Published online Jul 21, 2021. doi: 10.3748/wjg.v27.i27.4252
Peer-review started: January 28, 2021
First decision: March 29, 2021
Revised: April 12, 2021
Accepted: June 18, 2021
Article in press: June 18, 2021
Published online: July 21, 2021
Processing time: 172 Days and 7 Hours
Intrahepatic cholangiocarcinoma (iCCA) is a subgroup of cholangiocarcinoma that accounts for about 10%-20% of the total cases. Infection with hepatitis B virus (HBV) is one of the most important predisposing factors leading to the formation of iCCA. It has been recently estimated based on abundant epidemiological data that the association between HBV infection and iCCA is strong with an odds ratio of about 4.5. The HBV-associated mechanisms that lead to iCCA are under intense investigation. The diagnosis of iCCA in the context of chronic liver disease is challenging and often requires histological confirmation to distinguish from hepatocellular carcinoma. It is currently unclear whether antiviral treatment for HBV can decrease the incidence of iCCA. In terms of management, surgical resection remains the mainstay of treatment. There is a need for effective treatment modalities beyond resection in both first- and second-line treatment. In this review, we summarize the epidemiological evidence that links the two entities, discuss the pathogenesis of HBV-associated iCCA, and present the available data on the diagnosis and management of this cancer.
Core Tip: Cholangiocarcinoma (CCA) is the second most common primary liver cancer. Intrahepatic CCA (iCCA), a subgroup of CCA, has a pronounced association with hepatitis B virus (HBV) infection. Recent data have strengthened this association both in terms of epidemiology and pathogenesis. Moreover, the potential for the development of the combined hepatocellular-cholangiocarcinoma form poses additional difficulties in the successful management of iCCA. Herein, we discuss the available epidemiological data, present the current knowledge on the pathogenesis of HBV-associated iCCA, and review all of the available information on the diagnosis and management of this aggressive cancer.
- Citation: Fragkou N, Sideras L, Panas P, Emmanouilides C, Sinakos E. Update on the association of hepatitis B with intrahepatic cholangiocarcinoma: Is there new evidence? World J Gastroenterol 2021; 27(27): 4252-4275
- URL: https://www.wjgnet.com/1007-9327/full/v27/i27/4252.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i27.4252
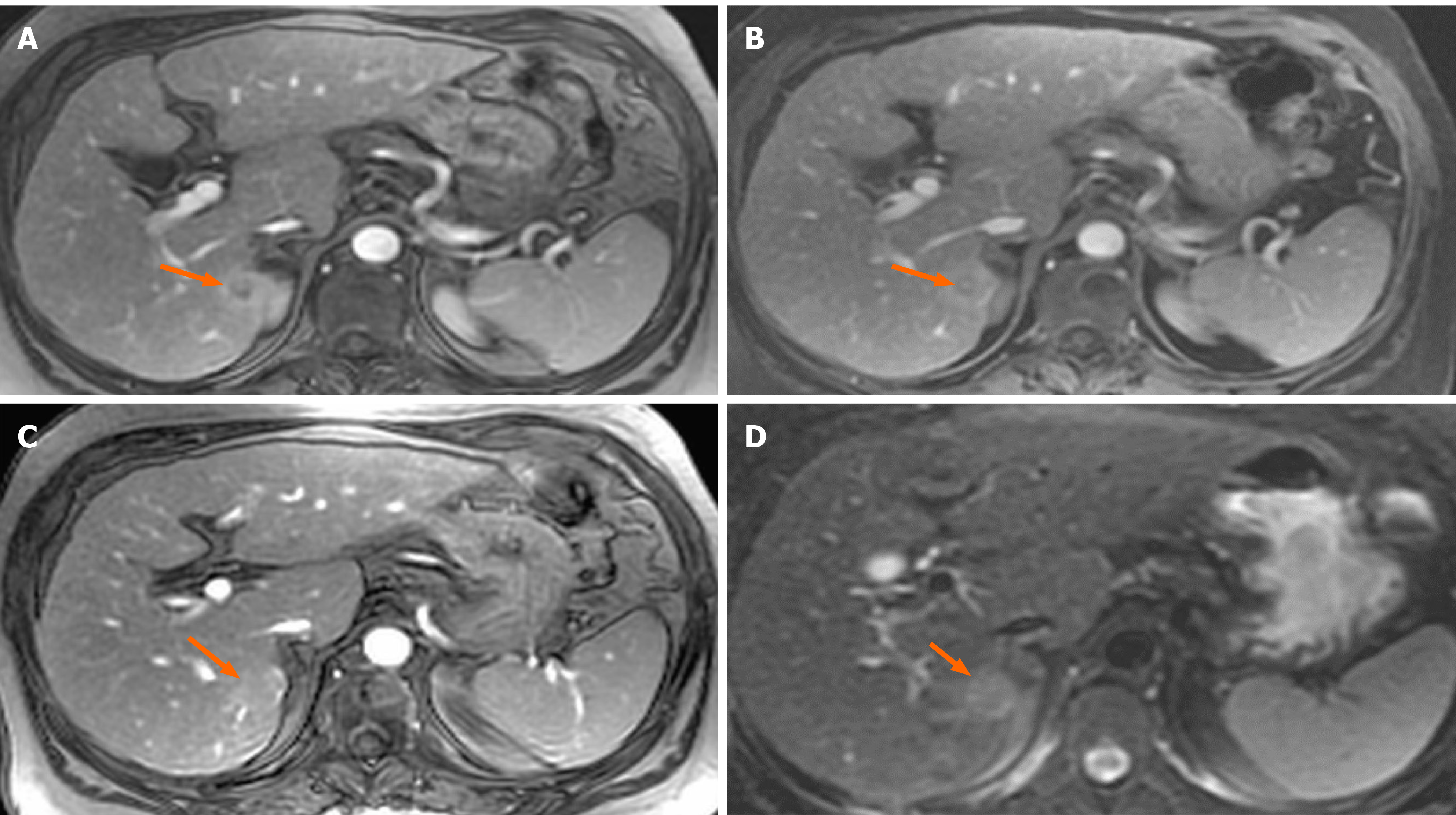
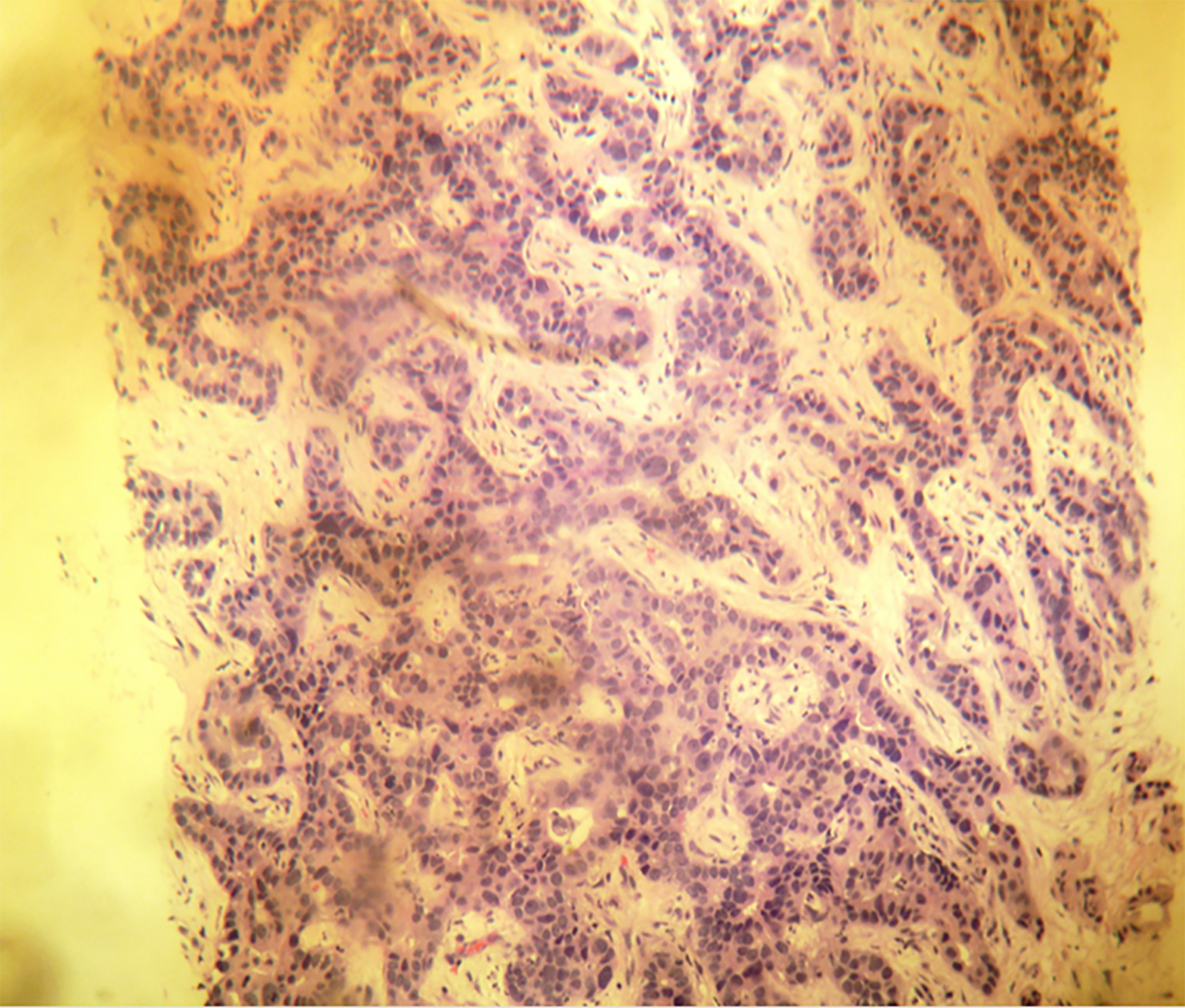
A 61-year-old woman with compensated liver cirrhosis due to chronic hepatitis B (CHB) presented to the outpatient clinic of our hospital for her semi-annual evaluation. She had started antiviral treatment with entecavir (0.5 mg per day) approximately 2 years prior. Her family history is significant for hepatocellular carcinoma (HCC), as her twin brother also suffered from liver cirrhosis due to CHB. On physical examination, no signs of liver decompensation were detected. Her liver synthetic function and alpha fetoprotein (aFP) value were completely normal. However, a small (1.8 cm) liver nodule with arterial enhancement was discovered in the left hepatic lobe during magnetic resonance imaging (MRI) (Figure 1). Hence, the patient underwent left hepatectomy without complications. Surprisingly, histological examination of the surgical specimen revealed a definite intrahepatic cholangiocarcinoma (CCA) (Figure 2).
This case vignette highlights the possible association between hepatitis B virus (HBV) infection and intrahepatic CCA (iCCA). iCCA is a subgroup of CCA that accounts for about 10%-20% of the total cases and classically arises above the second-order bile ducts[1]. iCCA was recently recognized as a distinct entity opposed to the extrahepatic type of CCA (eCCA)[2]. Several predisposing factors that contribute to the development of CCA have been described. A recent meta-analysis clearly showed that the degree to which these risk factors are associated with the development of this cancer significantly differ according to the tumor location[3]. Specifically for HBV infection, the odds ratio (OR) for the development of CCA is much greater for iCCA than for eCCA (4.57 vs 2.11). In view of these findings, several questions regarding this association arise such as how accurately can we distinguish between HCC and iCCA; what are the mechanisms that lead to the formation of iCCA; is there is a specific role for HBV in iCCA; and finally, can antiviral treatment against HBV reduce the incidence of iCCA. In this review, we summarize the epidemiological evidence that links the two entities, discuss the pathogenesis of HBV-associated iCCA, and present the available data on the diagnosis and management of this aggressive cancer.
To identify relevant epidemiological studies from geographically diverse regions, a comprehensive literature search of PubMed was conducted up to December 2020 without language restrictions. Studies were eligible for inclusion if they met the following criteria: were case-control or cohort study design, reported outcomes for the association between HBV infection and iCCA, and provided risk estimates with 95% confidence intervals (CIs) or sufficient data to calculate them. The following Medical Subject Headings were included: “intrahepatic cholangiocarcinoma” and “HBV.” Reference lists of the retrieved articles were also manually searched to identify additional studies.
The literature search identified a total of 417 references, of which 20 case-control[4-23] and 2 cohort studies[24,25] fulfilled the inclusion criteria (Table 1). The population of the included epidemiological studies originated not only from geographical regions of high or intermediate prevalence of cholangiocarcinoma (i.e. Thailand, South Korea, Japan, Mainland China and Taiwan area of China) but also from regions of low prevalence (i.e. the United States, Italy)[26,27]; with a predominance of studies performed in Eastern Asian countries. When needed, from these studies we extracted data for analyses that pertained only to the development of iCCA and not eCCA.
| Ref. | Study design | Region | Dates | Source | Age (yr) | Gender (% male) | Cases | Controls | OR (95%CI) for HBV infection | ||
| Total | With exposure (%) | Total | With exposure (%) | ||||||||
| Petrick et al[4], 2017 | Case-control | United States | 2000-2011 | Population | 78.0 | 46.6 | 2092 | 25 (1.2) | 323615 | 1200 (0.4%) | 2.97 (1.97-4.46) |
| Choi et al[5], 2016 | Case-control | United States | 2000-2014 | Hospital | 60.6 | 42.9 | 1169 | 10 (0.95) | 4769 | 8 (0.2%) | 12.9 (2.69-61.61) |
| Huang et al[6], 2017 | Case-control | Taiwan area of China | 2003-2009 | Population | 67.7 | 52.1 | 4695 | 138 (2.9) | 46942 | 513 (1.1%) | 2.23 (1.80-2.76) |
| Lee et al[7], 2015 | Case-control | South Korea | 2007-2013 | Hospital | 67.8 | 50.4 | 83 | 19 (22.9) | 166 | 9 (5.4%) | 4.58 (2.00-10.50) |
| Chaiteerakij et al[8], 2013 | Case-control | United States | 2000-2010 | Hospital | 61.2 | 50.3 | 612 | 3 (0.5) | 594 | 3 (0.5%) | 0.97 (0.20-4.80) |
| Chang et al[9], 2013 | Case-control | China | 2004-2008 | Population | NR | 56.1 | 2978 | 257 (8.6) | 11912 | 229 (1.9%) | 4.9 (4.1-5.9) |
| Wu et al[10], 2012 | Case-control | China | 2002-2008 | Hospital | 57.9 | 62.1 | 23 | 12 (52.2) | 52 | 2 (3.9%) | 38.9 (7.52-201.04) |
| Wu et al[11], 2012 | Case-control | China | 1998-2010 | Hospital | 58.7 | 55.9 | 102 | 28 (22.5) | 809 | 27 (3.3%) | 10.52 (5.94-18.62) |
| Peng et al[12], 2011 | Case-control | China | 2002-2009 | Hospital | 53.8 | 60.2 | 98 | 31 (31.6) | 196 | 25 (12.8%) | 2.75 (1.27-5.95) |
| Welzel et al[13], 2011 | Case-control | United States | 1994-2005 | Population | 76.4 | 52.5 | 743 | 11 (1.5) | 195953 | 442 (0.2%) | 3.07 (1.43-6.58) |
| Tao et al[14], 2010 | Case-control | China | 1998-2008 | Hospital | 58.7 | 60.7 | 61 | 17 (27.9) | 380 | 19 (5.0%) | 7.3 (3.1-17.2) |
| Zhou et al[15], 2010 | Case-control | China | 2003-2006 | Hospital | 53.1 | 70.3 | 317 | 154 (48.6) | 634 | 42 (6.6%) | 9.67 (6.33-14.77) |
| Lee et al[16], 2009 | Case-control | Taiwan area of China | 1991-2005 | Hospital | 61.5 | 63.1 | 160 | 60 (37.5) | 160 | 22 (13.8%) | 4.99 (2.78-8.95) |
| Lee et al[17], 2008 | Case-control | South Korea | 2000-2004 | Hospital | 60.7 | 69.1 | 622 | 84 (13.5) | 2488 | 125 (5.0%) | 2.3 (1.6-3.3) |
| Zhou et al[18], 2008 | Case-control | China | 2004-2006 | Hospital | 53.2 | 66.0 | 312 | 151 (48.4) | 438 | 42 (9.6%) | 8.88 (5.97-13.19) |
| Shaib et al[19], 2007 | Case-control | United States | 1992-2002 | Hospital | 59.8 | 55.4 | 83 | 1 (1.2) | 236 | 1 (0.4%) | 2.9 (0.1-236.8) |
| Choi et al[20], 2006 | Case-control | South Korea | 2003-2004 | Hospital | 64.0 | 62.2 | 51 | 4 (7.8) | 51 | 5 (9.8%) | 0.8 (0.19-3.02) |
| Shaib et al[21], 2005 | Case-control | United States | 1993-1999 | Population | 78.7 | 51.7 | 625 | 1 (0.2) | 90834 | 182 (0.2%) | 0.8 (0.1-5.9) |
| Yamamoto et al[22], 2004 | Case-control | Japan | 1991-2002 | Hospital | 64.6 | 58.0 | 50 | 2 (4.0) | 205 | 5 (2.5%) | 1.84 (0.34-10.11) |
| Donato et al[23], 2001 | Case-control | Italy | 1995-2000 | Hospital | 65.0 | 80.8 | 23 | 3 (13.0) | 824 | 45 (5.5%) | 2.7 (0.4-18.5) |
| Tanaka et al[24], 2010 | Cohort | Japan | 1991-1993 | Population | 40-60 | 54.4 | 9.08/100000 p-yrs | 0.66/100000 p-yrs | 8.56 (1.33-55.20) | ||
| Fwu et al[25], 2011 | Cohort | Taiwan area of China | 1983-2000 | Population | 28.29 | 0 | 0.43/100000 p-yrs | 0.09/100000 p-yrs | 4.80 (1.88-12.20) | ||
Twenty case-control studies were included (seven from Mainland China[9-12,14,15,18], six from the United States[4,5,8,13,19,21], three from South Korea[7,17,20], two from Taiwan area of China[6,16] and one each from Japan[22] and Italy[23]), involving 14899 patients and 681258 cases. Notably, only 4 of the 20 retrieved studies were published in the last 5 years. Population-based controls were employed in 3 studies from the United States[4,13,21] and 1 from Mainland China[9] and the Taiwan area of China[6], whereas the remaining 15 studies were hospital-based. HBV infection status was defined by the presence of hepatitis B surface antigen (HBsAg) in 15 studies[5,7,8,10-12,14-20,22,23] and by international classification of diseases-9 coding in 5 studies[4,6,9,13,21]. Seropositivity of hepatitis B core antibody (HBcAb) was assessed in five studies[10-12,14,19]. All of the included case-control studies reported adjustments for age and gender. Fourteen studies showed a statistically significant association between HBV infection and risk of iCCA (range of individual OR = 2.23-12.9)[4-7,9-18], whereas the rest of the studies (n = 6) did not[8,19-23]. These six studies were concluded prior to 2002 in geographically different areas. Geographical variations in iCCA incidence may be explained by differences in environmental and genetic risk factors. Moreover, observational studies are difficult to control for confounders, which could present an important risk factor and influence the prognostic value of HBV infection in iCCA.
Two large-scale cohort studies with a total of 1937215 participants evaluating the correlation between HBV infection and iCCA were identified (Table 1). A retrospective cohort study from Japan involved 154814 blood donors with a median follow-up period of 7.6 years and reported an iCCA incidence rate of 0.88 per 100000 person-years[24]. Compared with individuals negative for both HBsAg and hepatitis C virus antibody (anti-HCV), those who tested positive for HBsAg had a significantly higher risk of iCCA (hazard ratio [HR] = 8.56; 95%CI: 1.33-52.20). The latter study from Taiwan area of China included a cohort of 1782401 pregnant women with a median follow-up of 6.91 years and reported 18 cases of iCCA[25]. Women seropositive for HBsAg had significantly increased incidence rates of iCCA (0.43 per 100000 person-years) than HBsAg-negative women (0.09 per 100000 person-years) with an age-adjusted hazard ratio HR = 4.80; 95%CI: 1.88-12.20.
Occult HBV infection (OBI), which is defined by the detection of HBV DNA in the serum and liver tissue of HBsAg-negative individuals with or without serological markers of previous viral exposure[28], is an emerging risk factor for iCCA. HBV maintains its pro-oncogenic properties in patients with OBI[29]. Indeed, epidemiological and molecular studies have indicated that OBI represents an important risk factor for the development of cirrhosis and HCC[30,31]. Relevant evidence from pathological studies, investigating the presence of HBV DNA, genes, and proteins in iCCA tissue specimens, also suggests a possible etiological role of OBI in iCCA[32-34]. A case-control study of 183 cryptogenic iCCA patients and 549 healthy individuals confirmed that HBsAg seroclearance does not signify eradication of HBV and may not entirely prevent the development of iCCA in Chinese patients[32]. Pollicino et al[33] investigated the presence of HBV DNA in liver tissue specimens from 47 HBsAg-negative patients with iCCA and 41 paired non-tumor liver tissues. A high prevalence of OBI in iCCA patients, along with the presence of both free viral genomes and integrated HBV DNA in the tumor tissue, suggested an involvement of HBV in the carcinogenesis of iCCA not only in overt but also in occult infection[33].
To date, there are limited data on the relationship between iCCA and HBV genotype. Barusrux et al[35] investigated the prevalence of HBV and HCV infection among 295 CCA patients in northeast Thailand[35]. In this study, HBV genotypes C (73.3%) and B (26.7%) were detected, in line with the HBV genotype distribution in Thailand[36]. In a cohort of 11 cases of iCCA in the eastern United States, liver specimens were examined for HBV DNA and HCV RNA and the genotypes observed represented the dominant genotype (genotype A) in this area[37]. Currently, there is no sufficient evidence to support an etiological association between specific HBV genotypes and iCCA.
To address the inconsistent results reported in previous epidemiological studies, an overview of the most comprehensive meta-analyses exploring the association between HBV infection and iCCA is provided (Table 2). A total of six systematic reviews and meta-analyses were retrieved[3,38-42], two of which focused on the risk factors of iCCA alone[41,42]. The remaining studies assessed HBV infection as a sole factor or among other risk factors for both iCCA and eCCA[3,38-40]. Of these, two meta-analyses were published in 2020 (one from the United Kingdom[3] and the other from China[38]), one in 2016 from China[39] and three in 2012 (two from China[40,42] and one from the United States[41]). In four meta-analyses, the population of the primary epidemiological studies originated from both Eastern and Western countries[3,40-42], whereas the remaining two meta-analyses included studies only from Asia[38,39]. The period of data collection among these primary studies ranged from 1987 to 2014.
| Ref. | Number of included studies | Data collection period | Regions | Cases | Controls | Pooled OR (95%CI) for HBV infection | Heterogeneity | Publication bias |
| Clements et al[3], 2020 | Case-control = 18 | 1991-2014 | Eastern = 12. Western = 6 | 14825 | 681181 | 4.57 (3.43-6.09) | I2 = 83% Q = 100.88 (P < 0.001) | Funnel plot: Symmetrical. No bias |
| Tan et al[38], 2020 | Case-control = 6 | 1998-2013 | Eastern = 6 | 3623 | 13597 | 5.98 (4.08-8.76) | I2 = 69% Q = 16.01 (P = 0.07) | Funnel plot: Relatively symmetrical. Moderate bias |
| Zhang et al[39], 2016 | Case-control = 11 | 1991-2013 | Eastern = 11 | 4966 | 17752 | 3.18 (2.35-4.30) | I2 = 84% Q: P < 0.001 | Begg's test: P = 0.75, Egger's test: P = 0.42. No bias |
| Li et al[40], 2012 | Case-control = 11. Cohort = 2 | 1991-2008 | Eastern = 10. Western = 3 | 2402. NA | 96266. NA | 3.42 (2.46-4.74) | I2 = 75% Q = 48.37 (P < 0.001) | Funnel plot: Symmetrical Begg's test: P = 0.70, Egger's test: P = 0.26. No bias |
| Palmer et al[41], 2012 | Case-control = 8 | 1991-2008 | Eastern = 5. Western = 3 | 2753 | 291756 | 5.54 (3.19-9.63) | I2 = 86.3% Q = 51.2 (P < 0.001) | Funnel plot: Symmetrical. No bias |
| Zhou et al[42], 2012 | Case-control = 12. Cohort: = 2 | 1987-2009 | Eastern = 11. Western = 3 | 2362. NA | 96452. NA | 3.17 (1.88-5.34) | I2 = 81% Q: P < 0.001 | Funnel plot: Asymmetry. Moderate bias |
All of the included meta-analyses suggested that HBV infection was significantly associated with an increased risk of iCCA (range of pooled OR = 3.17-5.98)[3,38-42]. A moderate to severe degree of statistical heterogeneity was noted across the primary epidemiological studies, as assessed by Cochran’s Q and I2 statistic (Table 2). Publica
To the best of our knowledge, the most up-to-date systematic review and meta-analysis were performed by Clements et al[3] in 2020. A total of 13 risk factors, including HBV, were evaluated for iCCA and eCCA. Eighteen case-control studies were included to study the association between HBV and iCCA. The pooled risk estimate (OR = 4.57; 95%CI: 3.43-6.09) showed a statistically significant increased risk of iCCA with HBV infection. Furthermore, meta-regression analysis indicated that in Eastern countries, HBV conferred a greater risk of iCCA than in Western countries. Similar geographical differences for the association of HBV with iCCA have been reported by Li et al[40], however Palmer and Patel[41] did not reveal any significant difference between Eastern and Western populations.
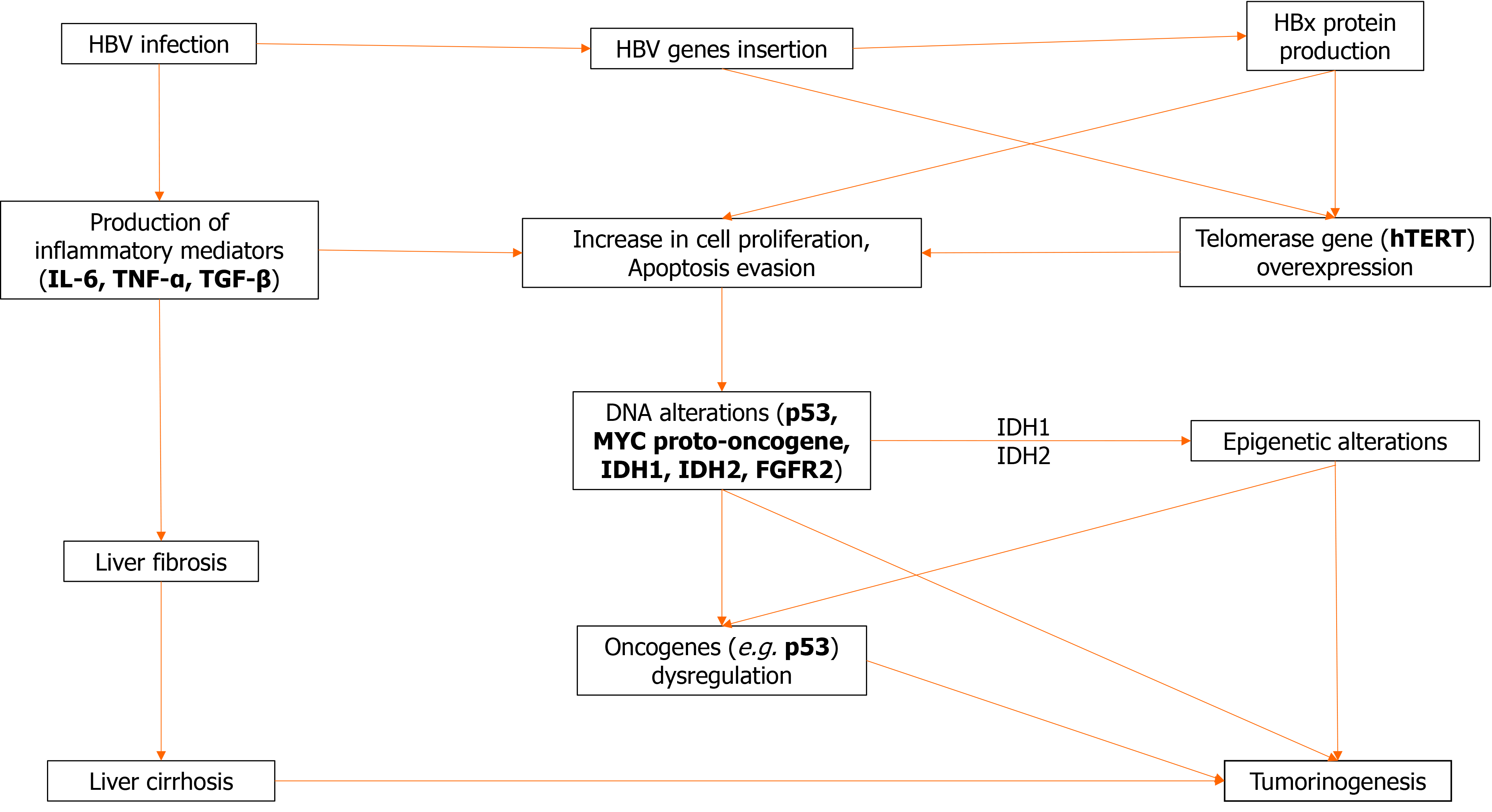
Cholangiocarcinoma is a multifactorial heterogeneous disease of diverse predisposing factors. However, regardless of underlying etiology, chronic inflammation seems to be a common denominator of most risk factors for CCA as they are usually associated with inflammation or cholestasis. Chronic inflammation leads to increased production of a multitude of inflammatory mediators such as interleukin-6 (IL-6), tumor necrosis factor (TNF), cyclooxygenase-2, and Wnt, resulting in an “activated” environment favoring successive mutations of tumor suppressor genes, and other oncogenes. Other mediators found to be upregulated in CCA include transforming growth factor beta (TGF-β), vascular endothelial growth factor, hepatocyte growth factor, and several microRNAs[43]. Most of those changes promote angiogenesis and cell proliferation.
In particular, liver cirrhosis regardless of etiology represents an inflammatory process that is clearly associated with the development of iCCA. Several population-based studies have confirmed cirrhosis as an important risk factor not only for HCC but also for iCCA[44]. A meta-analysis found that cirrhosis causes iCCA with an OR as high as 22[41]. Furthermore, a retrospective analysis of United States-based Surveil
Inflammation is a multifactorial and diffuse process. It involves multiple pathways acting through diverse mechanisms in an environment conducive of proliferation and oncogene mutations leading to malignant transformation. As a result, there has been no single molecular alteration characteristic of CCA. IL-6, a strong proinflammatory cytokine has a pleiotropic role, by increasing cell proliferation through activation of the Janus kinase-signal transducer and activator of transcription pathway; it also promotes expression of antiapoptotic myeloid leukemia cell differentiation protein and the activation of both the Akt and mitogen-activated protein kinase kinase (MEK)-extracellular signal-related kinase (ERK) pathways, which results in strong proliferative and anti-apoptotic effects[50-52]. In such an activated environment, increased DNA instability may lead to critical genetic alterations of the excited cells, and also increases the probability of mutations of important genes such as tumor suppressor gene p53 and the MYC proto-oncogene, among others[53]. The DNA instability is at least partially attributable to the effect of TNF-α, which among others, causes the upregulation of activation-induced cytidine deaminase, an enzyme that can create DNA mutations by converting cytosine to uracil.
In addition to the inflammatory changes caused in the liver tissues by the continuous presence of the virus, it seems that hepatitis viral components may act as oncogenes and promote oncogenicity per se. In particular, hepatitis B x (HBx) protein has been implicated in carcinogenesis. Experiments with transgenic mice have demonstrated that its expression enhances IL-6-dependent proinflammatory effects and causes malignant transformation towards both HCC and CCA[54].
The presence of HBV genes in the genome of CCA has been described. Although the exact effect on tumorigenesis is unknown, the observed proximity of the insertion sites to the gene encoding the tumor suppression p53 is intriguing. Wang et al[55] using in situ hybridization demonstrated the presence of HBV genes in 33 of 40 iCCA cases tested; the most common gene found was again HBx[55]. In a study by Pollicino et al[33] employing a PCR technique, incorporation of HBV genes in patients with iCCA and no overt viral infection was detected in tumor or non-tumorous liver cells in 29 of the 47 examined cases, suggesting the importance of occult viral infection in the development of CCA. Most cases involved the HBx gene.
Expression of the culprit HBx gene may be documented by immunohistochemistry in about 70% of patients with iCCA and correlates with peripheral tumors and higher aFP levels, but the exact percentage obviously depends on the epidemiology of the tested population[56]. Co-expression of HBx and HCV core proteins in transgenic zebra fish livers induces fibrosis and iCCA[57], and is associated with activation of the phosphorylated Smad3L oncogenic pathway with consequent phosphorylation and activation of MEK-ERK proteins. Bile duct proliferation, fibrosis, and iCCA were markedly reduced by knockdown of TGF-β, suggesting the important role of this mediator. Semaphorine 3B is a recently identified tumor suppressor gene encoded on chromosome 3p21.3 and is connected to p53 activity. Silencing of the locus due to gene or promoter hypermethylation or loss of homozygosity was detected in several CCA tumor cells, and as expected, was associated with the downregulation of mRNA transcripts[43].
These dysregulations result in a broad variety of somatic mutations that have been recently identified using next-generation sequencing, some of which impair the function of known oncogenes. Almost any gene implicated in carcinogenesis is abnormal in CCA, attesting to the heterogeneity of the disease and the lack of a pathognomonic driver mutation[58]. Thus, KRAS mutations may affect 20% of CCA. Mutations of isocitrate dehydrogenase 1 (IDH1) and IDH2 genes occur in approximate 20% of iCCA. IDH1 and IDH2 genes encode for enzymes active in the citric acid cycle (Krebbs cycle), and their loss of function mutations results in accumulation of an abnormal oncometabolite R-2-hydroxyglutarate which interferes with the function of several pathways and causes epigenetic DNA changes. Other alterations of iCCA include fibroblast growth factor receptor 2 (FGFR2) mutations. Translocation or amplification of the FGFR2 locus is found in 19% of 122 iCCA patients[59]. Tumors of such patients tend to be of the mass-forming type and compared to non-FGFR2 altered tumors, they secrete less mucus and are more commonly associated with HBV infection.
Although some of these alterations appear to be important for the malignant behavior of the tumor, they may not represent the initiating transforming event, which should be sought earlier in the pathogenesis of iCCA. There is enough evidence to suspect that chronic, overt or occult, infection with hepatitis virus may trigger the transformation of infected cells in the context of a conducive, inflammatory environ
It can be concluded that mounting evidence supports the theory that chronic, overt, or latent infection with HBV has direct causative effects on the development not only of HCC but also of iCCA, perhaps by acting on a common progenitor cell. The effect of certain viral genes, such as HBx, seems to have direct oncogenic capacity beyond the genetic instability produced by the chronic inflammation, perhaps aided by the latter. These observations support the epidemiologic data linking CHB to the development of iCCA and call for further studies to fully reveal the intricate interaction of the virus with the cellular mechanisms leading to carcinogenesis.
Patients with iCCA usually present with unspecific symptoms such as abdominal pain, malaise, night sweats, and cachexia[63]. Jaundice is usually a sign of advanced disease. In particular, patients with HBV-associated iCCA can be diagnosed before they develop any symptoms, due to the fact that they undergo annual or semi-annual surveillance for HCC, which enables iCCA diagnosis at a relatively early stage[64].
Imaging modalities, such as computed tomography (CT), MRI, and fluorodeoxyglucose positron emission tomography (FDG-PET) can be of great value in the management of iCCA in terms not only of diagnosis but also of staging, follow-up, and assessment of treatment response.
CT scan serves as the standard imaging method for the preoperative assessment of iCCA. It provides a comprehensive evaluation of the primary tumor, the relationship between the primary tumor and adjacent structures such as the hepatic artery or portal vein, and the whole abdomen surveillance for potential metastases[65]. On both contrast-enhanced CT and MRI scans, iCCA usually presents as a mass with irregular borders, peripheral rim enhancement on the arterial phase with progressive homogeneous contrast agent uptake until the delayed or stable uptake during late dynamic phases[66,67]. However, smaller tumors are less likely to demonstrate these characteristics and may be hard to distinguish from HCC, especially in the context of chronic liver diseases.
The utility of FDG-PET for iCCA diagnosis and management is still controversial. Although mass-forming iCCA can be detected with a reported sensitivity of 85% to 95%, it cannot be used for the diagnosis of iCCA as it is not specific enough[68]. However, it could prove useful in detecting distal, unexpected metastases. In fact, a recent meta-analysis suggested that it can bring changes to the management of a substantial portion of iCCA patients[69]. Therefore, it could be considered an option prior to surgical resection (SR) to rule out distant metastatic foci.
Tumor markers in serum are not specific for iCCA, but may be of diagnostic value in some cases. Widely used tumor markers such as CA 19-9 and carcinoembryonic antigen have significant overlap with other cholestatic diseases and low sensitivity for early stage disease, consequently limiting their use[70]. However, high values of these markers have been connected with a deterioration in overall survival (OS)[71,72]. As a result, high (> 500 ng/mL) levels of CA 19-9 in the absence of jaundice are considered a relative contraindication for resection of iCCA[73].
However, in cases of HBV-associated iCCA, these markers tend to be even less sensitive[48]. Instead, it has been suggested that such patients tend to have higher levels of aFP, a serum marker widely used for HCC diagnosis, probably because of the fact that both malignancies originate from hepatic progenitor cells, which have the ability to produce aFP[16,46,74,75].
Pathological diagnosis is recommended for all patients who will undergo systemic chemotherapy or radiation therapy, or take part in a therapeutic clinical trial[68,76]. It should be noted that although a positive result demonstrates the presence of the tumor, a negative result does not exclude it because of the potential sampling errors. In such cases, repeat sampling is often required to confirm the correct diagnosis[77].
According to the World Health Organization (WHO) classification for biliary tract cancer[78], iCCA can be further classified to two main subtypes according to the level or size of the affected duct; large duct type, which resembles eCCA and small duct type, which shares etiological, pathogenetic, and imaging characteristics with HCC and usually develops on a background of chronic liver disease (such as chronic viral hepatitis and cirrhosis)[58]. Small bile duct iCCA presents as a tubular or acinar adenocarcinoma with nodular growth invading the liver parenchyma, and with no or minimal mucin production[79-81]. Characteristic findings of small bile duct iCCA are IDH1 and IDH2 mutations or FGFR2 fusions or translocations[80-82]. In HBV-associated iCCA, there are data indicating the presence of hyperactivated program
iCCA is mainly staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) TNM system (Tables 3 and 4). Despite providing some meaningful contribution to the patient’s prognostication, it does have some rather important limitations. First, although the tumor size was introduced for the first time in the latest AJCC Cancer Staging Manual, the only discriminating size cut-off used is the one of < 5 cm encompassing all T1 tumors. However, there are some data indicating that patients with very small (< 2 cm) tumors have potentially better outcomes in terms of survival, rendering them eligible for different therapeutic modalities[83]. In addition, the latest TNM system fails to include parameters such as performance status (PS) or the severity of liver function impairment. Both of these limitations are clearly extremely important for assessing the prognosis of patients with HBV-associated iCCA, as very small tumors can be detected during surveillance programs and may also have advanced parenchymal liver disease.
| Staging classification | |
| T1a | Solitary tumor ≤ 5 cm without vascular invasion |
| T1b | Solitary tumor > 5 cm without vascular invasion |
| T2 | Solitary tumor with vascular invasion, or multiple tumors with or without vascular invasion |
| T3 | Tumor perforating the visceral peritoneum |
| T4 | Tumor involving the local extrahepatic structures by direct invasion |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis present |
| M0 | No distant metastasis |
| M1 | Distant metastasis present |
| Stage | T | N | M |
| Ia | T1a | N0 | M0 |
| Ib | T1b | N0 | M0 |
| II | T2 | N0 | M0 |
| IIIa | T3 | N0 | M0 |
| IIIb | T4 | N0 | M0 |
| Any T | N1 | M0 | |
| IV | Any T | Any N | M1 |
Combined HCC-CCA (cHCC-CCA) is a rare type of primary liver cancer (PLC), which has been defined as a distinct carcinoma displaying features of both hepatocytic and cholangiocytic differentiation[84]. Since the first description of this entity in 1949[85], significant terminology changes have been introduced. The 2010 WHO classification system distinguishes a classical form of cHCC-CCA and three variants with stem/progenitor cell features including typical, intermediate cell, and cholangiolocellular subtypes[86]. However, molecular advancements throughout the years have challenged the validity of stemness; thus, the term “typical subtype” has been abandoned; cholangiolocellular carcinoma is currently considered to be a subtype of iCCA, but it can still be within the spectrum of cHCC-CCA only if an HCC component is also involved and is currently addressed as “cholangiolocarcinoma”; PLCs consisting exclusively of intermediate cells are specified as intermediate cell carcinomas, while the role of intermediate cells in cHCC-CCA is still debated[84,87,88].
Large series have reported that cHCC-CCA represents 0.77%-5.8% of all PLCs[89,90]. Viral hepatitis (HBV, HCV), alcohol abuse, and cirrhosis have been associated with this cancer. Notably, cirrhosis and HBV infection are more commonly related with cHCC-CCA than iCCA, although with lower rates compared to HCC[91-94]. Most patients are aged between 50 and 60 years, with comparative studies claiming presentation in a younger age than iCCA[91,95], while others have not managed to point out significant age differences[92,93]. Male predominance is well documented (65%-80%)[89,90,95,96] and tends to be more notable opposed to iCCA patients[92]. Studies focusing on HBV-related cHCC-CCA present an even greater male to female ratio that reaches 9:1[97,98].
Imaging features may resemble patterns of HCC, CCA, or both, therefore rendering radiological modalities insufficient to ensure definite diagnosis. Arterial phase hyperenhancement and portal/delayed phase hypoenhancement (washout) are characteristic findings of HCC in CT, MRI, and contrast-enhanced ultrasound; however, they could also be displayed by cHCC-CCA lesions and lead to misdiagnosis[93,99]. Similarly, cHCC-CCA tumors can mimic CCA by displaying gradual enhancement and peripheral irregular rim-like contour. According to Wang et al[92], in the former case the presence of lymphadenopathy, elevation of CA 19-9 and/or biliary dilation are in favor of cHCC-CCA, whereas the presence of pseudocapsule and well-defined margin are more suggestive of HCC. In the latter case when cHCC-CCA resembles features of CCA, the presence of simultaneous cirrhosis, HBsAg positivity, pseudocapsule and/or portal vein thrombosis should raise suspicion of cHCC-CCA. Furthermore, concurrent elevation of both aFP and CA 19-9 or discordance between tumor markers and imaging features (i.e. radiological hallmarks of HCC accompanied by increased CA 19-9 or lesions resembling CCA with raised aFP) should prompt consideration of cHCC-CCA diagnosis[100,101]. Histopathological examination of biopsies or surgical specimens based on routine stains ultimately establishes the correct diagnosis. Immunohistochemical markers can provide additional information, but are of secondary importance[84].
Complete excision with negative margins and sufficient residual liver volume is the goal of treatment[102]. In a large series of 390 HBV-related cHCC-CCA, the median OS after SR was 20 mo and negative prognostic factors included male gender, number of tumors ≥ 2, major thrombus, gamma-glutamyl transferase (GGT) > 60 IU/L, and CA 19-9 levels[98]. In a recent systematic review, data analyses of 14 studies showed that patients with cHCC-CCA, HCC, and CCA undergoing SR had 5-year survival rates of 32.7%, 47.5%, and 30.3% respectively[96]. Similar outcomes have been published about liver transplantation (LT): HCC patients achieve considerably greater survival rates than cHCC-CCA and CCA subgroups[95,103]. The role of locoregional treatment and systematic chemotherapy in the management of cHCC-CCA remains uncertain. A retrospective analysis of 50 patients with unresectable cHCC-CCA undergoing transarterial chemoembolization (TACE) demonstrated that better response rates could be attained when careful selection is applied (i.e. hypovascular tumors respond poorly due to inability of chemotherapeutic agents to reach target)[94]. Limited data about chemotherapy are available; however, it is noteworthy to mention the results of two recent multicenter studies using platinum-based regimens, which were associated with favorable outcomes compared to sorafenib monotherapy[104] and could be potentially effective when used together with gemcitabine as first-line treatment for advanced, unresectable cHCC-CCA[105].
SR is the treatment of choice for all iCCA patients, since it is the only established therapy capable of achieving a possible cure[68,76]. Conventional surgical aim of a SR should be complete tumor resection with negative microscopic (R0) margins while preserving adequate liver volume to avoid post-operative liver failure[73].
The eligibility of a patient for curative surgery should always be determined by a multidisciplinary board on a case-by-case basis. Given the poor prognosis of patients who have multifocal disease or clinically evident lymph node metastasis, SR should be precluded for these patients[106,107]. Regarding macroscopic vascular invasion, although it should generally be considered a contraindication, there are some data from specialized centers supporting that major vascular resection is not associated with worse perioperative or oncologic outcomes[108]. Obviously, as with HCC[109], liver function should be assessed in patients with underlying liver cirrhosis. Restrictions for resection are recommended in cases where liver function is significan
As aforementioned, patients with HBV-associated iCCA are often diagnosed at a relatively early stage. This is probably the reason why the proportion of iCCA patients who undergo curative resection is significantly higher for HBV-positive than HBV-negative patients[110]. HBV infection has been identified by many studies as an independent factor associated with favorable outcomes, suggesting that better prognosis of HBV associated iCCA may be perhaps explained by less aggressive biological nature[111-115]. In fact, a meta-analysis showed that lymph node metastasis occurred less commonly in HBV-associated iCCA than iCCA of other etiology (OR = 0.39)[48]. In a large single-center retrospective study from China that included 1333 iCCA patients (608 HBsAg-positive and 725 HBsAg-negative) the median post-operative survival was 19 mo for HBsAg(+) compared to 12 mo for HBsAg(-) patients (P < 0.001)[116]. Factors that have been associated with better outcomes for patients with HBV-associated iCCA include tumor diameter < 5 cm, better tumor differentiation, lower serum levels of aFP or CA 19-9 and absence of vascular invasion, lymph node metastasis, extrahepatic metastasis or TP53 mutation[113,117,118].
Until a few years ago, the role of adjuvant chemotherapy in the setting of iCCA was not well defined because of a complete lack of data from randomized trials. However, a meta-analysis of retrospective cohorts indicated that administration of chemotherapy in the adjuvant setting provides a survival benefit to patients with iCCA[119]. This contrasted with the randomized PRODIGE 12[120] trial (gemcitabine and oxaliplatin [GEMOX] vs surveillance for patients with resected biliary tract cancer) which failed to produce statistically significant results. On the other hand, the BILCAP trial (capecitabine vs observation for patients with resected biliary tract cancer)[121], showed a benefit in terms of recurrence-free survival in the intention-to-treat analysis (HR = 0.75, P < 0.05) and also in terms of OS in the pre-planned sensitivity analysis (HR = 0.75, P < 0.05), both in the first 24 mo from randomization. However, no statistically significant benefit was observed in the intention-to-treat OS analysis of the whole population or the iCCA subgroup. Based on these partial benefits, the American Society of Clinical Oncology published a new guideline recommending that all patients undergoing SR of any biliary tract tumors should be offered adjuvant capecitabine chemotherapy for 6 mo[122]. More information is expected in the foreseeable future from the ACTICCA-1 study (NCT02170090), a phase III trial investigating the clinical performance of the GEMOX combination compared to capecitabine in the adjuvant setting.
There are not much data concerning the role of antiviral treatment in the management of HBV-associated iCCA. However, one retrospective study demonstrated that antiviral treatment, even when initiated post-operatively, can provide a significant benefit to the patients compared to patients with high (> 2000 IU/mL) HBV-DNA levels who are left untreated (5-year recurrence rate 70.5% vs 86.5%, P < 0.001 and 5-year OS rate 43% vs 20.5%, P < 0.001 respectively)[123].
In 2017, a large-scale retrospective study from Taiwan area of China that was based on data from the National Health Insurance Research Database showed that treatment with nucleos(t)ide analogues (NA) is an independent factor for the development of HBV-associated iCCA (HR 0.44, P = 0.004)[124]. In the population treated with NA, the 5-year cumulative incidence of iCCA was lower compared to the NA-untreated group (0.17% vs 0.39%, P = 0.005), stating that treatment with NA could contribute in preventing HBV-associated iCCA, in a similar way as it contributes to reducing the risk of HBV-associated HCC.
LT for iCCA is still a controversial issue. In the era of organ shortage, iCCA was traditionally considered a contraindication for LT, as both the OS and disease-free survival were disappointing. It should be noted that most of this information came from small, retrospective studies with much heterogeneity in transplant protocols. In certain studies, the 5-year OS rate ranged between 30% to 60%[125-128].
Studies focusing on favorable groups of patients who could potentially derive significant benefit from LT have been reported. In a multicenter retrospective study from Spain[129], a subgroup of patients (n = 8) with “very early” iCCA (single tumor, < 2 cm) was highlighted. The 5-year OS in this subgroup was 73% compared to 35% for the patients with single tumors > 2 cm or multinodular tumors (n = 21). Another international, multicenter study showed similar results, as the actuarial survival rate for “very early” iCCA was 65%[83]. A recent meta-analysis confirmed the results of such efforts (5-year pooled OS rate 71% vs 48% for advanced iCCA), stating that a solitary < 2 cm iCCA in a background of cirrhosis should not preclude consideration of LT[130]. It should be noted though, that the application of LT for cirrhotic patients, with “very early” iCCA unresectable due to impaired liver function still requires validation by a prospective study. Such a study (NCT02878473) is currently recruiting patients and is expected to provide primary results by 2026.
Another interesting subgroup of iCCA patients, who could substantially benefit from LT, are those with locally advanced, unresectable (due to tumor size, location, or multifocality) disease with a durable response or stability after neoadjuvant chemotherapy[131]. In fact, in an interim analysis of a small series of patients (n = 6), with large, unresectable iCCA subjected to LT after an at least 6-mo disease stability under neoadjuvant chemotherapy with gemcitabine-based regimens, the 5-year survival was 83%, although, of note, 5-year recurrence rate was 50%[132].
In light of this information, a recent consensus approved by the International Liver Transplant Society recommends that patients with “very early” iCCA may benefit from upfront LT, whereas those with advanced, unresectable iCCA in a non-cirrhotic state may become LT candidates if the disease remains stable after neoadjuvant therapy[133].
Unfortunately, many patients with iCCA present with advanced disease that is not amenable to surgery. When assessing a patient with unresectable iCCA, the medical team managing his care should first take into consideration the patient’s PS, as determined by the Eastern Cooperative Oncology Group PS (ECOG-PS) scale. Patients with ECOG-PS > 2 are unlikely to benefit from any treatment and should be managed with best supportive care. After that, the stage of the disease and the molecular profile of the tumor should be assessed in order to decide the most suitable therapeutic approach for each patient.
Systemic chemotherapy is generally recommended for patients with ECOG-PS 0-1[68]. The combination of gemcitabine and cisplatin (GEMCIS) is used as the first-line therapeutic regimen in patients with advanced iCCA[105,134]. In the ABC-02 trial (n = 410, all biliary tract cancers) the researchers observed a statistically significant difference in OS and in progression-free survival (11.7 mo vs 8.1 mo and 8 mo vs 5 mo, respectively) in favor of the GEMCIS combination compared to gemcitabine alone. The GEMOX combination may be used instead in patients with renal function impairment[76]. Additional combinations of chemotherapeutic agents are being investigated in the first-line setting, including more intensive, triple chemotherapy combinations like GEMCIS with nab-paclitaxel[135]. Acelarin is a NA which can achieve greater intracellular concentrations than gemcitabine and its efficacy in combination with cisplatin is currently being investigated in a phase III study as opposed to GEMCIS (NCT04163900).
After disease progression under first-line chemotherapy with GEMCIS, the next best choice is probably the combination of folinic acid, fluorouracil and oxaliplatin (FOLFOX). In the phase III ABC-06 trial, 162 patients who had already signs of disease progression under GEMCIS administration, were randomly assigned to active symptom control alone or concomitant FOLFOX[136]. In this study, differences in 6- and 12-mo survival (35% vs 50.6% and 11.4% vs 25.9%, respectively) were considered clinically important, although there was no significant difference in the median OS between the two arms (5.3 mo vs 6.2 mo).
A recent multicenter study used clinical and pathological data in addition to information from RNA sequencing in order to identify distinct subtypes of iCCA that might have favorable response to specific treatments. In the subtype that was associated with hepatitis B and C, an in vivo sensitivity to gemcitabine was observed. This was attributed to a higher expression of gemcitabine-response genes (SLC28A1 and SLC29A1)[137]. However, these findings should be confirmed in large clinical studies prior to establishing a recommendation for patients with hepatitis B.
Locoregional therapies have implicit appeal as a treatment option for iCCA, as it is a PLC and often the disease burden is either completely or largely confined to the liver[138]. The most widely used types of locoregional therapeutic regimens in iCCA are TACE, radiofrequency ablation (RFA) and microwave ablation (MWA) and transarterial radioembolization (TARE) with Yttrium90 (Y-90). The possible role of these modalities in the management of iCCA has mainly been investigated by retrospective studies in various settings. Consequently, the results of the studies are difficult to interpret, due to the high heterogeneity in terms of patient population (chemotherapy naïve or not) and type of treatment.
TACE is the most widely used treatment modality for patients with intermediate stage HCC not eligible for curative treatments. The rationale for TACE is based on the hypervascular nature of HCC that enables both strong cytotoxic and ischemic effects following the intra-arterial infusion of a cytotoxic agent and the selective embolization of the blood vessels feeding the tumor. However, iCCA rarely appears hypervascular on CT or MRI studies[68]. Despite that, a retrospective study showed that TACE (n = 72) could be beneficial in terms of OS to patients with unresectable iCCA when compared to supportive care (n = 83, OS = 12.2 vs 3.3 mo, respectively)[139]. However, in another multiinstitutional retrospective study of 188 patients with advanced-stage iCCA, TACE did not seem to have any significant benefit when compared to other arterial-based therapeutic interventions[138]. The efficacy of TACE has also been investigated in the adjuvant setting by a prospective study of 553 consecutive patients (122 TACE vs 431 non-TACE)[140]. Although in the whole cohort, the 5-year OS rates between groups were significantly different (38.4% vs 29.7) after 1:1 propensity score matching no significant differences were observed regarding OS or 5-year recurrence rate. Nevertheless, a recent systematic review and meta-analysis concluded that TACE in the adjuvant setting can actually prolong both the OS and the relapse-free survival[141]. A small, prospective study from China included 42 either HBsAg(+) or HBcAb(+) patients with iCCA who had received SR with curative intent and evaluated the prognostic impact of postoperative TACE[118]. TACE was performed in 9 patients who had demonstrated early recurrence of the tumor within 12 mo from the surgery. The OS of the patients in the TACE (n = 9) group was significantly prolonged (1-year, 88.9%; 3-year, 77.8%; 5-year, 66.7%) as compared to the non-TACE (n = 33) group (1-year, 63.6%; 3-year, 30.8%; 5-year, 13%), implying that TACE could have a role in the management of patients with HBV-associated iCCA when performed in the adjuvant setting.
A theoretical advantage of ablative therapies like RFA and MWA when compared to TACE, is that they are safe and effective for the local control of hepatic malignancies in patients considered unsuitable for SR without regard to the tumor’s vascular profile[142,143]. The efficacy of ablative therapies in the management of unresectable iCCA has been mostly studied in small-scale, retrospective studies. RFA and MWA seem to be helpful for smaller tumors (< 3 cm), but lose part of their effectiveness when it comes to bigger or irregularly shaped tumors resulting in higher recurrence rates[144,145]. This issue may be overcome by using stereotactic RFA, which combines pre-interventional three-dimensional imaging, computerized three-dimensional planning of electrode positions, and guided electrode placement at arbitrary angulations and orientations[146,147]. In a systematic review and meta-analysis regarding the efficacy of ablative therapies in the management of unresectable iCCA the pooled 1-, 3-, and 5-year OS rates were 76%, 33% and 16%, respectively[148]. It should be noted though, that this systematic review analyzed studies that included cases with various disease stages, such as recurrent or inoperable disease, thus resulting in a high degree of heterogeneity. To our knowledge, there are no published studies regarding the use of ablative therapies in the context of HBV-associated ICCA. It is obvious that in order to extract safer conclusions regarding the role of ablative therapies in the management of iCCA and HBV-associated iCCA in particular, well-designed prospective studies are needed.
In a systematic review and pooled analysis assessing the efficacy of Y-90 in the management of unresectable iCCA, the median OS was 15.5 mo, but the study highlighted the need for prospective studies on this subject[149]. In the MISPHEC study, a phase II single-arm trial where 41 patients were treated with Y-90 and GEMCIS, the results were rather promising, as median OS was 22 mo and 9 patients (22%) were even downstaged to surgery[150]. A phase III randomized trial investigating the efficacy of Y-90 with GEMCIS compared to GEMCIS alone is currently ongoing (NCT02807181). Unfortunately, there are no published studies regarding the use of TARE in the treatment of HBV-associated iCCA.
As our knowledge broadens regarding the DNA alterations and the molecular pathways that are involved in the pathogenesis of HBV-associated iCCA, more possible therapeutic targets are going to arise, until we reach a point where the treatment of each patient will be personalized. As mentioned earlier, some of the most common findings in the genome of viral hepatitis-associated iCCA, include IDH1, IDH2 and FGFR2 mutations. The efficacy of ivosidenib, a targeted inhibitor of IDH1 and IDH2, was investigated in the phase III ClarIDHy trial involving 185 patients with chemotherapy-refractory, IDH1 mutant CCA who were randomly assigned to ivosidenib or placebo[151]. The study managed to prove the clinical benefit of targeting IDH1 mutations in such tumors, as progression-free survival was signifi
The clinical data regarding immunotherapy in the management of CCA are limited. Although there is a lot of merit to the idea of a therapeutic approach that targets the immune checkpoint hyperactivation, which is common especially in HBV-associated iCCA, to date, the results from clinical trials are not promising. Pembrolizumab monotherapy provided antitumor activity in just 6% of patients with advanced biliary cancer enrolled in the KEYNOTE-158 study, with median progression-free survival of 2 mo[155]. Possibly, these therapeutic agents can provide better results when administered in combination with chemotherapy. There is an ongoing, phase II, single-arm trial assessing the efficacy of GEMCIS with pembrolizumab in patients with advanced biliary cancer (NCT03260712). Other studies have investigated the possible benefit of immune checkpoint inhibitors combined with locoregional therapeutic regimens (NCT02821754, NCT04299581).
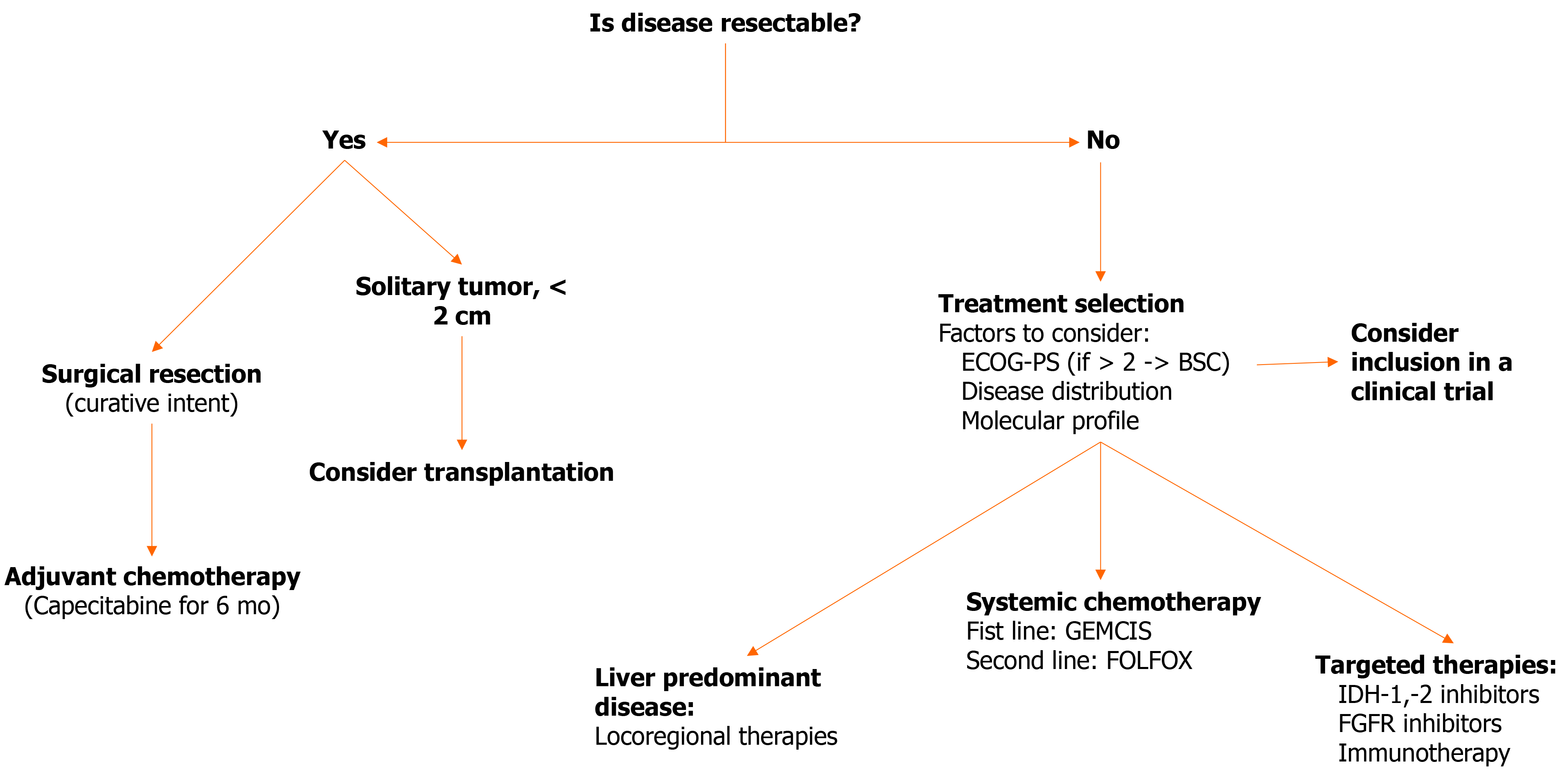
Figure 4 presents a therapeutic algorithm that encompasses all the above described treatment modalities for iCCA.
Increasing data during the last 5 years have strengthened the association between the infection with HBV and the development of iCCA. The epidemiological data unveiling this association are from both Eastern and Western countries and this linkage has been confirmed by multiple meta-analyses. In addition, the pathogenetic pathways through which HBV leads to the development of iCCA have been recently the targets of intense investigation. Furthermore, the diagnosis of iCCA in the setting of chronic liver disease is frequently challenging and often requires histological examination. Nonetheless, certain aspects of this association still remain ill-defined.
In terms of management, the need for effective treatment modalities beyond SR is critical both in the first and the second line treatment. The evolution of personalized approaches through the recognition of specific therapeutic targets will improve the effectiveness of our treatment approaches. It is also likely that the results of ongoing studies in the field of immunotherapy will allow the use of this promising treatment in patients with iCCA, as well. Furthermore, it is currently unclear whether antiviral treatment for HBV can decrease the incidence of iCCA. Large, well-designed studies should address this important question. Focused research on these aspects of the management of HBV-associated iCCA in the future will enhance our ability to manage successfully this dreadful cancer.
The authors want to thank Drs. Dimitrios Bekiaropoulos, Vasiliki Georgopoulou and Evangelia Katsiki for their help and encouragement in the process of preparing this work.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases, No. 118879; and European Association for the Study of the Liver, No. 43598.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chi G, Nakajima H, Santos-Laso A S-Editor: Fan JR L-Editor: Filipodia P-Editor: Xing YX
| 1. | Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463-73; discussion 473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 865] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 2. | Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 967] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 3. | Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol. 2020;72:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 343] [Article Influence: 68.6] [Reference Citation Analysis (1)] |
| 4. | Petrick JL, Yang B, Altekruse SF, Van Dyke AL, Koshiol J, Graubard BI, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS One. 2017;12:e0186643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 5. | Choi J, Ghoz HM, Peeraphatdit T, Baichoo E, Addissie BD, Harmsen WS, Therneau TM, Olson JE, Chaiteerakij R, Roberts LR. Aspirin use and the risk of cholangiocarcinoma. Hepatology. 2016;64:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Huang YJ, Wu AT, Chiou HY, Chuang MT, Meng TC, Chien LN, Yen Y. Interactive role of diabetes mellitus and female sex in the risk of cholangiocarcinoma: A population-based nested case-control study. Oncotarget. 2017;8:6642-6651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Lee BS, Park EC, Park SW, Nam CM, Roh J. Hepatitis B virus infection, diabetes mellitus, and their synergism for cholangiocarcinoma development: a case-control study in Korea. World J Gastroenterol. 2015;21:502-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Chaiteerakij R, Yang JD, Harmsen WS, Slettedahl SW, Mettler TA, Fredericksen ZS, Kim WR, Gores GJ, Roberts RO, Olson JE, Therneau TM, Roberts LR. Risk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer risk. Hepatology. 2013;57:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 9. | Chang JS, Tsai CR, Chen LT. Medical risk factors associated with cholangiocarcinoma in Taiwan: a population-based case-control study. PLoS One. 2013;8:e69981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Wu Y, Wang T, Ye S, Zhao R, Bai X, Wu Y, Abe K, Jin X. Detection of hepatitis B virus DNA in paraffin-embedded intrahepatic and extrahepatic cholangiocarcinoma tissue in the northern Chinese population. Hum Pathol. 2012;43:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Wu Q, He XD, Yu L, Liu W, Tao LY. The metabolic syndrome and risk factors for biliary tract cancer: a case-control study in China. Asian Pac J Cancer Prev. 2012;13:1963-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Peng NF, Li LQ, Qin X, Guo Y, Peng T, Xiao KY, Chen XG, Yang YF, Su ZX, Chen B, Su M, Qi LN. Evaluation of risk factors and clinicopathologic features for intrahepatic cholangiocarcinoma in Southern China: a possible role of hepatitis B virus. Ann Surg Oncol. 2011;18:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 423] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 14. | Tao LY, He XD, Qu Q, Cai L, Liu W, Zhou L, Zhang SM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver Int. 2010;30:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Zhou HB, Wang H, Zhou DX, Wang Q, Zou SS, Hu HP. Etiological and clinicopathologic characteristics of intrahepatic cholangiocarcinoma in young patients. World J Gastroenterol. 2010;16:881-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Lee CH, Chang CJ, Lin YJ, Yeh CN, Chen MF, Hsieh SY. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. Br J Cancer. 2009;100:1765-1770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, Kwon S, Lee SK, Seo DW, Kim MH, Suh DJ. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. 2008;103:1716-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, Wang YL, Li DQ. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2008;14:632-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, Hassan MM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102:1016-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, Jang KT, Lee NY, Kim S, Hong ST. Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. J Hepatol. 2006;44:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 385] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 22. | Yamamoto S, Kubo S, Hai S, Uenishi T, Yamamoto T, Shuto T, Takemura S, Tanaka H, Yamazaki O, Hirohashi K, Tanaka T. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci. 2004;95:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, Martelli C, Savio A, Trevisi P, Nardi G. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 186] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Tanaka M, Tanaka H, Tsukuma H, Ioka A, Oshima A, Nakahara T. Risk factors for intrahepatic cholangiocarcinoma: a possible role of hepatitis B virus. J Viral Hepat. 2010;17:742-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Fwu CW, Chien YC, You SL, Nelson KE, Kirk GD, Kuo HS, Feinleib M, Chen CJ. Hepatitis B virus infection and risk of intrahepatic cholangiocarcinoma and non-Hodgkin lymphoma: a cohort study of parous women in Taiwan. Hepatology. 2011;53:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RIR, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJG, Alvaro D. Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 972] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 27. | Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 502] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 28. | Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, Colombo M, Craxì A, Donato F, Ferrari C, Gaeta GB, Gerlich WH, Levrero M, Locarnini S, Michalak T, Mondelli MU, Pawlotsky JM, Pollicino T, Prati D, Puoti M, Samuel D, Shouval D, Smedile A, Squadrito G, Trépo C, Villa E, Will H, Zanetti AR, Zoulim F. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49:652-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 606] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 29. | Pollicino T, Squadrito G, Cerenzia G, Cacciola I, Raffa G, Craxi A, Farinati F, Missale G, Smedile A, Tiribelli C, Villa E, Raimondo G. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology. 2004;126:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 324] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 30. | Pollicino T, Saitta C. Occult hepatitis B virus and hepatocellular carcinoma. World J Gastroenterol. 2014;20:5951-5961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Makvandi M. Update on occult hepatitis B virus infection. World J Gastroenterol. 2016;22:8720-8734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 119] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 32. | Li Y, Wang H, Li D, Hu J, Zhou D, Li Q, Jiang X, Zhou H, Hu H. Occult hepatitis B virus infection in Chinese cryptogenic intrahepatic cholangiocarcinoma patient population. J Clin Gastroenterol. 2014;48:878-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Pollicino T, Musolino C, Saitta C, Tripodi G, Lanza M, Raffa G, Tocco FCD, Raggi C, Bragazzi MC, Barbera A, Navarra G, Invernizzi P, Alvaro D, Raimondo G. Free episomal and integrated HBV DNA in HBsAg-negative patients with intrahepatic cholangiocarcinoma. Oncotarget. 2019;10:3931-3938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Wang M, Wang Y, Feng X, Wang R, Zeng H, Qi J, Zhao H, Li N, Cai J, Qu C. Contribution of hepatitis B virus and hepatitis C virus to liver cancer in China north areas: Experience of the Chinese National Cancer Center. Int J Infect Dis. 2017;65:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Barusrux S, Nanok C, Puthisawas W, Pairojkul C, Poovorawan Y. Viral hepatitis B, C infection and genotype distribution among cholangiocarcinoma patients in northeast Thailand. Asian Pac J Cancer Prev. 2012;13 Suppl:83-87. [PubMed] |
| 36. | Tangkijvanich P, Mahachai V, Komolmit P, Fongsarun J, Theamboonlers A, Poovorawan Y. Hepatitis B virus genotypes and hepatocellular carcinoma in Thailand. World J Gastroenterol. 2005;11:2238-2243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Perumal V, Wang J, Thuluvath P, Choti M, Torbenson M. Hepatitis C and hepatitis B nucleic acids are present in intrahepatic cholangiocarcinomas from the United States. Hum Pathol. 2006;37:1211-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Tan JH, Zhou WY, Zhou L, Cao RC, Zhang GW. Viral hepatitis B and C infections increase the risks of intrahepatic and extrahepatic cholangiocarcinoma: Evidence from a systematic review and meta-analysis. Turk J Gastroenterol. 2020;31:246-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Zhang H, Zhu B, Zhang H, Liang J, Zeng W. HBV Infection Status and the Risk of Cholangiocarcinoma in Asia: A Meta-Analysis. Biomed Res Int. 2016;2016:3417976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Li M, Li J, Li P, Li H, Su T, Zhu R, Gong J. Hepatitis B virus infection increases the risk of cholangiocarcinoma: a meta-analysis and systematic review. J Gastroenterol Hepatol. 2012;27:1561-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? J Hepatol. 2012;57:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 42. | Zhou Y, Zhao Y, Li B, Huang J, Wu L, Xu D, Yang J, He J. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer. 2012;12:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Labib PL, Goodchild G, Pereira SP. Molecular Pathogenesis of Cholangiocarcinoma. BMC Cancer. 2019;19:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 44. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1382] [Article Influence: 125.6] [Reference Citation Analysis (1)] |
| 45. | Yu TH, Yuan RH, Chen YL, Yang WC, Hsu HC, Jeng YM. Viral hepatitis is associated with intrahepatic cholangiocarcinoma with cholangiolar differentiation and N-cadherin expression. Mod Pathol. 2011;24:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Tao LY, He XD, Xiu DR. Hepatitis B virus is associated with the clinical features and survival rate of patients with intrahepatic cholangiocarcinoma. Clin Res Hepatol Gastroenterol. 2016;40:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Tang ZH, Tong HJ, Quan ZW. [The research progress in clinicopathological characteristics and treatment of hepatitis B virus-associated intrahepatic cholangiocarcinoma]. Zhonghua Wai Ke Za Zhi. 2020;58:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Wang Z, Sheng YY, Dong QZ, Qin LX. Hepatitis B virus and hepatitis C virus play different prognostic roles in intrahepatic cholangiocarcinoma: A meta-analysis. World J Gastroenterol. 2016;22:3038-3051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Lu JC, Zeng HY, Sun QM, Meng QN, Huang XY, Zhang PF, Yang X, Peng R, Gao C, Wei CY, Shen YH, Cai JB, Dong RZ, Shi YH, Sun HC, Shi YG, Zhou J, Fan J, Ke AW, Yang LX, Shi GM. Distinct PD-L1/PD1 Profiles and Clinical Implications in Intrahepatic Cholangiocarcinoma Patients with Different Risk Factors. Theranostics. 2019;9:4678-4687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 50. | Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, Gores GJ. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132:384-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 51. | Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 52. | Frampton G, Invernizzi P, Bernuzzi F, Pae HY, Quinn M, Horvat D, Galindo C, Huang L, McMillin M, Cooper B, Rimassa L, DeMorrow S. Interleukin-6-driven progranulin expression increases cholangiocarcinoma growth by an Akt-dependent mechanism. Gut. 2012;61:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 53. | Komori J, Marusawa H, Machimoto T, Endo Y, Kinoshita K, Kou T, Haga H, Ikai I, Uemoto S, Chiba T. Activation-induced cytidine deaminase links bile duct inflammation to human cholangiocarcinoma. Hepatology. 2008;47:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 54. | Wang C, Yang W, Yan HX, Luo T, Zhang J, Tang L, Wu FQ, Zhang HL, Yu LX, Zheng LY, Li YQ, Dong W, He YQ, Liu Q, Zou SS, Lin Y, Hu L, Li Z, Wu MC, Wang HY. Hepatitis B virus X (HBx) induces tumorigenicity of hepatic progenitor cells in 3,5-diethoxycarbonyl-1,4-dihydrocollidine-treated HBx transgenic mice. Hepatology. 2012;55:108-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 55. | Wang W, Gu G, Hu M. [Expression and significance of hepatitis B virus genes in human primary intrahepatic cholangiocarcinoma and its surrounding tissue]. Zhonghua Zhong Liu Za Zhi. 1996;18:127-130. [PubMed] |
| 56. | Zhou YM, Cao L, Li B, Zhang XZ, Yin ZF. Expression of HBx protein in hepatitis B virus-infected intrahepatic cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2012;11:532-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Liu W, Chen JR, Hsu CH, Li YH, Chen YM, Lin CY, Huang SJ, Chang ZK, Chen YC, Lin CH, Gong HY, Lin CC, Kawakami K, Wu JL. A zebrafish model of intrahepatic cholangiocarcinoma by dual expression of hepatitis B virus X and hepatitis C virus core protein in liver. Hepatology. 2012;56:2268-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Rizvi S, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1551] [Article Influence: 310.2] [Reference Citation Analysis (0)] |
| 59. | Pu XH, Ye Q, Yang J, Wu HY, Ding XW, Shi J, Mao L, Fan XS, Chen J, Qiu YD, Huang Q. Low-level clonal FGFR2 amplification defines a unique molecular subtype of intrahepatic cholangiocarcinoma in a Chinese population. Hum Pathol. 2018;76:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Kew MC. Hepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26 Suppl 1:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 61. | Qu ZL, Zou SQ, Cui NQ, Wu XZ, Qin MF, Kong D, Zhou ZL. Upregulation of human telomerase reverse transcriptase mRNA expression by in vitro transfection of hepatitis B virus X gene into human hepatocarcinoma and cholangiocarcinoma cells. World J Gastroenterol. 2005;11:5627-5632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Li Y, Roberts ND, Wala JA, Shapira O, Schumacher SE, Kumar K, Khurana E, Waszak S, Korbel JO, Haber JE, Imielinski M; PCAWG Structural Variation Working Group; Weischenfeldt J, Beroukhim R, Campbell PJ; PCAWG Consortium. Patterns of somatic structural variation in human cancer genomes. Nature. 2020;578:112-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 536] [Cited by in RCA: 549] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 63. | Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 533] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 64. | Zhou HB, Hu JY, Hu HP. Hepatitis B virus infection and intrahepatic cholangiocarcinoma. World J Gastroenterol. 2014;20:5721-5729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Joo I, Lee JM, Yoon JH. Imaging Diagnosis of Intrahepatic and Perihilar Cholangiocarcinoma: Recent Advances and Challenges. Radiology. 2018;288:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 66. | Rimola J, Forner A, Reig M, Vilana R, de Lope CR, Ayuso C, Bruix J. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology. 2009;50:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 67. | Valls C, Gumà A, Puig I, Sanchez A, Andía E, Serrano T, Figueras J. Intrahepatic peripheral cholangiocarcinoma: CT evaluation. Abdom Imaging. 2000;25:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 68. | Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 1077] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 69. | Lamarca A, Barriuso J, Chander A, McNamara MG, Hubner RA, ÓReilly D, Manoharan P, Valle JW. 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) for patients with biliary tract cancer: Systematic review and meta-analysis. J Hepatol. 2019;71:115-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 70. | Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 285] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 71. | He C, Zhang Y, Song Y, Wang J, Xing K, Lin X, Li S. Preoperative CEA levels are supplementary to CA19-9 Levels in predicting prognosis in patients with resectable intrahepatic cholangiocarcinoma. J Cancer. 2018;9:3117-3128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 72. | Sasaki K, Margonis GA, Andreatos N, Chen Q, Barbon C, Bagante F, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Groot Koerkamp B, Guglielmi A, Endo I, Aucejo FN, Pawlik TM. Serum tumor markers enhance the predictive power of the AJCC and LCSGJ staging systems in resectable intrahepatic cholangiocarcinoma. HPB (Oxford). 2018;20:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:364-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 242] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 74. | Zhou YM, Yang JM, Li B, Yin ZF, Xu F, Wang B, Liu P, Li ZM. Clinicopathologic characteristics of intrahepatic cholangiocarcinoma in patients with positive serum a-fetoprotein. World J Gastroenterol. 2008;14:2251-2254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Zhou H, Wang H, Zhou D, Wang Q, Zou S, Tu Q, Wu M, Hu H. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. Eur J Cancer. 2010;46:1056-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 76. | Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D; ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28-v37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 484] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 77. | Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 202] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 78. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2442] [Article Influence: 488.4] [Reference Citation Analysis (3)] |
| 79. | Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, Laleman W, Pirenne J, Aerts R, Yano H, Nevens F, Topal B, Roskams T. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 251] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 80. | Liau JY, Tsai JH, Yuan RH, Chang CN, Lee HJ, Jeng YM. Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Mod Pathol. 2014;27:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 81. | Hayashi A, Misumi K, Shibahara J, Arita J, Sakamoto Y, Hasegawa K, Kokudo N, Fukayama M. Distinct Clinicopathologic and Genetic Features of 2 Histologic Subtypes of Intrahepatic Cholangiocarcinoma. Am J Surg Pathol. 2016;40:1021-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 82. | Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, Schenkein DP, Hezel AF, Ancukiewicz M, Liebman HM, Kwak EL, Clark JW, Ryan DP, Deshpande V, Dias-Santagata D, Ellisen LW, Zhu AX, Iafrate AJ. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 599] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 83. | Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, Vibert E, Cherqui D, Grant DR, Hernandez-Alejandro R, Dale CH, Cucchetti A, Pinna A, Hwang S, Lee SG, Agopian VG, Busuttil RW, Rizvi S, Heimbach JK, Montenovo M, Reyes J, Cesaretti M, Soubrane O, Reichman T, Seal J, Kim PT, Klintmalm G, Sposito C, Mazzaferro V, Dutkowski P, Clavien PA, Toso C, Majno P, Kneteman N, Saunders C, Bruix J; iCCA International Consortium. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology. 2016;64:1178-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 84. | Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, Gouw A, Kagen A, Klimstra D, Komuta M, Kondo F, Miksad R, Nakano M, Nakanuma Y, Ng I, Paradis V, Nyun Park Y, Quaglia A, Roncalli M, Roskams T, Sakamoto M, Saxena R, Sempoux C, Sirlin C, Stueck A, Thung S, Tsui WMS, Wang XW, Wee A, Yano H, Yeh M, Zen Y, Zucman-Rossi J, Theise N. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68:113-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 262] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 85. | ALLEN RA, LISA JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647-655. [PubMed] |
| 86. | Theise ND, Nakashima O, Park YN NY. Combined hepatocellular-cholangiocarcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND E. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: International Agency for Research on Cancer, 2010: 225-227. |
| 87. | Kim TH, Kim H, Joo I, Lee JM. Combined Hepatocellular-Cholangiocarcinoma: Changes in the 2019 World Health Organization Histological Classification System and Potential Impact on Imaging-Based Diagnosis. Korean J Radiol. 2020;21:1115-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 88. | Sempoux C, Kakar S, Kondo F SP. Combined hepatocellular-cholangiocarcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND E. WHO classification of tumours: digestive system tumours. 5th ed. Lyon: IARC. 2019: 260–262. |
| 89. | Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, Giardini V. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014;20:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 90. | Wang J, Wang F, Kessinger A. Outcome of Combined Hepatocellular and Cholangiocarcinoma of the Liver. J Oncol. 2010;2010:1-7. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Yin X, Zhang BH, Qiu SJ, Ren ZG, Zhou J, Chen XH, Zhou Y, Fan J. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol. 2012;19:2869-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 92. | Wang Y, Yang Q, Li S, Luo R, Mao S, Shen J. Imaging features of combined hepatocellular and cholangiocarcinoma compared with those of hepatocellular carcinoma and intrahepatic cholangiocellular carcinoma in a Chinese population. Clin Radiol 2019; 74: 407.e1-407. e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Chi CT, Chau GY, Lee RC, Chen YY, Lei HJ, Hou MC, Chao Y, Huang YH. Radiological features and outcomes of combined hepatocellular-cholangiocarcinoma in patients undergoing surgical resection. J Formos Med Assoc. 2020;119:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 94. | Kim JH, Yoon HK, Ko GY, Gwon DI, Jang CS, Song HY, Shin JH, Sung KB. Nonresectable combined hepatocellular carcinoma and cholangiocarcinoma: analysis of the response and prognostic factors after transcatheter arterial chemoembolization. Radiology. 2010;255:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 95. | Bergquist JR, Groeschl RT, Ivanics T, Shubert CR, Habermann EB, Kendrick ML, Farnell MB, Nagorney DM, Truty MJ, Smoot RL. Mixed hepatocellular and cholangiocarcinoma: a rare tumor with a mix of parent phenotypic characteristics. HPB (Oxford). 2016;18:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 96. | Gentile D, Donadon M, Lleo A, Aghemo A, Roncalli M, di Tommaso L, Torzilli G. Surgical Treatment of Hepatocholangiocarcinoma: A Systematic Review. Liver Cancer. 2020;9:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 97. | Cai X, Xiong J, Hu QG, Zhao QD, Wu D, Tang LG, Wan CD, Wei LX. Look into hepatic progenitor cell associated trait: Histological heterogeneity of hepatitis B-related combined hepatocellular-cholangiocarcinoma. J Huazhong Univ Sci Technolog Med Sci. 2017;37:873-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 98. | Chu KJ, Lu CD, Dong H, Fu XH, Zhang HW, Yao XP. Hepatitis B virus-related combined hepatocellular-cholangiocarcinoma: clinicopathological and prognostic analysis of 390 cases. Eur J Gastroenterol Hepatol. 2014;26:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 99. | Ye J, Xie X, Liu B, Zhang X, Wang W, Huang X, Lu M, Huang G. Imaging Features on Contrast-Enhanced Ultrasound and Clinical Characteristics of Hepatitis B Virus-Related Combined Hepatocellular-Cholangiocarcinoma: Comparison with Hepatitis B Virus-Related Hepatocellular Carcinoma. Ultrasound Med Biol. 2017;43:2530-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Li R, Yang D, Tang CL, Cai P, Ma KS, Ding SY, Zhang XH, Guo DY, Yan XC. Combined hepatocellular carcinoma and cholangiocarcinoma (biphenotypic) tumors: clinical characteristics, imaging features of contrast-enhanced ultrasound and computed tomography. BMC Cancer. 2016;16:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 101. | Gigante E, Paradis V, Ronot M, Cauchy F, Soubrane O, Ganne-Carrié N, Nault JC. New insights into the pathophysiology and clinical care of rare primary liver cancers. JHEP Rep. 2021;3:100174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 102. | Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract. 2008;62:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 103. | Vilchez V, Shah MB, Daily MF, Pena L, Tzeng CW, Davenport D, Hosein PJ, Gedaly R, Maynard E. Long-term outcome of patients undergoing liver transplantation for mixed hepatocellular carcinoma and cholangiocarcinoma: an analysis of the UNOS database. HPB (Oxford). 2016;18:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 104. | Kobayashi S, Terashima T, Shiba S, Yoshida Y, Yamada I, Iwadou S, Horiguchi S, Takahashi H, Suzuki E, Moriguchi M, Tsuji K, Otsuka T, Asagi A, Kojima Y, Takada R, Morizane C, Mizuno N, Ikeda M, Ueno M, Furuse J. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci. 2018;109:2549-2557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 105. | Salimon M, Prieux-Klotz C, Tougeron D, Hautefeuille V, Caulet M, Gournay J, Matysiak-Budnik T, Bennouna J, Tiako Meyo M, Lecomte T, Zaanan A, Touchefeu Y. Gemcitabine plus platinum-based chemotherapy for first-line treatment of hepatocholangiocarcinoma: an AGEO French multicentre retrospective study. Br J Cancer. 2018;118:325-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 106. | de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, Ferrone CR, Zhu AX, Bauer TW, Walters DM, Gamblin TC, Nguyen KT, Turley R, Popescu I, Hubert C, Meyer S, Schulick RD, Choti MA, Gigot JF, Mentha G, Pawlik TM. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 559] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 107. | Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014;149:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 596] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 108. | Reames BN, Ejaz A, Koerkamp BG, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Martel G, Marsh JW, Pawlik TM. Impact of major vascular resection on outcomes and survival in patients with intrahepatic cholangiocarcinoma: A multi-institutional analysis. J Surg Oncol. 2017;116:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 109. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1219] [Article Influence: 203.2] [Reference Citation Analysis (1)] |
| 110. | Wu ZF, Yang N, Li DY, Zhang HB, Yang GS. Characteristics of intrahepatic cholangiocarcinoma in patients with hepatitis B virus infection: clinicopathologic study of resected tumours. J Viral Hepat. 2013;20:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 111. | Zhou HB, Wang H, Li YQ, Li SX, Zhou DX, Tu QQ, Wang Q, Zou SS, Wu MC, Hu HP. Hepatitis B virus infection: a favorable prognostic factor for intrahepatic cholangiocarcinoma after resection. World J Gastroenterol. 2011;17:1292-1303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 112. | Jeong S, Cheng Q, Huang L, Wang J, Sha M, Tong Y, Xia L, Han L, Xi Z, Zhang J, Kong X, Gu J, Xia Q. Risk stratification system to predict recurrence of intrahepatic cholangiocarcinoma after hepatic resection. BMC Cancer. 2017;17:464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 113. | Wang Q, Li J, Lei Z, Wu D, Si A, Wang K, Wang Y, Wan X, Lau WY, Shen F. Prognosis of Intrahepatic Cholangiocarcinomas with HBV Infection is Better than Those with Hepatolithiasis After R0 Liver Resection: A Propensity Score Matching Analysis. Ann Surg Oncol. 2017;24:1579-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 114. | Ahn CS, Hwang S, Lee YJ, Kim KH, Moon DB, Ha TY, Song GW, Lee SG. Prognostic impact of hepatitis B virus infection in patients with intrahepatic cholangiocarcinoma. ANZ J Surg. 2018;88:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 115. | Jeong S, Luo G, Wang ZH, Sha M, Chen L, Xia Q. Impact of viral hepatitis B status on outcomes of intrahepatic cholangiocarcinoma: a meta-analysis. Hepatol Int. 2018;12:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 116. | Luo X, Yuan L, Wang Y, Ge R, Sun Y, Wei G. Survival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: a large single-center cohort study. J Gastrointest Surg. 2014;18:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 117. | Huang J, Wang X, Zhu Y, Wang Z, Li J, Xu D, Wang S, Li TE, Lu L. Specific prognostic factors in hepatitis B virus-related and non-hepatitis B virus-related intrahepatic cholangiocarcinoma after macroscopic curative resection. J Surg Oncol. 2019;119:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 118. | Jeong S, Zheng B, Wang J, Chi J, Tong Y, Xia L, Xu N, Zhang J, Kong X, Gu J, Xia Q. Transarterial Chemoembolization: A Favorable Postoperative Management to Improve Prognosis of Hepatitis B Virus-associated Intrahepatic Cholangiocarcinoma after Surgical Resection. Int J Biol Sci. 2017;13:1234-1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 119. | Ma KW, Cheung TT, Leung B, She BWH, Chok KSH, Chan ACY, Dai WC, Lo CM. Adjuvant chemotherapy improves oncological outcomes of resectable intrahepatic cholangiocarcinoma: A meta-analysis. Medicine (Baltimore). 2019;98:e14013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 120. | Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, Boudjema K, Fartoux L, Bouhier-Leporrier K, Jouve JL, Faroux R, Guerin-Meyer V, Kurtz JE, Assénat E, Seitz JF, Baumgaertner I, Tougeron D, de la Fouchardière C, Lombard-Bohas C, Boucher E, Stanbury T, Louvet C, Malka D, Phelip JM. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol. 2019;37:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 367] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 121. | Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J; BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 823] [Article Influence: 137.2] [Reference Citation Analysis (0)] |
| 122. | Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, El-Khoueiry A, Feng M, Katz MHG, Primrose J, Soares HP, Valle J, Maithel SK. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol. 2019;37:1015-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 306] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 123. | Lei Z, Xia Y, Si A, Wang K, Li J, Yan Z, Yang T, Wu D, Wan X, Zhou W, Liu J, Wang H, Cong W, Wu M, Pawlik TM, Lau WY, Shen F. Antiviral therapy improves survival in patients with HBV infection and intrahepatic cholangiocarcinoma undergoing liver resection. J Hepatol. 2018;68:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 124. | Lee TY, Hsu YC, Yu SH, Lin JT, Wu MS, Wu CY. Effect of Nucleos(t)ide Analogue Therapy on Risk of Intrahepatic Cholangiocarcinoma in Patients With Chronic Hepatitis B. Clin Gastroenterol Hepatol 2018; 16: 947-954. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 125. | Sapisochin G, de Lope CR, Gastaca M, de Urbina JO, López-Andujar R, Palacios F, Ramos E, Fabregat J, Castroagudín JF, Varo E, Pons JA, Parrilla P, González-Diéguez ML, Rodriguez M, Otero A, Vazquez MA, Zozaya G, Herrero JI, Antolin GS, Perez B, Ciria R, Rufian S, Fundora Y, Ferron JA, Guiberteau A, Blanco G, Varona MA, Barrera MA, Suarez MA, Santoyo J, Bruix J, Charco R. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann Surg. 2014;259:944-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 126. | Facciuto ME, Singh MK, Lubezky N, Selim MA, Robinson D, Kim-Schluger L, Florman S, Ward SC, Thung SN, Fiel M, Schiano TD. Tumors with intrahepatic bile duct differentiation in cirrhosis: implications on outcomes after liver transplantation. Transplantation. 2015;99:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 127. | Sotiropoulos GC, Kaiser GM, Lang H, Molmenti EP, Beckebaum S, Fouzas I, Sgourakis G, Radtke A, Bockhorn M, Nadalin S, Treckmann J, Niebel W, Baba HA, Broelsch CE, Paul A. Liver transplantation as a primary indication for intrahepatic cholangiocarcinoma: a single-center experience. Transplant Proc. 2008;40:3194-3195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 128. | Friman S, Foss A, Isoniemi H, Olausson M, Höckerstedt K, Yamamoto S, Karlsen TH, Rizell M, Ericzon BG. Liver transplantation for cholangiocarcinoma: selection is essential for acceptable results. Scand J Gastroenterol. 2011;46:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 129. | Sapisochin G, Rodríguez de Lope C, Gastaca M, Ortiz de Urbina J, Suarez MA, Santoyo J, Castroagudín JF, Varo E, López-Andujar R, Palacios F, Sanchez Antolín G, Perez B, Guiberteau A, Blanco G, González-Diéguez ML, Rodriguez M, Varona MA, Barrera MA, Fundora Y, Ferron JA, Ramos E, Fabregat J, Ciria R, Rufian S, Otero A, Vazquez MA, Pons JA, Parrilla P, Zozaya G, Herrero JI, Charco R, Bruix J. "Very early" intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant. 2014;14:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 130. | Ziogas IA, Giannis D, Economopoulos KP, Hayat MH, Montenovo MI, Matsuoka LK, Alexopoulos SP. Liver Transplantation for Intrahepatic Cholangiocarcinoma: A Meta-analysis and Meta-regression of Survival Rates. Transplantation. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 131. | Rana A, Hong JC. Orthotopic liver transplantation in combination with neoadjuvant therapy: a new paradigm in the treatment of unresectable intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2012;28:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 132. | Lunsford KE, Javle M, Heyne K, Shroff RT, Abdel-Wahab R, Gupta N, Mobley CM, Saharia A, Victor DW, Nguyen DT, Graviss EA, Kaseb AO, McFadden RS, Aloia TA, Conrad C, Li XC, Monsour HP, Gaber AO, Vauthey JN, Ghobrial RM; Methodist–MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC). Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 133. | Sapisochin G, Javle M, Lerut J, Ohtsuka M, Ghobrial M, Hibi T, Kwan NM, Heimbach J. Liver Transplantation for Cholangiocarcinoma and Mixed Hepatocellular Cholangiocarcinoma: Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1125-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 134. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine vs gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3169] [Article Influence: 211.3] [Reference Citation Analysis (1)] |
| 135. | Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, Raghav KPS, Iwasaki M, Masci P, Ramanathan RK, Ahn DH, Bekaii-Saab TS, Borad MJ. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 345] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 136. | Xu Z, Li H, Dong Y, Cheng P, Luo F, Fu S, Gao M, Kong L, Che N. Incidence and PD-L1 Expression of MET 14 Skipping in Chinese Population: A Non-Selective NSCLC Cohort Study Using RNA-Based Sequencing. Onco Targets Ther. 2020;13:6245-6253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 137. | Ahn KS, O'Brien D, Kang YN, Mounajjed T, Kim YH, Kim TS, Kocher JA, Allotey LK, Borad MJ, Roberts LR, Kang KJ. Prognostic subclass of intrahepatic cholangiocarcinoma by integrative molecular-clinical analysis and potential targeted approach. Hepatol Int. 2019;13:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 138. | Hyder O, Marsh JW, Salem R, Petre EN, Kalva S, Liapi E, Cosgrove D, Neal D, Kamel I, Zhu AX, Sofocleous CT, Geschwind JF, Pawlik TM. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol. 2013;20:3779-3786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 139. | Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization vs supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 140. | Li J, Wang Q, Lei Z, Wu D, Si A, Wang K, Wan X, Wang Y, Yan Z, Xia Y, Lau WY, Wu M, Shen F. Adjuvant Transarterial Chemoembolization Following Liver Resection for Intrahepatic Cholangiocarcinoma Based on Survival Risk Stratification. Oncologist. 2015;20:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 141. | Ke Q, Lin N, Deng M, Wang L, Zeng Y, Liu J. The effect of adjuvant therapy for patients with intrahepatic cholangiocarcinoma after surgical resection: A systematic review and meta-analysis. PLoS One. 2020;15:e0229292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 142. | Stang A, Fischbach R, Teichmann W, Bokemeyer C, Braumann D. A systematic review on the clinical benefit and role of radiofrequency ablation as treatment of colorectal liver metastases. Eur J Cancer. 2009;45:1748-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 143. | Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 144. | Carrafiello G, Laganà D, Cotta E, Mangini M, Fontana F, Bandiera F, Fugazzola C. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 145. | Kim JH, Won HJ, Shin YM, Kim KA, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011;196:W205-W209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 146. | Haidu M, Dobrozemsky G, Schullian P, Widmann G, Klaus A, Weiss H, Margreiter R, Bale R. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: a retrospective study. Cardiovasc Intervent Radiol. 2012;35:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 147. | Widmann G, Schullian P, Haidu M, Bale R. Stereotactic radiofrequency ablation (SRFA) of liver lesions: technique effectiveness, safety, and interoperator performance. Cardiovasc Intervent Radiol. 2012;35:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 148. | Zhang A, Liu B, Xu D, Sun Y. Advanced intrahepatic cholangiocarcinoma treated using anlotinib and microwave ablation: A case report. Medicine (Baltimore). 2019;98:e18435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 149. | Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau SS. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol. 2015;41:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 150. | Edeline J, Touchefeu Y, Guiu B, Farge O, Tougeron D, Baumgaertner I, Ayav A, Campillo-Gimenez B, Beuzit L, Pracht M, Lièvre A, Le Sourd S, Boudjema K, Rolland Y, Boucher E, Garin E. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020;6:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 151. | Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ, Bridgewater J, Harris WP, Murphy AG, Oh DY, Whisenant J, Lowery MA, Goyal L, Shroff RT, El-Khoueiry AB, Fan B, Wu B, Chamberlain CX, Jiang L, Gliser C, Pandya SS, Valle JW, Zhu AX. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 717] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 152. | Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson D, Murphy AG, Oh DY, Dotan E, Catenacci DV, Van Cutsem E, Ji T, Lihou CF, Zhen H, Féliz L, Vogel A. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 1074] [Article Influence: 214.8] [Reference Citation Analysis (0)] |
| 153. | Mazzaferro V, Shaib W, Rimassa L, Harris W, Personeni N, El-Rayes B, Tolcher A, Hall T, Wang Y, Schwartz B, Kazakin J, Droz Dit Busset M, Cotsoglou C, Papadopoulos K. PD-019 ARQ 087, an oral pan- fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced and/or metastatic intrahepatic cholangiocarcinoma (iCCA). Ann Oncol. 2016;27:ii109. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 154. | Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, Ramanathan RK, Goyal L, Sadeghi S, Macarulla T, El-Khoueiry A, Kelley RK, Borbath I, Choo SP, Oh DY, Philip PA, Chen LT, Reungwetwattana T, Van Cutsem E, Yeh KH, Ciombor K, Finn RS, Patel A, Sen S, Porter D, Isaacs R, Zhu AX, Abou-Alfa GK, Bekaii-Saab T. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol. 2018;36:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 518] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 155. | Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, Kelley RK, Ros W, Italiano A, Nakagawa K, Rugo HS, de Braud F, Varga AI, Hansen A, Wang H, Krishnan S, Norwood KG, Doi T. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147:2190-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 352] [Article Influence: 70.4] [Reference Citation Analysis (0)] |