Published online Jun 21, 2021. doi: 10.3748/wjg.v27.i23.3396
Peer-review started: March 8, 2021
First decision: March 27, 2021
Revised: April 17, 2021
Accepted: May 27, 2021
Article in press: May 27, 2021
Published online: June 21, 2021
Processing time: 102 Days and 3.5 Hours
Crohn’s disease (CD) and ulcerative colitis (UC) are inflammatory bowel diseases (IBDs) with a remission-relapsing presentation and symptomatic exacerbations that have detrimental impacts on patient quality of life and are associated with a high cost burden, especially in patients with moderate-to-severe disease. The Real-world Data of Moderate-to-Severe Inflammatory Bowel Disease in Brazil (RISE BR) study was a noninterventional study designed to evaluate disease control, treatment patterns, disease burden and health-related quality of life in patients with moderate-to-severe active IBD. We report findings from the prospective follow-up phase of the RISE BR study in patients with active UC or CD.
To describe the 12-mo disease evolution and treatment patterns among patients with active moderate-to-severe IBD in Brazil.
This was a prospective, noninterventional study of adult patients with active Crohn’s disease (CD: Harvey-Bradshaw Index ≥ 8, CD Activity Index ≥ 220), inadequate CD control (i.e., calprotectin > 200 µg/g or colonoscopy previous results), or active ulcerative colitis (UC: Partial Mayo score ≥ 5). Enrollment occurred in 14 centers from October 2016 to February 2017. The proportion of active IBD patients after 9-12 mo of follow-up, Kaplan-Meier estimates of the time to mild or no activity and a summary of treatment initiation, discontinuation and dose changes were examined.
The study included 118 CD and 36 UC patients, with mean ± SD ages of 43.3 ± 12.6 and 44.9 ± 16.5 years, respectively. The most frequent drug classes at index were biologics for CD (62.7%) and 5-aminosalicylate derivates for UC patients (91.7%). During follow-up, 65.3% of CD and 86.1% of UC patients initiated a new treatment at least once. Discontinuations/dose changes occurred in 68.1% of CD patients [median 2.0 (IQR: 2-5)] and 94.3% of UC patients [median 4.0 (IQR: 3-7)]. On average, CD and UC patients had 4.4 ± 2.6 and 5.0 ± 3.3 outpatient visits, respectively. The median time to first mild or no activity was 319 (IQR: 239-358) d for CD and 320 (IQR: 288-358) d for UC patients. At 9-12 mo, 22.0% of CD and 20.0% of UC patients had active disease.
Although a marked proportion of active IBD patients achieved disease control within one year, the considerable time to achieve this outcome represents an unmet medical need of the current standard of care in a Brazilian real-world setting.
Core Tip: This was a prospective, noninterventional study of 118 adult patients with active Crohn’s disease (CD) and 36 with active ulcerative colitis (UC). The aim was to describe the 12-mo disease evolution and treatment patterns of active moderate-to-severe inflammatory bowel disease (IBD) patients in Brazil. The median time to first mild or no activity was 319 (IQR: 239-358) d for CD and 320 (IQR: 288-358) d for UC patients. A marked proportion of active IBD patients achieved disease control within one year, which is considered a long time to achieve the goal of reducing inflammation.
- Citation: Sassaki LY, Miszputen SJ, Kaiser Junior RL, Catapani WR, Bafutto M, Scotton AS, Zaltman C, Baima JP, Ramos HS, Faria MAG, Gonçalves CD, Guimaraes IM, Flores C, Amarante HMBS, Nones RB, Parente JML, Lima MM, Chebli JM, Ferrari MLA, Campos JF, Sanna MGP, Ramos O, Parra RS, da Rocha JJR, Feres O, Feitosa MR, Caratin RF, Senra JT, Santana GO. Real-world treatment patterns and disease control over one year in patients with inflammatory bowel disease in Brazil. World J Gastroenterol 2021; 27(23): 3396-3412
- URL: https://www.wjgnet.com/1007-9327/full/v27/i23/3396.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i23.3396
Crohn’s disease (CD) and ulcerative colitis (UC) are inflammatory bowel diseases (IBDs) with a remission-relapsing presentation and symptomatic exacerbations that have a detrimental impact on patient quality of life and are associated with a high cost burden, especially in the case of moderate to severe disease[1,2]. The pharmacological treatment of IBD aims to achieve and maintain long-term remission and heal the gut mucosa, thus delaying the progression of disease and associated complications as well as the need for surgery[3]. For CD patients with moderate-to-severe disease activity, guidelines usually recommend the use of antitumor necrosis factor-α (anti-TNFα) either as a monotherapy or combined with an immunosuppressant to induce remission for steroid-refractory disease or steroid-intolerant patients[4]. With regard to moderate-to-severe active UC, an immunosuppressant and/or a biologic drug are recommended[5,6]. Systemic 5-aminosalicylate (5-ASA) agents are also indicated for maintenance treatment of UC[1]. Corticosteroids are recommended for short-term use (≤ 3 mo) during relapses of both CD and UC and are not recommended as main
In clinical practice, physicians often try several options and treatment sequences for IBD management, both in monotherapy or combined regimens[3]. Primary nonresponse, secondary loss of response and development of adverse reactions to anti-TNFα drugs may occur and lead to discontinuation, with the subsequent prescription of a second advanced therapy with a different mechanism of action (e.g., anti-integrins or JAK inhibitors)[7-11]. However, some studies have reported that up to half of IBD patients who discontinue their first-line biologic (infliximab or adalimumab) do not restart or switch to another therapy[3,12]. In Brazil, vedolizumab, ustekinumab and tofacitinib are available at private health services, the latter being approved only for UC treatment since 2018. Moreover, the VARSITY study recently demonstrated that the gut-selective anti-integrin vedolizumab was superior to the anti-TNFα adalimumab in achieving clinical remission in patients with moderate-to-severe active UC after one year[13].
Real-world data on IBD management could unveil unmet medical needs and treatment gaps[3]. This is of utmost relevance in developing countries such as Brazil, where the prevalence of IBD is increasing but access to biologic treatment may be restricted, and information on IBD treatment, in general, and associated outcomes is scarce[14,15]. The Real-world Data of Moderate-to-Severe Inflammatory Bowel Disease in Brazil (RISE BR) study was a noninterventional study designed to evaluate disease control, treatment patterns, burden of disease and health-related quality of life in patients with a previous diagnosis of moderate-to-severe active IBD[16,17]. Here, we report findings from the prospective follow-up phase of the RISE BR study in patients with active UC or CD and aim to describe the real-world treatment patterns and outcomes of IBD-related therapies during a 12-mo follow-up period.
The RISE BR study (ClinicalTrials.gov Identifier: NCT02822235) was a national, multicenter, noninterventional study designed to evaluate disease control, treatment patterns, burden of disease and health-related quality of life among IBD patients in Brazil. It involved a cross-sectional evaluation of IBD patients (index date) with a retrospective data collection component and a 12-mo prospective follow-up period of IBD patients who had active disease (at the index date). The cross-sectional evaluation was conducted at routine IBD outpatient visits between October 2016 and February 2017, and patients were consecutively recruited from 14 study sites (nine clinics from the public health system and five private clinics) covering Brazil’s most populated regions. In this article, we present data from the follow-up period. All participants provided written informed consent prior to the study procedures. The study was approved by the local ethics committees and was conducted according to the principles of the Helsinki Declaration.
At the index date, patients were included if they were aged 18 years or older and had a confirmed diagnosis of moderate-to-severe CD or UC at least 6 mo prior to index. Patients were excluded if they presented indeterminate/unclassified colitis, mental incapacity, unwillingness, or language barriers precluding adequate understanding or cooperation with the study, were hospitalized, participated in a clinical trial within the last 3 years, or received off-label treatment with vedolizumab. All patients presenting active disease at the index date were invited to participate in the prospective follow-up phase of the study. Active CD was defined as a Harvey-Bradshaw Index (HBI) ≥ 8 points or a Crohn’s Disease Activity Index (CDAI) ≥ 220 points or fecal calprotectin levels > 200 µg/g or colonoscopy results in the previous year suggestive of inadequate control (as per investigator criteria)[18]. Active UC was defined as a partial Mayo (pMayo) score ≥ 5 points.
The prospective observational period was defined as a 12-mo follow-up since the index date. Data were collected from patients’ medical records by staff personnel of the participating site. Sociodemographic characteristics (age, sex, educational level, and professional status), weight and body mass index (BMI), smoking habits, and family history of IBD were collected at the study appointment. Other clinical variables included the time since first IBD diagnosis, extension/severity and location of CD or UC (Montreal classification), corticosteroid dependency/intolerance status, and presence of extraintestinal manifestations. In addition, IBD treatment patterns and changes during the follow-up period (at the index date and the following medical appointments) were collected.
CD activity during follow-up was evaluated with the HBI[18] and/or CDAI[19], depending on local practice. Moderate-to-severe active CD was defined as patients with an HBI ≥ 8 or a CDAI ≥ 220, while CD disease control (i.e., mild or no disease activity) was defined as an HBI < 8 or a CDAI < 220[18-20]. UC disease activity during follow-up was assessed with the 9-item pMayo score; moderate-to-severe active UC was defined as pMayo ≥ 5, and UC disease control (i.e., mild or no UC activity) was defined as pMayo < 5[21]. Given the noninterventional design of the study, there were no predefined time points of IBD activity assessment, and measures were collected in the course of typical disease treatment.
Descriptive statistics [mean, median, standard deviation (SD) and minimum-maximum] were used to analyze sociodemographic, clinical, and treatment-related variables. Student’s t-test for independent samples or the Mann-Whitney test were used to compare CD and UC patients regarding quantitative variables. Fisher’s exact test and the chi-square test were used for qualitative variables.
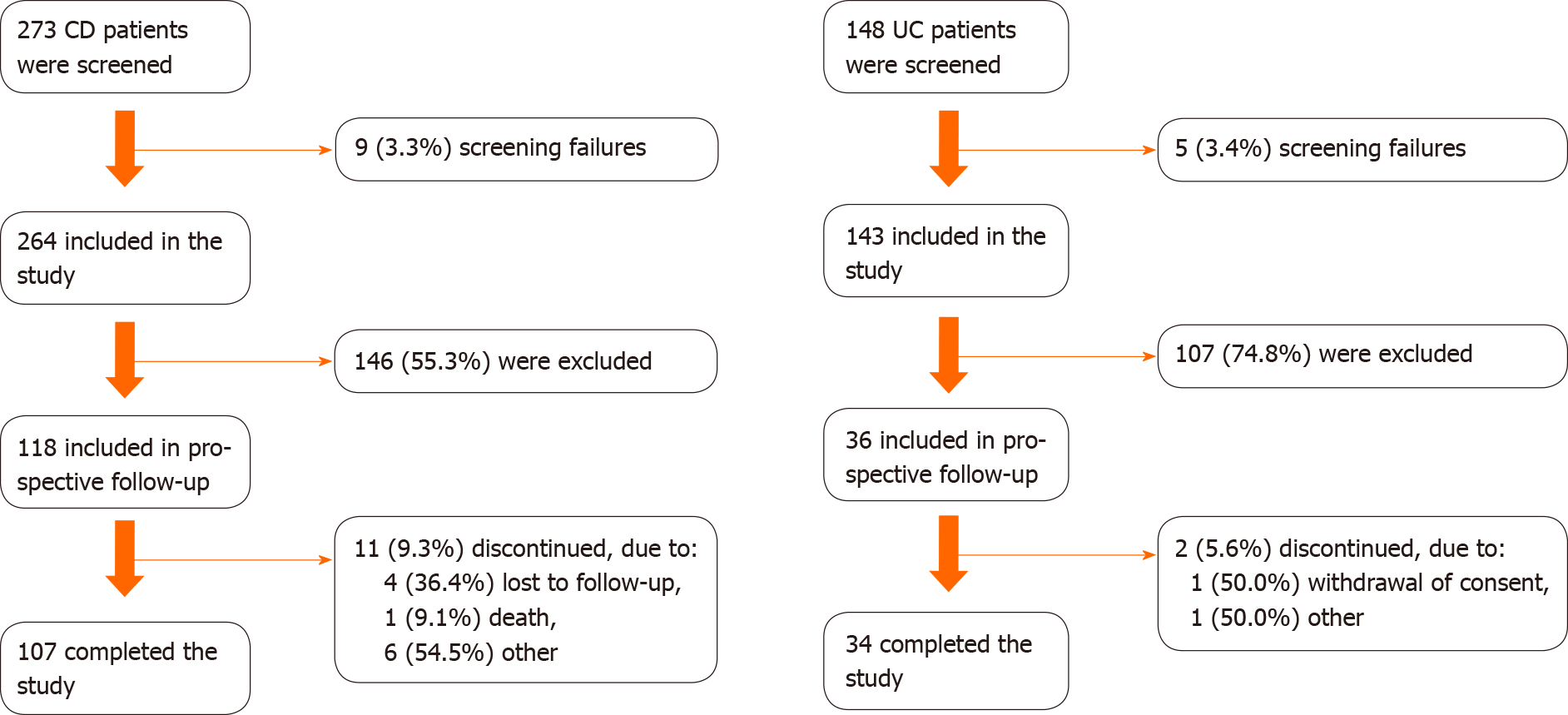
Overall, 407 patients (264 CD and 143 UC) were enrolled in the cross-sectional assessment. All patients with active IBD at the cross-sectional appointment were eligible for the prospective phase of the study, including 118 CD patients and 36 UC patients. These sample sizes allowed the use of a desirable central limit theorem in outcome inference, even accounting for sample losses [11/118 (9.3%) in CD; 2/36 (5.6%) in UC] after 12 mo of follow-up.
The time to disease control was calculated using the Kaplan-Meier approach, defined as the time (in days) from index date until the date of the first documentation of disease control [i.e., achieved disease control, mild or no active disease (defined as event)]. Patients who did not achieve disease control, died (without the event), discontinued the study or were lost to follow-up had their time censored at death or at the date of the last visit.
The proportion of patients with moderate-to-severe active IBD at 12 mo was calculated by IBD type at the last visit between 9 and 12 mo of follow-up.
Treatment changes were defined as drug discontinuations or dose changes (i.e., changes in frequency and/or dosage administered) during follow-up for ongoing medications or initiated at the index date and any new medication initiated after the index date. Patients who did not have any visits during follow-up or patients who had no ongoing treatments were considered missing in the survival analysis.
For each IBD type, bivariate analyses were conducted to evaluate sociodemographic, clinical and treatment-related variables between subjects who achieved disease control during the 12-mo follow-up period vs those who did not. Chi-square or Fisher’s exact tests were used to compare categorical variables, t-tests were used for independent samples, and the nonparametric Mann-Whitney test was used to compare quantitative variables. The incidence rate of treatment changes by person-years was also determined.
All statistical tests were two-tailed, with a significance level of 0.05, using SAS® (version 9.4, SAS Institute Inc., Cary).
Of 407 subjects (75.4%, n = 307, from public clinics) evaluated at the index date, 37.8% (n = 118 CD and 36 UC) were included in the prospective study phase (Figure 1). The majority of included patients were from public clinics: 74.6% (n = 88) CD and 72.2% (n = 26) UC patients. During the 12-mo follow-up, 11 (9.3%) CD patients and 2 (5.6%) UC patients discontinued the study, and one CD patient died. Overall, the mean ± SD follow-up times were 340.4 ± 59.2 d and 339.2 ± 64.8 d for CD and UC patients, respectively. A total of 107 CD patients and 34 UC patients completed the study and had a medical appointment between months 9 and 12, of which 82 CD patients and 25 UC patients had at least one evaluation of disease activity during this time window.
Most patients were female (58.5% and 78.8% of CD and UC patients, respectively), and the mean ± SD age at baseline was 43.3 ± 12.6 years for CD patients and 44.9 ± 16.5 years for UC patients (Table 1). A total of 45 (38.1%) CD patients and 22 (61.1%) UC patients had their first diagnosis of moderate-to-severe IBD within the past 5 years. The median CD disease duration was similar between patients receiving biologics and those not receiving biologics at the index date [11.0 (IQR: 0.5-11.0) vs 11.5 (IQR: 0.5-29.1) years]. UC patients on biologics at index had a shorter disease duration than patients not receiving biologics (6.0 years vs 10.0 years).
| CD patients (n = 118) | UC patients (n = 36) | |
| Age (yr), mean ± SD | 43.3 ± 12.6 | 44.9 ± 16.5 |
| Female, n (%) | 69 (58.5) | 26 (72.2) |
| Employed, n (%) | 36 (36.7) | 5 (16.1) |
| Attended higher education, n (%) | 32 (34.4) | 5 (23.8) |
| Body mass index (kg/m2), mean ± SD | 24.9 ± 4.7 | 24.9 ± 5.1 |
| Current smokers, n (%)1 | 7 (5.9) | 0 (0.0) |
| Family history of IBD, n (%) | 15 (12.7) | 2 (5.6) |
| IBD duration (yr), median (IQR) | 11.0 (0.5-11.0) | 6.5 (0.5-29.1) |
| Biologic group | 11.0 (5.0-16.0) | 6.0 (3.0-13.0) |
| Nonbiologic group | 11.5 (4.5-18.5) | 10.0 (4.0-17.0) |
| 5-ASA derivates group | 9.0 (4.0-13.0) | 5.5 (4.0-14.0) |
| Non-5-ASA derivates group | 11.5 (5.0-17.5) | 13.0 (4.0-15.0) |
| Corticosteroid status, n (%)2 | ||
| Steroid-dependent | 17 (14.4) | 10 (27.8) |
| Steroid-refractory disease | 5 (4.2) | 3 (8.3) |
| Not applicable (no previous use) | 33 (28.0) | 3 (8.3) |
| Unknown | 37 (31.4) | 15 (41.7) |
| Location of CD, n (%) | ||
| L1 ileal | 26 (22.0) | - |
| L2 colonic | 21 (17.8) | - |
| L3 ileocolonic | 71 (60.2) | - |
| CD Behavior, n (%) | ||
| B1 nonstricturing/penetrating | 31 (26.3) | - |
| B2 stricturing | 52 (44.1) | - |
| B3 penetrating | 35 (29.7) | - |
| Symptomatic perianal disease, n (%) | 37 (31.6) | - |
| HBI result (available for 101 CD patients), median (IQR) | 5.0 (1.0-9.0) | - |
| CDAI result (available for 43 CD patients), median (IQR) | 200.0 (114.0-274.0) | - |
| Fecal calprotectin level > 200 µg/g during previous year, n (%) | 40 (33.9) | - |
| Colonoscopy with CD activity 3 during previous year, n (%) | 69 (58.5) | - |
| Extent of UC inflammation - Montreal classification, n (%) | ||
| E1 ulcerative proctitis | - | 10 (27.8) |
| E2 left-sided UC | - | 8 (22.2) |
| E3 pancolitis | - | 18 (50.0) |
| Partial Mayo score, median (IQR) | - | 6.0 (5.0-6.0) |
| Total Mayo score (available for 13 UC patients) | - | 8.0 (7.0-8.0) |
| Extra intestinal manifestations4, n (%) | 29 (24.6) | 8 (22.2) |
| Patients with at least one IBD surgery during previous 3 yr, n (%) | 32 (27.1) | 1 (2.8) |
| Total colectomy | 0 (0.0) | 1 (2.8) |
| Fistulectomy | 7 (5.9) | 0 (0.0) |
| Enterostomy | 6 (5.1) | 0 (0.0) |
| Partial colectomy | 5 (4.2) | 0 (0.0) |
| Closure of enterostomy | 5 (4.2) | 0 (0.0) |
| Drainage of anorectal abscess | 3 (2.5) | 0 (0.0) |
| Jejunostomy/Ileostomy | 1 (0.8) | 0 (0.0) |
| Other | 15 (12.7) | 1 (2.8) |
| Patients with at least one IBD-related hospitalizations during previous 3 yr, n (%) | 51 (43.1) | 11 (30.6) |
At the index date, 46 (39.0%) CD patients had active disease according to the CDAI or HBI, of whom 24 were identified based on these indexes only, with six presenting severely active CD (HBI > 16 or CDAI > 450). Regarding disease behavior, 52 (44.1%) and 35 (29.7%) CD patients had B2 stricturing and B3 penetrating disease, respectively. In addition, 40 (33.9%) patients had fecal calprotectin levels > 200 µg/g (22 patients identified only by calprotectin levels), and 69 (58.5%) had a colonoscopy results suggestive of disease activity during the previous year (35 patients identified by this criterion only). UC patients with active disease had a median pMayo of 6.0 (IQR: 5.0-6.0) at baseline, four (11.1%) had severe UC based on pMayo > 7, and 50.0% of patients presented with pancolitis.
Biologics were the most frequent drug class that CD patients were receiving at the index date appointment (62.7%), and 5-ASA derivates (91.7%) were the most common for UC patients; however, it is important to highlight that biologics were not available as a UC treatment strategy in Brazil at the time of the study (Table 2). Approximately 48% of CD patients and 58.3% of UC patients were receiving corticosteroids at the index date. Half (50.0%) of UC patients had received at least one corticosteroid for a period of 3 mo or longer. At the start of the 12-mo follow-up period, 58.5% of CD patients maintained their current biologic therapy, while 5.1% initiated a new biologic. Regarding UC patients, 30.6% maintained their biologic therapy, while 8.3% initiated a new biologic treatment. Current 5-ASA agents were maintained by 14.4% of CD patients, while 55.6% of UC patients maintained the same therapy.
| CD patients (n = 118) | UC patients (n = 36) | |
| IBD treatment at index date appointment, n (%)1 | 111 (94.1) | 35 (97.2) |
| 5-ASA derivates | 36 (30.5) | 33 (91.7) |
| Corticosteroids | 56 (47.5) | 21 (58.3) |
| Immunosuppressants | 65 (55.1) | 21 (58.3) |
| Biologics | 74 (62.7) | 14 (38.9) |
| Patients who received at least one corticosteroid for ≥ 3 mo, n (%)2 | 31 (26.3) | 18 (50.0) |
| Treatments at the start of 12-mo follow-up (index date), n (%) | ||
| IBD treatment initiated or maintained | 112 (94.9) | 35 (97.2) |
| Biologic therapy | ||
| Maintained or initiated at index date | 75 (63.6) | 14 (38.9) |
| Maintained | 69 (58.5) | 11 (30.6) |
| Initiated new | 6 (5.1) | 3 (8.3) |
| Naïve to biologics3 | 1 (0.8) | 1 (2.8) |
| Discontinued at index date | 0 (0.0) | 0 (0.0) |
| Immunosuppressants | ||
| Maintained or initiated at index date | 73 (61.9) | 19 (52.8) |
| Maintained | 71 (60.2) | 14 (38.9) |
| Initiated new | 2 (1.7) | 5 (13.9) |
| Discontinued at index date | 1 (0.8) | 0 (0 .0) |
| 5-ASA compounds | ||
| Maintained or initiated at index date | 19 (16.1) | 27 (75.0) |
| Maintained | 17 (14.4) | 20 (55.6) |
| Initiated new | 2 (1.7) | 5 (13.9) |
| Naïve to 5-ASA compounds | 2 (1.7) | 5 (13.9) |
| Discontinued at index date | 1 (0.8) | 0 (0.0) |
| Corticosteroids | ||
| Maintained or initiated at index date | 16 (13.6) | 15 (41.7) |
| Maintained | 9 (7.6) | 6 (16.7) |
| Initiated new | 6 (5.1) | 8 (22.2) |
| Naïve to corticosteroids3 | 2 (1.7) | 1 (2.8) |
| Discontinued at index date | 2 (1.7) | 4 (11.1) |
| Any antibiotic initiated at index date | 12 (10.2) | 5 (13.9) |
| Treatment changes during 12-mo follow-up period | ||
| New treatment initiated during follow-up | 77 (65.3) | 31 (86.1) |
| Discontinuation or dose change during follow-up2 | 77 (68.1) | 33 (94.3) |
| Median (IQR) number of treatment changes by patient | 2 (2-5) | 4 (3-7) |
| Biologic therapy | ||
| Initiated | 45 (38.1) | 6 (16.7) |
| Naïve to biologics4 | 13 (11.0) | 1 (2.8) |
| Discontinued | 23 (19.5) | 5 (13.9) |
| Dose change | 14 (11.9) | 4 (11.1) |
| Immunosuppressants | ||
| Initiated | 24 (20.3) | 14 (38.9) |
| Naïve to immunosuppressants4 | 4 (3.4) | 2 (5.6) |
| Discontinued | 20 (16.9) | 11 (9.3) |
| Dose change | 7 (5.9) | 7 (19.4) |
| 5-ASA derivates | ||
| Initiated | 10 (8.5) | 21 (58.3) |
| Naïve to 5-ASA compounds4 | 1 (0.8) | 2 (5.6) |
| Discontinued | 7 (5.9) | 8 (22.2) |
| Dose change | 4 (3.4) | 14 (38.8) |
| Corticosteroids | ||
| Initiated | 24 (20.3) | 16 (44.4) |
| Naïve to corticosteroids4 | 5 (4.2) | 2 (5.6) |
| Discontinued | 23 (19.5) | 17 (47.2) |
| Any antibiotic initiated after index date | 23 (19.5) | 5 (13.9) |
During follow-up, 65.3% of CD and 86.1% of UC patients switched to a new treatment at least once. Discontinuation or dose changes (excluding antibiotics) of IBD-related treatments occurred in 68.1% of CD and 94.3% of UC patients.
The incidence rates of drug discontinuations and dose changes were 9.2 and 6.8 changes/person-years for CD and UC patients, respectively (data not shown). During follow-up, the median number of treatment changes among CD patients was 2.0 (IQR 2-5), with 26 (33.8%) patients having more than four changes. Regarding drug classes, 19.5% of CD patients discontinued biologic therapy, 16.9% discontinued immunosuppressants, and 5.9% discontinued 5-ASA derivates.
In UC patients, the median number of treatment changes was 4.0 (IQR 3-7), with 18 (54.5%) patients having more than four treatment changes. Regarding drug classes, 22.2% of UC patients discontinued 5-ASA derivates, 13.9% discontinued biologics, and 9.3% discontinued immunosuppressants.
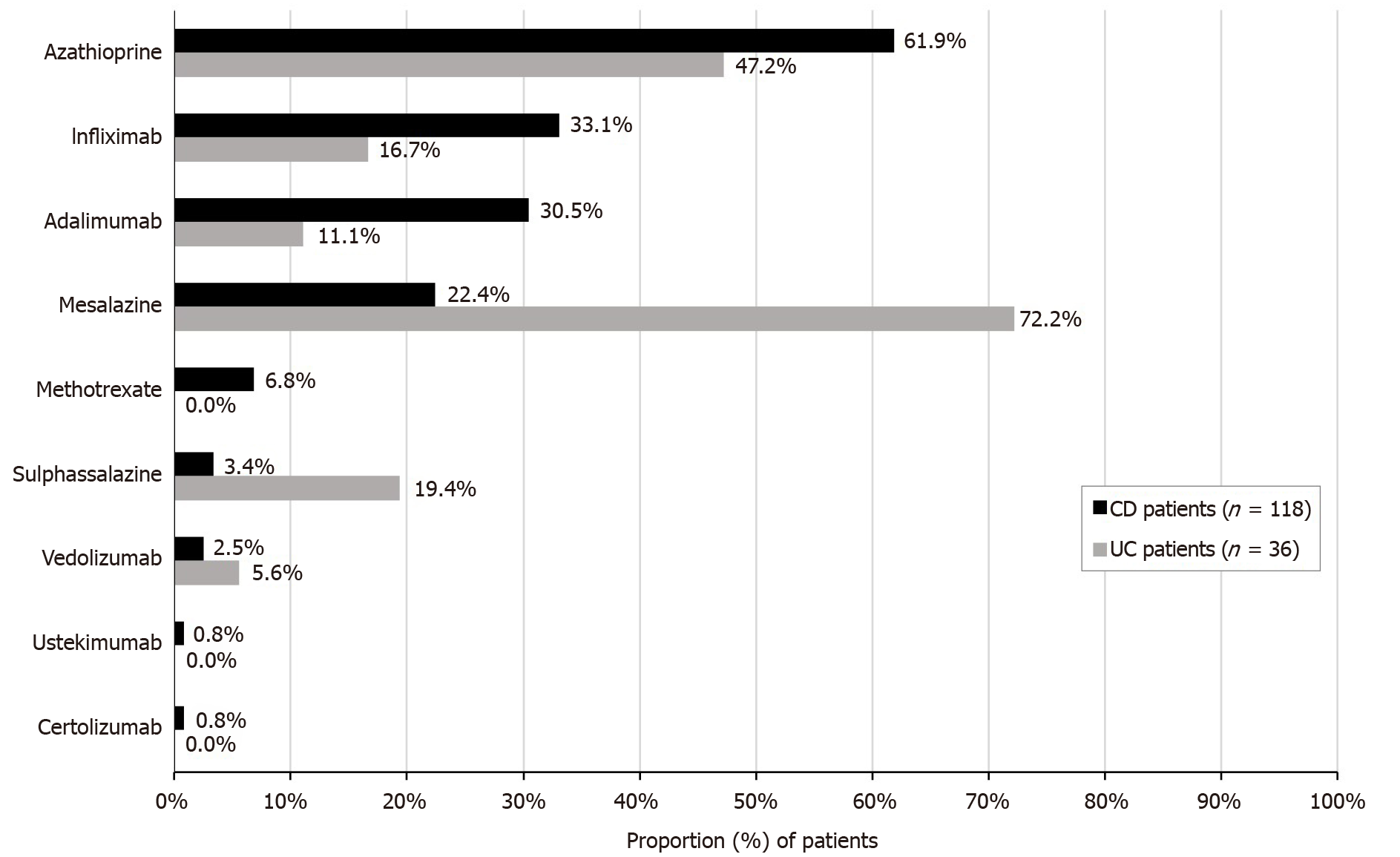
Azathioprine was the most frequently maintained or newly initiated agent in CD patients (61.9%), while mesalazine was the agent most maintained or newly initiated treatment by UC patients (72.2%), whether as monotherapy or combination therapy (Figure 2).
The vast majority (96.6%) of CD patients had at least one outpatient visit during the follow-up period, with a mean ± SD number of 4.4 ± 2.6 visits per patient and a total of 518 visits. Treatment changes were observed in 25.3% (n = 131) of CD outpatient visits, and disease activity was assessed by the HBI and/or CDAI scores in 12.6% (n = 51) and 6.4% (n = 26) of visits, respectively.
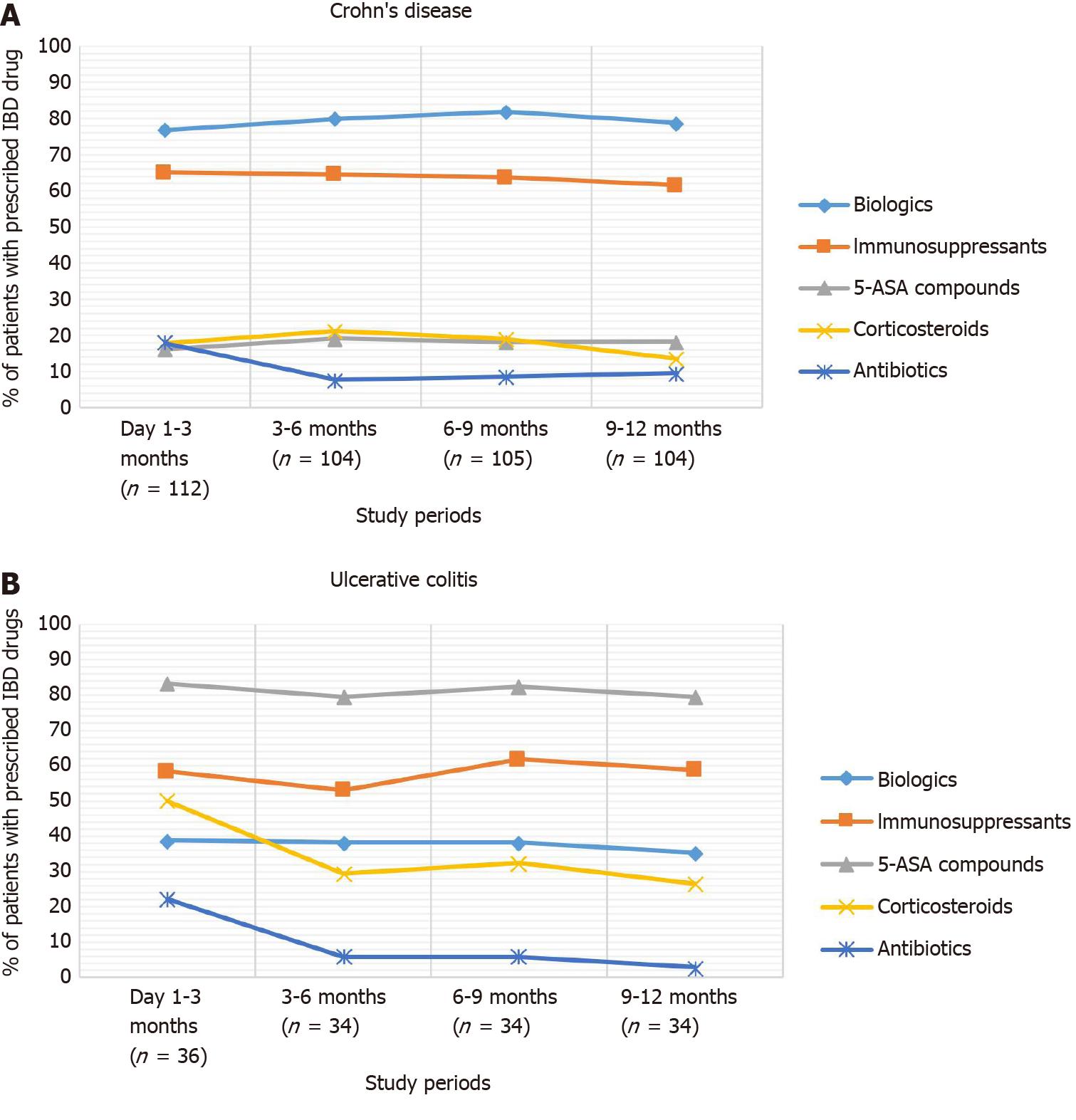
Biologics were the most frequent treatments used during follow-up (76.8% in the first quarter of the follow-up), followed by immunosuppressants (65.2%) and 5-ASA compounds (16.1%). The use of corticosteroids started to decrease from the third quarter of follow-up (Figure 3).
All UC patients had at least one visit during the follow-up period, with a mean ± SD number of 5.0 ± 3.3 visits per patient and a total of 179 visits. Treatment changes were observed among 40.2% (n = 72) of UC outpatient visits, and the assessment of UC activity with the Mayo score was performed in 11.2% (n = 16) of visits. The most prescribed agent during the first quarter of follow-up was 5-ASAs (83.3%), followed by immunosuppressants (58.3%) and biologics (38.9%). Corticosteroid use decreased from 50.0% in the first trimester to 26.5% in the last trimester of follow-up (Figure 3).
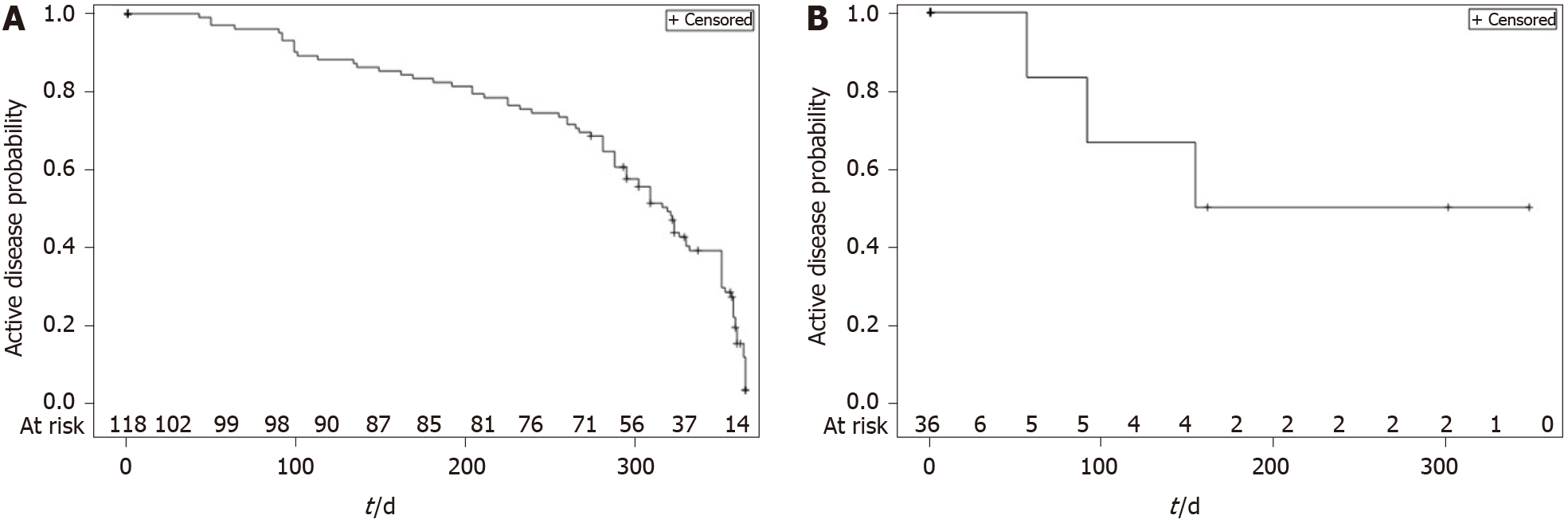
During the 12-mo follow-up period, 86 (72.9%) CD patients achieved disease control. The median time to first disease control for CD from the index date was 319 d (IQR 239-358) [95%CI: 295-330], i.e., approximately 10.6 mo (Figure 4A). There were five switches from “mild or no disease activity” to “moderate or severe disease” among the patients who had the event (control disease, n = 86).
Overall, 25 (69.4%) UC patients achieved disease control at least once, with 30.6% (n = 11) of patients remaining with “moderate or severe” disease at the end of the 12-mo period (censored observations). The median time for achieving the first episode of disease control from the index date was 320 d (IQR: 288-358) [95%CI: 302-358], i.e., approximately 10.7 mo (Figure 4B), and there were two changes from “mild or no disease activity” to “moderate or severe disease”.
Of the 107 patients who had a final evaluation during the remaining 90 d of the follow-up period, 23 (21.5%) showed moderate or severe disease activity. The proportions of CD and UC patients with active disease at the last follow-up visit were 22.0% (n = 15/82, 95%CI: 13.6%-32.5%) and 20.0% (n = 5/25, 95%CI: 6.8%-40.7%), respectively.
CD patients with active disease at the end of follow-up had a higher mean BMI at index than patients with ‘mild or no activity’ (27.5 ± 3.7 vs 24.2 ± 4.4; P = 0.007) (Table 3). Higher education was also more frequent among CD patients with mild or no disease activity at the end of follow-up than among patients with moderate-to-severe activity (42.6% vs 12.5%; P = 0.029).
| Characteristics at index date | CD patients (n = 82) | UC patients (n = 25) | ||||
| Moderate-to-severe activity (n = 18) | No or mildactivity (n = 64) | P value | Moderate-to-severe activity (n = 5) | No or mild activity (n = 20) | P value | |
| Age (yr), mean ± SD | 42.0 ± 13.0 | 43.1 ± 12.7 | 0.7323 | 38.0 ± 14.4 | 46.9 ± 17.5 | 0.3104 |
| Female, n (%) | 12 (66.7) | 39 (60.9) | 0.7865 | 4 (80.0) | 15 (75.0) | > 0.9995 |
| Employed, n (%) | 4 (23.5) | 22 (45.8) | 0.3286 | 1 (20.0) | 2 (10.5) | 0.9775 |
| Attended higher education, n (%) | 2 (12.5) | 20 (42.6) | 0.0296 | 1 (20.0) | 2 (18.2) | 0.4625 |
| Body Mass Index (kg/m2), mean ± SD | 27.5 ± 3.7 | 24.2 ± 4.4 | 0.0074 | 25.4 ± 7.5 | 24.8 ± 5.5 | 0.8383 |
| Current smokers, n (%)1 | 2 (12.5) | 4 (7.0) | 0.6075 | 0 (0.0) | 0 (0.0) | NA |
| IBD duration (yr), median (IQR) | 6.0 (1-27) | 13.0 (0-45) | 0.04333 | 5 (1-23) | 6.5 (1-23) | 0.70324 |
| Time since moderate to severe IBD (yr), median (IQR) | 5.5 (1-25) | 7.0 (0-26) | 0.88393 | 2 (0-18) | 3.5 (1-16) | 0.81013 |
| Extra intestinal manifestation, n (%) | 3 (50.0) | 17 (51.5) | 0.9466 | 1 (33.3) | 4 (40.0) | NA |
| HBI result, median (IQR) | 9.0 (7-13) | 4.0 (1-8) | NA | - | - | - |
| CDAI result, median (IQR) | 200.0 (114-311) | 224.0 (113-281) | NA | - | - | - |
| Previous calprotectin level > 200 µg/g, n (%) | 3 (16.7) | 24 (37.5) | 0.0976 | - | - | - |
| Previous colonoscopy with CD activity, n (%) | 12 (66.7) | 33 (51.6) | 0.2566 | - | - | - |
| Ileocolonic disease, n (%) | 13 (72.2) | 36 (56.3) | 0.2825 | - | - | - |
| Stricturing or penetrating CD behavior, n (%) | 11 (61.1) | 48 (75.0) | 0.2476 | - | - | - |
| Symptomatic perianal disease, n (%) | 10 (55.6) | 19 (30.2) | 0.0666 | - | - | - |
| Partial Mayo score, median (IQR) | - | - | - | 7.0 (5-8) | 6.0 (5-6) | NA |
| Left-sided or pancolitis UC, n (%) | - | - | - | 3 (60.0) | 15 (75.0) | |
| Current IBD treatment at baseline2, n (%) | 15 (83.3) | 61 (95.3) | 0.2335 | 5 (100.0) | 19 (95.0) | 0.8715 |
| Treatment ongoing or initiated at index, n (%) | 16 (88.9) | 62 (96.9) | 0.4165 | 5 (100.0) | 20 (100.0) | NA |
| 5-ASA compounds | 2 (12.5) | 10 (16.1) | 3 (60.0) | 16 (80.0) | ||
| Biologic therapy | 13 (81.3) | 41 (66.1) | 3 (60.0) | 4 (20.0) | ||
| Corticosteroids | 3 (18.8) | 9 (14.5) | 3 (60.0) | 9 (45.0) | ||
| Immunosuppressants | 5 (31.3) | 44 (71.0) | 3 (60.0) | 10 (50.0) | ||
Despite a higher proportion of CD patients with symptomatic perianal disease among patients with moderate to severe disease activity, the results were not statistically significant (55.6% vs 30.2% among patients with mild or no disease activity; P = 0.066).
No statistically significant differences were observed when comparing sociodemographic, clinical and treatment characteristics between UC patients with vs without disease activity at the end of follow-up.
This study aimed to provide a real-world perspective on the management of moderate-to-severe active IBD patients in Brazil, with a focus on treatment patterns and outcomes.
We found that more than two-thirds of IBD patients (73% and 69% for CD and UC patients) with active disease at the index date achieved disease control at least once during the 12-mo period. In addition, at the end of follow-up, approximately 20% of IBD patients had moderate-to-severe activity.
On average, almost 11 mo were required for half of UC and CD patients to achieve disease control. Considering that this is an observational study with no predefined visits, some patients did not undergo the 12-mo visit. Ideally, the control of disease activity should be achieved within 4-6 mo[22,23]. One possible explanation for this long period could be attributed to the difficulty in the public setting in Brazil to access fecal biomarkers (such as calprotectin) or routinely perform colonoscopy, which are essential assessments to evaluate the response to treatment[2,6,24]. These barriers can delay the assessment of treatment response and thus the optimization of or change in medications.
The lack of public access to serum and anti-drug antibody level testing (unless solicited and justified) could also contribute to this delay. Evidence shows the usefulness of these tests in the decision to proactively or reactively change or optimize biologic therapies[25,26]. During the follow-up period, a mean of 4.4 medical appointments were required for CD patients and 5.0 for UC patients. We observed that clinical scores to assess disease activity were not collected at the majority of appointments (the HBI was assessed only in 12.6%, the CDAI in 6.4% and the Mayo score in 11.2% of outpatient visits).
Since the assessment of symptoms is part of several disease activity scores and relevant for both patients and physicians[27-30], these data suggest that assessment of IBD activity can be improved.
For the purpose of this study, we grouped “no activity” and “mildly active” disease in the same category. The ultimate treatment goal in IBD consists of achieving mucosal healing; however, the definition for this outcome can vary. Some studies in UC consider an endoscopic response as a Mayo endoscopic score ≤ 1[6].
Biological agents were the most frequent treatment among CD patients, while 5-ASA compounds were largely the most common therapy among UC patients. Despite 5-ASA derivates not being recommended for routine use in CD patients for induction or maintenance therapy, 14.4% of CD patients received this treatment strategy. This highlights the need for continuing education of gastroenterologists in Brazil. In addition, the lack of other therapies in some regions of the country may have led to the prescription of salicylic derivatives as the only CD treatment option. In a systematic literature review conducted in 2019, the lack of evidence for conventional treatment effectiveness, especially for remission maintenance, mucosal healing and fecal calprotectin, was highlighted[31].
We observed that the majority (65.5%) of CD patients started a new therapy after the study appointment, and the most common new treatment was biologics. New treatments were found for 86.1% of UC patients, with 5-ASA compounds being the most frequent. The more recent treatment guidelines, which are also followed in Brazil, recommend 5-ASA compounds in UC treatment irrespective of disease activity, while CD patients with moderate-to-severe disease activity would benefit more from the early use of biologics and immunosuppressants[2,6]. However, we cannot exclude differences in accessibility to new and more effective drugs, as clinics from the public health system in Brazil have no reimbursement when prescribing biologics to UC patients. Furthermore, the use of immunosuppressants and biologics increased among UC patients during follow-up, suggesting that 5-ASA compounds may not be enough for achieving disease control in many patients.
A relevant proportion of CD and UC patients were receiving biologic therapy, not only at the time of the study appointment but also during the follow-up period. This finding suggests that despite difficulties in accessing biologic therapies in hospitals in Brazil, especially in UC, physicians prescribe these agents for patients with moderate-to-severe disease activity. At the time of data collection, biologic therapy was available for use but not reimbursed by public or private healthcare systems in Brazil (which would mean medication without cost for patient); however, patients with UC had access to biologics via other strategies such as judicial petition and out-of-pocket. Only from March 2020, was infliximab available for patients diagnosed with UC in the public healthcare system; infliximab, vedolizumab and golimumab were only available in the private setting in 2021. We observed a decrease in the use of corticosteroids during follow-up for both CD and UC, suggesting an adequate control of disease overtime. Nevertheless, approximately one-quarter of CD patients and half of UC patients required corticosteroids for a period longer than three months.
One of the strengths of this study was the inclusion of treated IBD patients from both public and private settings, allowing a broad characterization of this population.
Some study limitations should be recognized. The inclusion of prevalent IBD cases, with baseline variability in terms of time since diagnosis and previous treatments, was a barrier to the assessment of the association between treatment options and disease activity. Even though we found no statistically significant association between clinical and treatment variables and presenting disease activity at the end of follow-up, we cannot exclude that treatment at the index date and during follow-up may have contributed to this outcome, as we did not adjust for combined therapy, dosing information or duration of treatment (due to missing information). Moreover, the disease activity outcome was assessed at one time point only during the prospective phase (end of follow-up or month 12), making it difficult to determine whether it was a sustained control.
The inclusion of patients who had a previous event of moderate-to-severe IBD (irrespective of disease activity at index date) may affect the generalization of results for the whole IBD population in Brazil, even though these patients were selected for their higher burden of disease and thus needed closer medical follow-up. Disease activity was not assessed with the proposed scores in all outpatient medical appointments. Nevertheless, concerning disease activity, one limitation was the subjective assessment of colonoscopy, which was based on investigator criteria, which also impacted the selection of UC patients. The prospective design aimed to reduce missing data, but due to the observational character of the study, we did not proactively interfere with the usual practices for IBD activity evaluation. Finally, the sample size affected the group comparison regarding the main baseline and follow-up variables, especially for the UC patients. Furthermore, due to the small sample, one should be cautious when extrapolating the findings to a nationwide population. Additional studies are required to further understand the management of IBD in Brazil and understand the gaps and barriers in this setting.
In conclusion, to our knowledge, this was the first study aiming to characterize IBD evolution in a real-world setting in Brazil. Although a marked proportion of patients with active IBD achieved disease control within one year, the prolonged time to achieve this outcome suggests an area of unmet need with the current standard of care.
The Real-world Data of Moderate-to-Severe Inflammatory Bowel Disease in Brazil (RISE BR) study was a noninterventional study designed to evaluate disease control, treatment patterns, disease burden and health-related quality of life in patients with moderate-to-severe active inflammatory bowel diseases (IBD).
Real-world data on IBD management could unveil unmet medical needs and treatment gaps. This is of utmost relevance in developing countries such as Brazil, where the prevalence of IBD is increasing, but access to biologic treatment may be restricted, and information on IBD treatment, in general, and associated outcomes is scarce.
The aim was to describe the 12-mo disease evolution and treatment patterns among patients with active moderate-to-severe IBD in Brazil.
We report findings from the prospective follow-up phase of the RISE BR study in patients with active ulcerative colitis (UC) or Crohn’s disease (CD). This was a prospective, noninterventional study of adult patients with active CD, inadequate CD control or active UC. The proportion of active IBD patients after 9-12 mo of follow-up, the time to mild or no activity and a summary of treatment initiation, discontinuation and dose changes were evaluated.
The study included 118 CD and 36 UC patients. The most frequent drug classes at index were biologics for CD (62.7%) and 5-aminosalicylate derivates for UC patients (91.7%). During follow-up, 65.3% of CD and 86.1% of UC patients initiated a new treatment at least once. Considering the prospective follow-up period, discontinuations/dose changes occurred in 68.1% of CD patients [median 2.0 (IQR: 2-5)] and 94.3% of UC patients [median 4.0 (IQR: 3-7)]. On average, CD and UC patients had 4.4 ± 2.6 and 5.0 ± 3.3 outpatient visits, respectively. The median time to first mild or no activity was 319 (IQR: 239-358) d for CD and 320 (IQR: 288-358) d for UC patients. At 9-12 mo, 22.0% of CD and 20.0% of UC patients had active disease.
Although a marked proportion of active IBD patients achieved disease control within one year, the considerable time to achieve this outcome represents an unmet medical need of the current standard of care in a Brazilian real-world setting.
This was the first study aiming to characterize IBD evolution in a real-world setting in Brazil. Additional studies are required to further understand the management of IBD in Brazil and understand the gaps and barriers in this setting.
We would like to express our gratitude to the study participants and site staff that collaborated in the study. In addition, the authors acknowledge CTI Clinical Trial & Consulting Services for the study monitoring, statistical analysis and medical writing assistance (namely, Luís Veloso, Daniela Carvalho and Milene Fernandes) funded by Takeda Pharmaceuticals Brazil.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sipos F, Trifan A S-Editor: Gong ZM L-Editor: A P-Editor: Liu JH
| 1. | Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S; IBD Section of the British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 944] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 2. | Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1585] [Cited by in RCA: 1442] [Article Influence: 180.3] [Reference Citation Analysis (0)] |
| 3. | Brady JE, Stott-Miller M, Mu G, Perera S. Treatment Patterns and Sequencing in Patients With Inflammatory Bowel Disease. Clin Ther 2018; 40: 1509-1521. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 919] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 5. | Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501-23; quiz 524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 938] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 6. | Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnár T, Raine T, Sebastian S, de Sousa HT, Dignass A, Carbonnel F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 938] [Cited by in RCA: 866] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 7. | Lindsay JO, Armuzzi A, Gisbert JP, Bokemeyer B, Peyrin-Biroulet L, Nguyen GC, Smyth M, Patel H. Indicators of suboptimal tumor necrosis factor antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2017;49:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41:613-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 9. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1554] [Article Influence: 129.5] [Reference Citation Analysis (1)] |
| 10. | Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P, Ghosh S, de Villiers WJ, Panaccione R, Greenberg G, Schreiber S, Lichtiger S, Feagan BG; CERTIFI Study Group. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med. 2012;367:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 868] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 11. | Kirchgesner J, Lemaitre M, Rudnichi A, Racine A, Zureik M, Carbonnel F, Dray-Spira R. Therapeutic management of inflammatory bowel disease in real-life practice in the current era of anti-TNF agents: analysis of the French administrative health databases 2009-2014. Aliment Pharmacol Ther. 2017;45:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Null KD, Xu Y, Pasquale MK, Su C, Marren A, Harnett J, Mardekian J, Manuchehri A, Healey P. Ulcerative Colitis Treatment Patterns and Cost of Care. Value Health. 2017;20:752-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Schreiber S, Peyrin-Biroulet L, Loftus EV, Danese S, Colombel JF, Abhyankar B, Chen J, Rogers R, Lirio RA, Bornstein JD, Sands BE. OP34 VARSITY: A double-blind, double-dummy, randomised, controlled trial of vedolizumab vs adalimumab in patients with active ulcerative colitis. J Crohn’s Colitis. 2019;13 Suppl 1:S612-S613. [DOI] [Full Text] |
| 14. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4052] [Article Influence: 506.5] [Reference Citation Analysis (110)] |
| 15. | Lima Martins A, Volpato RA, Zago-Gomes MDP. The prevalence and phenotype in Brazilian patients with inflammatory bowel disease. BMC Gastroenterol. 2018;18:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Parra RS, Chebli JMF, Amarante HMBS, Flores C, Parente JML, Ramos O, Fernandes M, Rocha JJR, Feitosa MR, Feres O, Scotton AS, Nones RB, Lima MM, Zaltman C, Goncalves CD, Guimaraes IM, Santana GO, Sassaki LY, Hossne RS, Bafutto M, Junior RLK, Faria MAG, Miszputen SJ, Gomes TNF, Catapani WR, Faria AA, Souza SCS, Caratin RF, Senra JT, Ferrari MLA. Quality of life, work productivity impairment and healthcare resources in inflammatory bowel diseases in Brazil. World J Gastroenterol. 2019;25:5862-5882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 17. | Zaltman C, Parra RS, Sassaki LY, Santana GO, Ferrari MLA, Miszputen SJ, Amarante HMBS, Kaiser Junior RL, Flores C, Catapani WR, Parente JML, Bafutto M, Ramos O, Gonçalves CD, Guimaraes IM, da Rocha JJR, Feitosa MR, Feres O, Saad-Hossne R, Penna FGC, Cunha PFS, Gomes TN, Nones RB, Faria MAG, Parente MPPD, Scotton AS, Caratin RF, Senra J, Chebli JM. Real-world disease activity and sociodemographic, clinical and treatment characteristics of moderate-to-severe inflammatory bowel disease in Brazil. World J Gastroenterol. 2021;27:208-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 18. | Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1940] [Cited by in RCA: 2183] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 19. | Vilela EG, Torres HO, Martins FP, Ferrari Mde L, Andrade MM, Cunha AS. Evaluation of inflammatory activity in Crohn's disease and ulcerative colitis. World J Gastroenterol. 2012;18:872-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 20. | Lahiff C, Safaie P, Awais A, Akbari M, Gashin L, Sheth S, Lembo A, Leffler D, Moss AC, Cheifetz AS. The Crohn's disease activity index (CDAI) is similarly elevated in patients with Crohn's disease and in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2013;37:786-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 707] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 22. | Kuenzig ME, Benchimol EI, Lee L, Targownik LE, Singh H, Kaplan GG, Bernstein CN, Bitton A, Nguyen GC, Lee K, Cooke-Lauder J, Murthy SK. The Impact of Inflammatory Bowel Disease in Canada 2018: Direct Costs and Health Services Utilization. J Can Assoc Gastroenterol. 2019;2:S17-S33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Sales-Campos H, Basso PJ, Alves VB, Fonseca MT, Bonfá G, Nardini V, Cardoso CR. Classical and recent advances in the treatment of inflammatory bowel diseases. Braz J Med Biol Res. 2015;48:96-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Colombel JF, Narula N, Peyrin-Biroulet L. Management Strategies to Improve Outcomes of Patients With Inflammatory Bowel Diseases. Gastroenterology 2017; 152: 351-361. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 25. | Kopylov U, Ben-Horin S, Seidman E. Therapeutic drug monitoring in inflammatory bowel disease. Ann Gastroenterol. 2014;27:304-312. [PubMed] |
| 26. | López-Ibáñez M, Marín-Jiménez I. [Drugs and anti-drug antibody levels in the management of patients with inflammatory bowel disease]. Gastroenterol Hepatol. 2016;39:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Levesque BG, Sandborn WJ, Ruel J, Feagan BG, Sands BE, Colombel JF. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015; 148: 37-51. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 28. | Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D'Haens G, Dotan I, Dubinsky M, Feagan B, Fiorino G, Gearry R, Krishnareddy S, Lakatos PL, Loftus EV Jr, Marteau P, Munkholm P, Murdoch TB, Ordás I, Panaccione R, Riddell RH, Ruel J, Rubin DT, Samaan M, Siegel CA, Silverberg MS, Stoker J, Schreiber S, Travis S, Van Assche G, Danese S, Panes J, Bouguen G, O'Donnell S, Pariente B, Winer S, Hanauer S, Colombel JF. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 1399] [Article Influence: 139.9] [Reference Citation Analysis (115)] |
| 29. | Kim AH, Roberts C, Feagan BG, Banerjee R, Bemelman W, Bodger K, Derieppe M, Dignass A, Driscoll R, Fitzpatrick R, Gaarentstroom-Lunt J, Higgins PD, Kotze PG, Meissner J, O'Connor M, Ran ZH, Siegel CA, Terry H, van Deen WK, van der Woude CJ, Weaver A, Yang SK, Sands BE, Vermeire S, Travis SP. Developing a Standard Set of Patient-Centred Outcomes for Inflammatory Bowel Disease-an International, Cross-disciplinary Consensus. J Crohns Colitis. 2018;12:408-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 30. | Papay P, Ignjatovic A, Karmiris K, Amarante H, Milheller P, Feagan B, D'Haens G, Marteau P, Reinisch W, Sturm A, Steinwurz F, Egan L, Panés J, Louis E, Colombel JF, Panaccione R. Optimising monitoring in the management of Crohn's disease: a physician's perspective. J Crohns Colitis. 2013;7:653-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Damião AOMC, de Azevedo MFC, Carlos AS, Wada MY, Silva TVM, Feitosa FC. Conventional therapy for moderate to severe inflammatory bowel disease: A systematic literature review. World J Gastroenterol. 2019;25:1142-1157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |