Published online Jun 21, 2021. doi: 10.3748/wjg.v27.i23.3262
Peer-review started: January 28, 2021
First decision: February 24, 2021
Revised: March 9, 2021
Accepted: May 17, 2021
Article in press: May 17, 2021
Published online: June 21, 2021
Processing time: 140 Days and 21.8 Hours
Pancreatic cancer currently has no subtypes that inform clinical decisions; hence, there exists an opportunity to rearrange the morphological and molecular taxonomy that guides a better understanding of tumor characteristics. Nonethe
Core Tip: This review integrates the current knowledge about large-duct pattern invasive adenocarcinoma of the pancreas [large-duct pancreatic ductal adenocarcinoma (PDA)]. This subtype is a rare exocrine pancreatic neoplasm and often mimics intraductal papillary mucinous neoplasms (IPMNs). However, its prognosis is notably different from that of IPMNs, and distinguishing this subtype from IPMNs preoperatively is crucial. We summarized the morphological features and genetic landscape of large-duct PDA, with a primary focus on its differences from other types of pancreatic cystic neoplasms. The information aid in making appropriate decisions when tackling atypical pancreatic cystic neoplasms.
- Citation: Sato H, Liss AS, Mizukami Y. Large-duct pattern invasive adenocarcinoma of the pancreas–a variant mimicking pancreatic cystic neoplasms: A minireview. World J Gastroenterol 2021; 27(23): 3262-3278
- URL: https://www.wjgnet.com/1007-9327/full/v27/i23/3262.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i23.3262
Pancreatic cancer is one of the most devastating and fatal diseases in humans, with pancreatic ductal adenocarcinoma (PDA) being the most common exocrine pancreatic neoplasm[1]. With the increase in the number of patients, PDA is expected to become the second leading cause of cancer-related mortality worldwide[2,3]. Advances in multidisciplinary treatment have improved patient survival and have enhanced the effectiveness of modalities for the early detection and identification of cancer subtypes. Despite all efforts to prolong survival, PDA represents one of the worst tumors, with a 5-year overall survival rate of only 2%-9%[4]. Molecular subtyping of this tumor based on genetic signatures has been developed over the past 10 years; nevertheless, the correlation of such information with clinical phenotypes has been somewhat evasive.
Macroscopically, PDA is characterized by yellowish-white masses, usually without hemorrhagic necrosis[5,6]. On histological examination, typical PDA exhibits duct-like glandular structures infiltrating the pancreatic parenchyma, eliciting a strong desmoplastic stromal response[7,8]. The neoplastic cells are columnar to cuboidal and produce various mucins, depending on differentiation and histotypes. Several genetic alterations such as KRAS (chromosome 12p), SMAD4 (chromosome 18q), CDKN2A (chromosome 9p), and TP53 (chromosome 17p) are responsible for causing PDA[9-11] that progresses from low-grade pancreatic intraepithelial neoplasia (PanIN) to higher-grade PanIN and invasive PDA[12-16].
PDA accounts for up to 90% of pancreatic neoplasms and is typically visualized as a solid mass accompanied by abundant desmoplasia[17]. However, tumors with cystic components occasionally compose a small subset in approximately 4%-5% of patients. Colloid carcinoma, a subtype of PDA that is often conjugated to intraductal papillary mucinous neoplasms (IPMNs), is characterized by extracellular mucin pools. Morphologically, colloid carcinoma tends to exhibit a large, well-demarcated cystic lesion[18]. This subtype of PDA originates, at least in part, from IPMNs, which are well-known pancreatic cystic neoplasms[19-21]. IPMNs can be categorized into two morphologically distinct types — namely, the branched type and main duct type[22,23]. Furthermore, multiple subsets, including gastric, intestinal, and pancreatobiliary types, are histologically defined[24]. These types have unique paths during development and progression to invasive tumors[25], and specific genetic alterations observed in IPMNs may be responsible for tumor phenotypes and ultimately influence the patients’ outcome[26,27]. In most cases, other pancreatic lesions with cystic features, such as solid cystic neoplasm (SCN)[28,29], mucinous cystic neoplasm (MCN)[30], and solid pseudopapillary neoplasm (SPN)[28], can be easily distinguished from classic PDA[31]; however, atypical imaging findings of patients occasionally impair proper diagnosis. Knowledge and use of relevant genetic alterations unique to these cystic neoplasms may aid patients and physicians in making appropriate decisions.
Recent studies have revealed a histological subtype of PDA with cystic features mimicking pancreatic cystic diseases[32]. This PDA variant is referred to as large-duct pattern invasive adenocarcinoma of the pancreas (large-duct type) and is characterized by invasive PDA forming large, sometimes dilatated glands. Nevertheless, this entity is considered rare and often leads to the misdiagnosis of several pancreatic cystic neoplasms, including IPMNs. This review compares the clinical characteristics, gross morphology and histopathology, and genetic alterations of large-duct PDA and other pancreatic cystic neoplasms.
Large-duct PDA has been estimated to occur in < 7% of all PDA cases. It has been reported in only 63 patients in the past literature[33-35]. Large-duct PDA has been initially diagnosed as IPMN in most cases[34]; hence, the incidence of large-duct PDA has not been clearly recognized. Thus, regarding incidence, large-duct PDA of the pancreas is considered rare[36]. The median age of these patients with large-duct PDA was 63.0 years, and the sex ratio was female-dominant, with a female-to-male ratio of 1.74 (Table 1). The female-to-male ratio was higher in patients with large-duct PDA than in those without large-duct PDA[18]. A previous study reported no specific and unique risk factors.
Generally, 60% of classic PDAs develop in the pancreatic head[37], and the majority of the patients exhibit symptoms associated with obstructive jaundice. A summary of previously reported cases with large-duct PDA indicated similar tumor locations (Table 1). Kelly et al[34] reported that 2 out of 9 patients with large-duct PDA had jaundice. Other patients had acute pancreatitis (n = 1) and abdominal pain (n = 5).
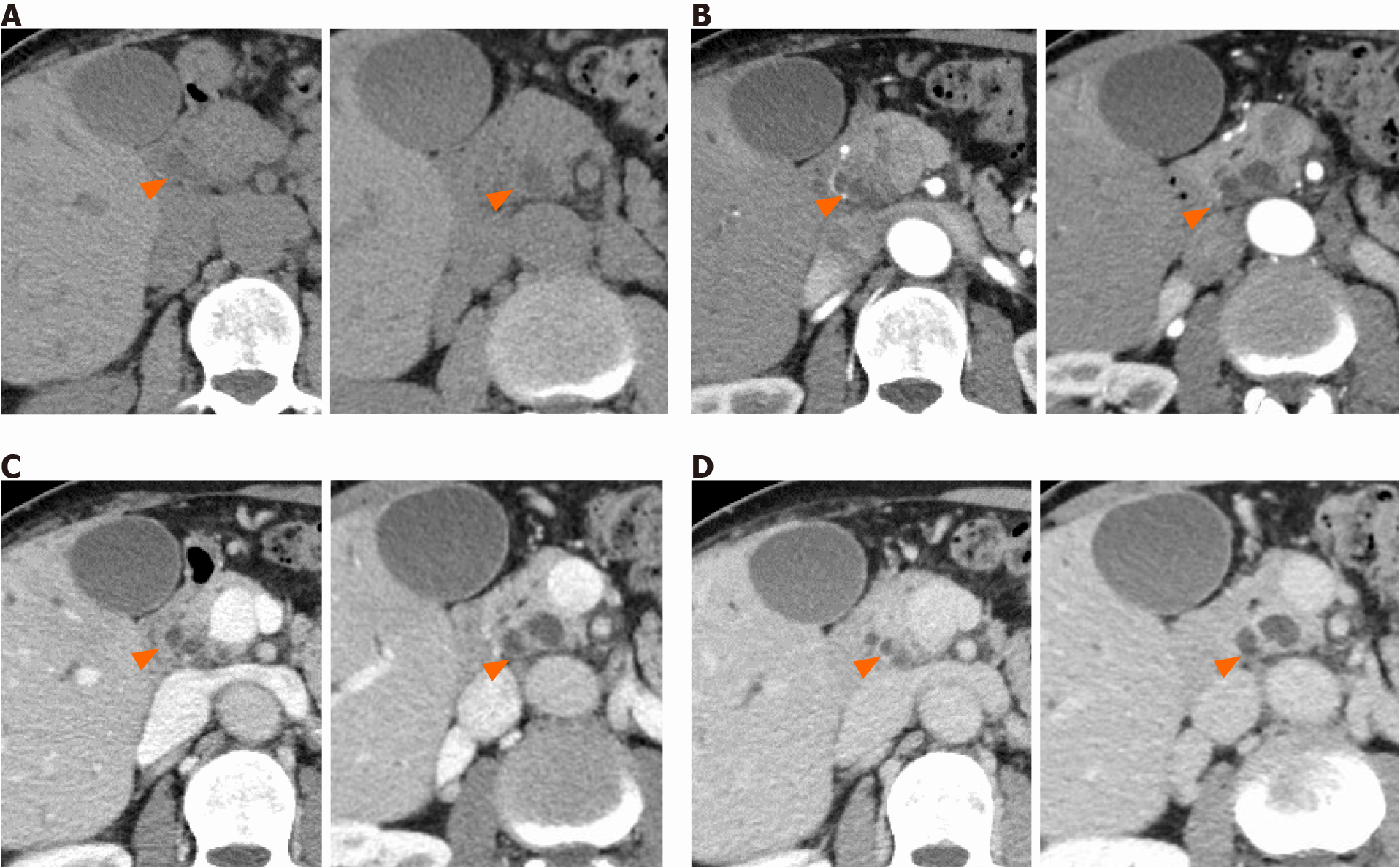
Dynamic contrast-enhanced computed tomography imaging is an essential modality for assessing cystic lesions in the pancreas. Typical PDA presents as a solid and hypovascular tumor, and the most common radiographic finding of PDA is dilatation or stricture of the main pancreatic duct. In contrast, large-duct PDA usually presents as a cluster of small cystic lesions. The diameter of cysts generally measures 5-7 mm, although they sometimes exceed 10 mm[38]. However, large-duct PDA does not have a “bunch-of-grapes” appearance[39], commonly detected in IPMNs. Given the multiple small cysts visualized on cross-sectional images, distinguishing them from SCN is more complicated, particularly in cases with macrocystic-type cysts[40]. SCN typically has a spongy or honeycomb-like appearance; one previous report described a large-duct PDA case showing a honeycomb-like morphology[36]. Large-duct PDA lacks either a star-shaped scar or calcification at the center of the cyst, commonly observed in SCN[38,41,42]. Considering the female-dominated epidemiology, MCN is also a significant differential diagnosis. MCN has an orange-like appearance and sometimes forms cystic lesions measuring over 10 cm; these features are not typical in large-duct PDA[42,43].
Another critical finding suggestive of the large-duct pattern is the slightly diminished enhancement of the parenchyma surrounding cystic components (Figure 1). Similar to typical PDA, such enhancement gradually increases with time after contrast media injection. Furthermore, walled-off necrosis (WON) should be excluded. WON often contains debris and hemorrhage in cysts, resulting in a mixed density change in the fluid[44,45]. These findings are not usually observed in large-duct PDA. Retention cysts often occur along with PDA and are also important in distinguishing it from large-duct PDA. Retention cysts are typically unilocular and tend to be larger than the cysts observed in large-duct PDA[46,47] (Table 2).
| Ref. | Diseases | Median age | Sex (%, females) | Tumor location (%, pancreatic head) | Multifocality | Gross appearance | Cyst diameter | Main pancreatic duct communication | Internal structure | Other features | Key genetic alterations |
| Bagci et al[33], 2012 | Large-duct PDA | 63 | 68.3 | 56.2 | No | Multiple small cysts | 0.5–0.7 cm in each cyst | No | Dilatated glandular cyst (duct) | Dilatated glandular cyst (duct) is > 0.5 mm in diameter. Easily invades to the perineural plexus and shows desmoplastic reaction | KRAS (codon 12) |
| Kelly et al[34], 2012 | |||||||||||
| Kosmahl et al[35], 2005 | |||||||||||
| Youn et al[38], 2018 | |||||||||||
| Seidel et al[18], 2002 | Colloid carcinoma | 61 | 52.9 | 66.7 | Infrequent | Single or multiple cysts, well demarcated | 1.2–16 cm | No | Well-defined pools of mucin, contained scanty malignant epithelial cells | Associated with intestinal-type IPMN | KRAS, BRAF, PIK3CA; MSIs are more frequently observed than non-colloid cancer PDA |
| Adsay et al[24], 2016 | |||||||||||
| Tanaka et al[41], 2012 | Branched duct-type IPMN | 65–70 | 55.2 | 62.3 | Yes | Bunch of grapes | Up to 30 mm for non-invasive IPMN; 30 mm or greater for IPMN with worrisome features | Yes | Cyst by cyst | Main pancreatic duct; normal or dilatated to > 5 mm, suggesting combined type with main duct IPMN | KRAS (codon 12), GNAS, RNF43 |
| Kim and Cho[46], 2015 | |||||||||||
| Laurent et al[51], 2016 | |||||||||||
| Hecht et al[19], 2021 | Main duct-type IPMN | 60 s | 41.3 | 59.6 | Yes | Bunch of grapes | Up to 30 mm for non-invasive IPMN; 30 mm or greater for IPMN with worrisome features | Yes | Cyst by cyst | Main pancreatic duct; partial or diffuse dilatation > 5 mm | KRAS (codon 12), GNAS, RNF43, TP53 |
| Tanaka et al[41], 2012 | |||||||||||
| Salvia et al[50], 2010 | |||||||||||
| Ånonsen et al[42], 2019 | SCN | 60–70 | 70 | 50 | No | Spongy or honeycomb-like | 3.7–5.1 cm | No | Microcystic or macrocystic | VHL | |
| Wu et al[80], 2011 | |||||||||||
| Ånonsen et al[42], 2019 | MCN | 40–50 | 95 | 5 | Infrequent | Orange-like | 1.0–26.4 cm | No | Cyst by cyst | Ovarian-like stroma | KRAS, RNF43 |
| Yamao et al[43], 2011 | |||||||||||
| Wu et al[80], 2011 | |||||||||||
| Kim and Cho[46], 2015 | Retention cyst | 60 s | ? | ? | No | Unilocular | 2.8–12 cm | Yes | No cellular dysplasia | Main pancreatic obstruction in should be observed downstream | ? |
| Assifi et al[47], 2014 | |||||||||||
| Singhi et al[61], 2012 | Pancreatic neuroendocrine neoplasms with cystic changes | 50 s | 42 | 24 | Infrequent, except MEN1-related neuroendocrine neoplasms | Pinkish-tan to yellowish in color, well demarcated | 0.8–18.0 cm | No | Well circumscribed and surrounded by a thin-to-thick fibrous capsule | Larger cysts tend to show hemorrhage | MEN1, PTEN, DAXX, ATRX, MUTYH, CHEK2 |
| Halfdanarson et al[62], 2008 | |||||||||||
| Scarpa et al[86], 2017 | |||||||||||
| Tanaka et al[41], 2012 | Walled-off necrosis | 40–50 | 25 | 45 | Rare | Variable | Variable | Yes | Unilocular | Main pancreatic duct; normal or irregularly dilatated. Observed no later than 6 wk after the occurrence of acute pancreatitis | |
| Cohen et al[45], 2003 |
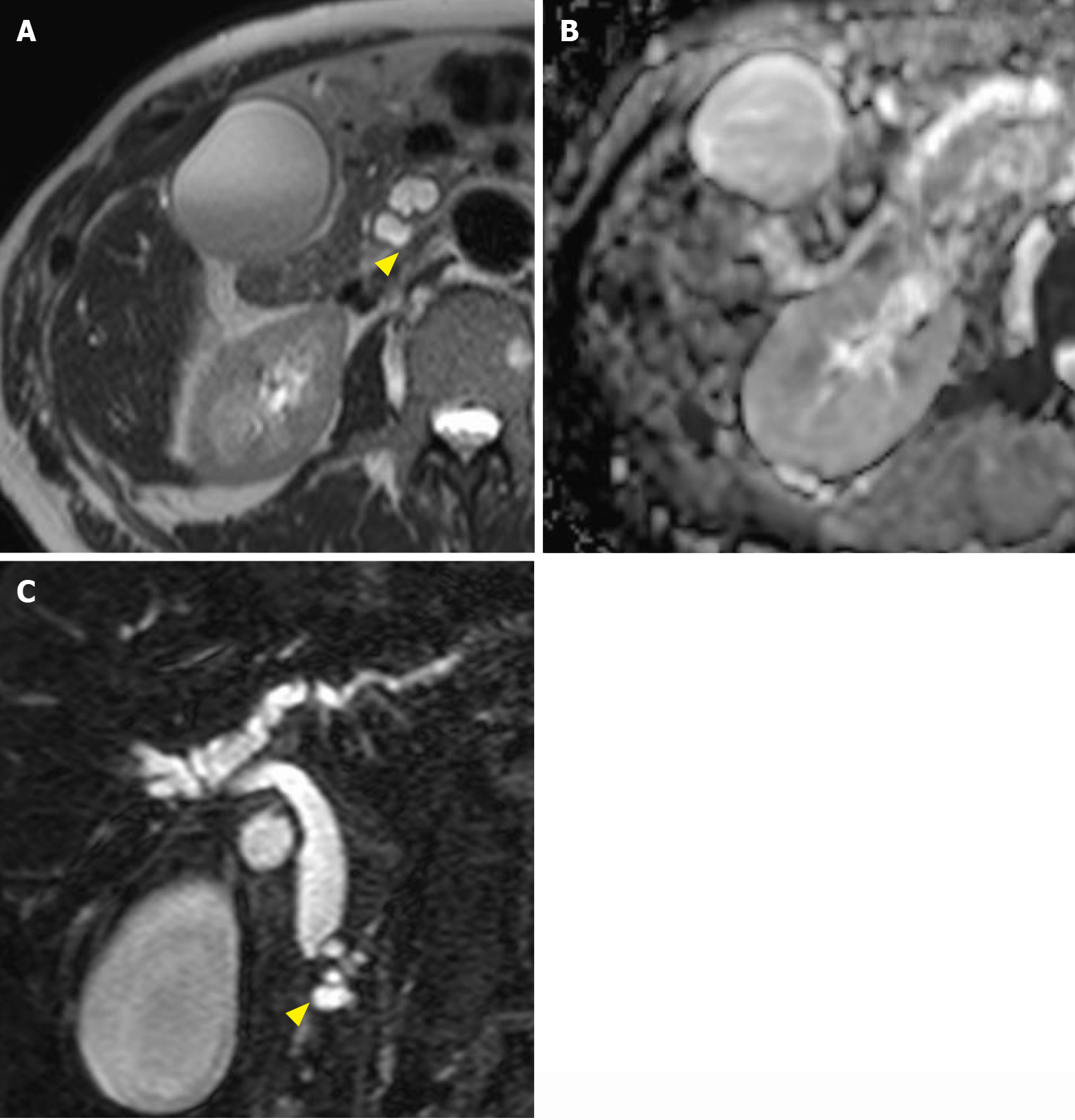
Magnetic resonance cholangiopancreatography is another essential modality for diagnosing pancreatic cystic diseases, except in claustrophobic individuals and those with implanted magnetic resonance imaging-unsafe foreign bodies. Crucial findings for large-duct PDA include small cysts in the pancreas[48], with each cyst usually having a diameter of 0.5-0.7 cm[49]. It should be noted that pancreatic duct stricture is more intense than that observed in other cystic neoplasms. Stricture of the main pancreatic duct and bile duct closely associated with a cluster of small cysts is another finding that we may consider when diagnosing large-duct PDA (Figure 2). In making a differential diagnosis of IPMNs, communication with the main pancreatic duct is crucial. Both typical main duct-type and branched duct-type IPMNs communicate with the main pancreatic duct and often show dilatation of the main pancreatic duct[41,50,51].
Moreover, diffusion-weighted imaging and apparent diffusion coefficient map on magnetic resonance cholangiopancreatography is helpful in diagnosing PDA. Considering the high intensity of cystic lesions on T2-weighted images[52,53], diffusion-weighted imaging may not help distinguish large-duct PDA from other pancreatic cystic diseases (Figure 2).
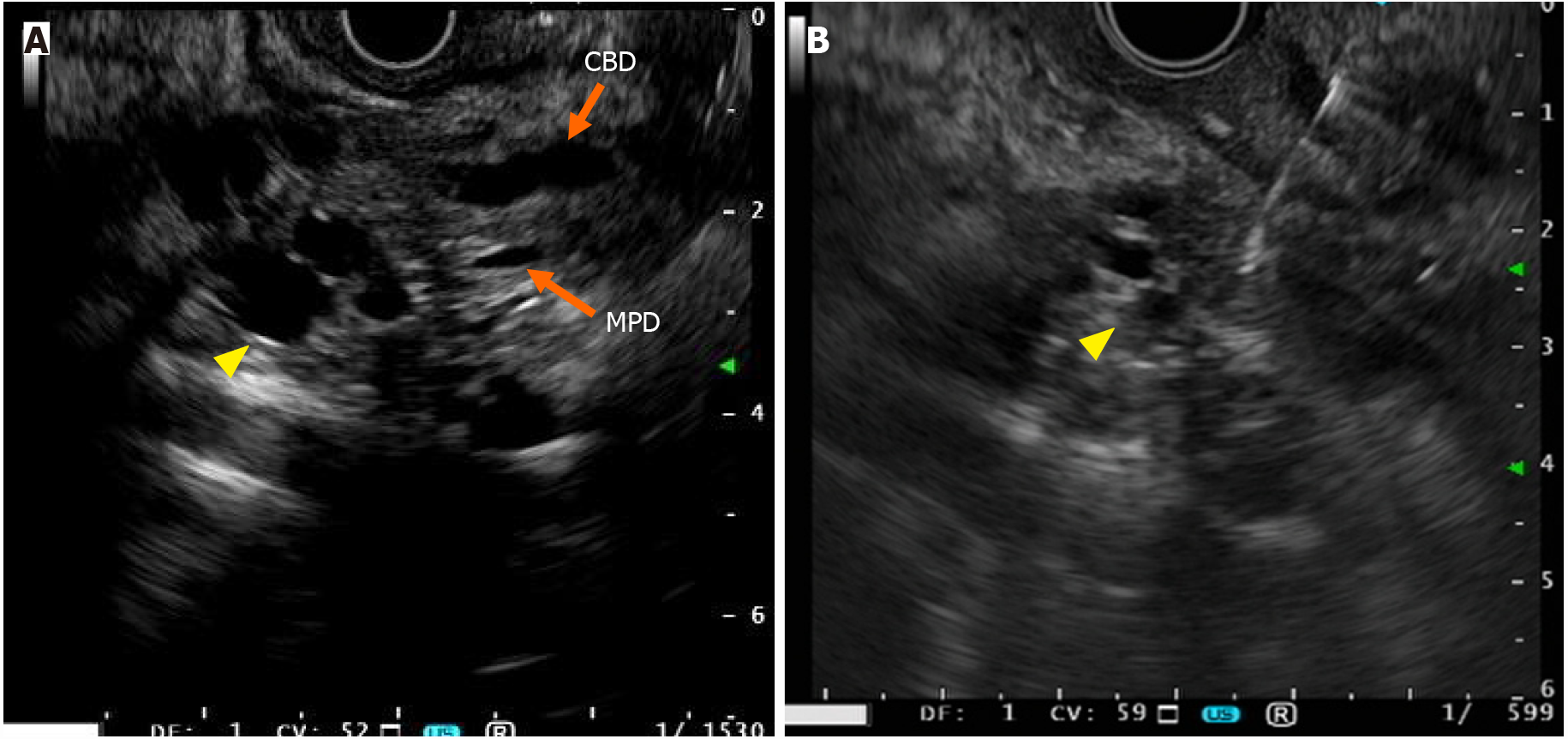
Endoscopic ultrasonography (EUS) has the highest sensitivity for detecting PDA lesions[54], specifically small-sized tumors. Irrespective of the size of PDA, non-neoplastic cystic changes associated with PDA include retention cysts caused by ductal obstruction, and pseudocysts attributable to tumor-associated pancreatitis may be considered to distinguish it from large-duct type tumors[55,56]. A “mucinous clot,” which is a hyperechoic lesion inside cysts, is sometimes visualized using EUS in patients with IPMNs, but not in those with large-duct PDAs[57,58]. Furthermore, large-duct PDAs typically lack mural nodules and papillary growth, signatures of high-risk stigmata, and worrisome features in the Fukuoka criteria[41,59]. Youn et al[38] described a case of large-duct PDA with a solid mass inside a dilatated glandular cyst[38]. Such atypical findings make a differential diagnosis between large-duct PDAs and IPMNs with high-risk stigmata confusing among endoscopists; nonetheless, surgical resection should be performed for either disease. Considering the multifocality of IPMNs, performing EUS for multifocal lesions, which are not observed in large-duct PDA, is crucial (Table 2)[41,51]. The morphology of “cyst by cyst” appea
No report has specifically described EUS-guided fine-needle aspiration (EUS-FNA) in patients with large-duct PDA. In the case of pancreatic tumors with cystic components, concerns related to tumor cell dissemination from the cystic fluid (needle tract seeding)[60] may need to be pointed out (Figure 3). Thus, drawing a distinction between large-duct PDA and IPMN-associated cancer using EUS-FNA can become highly challenging, which will be explained well in the “Pathology” section. The requirements for preoperative histological diagnosis of PDA have increased in the neoadjuvant chemotherapy era; therefore, an ingenious solution for the safe and accurate diagnosis of large-duct PDA using EUS-FNA needs to be considered. No report regarding endoscopic retrograde cholangiopancreatography has described specific findings for large-duct PDA. For tumors developing in the pancreatic head, transpapillary biopsy for histological diagnosis can be useful if massive bile duct invasion is evident.
Bagci et al[33] described 28 cases of large-duct PDA. Macroscopically, 10 patients had a cyst measuring < 10 mm, whereas 1 patient had a large cyst over 70 mm[33]. Pathologically, each small cyst is a dilatated glandular carcinoma. Similarly, Kosmahl et al[35]. described 24 cases of large-duct PDA and reported a diameter ranging from 4 to 18 mm for each cyst; the gross tumor size was not different from ordinary PDA[35]. The entire cystic lesion size is crucial, and the cystic component of large-duct PDA is less than 100 mm generally. In comparison, the size of cysts in MCN, SCN, or pancreatic neuroendocrine neoplasms (PanNENs) sometimes exceeds 100 mm (Table 2)[61,62].
Microscopically, large-duct PDA shows dilatated glands that range in size from 5 to 10 mm. The term “large duct” is defined as dilatation greater than 0.5 mm among ≥ 50% of glands in PDA[33]. Hematoxylin–eosin staining has revealed some essential pathological findings for large-duct PDA. The nuclei of the epithelial carcinoma (dilatated glands) are irregular, wrinkled, and basally located. The cytoplasm exhibits a foamy or microvesicular pattern, and the glands are jagged and irregular[34]. While a papillary growth pattern is typically absent, discrimination of large-duct PDA from other cystic neoplasms, especially gastric-type IPMNs, is crucial[63,64]. In contrast to mucinous carcinoma, large-duct PDA lacks signet ring cells or mucinous lakes[65,66].
Perineural invasion is another important finding for diagnosing large-duct PDA to discriminate from other cystic neoplasms. Bagci et al[33] reported that perineural invasion occurred in 88% of large-duct PDAs[33] but was less commonly observed in IPMNs, even though the tumor had invasive compartments. In large-duct PDA, the stroma has a rich desmoplastic appearance[33,34]. These features are commonly observed in PDA compared to IPMNs; however, they are more remarkable in large-duct PDA. Additionally, hypercellular stroma or ovarian-like stroma is present in some cases. As for the differential diagnosis of colloid carcinoma or PanNENs, the cysts in these lesions are relatively well-demarcated relative to those in large-duct PDA. PanNENs sometimes have a thick fibrous capsule with hemorrhage[61]. WON usually shows necrotic debris in cysts. Macroscopically, this finding is usually not observed in large-duct PDA and can be a point to distinguish one from other cystic lesions[67].
Immunohistochemistry (IHC) may be a crucial diagnostic tool that can be utilized to discriminate large-duct PDA from other pancreatic cystic neoplasms[36]. Among the immunostaining widely used in the diagnosis of tumors, mucin (MUC) staining is particularly important and may play a key role in diagnosing large-duct PDA. Gastric-type IPMN exhibits relatively low papillary growth[64] and is typically low grade. However, in tumors with high-grade gastric IPMN, distinction from the large-duct type PDA is sometimes uncertain pathologically. IPMNs present unique MUC staining patterns, depending on the epithelial subtypes[63]. Gastric-type IPMNs show MUC5AC-positive staining patterns and are negative for MUC1, MUC2, and MUC6. Kelly et al[34] and Kosmahl et al[35] reported MUC staining for large-duct PDAs (Table 3)[34,35]; the positivity rates of MUC1, MUC2, MUC5AC, and MUC6 immunostaining were 79.4%, 8.8%, 78.8%, and 60.6%, respectively. Thus, MUC1 and MUC6 IHC may help make to a differential diagnosis from IPMNs[68].
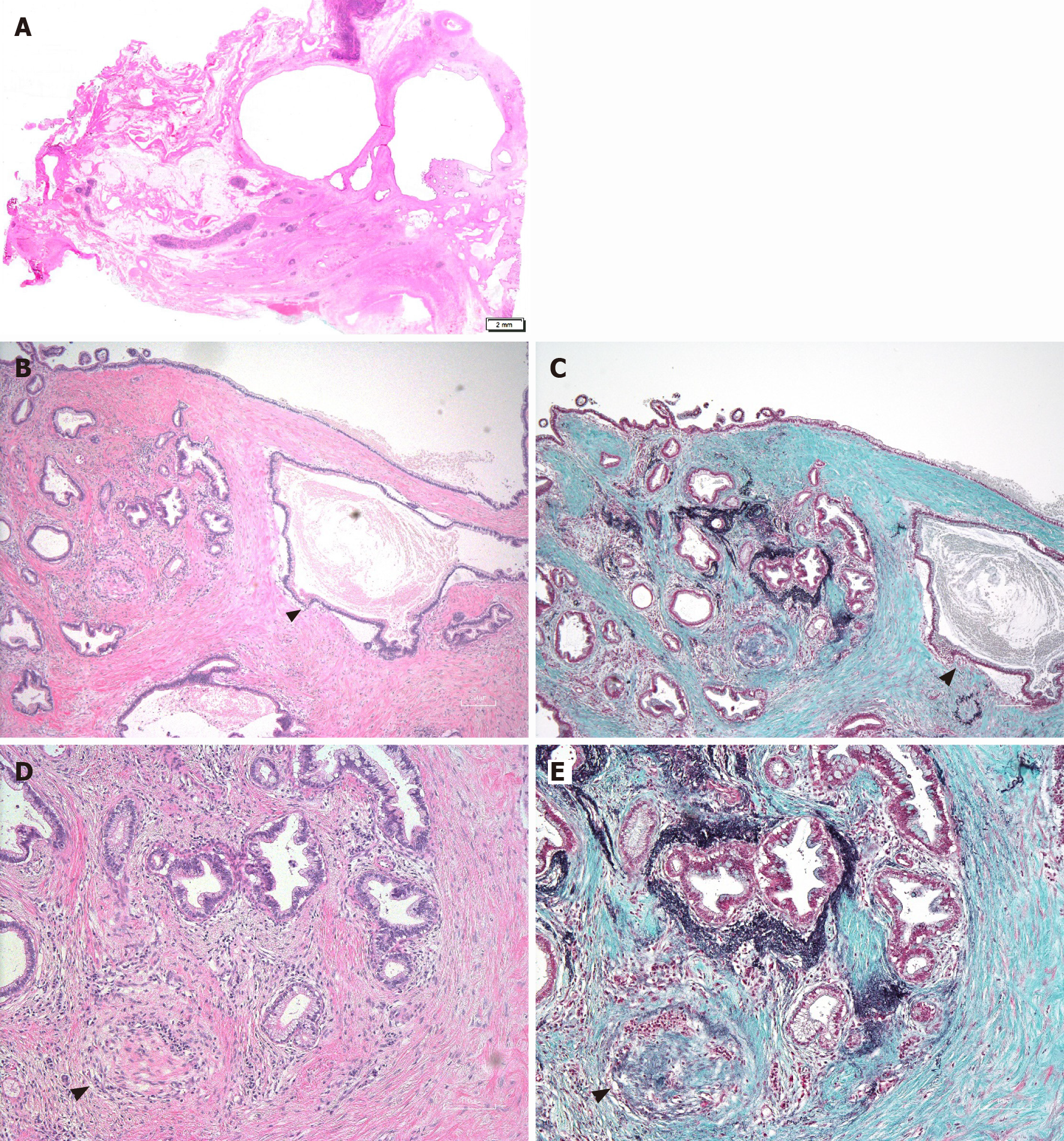
Furthermore, p53 IHC is beneficial in diagnosing lesions with malignant potential. Bagci et al[33] showed that 73% of all large-duct PDAs positively stained for p53. Another point of IHC for large-duct PDA is whether the dilatated glands are dilatated glandular carcinomas or dilatated glands resulting from the occlusion of upper-stream pancreatic ducts. To presume the origin of anatomical localization of the dilated pancreatic ducts, Elastica–Masson staining may be suitable (Figure 4). In addition to the main pancreatic duct, elastin fibers in these normal pancreatic ducts can be stained with Elastica–Masson. Typically, the dilatated glands in large-duct PDA are negative for the immunostaining, suggesting they were derived from tumor glands rather than the secondary dilation of the normal main pancreatic duct and associated large branch duct[69].
KRAS is mutated in the great majority of PDA, and approximately 90%-95% of PDA patients have major hot spot mutations in codons 12, 13, and 61[9]. This genetic event has been identified at the earliest stages of PanIN, and studies in genetically engineered mice models (GEMMs) indicate that PanINs arise from pancreatic acinar cells that incur Kras mutations (Ptf1a-induced Kras activation). In the KrasG12D-expressing mice, PanIN formation coincides with acinar-to-ductal metaplasia (ADM), characterized by the replacement of acinar cells with cells expressing ductal markers, such as CK19 and the ductal fate determinant Sox9[70]. Further, propensity of ductal and centroacinar cells to give rise to PanINs is demonstrated by GEMM with Sox9CreER-mediated Kras activation, although considerably less frequent relative to Ptf1aCreER-induced Kras activation[71].
Although observations in these mice models have not been validated in humans, multiple PanIN-like lesions with various grades of atypia can often be observed in the “normal area” of the resected specimens of pancreatic cancer. These lesions are usually solitary and localized in the normal acinar compartment without pancreatitis. However, PanINs associated with a cluster of ADM can also be seen[9]. Nevertheless, it remains to be determined if the PDA, including large-duct type, originated from either differentiated duct or duct-like cells associated with ADM. Such hypothesis may be clarified based on the particular mutation profiles and molecular signature related to cellular origin.
Moreover, genetic analysis is valuable in establishing a differential diagnosis between large-duct PDA and IPMNs. Bagci et al[33] conducted a mutation analysis of KRAS codon 12, which is the most frequent mutation point in PDA, using specimens from 28 large-duct PDA patients compared to ordinary ductal adenocarcinoma of the pancreas[33]. The results indicated 82% of large-duct PDAs harbored KRAS codon 12 mutations, a bit less frequency observed in classic PDAs (generally over 90%)[33,72]. Since the authors utilized slot-blot Southern analysis of polymerase chain reaction-amplified samples, KRAS mutation in codon 12 might be missed in case of the tumor with low tumor cellularity, and latest methods may also identify other hotspots at codons 13 and 61. Additionally, IPMN can be initiated by KRAS mutation[9], although less frequently than PanIN[73,74] . KRAS mutations are observed in 53%-87% of gastric-type IPMNs and approximately 40% in intestinal-type IPMNs. In contrast to PDAs including large-duct type, IPMNs frequently harbor multiple different types of KRAS mutations, suggesting poly-clonal diseases[25]. Therefore, specifying the variation in KRAS mutation types may be valuable in determining a subset of patients with large-duct PDA.
TP53 gene is also frequently mutated around 75% of pancreatic carcinoma patients[75], and Bagci et al[33] reported that abnormal immunostaining of p53 was observed in 73% of large-duct type PDA, a higher frequency relative to classic PDA (60%). Moreover, TP53 mutation can be seen in 10%-40% of high-grade IPMNs and 40%-60% of IPMN-associated invasive carcinomas[73,76]. Given the strong gain-of-function of particular missense mutations in TP53 relative to the truncating mutations[77,78], further studies will be required if such TP53 mutation types are associated with the tumor phenotypes. SMAD4 is also inactivated in 55% of PDA cases; of these, 35% are inactivated by homozygous deletion, whereas 20% show loss of one allele[10]. Inactivation of SMAD4 enhances the rapid progression of KrasG12D-initiated mice pancreatic neoplasms, showing IPMN-like phenotypes[79], and a significant fraction of IPMN-associated human pancreatic cancer harbors SMAD4 mutation and the abnormal immunostaining[25]. Whether SMAD4 inactivation can influence the progression or promotion of large-duct PDA remains unclear.
GNAS mutations are unique to IPMNs[80,81] and are more frequently identified in intestinal-type IPMNs than in gastric- and pancreatobiliary-type IPMNs[9]. The mutation can progress to IPMN-associated PDA, and recent studies demonstrated that about 8%-11% of pancreatic cancers[9], including cases considered not related explicitly to IPMN, harbor GNAS mutations or amplifications. Since GNAS mutation can lead to entirely distinct transcriptional and metabolic reprogramming to KRAS-driven circuitry[82], identification of GNAS mutations may discriminate large-duct PDA from IPMN-related pancreatic cancer.
RNF43 encodes an E3 ubiquitin ligase. RNF43 mutations are observed in IPMNs and MCNs and are detected in approximately 50% of all IPMNs[83]. Nonetheless, a recent meta-analysis has suggested that RNF43 mutations are not associated with clinicopathologic parameters in patients with IPMN[83,84]. Approximately 50% of MCNs present RNF43 mutations, including loss of heterozygosity[85]. Other cystic neoplasms in the pancreas exhibit specific genetic mutations. Genetic alterations such as VHL mutations and loss of heterozygosity in SCN, CTNNB1, and PIK3CA mutations in SPN and PanNEN-associated mutations (MEN1, PTEN, DAXX, and ATRX) have been speci
Further genetic analysis should be performed in the large-duct PDA, considering whether the cellular origin of the large-duct PDA was the branch or main pancreatic duct and pathways of carcinogenesis[87,88]. Whole-genome sequencing may be helpful in determining the genes responsible for forming large-duct glands[87,89].
Large-duct PDA has been recognized as an uncommon subtype of PDA. Hence, in previous reports, large-duct PDA was mainly diagnosed from surgically resected specimens[33,34]. Preoperative diagnosis was variable, including IPMN, MCN, and “PDA with solid and cystic mass.” Furthermore, the patients in these reports were diagnosed at an advanced stage, and the period of survival after resections was about 7-16 mo. These reports were published in the era when adjuvant or neoadjuvant chemotherapy for PDA was not established. The outcome is worse than that of surgically resected IPMNs, reported as 37.0 mo[90], and chemotherapy is not generally considered if the invasive component in IPMN-associated cancer is not evident. Therefore, distinguishing large-duct PDA from other cystic neoplasms, including IPMNs, is essential. Accurate histological and genomic information related to the tumor will help decide between appropriate therapeutic options before surgery[91-93]. Currently, how standard chemotherapy against PDAs, including both adjuvant and neoadjuvant chemotherapy, can effectively eradicate large-duct PDAs remains unclear.
Large-duct PDA is a subtype of PDA that mimics IPMNs or other pancreatic cystic neoplasms. Given the rarity of this disease, the diagnostic approach is sometimes challenging. Considering the estimated prognosis of the patients, it is crucial to distinguish large-duct PDAs from IPMNs using macroscopic and pathological findings. When an atypical cystic lesion is identified in the pancreas, both symptomatic and asymptomatic, the differential diagnosis should include large-duct PDA. Given their advantages, genetic analysis of PDAs and IPMNs, exploration of KRAS mutations, and mutation profiling of other cancer-related genes may help establish an accurate diagnosis.
We thank Hiroyuki Maguchi at Teine Keijinkai Hospital for providing support in the pathological and clinical diagnoses, as well as the members of the Department of Medicine at Asahikawa Medical University for carefully reading and examining the manuscript.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Japanese Society of Internal Medicine, No. 99021; Japanese Association for Medical Artificial Intelligence, No. 388; Japanese Board of Cancer Therapy, No. 1810053; Japan Gastroenterological Endoscopy Society, No. 20190318; The Japanese Society of Gastroenterology, No. 39625; and American Gastroenterological Association, No. 1156949.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ker CG, Neri V, Thosani N, Wang XB S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
| 1. | Fitzgerald TL, Hickner ZJ, Schmitz M, Kort EJ. Changing incidence of pancreatic neoplasms: a 16-year review of statewide tumor registry. Pancreas. 2008;37:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5134] [Article Influence: 466.7] [Reference Citation Analysis (0)] |
| 3. | Goess R, Friess H. A look at the progress of treating pancreatic cancer over the past 20 years. Expert Rev Anticancer Ther. 2018;18:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1262] [Article Influence: 180.3] [Reference Citation Analysis (39)] |
| 5. | Schlitter AM, Jesinghaus M, Jäger C, Konukiewitz B, Muckenhuber A, Demir IE, Bahra M, Denkert C, Friess H, Kloeppel G, Ceyhan GO, Weichert W. pT but not pN stage of the 8th TNM classification significantly improves prognostication in pancreatic ductal adenocarcinoma. Eur J Cancer. 2017;84:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | He J, Ahuja N, Makary MA, Cameron JL, Eckhauser FE, Choti MA, Hruban RH, Pawlik TM, Wolfgang CL. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB (Oxford). 2014;16:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 7. | Schlitter AM, Segler A, Steiger K, Michalski CW, Jäger C, Konukiewitz B, Pfarr N, Endris V, Bettstetter M, Kong B, Regel I, Kleeff J, Klöppel G, Esposito I. Molecular, morphological and survival analysis of 177 resected pancreatic ductal adenocarcinomas (PDACs): Identification of prognostic subtypes. Sci Rep. 2017;7:41064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am. 2007;36:831-849, vi. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Patra KC, Bardeesy N, Mizukami Y. Diversity of Precursor Lesions For Pancreatic Cancer: The Genetics and Biology of Intraductal Papillary Mucinous Neoplasm. Clin Transl Gastroenterol. 2017;8:e86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1669] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 11. | Caldas C, Hahn SA, da Costa LT, Redston MS, Schutte M, Seymour AB, Weinstein CL, Hruban RH, Yeo CJ, Kern SE. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994;8:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 754] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 12. | Real FX. A "catastrophic hypothesis" for pancreas cancer progression. Gastroenterology. 2003;124:1958-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Hata T, Suenaga M, Marchionni L, Macgregor-Das A, Yu J, Shindo K, Tamura K, Hruban RH, Goggins M. Genome-Wide Somatic Copy Number Alterations and Mutations in High-Grade Pancreatic Intraepithelial Neoplasia. Am J Pathol. 2018;188:1723-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Wilentz RE, Iacobuzio-Donahue CA, Argani P, McCarthy DM, Parsons JL, Yeo CJ, Kern SE, Hruban RH. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002-2006. [PubMed] |
| 15. | Cáceres M, Quesada R, Iglesias M, Real FX, Villamonte M, de Villarreal JM, Pérez M, Andaluz A, Moll X, Berjano E, Dorcaratto D, Sánchez-Velázquez P, Grande L, Burdío F. Pancreatic duct ligation reduces premalignant pancreatic lesions in a Kras model of pancreatic adenocarcinoma in mice. Sci Rep. 2020;10:18344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Kaneda A, Matsusaka K, Aburatani H, Fukayama M. Epstein-Barr virus infection as an epigenetic driver of tumorigenesis. Cancer Res. 2012;72:3445-3450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | Di Maggio F, El-Shakankery KH. Desmoplasia and Biophysics in Pancreatic Ductal Adenocarcinoma: Can We Learn From Breast Cancer? Pancreas. 2020;49:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Seidel G, Zahurak M, Iacobuzio-Donahue C, Sohn TA, Adsay NV, Yeo CJ, Lillemoe KD, Cameron JL, Hruban RH, Wilentz RE. Almost all infiltrating colloid carcinomas of the pancreas and periampullary region arise from in situ papillary neoplasms: a study of 39 cases. Am J Surg Pathol. 2002;26:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Hecht EM, Khatri G, Morgan D, Kang S, Bhosale PR, Francis IR, Gandhi NS, Hough DM, Huang C, Luk L, Megibow A, Ream JM, Sahani D, Yaghmai V, Zaheer A, Kaza R. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: recommendations for Standardized Imaging and Reporting from the Society of Abdominal Radiology IPMN disease focused panel. Abdom Radiol (NY). 2021;46:1586-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Nasca V, Chiaravalli M, Piro G, Esposito A, Salvatore L, Tortora G, Corbo V, Carbone C. Intraductal Pancreatic Mucinous Neoplasms: A Tumor-Biology Based Approach for Risk Stratification. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Zaccari P, Cardinale V, Severi C, Pedica F, Carpino G, Gaudio E, Doglioni C, Petrone MC, Alvaro D, Arcidiacono PG, Capurso G. Common features between neoplastic and preneoplastic lesions of the biliary tract and the pancreas. World J Gastroenterol. 2019;25:4343-4359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Lu C, Xu CF, Wan XY, Zhu HT, Yu CH, Li YM. Screening for pancreatic cancer in familial high-risk individuals: A systematic review. World J Gastroenterol. 2015;21:8678-8686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Chang YR, Park JK, Jang JY, Kwon W, Yoon JH, Kim SW. Incidental pancreatic cystic neoplasms in an asymptomatic healthy population of 21,745 individuals: Large-scale, single-center cohort study. Medicine (Baltimore). 2016;95:e5535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Adsay V, Mino-Kenudson M, Furukawa T, Basturk O, Zamboni G, Marchegiani G, Bassi C, Salvia R, Malleo G, Paiella S, Wolfgang CL, Matthaei H, Offerhaus GJ, Adham M, Bruno MJ, Reid MD, Krasinskas A, Klöppel G, Ohike N, Tajiri T, Jang KT, Roa JC, Allen P, Fernández-del Castillo C, Jang JY, Klimstra DS, Hruban RH; Members of Verona Consensus Meeting; 2013. Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting. Ann Surg. 2016;263:162-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 25. | Omori Y, Ono Y, Tanino M, Karasaki H, Yamaguchi H, Furukawa T, Enomoto K, Ueda J, Sumi A, Katayama J, Muraki M, Taniue K, Takahashi K, Ambo Y, Shinohara T, Nishihara H, Sasajima J, Maguchi H, Mizukami Y, Okumura T, Tanaka S. Pathways of Progression From Intraductal Papillary Mucinous Neoplasm to Pancreatic Ductal Adenocarcinoma Based on Molecular Features. Gastroenterology 2019; 156: 647-661. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 26. | Schönleben F, Qiu W, Bruckman KC, Ciau NT, Li X, Lauerman MH, Frucht H, Chabot JA, Allendorf JD, Remotti HE, Su GH. BRAF and KRAS gene mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/IPMC) of the pancreas. Cancer Lett. 2007;249:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Tan MC, Basturk O, Brannon AR, Bhanot U, Scott SN, Bouvier N, LaFemina J, Jarnagin WR, Berger MF, Klimstra D, Allen PJ. GNAS and KRAS Mutations Define Separate Progression Pathways in Intraductal Papillary Mucinous Neoplasm-Associated Carcinoma. J Am Coll Surg 2015; 220: 845-854. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Chagas VL, Rosman FC, Carvalho MDGDC. Solid pseudopapillary neoplasia of the pancreas: a review. Rev Assoc Med Bras (1992). 2020;66:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Gandhi D, Sharma P, Parashar K, Kochar PS, Ahuja K, Sawhney H, Sharma S. Solid pseudopapillary Tumor of the Pancreas: Radiological and surgical review. Clin Imaging. 2020;67:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Nilsson LN, Keane MG, Shamali A, Millastre Bocos J, Marijinissen van Zanten M, Antila A, Verdejo Gil C, Del Chiaro M, Laukkarinen J. Nature and management of pancreatic mucinous cystic neoplasm (MCN): A systematic review of the literature. Pancreatology. 2016;16:1028-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Hasan A, Visrodia K, Farrell JJ, Gonda TA. Overview and comparison of guidelines for management of pancreatic cystic neoplasms. World J Gastroenterol. 2019;25:4405-4413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (2)] |
| 32. | Nitta T, Mitsuhashi T, Hatanaka Y, Hirano S, Matsuno Y. Pancreatic ductal adenocarcinomas with multiple large cystic structures: a clinicopathologic and immunohistochemical study of seven cases. Pancreatology. 2013;13:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Bagci P, Andea AA, Basturk O, Jang KT, Erbarut I, Adsay V. Large duct type invasive adenocarcinoma of the pancreas with microcystic and papillary patterns: a potential microscopic mimic of non-invasive ductal neoplasia. Mod Pathol. 2012;25:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Kelly PJ, Shinagare S, Sainani N, Hong X, Ferrone C, Yilmaz O, Fernández-del Castillo C, Lauwers GY, Deshpande V. Cystic papillary pattern in pancreatic ductal adenocarcinoma: a heretofore undescribed morphologic pattern that mimics intraductal papillary mucinous carcinoma. Am J Surg Pathol. 2012;36:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Kosmahl M, Pauser U, Anlauf M, Klöppel G. Pancreatic ductal adenocarcinomas with cystic features: neither rare nor uniform. Mod Pathol. 2005;18:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Adsay V, Logani S, Sarkar F, Crissman J, Vaitkevicius V. Foamy gland pattern of pancreatic ductal adenocarcinoma: a deceptively benign-appearing variant. Am J Surg Pathol. 2000;24:493-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2959] [Cited by in RCA: 2761] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 38. | Youn SY, Rha SE, Jung ES, Lee IS. Pancreas ductal adenocarcinoma with cystic features on cross-sectional imaging: radiologic-pathologic correlation. Diagn Interv Radiol. 2018;24:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Kovač JD, Janković A, Mašulović D. The “bunch of grapes” pattern of branch-duct IPMN. Abdom Radiol (NY). 2019;45:249-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Chakravarthy S, Chakravarty R, Sarkar N, Chitrotpala R. Radiological diagnosis of rare pancreatic serous cystadenoma. J Family Med Prim Care. 2019;8:2744-2746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 42. | Ånonsen K, Sahakyan MA, Kleive D, Waage A, Verbeke C, Hauge T, Buanes T, Edwin B, Labori KJ. Trends in management and outcome of cystic pancreatic lesions - analysis of 322 cases undergoing surgical resection. Scand J Gastroenterol. 2019;54:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Yamao K, Yanagisawa A, Takahashi K, Kimura W, Doi R, Fukushima N, Ohike N, Shimizu M, Hatori T, Nobukawa B, Hifumi M, Kobayashi Y, Tobita K, Tanno S, Sugiyama M, Miyasaka Y, Nakagohri T, Yamaguchi T, Hanada K, Abe H, Tada M, Fujita N, Tanaka M. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan pancreas society. Pancreas. 2011;40:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 44. | Rana SS, Bhasin DK, Sharma R, Gupta R. Factors determining recurrence of fluid collections following migration of intended long term transmural stents in patients with walled off pancreatic necrosis and disconnected pancreatic duct syndrome. Endosc Ultrasound. 2015;4:208-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Cohen-Scali F, Vilgrain V, Brancatelli G, Hammel P, Vullierme MP, Sauvanet A, Menu Y. Discrimination of unilocular macrocystic serous cystadenoma from pancreatic pseudocyst and mucinous cystadenoma with CT: initial observations. Radiology. 2003;228:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 46. | Kim YS, Cho JH. Rare nonneoplastic cysts of pancreas. Clin Endosc. 2015;48:31-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Assifi MM, Nguyen PD, Agrawal N, Dedania N, Kennedy EP, Sauter PK, Prestipino A, Winter JM, Yeo CJ, Lavu H. Non-neoplastic epithelial cysts of the pancreas: a rare, benign entity. J Gastrointest Surg. 2014;18:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Young MR, Wagner PD, Ghosh S, Rinaudo JA, Baker SG, Zaret KS, Goggins M, Srivastava S. Validation of Biomarkers for Early Detection of Pancreatic Cancer: Summary of The Alliance of Pancreatic Cancer Consortia for Biomarkers for Early Detection Workshop. Pancreas. 2018;47:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Yoon SE, Byun JH, Kim KA, Kim HJ, Lee SS, Jang SJ, Jang YJ, Lee MG. Pancreatic ductal adenocarcinoma with intratumoral cystic lesions on MRI: correlation with histopathological findings. Br J Radiol. 2010;83:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Salvia R, Crippa S, Partelli S, Armatura G, Malleo G, Paini M, Pea A, Bassi C. Differences between main-duct and branch-duct intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2:342-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Laurent L, Vullierme MP, Rebours V, Maire F, Hentic O, Francoz C, Durand F, Ruszniewski P, Levy P. Estimation of the prevalence of intraductal papillary mucinous neoplasm of the pancreas in the French population through patients waiting for liver transplantation. United European Gastroenterol J. 2017;5:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Wang Y, Miller FH, Chen ZE, Merrick L, Mortele KJ, Hoff FL, Hammond NA, Yaghmai V, Nikolaidis P. Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. Radiographics. 2011;31:E47-E64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Morelli L, Guadagni S, Borrelli V, Pisano R, Di Franco G, Palmeri M, Furbetta N, Gambaccini D, Marchi S, Boraschi P, Bastiani L, Campatelli A, Mosca F, Di Candio G. Role of abdominal ultrasound for the surveillance follow-up of pancreatic cystic neoplasms: a cost-effective safe alternative to the routine use of magnetic resonance imaging. World J Gastroenterol. 2019;25:2217-2228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Kitano M, Yoshida T, Itonaga M, Tamura T, Hatamaru K, Yamashita Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 2019;54:19-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 55. | Kadiyala V, Lee LS. Endosonography in the diagnosis and management of pancreatic cysts. World J Gastrointest Endosc. 2015;7:213-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 56. | Will U, Reichel A, Fueldner F, Meyer F. Endoscopic ultrasonography-guided drainage for patients with symptomatic obstruction and enlargement of the pancreatic duct. World J Gastroenterol. 2015;21:13140-13151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Tsujino T, Chang KJ. Cyst-Within-Cyst Found on EUS-Guided Cystoscopy of a Pancreatic Mucinous Cystic Neoplasia. Am J Gastroenterol. 2016;111:1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Sbeit W, Kadah A, Farraj M, Shiller M. Pancreatic Cyst Mucin Ball Aspiration as a Diagnostic Maneuver. Isr Med Assoc J. 2018;20:394. [PubMed] |
| 59. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1156] [Article Influence: 144.5] [Reference Citation Analysis (1)] |
| 60. | Gao RY, Wu BH, Shen XY, Peng TL, Li DF, Wei C, Yu ZC, Luo MH, Xiong F, Wang LS, Yao J. Overlooked risk for needle tract seeding following endoscopic ultrasound-guided minimally invasive tissue acquisition. World J Gastroenterol. 2020;26:6182-6194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 61. | Singhi AD, Chu LC, Tatsas AD, Shi C, Ellison TA, Fishman EK, Kawamoto S, Schulick RD, Wolfgang CL, Hruban RH, Edil BH. Cystic pancreatic neuroendocrine tumors: a clinicopathologic study. Am J Surg Pathol. 2012;36:1666-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 62. | Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008;15:409-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 63. | Furukawa T, Klöppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y, Klimstra DS, Longnecker DS, Lüttges J, Offerhaus GJ, Shimizu M, Sunamura M, Suriawinata A, Takaori K, Yonezawa S. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 64. | Furukawa T, Hatori T, Fujita I, Yamamoto M, Kobayashi M, Ohike N, Morohoshi T, Egawa S, Unno M, Takao S, Osako M, Yonezawa S, Mino-Kenudson M, Lauwers GY, Yamaguchi H, Ban S, Shimizu M. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 65. | Adsay NV, Merati K, Nassar H, Shia J, Sarkar F, Pierson CR, Cheng JD, Visscher DW, Hruban RH, Klimstra DS. Pathogenesis of colloid (pure mucinous) carcinoma of exocrine organs: Coupling of gel-forming mucin (MUC2) production with altered cell polarity and abnormal cell-stroma interaction may be the key factor in the morphogenesis and indolent behavior of colloid carcinoma in the breast and pancreas. Am J Surg Pathol. 2003;27:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Adsay NV, Pierson C, Sarkar F, Abrams J, Weaver D, Conlon KC, Brennan MF, Klimstra DS. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 196] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 67. | Rana SS, Sharma V, Sharma R, Gupta R, Bhasin DK. Endoscopic ultrasound guided transmural drainage of walled off pancreatic necrosis using a "step - up" approach: A single centre experience. Pancreatology. 2017;17:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 68. | Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol. 2004;122:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Haeberle L, Esposito I. Pathology of pancreatic cancer. Transl Gastroenterol Hepatol. 2019;4:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 70. | Morris JP 4th, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 302] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 71. | Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JP 4th, Pan FC, Akiyama H, Wright CV, Jensen K, Hebrok M, Sander M. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 545] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 72. | Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, Goggins M. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012; 142: 730-733. e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 546] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 73. | Kuboki Y, Shimizu K, Hatori T, Yamamoto M, Shibata N, Shiratori K, Furukawa T. Molecular biomarkers for progression of intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2015;44:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 74. | Tsuda M, Fukuda A, Roy N, Hiramatsu Y, Leonhardt L, Kakiuchi N, Hoyer K, Ogawa S, Goto N, Ikuta K, Kimura Y, Matsumoto Y, Takada Y, Yoshioka T, Maruno T, Yamaga Y, Kim GE, Akiyama H, Wright CV, Saur D, Takaori K, Uemoto S, Hebrok M, Chiba T, Seno H. The BRG1/SOX9 axis is critical for acinar cell-derived pancreatic tumorigenesis. J Clin Invest. 2018;128:3475-3489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Redston MS, Caldas C, Seymour AB, Hruban RH, da Costa L, Yeo CJ, Kern SE. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res. 1994;54:3025-3033. [PubMed] |
| 76. | Furukawa T, Fujisaki R, Yoshida Y, Kanai N, Sunamura M, Abe T, Takeda K, Matsuno S, Horii A. Distinct progression pathways involving the dysfunction of DUSP6/MKP-3 in pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms of the pancreas. Mod Pathol. 2005;18:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Walerych D, Lisek K, Sommaggio R, Piazza S, Ciani Y, Dalla E, Rajkowska K, Gaweda-Walerych K, Ingallina E, Tonelli C, Morelli MJ, Amato A, Eterno V, Zambelli A, Rosato A, Amati B, Wiśniewski JR, Del Sal G. Proteasome machinery is instrumental in a common gain-of-function program of the p53 missense mutants in cancer. Nat Cell Biol. 2016;18:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 78. | Escobar-Hoyos LF, Penson A, Kannan R, Cho H, Pan CH, Singh RK, Apken LH, Hobbs GA, Luo R, Lecomte N, Babu S, Pan FC, Alonso-Curbelo D, Morris JP 4th, Askan G, Grbovic-Huezo O, Ogrodowski P, Bermeo J, Saglimbeni J, Cruz CD, Ho YJ, Lawrence SA, Melchor JP, Goda GA, Bai K, Pastore A, Hogg SJ, Raghavan S, Bailey P, Chang DK, Biankin A, Shroyer KR, Wolpin BM, Aguirre AJ, Ventura A, Taylor B, Der CJ, Dominguez D, Kümmel D, Oeckinghaus A, Lowe SW, Bradley RK, Abdel-Wahab O, Leach SD. Altered RNA Splicing by Mutant p53 Activates Oncogenic RAS Signaling in Pancreatic Cancer. Cancer Cell 2020; 38: 198-211. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 79. | Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, DePinho RA. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130-3146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 521] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 80. | Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA Jr, Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 596] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 81. | Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M, Shibata N, Shimizu K, Kamatani N, Shiratori K. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011;1:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 338] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 82. | Patra KC, Kato Y, Mizukami Y, Widholz S, Boukhali M, Revenco I, Grossman EA, Ji F, Sadreyev RI, Liss AS, Screaton RA, Sakamoto K, Ryan DP, Mino-Kenudson M, Castillo CF, Nomura DK, Haas W, Bardeesy N. Mutant GNAS drives pancreatic tumourigenesis by inducing PKA-mediated SIK suppression and reprogramming lipid metabolism. Nat Cell Biol. 2018;20:811-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 83. | Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, Fassan M, Antonello D, Sadakari Y, Castelli P, Zamboni G, Maitra A, Salvia R, Hruban RH, Bassi C, Capelli P, Lawlor RT, Goggins M, Scarpa A. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233:217-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 84. | Lee JH, Kim Y, Choi JW, Kim YS. KRAS, GNAS, and RNF43 mutations in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Springerplus. 2016;5:1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 85. | Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI, Schulick RD, Edil BH, Choti MA, Adsay V, Klimstra DS, Offerhaus GJ, Klein AP, Kopelovich L, Carter H, Karchin R, Allen PJ, Schmidt CM, Naito Y, Diaz LA Jr, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci USA. 2011;108:21188-21193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 482] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 86. | Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, Lawlor RT, Johns AL, Miller DK, Mafficini A, Rusev B, Scardoni M, Antonello D, Barbi S, Sikora KO, Cingarlini S, Vicentini C, McKay S, Quinn MCJ, Bruxner TJC, Christ AN, Harliwong I, Idrisoglu S, McLean S, Nourse C, Nourbakhsh E, Wilson PJ, Anderson MJ, Fink JL, Newell F, Waddell N, Holmes O, Kazakoff SH, Leonard C, Wood S, Xu Q, Hiriyur Nagaraj S, Amato E, Dalai I, Bersani S, Cataldo I, Dei Tos AP, Capelli P, Vittoria Davì M, Landoni L, Malpaga A, Miotto M, Whitehall VLJ, Leggett BA, Harris JL, Harris J, Jones MD, Humphris J, Chantrill LA, Chin V, Nagrial AM, Pajic M, Scarlett CJ, Pinho A, Rooman I, Toon C, Wu J, Pinese M, Cowley M, Barbour A, Mawson A, Humphrey ES, Colvin EK, Chou A, Lovell JA, Jamieson NB, Duthie F, Gingras MC, Fisher WE, Dagg RA, Lau LMS, Lee M, Pickett HA, Reddel RR, Samra JS, Kench JG, Merrett ND, Epari K, Nguyen NQ, Zeps N, Falconi M, Simbolo M, Butturini G, Van Buren G, Partelli S, Fassan M; Australian Pancreatic Cancer Genome Initiative; Khanna KK, Gill AJ, Wheeler DA, Gibbs RA, Musgrove EA, Bassi C, Tortora G, Pederzoli P, Pearson JV, Biankin AV, Grimmond SM. Corrigendum: Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;550:548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Pelosi E, Castelli G, Testa U. Pancreatic Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Biomedicines. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 88. | Lytle NK, Ferguson LP, Rajbhandari N, Gilroy K, Fox RG, Deshpande A, Schürch CM, Hamilton M, Robertson N, Lin W, Noel P, Wartenberg M, Zlobec I, Eichmann M, Galván JA, Karamitopoulou E, Gilderman T, Esparza LA, Shima Y, Spahn P, French R, Lewis NE, Fisch KM, Sasik R, Rosenthal SB, Kritzik M, Von Hoff D, Han H, Ideker T, Deshpande AJ, Lowy AM, Adams PD, Reya T. A Multiscale Map of the Stem Cell State in Pancreatic Adenocarcinoma. Cell 2019; 177: 572-586. e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 89. | Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3216] [Cited by in RCA: 3026] [Article Influence: 178.0] [Reference Citation Analysis (0)] |
| 90. | Okabayashi T, Shima Y, Kosaki T, Sumiyoshi T, Kozuki A, Iiyama T, Takezaki Y, Kobayashi M, Nishimori I, Ogawa Y, Hanazaki K. Invasive carcinoma derived from branch duct-type IPMN may be a more aggressive neoplasm than that derived from main duct-type IPMN. Oncol Lett. 2013;5:1819-1825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 91. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1361] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 92. | Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T, Ohashi Y; JASPAC 01 Study Group. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 784] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 93. | Eguchi H, Takeda Y, Takahashi H, Nakahira S, Kashiwazaki M, Shimizu J, Sakai D, Isohashi F, Nagano H, Mori M, Doki Y. A Prospective, Open-Label, Multicenter Phase 2 Trial of Neoadjuvant Therapy Using Full-Dose Gemcitabine and S-1 Concurrent with Radiation for Resectable Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol. 2019;26:4498-4505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |