondary to anerobic glycolysis. Complete ischemia may result in infarction of the bowel wall supplied by the blood vessel whereas relative ischemia generally causes cellular dysfunction[24]. If the vascular obstruction is relieved soon after ischemic injury, reversibly injured cells may recover but if reperfusion occurs late, irreversibly damaged cells may not recover and this may lead to inflammation and bleeding due to release of reactive oxygen species and endothelial damage that occurred during the ischemic period. The restored blood flow to the ischemic area can cause further tissue damage called reperfusion injury as the white blood cells (WBC) in the blood release free radicals and interleukins; oxygen delivery to the ischemic tissue damages plasma membrane, DNA and cellular protein; and plasma membrane injury may release more free radicals. WBC can cause further ischemia if they bind to the endothelial cells of capillaries[25]. The pathophysiological process correlates well with the clinical scenario. In the first phase, patient feels abdominal pain because of bowel ischemia and hyperperistalsis. In the second phase (after 3 to 6 h), patient feels better as the intestine becomes aperistaltic and the intramural pain receptors become damaged by ischemia. In the third phase, mucosal necrosis occurs followed by infarction of the bowel wall. Bacterial translocation leads to gangrene of the intestine, diffuse peritonitis, sepsis and multi-organ failure[26].
CI can cause reversible damage or irreversible damage[27]. Reversible damage occurs in 50% of cases and this includes: (1) Reversible ischemic colopathy due to submucosal or intramural hemorrhage in two-third of cases; and (2) Transient segmental IC in one-third of cases. Irreversible damage may cause chronic ulcerative IC in 20%-25% of cases, ischemic colonic stricture in 10%-15% of cases, colonic gangrene in about 15% of cases, and fulminant universal colitis or pancolitis in only 1% of cases[28]. Old age, long duration of subjective symptoms and prolonged Laboratory (Lab) abnormalities (elevated white blood cell count or erythrocyte sedimentation rate) may predict the development of chronic ulcerative IC or ischemic colonic stricture. 85% of cases of CI are non-gangrenous, transient and self-limiting[29]. In 95% of cases, CI occurs due to sudden but transient reduction in blood flow to the colon due to non-occlusive causes and as a result, the watershed zones are affected[30]. Most of the time, the cause of non-occlusive ischemia remains unidentified and it is thought to be due to localized non-occlusive ischemia secondary to small-vessel disease (Type I disease). Rarely, episodes of non-occlusive ischemia secondary to systemic hypotension are identified (Type II disease). Occlusive ischemia due to atheromatous emboli or vasculitis affects short segment of the colon. CI can affect any part of the colon but the left colon is involved in two-third of cases[31]. Segmental involvement of the colon is commonly observed, particularly the splenic flexure, the descending colon and the sigmoid colon are the commonest sites of involvement. Isolated right colon ischemia can occur in about 10% of cases. Right colonic involvement generally occurs in patients with chronic renal failure requiring hemodialysis and is associated with severe IC[32].
Ischemic injury first occurs in the colon mucosa and it is most commonly seen on the antimesenteric side. Subsequently the ischemic changes may extend to the outer layers of the colon wall.
CMI occurs as a result of gradual decrease in blood flow to the intestine. As a result, numerous collaterals develop in the mesenteric circulation[33]. CMI is almost always associated with athererosclerosis. Although the prevalence of asymptomatic chronic stenotic mesenteric disease varied from 3% (below the age of 65 years) to 18% (above the age of 65 years) on doppler sonography in some studies[34], the incidence of CMI is low as mentioned before. Asymptomatic single vessel disease is more common in CA (81%) than in the SMA (19%). Generally, two out of the three major blood vessels (CA, SMA and IMA) need to be stenotic to produce ischemic symptoms. But patients may develop symptoms even with single vessel involvement[35] and many patients with two vessel disease may remain asymptomatic for many years because of extensive collaterals in the mesenteric circulation[36]. The most common cause of single vessel involvement is CA compression syndrome or median arcuate ligament syndrome where eccentric compression of the CA and/or celiac ganglion occurs by the median arcuate ligament and diaphragmatic crura[37]. The pancreaticoduodenal artery arcades, GDA, dorsal pancreatic artery and occasionally the arc of Buhler (embryonic anastomotic branch between 10th and 13th ventral segmental arteries) are the most common collaterals between the CA and the SMA[38]. On the other hand, the marginal artery of Drummond (anastomosis between the terminal branches SMA and IMA forming a continuous arterial arcade along the inner border of the colon) and arc of Riolan/meandering mesenteric artery (arterioarterial anastomosis between the middle colic branch of SMA and left colic branch of IMA running close to the root of the mesentery) are the most common collaterals between the SMA and IMA[39]. The exact association of severity of mesenteric arterial stenosis with collaterals formation and symptom development is not known.
Chronic NOMI is another entity found in 13% to 16% of all patients with CMI[40]. Its clinical presentation is similar to chronic occlusive mesenteric ischemia but vascular occlusion is not identified. The exact etiology is not known but possible pathophy
AMI is a life threatening potentially fatal abdominal and vascular emergency due to diminished blood flow in the superior mesenteric blood vessels. Patients are generally above the age of 60 and women are 3 times more commonly affected as compared to men. Although the clinical presentation is similar, the etiology could be occlusive or non-occlusive ischemia. SMA embolism is the most common cause (40%-50%) followed by SMA thrombosis (15%-25%) followed by MVT (5%-15%)[41]. NOMI occurs in about 5 to 15% of cases AMI[42].
Patients generally present with sudden onset of severe sharp abdominal pain that is generally accompanied by nausea and vomiting. The pain is out proportion to physical signs. In the early phase, abdomen is generally soft and non-tender but auscultation may reveal hyperactive bowel sound. Tenderness to palpation occurs when the entire bowel wall becomes ischemic. In late phase (within 12 h of onset) rebound tenderness (Blumberg’s sign), rigidity and guarding indicate bowel wall infarction. At this stage, bowel sounds become hypoactive or absent, patient develops bloody diarrhea, fever and shock.
High degree of clinical suspicion is necessary to establish the diagnosis of AMI. A focused history is important to evaluate the underlying pathophysiology of AMI. Patients with atrial fibrillation, valvular heart disease, congestive heart failure, recent myocardial infarction and bacterial endocarditis are at increased risk of mesenteric embolism. About 50% of patients with embolic AMI have atrial fibrillation. Prior history of embolism is present in about one third of patients of embolic AMI[43]. The clinical triad of (1) Severe abdominal pain out of proportion to physical signs; (2) Spontaneous bowel movement (vomiting and/or non-bloody or bloody diarrhea); and (3) Obvious source of emboli such as atrial fibrillation or recent myocardial infarction may be present in 40% to 80% cases of SMA embolism[44]. History of chronic abdominal pain in the postprandial period, progressive weight loss and vascular procedures in the mesenteric arteries may be present in patients with mesenteric arterial thrombosis. Chronic and progressive symptoms appear in mesenteric arterial thrombosis is because of preexisting atherosclerosis plaque affecting mesenteric artery is the most common finding. NOMI should be considered in patients with hypotension and generalized (diffuse) abdominal pain. As NOMI generally occurs in critically ill patients on ventilator, the presentation can be silent. Gastrointestinal bleeding or unexplained abdominal distention can be the only presentation of NOMI. MVT is suspected when patients with thrombophilia (hereditary or acquired) or coagulopathy present with abdominal pain (84% of cases), diarrhea (42% of cases), nausea/vomiting (33% of cases) or gastrointestinal bleeding (10% of cases)[45]. The author finds these clinical suspicions very useful in categorizing the pathophysiologic form of AMI that can guide the investigation and therapy.
CI is mostly a benign and self-limiting disease in most of the time although a minority of cases can be severe. Most of the patients with non-iatrogenic causes are older than 60 years old with a slight female predilection. Rarely, IC can also occur in young individuals due to substance abuse, vasculitis, long distance running, thrombophilia, sickle cell crisis with microvascular occlusion, irritable bowel syndrome and iatrogenic causes as mentioned before. Chronic constipation and prior history of abdominal surgery are common in both young and old patients with CI[46]. One study found that 6.8% of patients had a recurrence of CI with a mean follow-up of 4.5 years[47]. The recurrence is more common in patients with hereditary thrombophilia (deficiencies of protein C, protein S, antithrombin III, and factor V Leiden mutation), anti-phospholipid syndrome, active smoking, abdominal aortic aneurysm, coronary artery disease and elevated serum creatinine[48,49].
Patients with CI generally present with sudden onset of mild to moderate cramping pain over left lower quadrant of the abdomen or hypogastrium, and urgency to defecate followed by hematochezia within 24 h. The bleeding is not severe enough to require blood transfusion. Patients with right colon ischemia usually present with hypogastric pain rather than hematochezia[50]. Physical examination may reveal mild to moderate tenderness over the left lower quadrant or over the involved segment. The usual course of the disease is benign in non-gangrenous and non-fulminant CI. Patients feel better with resolution of abdominal pain and hematochezia in few days time. Persistent symptoms more than few days may indicate development of chronic CI i.e. chronic ulcerative IC or ischemic colonic stricture. Gangrenous colitis or fulminant pancolitis is generally recognized by marked abdominal tenderness, rebound tenderness, hypotension or shock. Absence of hematochezia and development of hypoalbuminemia may predict the development of gangrenous changes of CI[51].
CMI also called abdominal angina or intestinal angina or migraine abdominale is mostly seen in patients older than 60 years of old and is 3 times more common in females than in males[52]. Patients generally present with the triad of sharp post-prandial epigastric or mid-abdominal pain, sitophobia (fear of eating food) and chronic weight loss[53]. Patient starts feeling abdominal pain 15 to 30 min after a meal and the pain lasts for about half an hour to 2 h[54]. As the diseases progress, abdominal pain becomes dull-aching and chronic. Patients may also develop nausea, vomiting, diarrhea or constipation[55]. Physical examination may reveal abdominal bruit. But only 16% to 22% of patients with CMI have the classic triad of post-prandial abdominal pain, weight loss and abdominal bruit[56].
AMI–At the present time, there is no specific Lab test which can identify AMI. But evidence of hemoconcentration, elevated white cell count, high anion gap lactic acidosis and positive D dimer test (fibrinolytic marker) in the presence severe abdominal pain disproportionate to physical sign may indicate AMI. Leucocytosis is the most common Lab finding seen in > 90% patients with AMI followed by lactic acidosis seen in 88% of cases of AMI[57]. But as the liver can metabolize large amount of L-lactic acid from the porto-mesenteric circulation and lactic acidosis can occur in dehydrated patients, it cannot reliably differentiate intestinal ischemia from infarction in the absence of appropriate clinical scenario. D-dimer can be considered as an exclusion test as in one study no patient with normal D-dimer had AMI[58]. Hyperamylasemia, hyperkalemia and hyperphosphatemia can occur when mesenteric ischemia progresses to mesenteric infarction[59,60]. Intestinal fatty acid binding protein (I-FABP) is a small, cytoplasmic, water soluble protein found abundantly in the epithelial cells of the small intestine. D-lactate has exclusive intestinal source and is produced by colonic bacterial metabolism. Glutathione S-transferase (GST) is a cytoplasmic enzyme and its alpha isoenzyme (alpha-GST) is almost exclusively located in the small intestine and liver. When binding of transition metals (copper, cobalt and nickel) to the N-terminal of Albumin is reduced in hypoxia or acidosis, it is called ischemia modified albumin (IMAL). I-FABP, D-lactate, alpha-GST and IMAL have been shown to be elevated in early AMI[61-63] but at the present time they are not being used in our clinical practice.
As neither symptoms nor Lab tests are specific for the diagnosis of AMI, imaging plays an important role. The recommended first line imaging study for the diagnosis of AMI is computed tomography angiography (CTA) or biphasic multidetector computed tomography of the abdomen and pelvis with IV contrast[64]. CTA is not only rapid, non-invasive, easily available and highly accurate (sensitivity 89%, specificity 99%) in diagnosing mesenteric ischemia but also helpful in detecting the underlying etiology of ischemia[65]. CTA can evaluate the bowel wall, vascular abnormalities and surrounding viscera. CTA may show bowel wall thickening, bowel wall hypoenhancement, intramural hemorrhage, fluid-filled dilated bowel loops, arterial or venous thrombus, decreased filling, narrowing, irregularity, spasm or engorgement of mesenteric vessels, pneumatosis intestinalis, portomesenteric venous gas, or infarction of other viscera[66]. Some of the findings may give a clue to the underlying cause of ischemia: Hypoenhanced bowel wall generally suggests arterial occlusion, bowel wall thickening may indicate MVT and patent SMA with diffuse narrowing of its branches (string of sausages sign) is seen in NOMI[67]. Considering serious and lethal consequences of missed diagnosis of AMI, CTA with intravenous contrast material should be done as soon as possible in patients with suspected AMI irrespective of renal function[68]. Traditional mesenteric angiogram or catheter-based angiogram is the gold standard considering its diagnostic and therapeutic potential but considering its invasiveness, expense and lack of wide availability it is now considered as the 2nd line modality in the evaluation of AMI[69].
Plain X-ray and ultrasound of abdomen have limited role in diagnosing AMI[70]. But plain X-ray abdomen may show free intraperitoneal air if bowel infarction and perforation occur. Mesenteric vessel duplex ultrasound scan (DUS) is useful in the evaluation of AMI. One multicenter study showed that DUS done at the Emergency Department can be helpful (sensitivity 100%, specificity 64%, negative predictive value 100%) in diagnosing and excluding occlusive AMI[71]. DUS is also very helpful in direct evaluation of MVT in the setting of AMI. Although it is specific and can be done at the bedside, it is not as sensitive as CTA or MRA[72]. DUS has also certain limitations that it is operator dependent, not useful in presence of dilated bowel with gas, cannot detect thrombus or embolus beyond the major vessels, and it cannot identify NOMI.
Contrast enhanced MRA is an excellent radiation-free non-invasive test with a sensitivity of 100% and a specificity of 91% in diagnosing AMI. But it is not considered as a practical test in diagnosing AMI considering its long time to do the test and high expense.
Once the diagnosis of MVT is made on imaging, the underlying cause of MVT should be investigated particularly for congenital or acquired thrombophilia. Patients should be evaluated for protein C, protein S and anti-thrombin III deficiency, factor V Leiden mutation, myeloproliferative disorders, homocystinuria, paroxysmal nocturnal hemoglobinuria as well as inflammatory bowel disease when more stable.
CI: There is no Lab test diagnostic of CI. Complete blood count, complete metabolic profile, serum lactate, lactate dehydrogenase (LDH), creatinine phosphokinase (CPK), amylase and coagulation studies are helpful assessing the severity of CI. Low hemoglobin may indicate the amount of colonic blood loss. Leucocytosis, lactic acidosis, hypoalbuminemia, elevated LDH, CPK and amylase may suggest colonic infarction[73]. Plain X-ray abdomen is normal in most of the time but rarely it may show ‘thumbprinting’ due to submucosal edema/hemorrhage, pneumatosis linearis due to necrosis of the colon wall, portal venous gas, colonic ileus or pneumoperitoneum due to perforation[74]. CT abdomen and pelvis with oral and intravenous contrast is the imaging study of choice to evaluate CI[75]. But as patients with right-sided CI (RSCI) can have large visceral artery occlusion, CTA should be done in case of suspected RSCI or when the diagnosis of AMI needs to be ruled out[76].
Colonoscopy with biopsy within 48 h is the next step to conform the diagnosis of CI. In non-gangrenous IC, colonoscopy may show a highly specific sign like a single linear ulcer running longitudinally along the antimesenteric colonic wall (colon single-strap sign) or non-specific signs like segmental erythema, fragile and edematous colon mucosa, scattered hemorrhagic erosions, scattered petechial hemorrhages with pale areas, bluish hemorrhagic nodules due to submucosal hemorrhage and sometimes mass-like lesions mimicking malignancy[77,78]. In gangrenous IC, colon mucosa will appear as black or gray-green. Histology is nonspecific most of the time. It may show focal crypt drop out, lamina propria and submucosal hemorrhage and edema, hemosiderin laden macrophages in the submucosa, fibrin thrombi in capillaries with infiltration of neutrophils, erosion and granulation tissue hyperplasia[79]. Necrosis and ghost cells i.e. preserved cellular outlines with empty cell contents are pathog
CMI: Lab tests are generally non-specific but may show anemia, leukopenia, lymphopenia and hypoalbuminemia indicating underlying malnutrition due to CMI. There are various imaging studies available to diagnose CMI. According to American College of Radiology, CTA should be the first-line test to evaluate CMI[80]. Gadolonium-enhanced MRA is highly sensitive in detecting stenosis at the origin of CA, SMA and IMA. One disadvantage of MRA is that it may not be able to evaluate the IMA well[81]. Duplex ultrasound is cost-effective and may play an important role in the diagnosis of CMI. Fasting peak systolic velocity ≥ 275 cm/s in the SMA and ≥ 200 cm/s in the CA can reliably (sensitivity and specificity around 90%) indicate ≥ 70% stenosis[82]. But the study is time consuming and can be unsatisfactory due to bowel gas, obesity, body habitus, complex anatomy, post-operative changes and operator dependency[83]. Catheter-based angiography had been the gold standard for diagnosing CMI for many years[84]. Now-a-days it is done mainly for therapeutic intervention and also for diagnostic confirmation when the results of other imaging modalities are inconclusive.
The main goal of managing AMI is not only prompt diagnosis but also resuscitation of the patient and revascularization of the bowel before irreversible damage occurs. As soon as AMI is suspected, the patient should be given supplemental oxygen and adequate hydration with crystalloid infusion (up to 100 mL/kg) to restore tissue perfusion. Hemodynamic monitoring including mental status and urine output should be done[85]. If the patient still remains hypotensive despite adequate fluid resuscitation, vasopressor pressor agents should be considered. But catecholamines should be avoided as they decrease splanchnic blood flow. Vasopressor agent like dobutamine, low dose dopamine or milrinone can be used to maintain blood pressure as they have less vasoconstrictive effect on mesenteric vessels. Many patients with atrial fibrillation/flutter will need medications to control the ventricular rate. In those cases, medications other than cardiac glycosides should be used as they can also reduce splanchnic blood flow. Any acid-base and electrolyte abnormalities (metabolic acidosis, hyperkalemia) should be corrected. Nasogastric tube should be placed for decompression. Broad spectrum antibiotics and heparin infusion should be started. Both general surgery and vascular surgery teams should be consulted and after resuscitation, laparotomy should be done promptly if the patient has signs of peritonitis (rebound tenderness) indicating bowel infarction. During laparotomy, the whole bowel should be inspected carefully and only the clearly defined infarcted bowel should be resected. According to Bulkley et al[86], signs of intestinal viability include normal looking bowl wall without bleeding, pulsatile artery and stimulation of intestinal peristalsis by mechanical factors or heat[86]. If there is any suspicion, intraoperative Doppler ultrasound should be done to find out the presence of Doppler flow over the distal branches of SMA in order to preserve all the viable bowels. Potentially viable bowel should be inspected for 30 min after revascularization. Revascularization technique depends on the underlying pathophysiologic form. In case of SMA embolism, a standard embolectomy is performed by doing a tranverse arteriotomy after clamping the SMA proximal and distal to the embolus[87]. The arteriotomy site is then closed with nonabsorbable interrupted sutures. In case of SMA thrombosis, a longitudinal arteriotomy is done followed by revascularization either by thromboendartectomy with a Fogarty catheter[88] or a bypass procedure. After thromboendartectomy, heparinized saline is used to flush the artery. If thromboar
Over the last 15 years there has been tremendous interest in minimally invasive endovascular treatment of embolic or thrombotic AMI. This should be considered when there is no clinical, serological or imaging evidence of necrosis or infarction of the bowel wall. Endovascular treatment involves less surgical trauma and less intensive unit care in the elderly patients with multiple comorbidities as compared to open surgical procedures. The endovascular interventions include endovascular embolectomy by transcutaneous mechanical aspiration, percutaneous aspiration thrombectomy, intraarterial pharmacological thrombolysis with infusion of urokinase for 2 to 12 h, and percutaneous transluminal angioplasty with or without stenting[90-93]. Intraarterial infusion of papaverine (30 to 60 mg/dL) is also considered when there is evidence of vasospasm in embolic or thrombotic AMI and the infusion is generally continued for 24 h. Intraarterial bolus followed by infusion of heparin is continued throughout the procedure. But heparin infusion should not be mixed with papaverine (a phosphodiesterase inhibitor) because of the risk of precipitation. After endovascular treatment, patient should receive low molecular weight heparin for 5 d and then anticoagulation should be continued with warfarin. In patients with stenting, in-stent restenosis and high symptom recurrence have been reported. So, if no contraindication exists, patients should be instructed to take daily antiplatelet indefinitely. Although the technical success rate of endovascular treatment in acute arterial mesenteric ischemia can be as high as 100%, intestinal necrosis can occur following the treatment requiring laparotomy and intestinal resection[94]. Other potential complications of endovascular treatment include vascular access-related bleeding, hematoma or pseudoaneurysm formation, and trauma to the vessels leading to arterial dissection or atheroembolization. One study compared endovascular treatment with open surgical treatment. Endovascular treatment group needed less laparotomy (33.3% vs 58.3%), had significantly shorter length of bowel resection (88 ± 44 cm vs 253 ± 103 cm) and less mortality (16.7% vs 33.3%)[95]. A completion angiogram is necessary following endovascular treatment to assess revascularization[96]. Revascularization is required in more than 70% of patients with occlusive AMI while bowel resection alone can be life saving in 30% of patients with occlusive AMI[97]. Hybrid technique (i.e. combination of endovascular surgery and open vascular surgery) is also widely used in the treatment of occlusive AMI. Intra-arterial catheter-directed thrombolysis following SMA recanalization by intraoperative retrograde balloon angioplasty, retrograde open mesenteric stenting or open embolectomy during laparotomy has been found to prevent necrosis of ischemic bowel and decrease the occurrence of short bowel syndrome[98].
The main principle of management of NOMI is reversal of mesenteric arterial spasm and restoration of mesenteric blood flow as fast as possible[99]. As soon as the diagnosis of NOMI is made by CTA, patients with NOMI should be resuscitated. The precipitating factors should be addressed to improve mesenteric perfusion. Measures should be taken to correct hypovolumia and hypotension, improve cardiac function in congestive heart failure, treat cardiac arrhythmias, correct metabolic acidosis, initiate broad-spectrum antibiotics, and place a nasogastric tube for gastric decompression. The next step of management depends on the stage of mesenteric ischemia. If the patient has developed signs of bowel infarction, surgical intervention should be done to resect the infarcted bowel and second look surgery should be done to save as much bowel as possible[100]. If there is no sign of bowel infarction, prompt catheter-based mesenteric arteriogram should be done to start continuous intra-arterial vasodilator infusion (papaverine 30-60 mg/h, prostaglandin PGE1 alprostadil 20 mcg bolus followed by infusion 60-80 mcg/24 h) for about 48 h by placing the catheter into the proximal SMA[101]. Frequently arterial vasospasm responds to this direct vasodilator treatment and occasionally within minutes[102]. One study showed that the shorter the time from CTA to direct vasodilator infusion, the better the chance of survival[103].
Treatment of MVT again depends on the presence or absence of bowel infarction. In the absence of bowel infarction, the main stay of treatment is anticoagulation with continuous heparin infusion which has been shown to cause frequent recanalization with better outcome[104]. Systemic thrombolysis with recombinant tissue–type plasminogen activator or transcatheter thrombolysis with urokinase can also be an initial effective treatment for acute superior MVT[105,106]. Anticoagulation with warfarin or direct oral anticoagulants (DOAC) should be continued for 3 to 6 mo in case of reversible causes, and for indefinite period in case of irreversible causes[107]. Patients with bowel infarction should have emergency surgery with resection of the infarcted bowel followed by second look surgery. Transjugular intrahepatic portosystemic shunt can be placed in case of extensive MVT to reduce the portal pressure and to increase the effectiveness of thrombolytic therapy[108].
CI is managed by supportive care in most of the cases in clinical practice. But risk stratification is extremely important in deciding whether supportive care only or surgical intervention is needed. Broadly, CI can be classified as non-gangrenous and gangrenous forms. But CI can also be classified as mild, moderate and severe disease depending on the presence or absence of certain risk factors associated with poor outcome as proposed by the American College of Gastroenterology (ACG) in 2015[109]. The risk factors include: (1) Male sex; (2) Abdominal pain without rectal bleeding; (3) Tachycardia (heart rate > 100/min); (4) Hypotension (systolic blood pressure < 90 mmHg); (5) Leukocytosis (white blood cell count > 15000/cmm; (6) Anemia (hemoglobin < 12 gm/dL); hyponatremia (< 136 meq/L); (7) Azotemia (blood urea nitrogen > 20 mg/dL; and (8) High serum LDH level (> 350 units/L). Mild CI: No risk factor associated with poor outcome. Moderate CI: Up to 3 risk factors associated with poor outcome. Severe CI: More than 3 risk factors associated with poor outcome or any of the following: (1) Signs of peritonitis on physical examination; (2) Pneumoperitoneum, portal venous gas or pneumatosis on imaging; or (3) Gangrenous colitis on colonoscopy.
Patients with mild to moderate CI should be managed conservatively with bowel rest, intravenous fluid infusion to improve colonic perfusion and nasogastric tube placement if there is ileus. Any precipitating condition like hypotension or cardiac arrhythmia should be treated. Offending medications like mesenteric vasospastic drugs should be discontinued immediately. Most of the patients with mild to moderate CI improve symptomatically in one to two days and recover completely in one to two weeks[110]. But if the patients continue to have symptoms (abdominal pain, hematochezia) with fever, leukocytosis or develop rebound tenderness, surgical exploration should be considered. Patients with severe CI without perforation or gangrenous colitis also need supportive care but they should have close clinical follow up for development of late complications like chronic IC presenting as chronic diarrhea or ischemic colonic stricture presenting as colonic obstruction. In those cases, patients may need elective segmental colonic resection with primary anastomosis. Patients with gangrenous colitis or colonic perforation should undergo surgical exploration for colectomy.
Patients with CMI should have mesenteric revascularization either surgically or endovascularly. Patient’s comorbidities, nutritional status and renal function should be assessed before selecting the revascularization technique. Open surgical procedures include antegrade aorto-mesenteric or aorto-celiac bypass graft, retrograde bypass graft from infrarenal aorta and iliac arteries to distal SMA, endarterctomy and reimplantation of SMA directly into the infra-renal aorta[111-113]. The bypass graft can be synthetic (Polytetrafluoroethylene or Dacron grafts) or autologous (femoral vein or sephanous vein) graft and the type of surgery depends on the location of the stenosis[114]. Open surgical procedures for revascularization are highly successful in symptomatic improvement in 90% to 100% of cases[115] but associated with signi
AMI: Despite the advancement in diagnostic modalities and treatment options, the prognosis of AMI remains grave. Adaba et al[122] did a meta-analysis of observational studies in patients with acute mesenteric infarction and found that the pooled in-hospital mortality was 63%. Mortality was higher in acute arterial mesenteric infarction (73.9%) and NOMI (68.5% as compared to acute mesenteric venous infarction (44.2%)[122]. Risk factors for increased mortality include advanced age, leukocytosis, high arterial lactate level in the first 24 h of admission, metabolic acidosis, bandemia, sepsis, hypoxia, hyperamylasemia, intramural pneumatosis, elapsed time between onset of symptoms and surgery more than 24 h, colon involvement, hepatic and renal impairment and multi-organ failure[123-125]. Mortality can be reduced substantially if diagnosis can be made and treatment given before the development of mesenteric infarction[126]. Patients who survive from extensive bowel resection suffer from short gut syndrome and become dependent on total parenteral nutrition. Klempnauer et al[127] did a long-term study on those patients and found that their 2-year and 5-year survival rates were 70% and 50% respectively[127].
CI: Most of the patients with CI recover quickly within 1 to 2 wk and the overall mortality is almost 10%. Bad prognostic factors include absence of hematochezia, signs of peritonitis (Blumberg’s sign: Rigidity and rebound tenderness), tachycardia, anemia, hyponatremia, right sided colonic involvement and stenosis/stricture seen during colonoscopy[128,129].
CMI: Patients with CMI are at risk of developing acute mesenteric artery thrombosis or embolism that can cause increased morbidity and mortality. Patients with malnutrition due to sitophobia may have to stay at the hospital for prolonged period of time. There are certain prognostic factors associated with bad long-term outcome after open mesenteric revascularization. These include body mass index > 25 kg/m, smoking, peripheral artery disease, hypertensive chronic kidney disease, and open mesenteric bypass with a venous graft[130]. Blauw et al[131] did a retrospective study on the impact of revascularization in patients with CMI and found that mesenteric artery revascularization significantly improved their quality of life[131].
ISBODI is an important clinical entity we come across in our day-to-day clinical practice. Among the 3 forms of ISBODI (AMI, CMI, CI), CI is the most common form.
CI occurs as a result of non-occlusive ischemia due to small vessel disease in most of the time. Rarely, CI is due to systemic hypotension or vascular occlusion due to atheromatous emboli, vasculitis or vascular spasm. Although CI can occur in a well-known clinical setting, in a vast majority of cases, the risk factor remains unidentified. Patients with CI should be stratified into mild, moderate and severe cases depending on the presence or absence of the risk factors associated poor outcome as proposed by the ACG. Management will depend on the severity of the cases. Patients generally present with lower abdominal pain and hematochezia. Segmental involvements of the splenic flexure, rectosigmoid area and ileocecal region are most commonly seen. Imaging with CT followed by colonoscopy with biopsy clinches the diagnosis. Most of the patients belong to mild to moderate severity and CI carries a benign course in those cases. Surgical intervention is rarely required and indicated for fulminant, gangrenous or perforated CI, non-responders to conservative management, chronic ulcerative IC and ischemic colonic structure. Almost 10% of patients can die from CI. AMI is the most severe and devastating form of ISBODI. It has four pathophysiological forms which include SMA embolism, SMA thrombosis, MVT and NOMI. Irrespective of these pathophysiological forms, in the early phase, patients present with sudden onset of severe abdominal pain without much physical sign. As the mesenteric ischemia progresses to mesenteric infarction, patients develop Blumberg’s sign. High clinical suspicion and a focused history are helpful in establishing the diagnosis and suspecting the underlying pathophysiologic form of AMI. Evidence of hemoconcentration, leukocytosis, lactic acidosis and positive D-dimer test may suggest the presence of AMI but the diagnosis is generally confirmed by contrast enhanced CTA. Resuscitation of the patient and revascularization of the bowel are the main aims of treatment of AMI. SMA embolism and SMA thrombosis can be treated by open surgery or PEVT. Combination of both treatments (hybrid technique) is also widely used. NOMI can be treated by intra-arterial vasodilator infusion therapy by placing a catheter into the SMA. In case of MVT, systemic anticoagulation or thrombolytic therapy followed by coumidine or DOAC should be given. If the patient has developed bowel wall necrosis in any form of AMI, open surgery should be done to resect the necrotic bowel and then revascularization should be done. Second look surgery should be done after 24 to 48 h to evaluate the potentially viable bowel and to preserve the bowel as much as possible to prevent short bowel syndrome. CMI occurs when patients develop ischemic symptoms due to stenosis of two of the three major mesenteric arteries. Almost always it develops slowly over the years due to atherosclerotic narrowing of those arteries. Patients generally present with post-prandial intestinal angina. The classic triad of post-prandial upper or mid-abdominal pain, sitophobia and weight loss is present in less than one quarter of cases. CTA is the diagnostic investigation of choice. Revascularization can be done either surgically or by PEVT. Currently, PEVT is widely used as the first line treatment in high-risk patients and open surgical treatment is reserved for patients with less comoribidities. Revascularization can greatly improve the quality of life in patients with CMI.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nah YW S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | Yadav S, Dave M, Edakkanambeth Varayil J, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sweetser SR, Melton LJ 3rd, Sandborn WJ, Loftus EV Jr. A population-based study of incidence, risk factors, clinical spectrum, and outcomes of ischemic colitis. Clin Gastroenterol Hepatol. 2015;13:731-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (2)] |
| 2. | Silva JA, White CJ. Ischemic bowel syndromes. Prim Care. 2013;40:153-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Kolkman JJ, Reeders JW, Geelkerken RH. [Gastrointestinal surgery and gastroenterology. VIII. Gastroenterologic aspects of chronic gastrointestinal ischemia]. Ned Tijdschr Geneeskd. 2000;144:792-797. [PubMed] |
| 4. | Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, Ben-Ishay O, Rubinstein C, Balogh ZJ, Civil I, Coccolini F, Leppaniemi A, Peitzman A, Ansaloni L, Sugrue M, Sartelli M, Di Saverio S, Fraga GP, Catena F. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2017;12:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 307] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 5. | Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am. 2001;30:445-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Acosta S, Alhadad A, Svensson P, Ekberg O. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. Br J Surg. 2008;95:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Vodusek Z, Feuerstadt P, Brandt LJ. Review article: the pharmacological causes of colon ischaemia. Aliment Pharmacol Ther. 2019;49:51-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Patil P, Panarelli NC. Educational Case: Ischemic Disorders of the Gut in Adult Patients. Acad Pathol. 2019;6:2374289519888709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Uberti G, Goldblum JR, Allende DS. Ischemic enterocolitis and its differential diagnosis. Semin Diagn Pathol. 2014;31:152-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Toner M, Condell D, O'Briain DS. Obstructive colitis. Ulceroinflammatory lesions occurring proximal to colonic obstruction. Am J Surg Pathol. 1990;14:719-728. [PubMed] |
| 11. | Andersson PO. Vascular control in the colon and rectum. Scand J Gastroenterol Suppl. 1984;93:65-78. [PubMed] |
| 12. | Kvietys PR. The Gastrointestinal Circulation. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 2, Anatomy. [cited 10 January 2021]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53099/. |
| 13. | Shaikh H, Wehrle CJ, Khorasani-Zadeh A. Anatomy, Abdomen and Pelvis, Superior Mesenteric Artery. 2020 Aug 15. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–.. [PubMed] |
| 14. | Yamazaki T, Shirai Y, Tada T, Sasaki M, Sakai Y, Hatakeyama K. Ischemic colitis arising in watershed areas of the colonic blood supply: a report of two cases. Surg Today. 1997;27:460-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Robinson JW, Mirkovitch V, Winistörfer B, Saegesser F. Response of the intestinal mucosa to ischaemia. Gut. 1981;22:512-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | McFadden DW, Rongione AJ. Intestinal circulation and vascular disorders. Miller TA ed. Modern Surgical Care Physiologic Foundation & Clinical Applications. 2nd ed. St Louis, Mo Quality Medical Publishing, 1998: 443-463. |
| 17. | Rosenblum JD, Boyle CM, Schwartz LB. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am. 1997;77:289-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Haglund U, Bergqvist D. Intestinal ischemia -- the basics. Langenbecks Arch Surg. 1999;384:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Kahai P, Mandiga P, Wehrle CJ, Lobo S. Anatomy, Abdomen and Pelvis, Large Intestine. 2020 Aug 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–.. [PubMed] |
| 20. | Paterno F, Longo WE. The etiology and pathogenesis of vascular disorders of the intestine. Radiol Clin North Am. 2008;46:877-885, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Cappell MS. Intestinal (mesenteric) vasculopathy. I. Acute superior mesenteric arteriopathy and venopathy. Gastroenterol Clin North Am. 1998;27:783-825, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Franca E, Shaydakov ME, Kosove J. Mesenteric Artery Thrombosis. 2021 May 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–.. [PubMed] |
| 23. | Singal AK, Kamath PS, Tefferi A. Mesenteric venous thrombosis. Mayo Clin Proc. 2013;88:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | King TC. Cell Injury, Cellular Responses to Injury, and Cell Death. Elsevier's Integr Pathol. 2006;. |
| 25. | Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 26. | Luther B, Mamopoulos A, Lehmann C, Klar E. The Ongoing Challenge of Acute Mesenteric Ischemia. Visc Med. 2018;34:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Brandt LJ, Boley SJ. Colonic ischemia. Surg Clin North Am. 1992;72:203-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 123] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med. 2003;70:920-921, 925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Mohanapriya T, Singh KB, Arulappan T, Shobhana R. Ischemic colitis. Indian J Surg. 2012;74:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Brandt LJ, Feuerstadt P, Longstreth GF, Boley SJ; American College of Gastroenterology. ACG clinical guideline: epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI). Am J Gastroenterol. 2015;110:18-44; quiz 45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 31. | Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum. 1996;39:88-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 159] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Flobert C, Cellier C, Berger A, Ngo A, Cuillerier E, Landi B, Marteau P, Cugnenc PH, Barbier JP. Right colonic involvement is associated with severe forms of ischemic colitis and occurs frequently in patients with chronic renal failure requiring hemodialysis. Am J Gastroenterol. 2000;95:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | van Petersen AS, Kolkman JJ, Meerwaldt R, Huisman AB, van der Palen J, Zeebregts CJ, Geelkerken RH. Mesenteric stenosis, collaterals, and compensatory blood flow. J Vasc Surg. 2014;60:111-119, 119.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Roobottom CA, Dubbins PA. Significant disease of the celiac and superior mesenteric arteries in asymptomatic patients: predictive value of Doppler sonography. AJR Am J Roentgenol. 1993;161:985-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 91] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Mensink PB, van Petersen AS, Geelkerken RH, Otte JA, Huisman AB, Kolkman JJ. Clinical significance of splanchnic artery stenosis. Br J Surg. 2006;93:1377-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Walker TG. Mesenteric vasculature and collateral pathways. Semin Intervent Radiol. 2009;26:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Kim EN, Lamb K, Relles D, Moudgill N, DiMuzio PJ, Eisenberg JA. Median Arcuate Ligament Syndrome-Review of This Rare Disease. JAMA Surg. 2016;151:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 38. | Kageyama Y, Kokudo T, Amikura K, Miyazaki Y, Takahashi A, Sakamoto H. The arc of Buhler: special considerations when performing pancreaticoduodenectomy. Surg Case Rep. 2016;2:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | van Bockel JH, Geelkerken RH, Wasser MN. Chronic splanchnic ischaemia. Best Pract Res Clin Gastroenterol. 2001;15:99-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Otte JA, Geelkerken RH, Oostveen E, Mensink PB, Huisman AB, Kolkman JJ. Clinical impact of gastric exercise tonometry on diagnosis and management of chronic gastrointestinal ischemia. Clin Gastroenterol Hepatol. 2005;3:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Clair DG, Beach JM. Mesenteric Ischemia. N Engl J Med. 2016;374:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 329] [Article Influence: 36.6] [Reference Citation Analysis (1)] |
| 42. | Howard TJ, Plaskon LA, Wiebke EA, Wilcox MG, Madura JA. Nonocclusive mesenteric ischemia remains a diagnostic dilemma. Am J Surg. 1996;171:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 95] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Park WM, Gloviczki P, Cherry KJ Jr, Hallett JW Jr, Bower TC, Panneton JM, Schleck C, Ilstrup D, Harmsen WS, Noel AA. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg. 2002;35:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 44. | Block T. Acute occlusion of the superior mesenteric artery: Diagnosis and treatment. Acta Univ Upps. 2010;. |
| 45. | Morasch MD, Ebaugh JL, Chiou AC, Matsumura JS, Pearce WH, Yao JS. Mesenteric venous thrombosis: a changing clinical entity. J Vasc Surg. 2001;34:680-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Habu Y, Tahashi Y, Kiyota K, Matsumura K, Hirota M, Inokuchi H, Kawai K. Reevaluation of clinical features of ischemic colitis. Analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol. 1996;31:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Huguier M, Barrier A, Boelle PY, Houry S, Lacaine F. Ischemic colitis. Am J Surg. 2006;192:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Sherid M, Sifuentes H, Samo S, Sulaiman S, Husein H, Tupper R, Spurr C, Vainder J, Sridhar S. Risk factors of recurrent ischemic colitis: a multicenter retrospective study. Korean J Gastroenterol. 2014;63:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Mosli M, Parfitt J, Gregor J. Retrospective analysis of disease association and outcome in histologically confirmed ischemic colitis. J Dig Dis. 2013;14:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Washington C, Carmichael JC. Management of ischemic colitis. Clin Colon Rectal Surg. 2012;25:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 51. | Kwak HD, Kang H, Ju JK. Fulminant gangrenous ischemic colitis: is it the solely severe type of ischemic colitis? Int J Colorectal Dis. 2017;32:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Kougias P, El Sayed HF, Zhou W, Lin PH. Management of chronic mesenteric ischemia. The role of endovascular therapy. J Endovasc Ther. 2007;14:395-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Musil F, Kouhout P, Eliás P, Krajina A, Podhola M. [Chronic mesenteric ischemia]. Vnitr Lek. 2000;46:418-422. [PubMed] |
| 54. | Ujiki M, Kibbe MR. Mesenteric ischemia. Perspect Vasc Surg Endovasc Ther. 2005;17:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Björck M, Koelemay M, Acosta S, Bastos Goncalves F, Kölbel T, Kolkman JJ, Lees T, Lefevre JH, Menyhei G, Oderich G; Esvs Guidelines Committee; Kolh P, de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Sanddal Lindholt J, Vega de Ceniga M, Vermassen F, Verzini F, Document Reviewers, Geelkerken B, Gloviczki P, Huber T, Naylor R. Editor's Choice - Management of the Diseases of Mesenteric Arteries and Veins: Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53:460-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 428] [Article Influence: 61.1] [Reference Citation Analysis (1)] |
| 56. | Sana A, Vergouwe Y, van Noord D, Moons LM, Pattynama PM, Verhagen HJ, Kuipers EJ, Mensink PB. Radiological imaging and gastrointestinal tonometry add value in diagnosis of chronic gastrointestinal ischemia. Clin Gastroenterol Hepatol. 2011;9:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg. 2007;46:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 58. | Block T, Nilsson TK, Björck M, Acosta S. Diagnostic accuracy of plasma biomarkers for intestinal ischaemia. Scand J Clin Lab Invest. 2008;68:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 59. | Wilson C, Imrie CW. Amylase and gut infarction. Br J Surg. 1986;73:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | May LD, Berenson MM. Value of serum inorganic phosphate in the diagnosis of ischemic bowel disease. Am J Surg. 1983;146:266-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Evennett NJ, Petrov MS, Mittal A, Windsor JA. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J Surg. 2009;33:1374-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 62. | Delaney CP, O'Neill S, Manning F, Fitzpatrick JM, Gorey TF. Plasma concentrations of glutathione S-transferase isoenzyme are raised in patients with intestinal ischaemia. Br J Surg. 1999;86:1349-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Yeniocak S, Saraç F, Yazıcıoğlu M, Karabulut N, Ünal A, Yücetaş E, Koldaş M, Akkoç İ, Ekici M, Evrin T. The Diagnostic Values of Ischemia-Modified Albumin in Patients with Acute Abdominal Pain and Its Role in Differentiating Acute Abdomen. Emerg Med Int. 2020;2020:7925975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Ginsburg M, Obara P, Lambert DL, Hanley M, Steigner ML, Camacho MA, Chandra A, Chang KJ, Gage KL, Peterson CM, Ptak T, Verma N, Kim DH, Carucci LR, Dill KE. American College of Radiology. ACR Appropriateness Criteria® Imaging of Mesenteric Ischemia. American College of Radiology. [cited 10 January 2021]. Available from: https://acsearch.acr.org/docs/70909/Narrative/. |
| 65. | Henes FO, Pickhardt PJ, Herzyk A, Lee SJ, Motosugi U, Derlin T, Lubner MG, Adam G, Schön G, Bannas P. CT angiography in the setting of suspected acute mesenteric ischemia: prevalence of ischemic and alternative diagnoses. Abdom Radiol (NY). 2017;42:1152-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 438] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 67. | Pérez-García C, de Miguel Campos E, Fernández Gonzalo A, Malfaz C, Martín Pinacho JJ, Fernández Álvarez C, Herranz Pérez R. Non-occlusive mesenteric ischaemia: CT findings, clinical outcomes and assessment of the diameter of the superior mesenteric artery. Br J Radiol. 2018;91:20170492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Olson MC, Fletcher JG, Nagpal P, Froemming AT, Khandelwal A. Mesenteric ischemia: what the radiologist needs to know. Cardiovasc Diagn Ther. 2019;9:S74-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Rosow DE, Sahani D, Strobel O, Kalva S, Mino-Kenudson M, Holalkere NS, Alsfasser G, Saini S, Lee SI, Mueller PR, Fernández-del Castillo C, Warshaw AL, Thayer SP. Imaging of acute mesenteric ischemia using multidetector CT and CT angiography in a porcine model. J Gastrointest Surg. 2005;9:1262-74; discussion 1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Oliva IB, Davarpanah AH, Rybicki FJ, Desjardins B, Flamm SD, Francois CJ, Gerhard-Herman MD, Kalva SP, Ashraf Mansour M, Mohler ER 3rd, Schenker MP, Weiss C, Dill KE. ACR Appropriateness Criteria ® imaging of mesenteric ischemia. Abdom Imaging. 2013;38:714-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 71. | Sartini S, Calosi G, Granai C, Harris T, Bruni F, Pastorelli M. Duplex ultrasound in the early diagnosis of acute mesenteric ischemia: a longitudinal cohort multicentric study. Eur J Emerg Med. 2017;24:e21-e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 73. | Theodoropoulou A, Koutroubakis IE. Ischemic colitis: clinical practice in diagnosis and treatment. World J Gastroenterol. 2008;14:7302-7308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 159] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (2)] |
| 74. | Trotter JM, Hunt L, Peter MB. Ischaemic colitis. BMJ. 2016;355:i6600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology. 1999;211:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 76. | Longstreth GF, Hye RJ. Right-Side Colon Ischemia: Clinical Features, Large Visceral Artery Occlusion, and Long-Term Follow-Up. Perm J. 2015;19:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Zuckerman GR, Prakash C, Merriman RB, Sawhney MS, DeSchryver-Kecskemeti K, Clouse RE. The colon single-stripe sign and its relationship to ischemic colitis. Am J Gastroenterol. 2003;98:2018-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 78. | Deepak P, Devi R. Ischemic colitis masquerading as colonic tumor: case report with review of literature. World J Gastroenterol. 2011;17:5324-5326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 79. | Zou X, Cao J, Yao Y, Liu W, Chen L. Endoscopic findings and clinicopathologic characteristics of ischemic colitis: a report of 85 cases. Dig Dis Sci. 2009;54:2009-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 80. | Expert Panel on Interventional Radiology, Fidelman N, AbuRahma AF, Cash BD, Kapoor BS, Knuttinen MG, Minocha J, Rochon PJ, Shaw CM, Ray CE Jr, Lorenz JM. ACR Appropriateness Criteria® Radiologic Management of Mesenteric Ischemia. J Am Coll Radiol. 2017;14:S266-S271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Hohenwalter EJ. Chronic mesenteric ischemia: diagnosis and treatment. Semin Intervent Radiol. 2009;26:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Mitchell EL, Moneta GL. Mesenteric duplex scanning. Perspect Vasc Surg Endovasc Ther. 2006;18:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Eidt JF, Harward T, Cook JM, Kahn MB, Troillett R. Current status of duplex Doppler ultrasound in the examination of the abdominal vasculature. Am J Surg. 1990;160:604-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Char D, Hines G. Chronic mesenteric ischemia: diagnosis and treatment. Heart Dis. 2001;3:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Wyers MC. Acute mesenteric ischemia: diagnostic approach and surgical treatment. Semin Vasc Surg. 2010;23:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 86. | Bulkley GB, Zuidema GD, Hamilton SR, O'Mara CS, Klacsmann PG, Horn SD. Intraoperative determination of small intestinal viability following ischemic injury: a prospective, controlled trial of two adjuvant methods (Doppler and fluorescein) compared with standard clinical judgment. Ann Surg. 1981;193:628-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 141] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Bradbury AW, Brittenden J, McBride K, Ruckley CV. Mesenteric ischaemia: a multidisciplinary approach. Br J Surg. 1995;82:1446-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 149] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 88. | Romano N, Prosperi V, Basili G, Lorenzetti L, Gentile V, Luceretti R, Biondi G, Goletti O. Acute thrombosis of the superior mesenteric artery in a 39-year-old woman with protein-S deficiency: a case report. J Med Case Rep. 2011;5:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Yanar H, Taviloglu K, Ertekin C, Ozcinar B, Yanar F, Guloglu R, Kurtoglu M. Planned second-look laparoscopy in the management of acute mesenteric ischemia. World J Gastroenterol. 2007;13:3350-3353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 90. | Kim BG, Ohm JY, Bae MN, Kim HN, Kim YJ, Chung MH, Park CS, Ihm SH, Kim HY. Successful percutaneous aspiration thrombectomy for acute mesenteric ischemia in a patient with atrial fibrillation despite optimal anticoagulation therapy. Can J Cardiol. 2013;29:1329.e5-1329.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 91. | Schoenbaum SW, Pena C, Koenigsberg P, Katzen BT. Superior mesenteric artery embolism: treatment with intraarterial urokinase. J Vasc Interv Radiol. 1992;3:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | Jia Z, Jiang G, Tian F, Zhao J, Li S, Wang K, Wang Y, Jiang L, Wang W. Early endovascular treatment of superior mesenteric occlusion secondary to thromboemboli. Eur J Vasc Endovasc Surg. 2014;47:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 93. | Erdogan E, Turfan M, Akkaya M, Bacaksız A, Tasal A, Ergelen M, Goktekin O. Successful recanalization of acute superior mesenteric artery ischemia with balloon angioplasty and aspiration embolectomy. Eur Geriatr Med. 2013;4:350-351. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 94. | Ierardi AM, Tsetis D, Sbaraini S, Angileri SA, Galanakis N, Petrillo M, Patella F, Panella S, Balestra F, Lucchina N, Carrafiello G. The role of endovascular therapy in acute mesenteric ischemia. Ann Gastroenterol. 2017;30:526-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | Zhang Z, Wang D, Li G, Wang X, Wang Y, Jiang T. Endovascular Treatment for Acute Thromboembolic Occlusion of the Superior Mesenteric Artery and the Outcome Comparison between Endovascular and Open Surgical Treatments: A Retrospective Study. Biomed Res Int. 2017;2017:1964765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 96. | Björck M, Orr N, Endean ED. Debate: Whether an endovascular-first strategy is the optimal approach for treating acute mesenteric ischemia. J Vasc Surg. 2015;62:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 97. | Alhan E, Usta A, Çekiç A, Saglam K, Türkyılmaz S, Cinel A. A study on 107 patients with acute mesenteric ischemia over 30 years. Int J Surg. 2012;10:510-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 98. | Chen Y, Zhu J, Ma Z, Dai X, Fan H, Feng Z, Zhang Y, Luo Y. Hybrid technique to treat superior mesenteric artery occlusion in patients with acute mesenteric ischemia. Exp Ther Med. 2015;9:2359-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Mitsuyoshi A, Obama K, Shinkura N, Ito T, Zaima M. Survival in nonocclusive mesenteric ischemia: early diagnosis by multidetector row computed tomography and early treatment with continuous intravenous high-dose prostaglandin E(1). Ann Surg. 2007;246:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 100. | Sise MJ. Mesenteric ischemia: the whole spectrum. Scand J Surg. 2010;99:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 101. | Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss L, Golzarian J, Gornik HL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE; American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:1555-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 102. | Trompeter M, Brazda T, Remy CT, Vestring T, Reimer P. Non-occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. Eur Radiol. 2002;12:1179-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 103. | Miyazawa R, Kamo M. What affects the prognosis of NOMI patients? Surg Endosc. 2020;34:5327-5330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Condat B, Pessione F, Helene Denninger M, Hillaire S, Valla D. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology. 2000;32:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 330] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 105. | Robin P, Gruel Y, Lang M, Lagarrigue F, Scotto JM. Complete thrombolysis of mesenteric vein occlusion with recombinant tissue-type plasminogen activator. Lancet. 1988;1:1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 106. | Yang SF, Liu BC, Ding WW, He CS, Wu XJ, Li JS. Initial transcatheter thrombolysis for acute superior mesenteric venous thrombosis. World J Gastroenterol. 2014;20:5483-5492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 107. | Chawla YK, Bodh V. Portal vein thrombosis. J Clin Exp Hepatol. 2015;5:22-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 108. | Sehgal M, Haskal ZJ. Use of transjugular intrahepatic portosystemic shunts during lytic therapy of extensive portal splenic and mesenteric venous thrombosis: long-term follow-up. J Vasc Interv Radiol. 2000;11:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 109. | Oglat A, Quigley EM. Colonic ischemia: usual and unusual presentations and their management. Curr Opin Gastroenterol. 2017;33:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 110. | Beck DE, de Aguilar-Nascimento JE. Surgical management and outcome in acute ischemic colitis. Ochsner J. 2011;11:282-285. [PubMed] |
| 111. | Jimenez JG, Huber TS, Ozaki CK, Flynn TC, Berceli SA, Lee WA, Seeger JM. Durability of antegrade synthetic aortomesenteric bypass for chronic mesenteric ischemia. J Vasc Surg. 2002;35:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 112. | Bakoyiannis C, Mylonas KS, Davakis S, Tsaples G, Karaolanis G, Liakakos T. Superior mesenteric artery endarterectomy for chronic mesenteric ischemia: A viable alternative in poor candidates for endovascular interventions. Vascular. 2020;28:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 113. | Kieny R, Batellier J, Kretz JG. Aortic reimplantation of the superior mesenteric artery for atherosclerotic lesions of the visceral arteries: sixty cases. Ann Vasc Surg. 1990;4:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 114. | Keese M, Schmitz-Rixen T, Schmandra T. Chronic mesenteric ischemia: time to remember open revascularization. World J Gastroenterol. 2013;19:1333-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 115. | Medical Advisory Secretariat. . Fenestrated endovascular grafts for the repair of juxtarenal aortic aneurysms: an evidence-based analysis. Ont Health Technol Assess Ser. 2009;9:1-51. [PubMed] |
| 116. | Touloumtzidis A, Mamopoulos AT, Lilien-Waldau V, Zapenko A, Gäbel G, Luther B. Chronic Mesenteric Ischemia: Long Term Results after Open Surgical Revascularization. Clin Surg. 2018;3:2080. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 117. | Matsumoto AH, Angle JF, Spinosa DJ, Hagspiel KD, Cage DL, Leung DA, Kern JA, Tribble CG, Kron IL. Percutaneous transluminal angioplasty and stenting in the treatment of chronic mesenteric ischemia: results and longterm followup. J Am Coll Surg. 2002;194:S22-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 118. | Landis MS, Rajan DK, Simons ME, Hayeems EB, Kachura JR, Sniderman KW. Percutaneous management of chronic mesenteric ischemia: outcomes after intervention. J Vasc Interv Radiol. 2005;16:1319-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 119. | Gibbons CP, Roberts DE. Endovascular treatment of chronic arterial mesenteric ischemia: a changing perspective? Semin Vasc Surg. 2010;23:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 120. | Kasirajan K, O'Hara PJ, Gray BH, Hertzer NR, Clair DG, Greenberg RK, Krajewski LP, Beven EG, Ouriel K. Chronic mesenteric ischemia: open surgery versus percutaneous angioplasty and stenting. J Vasc Surg. 2001;33:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 164] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 121. | GS, Bower TC, Sullivan TM, Bjarnason H, Cha S, Gloviczki P. Open versus endovascular revascularization for chronic mesenteric ischemia: risk-stratified outcomes. J Vasc Surg. 2009;49:1472-9.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 122. | Adaba F, Askari A, Dastur J, Patel A, Gabe SM, Vaizey CJ, Faiz O, Nightingale JM, Warusavitarne J. Mortality after acute primary mesenteric infarction: a systematic review and meta-analysis of observational studies. Colorectal Dis. 2015;17:566-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 123. | Aliosmanoglu I, Gul M, Kapan M, Arikanoglu Z, Taskesen F, Basol O, Aldemir M. Risk factors effecting mortality in acute mesenteric ischemia and mortality rates: a single center experience. Int Surg. 2013;98:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 124. | Caluwaerts M, Castanares-Zapatero D, Laterre PF, Hantson P. Prognostic factors of acute mesenteric ischemia in ICU patients. BMC Gastroenterol. 2019;19:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 125. | Huang HH, Chang YC, Yen DH, Kao WF, Chen JD, Wang LM, Huang CI, Lee CH. Clinical factors and outcomes in patients with acute mesenteric ischemia in the emergency department. J Chin Med Assoc. 2005;68:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 126. | Boley SJ, Sprayregen S, Veith FJ, Siegelman SS. An aggressive roentgenologic and surgical approach to acute mesenteric ischemia. Surg Annu. 1973;5:355-378. [PubMed] |
| 127. | Klempnauer J, Grothues F, Bektas H, Pichlmayr R. Long-term results after surgery for acute mesenteric ischemia. Surgery. 1997;121:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 128. | Añón R, Boscá MM, Sanchiz V, Tosca J, Almela P, Amorós C, Benages A. Factors predicting poor prognosis in ischemic colitis. World J Gastroenterol. 2006;12:4875-4878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 129. | Cosme A, Montoro M, Santolaria S, Sanchez-Puertolas AB, Ponce M, Durán M, Cabriada JL, Borda N, Sarasqueta C, Bujanda L. Prognosis and follow-up of 135 patients with ischemic colitis over a five-year period. World J Gastroenterol. 2013;19:8042-8046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 130. | Mansukhani NA, Hekman KE, Yoon DY, Helenowski IB, Hoel AW, Rodriguez HE, Pearce WH, Eskandari MK, Tomita TM. Impact of Body Mass Index on Outcomes after Mesenteric Revascularization for Chronic Mesenteric Ischemia. Ann Vasc Surg. 2018;48:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 131. | Blauw JTM, Pastoors HAM, Brusse-Keizer M, Beuk RJ, Kolkman JJ, Geelkerken RH; For The Dutch Mesenteric Ischemia Study Group. The Impact of Revascularisation on Quality of Life in Chronic Mesenteric Ischemia. Can J Gastroenterol Hepatol. 2019;2019:7346013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |