Published online Jun 21, 2021. doi: 10.3748/wjg.v27.i23.3413
Peer-review started: March 7, 2021
First decision: March 27, 2021
Revised: March 28, 2021
Accepted: May 8, 2021
Article in press: May 8, 2021
Published online: June 21, 2021
Processing time: 102 Days and 9.8 Hours
Currently, the technologies most commonly used to treat locally advanced pancreatic cancer are radiofrequency ablation (RFA), microwave ablation, and irreversible (IRE) or reversible electroporation combined with low doses of chemotherapeutic drugs.
To report an overview and updates on ablative techniques in pancreatic cancer.
Several electronic databases were searched. The search covered the years from January 2000 to January 2021. Moreover, the reference lists of the found papers were analysed for papers not indexed in the electronic databases. All titles and abstracts were analysed.
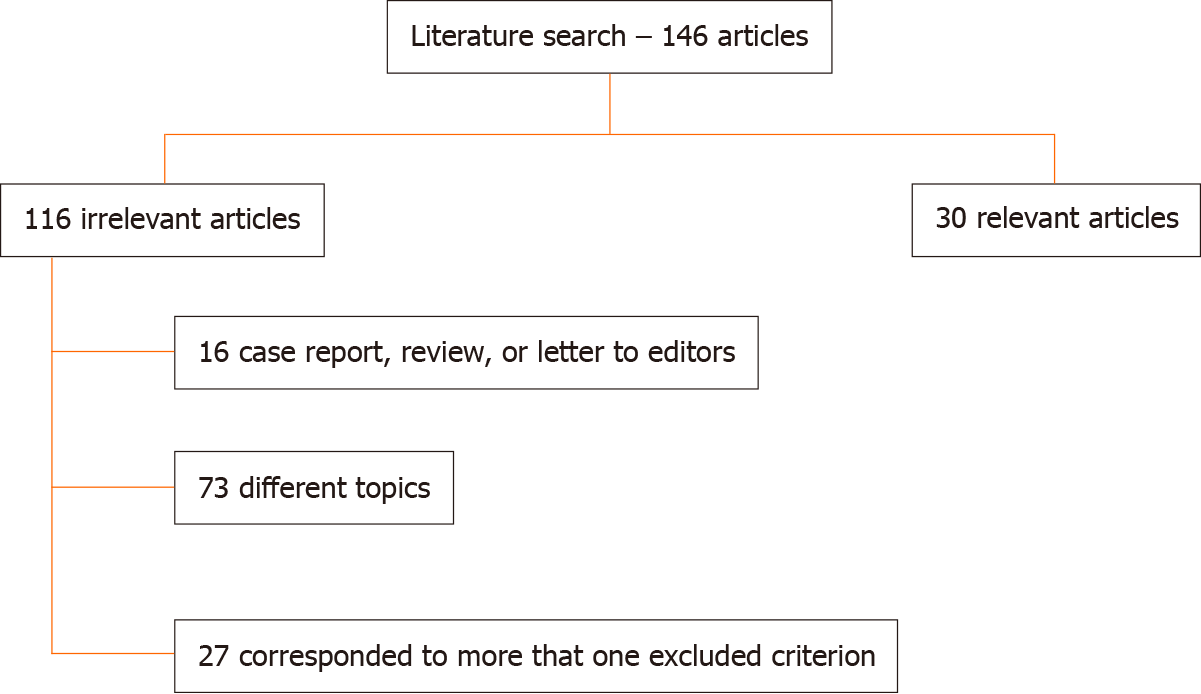
We found 30 studies (14 studies for RFA, 3 for microwave therapy, 10 for IRE, and 3 for electrochemotherapy), comprising 1047 patients, which were analysed further. Two randomized trials were found for IRE. Percutaneous and laparotomy approaches were performed. In the assessed patients, the median maximal diameter of the lesions was in the range of 2.8 to 4.5 cm. All series included patients unfit for surgical treatment, but Martin et al assessed a subgroup of patients with borderline resectable tumours who underwent resection with margin attenuation with IRE. Most studies administered chemotherapy prior to ablative therapies. However, several studies suggest that the key determinant of improved survival is attributable to ablative treatment alone. Nevertheless, the authors suggested chemotherapy before local therapies for several reasons. This strategy may not only downstage a subgroup of patients to curative-intent surgery but also support to recognize patients with biologically unfavourable tumours who would likely not benefit from ablation treatments. Ablation therapies seem safe based on the 1047 patients assessed in this review. The mortality rate ranged from 1.8% to 2%. However, despite the low mortality, the reported rates of severe post procedural complications ranged from 0%-42%. Most reported complications have been self-limiting and manageable. Median overall survival varied between 6.0 and 33 mo. Regarding the technical success rate, assessed papers reported an estimated rate in the range of 85% to 100%. However, the authors reported early recurrence after treatment. A distinct consideration should be made on whether local treatments induce an immune response in the ablated area. Preclinical and clinical studies have shown that RFA is a promising mechanism for inducing antigen-presenting cell infiltration and enhancing the systemic antitumour T-cell immune response and tumour regression.
In the management of patients with pancreatic cancer, the possibility of a multimodal approach should be considered, and conceptually, the combination of RFA with immunotherapy represents a novel angle of attack against this tumour.
Core Tip: In the current state of knowledge, the most commonly used technologies in locally advanced pancreatic cancer are radiofrequency ablation, microwave ablation, and irreversible or reversible electroporation combined with low doses of chemotherapeutic drugs. Our purpose is to report an updated overview of these techniques, highlighting the advantages and limitations of each technology.
- Citation: Granata V, Grassi R, Fusco R, Belli A, Palaia R, Carrafiello G, Miele V, Grassi R, Petrillo A, Izzo F. Local ablation of pancreatic tumors: State of the art and future perspectives. World J Gastroenterol 2021; 27(23): 3413-3428
- URL: https://www.wjgnet.com/1007-9327/full/v27/i23/3413.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i23.3413
Worldwide, an estimated 19.3 million new cancer cases and almost 10.0 million cancer deaths occurred in 2020[1]. Pancreatic cancer accounts for almost as many deaths (466000) as cases (496000) because of its poor prognosis, and it is the seventh leading cause of cancer death in both sexes. Both incidence and mortality rates have been stable or slightly increased in many countries, likely reflecting the increasing prevalence of obesity, diabetes, and alcohol consumption, although improvements in diagnostic and cancer registration practices may also be factors in some countries. Given that the rates of this disease are rather stable relative to the declining rates of breast cancer, it has been projected in a study of 28 European countries that pancreatic cancer will surpass breast cancer as the third leading cause of cancer death by 2025[1].
The only curative treatment is surgery; however, many patients have locally advanced or metastatic disease at diagnosis, and systemic chemotherapy is usually the main treatment[2-6]. The median survival of patients with metastatic disease treated with FOLFIRINOX therapy is only 3 mo[2,5]. FOLFIRINOX or modified FOLFIRINOX and gemcitabine/albumin-bound nab-paclitaxel remain the first-line treatment regimens, and for patients with BRCA1/2 and PALB2 mutations, FOLFIRINOX or modified FOLFIRINOX and gemcitabine/cisplatin are a second option[2,3]. Despite the recent introduction of novel chemotherapeutic schemes, these treatments still correlate with inadequate survival and significant systemic complications. Additionally, only one-third of patients are responsive to chemotherapy[6,7].
Local ablation treatment is considered in some centres for patients with persistent locally advanced disease after chemotherapy. Although randomized trials to establish the role of ablation treatments in addition to chemotherapy alone are absent and there are no concluded trials that have compared various ablative modalities[8], patients with persistent locally advanced disease who are in good clinical condition (World Health Organization performance status 0-1) and Response Evaluation Criteria in Solid Tumours (RECIST) stable disease after 2-4 mo of chemotherapy can be treated by local ablation therapies. Moreover, there is a growing interest in these techniques related to the fact that they can encourage a systemic antitumour response. Therefore, it is proposed to combine ablative treatments with immunotherapy to improve disease control[8]. Nonetheless, ablative treatments should be employed in pancreatic cancers that show a local growth pattern without systemic involvement and should be chosen as consolidative treatments in a multimodal approach. The superior technique between the two remains unknown; therefore, the choice to employ one or the other should be reserved for a multidisciplinary team, considering the patients’ comorbidities, the tumour characteristics and, particularly, the response to medical therapies[9-19].
In the current state of knowledge, the most commonly used technologies in locally advanced pancreatic cancer (LAPC) are radiofrequency ablation (RFA), microwave ablation (MWA), and irreversible electroporation (IRE) or reversible electroporation combined with low-dose chemotherapeutic drugs (ECT).
We report an overview and an update of these procedures, highlighting the advantages and limitations of each technology.
This study is an autonomous study with no protocol or registration number.
The following electronic databases were used for search: PubMed (United States National Library of Medicine, http://www.ncbi.nlm.nih.gov/pubmed), Scopus (Elsevier, http://www.scopus.com/), Web of Science (Thomson Reuters, http://apps.webofknowledge.com/), and Google Scholar (https://scholar.google.it/). The following search criteria were used: “Pancreatic Cancer” AND “Ablative Therapies”; “Pancreatic Cancer” AND “RFA”; “Pancreatic Cancer” AND “MWA”; “Pancreatic Cancer” AND “IRE”; “Pancreatic Cancer” AND “ECT”.
The search covered the years from January 2000 to January 2021. Reference lists of the found papers were analysed for papers not indexed in the electronic databases. The included papers were required to be clinical studies (e.g., retrospective analyses, case series, and prospective cohort studies) evaluating the safety and efficacy of ablative therapies in pancreatic adenocarcinoma. Articles published in the English language from January 2000 to January 2021 were included. The exclusion criteria were: Different topics, unavailability of full text, insufficient data, and case reports, reviews, or letters to editors.
The search strategy resulted in 30 studies [14 studies for RFA, 3 for MWA, 10 for IRE, and 3 for electrochemotherapy (ECT)] (Figure 1), including 1047 patients, which were further assessed. We found two randomized trials for IRE. Percutaneous and laparotomy approaches were performed. In the assessed patients, the median maximal diameter of the lesions was in the range of 2.8-4.5 cm, including patients unfit for surgical treatment. Additionally, Martin et al[19] evaluated patients with borderline resectable tumours who underwent resection with margin attenuation with IRE. Most series administered chemotherapy prior to IRE. The specific types of drugs varied between series, but gemcitabine- or 5-FU-based regimens were common.
In Table 1, we report the sample size, overall survival (OS), major complication rate, minor complication rate, and mortality rate in pancreatic cancer treated with RFA according to the studies assessed.
| Ref. | Sample size | Overall survival | Major complication rate | Minor complication rate | Mortality rate |
| D'Onofrio et al[28], 2016 | 51 | - | - | - | - |
| D'Onofrio et al[29], 2017 | 18 | Median, 185 d (range, 62-398 d) | 0% | 0% | 0% |
| Giardino et al[30], 2013 | 168 | 34.0 mo | 3.70% | 17.70% | 1.80% |
| Hadjicostas et al[31], 2006 | 4 | 6 mo | 0% | 25% | 0% |
| Kallis et al[33], 2015 | 23 | 226 d (range, 140-526 d) | 0% | 4.30% | 0% |
| Song et al[37], 2016 | 6 | NR | 0% | 33.30% | 0% |
| Spiliotis et al[38], 2007 | 25 | 33 mo | 0% | - | 0% |
| Varshney et al[39], 2006 | 3 | - | 0% | 66.70% | 0% |
| Zou et al[41], 2010 | 32 | 17.5 mo | 3.10% | 0% | 0% |
| Giardino et al[44], 2017 | 10 | NR | 30% | 0% | 0% |
| D’Onofrio et al[46], 2020 | 35 | 310 (65–718) d | 0% | 0% | 0% |
| Wang et al[48], 2020 | 11 | 12 mo | 0% | 0% | 0% |
| He et al[50], 2020 | 18 | 1-yr 40.5%; 2-yr 27.0% | 22.20% | 50% | 0% |
| Fegrachi et al[52], 2019 | 17 | 9 mo (range, 5-11 mo) | 6% | 24% | 0% |
In Table 2, we report the sample size, OS, major complication rate, minor complication rate, and mortality rate in pancreatic cancer treated with MWA according to the studies assessed.
In Table 3, we report the sample size, OS, major complication rate, minor com
| Ref. | Sample size | Overall survival | Major complication rate | Minor complication rate | Mortality rate |
| Vroomen et al[14], 2017 | 25 | - | 8% | 20% | 0% |
| Martin et al[19], 2015 | 200 | 24.9 mo (range, 4.9–85 mo) | 18.50% | 50,5% | 2% |
| Martin et al[20], 2013 | 54 | 20 mo | 24% | 55,5% | 2% |
| Lambert et al[23], 2016 | 21 | 10.2 mo | 23.80% | - | 0% |
| Yan et al[24], 2016 | 25 | - | 36% | 16% | 0% |
| Scheffer et al[26], 2017 | 25 | 11 mo | 40% | 40% | 0% |
| Ruarus et al[65], 2020 | 50 | 11.6 mo (no induction chemotherapy or gemcitabine-based induction chemotherapy) and 14.9 mo (FOLFIRINOX) | 42% | 28% | 2% |
| van Veldhuisen et al[66], 2020 | 30 | 17.0 (range, 5-35 mo) | 20% | 23% | 0% |
| Narayananet al[67], 2017 | 50 | 27 mo | 20% | - | 0% |
| Liu et al[68], 2019 | 54 | 16.2 and 20.3 mo in the IRE and IRE + chemo groups | 7.40% | 81% | 0% |
In Table 4, we report the sample size, OS, major complication rate, minor com
| Ref. | Sample size | Overall survival | Major complication rate | Minor complication rate | Mortality rate |
| Granata et al[11], 2015 | 13 | - | 0% | 23% | 0% |
| Granata et al[12], 2017 | 19 | - | - | - | - |
| Granata et al[71], 2020 | 25 | In fixed geometry, treated patients 6 mo (range, 1-74 mo); in variable geometry treated patients 12 mo (range, 2-50 mo) | 0% | 23% | 0% |
RFA and MWA are hyper-thermic techniques that utilize energy to heat the lesions to at least 60°C[16].
RFA produces necrosis due to thermocoagulation. With this treatment, the area of active tissue heating is limited to a few millimetres near the electrode[16]. Con
MWA uses the dielectric effect, which occurs when an imperfect dielectric material is subjected to an alternating electromagnetic field, to generate a larger area of active heating (up to 2 cm close to the antenna), allowing more homogeneous necrosis in the target zone compared to RFA[16]. Additionally, MWA has several supposed improvements with respect to RFA: The target can be greater given that it generates a larger area of necrosis; the treatment time is quicker; and it is less influenced by the defence of the neighbouring tissues, which is due to vaporization and charring, so the heat-sink effect impacts the efficacy of MWA[16].
In contrast to RFA and MWA, IRE and ECT are non-thermal techniques that cause ablation, changing cell membrane permeability through an induced electric field (electroporation). IRE is considered a direct ablation tool since electroporation is used in an irreversible manner[17-26]. The use of short high-voltage electric current fields (up to 3000 V and 50 A for milliseconds) cause irreversible permeabilization of the lipid bilayer, disruption of cellular homeostasis, and stimulation of apoptotic pathways, causing death of neoplastic cells[9-17,26]. Taking into account its mecha
RFA: RFA is the typical treatment worldwide for the treatment of LAPC if further benefit from chemotherapy is expected. Several studies have evaluated the role of RFA in metastatic pancreatic cancer[9]. To the best of our knowledge, 14 studies assessed the safety and efficacy of RFA in pancreatic cancer[28-52]. In several studies, the use of RFA was limited to patients with locally advanced cancer and/or metastatic cancer. Only in cases where the patients were unfit for surgery was RFA used in resectable cancer[25-52]. Recently, RFA has been used as an upfront option at the time of diagnosis[9], justified based on immunological antitumoral stimulation[43,44].
The results of the assessed studies in terms of OS, major and minor complication rates, and mortality rates are reported in Table 1.
In the treatment of pancreatic adenocarcinoma, RFA has been mainly applied during laparotomy or with an endoscopic approach. The percutaneous approach has rarely been described in the literature, and worldwide experience is still limited[46]. However, the percutaneous approach should be favoured to avoid the invasiveness of the intraoperative approach. If percutaneous RFA is feasible, it could avoid unnecessary laparotomy, thus reducing the risk of surgical complications as well as the time and costs of the treatment[46]. Moreover, while surgery involves an impaired immune response, enhanced immune system stimulation and immune response against the tumour have been described in a percutaneously treated patient[43,47].
Nonrandomized studies showed a promising OS up to 25.6 mo after RFA for LAPC[53]. However, no randomized controlled trials have been performed, so the true effectiveness of RFA combined with systemic chemotherapy regimens remains unknown. Several studies[9] reported an excellent outcome with a median OS of 30 mo for patients subjected to RFA and a median OS of 25.6 mo in the patients subjected to primary treatments plus RFA plus further systemic treatments. Few studies have assessed the efficacy of RFA compared to other treatments[50]. He et al[50] compared the efficacy of IRE with RFA in patients with LAPC, showing that IRE after induction chemotherapy is superior to RFA after induction chemotherapy for treating LAPC, while these two therapies have comparable efficacy for tumours that were larger than 4 cm. However, the study was not a randomized controlled trial but a retrospective study.
Morbidity rates range from 14% to 28% and seem to depend on RFA temperature settings, preventive duodenal cooling, and safety margins from vital structures[52]. Since pancreatic tissue is sensitive to heat and rich in blood vessels and since the anatomical position is close to arteries and bile ducts, the application of thermotherapy techniques carries a high risk. However, as RFA application becomes increasingly mature, the incidence of postoperative complications has decreased significantly[52]. Complications related to RFA included pancreatic fistulae, portal vein thrombosis, gastrointestinal bleeding, and acute pancreatitis. The rates of RFA-related mortality ranged from 0% to 19%[51]. The RFA-related complications that resulted in patient deaths included gastrointestinal bleeding and sepsis. The rates of overall complications ranged from 10% to 43%[51]. The types of complications reported varied widely and included pneumonia, peritoneal cavity abscess, acute renal failure, transient ascites, hepatic insufficiency, pseudomembrane colitis, hemoperitoneum, abdominal fluid collection, gastric bypass fistula, gastric ulcer, and choledocholithiasis.
Computed tomography (CT) is the diagnostic tool most often employed to evaluate treatment in terms of efficacy and safety. Although CT is best known for its role in the evaluation of abdominal emergencies, it is also an excellent tool in the evaluation of posttreatment complications[54-58]. However, CT has significant limitations in assessing treatment effectiveness[6]. RFA produces side effects such as interstitial oedema, haemorrhage, carbonization, necrosis, and fibrosis. These are responsible for heterogeneous appearances on imaging. The assessment of the treatment response in terms of dimensional criteria, according to RECIST 1.1[15] criteria, is not applicable because effectiveness is not always correlated to a size decrease[6]. Nevertheless, the assessed studies evaluated short- and long-term RFA efficacy according to dimensional criteria[15]. The assessment time was between 7 and 34 mo considering only dimensional criteria. According to Paiella et al[9] for RFA the technique to choose is CT, and the effectiveness is related to a post treatment hypodense zone. However, pancreatic tumours are also hypodense, so a “qualitative evaluation” based on a visual assessment could cause misdiagnosis. A quantitative evaluation founded on functional analysis allows a more objective assessment and a more correct diagnosis[6].
Distinct consideration should be made regarding whether RFA induces an immune response in the ablated area. Preclinical and clinical studies have shown that RFA is a favourable tool to induce antigen-presenting cell infiltration and to enhance the systemic antitumour T-cell immune response and tumour regression. The treatment is followed by a significant inflammatory response with intense T-cell infiltration[43,45].
Therefore, in the management of patients with pancreatic cancer, the possibility of a multimodal approach should be considered, and theoretically, the association of RFA with immunotherapy is a novel strategy against this tumour.
The results of the assessed studies in terms of OS, major and minor complication rates, and mortality rates are reported in Table 2.
Carrafiello et al[59] evaluated the efficacy of MWA in ten unresectable pancreatic head adenocarcinomas. The mean follow-up was 9.2 mo (range, 3 to 16 mo). The rate of MWA-related morbidity was 30% (3 patients). The authors found pancreatitis in two patients and gastroduodenal artery pseudoaneurysm in one patient. CT was executed up to 15 mo after the procedure. At the first follow-up, the researchers found one case with partial response (PR), eight with stable disease (SD), and one with progressive disease.
Ierardi et al[60] evaluated the feasibility and safety of MWA in five head pancreatic locally advanced cancer patients using a new technology with a high power and frequency of 2450 MH. CT was performed after 1, 3, 6, and 12 mo. No major complications were reported with a safe treatment in all patients (100%). Minor complications resolved during hospitalization (median, 4 d)[60].
Vogl et al[61] cured 22 lesions: In 17 (77.3%) patients, the tumour was in the pancreatic head and in 5 (22.7%) in the pancreatic tail. The rate of MWA efficacy was 100%. No major complications were reported; however, in two (9.1%) cases, minor complications were found because of severe local pain post-MWA treatment. Only ten patients underwent follow-up magnetic resonance imaging (MRI) examinations (median, 3 mo); local tumour progression was reported in one (10%) case.
Unlike RFA, the percutaneous approach was the most commonly used during MWA treatment, probably explaining the lower complication and death rates. However, the complication rates of MWA varied among the assessed studies. This finding might be due to the heterogeneity and the sample size of the studies assessed. In fact, Carrafiello et al[59] treated ten unresectable head pancreatic adenocarcinomas, Ierardi et al[60] treated five locally advanced head pancreatic cancers, and Vogl et al[61] treated 22 patients: In 17 (77.3%) patients, the tumour was in the pancreatic head and in five (22.7%) in the pancreatic tail.
CT is the diagnostic tool most often employed to evaluate the treatment, for either efficacy and/or safety. However, an assessment centred only on dimensional criteria is inappropriate to evaluate this procedure.
No data on MWA and immune response in the ablated area are described in the literature.
Although MWA is a promising treatment for LAPC, further studies are needed to increase the data about its safety and efficacy as well as the oncological outcome.
Currently, IRE is applied in stage III LAPC[17,26], even if several studies have reported three cases of IRE in stage IV with liver metastases[62] and the option to employ IRE as a technique to reduce the rate of R1 resections[19,21,63]. According to the reported data[17-26,62,64], IRE works better on target areas not larger than 3-3.5 cm; in addition, IRE should be more suitable than thermal tools when the tumour encapsulates the superior mesenteric artery. However, IRE has the disadvantage of necessitating general anaesthesia.
The results of the assessed studies in terms of OS, major and minor complication rates, and mortality rates are reported in Table 3.
Rombouts et al[53] described an IRE-related complication rate of 13%, with a mortality of 2%. The complication rate increases with the percutaneous treatment (29% vs 13%)[53]. Martin et al[19], evaluating 200 cured lesions, reported an overall rate of adverse events of 37% and a mortality rate of 2%. The most common complications reported are abdominal pain as a minor complication and pancreatitis, bile leakage, pancreatic leakage, duodenal leakage, duodenal ulcer, pneumothorax, haematoma, and deep vein thrombosis as major complications[6]. Several studies have confirmed the safety profile of IRE, with encouraging survival outcomes. Most studies, however, were retrospective, had limited sample sizes, and had a relatively short follow-up time. The aim of the PANFIRE study was to evaluate the efficacy and safety of percutaneous IRE for LAPC and isolated local recurrence following surgical resection of pancreatic cancer[65]. In this prospective single-arm phase II study, 40 patients with LAPC and 10 with local recurrence after resection were treated. The primary endpoint was the median survival times with primary LAPC (median OS, 17 mo) and with local recurrence (median OS, 16 mo), which exceeded the target median survival times based on chemotherapy alone. These results show a survival benefit compared with the current standard of care[65]. The reported overall complication rate was of 58%, including 21 major adverse events and two deaths within 90 d of the treatment. In addition, 13 (33%) patients were treated upfront with IRE. No survival benefit was demonstrated for patients receiving a 5-FU-based regimen. This finding suggests that the key determinant of improved survival is attributable to IRE treatment alone. Nevertheless, the authors recommend at least four cycles of a 5-FU-based regimen before IRE for several reasons. First, a 5-FU-based regimen enables the identification of patients with aggressive tumour biologic features, allowing the exclusion of those who would not benefit from IRE. Second, a 5-FU-based regimen can result in downstaging, potentially rendering 15%–25% of patients with resectable disease. Last, an upfront 5-FU-based regimen has the potential to result in a longer OS[65]. The combination of systemic chemotherapy and cytoreductive ablation using IRE may prove synergistic for several reasons[66]. Induction chemotherapy may not only downstage a subgroup of lesions to curative-intent surgery but also identify biologically unfavourable tumours with rapid progression that would likely not benefit from ablative treatment[66]. As systemic chemotherapy remains the only treatment for LAPC proven to be beneficial, patients should first receive systemic chemotherapy followed by experimental treatment in the setting of a clinical trial[66-69].
IRE can be implemented successfully as an adjunctive measure for attempting to achieve negative microscopic operative margins in selected patients[21]. This treatment is limited to patients who have generally stable disease at the time of resection. To date, there are few options for effective therapy to facilitate microscopically negative margin resections outside of patient selection and meticulous operative dissection. Accepting that true margin-positive rates are significantly high (> 75%) in resected pancreatic cancers, intraoperative IRE could accentuate negative-margin dissection of the retroperitoneal margin and its surrounding perivascular soft tissue, primarily the perineural and mesenteric tissue adjacent to critical vascular structures[21].
MRI and CT are the diagnostic tools mostly used to assess IRE.
IRE produces the formation of nanoscale pores within the cell membrane, changing the transmembrane potential and causing cell death. Experimental models showed that diffusion-weighted imaging (DWI) could be used to assess therapeutic effects[14,70]. Vroomen et al[14] evaluated specific imaging parameters with contrast-enhanced (ce) MRI and ce-CT. The authors evaluated pre- and post-treatment, for MRI, the signal intensity (SI) on T2-weighted (W) images, on T1-W images before and after contrast medium, on DWI, and on apparent coefficient of diffusion (ADC) maps and for CT attenuation in the arterial and portal-venous phases. These authors showed that the most significant features to evaluate efficacy and outcome were SI on images with b = 800s/mm2 and contrast-enhanced MRI.
Only two studies[19,67] reported an outstanding median OS of 24.9 and 27 mo, respectively. Consequently, there is a need for a greater number of studies that assess efficacy in terms of oncological outcomes.
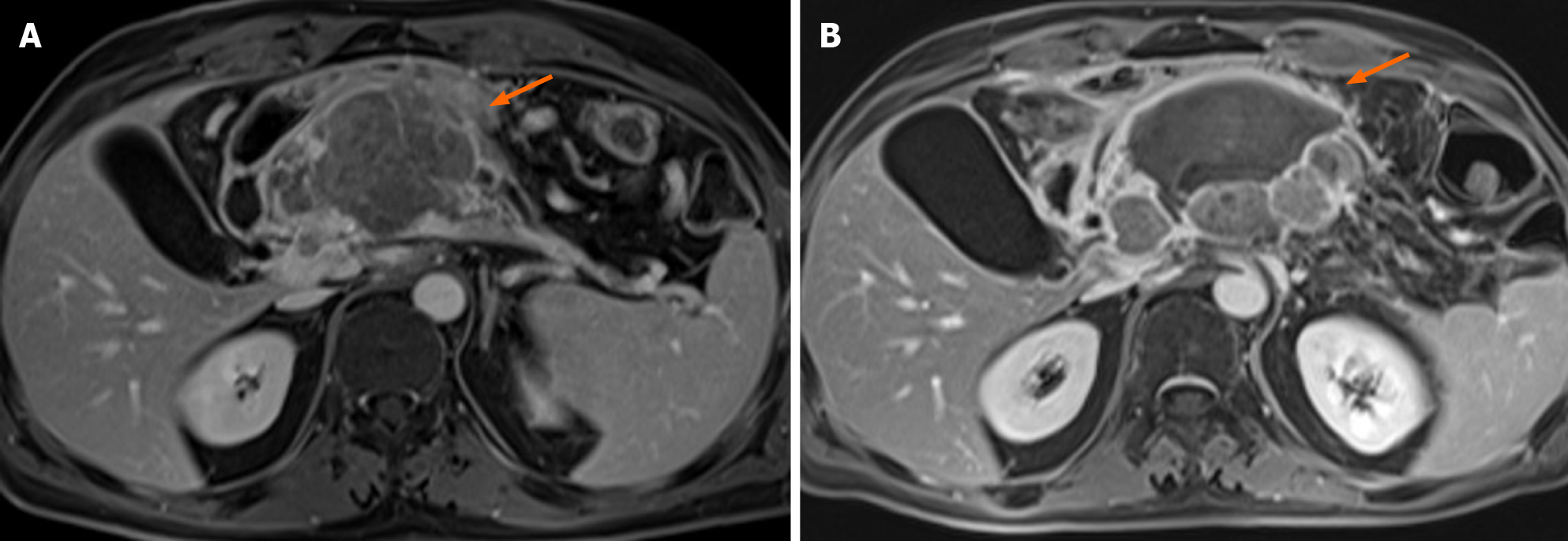
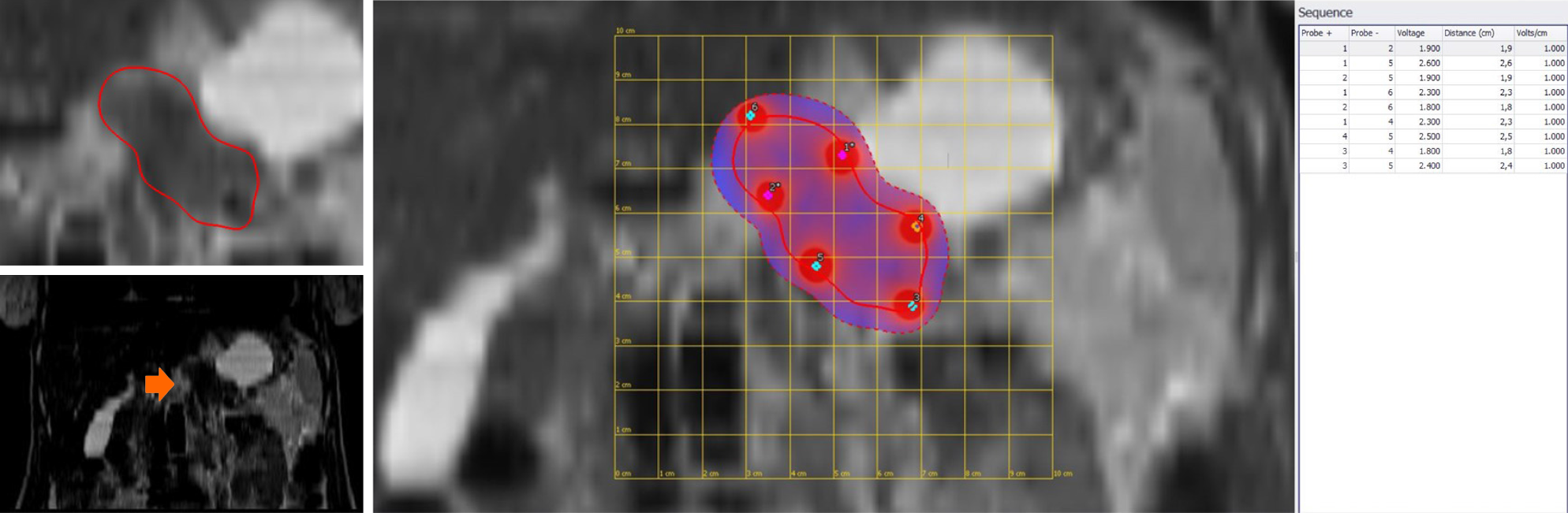
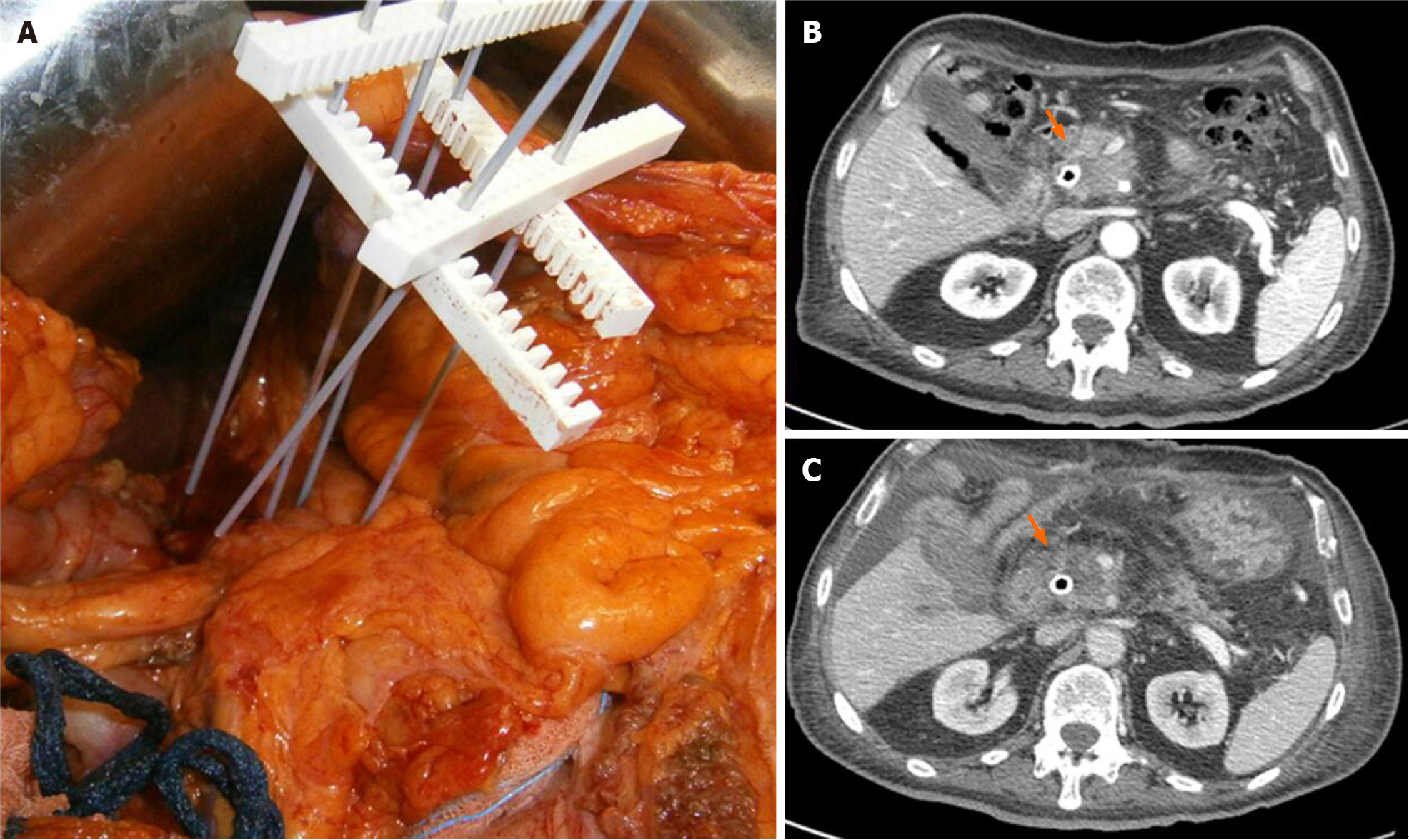
Today, few studies have evaluated the role of ECT in LAPC[11-13,71]. In our previous study, we assessed 13 patients with LAPC. In seven (53.8%) patients, the tumour was localized in the head, and in six (46.2%), it was localized in the body tail (Figure 2). The treatment was safe in all patients without major complications. The types of minor complications reported varied widely and included transient ascites, transient pleural effusion, and gastric emptying documented by radiological studies, without clinically significant signs. The mean duration of hospitalization was 12 d. CT and MR were utilized for the follow-up[11]. In an ongoing study, we found that the median OS was 11.5 mo with a range in values of 73 mo. At 1 mo after ECT, 76.0% of patients were in PR, and 20.0% were in SD. Moreover, we found that the use of pre-treatment planning (Figure 3), which optimizes the multiple insertions of single electrodes, increases the local disease control rate (LDCR) and the OS compared with the use of fixed-geometry electrodes (hexagonal or linear). The patients treated with fixed geometry had an LDCR of 46.1%, whereas the group treated with variable geometry (Figure 4) had an LDCR of 66.7%. For the 13 patients treated with fixed geometry, the median OS was 6 mo (range, 1–74 mo), whereas for the 12 patients treated with variable geometry, the median OS was 12 mo (range, 2 to 50 mo)[71].
Although ECT is a promising tool for cancer treatment, how to assess tumour treatment response is still a problem. In fact, as highlighted in our preliminary experience, the RECIST 1.1 criterion, using the variation of the largest diameter on both CT and MRI images, does not provide appropriate patient stratification in responders or non-responders[12,13]. It is clear that when considering therapeutic effects on tumours, imaging observations are sometimes difficult to interpret, so functional imaging should resolve this problem. We evaluated several functional features as follows: for MRI, wash-in slope and wash-out slope by dynamic contrast-enhanced MRI, pseudo-diffusivity, perfusion fraction, and tissue diffusivity by the intravoxel incoherent motion model, ADC by the conventional mono-exponential approach, and the mean of the diffusion coefficient and the mean of diffusional kurtosis by diffusion kurtosis imaging. In addition, for positron emission tomography, maximum standardized uptake value was assessed and for CT, lesion density was evaluated. We found that conventional morphologic criteria were not able to differentiate partial, complete, or incomplete responses after ECT, while changes in functional parameters could be more suitable to assess ECT responses[11,13].
Today, ECT is recommended during clinical trials in dedicated centres[11-13].
Several studies have suggested that the key determinant of improved survival is attributable to ablative treatment alone[72-75]. Nevertheless, the authors recommend at least four cycles of a 5-FU-based regimen before local therapy for several reasons. Induction chemotherapy may not only downstage a subgroup of lesions to curative-intent surgery but also identify biologically unfavourable tumours with rapid progression that would likely suffer from ablation treatment[66,76-80]. As systemic chemotherapy remains the only treatment for LAPC proven to be beneficial, patients should first receive systemic chemotherapy followed by experimental treatment in the setting of a clinical trial[66].
Ablation therapies seem to be safe in 1047 patients assessed in this study. The mortality rate ranged from 1.8% to 2%. However, despite the low mortality, the reported rates of severe post procedural complications ranged from 0%–42%. Additionally, for laparotomy, the series reported by Martin et al[19] had more severe complications, including procedure-related deaths. The major drawback inherent to all thermal ablation techniques is the fact that these therapies comprise the risk of the heat-sink effect[69]. This issue is particularly important, as the pancreas is an organ with a peculiar position that is closely related to the duodenum, bile duct, and major vessels. This feature turned IRE into an attractive tool for LAPC ablation[69]. Taken together, pathological studies revealed that one-third of patients died of PC as a result of local tumour infiltration, without evidence of metastatic disease. This population appears to be ideal for IRE to increase patient survival and, importantly, quality of life[69]. A registry-based study showed a high rate of complications (42%) post-IRE. An important point demonstrated in this study is the correlation of the learning curve to the rate of complications, which seems to drop after a cumulative experience of a minimal of five IRE cases in PC[69].
Median OS varied between 6.0 and 33 mo. However, these data are very problematic to understand because of the heterogeneity between the series.
Regarding the technical success rate, several studies reported an estimated technical success rate in the range of 85%–100%. However, the authors reported early recurrence after treatment, indicating the limitations of the radiological assessment post-treatment[16]. In addition, none of these studies assessed the relationship between the technical success rate and tumour size.
A distinct consideration should be made on whether local treatments induce an immune response in the ablated area. Preclinical and clinical studies have shown that RFA is an interesting tool to induce antigen-presenting cell infiltration and to enhance the systemic antitumour response. To the best of our knowledge, no data on other local treatments are available; therefore, studies that also evaluate this aspect for the other methods would be interesting.
Therefore, in the management of patients with pancreatic cancer, the possibility of a multimodal approach should be considered, and theoretically, the association of RFA with immunotherapy is a novel strategy against this tumour.
In conclusion, ablation therapies seem effective and safe with low post-treatment mortality. Although complications are mostly self-limiting, severe complications do occur. The technical success rate is high at 85%–100%, but this feature may be an over-estimation. Further efforts are also needed to address patient selection, as well as the use of IRE for “margin accentuation” during surgical resection, so the combination of RFA with immunotherapy represents a novel strategy against this tumour.
In the current state of knowledge, the most commonly used technologies in locally advanced pancreatic cancer (LAPC) are radiofrequency ablation (RFA), microwave ablation, and irreversible electroporation (IRE) or reversible electroporation combined with low doses of chemotherapeutic drugs.
In the management of patients with pancreatic cancer, the possibility of a multimodal approach should be considered.
The research purpose was to report an overview and an update on ablation techniques, highlighting the advantages and limitations of each technology.
The search covered the years from January 2000 to January 2021 and was performed using data from several electronic databases.
Ablation therapies seem effective and safe with low post-treatment mortality. Although complications are mostly self-limiting, severe complications do occur.
Overall survival varies widely between different studies, and the additional value of ablation treatments for LAPC needs to be further explored.
Further efforts are also needed to address patient selection, as well as the use of IRE for “margin accentuation” during surgical resection, so the combination of RFA with immunotherapy represents a novel angle of attack against this tumour type.
The authors are grateful to Alessandra Trocino, librarian at the National Cancer Institute of Naples, Italy. Moreover, the authors are grateful to Assunta Zazzaro for the collaboration.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sato H S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64640] [Article Influence: 16160.0] [Reference Citation Analysis (176)] |
| 2. | NCCN Guidelines Updates: Pancreatic Cancer. [cited 28 March 2021]. Available from: https://www.nccn.org/patients/guidelines/content/PDF/pancreatic-patient.pdf. |
| 3. | Tempero MA. NCCN Guidelines Updates: Pancreatic Cancer. J Natl Compr Canc Netw. 2019;17:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 123] [Reference Citation Analysis (0)] |
| 4. | Alistar A, Morris BB, Desnoyer R, Klepin HD, Hosseinzadeh K, Clark C, Cameron A, Leyendecker J, D'Agostino R Jr, Topaloglu U, Boteju LW, Boteju AR, Shorr R, Zachar Z, Bingham PM, Ahmed T, Crane S, Shah R, Migliano JJ, Pardee TS, Miller L, Hawkins G, Jin G, Zhang W, Pasche B. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017;18:770-778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 5. | Usón Junior PLS, Rother ET, Maluf FC, Bugano DDG. Meta-analysis of Modified FOLFIRINOX Regimens for Patients With Metastatic Pancreatic Cancer. Clin Colorectal Cancer. 2018;17:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Granata V, Grassi R, Fusco R, Setola SV, Palaia R, Belli A, Miele V, Brunese L, Petrillo A, Izzo F. Assessment of Ablation Therapy in Pancreatic Cancer: The Radiologist's Challenge. Front Oncol. 2020;10:560952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 7. | Chin V, Nagrial A, Sjoquist K, O'Connor CA, Chantrill L, Biankin AV, Scholten RJ, Yip D. Chemotherapy and radiotherapy for advanced pancreatic cancer. Cochrane Database Syst Rev. 2018;3:CD011044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | van Veldhuisen E, van den Oord C, Brada LJ, Walma MS, Vogel JA, Wilmink JW, Del Chiaro M, van Lienden KP, Meijerink MR, van Tienhoven G, Hackert T, Wolfgang CL, van Santvoort H, Groot Koerkamp B, Busch OR, Molenaar IQ, van Eijck CH, Besselink MG; Dutch Pancreatic Cancer Group and International Collaborative Group on Locally Advanced Pancreatic Cancer. Locally Advanced Pancreatic Cancer: Work-Up, Staging, and Local Intervention Strategies. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (2)] |
| 9. | Paiella S, Salvia R, Ramera M, Girelli R, Frigerio I, Giardino A, Allegrini V, Bassi C. Local Ablative Strategies for Ductal Pancreatic Cancer (Radiofrequency Ablation, Irreversible Electroporation): A Review. Gastroenterol Res Pract. 2016;2016:4508376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Testoni SGG, Healey AJ, Dietrich CF, Arcidiacono PG. Systematic review of endoscopy ultrasound-guided thermal ablation treatment for pancreatic cancer. Endosc Ultrasound. 2020;9:83-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Granata V, Fusco R, Piccirillo M, Palaia R, Petrillo A, Lastoria S, Izzo F. Electrochemotherapy in locally advanced pancreatic cancer: Preliminary results. Int J Surg. 2015;18:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Granata V, Fusco R, Setola SV, Piccirillo M, Leongito M, Palaia R, Granata F, Lastoria S, Izzo F, Petrillo A. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J Gastroenterol. 2017;23:4767-4778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Granata V, Fusco R, Setola SV, Palaia R, Albino V, Piccirillo M, Grimm R, Petrillo A, Izzo F. Diffusion kurtosis imaging and conventional diffusion weighted imaging to assess electrochemotherapy response in locally advanced pancreatic cancer. Radiol Oncol. 2019;53:15-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Vroomen LGPH, Scheffer HJ, Melenhorst MCAM, de Jong MC, van den Bergh JE, van Kuijk C, van Delft F, Kazemier G, Meijerink MR. MR and CT imaging characteristics and ablation zone volumetry of locally advanced pancreatic cancer treated with irreversible electroporation. Eur Radiol. 2017;27:2521-2531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Pelle E, Al-Toubah T, Morse B, El-Haddad G, Strosberg J. Desmoplastic mesenteric lesions do not respond radiographically to peptide receptor radionuclide therapy. J Neuroendocrinol. 2021;33:e12936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 2019;24:e990-e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 360] [Article Influence: 60.0] [Reference Citation Analysis (1)] |
| 17. | Granata V, de Lutio di Castelguidone E, Fusco R, Catalano O, Piccirillo M, Palaia R, Izzo F, Gallipoli AD, Petrillo A. Irreversible electroporation of hepatocellular carcinoma: preliminary report on the diagnostic accuracy of magnetic resonance, computer tomography, and contrast-enhanced ultrasound in evaluation of the ablated area. Radiol Med. 2016;121:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Ierardi AM, Lucchina N, Petrillo M, Floridi C, Piacentino F, Bacuzzi A, Fonio P, Fontana F, Fugazzola C, Brunese L, Carrafiello G. Systematic review of minimally invasive ablation treatment for locally advanced pancreatic cancer. Radiol Med. 2014;119:483-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Martin RC 2nd, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, McMasters KM, Watkins K. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg. 2015;262:486-494; discussion 492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 294] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 20. | Martin RC 2nd, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol. 2013;20 Suppl 3:S443-S449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 199] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Marsanic P, Mellano A, Sottile A, De Simone M. Irreversible electroporation as treatment of locally advanced and as margin accentuation in borderline resectable pancreatic adenocarcinoma. Med Biol Eng Comput. 2017;55:1123-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Ansari D, Kristoffersson S, Andersson R, Bergenfeldt M. The role of irreversible electroporation (IRE) for locally advanced pancreatic cancer: a systematic review of safety and efficacy. Scand J Gastroenterol. 2017;52:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Lambert L, Horejs J, Krska Z, Hoskovec D, Petruzelka L, Krechler T, Kriz P, Briza J. Treatment of locally advanced pancreatic cancer by percutaneous and intraoperative irreversible electroporation: general hospital cancer center experience. Neoplasma. 2016;63:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Yan L, Chen YL, Su M, Liu T, Xu K, Liang F, Gu WQ, Lu SC. A Single-institution Experience with Open Irreversible Electroporation for Locally Advanced Pancreatic Carcinoma. Chin Med J (Engl). 2016;129:2920-2925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Zhang Y, Shi J, Zeng J, Alnagger M, Zhou L, Fang G, Long X, Pan Z, Li Y, Chen J, Xu K, Qian W, Niu L. Percutaneous Irreversible Electroporation for Ablation of Locally Advanced Pancreatic Cancer: Experience From a Chinese Institution. Pancreas. 2017;46:e12-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Scheffer HJ, Vroomen LG, de Jong MC, Melenhorst MC, Zonderhuis BM, Daams F, Vogel JA, Besselink MG, van Kuijk C, Witvliet J, de van der Schueren MA, de Gruijl TD, Stam AG, van den Tol PM, van Delft F, Kazemier G, Meijerink MR. Ablation of Locally Advanced Pancreatic Cancer with Percutaneous Irreversible Electroporation: Results of the Phase I/II PANFIRE Study. Radiology. 2017;282:585-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Izzo F, Ionna F, Granata V, Albino V, Patrone R, Longo F, Guida A, Delrio P, Rega D, Scala D, Pezzuto R, Fusco R, Di Bernardo E, D'Alessio V, Grassi R, Contartese D, Palaia R. New Deployable Expandable Electrodes in the Electroporation Treatment in a Pig Model: A Feasibility and Usability Preliminary Study. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | D'Onofrio M, Barbi E, Girelli R, Tinazzi Martini P, De Robertis R, Ciaravino V, Salvia R, Butturini G, Frigerio I, Milazzo T, Crosara S, Paiella S, Pederzoli P, Bassi C. Variation of tumoral marker after radiofrequency ablation of pancreatic adenocarcinoma. J Gastrointest Oncol. 2016;7:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 29. | D'Onofrio M, Crosara S, De Robertis R, Butturini G, Salvia R, Paiella S, Bassi C, Mucelli RP. Percutaneous Radiofrequency Ablation of Unresectable Locally Advanced Pancreatic Cancer: Preliminary Results. Technol Cancer Res Treat. 2017;16:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Giardino A, Girelli R, Frigerio I, Regi P, Cantore M, Alessandra A, Lusenti A, Salvia R, Bassi C, Pederzoli P. Triple approach strategy for patients with locally advanced pancreatic carcinoma. HPB (Oxford). 2013;15:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Hadjicostas P, Malakounides N, Varianos C, Kitiris E, Lerni F, Symeonides P. Radiofrequency ablation in pancreatic cancer. HPB (Oxford). 2006;8:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Ikuta S, Kurimoto A, Iida H, Aihara T, Takechi M, Kamikonya N, Yamanaka N. Optimal combination of radiofrequency ablation with chemoradiotherapy for locally advanced pancreatic cancer. World J Clin Oncol. 2012;3:12-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Kallis Y, Phillips N, Steel A, Kaltsidis H, Vlavianos P, Habib N, Westaby D. Analysis of Endoscopic Radiofrequency Ablation of Biliary Malignant Strictures in Pancreatic Cancer Suggests Potential Survival Benefit. Dig Dis Sci. 2015;60:3449-3455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Lakhtakia S, Ramchandani M, Galasso D, Gupta R, Venugopal S, Kalpala R, Reddy DN. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest Endosc. 2016;83:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 35. | Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M, Brugge W. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 167] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 36. | Rossi S, Viera FT, Ghittoni G, Cobianchi L, Rosa LL, Siciliani L, Bortolotto C, Veronese L, Vercelli A, Gallotti A, Ravetta V. Radiofrequency ablation of pancreatic neuroendocrine tumors: a pilot study of feasibility, efficacy, and safety. Pancreas. 2014;43:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Song TJ, Seo DW, Lakhtakia S, Reddy N, Oh DW, Park DH, Lee SS, Lee SK, Kim MH. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest Endosc. 2016;83:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 38. | Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Varshney S, Sewkani A, Sharma S, Kapoor S, Naik S, Sharma A, Patel K. Radiofrequency ablation of unresectable pancreatic carcinoma: feasibility, efficacy and safety. JOP. 2006;7:74-78. [PubMed] |
| 40. | Waung JA, Todd JF, Keane MG, Pereira SP. Successful management of a sporadic pancreatic insulinoma by endoscopic ultrasound-guided radiofrequency ablation. Endoscopy. 2016;48 Suppl 1:E144-E145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Zou YP, Li WM, Zheng F, Li FC, Huang H, Du JD, Liu HR. Intraoperative radiofrequency ablation combined with 125 iodine seed implantation for unresectable pancreatic cancer. World J Gastroenterol. 2010;16:5104-5110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Saccomandi P, Lapergola A, Longo F, Schena E, Quero G. Thermal ablation of pancreatic cancer: A systematic literature review of clinical practice and pre-clinical studies. Int J Hyperthermia. 2018;35:398-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Waitz R, Solomon SB. Can local radiofrequency ablation of tumors generate systemic immunity against metastatic disease? Radiology. 2009;251:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Giardino A, Innamorati G, Ugel S, Perbellini O, Girelli R, Frigerio I, Regi P, Scopelliti F, Butturini G, Paiella S, Bacchion M, Bassi C. Immunomodulation after radiofrequency ablation of locally advanced pancreatic cancer by monitoring the immune response in 10 patients. Pancreatology. 2017;17:962-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 45. | Fei Q, Pan Y, Lin W, Zhou Y, Yu X, Hou Z, Lin X, Lin R, Lu F, Guan H, Huang H. High-dimensional single-cell analysis delineates radiofrequency ablation induced immune microenvironmental remodeling in pancreatic cancer. Cell Death Dis. 2020;11:589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | D'Onofrio M, Beleù A, Sarno A, De Robertis R, Paiella S, Viviani E, Frigerio I, Girelli R, Salvia R, Bassi C. US-Guided Percutaneous Radiofrequency Ablation of Locally Advanced Pancreatic Adenocarcinoma: A 5-Year High-Volume Center Experience. Ultraschall Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Gao S, Pu N, Yin H, Li J, Chen Q, Yang M, Lou W, Chen Y, Zhou G, Li C, Li G, Yan Z, Liu L, Yu J, Wang X. Radiofrequency ablation in combination with an mTOR inhibitor restrains pancreatic cancer growth induced by intrinsic HSP70. Ther Adv Med Oncol. 2020;12:1758835920953728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Wang J, Wang Y, Zhao Y, Wu X, Zhang M, Hou W, Chen Q, Cheng B. Endoscopic ultrasound-guided radiofrequency ablation of unresectable pancreatic cancer with low ablation power and multiple applications: a preliminary study of 11 patients. Ann Palliat Med. 2021;10:1842-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Al-Jumah R, Urits I, Viswanath O, Kaye AD, Hasoon J. Radiofrequency Ablation and Alcohol Neurolysis of the Splanchnic Nerves for a Patient With Abdominal Pain From Pancreatic Cancer. Cureus. 2020;12:e10758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | He C, Wang J, Zhang Y, Cai Z, Lin X, Li S. Comparison of combination therapies in the management of locally advanced pancreatic cancer: Induction chemotherapy followed by irreversible electroporation vs radiofrequency ablation. Cancer Med. 2020;9:4699-4710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Fegrachi S, Besselink MG, van Santvoort HC, van Hillegersberg R, Molenaar IQ. Radiofrequency ablation for unresectable locally advanced pancreatic cancer: a systematic review. HPB (Oxford). 2014;16:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Fegrachi S, Walma MS, de Vries JJJ, van Santvoort HC, Besselink MG, von Asmuth EG, van Leeuwen MS, Borel Rinkes IH, Bruijnen RC, de Hingh IH, Klaase JM, Molenaar IQ, van Hillegersberg R. Safety of radiofrequency ablation in patients with locally advanced, unresectable pancreatic cancer: A phase II study. Eur J Surg Oncol. 2019;45:2166-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Rombouts SJ, Vogel JA, van Santvoort HC, van Lienden KP, van Hillegersberg R, Busch OR, Besselink MG, Molenaar IQ. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br J Surg. 2015;102:182-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 54. | Trinci M, Cirimele V, Cozzi D, Galluzzo M, Miele V. Diagnostic accuracy of pneumo-CT-cystography in the detection of bladder rupture in patients with blunt pelvic trauma. Radiol Med. 2020;125:907-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Giannitto C, Campoleoni M, Maccagnoni S, Angileri AS, Grimaldi MC, Giannitto N, De Piano F, Ancona E, Biondetti PR, Esposito AA. Unindicated multiphase CT scans in non-traumatic abdominal emergencies for women of reproductive age: a significant source of unnecessary exposure. Radiol Med. 2018;123:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Miele V, Piccolo CL, Trinci M, Galluzzo M, Ianniello S, Brunese L. Diagnostic imaging of blunt abdominal trauma in pediatric patients. Radiol Med. 2016;121:409-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Schueller G, Scaglione M, Linsenmaier U, Schueller-Weidekamm C, Andreoli C, De Vargas Macciucca M, Gualdi G. The key role of the radiologist in the management of polytrauma patients: indications for MDCT imaging in emergency radiology. Radiol Med. 2015;120:641-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Sessa B, Trinci M, Ianniello S, Menichini G, Galluzzo M, Miele V. Blunt abdominal trauma: role of contrast-enhanced ultrasound (CEUS) in the detection and staging of abdominal traumatic lesions compared to US and CE-MDCT. Radiol Med. 2015;120:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 59. | Carrafiello G, Ierardi AM, Fontana F, Petrillo M, Floridi C, Lucchina N, Cuffari S, Dionigi G, Rotondo A, Fugazzola C. Microwave ablation of pancreatic head cancer: safety and efficacy. J Vasc Interv Radiol. 2013;24:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Ierardi AM, Biondetti P, Coppola A, Fumarola EM, Biasina AM, Alessio Angileri S, Carrafiello G. Percutaneous microwave thermosphere ablation of pancreatic tumours. Gland Surg. 2018;7:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Vogl TJ, Panahi B, Albrecht MH, Naguib NNN, Nour-Eldin NA, Gruber-Rouh T, Thompson ZM, Basten LM. Microwave ablation of pancreatic tumors. Minim Invasive Ther Allied Technol. 2018;27:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Narayanan G, Hosein PJ, Arora G, Barbery KJ, Froud T, Livingstone AS, Franceschi D, Rocha Lima CM, Yrizarry J. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol. 2012;23:1613-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 63. | Weiss MJ, Wolfgang CL. Irreversible electroporation: a novel pancreatic cancer therapy. Curr Probl Cancer. 2013;37:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Scheffer HJ, Nielsen K, de Jong MC, van Tilborg AA, Vieveen JM, Bouwman AR, Meijer S, van Kuijk C, van den Tol PM, Meijerink MR. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vasc Interv Radiol. 2014;25:997-1011; quiz 1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 65. | Ruarus AH, Vroomen LGPH, Geboers B, van Veldhuisen E, Puijk RS, Nieuwenhuizen S, Besselink MG, Zonderhuis BM, Kazemier G, de Gruijl TD, van Lienden KP, de Vries JJJ, Scheffer HJ, Meijerink MR. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): A Multicenter, Prospective, Single-Arm, Phase II Study. Radiology. 2020;294:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 66. | van Veldhuisen E, Vroomen LG, Ruarus AH, Derksen TC, Busch OR, de Jong MC, Kazemier G, Puijk RS, Sorgedrager NS, Vogel JA, Scheffer HJ, van Lienden KP, Wilmink JW, Besselink MG, Meijerink MR. Value of CT-Guided Percutaneous Irreversible Electroporation Added to FOLFIRINOX Chemotherapy in Locally Advanced Pancreatic Cancer: A Post Hoc Comparison. J Vasc Interv Radiol. 2020;31:1600-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Narayanan G, Hosein PJ, Beulaygue IC, Froud T, Scheffer HJ, Venkat SR, Echenique AM, Hevert EC, Livingstone AS, Rocha-Lima CM, Merchan JR, Levi JU, Yrizarry JM, Lencioni R. Percutaneous Image-Guided Irreversible Electroporation for the Treatment of Unresectable, Locally Advanced Pancreatic Adenocarcinoma. J Vasc Interv Radiol. 2017;28:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 68. | Liu S, Qin Z, Xu J, Zeng J, Chen J, Niu L, Xu M. Irreversible electroporation combined with chemotherapy for unresectable pancreatic carcinoma: a prospective cohort study. Onco Targets Ther. 2019;12:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Linecker M, Pfammatter T, Kambakamba P, DeOliveira ML. Ablation Strategies for Locally Advanced Pancreatic Cancer. Dig Surg. 2016;33:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Zhang Z, Li W, Procissi D, Tyler P, Omary RA, Larson AC. Rapid dramatic alterations to the tumor microstructure in pancreatic cancer following irreversible electroporation ablation. Nanomedicine (Lond). 2014;9:1181-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Granata V, Fusco R, Palaia R, Belli A, Petrillo A, Izzo F. Comments on "Electrochemotherapy with Irreversible Electroporation and FOLFIRINOX Improves Survival in Murine Models of Pancreatic Adenocarcinoma". Ann Surg Oncol. 2020;27:954-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | De Filippo M, Bozzetti F, Martora R, Zagaria R, Ferretti S, Macarini L, Brunese L, Rotondo A, Rossi C. Radiofrequency thermal ablation of renal tumors. Radiol Med. 2014;119:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Vogl TJ, Farshid P, Naguib NN, Darvishi A, Bazrafshan B, Mbalisike E, Burkhard T, Zangos S. Thermal ablation of liver metastases from colorectal cancer: radiofrequency, microwave and laser ablation therapies. Radiol Med. 2014;119:451-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Cornalba G, Melchiorre F. Interventional oncology: state of the art. Radiol Med. 2014;119:449-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 75. | Garetto I, Busso M, Sardo D, Filippini C, Solitro F, Grognardi ML, Veltri A. Radiofrequency ablation of thoracic tumours: lessons learned with ablation of 100 lesions. Radiol Med. 2014;119:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Kaufman JD, Fesmire CC, Petrella RA, Fogle CA, Xing L, Gerber D, Sano MB. High-Frequency Irreversible Electroporation Using 5,000-V Waveforms to Create Reproducible 2- and 4-cm Ablation Zones-A Laboratory Investigation Using Mechanically Perfused Liver. J Vasc Interv Radiol 2020; 31: 162-168. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 77. | Timmer FEF, Geboers B, Ruarus AH, Schouten EAC, Nieuwenhuizen S, Puijk RS, de Vries JJJ, Meijerink MR, Scheffer HJ. Irreversible Electroporation for Locally Advanced Pancreatic Cancer. Tech Vasc Interv Radiol. 2020;23:100675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 78. | Reames BN, Blair AB, Krell RW, Groot VP, Gemenetzis G, Padussis JC, Thayer SP, Falconi M, Wolfgang CL, Weiss MJ, Are C, He J. Management of Locally Advanced Pancreatic Cancer: Results of an International Survey of Current Practice. Ann Surg. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 79. | Tartaglia E, Fabozzi M, Rizzuto A, Settembre A, Abete R, Guerriero L, Favoriti P, Cuccurullo D, Corcione F. Irreversible electroporation for locally advanced pancreatic cancer through a minimally invasive surgery supported by laparoscopic ultrasound. Int J Surg Case Rep. 2018;42:290-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Latouche EL, Sano MB, Lorenzo MF, Davalos RV, Martin RCG 2nd. Irreversible electroporation for the ablation of pancreatic malignancies: A patient-specific methodology. J Surg Oncol. 2017;115:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |