Published online Jul 7, 2021. doi: 10.3748/wjg.v27.i25.3748
Peer-review started: January 27, 2021
First decision: February 25, 2021
Revised: March 5, 2021
Accepted: April 13, 2021
Article in press: April 13, 2021
Published online: July 7, 2021
Processing time: 159 Days and 13.5 Hours
Since the initial coronavirus disease 2019 (COVID-19) outbreak in China in December 2019, the infection has now become the biggest medical issue of modern medicine. Two major contributors that amplified the impact of the disease and subsequently increased the burden on health care systems were high mortality among patients with multiple co-morbidities and overcapacity of intensive care units. Within the gastroenterology-related community, particular concern was raised with respect to patients with inflammatory bowel disease (IBD), as those patients are prone to opportunistic infections mainly owing to their immunosuppressive-based therapies. Hence, we sought to summarize current knowledge regarding COVID-19 infection in patients with IBD. Overall, it seems that IBD is not a comorbidity that poses an increased risk for COVID-19 acqui
Core Tip: Coronavirus disease 2019 (COVID-19) is the biggest medical issue of the 21st century so far. Within the gastroenterology-related community, COVID-19 is a concern in patients with inflammatory bowel disease (IBD), as those patients are prone to opportunistic infections owing to their immunosuppressive-based therapies. Hence, in this review, we summarized currently available data and concluded that patients with IBD are not at a higher risk for COVID-19 development, unless treated with 5-aminosalicylates, and that the outcomes of infected patients depend on their respective therapeutic modalities. Finally, we discussed the impact of the COVID-19 pandemic and the concomitantly increased health care burden on IBD-management.
- Citation: Kumric M, Ticinovic Kurir T, Martinovic D, Zivkovic PM, Bozic J. Impact of the COVID-19 pandemic on inflammatory bowel disease patients: A review of the current evidence. World J Gastroenterol 2021; 27(25): 3748-3761
- URL: https://www.wjgnet.com/1007-9327/full/v27/i25/3748.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i25.3748
With the immense burden that coronavirus disease 2019 (COVID-19) posited on health care systems and the global economy in general, the disease is unequivocally the biggest medical concern of the 21st century so far[1]. Globally, by January 2021, there have been over 84 million confirmed cases of COVID-19, with more than 1.8 million deaths reported by the World Health Organization (WHO)[2]. The causative agent of the pandemic, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), belongs to the family of Coronaviridae, a group of viruses which have already been associated with epidemics in the early 2000s, i.e., severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS)[3,4]. Similar to its related viruses, SARS-CoV-2 primary pathologic manifestations occur in the respiratory system[5,6]. Initial clinical presentation is usually characterized by fever, cough, shortness of breath and specific loss of smell and taste, whereas in a smaller, yet significant amount of patients, the disease progresses to severe lung injury, resulting in the need for intensive care support and concomitant tertiary care equipment[7,8]. This raises two principal issues surrounding COVID-19 and explains why it has caused such a huge global impact. The issue first is high mortality in those patients and the other is the overcapacity of intensive care units (ICU), subsequently burdening health care systems. In the early phases of the pandemic, based on initial observations and knowledge about communicable diseases, particular concern was raised with respect to patients who were at high risk of acquiring severe illness. High risk patients mainly consist of the elderly, obese and patients with pre-existing comorbidities, especially those who are immunocompromised and immunosuppressed[9-11].
Therefore, within the gastroenterology-related community consisting of both medical staff and patients, inflammatory bowel disease (IBD) emerged as an important concern, mainly owing to the IBD therapeutic approach as opposed to the disease itself. Although IBD pathophysiology includes immune dysregulation, the available data does not support the notion that patients with IBD are at a higher risk of acquiring communicable diseases[12-14]. Nevertheless, the IBD therapeutic approach is mainly based on a palette of immunosuppressants, medications whose role in promoting opportunistic infections has been well-established[15-18]. Hence, major organizations instantly provided recommendations for the management of patients with IBD[19-21]. However, knowledge regarding SARS-CoV-2 evolves on a daily basis, resulting in updates to recommendations in order to reach an optimal approach for patients with IBD. In this review, we sought to address the main concerns regarding the relationship between of IBD and COVID-19. Specifically, we summa
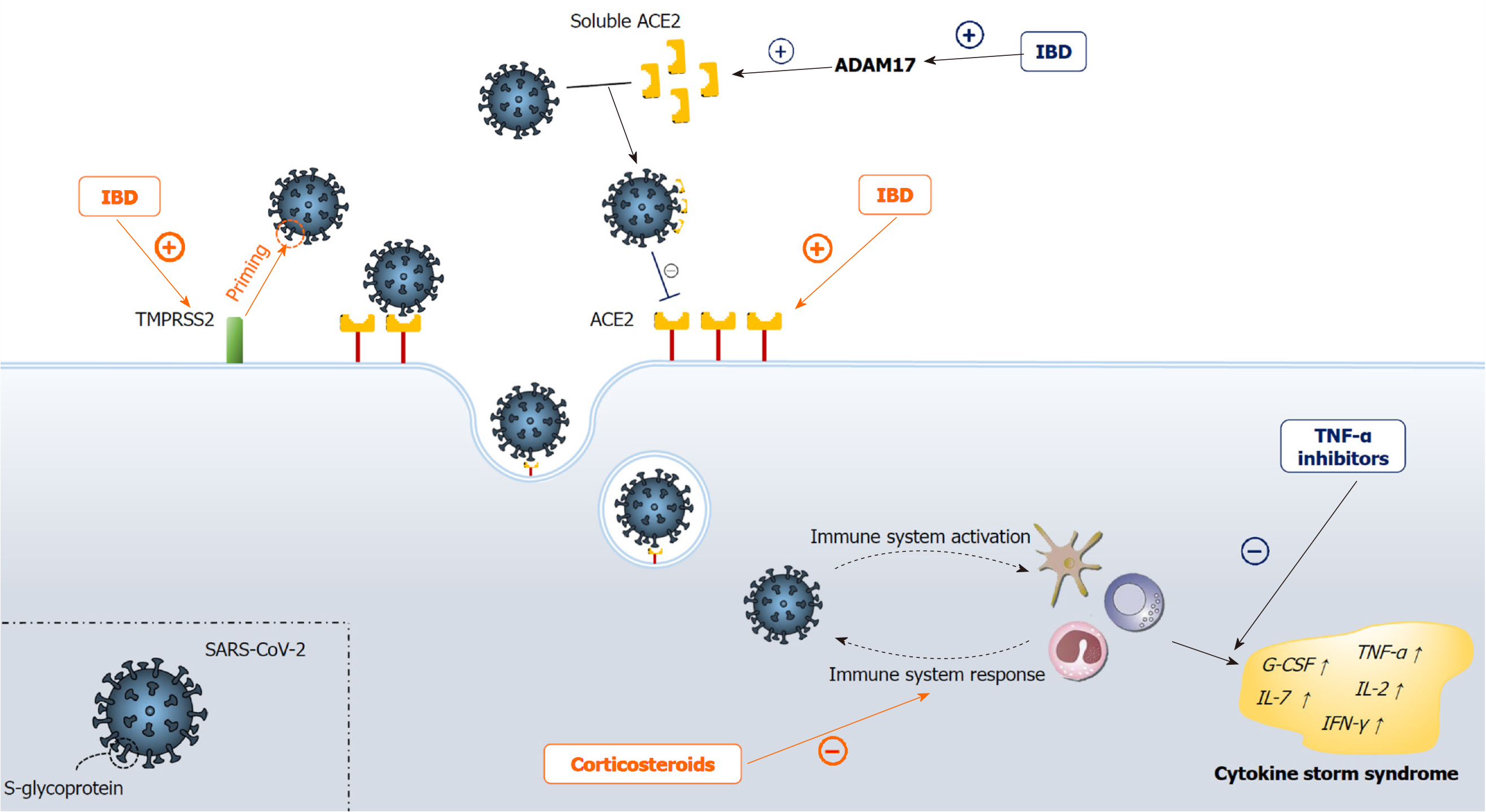
Early in the pandemic, a major concern among gastroenterologists was about the occurrence of COVID-19 among patients with IBD for several reasons. SARS-CoV-2 binds to targeted cells via angiotensin-converting enzyme 2 (ACE2), a protein constitutively expressed by epithelial cells of the blood vessels, lung, kidney and especially the intestines, where ACE2 expression is among the highest in humans[22-24]. Moreover, as shown by proteomic tissue analysis, ACE2 gastrointestinal expression is increased in IBD patients, especially among the Crohn's disease (CD) subgroup, where expression is markedly higher than in ulcerative colitis (UC)[25]. ACE2 has been also implicated in the pathophysiology of IBD, having a dual-role: aggravation of colitis via the classical renin/angiotensin II/aldosterone pathway and amelioration of colitis via the ACE2/MAS-1 receptor pathway[26-29]. Except for ACE2, SARS-CoV-2 pathogenesis depends on the specific "spike" glycoprotein that mediates fusion of the coronavirus envelope with the host cell membrane[30]. This protein is activated through the trypsin-like protease, the transmembrane protease serine 2 (TMPRSS2), the activity of which has been shown to be up-regulated in IBD[31]. Furthermore, in up to 50% of COVID-19 patients, fecal samples were positive for SARS-CoV-2 virus, with more than one-fifth of the samples testing positive even after subjects tested negative from respiratory samples, implicating the fecal route of SARS-CoV-2 transmission[32,33]. This could be even more important, as patients with IBD are more frequently assessed with invasive gastrointestinal procedures such as esophagogastroduodenoscopy and ileocolonoscopy compared with the non-IBD population, subsequently exposing both the patient and the examiner to a higher risk of infection[34]. Finally, the use of IBD immunosuppressive therapies has been associated with an increased risk of infections[13,14]. All of these findings suggest that patients with IBD should be the "perfect" host for SARS-CoV-2 viral infection (Figure 1). However, results from a recent systematic review that comprised 13 cohort studies and 5 single case reports from all around the world suggest that patients with IBD do not seem to have a higher risk of COVID-19 infection with respect to the general population, not even in IBD patients treated with immunosuppressive drugs[35]. Another systematic review and meta-analysis by Singh et al[36] concluded similarly and additionally determined that there was no difference in COVID-19 occurrence between IBD subgroups, i.e., between CD and UC. Regarding the IBD therapeutic strategies, Singh et al[36] demonstrated that no use of therapeutics was associated with an increased risk of COVID-19 acquisition aside from the use of 5-aminosalicylic acid (5-ASA). In fact, Taxonera et al[37] evaluated the age-standardized incidence of COVID-19 in IBD patients, and suggested that COVID-19 incidence might be overestimated in the IBD population. Unfortunately, larger studies did not conduct a comparable evaluation.
Multiple authors struggled to explain the discrepancy between the expected and evidence-based COVID-19 incidence among populations with IBD[36,38]. Firstly, a major determinant to reduced COVID-19 incidence could be the tighter containment of patients with IBD, since people suffering from chronic diseases, especially patients treated with immunosuppressants, were warned by experts to follow strict social distancing measures, known as shielding, from the beginning of the pandemic. Furthermore, ACE2, the above-noted protein that is up-regulated in IBD, has two distinct functional forms. The full-length form of ACE2 possesses an extracellular domain that binds to the SARS-CoV-2 virus and transmembrane domain, which anchors the first domain to the plasma membrane and aids viral entry into the cell[39]. Conversely, the soluble form of ACE2 lacks a transmembrane domain and it is therefore a sort of a decoy receptor for SARS-CoV-2 virus in the blood[40,41]. Notably, the latter form is up-regulated in IBD patients, as a consequence of ACE2 membrane cleavage into the soluble form, in a process regulated by the tumor necrosis factor-alpha (TNF-α) convertase ADAM17 (a disintegrin and metalloproteinase 17), the protease is up-regulated in patients with active IBD[42,43]. Furthermore, although SARS-CoV-2 is detectable in fecal samples and active viral replication in the enterocytes of the small intestine has been reported[33,44], to this day there is no firm evidence to imply that increased SARS-CoV-2 replication in intestines is proportional to intestinal ACE2 expression. This is substantiated by the fact that SARS coronavirus, a SARS-CoV-2 close relative, spreads through the upper respiratory tract (URT) very effectively despite only modest ACE2 expression in the URT[24]. Overall, it seems that SARS-CoV-2 also needs the presence of a co-receptor for host cell infection, similarly to HIV infection[45]. However, the hypothesized co-receptor that synergistically with ACE2 leads to SARS-CoV-2 infection has yet to be determined in future studies. The reasons for the increased risk of COVID-19 acquisition with 5-ASA are still unclear but, as discussed by Singh et al[36], the observed increase could be related to the fact that 5-ASA use may be a proxy for underlying UC in these circumstances. Namely, patients with UC have higher ACE2 levels (although, as we discussed, this is not a reliable indicator) and a population with UC tends to be older than a CD population[46], hence they are more prone to get tested, as Singh et al[36] argue. Despite the sensitivity of the fecal reverse transcription polymerase chain reaction (RT-PCR) test for the diagnosis of COVID-19, its diagnostic power still needs to be elucidated. D'Amico et al[47] hypothesized that fecal RT-PCR testing may be useful in IBD patients to distinguish disease re-exacerbation from SARS-CoV-2 superinfection, allowing better patient management and targeted therapy.
Since the detrimental effects of immunosuppressive agents on the host-cell defense against pathogens have been well-established, particular concern was raised with respect to the clinical course of COVID-19 in patients on various immunosuppressive therapies and ubiquitous therapeutic strategies for patients with IBD[15-17,48]. Furthermore, active IBD itself might worsen COVID-19 outcomes, as those patients are markedly frailer and more prone to adverse outcomes by virtually any infection[49,50]. Finally, non-IBD patients with COVID-19 have high fecal calprotectin even after diarrhea resolves and COVID-19 patients with ongoing diarrhea have even higher levels in comparison with COVID-19 patients without diarrhea[51,52], suggesting that the presence of SARS-CoV-2 in the gastrointestinal tract is associated with greater intestinal inflammation. This indicates that COVID-19 could exacerbate inflammation and, subsequently, symptoms in IBD patients. However, it is very challenging to assign a symptom to the underlying disease, its exacerbation, or the concomitant infection, making these characteristics difficult to interpret. Of note, in the aforementioned studies[51,52], patients with diarrhea exhibited higher serum interleukin 6 concentrations, raising the possibility of more severe systemic inflammation in this group of patients[53].
Fortunately, an abundance of clinical studies demonstrated that most of the hypothesized adverse outcomes were not observed in IBD patients that acquired COVID-19. Nonetheless, in some cases this comorbidity seems to even dampen the deleterious effects of COVID-19. Considering the differences in the initial clinical presentation, although in concordance with the non-IBD population, fever and cough were the most common clinical findings. Further, COVID-19 positive IBD patients presented with diarrhea significantly more often than the general population[54-57]. This disparity could be associated with the influence of the underlying disease on the number of evacuations, justifying the greater percentage of diarrhea in IBD patients than in the general population. In contrast, the observed difference could also be due to the aforementioned exacerbation of IBD as a result of COVID-19 infection. The risk of severe COVID-19 outcomes, i.e., the need for hospitalization, admission to the ICU, mechanical ventilation or death, were not higher among IBD patients in comparison to the general population, as demonstrated in multiple systematic reviews and meta-analyses[35,36,47]. These results are also in accordance with the latest data from the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD), an international web-based database where physicians are encouraged to report all cases of COVID-19 in patients with IBD[58]. A total of 4038 IBD patients with confirmed COVID-19 were reported in the SECURE-IBD database as of January 6, 2021 with 19% of patients in need of hospitalization, 3% admitted to the ICU, and 3% in need of mechanical ventilation, where the case fatality rate was 2%. Of note, in a study by Lukin et al[59], the authors included a control group consisting of non-IBD patients with COVID-19. Rather interestingly, death and ICU admission were numerically lower in the IBD group than in the control group. Although these results should be taken with caution, it is possible that certain IBD medications led to the blunting of the cytokine release syndrome and subsequently to more favorable outcomes.
The therapeutic choice emerged as a major determinant for COVID-19 prognosis in patients with IBD. Accumulating data implies that the use of systemic corticosteroids is associated with the highest risk of severe COVID-19 outcomes[35,36,47,58]. It is well known that corticosteroids affect the immune system via multiple mechanisms, including the inhibition of adhesion molecules, decreasing the expression of inflammatory cytokines and inducing apoptosis of activated lymphocytes. Moreover, in studies that tested the use of corticosteroids on MERS and SARS patients, authors have demonstrated a delayed viral clearance in patients receiving high-dose corticosteroids[60]. However, the effects of corticosteroid use on COVID-19 adverse outcomes in IBD is not as clear as it may seem. In a report from the large RECOVERY trial, in which the effects of dexamethasone on hospitalized COVID-19 patients were assessed, authors concluded that the use of dexamethasone resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone at the time of randomization but not among those receiving no respiratory support[61]. Furthermore, in a recently published meta-analysis by van Paassen et al[62], which included the largest number of studies and COVID-19 patients, the authors demonstrated the beneficial effects of corticosteroids use on short-term mortality and a reduction in the need for mechanical ventilation. Notably, the authors also found a signal of delayed viral clearance, but data in the studies were too uncertain to reach any firm conclusions. Three other meta-analyses that were conducted on this topic have rather conflicting reports. The WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group[63] concluded similarly to van Paassen et al[62], reporting that the administration of systemic corticosteroids was associated with lower 28-day all-cause mortality in comparison to placebo or usual care. Tlayjeh et al[64] found no difference in mortality or the necessity for mechanical ventilation, yet similarly to van Paassen et al[62], they observed a prolonged viral clearance time. Sarkar et al[65] demonstrated that in patients with COVID-19, corticosteroids may be associated with a twofold increase in mortality, yet their analysis was based on low-quality evidence with high variability. Considering the beneficial effects of corticosteroid use in COVID-19 patients, doubt was raised with respect to poor outcomes of their use in COVID-19 patients with concomitant IBD. It is possible that the corticosteroid use in these circumstances is merely an indicator from the subset of patients with active IBD who are predisposed to adverse outcomes. In fact, in a retrospective cohort study, Singh et al[66] reported that IBD patients who received corticosteroids up to 3 months before the diagnosis of COVID-19 had a higher risk of severe COVID-19 in comparison to patients who did not receive corticosteroids. Although the authors conducted an unadjusted analysis, because corticosteroid use in IBD is associated to worsening of the disease, these results could imply that in this setting corticosteroids were not the cause, but an indicator of higher risk for a severe COVID-19 clinical course[67].
Apart from corticosteroid use, as shown by multiple studies, the use of 5-ASA has been also associated with more severe COVID-19 outcomes[35,36,47,58,68]. This finding persisted even after controlling for confounding factors such as age, co-morbidities, IBD disease characteristics and corticosteroid use. Since mechanisms by which 5-ASA exerts its anti-inflammatory effect are rather disperse and include peroxisome proliferator-activated receptor-γ up-regulation, cyclooxygenase 2/prosta
Immunomodulators, a group of medications used in IBD treatment that includes azathioprine, 6-mercaptopurine, and methotrexate have been known to inhibit the immune response to viral infections by multiple mechanisms[70,71]. However, the data regarding the role of immunomodulators in COVID-19 is quite reassuring, as conducted studies do not seem to demonstrate any difference in severe outcomes in comparison to the general population[35,36,47,58,68]. Since the effects of immunomodulators and corticosteroids on the suppression of the immune system are in part overlapping[72,73], we hypothesize that this provides further evidence toward the notion that the use of corticosteroids itself does not result in a more severe form of COVID-19. In fact, different results between the use of corticosteroids and immuno
The most ambiguous results that emerged from observational studies is in relation to the use of biological agents, an immunosuppressive medication group used in the management of IBD which includes infliximab, adalimumab, golimumab, certolizumab pegol, ustekinumab and vedolizumab[74]. Biological agents, particularly TNF-α inhibitors, have been known to mitigate the host immune system response against infectious organisms, especially intracellular pathogens, such as mycobacterial, fungal and viral infections[75,76]. However, aside from the systematic review by Macaluso and Orlando[35] which showed no difference as opposed to the general population, available data suggests that patients treated with biological agents are significantly less prone to develop severe forms of COVID-19, distinctly in terms of mortality[36,58,68]. Accumulating evidence implies that COVID-19 severity is associated with a cytokine storm syndrome, an immune-mediated process characterized by hyperacti
Between the IBD subgroups, in all of the aforementioned reports[35,36,47,58,68], patients with UC had markedly worse outcomes than patients with CD. Singh et al[36] attribute this risk incrementation to the fact that patients with UC are more likely to be older and undergo different therapeutic modalities between the two groups[36,80]. However, since Singh et al[36] did not provide the age-adjusted comparison of outcomes, whether the poor outcomes of UC in contrast to CD are related to old age remains unclear. Regarding the difference in therapeutic modalities, patients with UC are more likely to be treated with 5-ASA, a treatment shown to pose a risk for more severe outcomes, whereas patients with CD are more likely to be treated with biological agents, treatment that seem to have a protective role in COVID-19 infection[81,82]. In addition, it is unclear if the observed disparity is due to the pathobiological differences between the two types of IBD, including the variability in expression of ACE2 and TMPRSS2.
Two major organizations that cover the issues regarding IBD, the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) and European Crohn’s and Colitis Organization (ECCO) have partnered and provided a set of guidelines regarding the management of IBD in relation to COVID-19[83]. However, there are two important notions to accentuate regarding the present guidelines. Firstly, as currently there is no adequate evidence-based data, the recommendations are based on a consensus between a group of international IBD and infectious disease experts. Secondly, since the speed of publishing is slower than the amount of emerging data, organizations urged physicians to continue to check the IOIBD or the ECCO websites for the most up-to-date information.
The most important question that was raised in the guidelines concerns the IBD immunosuppressive therapies, i.e., whether the infected patients should discontinue these therapies and if should, for how long. The current consensus is that recommencing these therapies should be influenced by the clinical severity of both IBD and COVID-19. Conceptually speaking, the greater the severity of IBD and the lesser the severity of COVID-19, the discontinuation of therapy should be shorter and vice versa. Experts suggest that for most patients, a symptom-based strategy is suitable[84]. According to this strategy, COVID-19 resolution is evaluated according to symptom onset (≥ 10 d) and clinical improvement. Current expert recommendations regarding immunosuppressive therapies are summarized in Table 1. Other aspects regarding recommendations, such as care for patients with IBD requiring hospitalization, priority for endoscopy, guidance for the infusion centers, management of pregnant IBD patients and a very practical set of ten “Do's” and “Don'ts” for IBD management during the COVID-19 outbreak were further discussed in the aforementioned IOIBD/ECCO guidelines[85-89]. Following the approval of several COVID-19 vaccines, the IOIBD experts have recently issued a statement regarding vaccination[90]. The expert group advised vaccinating all patients with IBD as soon as they are able to receive a vaccine, regardless of their immune-modifying therapies.
| COVID-19 symptomatology | SARS-CoV-2 status | Recommendation |
| Asymptomatic | Non-tested | Do not withhold therapy (reduce corticosteroid use if possible) |
| Asymptomatic | Positive | Withhold therapy for 10 days1 |
| Symptomatic | Positive | Withhold therapy until all of the following is fulfilled1: (1) At least 10 days has passed since symptoms onset; (2) Improvement in respiratory symptoms; and (3) Days without fever (without the use of antipyretics) |
There is a scarcity of data regarding the post-lockdown phase in terms of health-care procedures[91,92], let alone in the IBD population[93-96]. Early into the pandemic, hospitals were urged to restructure their daily activities to meet the needs of health care practitioners and to provide the facilities to treat COVID-19 patients. The restructuring of the health care system did not circumvent IBD management. Consequently, the risks of secondary harm emerged, as the latter resulted in reduced access to diagnostic endoscopy, lack of face-to-face clinics, difficulties in continuing day-case infusions, issues in performing routine blood and/or stool monitoring as well as patients' fears which may have reduced their attendance in hospitals[93]. Particular problems emerged in the pediatric population, since a delay in diagnosis and delayed treatment has the potential to result in serious repercussions, such as an impact on children’s growth[97,98]. Recent Italian and Spanish surveys both demonstrated that the management of urgent activities and administration of biological therapies in both the lockdown and post-lockdown periods substantially maintained the pre-pandemic standards of care[93,94]. However, the surveys also highlighted that the reduction in number of visits, endoscopies and gastrointestinal ultrasounds observed in the lockdown but also in the post-lockdown phase could result in worse long-term outcomes[93,94]. A study in the pediatric population had similar conclusions regarding the quality of care[93]. This study accentuated concerns with respect to newly diagnosed IBD patients as this subgroup was diagnosed without a histological confirmation of the disease, which is a controversial exception that had to be adopted given the present special circumstances[93]. These patients were diagnosed via a combination of blood tests, radiological imaging, fecal calprotectin and exclusion of infectious causes, followed by multidisciplinary discussion. The commencement of the systemic immunosuppression in children without endoscopic or histological diagnosis was an additional concern, yet physicians could adapt in the beginning exclusive enteral nutrition as a first-line therapy as an induction strategy with multiple benefits[99]. Although a delay in exposing patients to systemic immunosuppression for 4-8 weeks is beneficial, after this time patients enter a period where immunomodulatory and biological therapy should commence, while simultaneously, the ability to conduct a full disease assessment beforehand may continue to be limited. Overall, experts agree that the implementation of a telemedicine approach has played an important role in maintaining the standards of quality of care in IBD management during the pandemic[93,94,100].
Even though COVID-19 had a range of detrimental effects on health care systems globally, it also opened a space for improvements in clinical practice, which could be used long after the COVID-19 pandemic resolves. One of those is the use of telemedicine, i.e., the implementation of virtual technologies in routine clinical practice. An expansion in technological solutions and loosening restrictions on how telemedicine can be deployed and reimbursed have opened the way for telemedicine to become an integral part of clinical practice both now and in the future. In the IBD population, virtual appointments, multidisciplinary discussions, and improvement of networks by remote collaboration all provide the opportunity for better care within specific situations, with the simultaneous reduction of the transmission of infectious diseases. Today it is COVID-19, but in the future, it could be some other virus, especially as we are now more than ever aware of our susceptibility to a viral pandemic. IOIBD/ECCO issued a summary of the best strategies for IBD management via telemedicine, carefully covering every aspect of the patient-physician relationship[101]. Another very important aspect of telemedicine is its inexpensiveness. As a global economic crisis is imminent, this will actually become the critical reason for widespread implementation of telemedicine. Regarding the disease itself, although for now a lot of data substantiates the fact that COVID-19 does not influence the short-term prognosis for IBD patients, the long-term effects are quite unknown. We believe that poorer long-term outcomes will be mainly due to delayed diagnostic (especially endoscopy) therapeutic procedures and not COVID-19 itself. However, Gower-Rousseau et al[102] argue that most of the researchers too hastily concluded that the COVID-19 pandemic is relatively safe for IBD patients. They highlighted that the recently published, underpowered studies cannot provide answers for patients with IBD, or other infrequent diseases for that matter. Further, Gower-Rousseau et al. asserted that low-quality studies might even prompt misguided and harmful treatment decisions. On the contrary, they argue that well-grounded answers to these questions require complex epidemiologic risk and benefit analyses with an a priori sample size calculation and a removal of unwanted biases.
The initial fear of COVID-19 infection among patients in the IBD community that was based on available knowledge, now seems unnecessary. Accumulating data suggests that IBD is not a comorbidity that poses an increased risk for COVID-19 acquisition, except in patients treated with 5-ASA. Furthermore, although the outcomes of infected patients are largely dependent on the therapeutic modality by which they are treated, overall, IBD patients seem to have COVID-19 outcomes similar to the general population. This is in contrast to those on corticosteroids, as they currently seem to have a less favorable prognosis. Biological agents even dampen the detrimental effects of COVID-19 by inhibiting cytokine storm syndrome, according to the available data. However, preliminary data must be interpreted with caution, as the long-term effects of both COVID-19 and IBD management during the COVID-19 outbreak are quite unknown. Finally, the COVID-19 outbreak could also change the future of IBD management, and management of the diseases in general, as telemedicine could dethrone face-to-face examinations in the following years.
The authors thank D. Behmen and S. Pranic for language proofreading and editing the paper.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Islam MM S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Pak A, Adegboye OA, Adekunle AI, Rahman KM, McBryde ES, Eisen DP. Economic Consequences of the COVID-19 Outbreak: the Need for Epidemic Preparedness. Front Public Health. 2020;8:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. Coronavirus disease 2019 (COVID-19): situation report. [cited 5 January 2021]. In: World Health Organization [Internet]. Available from: https://covid19.who.int/. |
| 3. | Vijayanand P, Wilkins E, Woodhead M. Severe acute respiratory syndrome (SARS): a review. Clin Med (Lond). 2004;4:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2157] [Cited by in RCA: 2280] [Article Influence: 253.3] [Reference Citation Analysis (0)] |
| 5. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18867] [Article Influence: 3773.4] [Reference Citation Analysis (7)] |
| 6. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30098] [Article Influence: 6019.6] [Reference Citation Analysis (3)] |
| 7. | Gibson PG, Qin L, Puah SH. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust. 2020;213:54-56.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 428] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 8. | Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia. 2020;75:1340-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 288] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 9. | Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A; COVID-19 Lombardy ICU Network. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3537] [Cited by in RCA: 3825] [Article Influence: 765.0] [Reference Citation Analysis (0)] |
| 10. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11504] [Article Influence: 2300.8] [Reference Citation Analysis (0)] |
| 11. | Fung M, Babik JM. COVID-19 in Immunocompromised Hosts: What We Know So Far. Clin Infect Dis. 2021;72:340-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 367] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 12. | Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R, Esteve M, Katsanos K, Lees CW, Macmahon E, Moreels T, Reinisch W, Tilg H, Tremblay L, Veereman-Wauters G, Viget N, Yazdanpanah Y, Eliakim R, Colombel JF; European Crohn's and Colitis Organisation (ECCO). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 746] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 13. | Vrdoljak J, Vilović M, Živković PM, Tadin Hadjina I, Rušić D, Bukić J, Borovac JA, Božić J. Mediterranean Diet Adherence and Dietary Attitudes in Patients with Inflammatory Bowel Disease. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Brnic D, Martinovic D, Zivkovic PM, Tokic D, Vilovic M, Rusic D, Tadin Hadjina I, Libers C, Glumac S, Supe-Domic D, Tonkic A, Bozic J. Inactive matrix Gla protein is elevated in patients with inflammatory bowel disease. World J Gastroenterol. 2020;26:4866-4877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Zabana Y, Rodríguez L, Lobatón T, Gordillo J, Montserrat A, Mena R, Beltrán B, Dotti M, Benitez O, Guardiola J, Domènech E, Garcia-Planella E, Calvet X, Piqueras M, Aceituno M, Fernández-Bañares F, Esteve M. Relevant Infections in Inflammatory Bowel Disease, and Their Relationship With Immunosuppressive Therapy and Their Effects on Disease Mortality. J Crohns Colitis. 2019;13:828-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 16. | Luthra P, Peyrin-Biroulet L, Ford AC. Systematic review and meta-analysis: opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013;108:1268-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 18. | Bonovas S, Fiorino G, Allocca M, Lytras T, Nikolopoulos GK, Peyrin-Biroulet L, Danese S. Biologic Therapies and Risk of Infection and Malignancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1385-1397.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 295] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 19. | Kennedy NA, Jones GR, Lamb CA, Appleby R, Arnott I, Beattie RM, Bloom S, Brooks AJ, Cooney R, Dart RJ, Edwards C, Fraser A, Gaya DR, Ghosh S, Greveson K, Hansen R, Hart A, Hawthorne AB, Hayee B, Limdi JK, Murray CD, Parkes GC, Parkes M, Patel K, Pollok RC, Powell N, Probert CS, Raine T, Sebastian S, Selinger C, Smith PJ, Stansfield C, Younge L, Lindsay JO, Irving PM, Lees CW. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2020;69:984-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 20. | D'Amico F, Danese S, Peyrin-Biroulet L; ECCO COVID taskforce. Inflammatory Bowel Disease Management During the Coronavirus-19 Outbreak: A Survey From the European Crohn's and Colitis Organization. Gastroenterology. 2020;159:14-19.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Rubin DT, Abreu MT, Rai V, Siegel CA; International Organization for the Study of Inflammatory Bowel Disease. Management of Patients With Crohn's Disease and Ulcerative Colitis During the Coronavirus Disease-2019 Pandemic: Results of an International Meeting. Gastroenterology. 2020;159:6-13.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 22. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14257] [Article Influence: 2851.4] [Reference Citation Analysis (0)] |
| 23. | Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 655] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 24. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 25. | Garg M, Royce SG, Tikellis C, Shallue C, Batu D, Velkoska E, Burrell LM, Patel SK, Beswick L, Jackson A, Britto K, Lukies M, Sluka P, Wardan H, Hirokawa Y, Tan CW, Faux M, Burgess AW, Hosking P, Monagle S, Thomas M, Gibson PR, Lubel J. Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: a novel therapeutic target? Gut. 2020;69:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 26. | Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838-14843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1073] [Cited by in RCA: 1119] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 27. | Khajah MA, Fateel MM, Ananthalakshmi KV, Luqmani YA. Anti-Inflammatory Action of Angiotensin 1-7 in Experimental Colitis. PLoS One. 2016;11:e0150861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Cerniello FM, Carretero OA, Longo Carbajosa NA, Cerrato BD, Santos RA, Grecco HE, Gironacci MM. MAS1 Receptor Trafficking Involves ERK1/2 Activation Through a β-Arrestin2-Dependent Pathway. Hypertension. 2017;70:982-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1522] [Cited by in RCA: 1709] [Article Influence: 341.8] [Reference Citation Analysis (0)] |
| 30. | Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect. 2020;80:554-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 367] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 31. | Jablaoui A, Kriaa A, Mkaouar H, Akermi N, Soussou S, Wysocka M, Wołoszyn D, Amouri A, Gargouri A, Maguin E, Lesner A, Rhimi M. Fecal Serine Protease Profiling in Inflammatory Bowel Diseases. Front Cell Infect Microbiol. 2020;10:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 32. | Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 2658] [Article Influence: 531.6] [Reference Citation Analysis (0)] |
| 33. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1993] [Article Influence: 398.6] [Reference Citation Analysis (1)] |
| 34. | Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 949] [Article Influence: 189.8] [Reference Citation Analysis (1)] |
| 35. | Macaluso FS, Orlando A. COVID-19 in patients with inflammatory bowel disease: A systematic review of clinical data. Dig Liver Dis. 2020;52:1222-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Singh AK, Jena A, Kumar-M P, Sharma V, Sebastian S. Risk and outcomes of coronavirus disease (COVID-19) in patients with inflammatory bowel disease: a systematic review and meta-analysis. United European Gastroenterol J. 2020;2050640620972602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 37. | Taxonera C, Sagastagoitia I, Alba C, Mañas N, Olivares D, Rey E. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2020;52:276-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (1)] |
| 38. | Monteleone G, Ardizzone S. Are Patients with Inflammatory Bowel Disease at Increased Risk for Covid-19 Infection? J Crohns Colitis. 2020;14:1334-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 39. | Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1393] [Cited by in RCA: 1205] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 40. | Wysocki J, Ye M, Rodriguez E, González-Pacheco FR, Barrios C, Evora K, Schuster M, Loibner H, Brosnihan KB, Ferrario CM, Penninger JM, Batlle D. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010;55:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 41. | Batlle D, Wysocki J, Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond). 2020;134:543-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 323] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 42. | Garg M, Burrell LM, Velkoska E, Griggs K, Angus PW, Gibson PR, Lubel JS. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: A pilot study. J Renin Angiotensin Aldosterone Syst. 2015;16:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Wong E, Cohen T, Romi E, Levin M, Peleg Y, Arad U, Yaron A, Milla ME, Sagi I. Harnessing the natural inhibitory domain to control TNFα Converting Enzyme (TACE) activity in vivo. Sci Rep. 2016;6:35598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 44. | Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 295] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 45. | Zhang L, Huang Y, He T, Cao Y, Ho DD. HIV-1 subtype and second-receptor use. Nature. 1996;383:768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 166] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Windsor JW, Kaplan GG. Evolving Epidemiology of IBD. Curr Gastroenterol Rep. 2019;21:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 226] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 47. | D'Amico F, Danese S, Peyrin-Biroulet L. Systematic Review on Inflammatory Bowel Disease Patients With Coronavirus Disease 2019: It Is Time to Take Stock. Clin Gastroenterol Hepatol. 2020;18:2689-2700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Mazzola G, Macaluso FS, Adamoli L, Renna S, Cascio A, Orlando A. Diagnostic and vaccine strategies to prevent infections in patients with inflammatory bowel disease. J Infect. 2017;74:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Kochar B, Cai W, Cagan A, Ananthakrishnan AN. Pretreatment Frailty Is Independently Associated With Increased Risk of Infections After Immunosuppression in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2020;158:2104-2111.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 50. | Kucharzik T, Maaser C. Infections and Chronic Inflammatory Bowel Disease. Viszeralmedizin. 2014;30:326-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Reuken PA, Wüst M, Löffler B, Bauer M, Stallmach A. Letter: SARS-CoV-2-induced gastrointestinal inflammation. Aliment Pharmacol Ther. 2020;52:1748-1749. [PubMed] |
| 52. | Effenberger M, Grabherr F, Mayr L, Schwaerzler J, Nairz M, Seifert M, Hilbe R, Seiwald S, Scholl-Buergi S, Fritsche G, Bellmann-Weiler R, Weiss G, Müller T, Adolph TE, Tilg H. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut. 2020;69:1543-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 53. | Taxonera C, Alba C. Letter: SARS-CoV-2 induced gastrointestinal inflammation-authors' reply. Aliment Pharmacol Ther. 2020;52:1750-1751. [PubMed] |
| 54. | Gajendran M, Perisetti A, Aziz M, Raghavapuram S, Bansal P, Tharian B, Goyal H. Inflammatory bowel disease amid the COVID-19 pandemic: impact, management strategies, and lessons learned. Ann Gastroenterol. 2020;33:591-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | D'Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin Gastroenterol Hepatol. 2020;18:1663-1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 378] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 56. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 752] [Article Influence: 150.4] [Reference Citation Analysis (0)] |
| 57. | Parasa S, Desai M, Thoguluva Chandrasekar V, Patel HK, Kennedy KF, Roesch T, Spadaccini M, Colombo M, Gabbiadini R, Artifon ELA, Repici A, Sharma P. Prevalence of Gastrointestinal Symptoms and Fecal Viral Shedding in Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3:e2011335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 302] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 58. | Brenner EJ, Ungaro RC, Colombel JF, Kappelman MD. Current data. 2020 June 14 [cited 5 January 2021]. In: Secure-IBD Database Public Data [Internet]. Available from: https://covidibd.org/current-data/. |
| 59. | Lukin DJ, Kumar A, Hajifathalian K, Sharaiha RZ, Scherl EJ, Longman RS; Jill Roberts Center Study Group Study Group; Weill Cornell Medicine-Gastrointestinal Study Group. Baseline Disease Activity and Steroid Therapy Stratify Risk of COVID-19 in Patients With Inflammatory Bowel Disease. Gastroenterology. 2020;159:1541-1544.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 60. | Neurath MF. COVID-19 and immunomodulation in IBD. Gut. 2020;69:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 61. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7376] [Article Influence: 1844.0] [Reference Citation Analysis (1)] |
| 62. | van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24:696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 63. | WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Jüni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Møller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324:1330-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 1678] [Article Influence: 335.6] [Reference Citation Analysis (0)] |
| 64. | Tlayjeh H, Mhish OH, Enani MA, Alruwaili A, Tleyjeh R, Thalib L, Hassett L, Arabi YM, Kashour T, Tleyjeh IM. Association of corticosteroids use and outcomes in COVID-19 patients: A systematic review and meta-analysis. J Infect Public Health. 2020;13:1652-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 65. | Sarkar S, Khanna P, Soni KD. Are the steroids a blanket solution for COVID-19? J Med Virol. 2021;93:1538-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 66. | Singh S, Khan A, Chowdhry M, Bilal M, Kochhar GS, Clarke K. Risk of Severe Coronavirus Disease 2019 in Patients With Inflammatory Bowel Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:1575-1578.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 67. | Waljee AK, Wiitala WL, Govani S, Stidham R, Saini S, Hou J, Feagins LA, Khan N, Good CB, Vijan S, Higgins PD. Corticosteroid Use and Complications in a US Inflammatory Bowel Disease Cohort. PLoS One. 2016;11:e0158017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 68. | Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, Ng SC, Rahier JF, Reinisch W, Ruemmele FM, Steinwurz F, Underwood FE, Zhang X, Colombel JF, Kappelman MD. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology. 2020;159:481-491.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 580] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 69. | Perrotta C, Pellegrino P, Moroni E, De Palma C, Cervia D, Danelli P, Clementi E. Five-aminosalicylic Acid: an update for the reappraisal of an old drug. Gastroenterol Res Pract. 2015;2015:456895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Bleyer WA. The clinical pharmacology of methotrexate: new applications of an old drug. Cancer. 1978;41:36-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 71. | Ibrahim A, Ahmed M, Conway R, Carey JJ. Risk of Infection with Methotrexate Therapy in Inflammatory Diseases: A Systematic Review and Meta-Analysis. J Clin Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 72. | Bedoui Y, Guillot X, Sélambarom J, Guiraud P, Giry C, Jaffar-Bandjee MC, Ralandison S, Gasque P. Methotrexate an Old Drug with New Tricks. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 255] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 73. | Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1343] [Cited by in RCA: 1212] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 74. | Algood HM, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41 Suppl 3:S189-S193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 75. | Kaneko H, Yamada H, Mizuno S, Udagawa T, Kazumi Y, Sekikawa K, Sugawara I. Role of tumor necrosis factor-alpha in Mycobacterium-induced granuloma formation in tumor necrosis factor-alpha-deficient mice. Lab Invest. 1999;79:379-386. [PubMed] |
| 76. | Murdaca G, Spanò F, Contatore M, Guastalla A, Penza E, Magnani O, Puppo F. Infection risk associated with anti-TNF-α agents: a review. Expert Opin Drug Saf. 2015;14:571-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 77. | Chen C, Zhang XR, Ju ZY. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua Shaoshang Zazhi. 2020;36:E005. |
| 78. | Dolinger MT, Person H, Smith R, Jarchin L, Pittman N, Dubinsky MC, Lai J. Pediatric Crohn Disease and Multisystem Inflammatory Syndrome in Children (MIS-C) and COVID-19 Treated With Infliximab. J Pediatr Gastroenterol Nutr. 2020;71:153-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 79. | Bezzio C, Manes G, Bini F, Pellegrini L, Saibeni S. Infliximab for severe ulcerative colitis and subsequent SARS-CoV-2 pneumonia: a stone for two birds. Gut. 2021;70:623-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 80. | Duricova D, Burisch J, Jess T, Gower-Rousseau C, Lakatos PL; ECCO-EpiCom. Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J Crohns Colitis. 2014;8:1351-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 81. | Williams C, Panaccione R, Ghosh S, Rioux K. Optimizing clinical use of mesalazine (5-aminosalicylic acid) in inflammatory bowel disease. Therap Adv Gastroenterol. 2011;4:237-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 82. | Hanauer SB. The expanding role of biologic therapy for IBD. Nat Rev Gastroenterol Hepatol. 2010;7:63-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Abreu MT, Peyrin-Biroulet L. Providing Guidance During a Global Viral Pandemic for the Care of Patients With Inflammatory Bowel Disease. J Crohns Colitis. 2020;14:S767-S768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Centers for Disease Control and Prevention. Discontinuation of Transmission-Based Precautions and Disposition of Patients with COVID-19 in Healthcare Settings. [cited 5 January 2021]. In: Centers for Disease Control and Prevention [Internet]. Available from: http://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html/. |
| 85. | Allez M, Fleshner P, Gearry R, Lakatos PL, Rubin DT. Care of the Patient With IBD Requiring Hospitalisation During the COVID-19 Pandemic. J Crohns Colitis. 2020;14:S774-S779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 86. | Ng SC, Mak JWY, Hitz L, Chowers Y, Bernstein CN, Silverberg MS. COVID-19 Pandemic: Which IBD Patients Need to Be Scoped-Who Gets Scoped Now, Who Can Wait, and how to Resume to Normal. J Crohns Colitis. 2020;14:S791-S797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | De Lima-Karagiannis A, Juillerat P, Sebastian S, Pedersen N, Bar-Gil Shitrit A, van der Woude CJ. Management of Pregnant Inflammatory Bowel Disease Patients During the COVID-19 Pandemic. J Crohns Colitis. 2020;14:S807-S814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 88. | Magro F, Rahier JF, Abreu C, MacMahon E, Hart A, van der Woude CJ, Gordon H, Adamina M, Viget N, Vavricka S, Kucharzik T, Leone S, Siegmund B, Danese S, Peyrin-Biroulet L. Inflammatory Bowel Disease Management During the COVID-19 Outbreak: The Ten Do's and Don'ts from the ECCO-COVID Taskforce. J Crohns Colitis. 2020;14:S798-S806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 89. | Dotan I, Panaccione R, Kaplan GG, O'Morain C, Lindsay JO, Abreu MT. Best Practice Guidance for Adult Infusion Centres during the COVID-19 Pandemic: Report from the COVID-19 International Organization for the Study of IBD [IOIBD] Task Force. J Crohns Colitis. 2020;14:S785-S790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 90. | Siegel CA, Melmed GY, McGovern DP, Rai V, Krammer F, Rubin DT, Abreu MT, Dubinsky MC; International Organization for the Study of Inflammatory Bowel Disease (IOIBD); International Organization for the Study of Inflammatory Bowel Diseases (IOIBD). SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70:635-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 91. | Beddok A, Calugaru V, Minsat M, Dendale R, De Oliveira A, Costa É, Goudjil F, Belshi R, Pierrat N, Rochas C, Gravigny AC, Soisick L, Colella Fleury H, Créhange G. Post-lockdown management of oncological priorities and postponed radiation therapy following the COVID-19 pandemic: Experience of the Institut Curie. Radiother Oncol. 2020;150:12-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 92. | Bilato C, Roncon L, Anselmi M, Valle R, Perrone C, Mecenero A, Zuin M, Themistoclakis S; a nome dell’ANMCO Veneto. [Managing cardiac patients post-COVID-19 pandemic: a proposal by the ANMCO Veneto Region]. G Ital Cardiol (Rome). 2020;21:408-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 93. | Saibeni S, Scucchi L, Dragoni G, Bezzio C, Miranda A, Ribaldone DG, Bertani A, Bossa F, Allocca M, Buda A, Mocci G, Soriano A, Mazzuoli S, Bertani L, Baccini F, Loddo E, Privitera AC, Sartini A, Viscido A, Grossi L, Casini V, Gerardi V, Ascolani M, Ruscio MD, Casella G, Savarino E, Stradella D, Pumpo R, Cortelezzi CC, Daperno M, Ciardo V, Nardone OM, Caprioli F, Vitale G, Cappello M, Comberlato M, Alvisi P, Festa S, Campigotto M, Bodini G, Balestrieri P, Viola A, Pugliese D, Armuzzi A, Fantini MC, Fiorino G; IG-IBD (Italian Group for the study of Inflammatory Bowel Disease). Activities related to inflammatory bowel disease management during and after the coronavirus disease 2019 Lockdown in Italy: How to maintain standards of care. United European Gastroenterol J. 2020;8:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Martin Arranz E, Suarez Ferrer C, García Ramírez L, Rueda García JL, Sánchez-Azofra M, Poza Cordón J, Noci J, Zabana Y, Barreiro-de Acosta M, Martín-Arranz MD. Management of COVID-19 Pandemic in Spanish Inflammatory Bowel Disease Units: Results From a National Survey. Inflamm Bowel Dis. 2020;26:1149-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Bai X, Yang H, Qian J. COVID-19 Outbreak and Inflammatory Bowel Disease Management: A Questionnaire Survey From Realistic Practice. J Crohns Colitis. 2020;14:1494-1495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Allocca M, Fiorino G, Furfaro F, Gilardi D, Radice S, D'Amico F, Zilli A, Danese S. Maintaining the Quality Standards of Care for Inflammatory Bowel Disease Patients During the COVID-19 Pandemic. Clin Gastroenterol Hepatol. 2020;18:1882-1883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 97. | Ashton JJ, Harden A, Beattie RM. Paediatric inflammatory bowel disease: improving early diagnosis. Arch Dis Child. 2018;103:307-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Ricciuto A, Fish JR, Tomalty DE, Carman N, Crowley E, Popalis C, Muise A, Walters TD, Griffiths AM, Church PC. Diagnostic delay in Canadian children with inflammatory bowel disease is more common in Crohn's disease and associated with decreased height. Arch Dis Child. 2018;103:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 99. | Ashton JJ, Gavin J, Beattie RM. Exclusive enteral nutrition in Crohn's disease: Evidence and practicalities. Clin Nutr. 2019;38:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 100. | Fiorino G, Lytras T, Younge L, Fidalgo C, Coenen S, Chaparro M, Allocca M, Arnott I, Bossuyt P, Burisch J, Campmans-Kuijpers M, de Ridder L, Dignass A, Drohan C, Feakins R, Gilardi D, Grosek J, Groß E, Hart A, Jäghult S, Katsanos K, Lönnfors S, Panis Y, Perovic M, Pierik M, Rimola J, Tulchinsky H, Gisbert JP. Quality of Care Standards in Inflammatory Bowel Diseases: a European Crohn's and Colitis Organisation [ECCO] Position Paper. J Crohns Colitis. 2020;14:1037-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 101. | Lewin S, Lees C, Regueiro M, Hart A, Mahadevan U. International Organization for the Study of Inflammatory Bowel Disease: Global Strategies for Telemedicine and Inflammatory Bowel Diseases. J Crohns Colitis. 2020;14:S780-S784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 102. | Gower-Rousseau C, Fumery M, Pariente B; EPIMAD Registry Group. Inflammatory Bowel Disease and the SARS-CoV-2 Pandemic: More Speed, Less Haste. Gastroenterology. 2021;160:473-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |