Published online Jul 7, 2021. doi: 10.3748/wjg.v27.i25.3888
Peer-review started: January 29, 2021
First decision: February 25, 2021
Revised: March 11, 2021
Accepted: June 4, 2021
Article in press: June 4, 2021
Published online: July 7, 2021
Processing time: 157 Days and 23.9 Hours
Abnormal expression patterns of mucin 2 (MUC2) have been reported in a variety of malignant tumors and precancerous lesions. Reduced MUC2 expression in the intestinal mucosa, caused by various pathogenic factors, is related to mechanical dysfunction of the intestinal mucosa barrier and increased intestinal mucosal permeability. However, the relationship between MUC2 and the intestinal mucosal barrier in patients with colorectal cancer (CRC) is not clear.
To explore the relationship between MUC2 and intestinal mucosal barrier by characterizing the multiple expression patterns of MUC2 in CRC.
Immunohistochemical staining was performed on intestinal tissue specimens from 100 CRC patients, including both cancer tissues and adjacent normal tissues. Enzyme-linked immunosorbent assays were performed on preoperative sera from 66 CRC patients and 20 normal sera to detect the serum levels of MUC2, diamine oxide (DAO), and D-lactate (D-LAC). The relationship between MUC2 expression and clinical parameters was calculated by the χ2 test or Fisher’s exact test. Prognostic value of MUC2 was evaluated by Kaplan-Meier curve and log-rank tests.
Immunohistochemical staining of 100 CRC tissues showed that the expression of MUC2 in cancer tissues was lower than that in normal tissues (54% vs 79%, P < 0.05), and it was correlated with tumor-node-metastasis (TNM) stage and lymph node metastasis in CRC patients (P < 0.05). However, the serum level of MUC2 in CRC patients was higher than that in normal controls, and was positively associated with serum levels of human DAO (χ2 = 3.957, P < 0.05) and D-LAC (χ2 = 7.236, P < 0.05), which are the biomarkers of the functional status of the intestinal mucosal barrier. And the serum level of MUC2 was correlated with TNM stage, tumor type, and distant metastasis in CRC patients (P < 0.05). Kaplan-Meier curves showed that decreased MUC2 expression in CRC tissues predicted a poor survival.
MUC2 in tissues may play a protective role by participating in the intestinal mucosal barrier and can be used as an indicator to evaluate the prognosis of CRC patients.
Core Tip: This study found that mucin 2 (MUC2) in intestinal tissues may play a protective role on the intestine and can be used as one of the indicators to evaluate the prognosis of patients with colorectal cancer (CRC). When the intestinal mucosal barrier function of patients with CRC is impaired, the serum level of MUC2 can reflect the severity of the damage. Therefore, in CRC patients with impaired intestinal mucosal barrier function, the serum level of MUC2 could reflect the severity of the damage, providing a potential mechanism for the development of therapeutic strategies for CRC patients.
- Citation: Gan GL, Wu HT, Chen WJ, Li CL, Ye QQ, Zheng YF, Liu J. Diverse expression patterns of mucin 2 in colorectal cancer indicates its mechanism related to the intestinal mucosal barrier. World J Gastroenterol 2021; 27(25): 3888-3900
- URL: https://www.wjgnet.com/1007-9327/full/v27/i25/3888.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i25.3888
The incidence of colorectal cancer (CRC) ranks third in the world among the common malignant tumors, and the mortality ranks second[1]. Due to large changes in lifestyle and dietary habits, the incidence and mortality of CRC will continue to rise. In China, there are 300000 new CRC cases each year, with an annual average increase of 4.2%[2]. There are obvious gender and regional differences in the incidence and mortality rate of CRC cases, with the overall distribution being more in males than in females, and more in urban areas than in rural areas[3]. Therefore, CRC is one of the major diseases that seriously threatens human life and health, and the situation is quite serious.
The etiological mechanism of CRC tumorigenesis and development is extremely complex, involving a variety of genetic and environmental factors[4]. Among them, the impairment of intestinal barrier structure and function leads to a series of pathophysiological changes in the intestinal mucosa, which eventually evolves into tumor malignancy. Mucins (MUCs) are the main components of the mucus layer, which provides the first-line defense against infection and participates in the process of intercellular adhesion, intercellular communication, and immune regulation[5].
Among 27 reported MUC proteins, MUC2 is a secretory mucin involved in the formation of mucus, and is mainly secreted by goblet cells[6]. Reduced MUC2 expre
To verify the diverse expression patterns of MUC2 in CRC patients, the current study enrolled CRC patients and investigated MUC2 levels in both malignant tissue and serum to provide an underlying mechanism of MUC2 related to intestinal barrier function, and lay a foundation for further revealing the molecular mechanism of MUC2 involvement in the malignant biological behavior of CRC.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College. All patients or their guardians signed written informed consent before the study. All experiments were conducted following the guidelines of the Ethics Review Committee.
Cancer tissues and adjacent normal intestinal mucosal tissues (> 5 cm away from the tumor) were collected from 100 CRC patients who underwent radical resection at the Department of Gastrointestinal Surgery of the First Affiliated Hospital of Shantou University Medical College from January 2015 to December 2016. The inclusion criteria included: (1) Age from 18 to 90; (2) No medical history of blood, cardiovascular, or immune disease, or inflammatory bowel disease; (3) Pathological diagnosis of CRC; (4) Tumor-node-metastasis (TNM) stages I to III; and (5) Patients who underwent radical surgery. The exclusion criteria included: (1) Preoperative history of neoadjuvant chemotherapy or radiotherapy; (2) Binary or multivariate cancer; (3) Preoperative complications, such as malignant intestinal obstruction, perforation, or bleeding; (4) No standardized chemotherapy after the radical surgery; and (5) Incomplete clinico
Peripheral blood was collected before surgery from 66 CRC patients who were diagnosed with CRC at the Department of Gastrointestinal Surgery of the First Affiliated Hospital of Medical College of Shantou University from January 2018 to December 2019. The inclusion criteria were almost the same as above, except that patients at stage IV were also recruited. The exclusion criteria were the same. For comparison, 20 normal subjects in the same period were recruited and their sera were collected in the Health Management Center of the First Affiliated Hospital of Shantou University Medical College.
Formalin-fixed and paraffin-embedded CRC tissues were cut into 4-μm sections and stained with hematoxylin and eosin. Histopathological differentiation was made by two pathologists based on the World Health Organization criteria. TNM pathological staging was determined according to the staging manual of the American Joint Committee on Cancer[11].
Immunohistochemistry (IHC) was conducted as described before[12]. CRC and adjacent normal tissues were dewaxed in xylene, hydrated in a series of graded alcohols, and placed in a citric acid buffer for epitope retrieval. After immersion in 3% H2O2 solution to block endogenous peroxidase, the slides were incubated with anti-MUC2 monoclonal antibody (ab118964, Abcam, United Kingdom) at 4 ℃ overnight. Negative controls were treated with PBS instead of primary antibody. Haematoxylin was used for counterstaining.
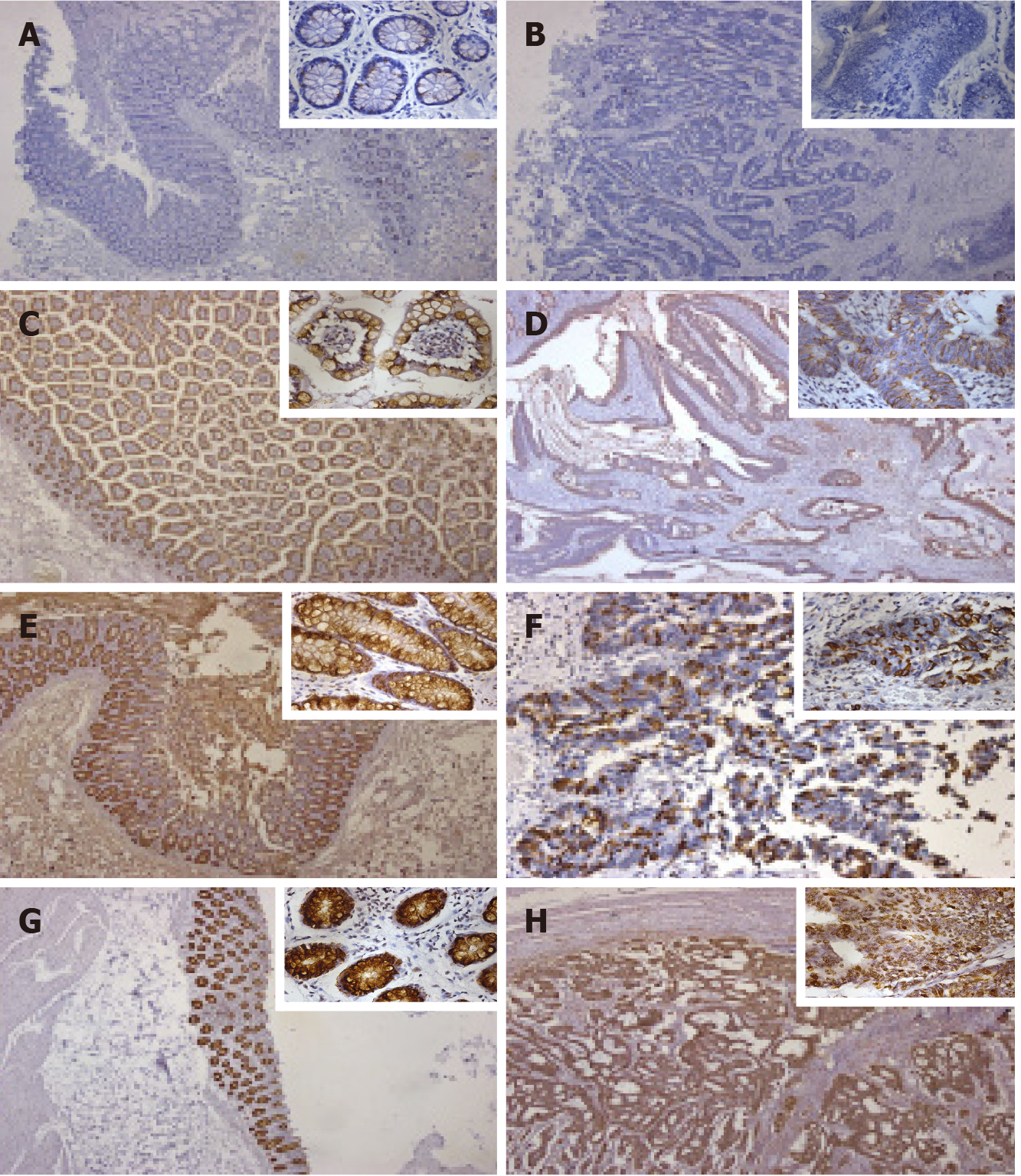
Sections were visualized under a bright-field microscope (CKX41, Olympus, Japan) and evaluated independently by two investigators with no prior knowledge of the patient information. For tissue expression of MUC2, staining intensity was recorded as 0, 1, 2 and 3 for colorless, light yellow, brown yellow, and dark brown, respectively, and the percentage of positive cells < 5%, 6%-30%, 31%-60%, and 61%-100% was scored as 0, 1, 2, and 3 points, respectively. To evaluate the expression level of MUC2, the scores of staining intensity and the percentage of positive cells were added and defined as low expression (0-3) and high expression (4-6).
The collected peripheral blood samples were centrifuged at 2000-3000 rpm for 15 min, and the supernatant serum was collected carefully and stored in -80 ℃. Human MUC2, human diamine oxide (DAO), and human D-lactate (D-LAC) enzyme-linked immunosorbent assay detection kits were purchased from Nanjing Senberga Biotechnology Co., Ltd., China. Experiments were carried out as previously described[13]. The standard curve was determined by the standard concentration and their corresponding absorbance (OD value). The actual concentrations of MUC2, DAO, and D-LAC were calculated based on the standard curves.
Overall survival (OS) time was calculated in months from the date of diagnosis to the date of death of the patient or the last follow-up visit. Disease-free survival (DFS) time was determined by the date of relapse.
SPSS 21.0 statistical software was used to analyze the data. Enumerated data are expressed by the number of cases (N), and the measurement data that conformed to a normal distribution are expressed as the mean ± SD. The relationship between MUC2 and the patient’s clinicopathological data was tested by χ2 and Fisher’s exact probability tests. The relationship between serum MUC2, DAO, and D-LAC was analyzed by the χ2 test. The Kaplan-Meier survival curve and log-rank test were used to evaluate the association of MUC2 expression with prognosis. The difference between two groups was statistically significant at P < 0.05.
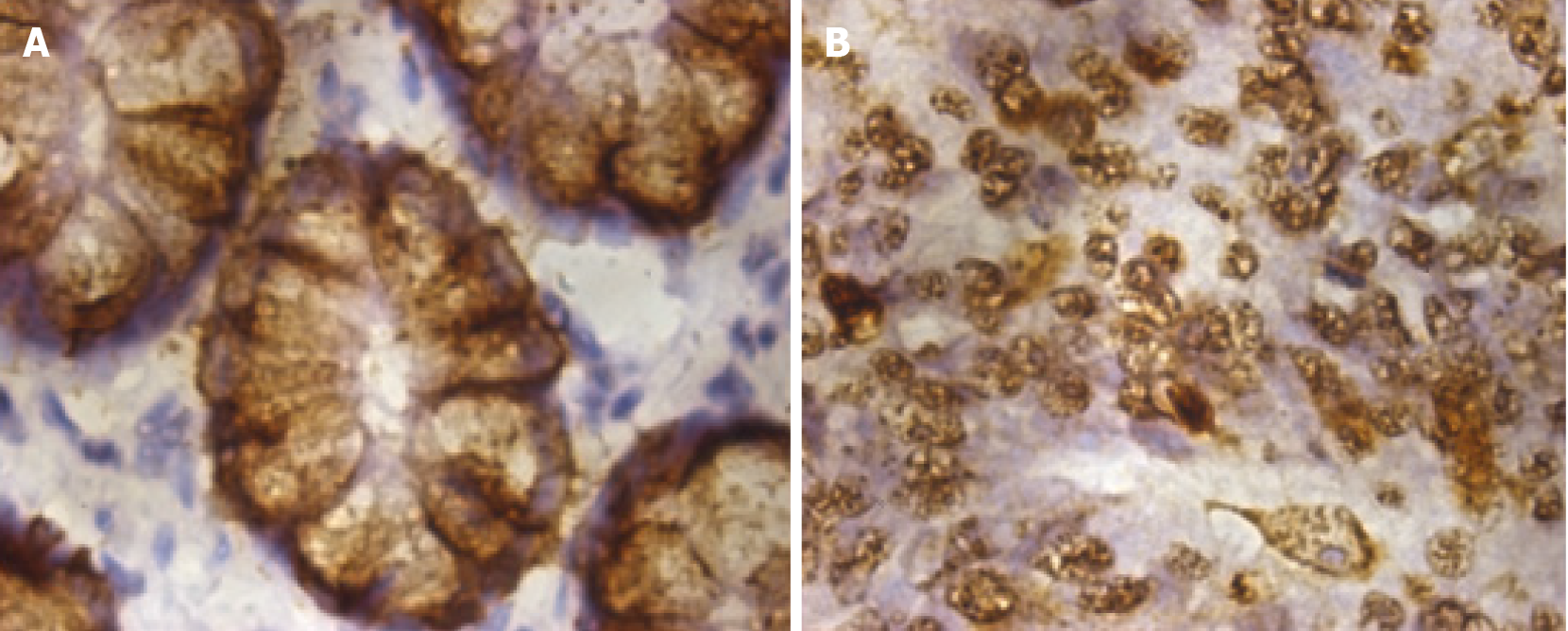
To detect the location of MUC2 in intestinal tissues, IHC was performed and revealed that MUC2-positive staining was mainly located in the cytoplasm, both in normal tissues and CRC tissues (Figure 1). In normal tissues, the cytoplasm was diffusely and homogeneously positive, and the nucleus was not stained, while in cancer tissues, cells lost their normal morphology, adenoid structures were destroyed or had even disappeared, and heterotypic cancer cells could be detected.
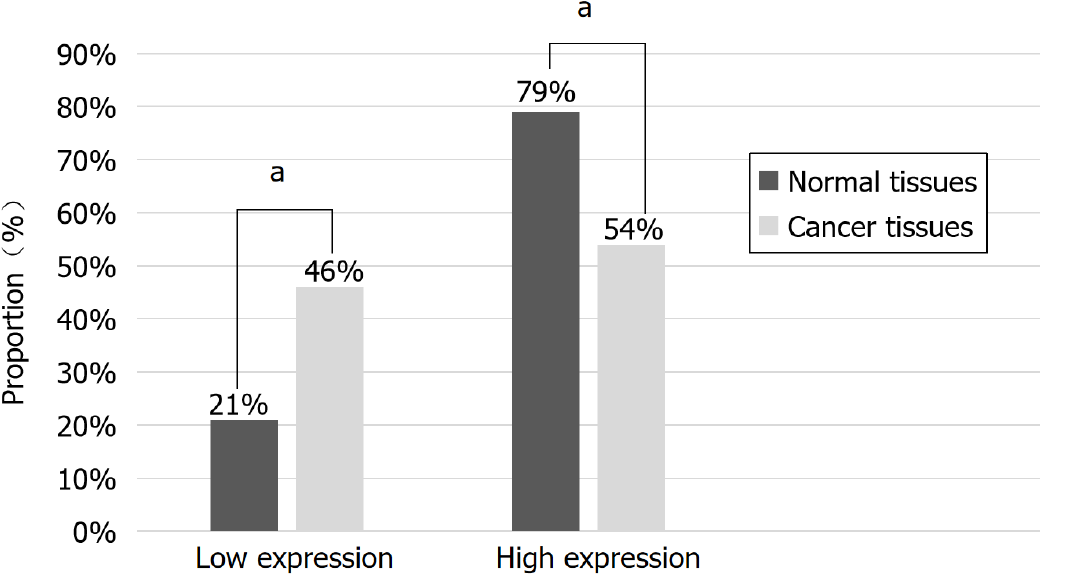
Representative images of MUC2 staining in adjacent and cancer tissues are shown in Figure 2. Interestingly, the percentage of tissues with high MUC2 expression was significantly decreased from 79% in adjacent normal tissues to 54% in CRC tissues (Figure 3). The difference in MUC2 expression between normal and cancer tissues was statistically significant (P < 0.01; Table 1)
Clinicopathological analysis showed that tissue MUC2 expression (low vs high) was not significantly associated with the age at diagnosis, gender, tumor location, tumor size, depth of invasion, degree of differentiation, or tumor type (Table 2). In compari
| Clinicopathological parameter | n (%) | MUC2 expression | χ2 | P value | |
| Low, n (%) | High, n (%) | ||||
| Age, yr | |||||
| ≤ 60 | 48 | 22 (45.8) | 26 (54.2) | 0.001 | 0.974 |
| > 60 | 52 | 24 (46.2) | 28 (53.8) | ||
| Gender | |||||
| Male | 52 | 23 (44.2) | 29 (55.8) | 0.137 | 0.712 |
| Female | 48 | 23 (47.9) | 25 (52.1) | ||
| Tumor location | |||||
| Rectum and anus | 48 | 21 (43.8) | 27 (56.3) | 0.188 | 0.664 |
| Colon | 52 | 25 (48.1) | 27 (51.9) | ||
| TNM stage | |||||
| I-II | 57 | 17 (29.8) | 40 (70.2) | 13.963 | < 0.01 |
| III | 43 | 29 (67.4) | 14 (32.6) | ||
| Maximum tumor diameter | |||||
| < 5 cm | 49 | 23 (46.9) | 26 (53.1) | 0.034 | 0.854 |
| ≥ 5 cm | 51 | 23 (45.1) | 28 (54.9) | ||
| Depth of invasion | |||||
| Non-immersed serosa | 22 | 8 (36.4) | 14 (63.6) | 1.054 | 0.304 |
| Immersed serosa | 78 | 38 (48.7) | 40 (51.3) | ||
| Degree of differentiation | |||||
| High-moderate | 93 | 42 (45.2) | 51 (54.8) | F1 | 0.700 |
| Low | 7 | 4 (57.1) | 3 (42.9) | ||
| Tumor type | |||||
| Mucinous adenocarcinoma | 13 | 5 (38.5) | 8 (61.5) | 0.342 | 0.559 |
| Non-mucinous adenocarcinoma | 87 | 41 (47.1) | 46 (52.9) | ||
| Lymph node metastasis | |||||
| No | 56 | 17 (30.4) | 39 (69.6) | 12.538 | < 0.01 |
| Yes | 44 | 29 (65.9) | 15 (34.1) | ||
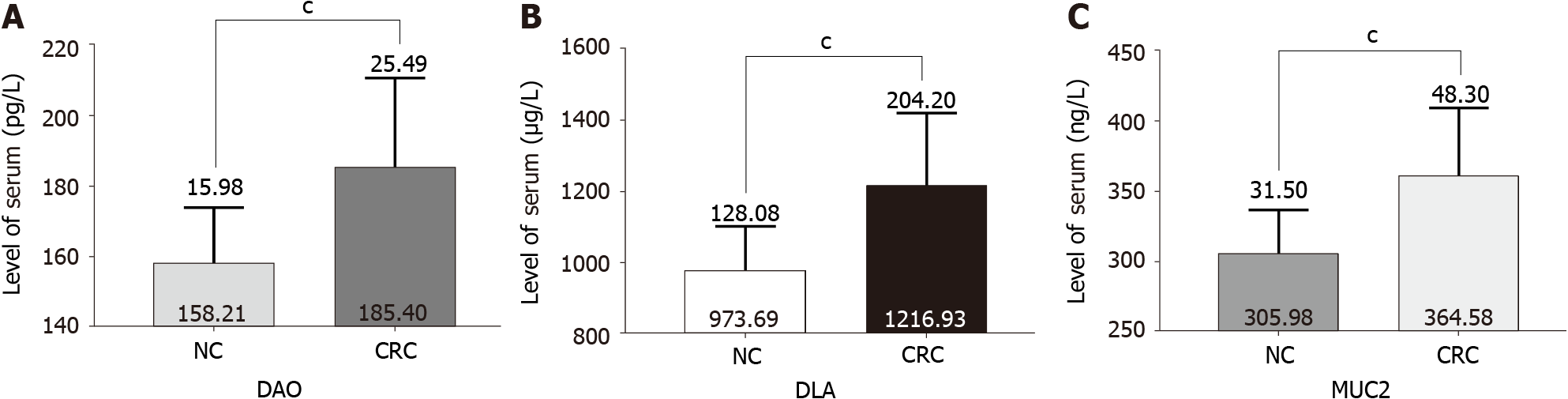
Serum DAO and D-LAC are indicators of intestinal mucosal barrier permeability and integrity[14,15]. As expected, in patients with CRC, the serum levels of DAO and D-LAC were significantly increased compared with those in normal controls (Figure 4A and B), indicating impaired intestinal mucosal barrier function and increased intestinal permeability in CRC patients (Table 3). Interestingly, serum levels of MUC2 were also higher than those in normal controls (Figure 4C) and closely related to the serum levels of DAO and D-LAC (Table 4).
| Group | n (%) | DAO (pg/mL) | D-LAC (μg/L) | MUC2 (ng/L) |
| Normal control | 20 | 158.21 ± 15.98 | 973.69 ± 128.08 | 305.98 ± 31.50 |
| CRC | 66 | 185.40 ± 25.49 | 1216.93 ± 204.20 | 364.58 ± 48.30 |
| P value | < 0.01 | < 0.01 | < 0.01 |
| MUC2 | DAO | D-LAC | ||
| Low | High | Low | High | |
| Low | 15 | 8 | 16 | 7 |
| High | 17 | 26 | 15 | 28 |
| χ2 | 3.957 | 7.236 | ||
| P value | 0.047 | 0.007 | ||
As shown in Table 5, the higher the TNM stage in CRC patients, the higher the serum MUC2 level (P = 0.033). Importantly, the percentage of patients with high serum levels of MUC2 was dramatically increased in CRC patients with distant metastasis com
| Clinicopathological parameter | n (%) | Serum MUC2 expression | χ2 | P value | |
| Low, n (%) | High, n (%) | ||||
| Age, yr | |||||
| ≤ 60 | 30 | 11 (36.7) | 19 (63.3) | 0.080 | 0.777 |
| > 60 | 36 | 12 (33.3) | 24 (66.7) | ||
| Gender | |||||
| Male | 38 | 12 (31.6) | 26 (68.4) | 0.422 | 0.516 |
| Female | 28 | 11 (39.3) | 17 (60.7) | ||
| Tumor location | |||||
| Rectum and anus | 24 | 10 (47.1) | 14 (58.3) | 0.772 | 0.380 |
| Colon | 42 | 13 (31.0) | 29 (65.2) | ||
| TNM stage | |||||
| I-II | 36 | 16 (44.4) | 20 (55.6) | 6.687 | 0.033 |
| III | 21 | 7 (33.3) | 14 (66.7) | ||
| IV | 9 | 0 (0.0) | 9 (100.0) | ||
| Maximum tumor diameter | |||||
| < 5 cm | 33 | 12 (36.4) | 21 (63.6) | 0.067 | 0.796 |
| ≥ 5 cm | 33 | 11 (33.3) | 22 (66.7) | ||
| Depth of invasion | |||||
| Non-immersed serosa | 12 | 5 (41.7) | 7 (58.3) | 0.300 | 0.584 |
| Immersed serosa | 54 | 18 (33.3) | 36 (66.7) | ||
| Degree of differentiation | |||||
| High-moderate | 60 | 21 (38.3) | 39 (61.7) | F1 | 1.000 |
| Low | 6 | 2 (33.3) | 4 (66.7) | ||
| Tumor type | |||||
| Mucinous adenocarcinoma | 14 | 8 (57.1) | 6 (42.9) | 21.241 | < 0.01 |
| Non-mucinous adenocarcinoma | 52 | 15 (32.7) | 37 (67.3) | ||
| Lymph node metastasis | |||||
| No | 36 | 16 (44.4) | 20 (55.6) | 3.212 | 0.073 |
| Yes | 30 | 7 (23.3) | 23 (76.7) | ||
| Distant metastasis | |||||
| No | 57 | 23 (40.4) | 34 (59.6) | F1 | 0.022 |
| Yes | 9 | 0 (0.0) | 9 (100.0) | ||
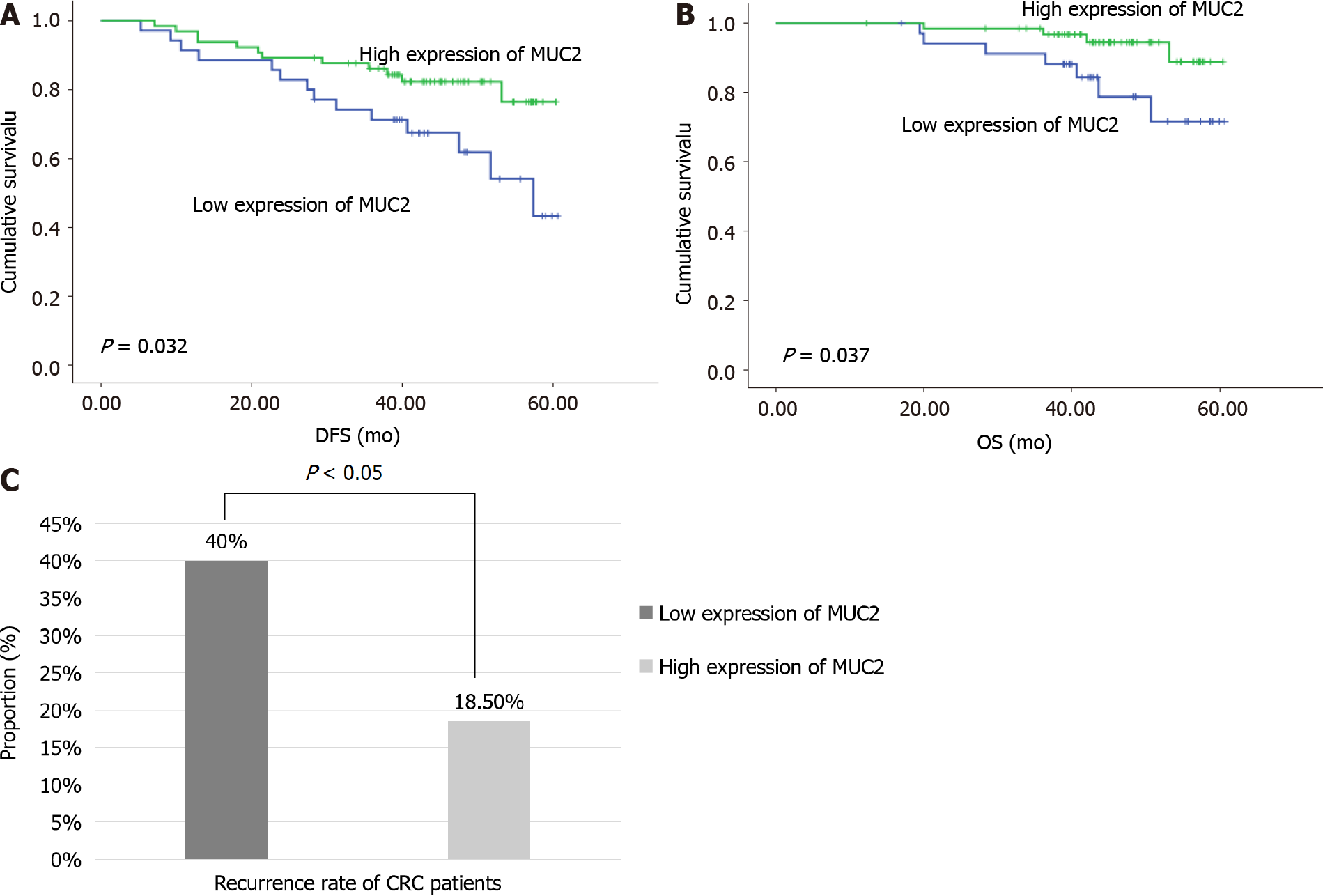
Kaplan-Meier curve analyses with log-rank test revealed that tissue MUC2 expression was significantly associated with DFS (P = 0.032) (Figure 5A) and OS (P = 0.037) (Figure 5B) in all CRC patients. Decreased tissue MUC2 level predicted a poor prognosis of CRC patients. During the 5-year follow-up period, the recurrence was 40.0% in patients with low expression of MUC2 and 18.5% in patients with high expression of MUC2 (χ2 = 5.485, P < 0.05) (Figure 5C).
The protein encoded by the MUC2 gene is the most abundant secreted mucin, covering the surface of the intestinal mucosa in the form of a gel and forming the skeleton of the mucus layer, protecting the intestine in many ways[16]. Recently, Javitt et al[17] presented an integrated structural analysis of the intestinal mucin MUC2, and revealed that the mucin assembly mechanism and its adaptation for hemostasis provide the foundation for rational manipulation of barrier function and coagulation[17]. On the other hand, CRC shows multiple complex pathologies based on the impaired structure and function of the intestinal mucosal barrier, and is associated with the disordered expression and dysfunction of mucins[18]. However, controversial findings of MUC2 function in the occurrence and development of CRC have required further investigation to uncover the underlying mechanisms. We showed that MUC2 expression was decreased in carcinomas, compared with adjacent normal tissues, but CRC patients had higher serum levels of MUC2 compared with normal controls.
MUC2 plays an important role in maintaining the homeostasis of the intestinal environment and protecting susceptible bacteria from pathogenic microorganisms and/or toxic substances[19]. We detected decreased MUC2 in CRC tissue, compared to adjacent intestinal tissues, which may be related to the suppressed immune function in intestinal homeostasis. Importantly, decreased MUC2 expression was found to be associated with advanced CRC stage, suggesting a tumor-suppressive role in the development of CRC. In CRC patients with lymph node metastasis, the tissue MUC2 level was also lower than that in CRC patients without lymph node metastasis, further suggesting a potential protective role of MUC2 in lymph node metastasis in CRC patients. Although statistical significance was not found in the relationship between tissue MUC2 expression and tumor size, depth of invasion, or degree of differentiation, our results indicate that a more severe malignant biological behavior was more likely found in CRC tissues with low MUC2 expression, demonstrating a potential protective role of MUC2 in the development of CRC.
The expression of MUC2 in CRC tissues is reported to be related to the histopathological types of CRC. The expression of MUC2 in mucinous adenocarcinoma is increased, while that in non-mucinous adenocarcinoma is decreased[8,20]. In this study, patients with mucinous adenocarcinoma tended to have a higher proportion of patients with high expression of MUC2 than those with non-mucinous adenocarcinoma. However, the underlying roles of MUC2 in different types of CRC are still unclear and need further investigation.
Li et al[21] reviewed the prognostic and clinicopathological significance of MUCs in CRC, and demonstrated that upregulated MUC2 expression is associated with a better OS, while upregulated MUC1 expression is associated with a poor OS[21]. Elzagheid et al[22] reported that loss of MUC2 expression is associated with disease recurrence and tumor location. However, in multivariate survival analysis, MUC2 lost its power as an independent predictor of DFS and disease-specific survival[22]. To verify the prog
To investigate the potential function of secreted MUC2 in CRC patients, this study further analyzed the expression of serum MUC2 in CRC patients and normal controls. As serum DAO and D-LAC are biomarkers of the functional status of the intestinal mucosal barrier[23], we also monitored serum DAO and D-LAC levels. Interestingly, the serum level of MUC2 was positively related with serum DAO and D-LAC levels, which indicates dysfunction of intestinal mucosal barriers and increased intestinal permeability in CRC patients. The increased serum level of MUC2 may be associated with the progress of tumor infiltration.
The increased serum levels of MUC2 may be related to the fact that cancer cells gradually destroy the mucus layer, intestinal mucosal epithelial cells, and tight junctions, which result in the destruction of the MUC2 skeletal structure in the mucus layer, the apoptosis of intestinal mucosal epithelial cells, and abnormal expression and distribution of tight junction proteins. Decreased MUC2 in the intestinal mucosa and the damage of the intestinal barrier could result in invasion of various pathogenic microorganisms and toxic substances in the intestinal cavity to further aggravate the damage of the intestinal barrier and constitute a vicious circle, increasing intestinal mucosal permeability and promoting the translocation of MUC2 from epithelial cells to the blood.
This study found that MUC2 in intestinal tissues may play a protective role in the intestine and can be used as one of the indicators to evaluate the prognosis of patients with CRC. When the intestinal mucosal barrier function of patients with CRC is impaired, the serum level of MUC2 can reflect the severity of damage. Subsequent studies can further investigate the role of MUC2 in the malignant transformation of colorectal inflammatory diseases, cancer cell proliferation, invasion, metastasis, and the mechanism of resistance to chemotherapeutic drugs at the molecular level.
At present, several studies have reported abnormal expression patterns of mucin 2 (MUC2) in cancerous lesions, including colorectal cancer (CRC). However, as a member of the intestinal mucosal mechanical barrier, the relationship between MUC2 and the intestinal mucosal barrier in patients with CRC remains unclear. Revealing this association will help us more fully understand the role of MUC2 in CRC.
Although many studies have proved that intestinal mucosal barrier function is impair
This study aimed to explore abnormal expression patterns of MUC2 and the rela
Immunohistochemical staining was performed on cancer tissue and normal tissue samples from 100 patients with CRC to evaluate the expression of MUC2 in two different tissues, and these patients were followed for 12-60 mo to understand the overall survival (OS) and disease-free survival (DFS). Preoperative serum levels of MUC2, diamine oxide (DAO), and D-lactate (D-LAC) in 66 patients with CRC were detected by enzyme-linked immunosorbent assay and compared with those in 20 normal controls, so as to evaluate the damage of intestinal mucosal barrier in patients with CRC. The statistical methods involved in this study include χ2 test, Fisher’s exact test, Kaplan-Meier curve, and log-rank tests.
Immunohistochemical staining results showed that the expression of MUC2 in cancer tissues was lower than that in normal tissues (54% vs 79%, P < 0.05), and the expression of MUC2 was correlated with tumor-node-metastasis (TNM) stage and lymph node metastasis in CRC patients (P < 0.05), but not significantly related to the patient’s age, sex, tumor location, size, depth of invasion, or degree of differentiation. The serum levels of MUC2, DAO, and D-LAC in patients with CRC were higher than those in normal people (P < 0.05), and were positively associated with serum levels of human DAO (χ2 = 3.957, P < 0.05) and D-LAC (χ2 = 7.236, P < 0.05), which are the biomarkers of the functional status of the intestinal mucosal barriers. It was suggested that the intestinal mucosal barrier was damaged, and MUC2 can also be used as a new evaluation index. The serum levels of MUC2 were correlated with TNM stage, tumor type, and distant metastasis in CRC patients (P < 0.05). It seems to be a trend that patients with higher malignancy and later stage of tumors have higher serum MUC2 levels. Survival analysis showed that decreased expression of MUC2 in CRC tissues predicted a poor survival. The expression of MUC2 in tissues was significantly correlated with DFS (P = 0.032) and OS (P = 0.037). And the recurrence rate of patients with low expression of MUC2 was higher than that of patients with high expression of MUC2 (40% vs 18.5%, χ2 = 5.485, P < 0.05).
MUC2 in the intestinal tissue may play a protective role on the intestine, which can be used as an indicator to evaluate the prognosis of CRC patients. Intestinal mucosal barrier function of CRC patients is impaired, and the serum MUC2 level can reflect the severity of the damage.
Future researchers can further study the molecular mechanism of MUC2 in the process of intestinal mucosal barrier damage, which may reveal the pathological mechanism of CRC from a new perspective and provide a basis for the development of new targeted therapy drugs. In addition, related research can also be carried out in inflammatory bowel disease.
We are thankful to Prof. Shanley Lin for his critical and careful editing and proo
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang Y S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 914] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 2. | Xing XL, Yao ZY, Zhang T, Zhu N, Liu YW, Peng J. MicroRNA-Related Prognosis Biomarkers from High-Throughput Sequencing Data of Colorectal Cancer. Biomed Res Int. 2020;2020:7905380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Zahnd WE, Josey MJ, Schootman M, Eberth JM. Spatial accessibility to colonoscopy and its role in predicting late-stage colorectal cancer. Health Serv Res. 2021;56:73-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Gonzalez-Villarreal CA, Quiroz-Reyes AG, Islas JF, Garza-Treviño EN. Colorectal Cancer Stem Cells in the Progression to Liver Metastasis. Front Oncol. 2020;10:1511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Loktionov A, Soubieres A, Bandaletova T, Mathur J, Poullis A. Colorectal cancer detection by biomarker quantification in noninvasively collected colorectal mucus: preliminary comparison of 24 protein biomarkers. Eur J Gastroenterol Hepatol. 2019;31:1220-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Pigny P, Van Seuningen I, Desseyn JL, Nollet S, Porchet N, Laine A, Aubert JP. Identification of a 42-kDa nuclear factor (NF1-MUC5B) from HT-29 MTX cells that binds to the 3' region of human mucin gene MUC5B. Biochem Biophys Res Commun. 1996;220:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Liu Y, Yu X, Zhao J, Zhang H, Zhai Q, Chen W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int J Biol Macromol. 2020;164:884-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 8. | Kasprzak A, Siodła E, Andrzejewska M, Szmeja J, Seraszek-Jaros A, Cofta S, Szaflarski W. Differential expression of mucin 1 and mucin 2 in colorectal cancer. World J Gastroenterol. 2018;24:4164-4177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 10. | Pyo JS, Ko YS, Kang G, Kim DH, Kim WH, Lee BL, Sohn JH. Bile acid induces MUC2 expression and inhibits tumor invasion in gastric carcinomas. J Cancer Res Clin Oncol. 2015;141:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Hou Y, Hou L, Liang Y, Zhang Q, Hong X, Wang Y, Huang X, Zhong T, Pang W, Xu C, Zhu L, Li L, Fang J, Meng X. The p53-inducible CLDN7 regulates colorectal tumorigenesis and has prognostic significance. Neoplasia. 2020;22:590-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Liu J, Wei XL, Huang WH, Chen CF, Bai JW, Zhang GJ. Cytoplasmic Skp2 expression is associated with p-Akt1 and predicts poor prognosis in human breast carcinomas. PLoS One. 2012;7:e52675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Liu C, Zha Z, Zhou C, Chen Y, Xia W, Wang YN, Lee HH, Yin Y, Yan M, Chang CW, Chan LC, Qiu Y, Li H, Li CW, Hsu JM, Hsu JL, Wang SC, Ren N, Hung MC. Ribonuclease 7-driven activation of ROS1 is a potential therapeutic target in hepatocellular carcinoma. J Hepatol. 2021;74:907-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Wang H, He C, Liu Y, Zhao H, Long L, Gai X. Soluble dietary fiber protects intestinal mucosal barrier by improving intestinal flora in a murine model of sepsis. Biomed Pharmacother. 2020;129:110343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Honzawa Y, Nakase H, Matsuura M, Chiba T. Clinical significance of serum diamine oxidase activity in inflammatory bowel disease: Importance of evaluation of small intestinal permeability. Inflamm Bowel Dis. 2011;17:E23-E25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Cobo ER, Kissoon-Singh V, Moreau F, Holani R, Chadee K. MUC2 Mucin and Butyrate Contribute to the Synthesis of the Antimicrobial Peptide Cathelicidin in Response to Entamoeba histolytica- and Dextran Sodium Sulfate-Induced Colitis. Infect Immun. 2017;85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Javitt G, Khmelnitsky L, Albert L, Bigman LS, Elad N, Morgenstern D, Ilani T, Levy Y, Diskin R, Fass D. Assembly Mechanism of Mucin and von Willebrand Factor Polymers. Cell. 2020;183:717-729.e16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 18. | Gan GL, Liu J, Chen WJ, Ye QQ, Xu Y, Wu HT, Li W. The Diverse Roles of the Mucin Gene Cluster Located on Chromosome 11p15.5 in Colorectal Cancer. Front Cell Dev Biol. 2020;8:514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Cobo ER, Kissoon-Singh V, Moreau F, Chadee K. Colonic MUC2 mucin regulates the expression and antimicrobial activity of β-defensin 2. Mucosal Immunol. 2015;8:1360-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Shen P, Yang S, Sun H, Li G, Wu B, Ji F, Sun T, Zhou D. SCF/c-KIT Signaling Increased Mucin2 Production by Maintaining Atoh1 Expression in Mucinous Colorectal Adenocarcinoma. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Li C, Zuo D, Liu T, Yin L, Li C, Wang L. Prognostic and Clinicopathological Significance of MUC Family Members in Colorectal Cancer: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2019;2019:2391670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Elzagheid A, Emaetig F, Buhmeida A, Laato M, El-Faitori O, Syrjänen K, Collan Y, Pyrhönen S. Loss of MUC2 expression predicts disease recurrence and poor outcome in colorectal carcinoma. Tumour Biol. 2013;34:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Guo YY, Liu ML, He XD, Jiang CQ, Liu RL. Functional changes of intestinal mucosal barrier in surgically critical patients. World J Emerg Med. 2010;1:205-208. [PubMed] |