Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3654
Peer-review started: December 15, 2020
First decision: January 6, 2021
Revised: January 20, 2021
Accepted: February 24, 2021
Article in press: February 24, 2021
Published online: June 28, 2021
Processing time: 189 Days and 21.3 Hours
The procedure for lateral lymph node (LLN) dissection (LLND) is complicated and can result in complications. We developed a technique for laparoscopic LLND based on two fascial spaces to simplify the procedure.
To clarify the anatomical basis of laparoscopic LLND in two fascial spaces and to evaluate its efficacy and safety in treating locally advanced low rectal cancer (LALRC).
Cadaveric dissection was performed on 24 pelvises, and the fascial composition related to LLND was observed and described. Three dimensional-laparoscopic total mesorectal excision with LLND was performed in 20 patients with LALRC, and their clinical data were analyzed.
The cadaver study showed that the fascia propria of the rectum, urogenital fascia, vesicohypogastric fascia and parietal fascia lie side by side in a medial-lateral direction constituting the dissection plane for curative rectal cancer surgery, and the last three fasciae formed two spaces (Latzko's pararectal space and paravesical space) which were the surgical area for LLND. Laparoscopic LLND in two fascial spaces was performed successfully in all 20 patients. The median operating time, blood loss and postoperative hospitalization were 178 (152-243) min, 55 (25-150) mL and 10 (7-20) d, respectively. The median number of harvested LLNs was 8.6 (6-12), and pathologically positive LLN metastasis was confirmed in 7 (35.0%) cases. Postoperative complications included lower limb pain in 1 case and lymph leakage in 1 case.
Our preliminary surgical experience suggests that laparoscopic LLND based on fascial spaces is a feasible, effective and safe procedure for treating LALRC.
Core Tip: The procedure for lateral lymph node dissection (LLND) is complicated, with a high incidence of complications. We developed a technique of laparoscopic LLND based on two fascial spaces to simplify the procedure. By cadaveric dissection, we found that urogenital fascia, vesicohypogastric fascia and parietal fascia lie side by side and formed two spaces (Latzko's pararectal space and paravesical space) which were the surgical area for LLND. 3D-Laparoscopic LLND in two fascial spaces was performed successfully in 20 patients with locally advanced low rectal cancer, and the results showed that it was a feasible, effective and safe procedure.
- Citation: Jiang HH, Liu HL, Li AJ, Wang WC, Lv L, Peng J, Pan ZH, Chang Y, Lin MB. Laparoscopic lateral lymph node dissection in two fascial spaces for locally advanced lower rectal cancer. World J Gastroenterol 2021; 27(24): 3654-3667
- URL: https://www.wjgnet.com/1007-9327/full/v27/i24/3654.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i24.3654
For rectal cancers below the peritoneal reflection, the incidence of lateral lymph node (LLN) metastasis is reported to be 15%-20%[1-3]. However, routine LLN dissection (LLND) for locally advanced low rectal cancer (LALRC) is still a controversial issue. In addition to the controversy on the indication for LLND, another argument against LLND is that the procedure is complicated, and consequently results in a high incidence of complications[4]. Earlier studies showed that LLND was associated with 30%-70% of urinary disorders and 80%-100% of sexual dysfunction at the initial stage[5]. Despite the introduction of pelvic autonomic nerve preservation, a Japanese clinical trial showed that urinary dysfunction and postoperative complications occurred in 59% and 5% of patients, respectively[6]. In this study, we do not discuss the indications for LLND but focus on how to improve the accuracy and safety of the procedure for laparoscopic LLND based on the fascial space approach.
Total mesorectal excision (TME) is the gold standard surgery for rectal cancer and has stimulated a tremendous upsurge of interest in “fascia anatomy” in colorectal surgery[7-10]. The theory of “fascia anatomy” has changed the traditional view of surgical anatomy focusing on organs and blood vessels, and holds that surgical dissection should be performed along a pre-existing embryological plane (space) formed by fasciae. However, the detailed anatomy of fasciae and spaces related to LLND is not clear, and therefore standard surgical procedures have not been established. In the last few years, there have been numerous anatomical research studies on pelvic fascia[11,12]. Based on the new understanding of fascial composition in the pelvis, we have developed a laparoscopic LLND in two fascial spaces formed by three layers of fasciae to treat LALRC. We describe the anatomical basis, surgical procedure and summarize our initial clinical results.
Twenty-three formalin-preserved and 1 fresh cadavers (12 males and 12 females) were dissected at the Anatomy Department of Shanghai Jiaotong University School of Medicine. Eighteen specimens (9 males and 9 females) were separated into two hemi-pelvises through the mid-sagittal plane. Detailed dissections were performed macroscopically with the assistance of binocular loupes (Heine, Germany, HR 2.5 mm × 340 mm). Cadavers with malformations, abdominal or pelvic adhesions, or a history of abdominal or pelvic surgery were excluded.
From July 2018 to October 2020, a total of 20 patients with LALRC underwent 3D-laparoscopic TME with LLND at Yangpu Hospital affiliated to Tongji University School of Medicine. There were 16 males and 4 females, with a median age of 58.2 years (range: 48-68 years). All patients were pathologically diagnosed by endoscopic biopsy and evaluated by computed tomography (CT) and magnetic resonance imaging (MRI) before surgery. All tumors were located below the peritoneal reflection, and the median distance between the inferior margin of the tumor and anal verge was 4.7 cm (range: 2.5-6.8 cm). The preoperative T stage was T3 in 13 patients and T4 in 7 patients. Preoperative MRI showed uni-LLN enlargement (the largest short diameter > 7 mm) in all cases.
This was a descriptive study, and was approved by the local Ethics Committee (LL-2020-KXJS-004) and performed in accordance with the Declaration of Helsinki[13].
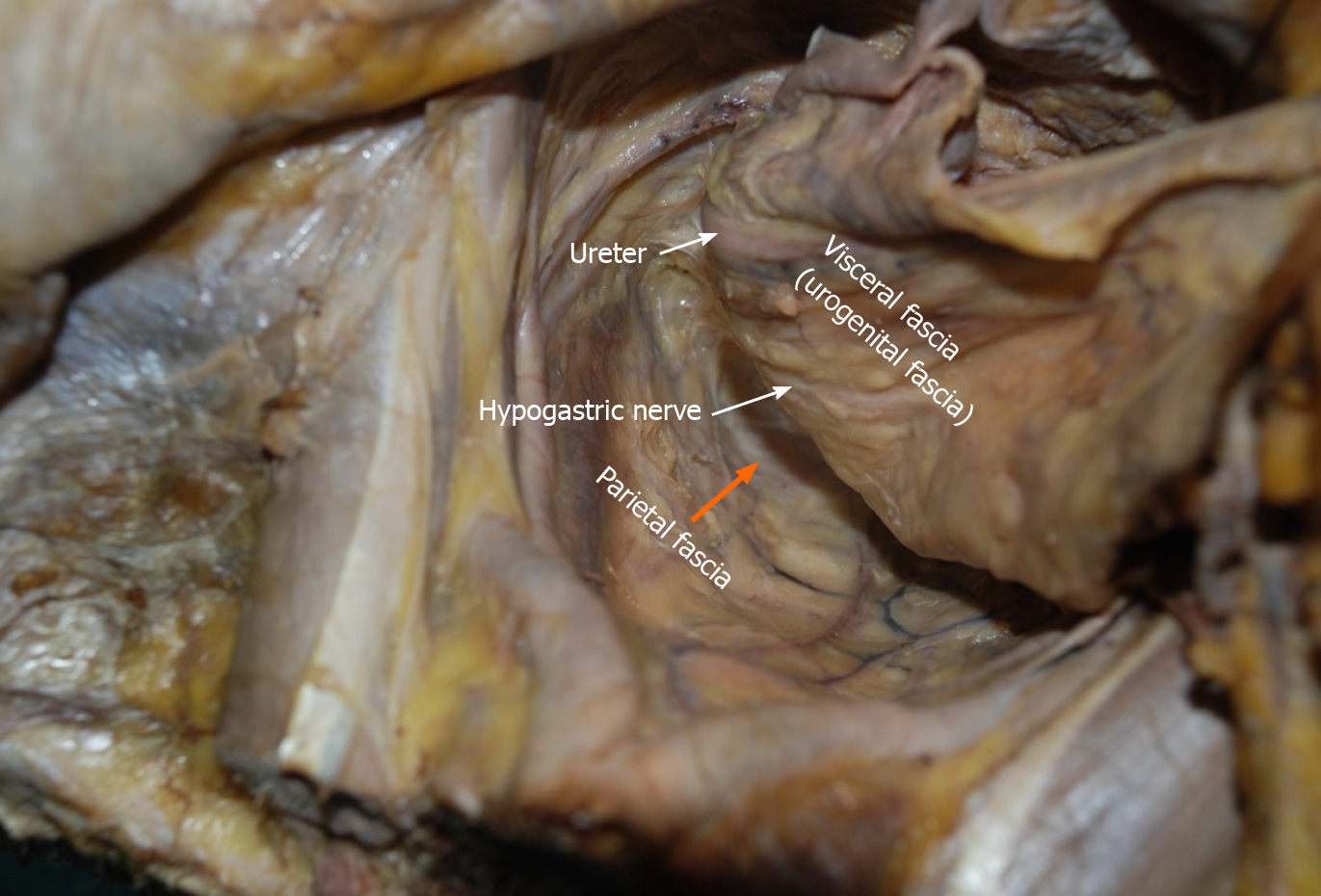
Cadaver dissection: The details of the dissection procedure were described in our previous studies[11,12]. The main observation points were as follows: (1) The distribution of visceral fascia in the pelvic cavity and its association with the urogenital and vesicohypogastric fasciae; (2) The association of the hypogastric nerve and ureter with the urogenital fascia; (3) The association of the urogenital and vesicohypogastric fasciae with the cardinal ligament; (4) The association of Denonvilliers’ fascia with the pelvic plexus; (5) The association of internal iliac blood vessels and their branches with the vesicohypogastric fascia; and (6) The distribution and variation of the branches of the internal iliac artery.
Surgical technique: TME: All operations were performed by the same experienced surgical team using the Karl Storz Image1 S™ 3D system. The patient was placed in a modified lithotomy position. Laparoscopic TME was performed using a five-trocar method through the traditional medial-to-lateral approach, and a detailed description of this surgical procedure was given previously[14].
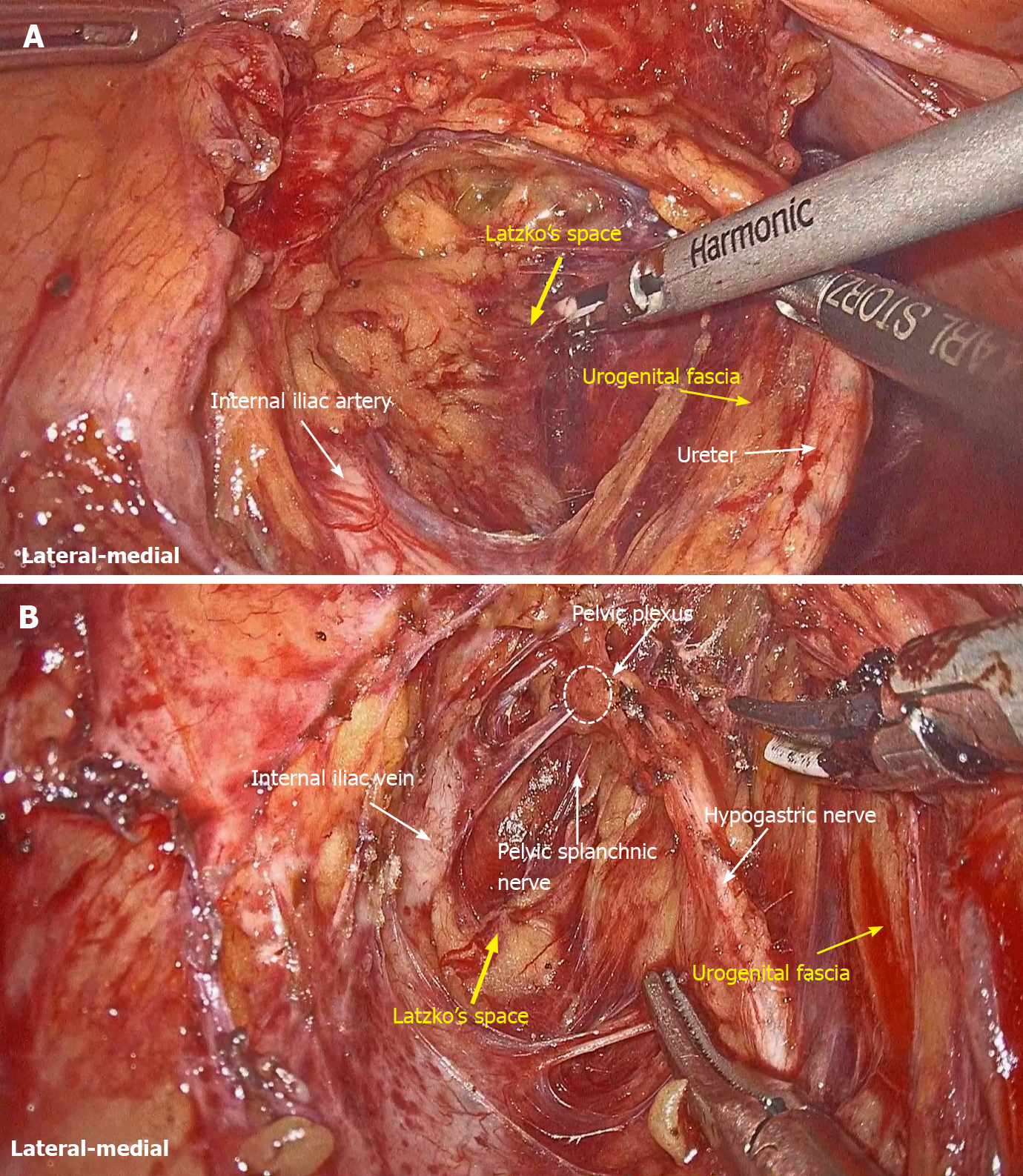
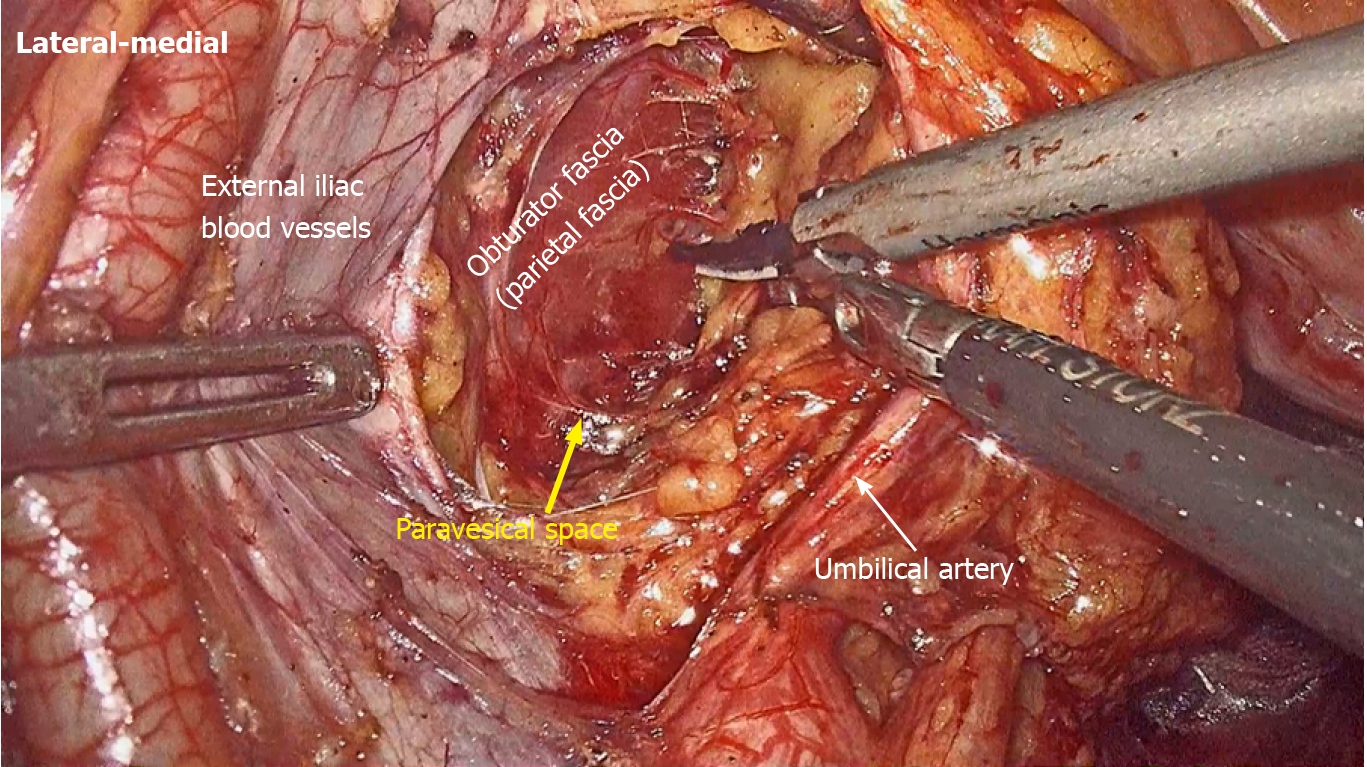
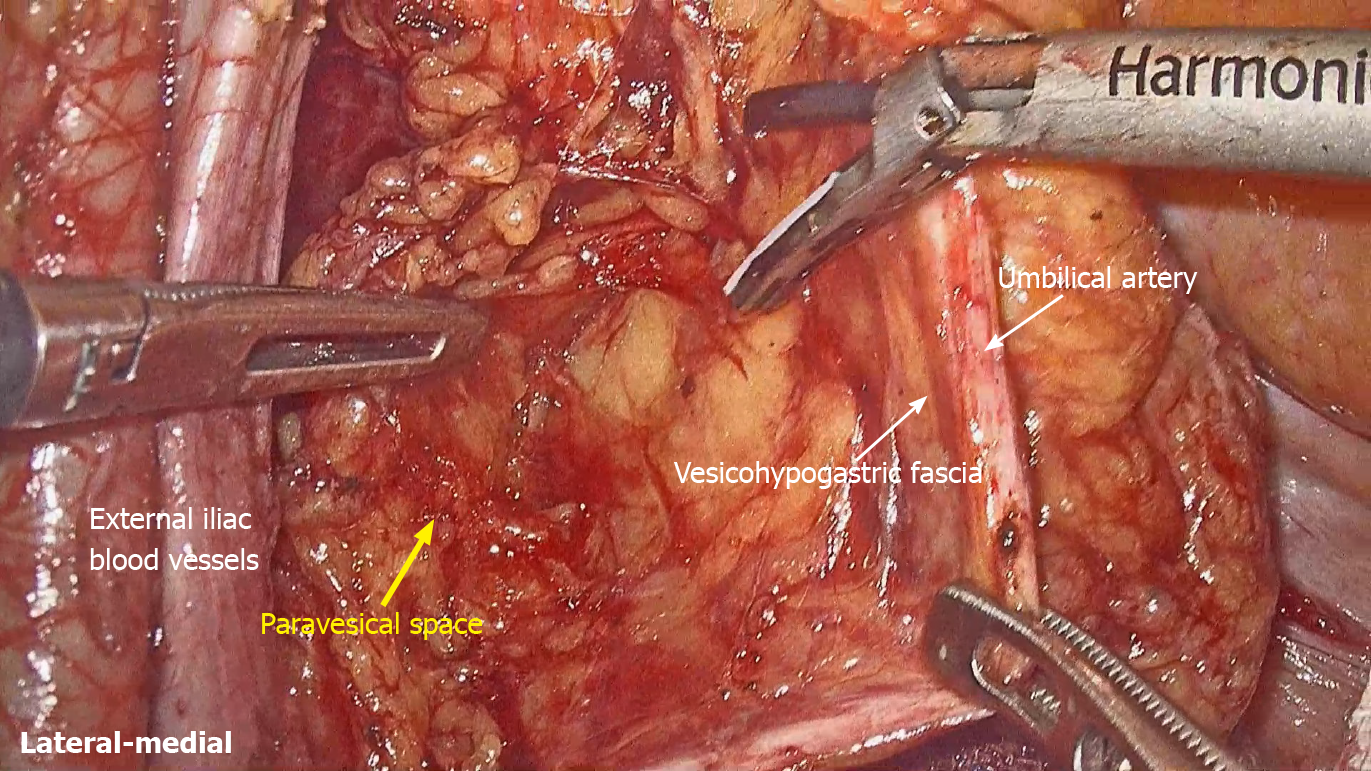
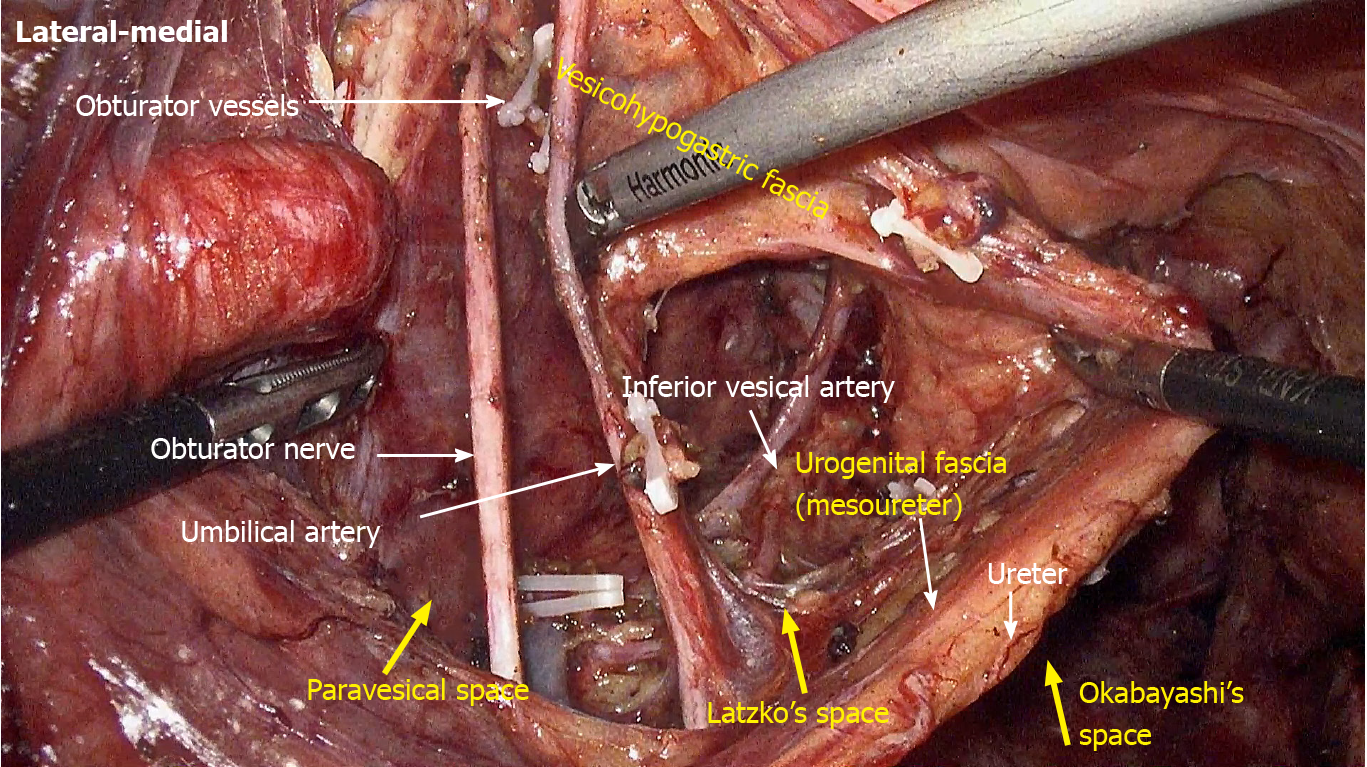
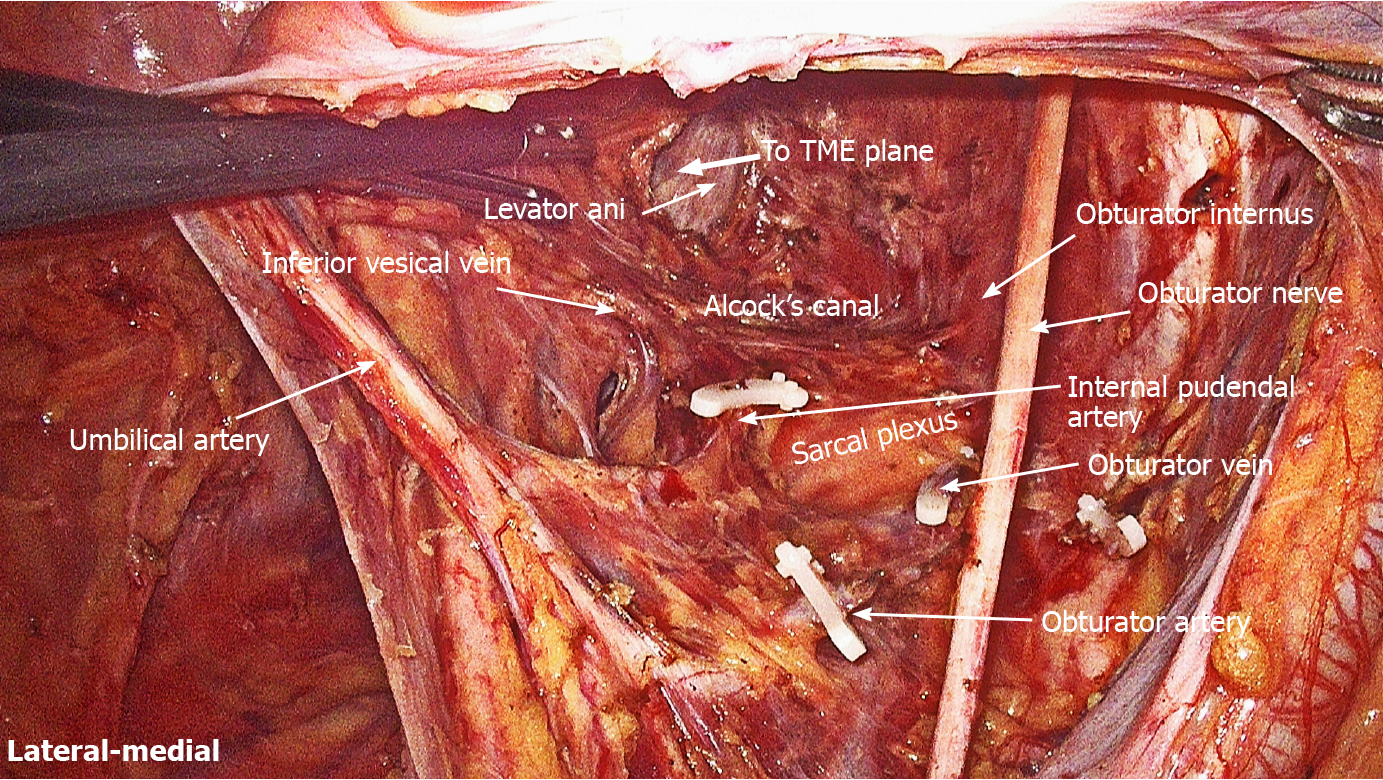
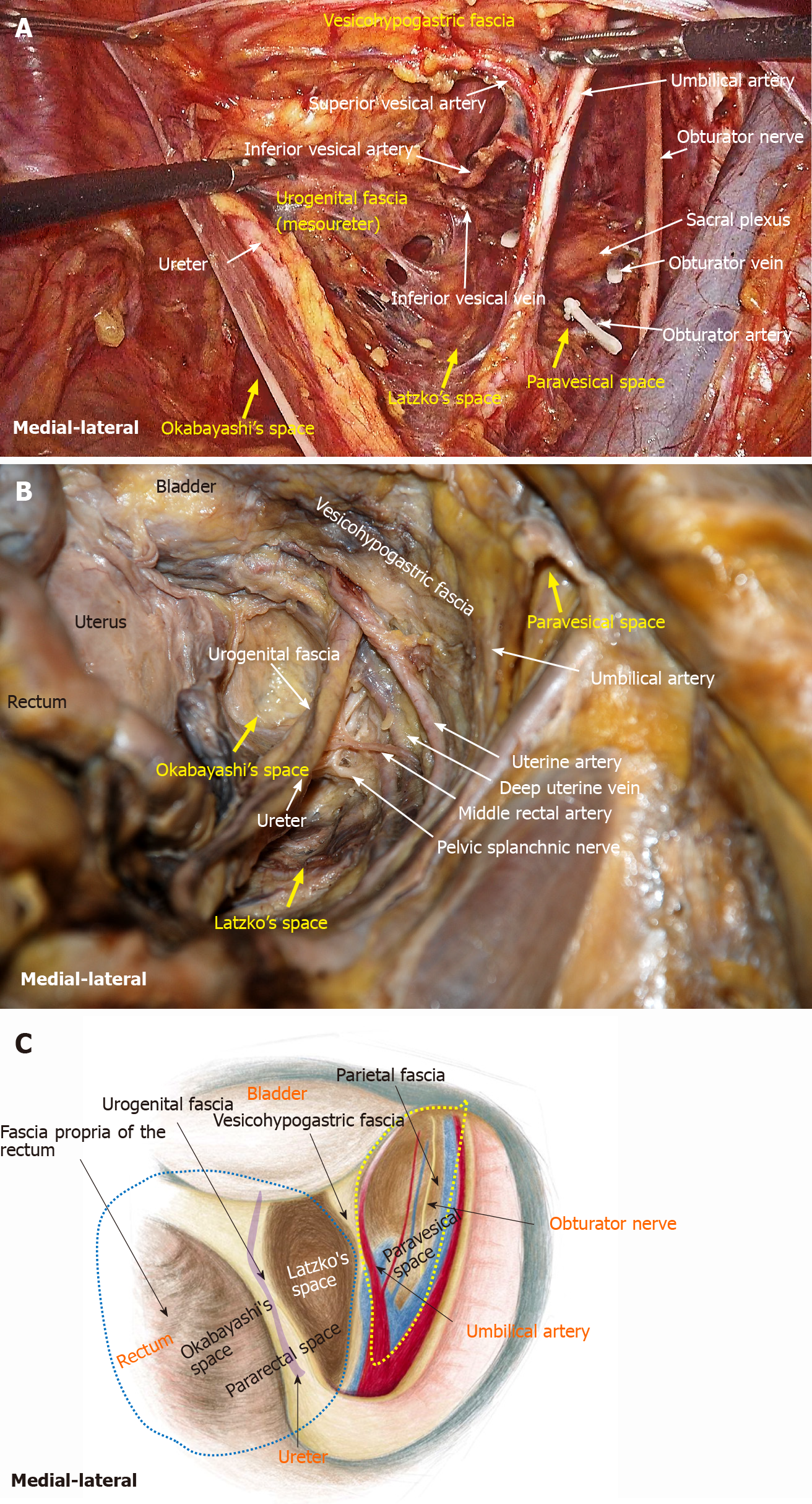
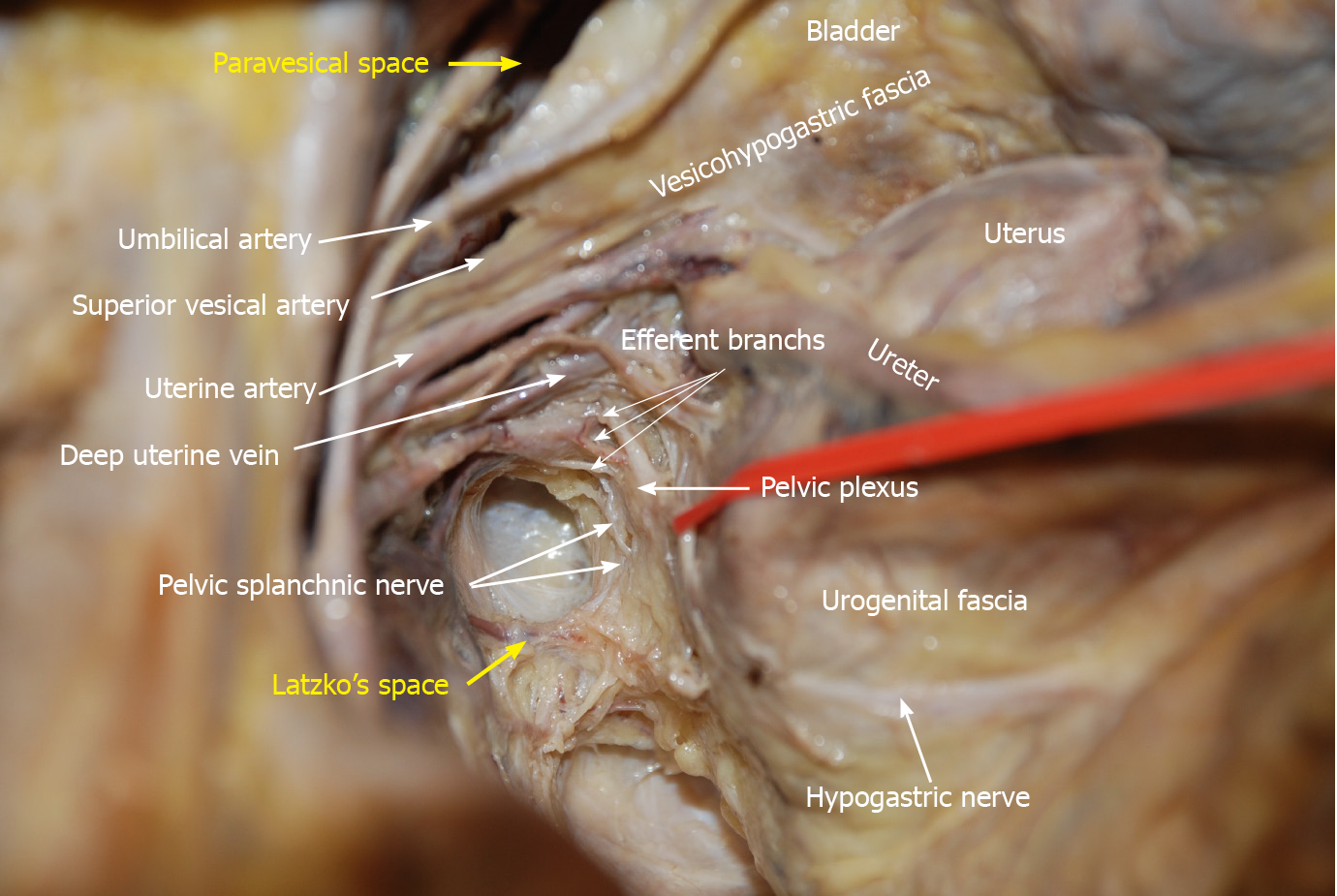
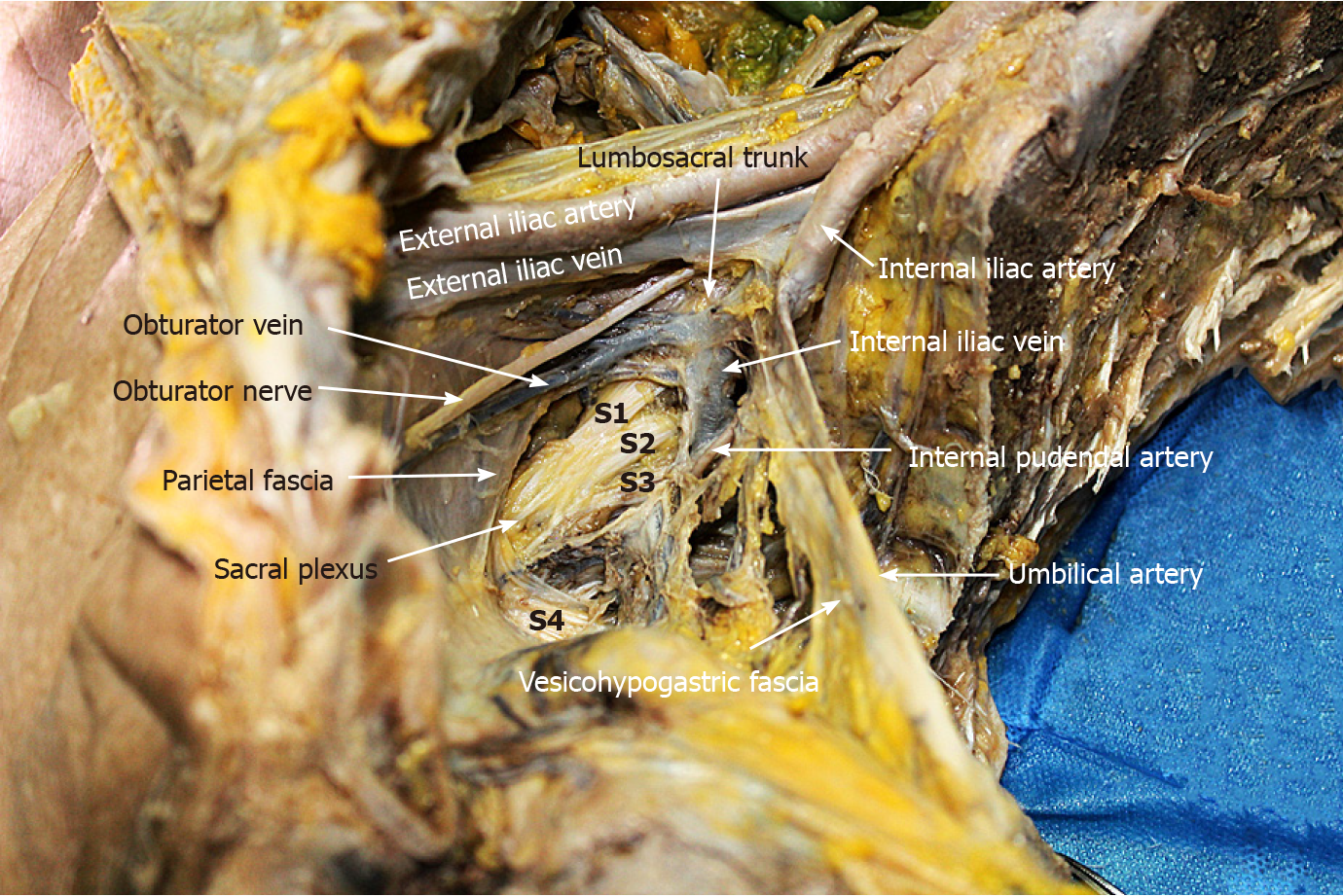
LLND: (1) Separation of the urogenital fascia to expose the medial boundary of Latzko's pararectal space (Figure 1). The peritoneum was incised lateral to the ureter, and the dissection continued to the vas deferens (male) or the round ligament of the uterus (female). The ureter was pulled medially to expose the urogenital fascia containing the hypogastric nerve. There is an embryonic plane between the urogenital fascia and internal iliac lymph nodes. Dissection was continued along the surface of the urogenital fascia, and was stopped at the level of the pelvic plexus to avoid injuring the neurovascular bundle; (2) Separation of the obturator fascia (parietal fascia) to expose the lateral boundary of the paravesical space (Figure 2). The external iliac lymph nodes were removed along the external iliac artery, and the dissection was continued along the medial surface of the iliopsoas muscle deep into the obturator fascia. An avascular plane exists between the obturator lymph nodes and the obturator fascia. The obturator nerve and vessels were identified at the obturator. A further dissection along the obturator fascia was performed to expose the parietal fascia covering the levator ani muscle (superior fascia of the pelvic diaphragm), and then the dissection plane was connected to the TME surgical plane; (3) Separation of the vesicohypogastric fascia to expose the medial boundary of the paravesical space (Figure 3). The umbilical artery was identified, and the dissection was continued along the lateral border of the umbilical artery to expose the vesicohypogastric fascia. The vesicohypogastric fascia covers the lateral side of the branches of the internal iliac artery. An avascular plane also exists between the obturator lymph nodes and the vesicohypogastric fascia. The superior border of the distal part of the vesicohypogastric fascia fuses with the obturator fascia where the obturator was easily identified, and the obturator nerve and vessels were exposed. The inferior border of the vesicohypogastric fascia reaches the superior fascia of the pelvic diaphragm; (4) Dissection of the obturator lymph nodes in the paravesical space between the vesicohypogastric and parietal fasciae (Figure 4). Dissection of the paravesical space included three steps. At the lateral side, the obturator nerve was skeletonized, and the distal obturator vessels were cut and ligated at the obturator canal. At the medial side, the dissection began with removal of the common iliac lymph nodes, followed by dissection along the bifurcation of the internal and external blood vessels and the proximal vesicohypogastric fascia. The proximal obturator vessels were ligated and cut at their origins. At the dorsal side, after exposing the lumbosacral trunk, the dissection started from the bottom of the obturator fossa, and then extended ventrally, to expose Alcock’s canal (pudendal canal) and the superior fascia of the pelvic diaphragm. The dissection also allowed the paravesical space to connect to the surgical plane of TME; and (5) Dissection of the internal iliac lymph nodes in Latzko's pararectal space between the urogenital and vesicohypogastric fasciae (Figure 5). The medial side of the vesicohypogastric fascia was dissected to expose the lateral boundary of Latzko's pararectal space. After identifying the trunks of the internal iliac vessels, the dissection was performed along the lateral surface of the vesicohypogastric fascia, in order to sever the branches of the internal iliac vessels at their origins. It should be noted that there was no need to identify each branch. Dissection around the umbilical artery, superior vesical artery, internal pudendal artery, and inferior vesical artery (male) or uterine artery (female) was required for internal iliac lymph nodes cleaning.
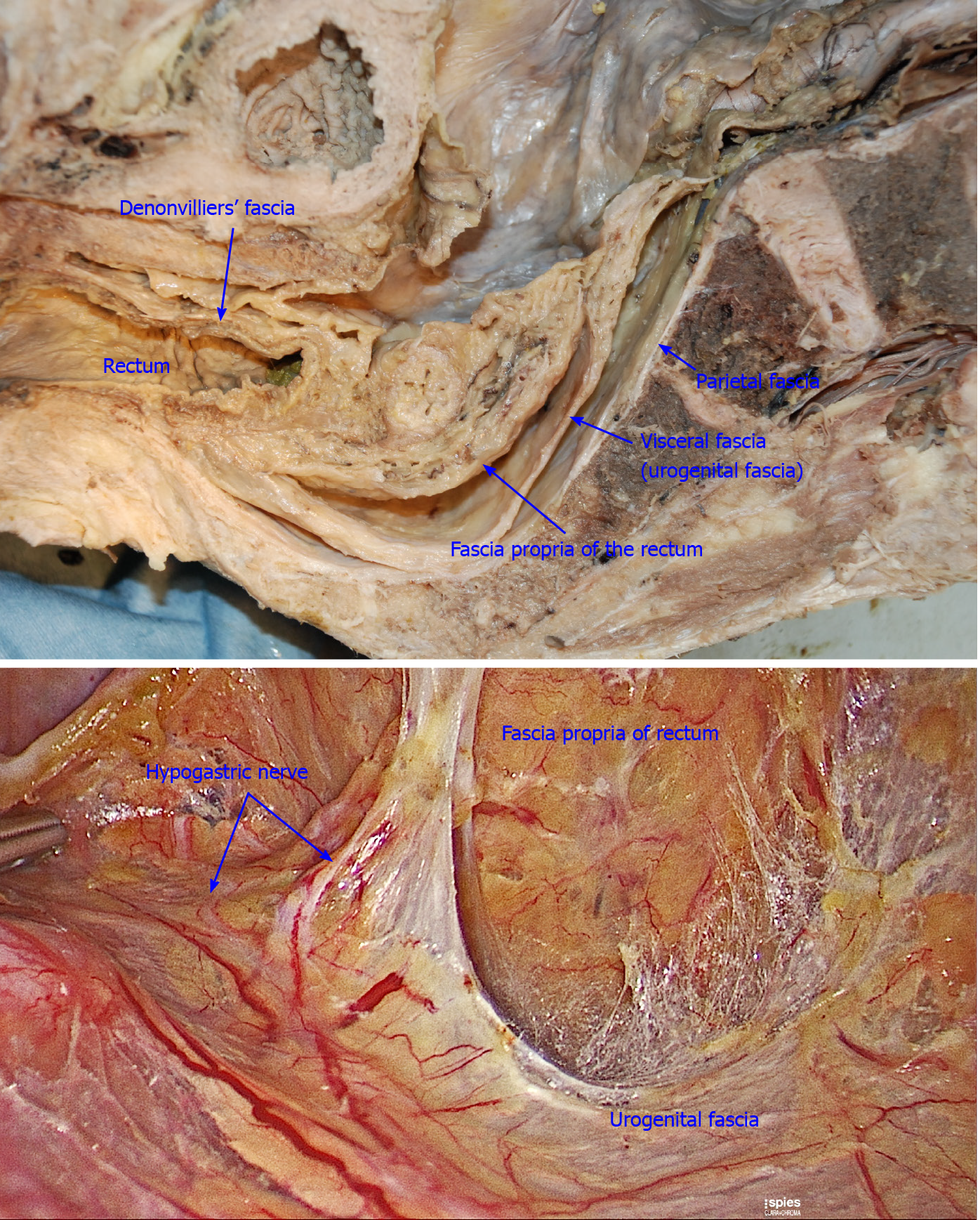
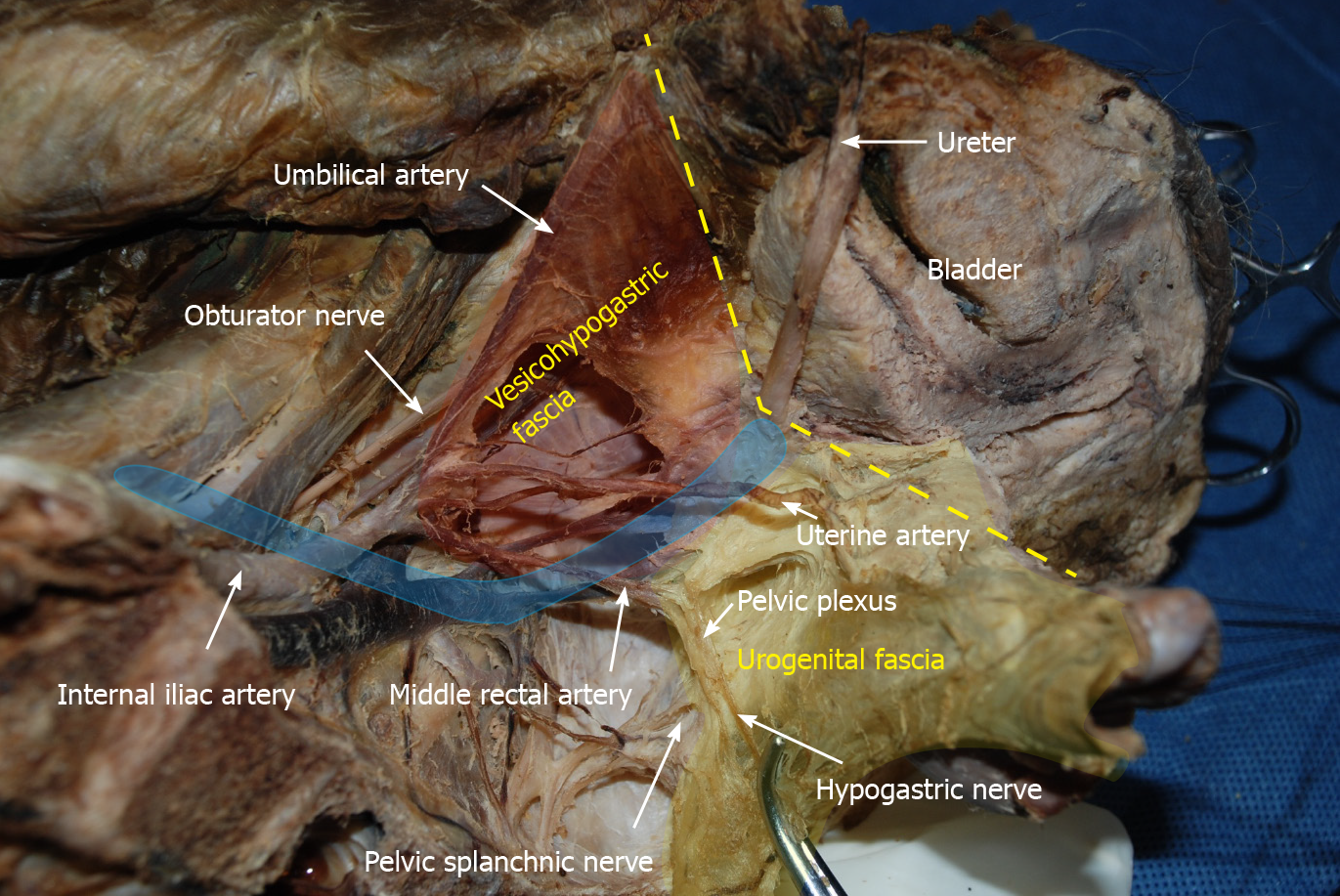
Fasciae related to LLND: (1) Fascia propria of the rectum (Figure 6). The fascia propria of the rectum is a thin fascia layer surrounding the mesorectum. It extends downward and shows tight contact with the visceral fascia below the level of Waldeyer's fascia; (2) Urogenital fascia (Figure 7). The urogenital fascia extends posterolateral to the rectum, providing a hammock-like support to the rectum. It can be recognized as the dense connective tissue which contains the hypogastric nerve and ureter. The urogenital fascia connects with Denonvilliers’ fascia anterior to the rectum. The urogenital fascia is actually the visceral fascia described by Heald. The mesoureter is defined as a sheet of connective tissue surrounding the ureter. Actually, it is part of the urogenital fascia suspended below the ureter (Figure 4); and (3) Vesicohypogastric fascia (Figure 8). The vesicohypogastric fascia is a triangle-shaped structure, and its boundaries are formed by the umbilical artery, the tendinous arch of the pelvic fascia and the lateral surface of the bladder. The urogenital and vesicohypogastric fasciae blend with each other in the tendinous arch of the pelvic fascia, and present in a V-shape relationship.
Spaces related to LLND: (1) Pararectal space (Figure 9). The pararectal space is a potential space between the rectum and internal iliac vessels, and is divided into Latzko's and Okabayashi's pararectal spaces by the mesoureter. In the view of “fascia anatomy”, Latzko's pararectal space is the space between the urogenital fascia and vesicohypogastric fascia, and Okabayashi's pararectal space is the space between the fascia propria of the rectum and the urogenital fascia; and (2) Paravesical space (Figure 9). The paravesical space is a potential space between the umbilical artery and the external iliac vein. From the perspective of “fascia anatomy”, the paravesical space is the space between the vesicohypogastric fascia and parietal fascia.
Vascular anatomy related to LLND: As shown in Figure 10, the anatomical relati
Nerve anatomy related to LLND: As shown in Figure 10, the hypogastric nerve is found within the urogenital fascia, which passes from the posterior to the anterior-lateral aspect of the rectum and fuses with Denonvilliers’ fascia in a fan shape. The pelvic plexus is located exactly external to the junction of the urogenital and Denonvilliers’ fascia. The pelvic plexus is made up of the hypogastric nerve and the pelvic splanchnic nerve, and give off branches to the bladder and the uterus. All these nerves are located in the same connective tissue plane and presents as a T-shape “pelvic nerve plate”.
3D-Laparoscopic LLND was performed successfully in all 20 patients without conversion to laparotomy. Of these patients, 12 underwent low anterior resection, 7 cases underwent intersphincteric resection, and 1 case underwent abdominoperineal resection. The median operating time for LLND was 178 (range: 152-243) min, with a median blood loss of 55 (range: 25-150) mL. The median length of postoperative hospitalization was 10 (range: 7-20) d.
All patients received uni-LLND, and the postoperative pathology showed that LLN metastasis was present in 7 (35.0%) cases. Of these, 6 cases had both mesenteric and internal iliac lymph node metastases, and 1 case had only internal iliac lymph node metastasis. The median number of harvested LLNs was 8.6 (range: 6-12).
Postoperative complications included lymph leakage in 1 case and lower limb pain in 1 case (grade I based on Clavien-Dindo classification). No other postoperative complication was observed. The former was cured after fasting for 4 d, and the latter resolved spontaneously 1 mo after hospital discharge.
With the application of TME in clinical practice, the concept of performing surgery along the embryological space formed by fasciae has been established in colorectal surgery[15]. However, there is still a lack of data to illustrate fascial composition related to LLND; therefore, it is impossible to establish standard LLND procedures based on fascial anatomy. This is the first study to outline the terminology of fasciae and spaces used in colorectal surgery and gynecology to clarify the surgical plane of LLND. We have put forward the concept of two-space dissection for LLND, and located important blood vessels and nerves using landmark fasciae in each space. By applying these anatomical findings in practice, the initial clinical results showed that the fascial space approach can significantly reduce intraoperative blood loss and maintain a clear surgical field. Moreover, this approach can easily expose and protect the ureter, the hypogastric nerve, pelvic plexus, lumbosacral trunk and sacral plexus, which significantly decreased the incidence of postoperative urogenital dysfunction.
There are many diverse descriptions on the fascia composition related to rectal cancer surgery[15]. As described in the literature, the fascia propria of the rectum is part of the visceral fascia, and the visceral fascia together with Denonvilliers’ fascia constitute the morphology of the “mesorectum”[16]. This has become the knowledge on classical pelvic anatomy and has been widely accepted by colorectal surgeons. However, we found that the fascia propria of the rectum and visceral fascia are two independent layers of fascia. The fascia propria of the rectum presents as a barely visible translucent layer which is the innermost layer of fascia surrounding the rectum and perirectal fat (Figure 6). The visceral fascia was the densest portion of pelvic fascia, which stretched “like a hammock” posterolateral to the rectum and fused anteriorly with Denonvilliers’ fascia (Figure 7). In fact, “visceral fascia” is a general term used to describe the fascia ensheathing organs[17], and therefore, it is inappropriately used to describe specific fascia. Actually, the visceral fascia described by Heald et al[7] in TME is just the urogenital fascia. The urogenital fascia originates from the fusion of the anterior renal fascia and posterior renal fascia below the inferior pole of the kidney. Muntean et al[18] described the hypogastric nerve and the ureter embedded in this fascia, hence the name urogenital fascia. However, Kinugasa demonstrated a protective fascia overlying the hypogastric nerves and proposed that “prehypogastric nerve fascia” seemed more appropriate[19]. In some patients with an enlarged hypogastric nerve, the hypogastric nerve was identified posterior to the urogenital fascia in this study (Figure 1B). The urogenital fascia carries the ureter and the hypogastric nerve to the bladder and continues with the vesicohypogastric fascia, and the two fasciae blend with each other anterior-laterally in the tendinous arch of the pelvic fascia (Figure 8). The obturator fascia is one of three parts of the parietal fascia[20]. In summary, the fascia propria of the rectum, urogenital fascia, vesicohypogastric fascia and parietal fascia lie side by side in a medial-lateral direction, and constitute the dissection plane for curative rectal cancer surgery (Figure 9).
In gynecological anatomy, the paravesical space is a potential space between the umbilical artery and the external iliac vein[21]. Given that the umbilical artery is the landmark of the vesicohypogastric fascia and the obturator fascia (parietal fascia) follow the same plane as the external iliac vein, the paravesical space can be considered to be located between the vesicohypogastric fascia and the parietal fascia (Figure 9). Gynecological anatomy also defines the pararectal space as a loose connective tissue space between the rectum and the internal iliac vessels, and is further divided into the medial Okabayashi's space and lateral Latzko's space by the ureter[21]. According to our findings, the fascia propria of the rectum is the innermost layer covering the rectum, the mesoureter is a part of the urogenital fascia, and the umbilical artery constituting the superior border of the vesicohypogastric fascia is the first branch of the internal iliac artery. Therefore, in the view of fascia anatomy, Okabayashi's space can be described as the space between the fascia propria of the rectum and the urogenital fascia, and Latzko's space lies between the urogenital fascia and the vesicohypogastric fascia (Figure 9). Okabayashi's space corresponds to the resection area of TME, while Latzko's pararectal space and the paravesical space constitute the surgical field for LLND. Moreover, the vesicohypogastric fascia divides the LLNs into obturator lymph nodes and internal iliac lymph nodes.
The advantage of the fascial space approach lies in its ability to accurately locate important anatomical structures, for example, the hypogastric nerve is located in Latzko's space, while the lumbosacral trunk and sacral plexus are located in the paravesical space (Figures 1B and 5). Dissection of Latzko's space mainly involves the hypogastric nerve and inferior vesical artery. As the hypogastric nerve runs in the urogenital fascia, the urogenital fascia is recommended to be separated first and pulled medially to protect the hypogastric nerve. Separation of the urogenital fascia is always stopped at the level of the pelvic plexus to avoid damage to the neurovascular bundle. In fact, the hypogastric nerve and the pelvic plexus are easily exposed and protected using the fascial anatomy approach. Therefore, we consider that possible nerve damage depends more on the surgical procedures for TME rather than those for LLND. In TME, if we follow the traditional view to dissect in the “Holy plane” between the visceral fascia (urogenital fascia) and parietal fascia, the hypogastric nerve will undoubtedly be impaired (Figure 7). According to our viewpoint, the appropriate plane for TME is between the fascia propria of the rectum and the visceral fascia (urogenital fascia) (Figure 6), which is consistent with the opinions of Kinugasa et al[19]. We also showed that the mesoureter is a part of the urogenital fascia (Figure 9B); thus, care should be taken to preserve the integrity of the mesoureter to avoid damage to the hypogastric nerve, and the traditional procedure such as isolating the ureter and picking it up by a rubber band should be abandoned.
The inferior vesical artery is a common site for internal iliac lymph node metastasis[22]. It is the last branch of the internal iliac artery, and located just above the infrapiriformis foramen. Therefore, the inferior vesical artery can be considered the distal end of the vascular dissection in LLND. It should also be noted that the branches of the internal iliac artery are varied in their number, origin, location and course. For instance, our previous study showed that the middle rectal artery was only observed in 29.2% (7/24) specimens, and its diameter was mostly less than 1.5 mm[12]. As a result, we suggest that it is unnecessary to expose each branch of the internal iliac vessels in LLND. The blood vessels requiring dissection include the umbilical artery, superior vesical artery, internal pudendal artery, and inferior vesical artery (male) or uterine artery (female). Many classic anatomical works believe that the inferior vesical artery does not exist in females. In “Gray’s anatomy”, the inferior vesical artery is described as being replaced by the ovarian artery or uterine artery[23]. However, a cadaver study by de Treigny et al[24] indicated that the inferior vesical artery does exist in females, and originates mostly from the common trunk with the umbilical or uterine arteries. Whichever is the case, it is clear that it is difficult to identify the inferior vesical artery in females during LLND.
Dissection of the paravesical space can be completed from three directions. The lateral dissection begins at the external iliac lymph nodes and extends downward to the obturator fascia (parietal fascia). The medial dissection is performed along the vesicohypogastric fascia. The vesical veins change direction sagittally and are distributed in the distal part of the vesicohypogastric fascia. Therefore, special attention should be paid to avoid damage to the vesical veins when the distal end of the obturator vessels (at the obturator) is isolated and ligated. The proximal dissection begins at the bifurcation of the internal and external iliac vessels, followed by exposing the lumbosacral trunk. After dissecting the bottom of the obturator fossa, Alcock’s canal and the superior fascia of the pelvic diaphragm are exposed, forming a connection between the paravesical space and the TME surgical plane. Although the sacral plexus is formed by the lumbosacral trunk and other rami of S1-S4, they have different anatomical locations. The sacral plexus is located between the obturator vein and internal pudendal artery (Figure 11). The sacral plexus is usually covered by the parietal fascia, which should be preserved to avoid postoperative limb pain. This might be the reason why one patient in this study developed limb pain after surgery. In addition, the lymphatic chain connects with the inguinal lymph nodes and the obturator nodes at the distal external iliac vein; therefore, the distal end should be ligated to avoid lymph leakage. In this study, one patient suffered postoperative lymph leakage, possibly because the lymphatic vessels were not securely ligated when dissecting the superior inguinal lymph node (stated by Fujii et al[25]) located in the angle between the external iliac vein and pubis bone.
In conclusion, laparoscopic LLND in two fascial spaces has several significant advantages, including easy identification of the surgical plane and clear location of nerves and blood vessels. Our preliminary surgical experience has shown that it is a feasible surgical approach for treating LALRC, which not only improves surgical safety but also ensures radical resection of the tumor. However, further studies are warranted to validate these results and optimize the surgical procedure.
Lateral lymph node (LLN) metastasis is a major cause for local recurrence in rectal cancer. In Japan, LLN dissection (LLND) is the standard treatment for locally advanced low rectal cancer (LALRC). However, the procedure is complicated with significant morbidity. In recent years, with the rise of “fascia anatomy”, more and more surgeons began to explore LLND based on the fascial space approach.
LLND is a challenging procedure due to its technical difficulty and higher incidence of complications. The development of “fascia anatomy” provides a new sight for improving the accuracy and safety of laparoscopic LLND. However, the detailed anatomy is not clear and a standard surgical procedure has not yet been established.
We developed a technique of laparoscopic LLND in two fascial spaces formed by three layers of fasciae. This study aimed to describe the surgical procedure on an anatomical basis and to summarizes our preliminary surgical experiences in the treatment of LALRC.
Detailed pelvic dissections were performed in 24 cadavers, and the fasciae and spaces related to LLND were observed and described. 20 patients with LALRC received 3D-laparoscopic total mesorectal excision with LLND at our hospital from July 2018 to October 2020, and their surgical videos and clinical data were analyzed.
The urogenital fascia lies posterolateral to the rectum, and the hypogastric nerve and ureter are observed to be enveloped in it; vesicohypogastric fascia shows a triangle shape formed by the umbilical artery, the tendinous arch of the pelvic fascia and the lateral border of the bladder. In all 24 cadavers, urogenital fascia, vesicohypogastric fascia and obturator fascia (parietal fascia) were located lateral to the rectum in a medial-to-lateral direction and form the Okabayashi's pararectal space and paravesical space, respectively, which were the surgical area for LLND. Laparoscopic LLND was performed successfully in all 20 LALRC patients with a median postoperative hospitalization of 10 (7-20) d. The median operating time was 178 (152-243) min, with a median blood loss of 55 (25-150) mL. The median number of harvested LLNs was 8.6 (6-12), and 7 patients (35.0 %) had LLN metastasis. Postoperative complications included lymph leakage and lower limb pain in 1 case, respectively.
This study indicated that urogenital fascia, vesicohypogastric fascia and parietal fascia lie side by side in the pelvis and formed two spaces (Latzko's pararectal space and paravesical space), which were the surgical area for LLND. Performing LLND in two fascial spaces is a feasible surgical approach, which improves surgical safety while ensuring radical tumor resection.
The present study preliminarily explored the clinical significance of laparoscopic LLND in two fascial spaces for treating LALRC. However, large studies with long-term follow-up and more detailed clinical data are needed to confirm these findings.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hendi M, Kinoshita T, Masubuchi S S-Editor: Zhang H L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Akiyoshi T, Watanabe T, Miyata S, Kotake K, Muto T, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg. 2012;255:1129-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | Ueno H, Mochizuki H, Hashiguchi Y, Ishiguro M, Miyoshi M, Kajiwara Y, Sato T, Shimazaki H, Hase K. Potential prognostic benefit of lateral pelvic node dissection for rectal cancer located below the peritoneal reflection. Ann Surg. 2007;245:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Takahashi T, Ueno M, Azekura K, Ohta H. Lateral node dissection and total mesorectal excision for rectal cancer. Dis Colon Rectum. 2000;43:S59-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 188] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Nakamura T, Watanabe M. Lateral lymph node dissection for lower rectal cancer. World J Surg. 2013;37:1808-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Hojo K, Koyama Y. The effectiveness of wide anatomical resection and radical lymphadenectomy for patients with rectal cancer. Jpn J Surg. 1982;12:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Ito M, Kobayashi A, Fujita S, Mizusawa J, Kanemitsu Y, Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, Yamaguchi T, Akasu T, Moriya Y; Colorectal Cancer Study Group of Japan Clinical Oncology Group. Urinary dysfunction after rectal cancer surgery: Results from a randomized trial comparing mesorectal excision with and without lateral lymph node dissection for clinical stage II or III lower rectal cancer (Japan Clinical Oncology Group Study, JCOG0212). Eur J Surg Oncol. 2018;44:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med. 1988;81:503-508. [PubMed] |
| 8. | Bissett IP, Hill GL. Extrafascial excision of the rectum for cancer: a technique for the avoidance of the complications of rectal mobilization. Semin Surg Oncol. 2000;18:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Shen J, Dong X, Liu Z, Wang G, Yang J, Zhou F, Lu M, Ma X, Li Y, Tang C, Luo X, Zhao Q, Zhang J. Modularized laparoscopic regional en bloc mesogastrium excision (rEME) based on membrane anatomy for distal gastric cancer. Surg Endosc. 2018;32:4698-4705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Coffey JC, Dillon M, Sehgal R, Dockery P, Quondamatteo F, Walsh D, Walsh L. Mesenteric-Based Surgery Exploits Gastrointestinal, Peritoneal, Mesenteric and Fascial Continuity from Duodenojejunal Flexure to the Anorectal Junction--A Review. Dig Surg. 2015;32:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Lin M, Chen W, Huang L, Ni J, Ding W, Yin L. The anatomic basis of total mesorectal excision. Am J Surg. 2011;201:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Lin M, Chen W, Huang L, Ni J, Yin L. The anatomy of lateral ligament of the rectum and its role in total mesorectal excision. World J Surg. 2010;34:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14-18. [PubMed] |
| 14. | Han Y, He YG, Zhang HB, Lv KZ, Zhang YJ, Lin MB, Yin L. Total laparoscopic sigmoid and rectal surgery in combination with transanal endoscopic microsurgery: a preliminary evaluation in China. Surg Endosc. 2013;27:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Mike M, Kano N. Laparoscopic surgery for colon cancer: a review of the fascial composition of the abdominal cavity. Surg Today. 2015;45:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Chapuis P, Bokey L, Fahrer M, Sinclair G, Bogduk N. Mobilization of the rectum: anatomic concepts and the bookshelf revisited. Dis Colon Rectum. 2002;45:1-8; discussion 8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Stecco C, Sfriso MM, Porzionato A, Rambaldo A, Albertin G, Macchi V, De Caro R. Microscopic anatomy of the visceral fasciae. J Anat. 2017;231:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Muntean V. The surgical anatomy of the fasciae and the fascial spaces related to the rectum. Surg Radiol Anat. 1999;21:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Kinugasa Y, Murakami G, Suzuki D, Sugihara K. Histological identification of fascial structures posterolateral to the rectum. Br J Surg. 2007;94:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Ercoli A, Delmas V, Fanfani F, Gadonneix P, Ceccaroni M, Fagotti A, Mancuso S, Scambia G. Terminologia Anatomica versus unofficial descriptions and nomenclature of the fasciae and ligaments of the female pelvis: a dissection-based comparative study. Am J Obstet Gynecol. 2005;193:1565-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Ceccaroni M, Clarizia R, Roviglione G, Ruffo G. Neuro-anatomy of the posterior parametrium and surgical considerations for a nerve-sparing approach in radical pelvic surgery. Surg Endosc. 2013;27:4386-4394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Konishi T. Laparoscopic Lateral Pelvic Lymph Node Dissection. In: Kim J, Garcia-Aguilar J. Minimally Invasive Surgical Techniques for Cancers of the Gastrointestinal Tract 2020. Springer, Cham; 2020. |
| 23. | Standring S. Gray’s Anatomy. 41th ed. Elsevier Churchill Livingstone: London; 2016. |
| 24. | de Treigny OM, Roumiguie M, Deudon R, de Bonnecaze G, Carfagna L, Chaynes P, Rimailho J, Chantalat E. Anatomical study of the inferior vesical artery: is it specific to the male sex? Surg Radiol Anat. 2017;39:961-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Fujii S, Sekiyama K. Precise Neurovascular Anatomy for Radical Hysterectomy 2002. Springer Nature Singapore Pte Ltd: Singapore. |