Published online Jun 21, 2021. doi: 10.3748/wjg.v27.i23.3148
Peer-review started: January 25, 2021
First decision: February 27, 2021
Revised: March 13, 2021
Accepted: May 8, 2021
Article in press: May 8, 2021
Published online: June 21, 2021
Processing time: 143 Days and 14 Hours
Acute pancreatitis (AP), chronic pancreatitis (CP) and pancreatic cancer are three distinct pancreatic diseases with different prognoses and treatment options. However, it may be difficult to differentiate between benign and malignant disease. AP may be a first symptom of pancreatic cancer, particularly in patients between the ages of 56 and 75 with presumed idiopathic AP who had a concomitant diagnosis of new-onset diabetes mellitus or patients who present with CP at diagnosis of AP. In these patients, additional imaging is warranted, preferably by endoscopic ultrasonography. CP may lead to pancreatic cancer through oncogenic mutations, mostly in patients with hereditary CP, and in patients in whom risk factors for pancreatic cancer (e.g., nicotine and alcohol abuse) are also present. Patients with PRSS1-mediated CP and patients with a history of autosomal dominant hereditary CP without known genetic mutations may be considered for surveillance for pancreatic cancer. Pancreatic inflammation may mimic pancreatic cancer by appearing as a focal mass-forming lesion on imaging. Differentiation between the above mentioned benign and malignant disease may be facilitated by specific features like the duct-penetrating sign and the duct-to-parenchyma ratio. Research efforts are aimed towards developing a superior discriminant between pancreatitis and pancreatic cancer in the form of imaging modalities or biomarkers. This may aid clinicians in timely diagnosing pancreatic cancer in a potentially curable stage.
Core Tip: It is essential to distinguish acute pancreatitis and chronic pancreatitis (CP) from pancreatic ductal adenocarcinoma (PDAC), as these diseases have different treatment options and prognoses. Idiopathic acute pancreatitis may be a first symptom of PDAC, and endoscopic ultrasonography should therefore be considered. CP may obscure PDAC, and long-standing inflammation may cause PDAC, especially in hereditary CP and patients with a history of nicotine and alcohol abuse. Patients should be counselled in cessation of tobacco and alcohol use. Pancreatitis may mimic PDAC by presenting as a mass on imaging. Specific imaging features can aid in the differentiation between benign and malignant disease.
- Citation: Umans DS, Hoogenboom SA, Sissingh NJ, Lekkerkerker SJ, Verdonk RC, van Hooft JE. Pancreatitis and pancreatic cancer: A case of the chicken or the egg. World J Gastroenterol 2021; 27(23): 3148-3157
- URL: https://www.wjgnet.com/1007-9327/full/v27/i23/3148.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i23.3148
Acute pancreatitis (AP) and chronic pancreatitis (CP) are inflammatory diseases of the pancreas. While AP is sudden and transient, CP is a longstanding inflammation of the pancreas characterized by pancreatic calcifications and exocrine pancreatic insufficiency. In CP, bouts of so-called acute-on-chronic episodes of pancreatitis may also occur.
Both AP and CP are benign diseases that are, in different ways, associated with pancreatic ductal adenocarcinoma (PDAC). Although these are three separate disease entities, inflammation and cancer can co-exist, and inflammation may cause cancer while cancer may also cause inflammation. This entails they are not always easy to distinguish from each other. As the treatment and prognosis of AP, CP and PDAC are completely different, it is important to understand the nature of the association between these diseases and be able to take the proper diagnostic steps to gather the correct diagnosis and subsequent treatment options.
In this review, we will focus on the relationship between AP, CP and PDAC and how to distinguish between these three disease entities.
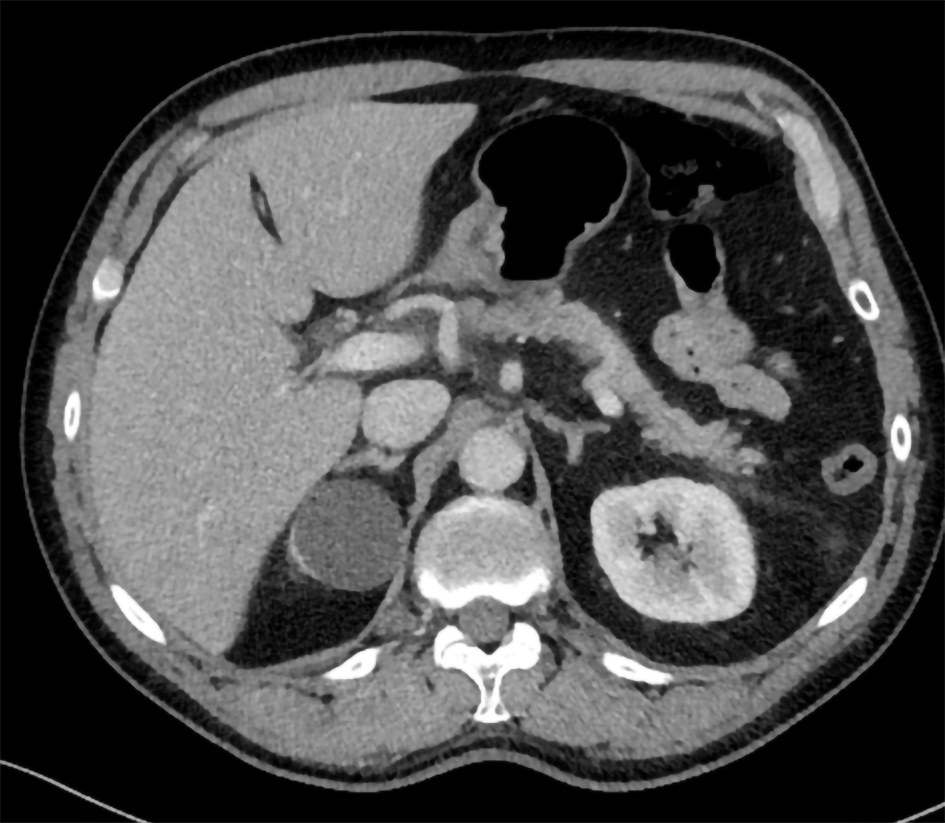
A 64-year-old man visited the emergency department with acute abdominal pain in the epigastric region. Laboratory tests revealed an amylase level of 2100 U/L (normal < 107 U/L), a lipase level of 5548 U/L (normal 13-60 U/L) and normal liver enzymes, calcium and triglycerides. Computed tomography (CT) showed inflammation of the head of the pancreas (Figure 1). The patient was admitted with AP, received fluid resuscitation therapy and analgesics. Transabdominal ultrasound showed no cause for the AP episode. After 3 d, the patient was discharged with a diagnosis of idiopathic AP.
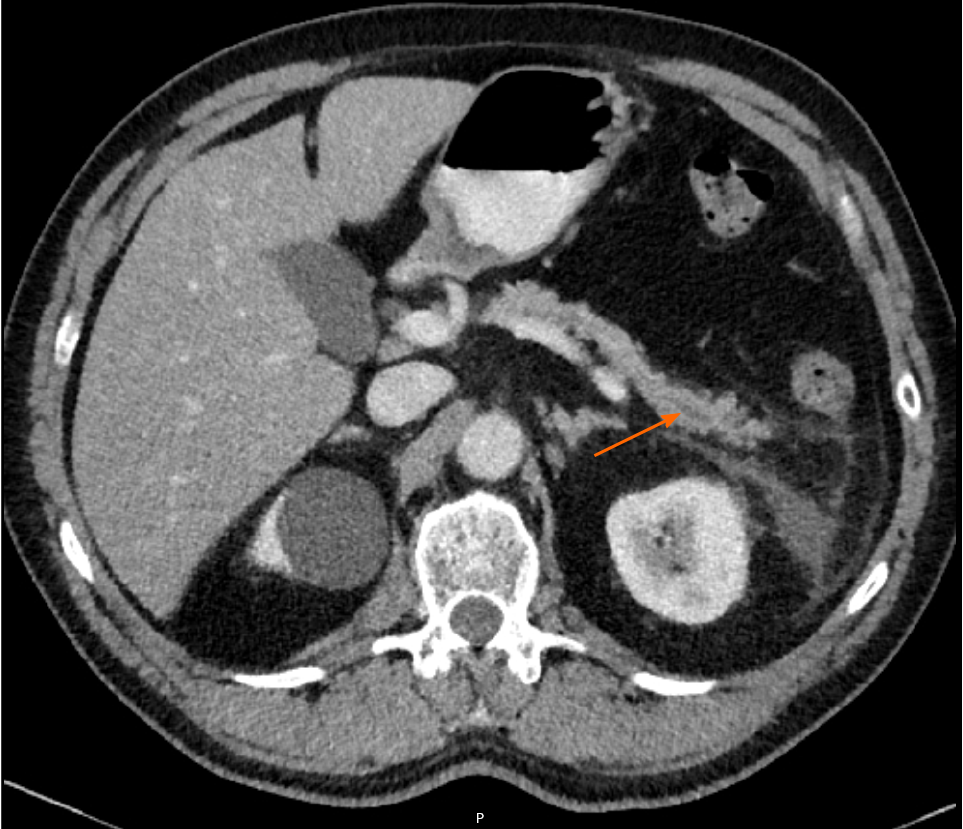
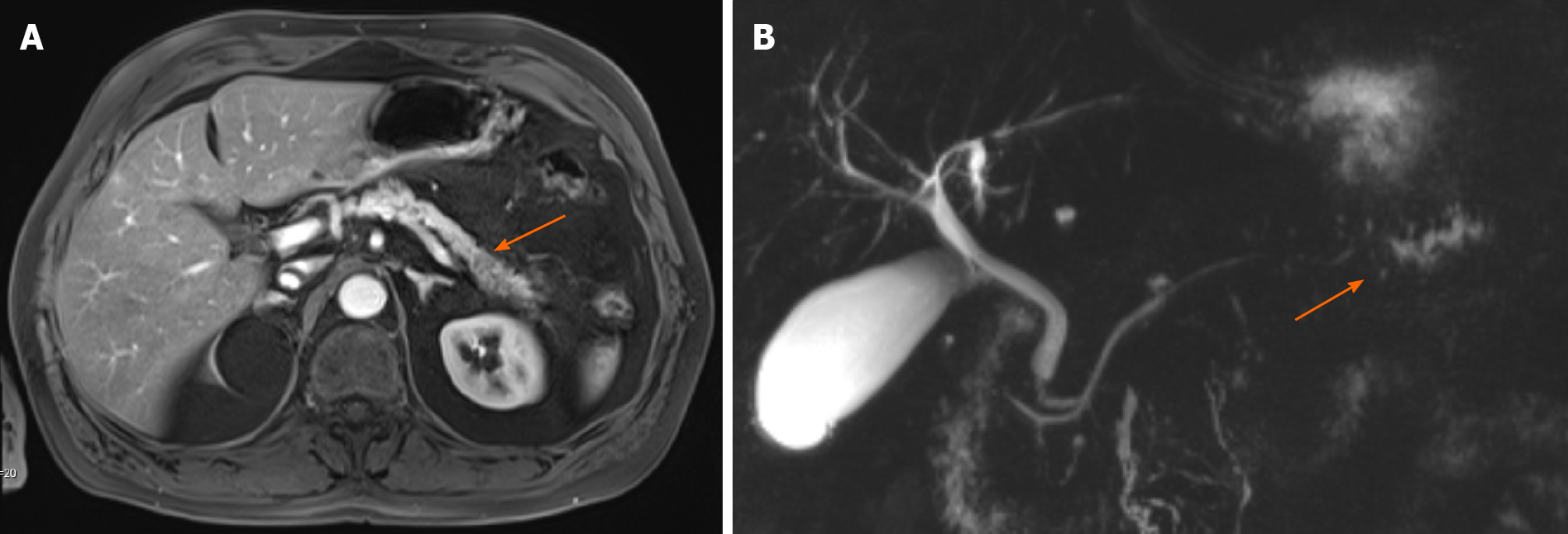
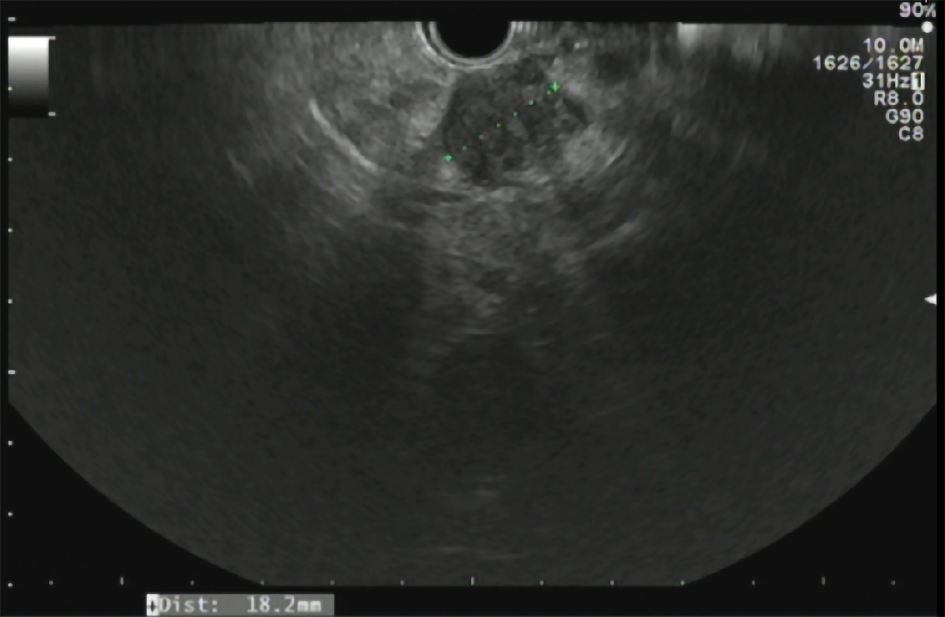
Approximately 1.5 years later, this patient was readmitted with a recurrent AP episode. CT of the abdomen revealed inflammation of the pancreas with a splenorenal fluid collection and slight dilatation of the pancreatic duct in the tail of the pancreas (Figure 2). Additional magnetic resonance imaging (MRI) showed a hypo-intense lesion in the pancreatic tail, causing a stenosis of the pancreatic duct (Figure 3). Endoscopic ultrasonography (EUS) with biopsy confirmed the diagnosis of PDAC (Figure 4). The patient underwent a pancreatic tail and spleen resection, and pathology revealed a radically resected T3N0 PDAC.
The incidence of AP is rising, with a median increase of 3.4% per year[1]. Reported incidence rates ranges from 9.8 to 100 per 100000 in Europe and rise with age[1-3]. AP is often caused by alcohol or gallstone disease, although the incidence of these etiologies differ geographically[1].
A strong association between AP and PDAC has been established through multiple studies. A recent systematic review and meta-analysis by Liu et al[4] included 11 studies reporting on the risk of PDAC after an episode of AP and found an effect estimate of 2.07 [95% confidence interval (CI): 1.36-2.78] during a follow-up time exceeding 10 years. In a subgroup analysis of 103961 AP patients and 1442158 control subjects from five prospective studies in this systematic review, a relative risk of 7.81 (95%CI: 5.00-12.19) for PDAC was observed in patients with AP as compared to healthy control subjects.
Multiple hypotheses have been raised to explain this apparent association between AP and PDAC. Firstly, it has been suggested that risk factors for AP are similar to risk factors for PDAC. For instance, alcohol abuse is a prevalent etiology of AP and also a known risk factor for pancreatic cancer. Although the etiological value of nicotine abuse in AP is still a topic of discussion, it is an established risk factor for recurrent AP, while also being a strong risk factor for PDAC[5-7]. Secondly, in recurrent AP, the accumulating inflammatory states of the pancreas are thought to increase the chance of oncogenic mutations in pancreatic cells, leading to carcinogenesis in a similar fashion as CP, as elaborated below in the section on CP[8].
However, the most prevailing hypothesis has been that a single episode of AP cannot cause PDAC but that PDAC may cause AP. Although this is rare, it has been acknowledged that PDAC can cause an episode of AP by ductal obstruction before the PDAC has even been diagnosed[9]. Temporal data on the association of PDAC and AP endorse this hypothesis. In the previously mentioned meta-analysis by Liu et al[4], the strongest association between AP and PDAC was found in the first year after AP [effect estimate 23.47 (95%CI: 3.26-43.68)], while this association diminished over a course of 2 years after AP [effect estimate 9.82 (95%CI: 3.01-16.64)], 5 years [effect estimate 2.47 (95%CI: 1.93-3.02)], 10 years [effect estimate 1.69 (95%CI: 1.26; 2.11)] and over 10 years after AP [effect estimate 1.17 (95%CI: 0.78-1.57)]. A Danish matched-cohort study, which was included in this meta-analysis, also adjusted for alcohol- and smoking-related conditions and the Charlson Comorbidity Index score and excluded patients who developed CP or other exocrine pancreatic disease during follow-up[10]. This well-designed study found that a presumed idiopathic etiology appeared to be associated with the highest risk of PDAC [adjusted hazard ratio of 2.52 (95%CI: 1.83-3.47)]. This finding was supported by a recent cohort study, which found that biliary AP and a history of alcohol-related disease were predictors for no underlying PDAC[11]. In this study of 28231 patients with AP, 283 received the diagnosis PDAC during follow-up (overall risk 1.0%). They found several predictors for PDAC in patients with AP, including age between 56 and 75 at diagnosis of AP (risk approximately 1.6%), new-onset diabetes mellitus (DM) and new-onset CP [risk 2.0% (95%CI: 1.1-3.3%)] at diagnosis of AP. Interestingly, severe AP, smoking-related diseases, venous thromboembolism and previous malignancies were not associated with a higher risk for PDAC. A Swedish matched-cohort study of 49749 AP patients and 138750 matched subjects also identified recurrent pancreatitis as a risk factor for PDAC [hazard ratio 4.44 (95%CI: 1.181-10.89) after censoring for diagnosis of CP)][12].
It has become more and more apparent that PDAC is a noteworthy etiology of AP, particularly in patients with presumed idiopathic etiology after standard diagnostic work-up. In these patients with idiopathic AP, primarily in patients aged 56-75, it is imperative to exclude the presence of an occult pancreatic tumor by providing additional imaging. Commonly, standard diagnostic work-up includes a personal history, laboratory tests (including liver enzymes, calcium and triglycerides) and transabdominal ultrasonography. EUS has been proven to be more sensitive for the detection of PDAC than CT (98 vs 74%) and transabdominal ultrasonography (94% vs 67%), especially for small lesions[13]. A meta-analysis comparing the diagnostic yield of EUS vs magnetic resonance cholangiopancreatography (MRCP) showed that EUS is superior to MRCP in detecting all etiologies in idiopathic AP[14]. Thus, mainly in patients aged 56-75 with idiopathic AP, recurring AP or new-onset DM or CP concomitant with their AP diagnosis, additional imaging is warranted, preferably using EUS.
After a first episode of AP, 17% of patients have at least one recurrent attack, and 8% develop CP[5]. CP is a progressive inflammatory disease that often leads to pain, exocrine pancreatic insufficiency and DM. It is characterized by a pathologic response to pancreatic injury leading to irreversible parenchymal damage[15]. Estimates for global CP incidence range from 5.0 to 10.0 cases per 100000 person-years[16]. In the majority of cases, CP develops after a history of recurrent AP in patients with multiple environmental and genetic risk factors[17]. Alcohol and nicotine are the most important environmental risk factors for CP[5]. Genetic risk factors include mutations in the protease serine 1 (PRSS1) gene, which cause hereditary CP, and in the serine protease inhibitor Kazal-type 1 (SPINK1) and cystic fibrosis chymotrypsinogen C (CFTR) genes[18].
Lowenfels et al[19] were the first to describe the link between CP and progression to PDAC. In this study including 2015 CP patients, a cumulative risk for PDAC was observed in 1.8% (95%CI: 1.0-2.6) and 4.0% (95%CI: 2.0-5.9) 10 and 20 years after diagnosis, respectively[19]. To date, the increased risk of PDAC in CP patients has been confirmed by several meta-analyses[20-22]. However, the exact risk remains debated, mainly because different factors contribute to PDAC progression.
The risk of PDAC is much higher for patients with hereditary CP from PRSS1 mutations [relative risk 69.0 (95%CI: 56.4-84.4)] and tropical CP [relative risk 100 (95%CI: 37.0-218)], when compared to patients with sporadic CP [relative risk 5.1 (95%CI: 3.5-7.3)][21,23-25]. Additionally, alcohol consumption and smoking, both well-known risk factors for PDAC, form important confounding factors as patients with CP often have a history of alcohol- and nicotine abuse[26]. Other risk factors are older age at disease onset, obesity, concurrent DM and pancreatic duct dilatation[27,28].
Even though many contributing factors play a role in the association between CP and PDAC, evidence suggests that a causal relationship is plausible. The pathway from CP to PDAC is not yet entirely understood. The prevailing hypothesis is that the long-standing inflammatory state of the pancreas associated with CP leads to oncogenic mutations. Several studies demonstrated that the pancreas of a notable proportion of CP patients harbor downregulating mutations of the tumor suppressing p16, p53 and SMAD4 genes and upregulating mutations of the oncogenic K-ras, tumor necrosis factor-α and nuclear factor κ B genes, which are also present in the majority of PDAC[8,29-31]. These mutations could subsequently facilitate carcinogenesis in patients who may already have a genetic disposition or environmental risk factors (e.g., nicotine abuse) for developing PDAC.
Additional to the risk of progression to PDAC in CP, there is a risk of PDAC being misdiagnosed as CP because discrimination between these diseases can be difficult. A retrospective study found that 5% of PDAC patients were misdiagnosed with CP, while actually having PDAC[32]. This finding has been supported by the temporal data of a recent meta-analysis of 13 studies[20]. This meta-analysis found a decrease in PDAC risk between a 2-year lag-period [effect estimate 16.16 (95%CI: 12.59-20.73)] and a 5- and 9-year lag-period [effect estimate 7.90 (95%CI: 4.26-14.66) and 3.53 (95%CI: 1.69-7.38), respectively][20]. The peak of diagnosis of PDAC in the first years after diagnosis of CP suggests that these were either patients with PDAC who were initially wrongfully diagnosed with CP or patients with CP and concomitant PDAC in whom the PDAC was not recognized at diagnosis of CP.
Special attention should be paid to the presence of PDAC in newly diagnosed CP patients, given the high risk of PDAC in the first years after CP diagnosis. Although the discussion is ongoing, international guidelines currently only consider surveillance of PDAC justified in patients with PRSS1-mediated CP and patients with a history of autosomal dominant hereditary CP without known genetic mutations, from the age of 40 until the patient is no longer suitable for (surgical) intervention. As small tumor lesions may be obscured by inflammation, fibrosis and calcification on EUS, it is advised to screen using CT imaging or MRI, although high quality evidence on the accuracy of different imaging modalities in detection of DPAC in CP patients is sparse[33]. Other contributing factors for PDAC progression that justify screening in patients with CP should be further clarified.
Additionally, time and effort should be invested in reducing environmental risk factors for PDAC, such as nicotine and alcohol abuse. These risk factors are prevalent among patients with CP and counselling and support should be provided to patients willing to quit smoking or drinking.
Inflammatory diseases of the pancreas can sometimes mimic PDAC by the appearance of a focal, mass-forming pancreatitis. Although diffuse forms of pancreatitis are more common, some patients present with a focal pancreatitis, in particular autoimmune pancreatitis, groove pancreatitis and CP. Clinical symptoms of mass-forming pancreatitis often overlap with symptoms related to PDAC, including abdominal pain, weight loss and jaundice. In patients undergoing surgery for suspected (periampullary) cancer, up to 23% turn out to be chronic inflammatory lesions[34].
Similar to PDAC, focal CP can lead to focal parenchymal atrophy or enlargement and appears as a hypovascular mass on imaging, accompanied by upstream ductal dilation and atrophy[35]. Imaging features that point towards an inflammatory cause include an unobstructed or smooth tapering pancreatic duct through the hypovascular mass (duct-penetrating sign), while PDAC usually demonstrates an abrupt or irregular obstruction (Figure 2)[36-38]. The duct-penetrating sign is highly specific for an inflammatory mass (96%), with a sensitivity of 85%, and can be best visualized during MRCP[39]. In addition, collateral duct dilation or side-branch dilation in the upstream pancreas favors CP, due to traction effect of parenchymal fibrosis on the upstream healthy parenchyma[39,40]. In contrast, side-branches adjacent to malignancy are usually obliterated owing to mass-effect[39,41]. The presence of (pseudo)cysts and parenchymal or intraductal calcifications may suggest an inflammatory cause, although these findings can also sometimes be found in intraductal papillary mucinous neoplasms, especially with main duct involvement[39,42]. Peripheral displacement of calcifications can be seen in patients with a malignant mass in the background of CP[43]. Another imaging feature that can be used to differentiate between malignancy and inflammation is the extent of ductal dilation and parenchymal atrophy, expressed by the pancreatic duct-to-parenchyma ratio. A duct-to-parenchyma ratio ≥ 0.34 on EUS, indicating pronounced ductal dilation and parenchymal atrophy, is highly suggestive for PDAC instead of CP, with a diagnostic accuracy of 97%[44]. Signs that are more often observed in advanced PDAC compared to pancreatitis are vascular encasement by attenuating soft-tissue, caliber narrowing and occlusion[39]. Interestingly, a superior mesenteric artery-to-superior mesenteric vein ratio ≥ 1.0 favors the diagnosis of an malignancy[45]. Although not well-understood, enlargement of the superior mesenteric artery is probably caused by an increased resistance to blood flow in the pancreas of patients with PDAC, while in inflammatory conditions an increase in the diameter of the superior mesenteric vein can be observed secondary to the release of pancreatitis-induced vasoactive agents[39]. Imaging findings are summarized in Table 1.
| Inflammatory cause | Malignant cause |
| Penetrating duct sign | Abrupt cutoff of the pancreatic duct |
| Collateral or side branch dilation | Upstream obliteration of side branches |
| Duct-to-parenchyma ratio < 0.34 | Duct-to-parenchyma ratio ≥ 0.34 |
| AIP: occasionally perivascular involvement | Vessel encasement and caliber changes |
| SMA-to-SMV ratio < 1.0 | SMA-to-SMV ratio ≥ 1.0 |
Even though secondary imaging features can provide valuable information for the differentiation between pancreatitis and PDAC, none of them are 100% specific or sensitive. The two conditions may not only have overlapping clinical and imaging features but can also coexist, and the diagnostic performance of imaging modalities remains operator-dependent. Recent advances in machine learning-based diagnostic models may overcome part of these limitations. Preliminary studies using machine-learning methods reported promising results in differentiating inflammatory from malignant lesions on EUS but need to be further evolved before implementation in clinical practice[46,47].
Serum carbohydrate antigen 19-9 (CA 19-9) is a widely used diagnostic tool for PDAC diagnosis. However, patients with CP often have elevation of serum CA 19-9 that is not indicative of malignancy but a result of pancreatic inflammation[48]. Therefore, the international consensus guideline do not recommend CA 19-9 as a diagnostic tool for PDAC in the CP population[33]. Over the last decades, several studies have investigated the use of other early predictive biomarkers (i.e. K-ras, interleukins, micro-RNAs and proteomics) in CP patients, however, no accurate biomarker is yet available for clinical use[49].
Many questions regarding the exact relative risk estimates of PDAC in CP patients remain, but most importantly, we need an adequate strategy for differentiation between CP and PDAC. Future research should devise the most optimal imaging methods and early detection biomarker to prevent misdiagnosis of PDAC as CP and, hence, delay of cancer diagnosis. As this delay compromises diagnosis of early-stage tumors and consequently resectable treatment options, optimizing diagnostic methods is clinically relevant.
AP can be a first symptom of underlying PDAC, especially in patients with presumed idiopathic AP, between the ages of 56 and 75, and those who had a diagnosis of new-onset DM or CP. Additional imaging to exclude PDAC in these patients should at least be considered. EUS seems to be the preferred imaging modality.
CP, particularly hereditary CP, may lead to PDAC through oncogenic mutations caused by long-standing pancreatic inflammation, and CP patients may be exposed to overlapping risk factors for CP and PDAC. In patients with PRSS1-mediated CP or a history of autosomal dominant hereditary CP without known mutations, surveillance for PDAC can be considered, although the efficacy and modalities of surveillance are still up for debate.
Chronic pancreatic inflammation may present as a focal mass on imaging. Specific findings, such as the duct-penetrating sign (MRCP) and the duct-to-parenchyma ratio (EUS), may aid in the differentiation between pancreatitis and PDAC.
Currently, considerable effort is focused on finding a biomarker or machine-learning methods as a superior discriminant between CP and PDAC. Unfortunately, no clinically useful technique has yet emerged. Improving the possibility to differentiate between CP and PDAC, as well as identifying patients at risk of underlying or future PDAC, may give clinicians the opportunity to enhance the diagnostic process.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: The Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li JT, Meyer A, Suzuki R S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 2017;17:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 243] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 2. | Lankisch PG, Karimi M, Bruns A, Maisonneuve P, Lowenfels AB. Temporal trends in incidence and severity of acute pancreatitis in Lüneburg County, Germany: a population-based study. Pancreatology. 2009;9:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Głuszek S, Kozieł D. Prevalence and progression of acute pancreatitis in the Świętokrzyskie Voivodeship population. Pol Przegl Chir. 2012;84:618-625. [PubMed] |
| 4. | Liu J, Wang Y, Yu Y. Meta-analysis reveals an association between acute pancreatitis and the risk of pancreatic cancer. World J Clin Cases. 2020;8:4416-4430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 5. | Ahmed Ali U, Issa Y, Hagenaars JC, Bakker OJ, van Goor H, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Brink MA, Schaapherder AF, Dejong CH, Spanier BW, Heisterkamp J, van der Harst E, van Eijck CH, Besselink MG, Gooszen HG, van Santvoort HC, Boermeester MA; Dutch Pancreatitis Study Group. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clin Gastroenterol Hepatol. 2016;14:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 6. | Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 368] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 7. | Lynch SM, Vrieling A, Lubin JH, Kraft P, Mendelsohn JB, Hartge P, Canzian F, Steplowski E, Arslan AA, Gross M, Helzlsouer K, Jacobs EJ, LaCroix A, Petersen G, Zheng W, Albanes D, Amundadottir L, Bingham SA, Boffetta P, Boutron-Ruault MC, Chanock SJ, Clipp S, Hoover RN, Jacobs K, Johnson KC, Kooperberg C, Luo J, Messina C, Palli D, Patel AV, Riboli E, Shu XO, Rodriguez Suarez L, Thomas G, Tjønneland A, Tobias GS, Tong E, Trichopoulos D, Virtamo J, Ye W, Yu K, Zeleniuch-Jacquette A, Bueno-de-Mesquita HB, Stolzenberg-Solomon RZ. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170:403-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 8. | Kolodecik T, Shugrue C, Ashat M, Thrower EC. Risk factors for pancreatic cancer: underlying mechanisms and potential targets. Front Physiol. 2013;4:415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Minato Y, Kamisawa T, Tabata T, Hara S, Kuruma S, Chiba K, Kuwata G, Fujiwara T, Egashira H, Koizumi K, Saito I, Endo Y, Koizumi S, Fujiwara J, Arakawa T, Momma K, Kurata M, Honda G. Pancreatic cancer causing acute pancreatitis: a comparative study with cancer patients without pancreatitis and pancreatitis patients without cancer. J Hepatobiliary Pancreat Sci. 2013;20:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Kirkegård J, Cronin-Fenton D, Heide-Jørgensen U, Mortensen FV. Acute Pancreatitis and Pancreatic Cancer Risk: A Nationwide Matched-Cohort Study in Denmark. Gastroenterology. 2018;154:1729-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 11. | Kirkegård J, Mortensen FV, Heide-Jørgensen U, Cronin-Fenton D. Predictors of underlying pancreatic cancer in patients with acute pancreatitis: a Danish nationwide cohort study. HPB (Oxford). 2020;22:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic Cancer Following Acute Pancreatitis: A Population-based Matched Cohort Study. Am J Gastroenterol. 2018;113:1711-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Kitano M, Yoshida T, Itonaga M, Tamura T, Hatamaru K, Yamashita Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 2019;54:19-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 14. | Wan J, Ouyang Y, Yu C, Yang X, Xia L, Lu N. Comparison of EUS with MRCP in idiopathic acute pancreatitis: a systematic review and meta-analysis. Gastrointest Endosc 2018; 87: 1180-1188. e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Löhr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, Haas S, Akisik F, Kartalis N, Iglesias-Garcia J, Keller J, Boermeester M, Werner J, Dumonceau JM, Fockens P, Drewes A, Ceyhan G, Lindkvist B, Drenth J, Ewald N, Hardt P, de Madaria E, Witt H, Schneider A, Manfredi R, Brøndum FJ, Rudolf S, Bollen T, Bruno M; HaPanEU/UEG Working Group. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J. 2017;5:153-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 415] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 16. | Lévy P, Domínguez-Muñoz E, Imrie C, Löhr M, Maisonneuve P. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United European Gastroenterol J. 2014;2:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 757] [Article Influence: 31.5] [Reference Citation Analysis (1)] |
| 18. | Whitcomb DC. Value of genetic testing in the management of pancreatitis. Gut. 2004;53:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1138] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 20. | Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112:1366-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 341] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 21. | Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 432] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 22. | Tong GX, Geng QQ, Chai J, Cheng J, Chen PL, Liang H, Shen XR, Wang DB. Association between pancreatitis and subsequent risk of pancreatic cancer: a systematic review of epidemiological studies. Asian Pac J Cancer Prev. 2014;15:5029-5034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Lowenfels AB, Maisonneuve P, DiMagno EP, Elitsur Y, Gates LK Jr, Perrault J, Whitcomb DC. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 609] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 24. | Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P, Truninger K, Ammann R, Cavallini G, Charnley RM, Uomo G, Delhaye M, Spicak J, Drumm B, Jansen J, Mountford R, Whitcomb DC, Neoptolemos JP; European Registry of Hereditary Pancreatitis and Pancreatic Cancer (EUROPAC). Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 373] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 25. | Chari ST, Mohan V, Pitchumoni CS, Viswanathan M, Madanagopalan N, Lowenfels AB. Risk of pancreatic carcinoma in tropical calcifying pancreatitis: an epidemiologic study. Pancreas. 1994;9:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 100] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1336] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 27. | Korpela T, Udd M, Mustonen H, Ristimäki A, Haglund C, Seppänen H, Kylänpää L. Association between chronic pancreatitis and pancreatic cancer: A 10-year retrospective study of endoscopically treated and surgical patients. Int J Cancer. 2020;147:1450-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Jeon CY, Chen Q, Yu W, Dong EY, Chung J, Pandol SJ, Yadav D, Conwell DL, Wu BU. Identification of Individuals at Increased Risk for Pancreatic Cancer in a Community-Based Cohort of Patients With Suspected Chronic Pancreatitis. Clin Transl Gastroenterol. 2020;11:e00147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Lüttges J, Diederichs A, Menke MA, Vogel I, Kremer B, Klöppel G. Ductal lesions in patients with chronic pancreatitis show K-ras mutations in a frequency similar to that in the normal pancreas and lack nuclear immunoreactivity for p53. Cancer. 2000;88:2495-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Maire F, Micard S, Hammel P, Voitot H, Lévy P, Cugnenc PH, Ruszniewski P, Puig PL. Differential diagnosis between chronic pancreatitis and pancreatic cancer: value of the detection of KRAS2 mutations in circulating DNA. Br J Cancer. 2002;87:551-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Kong X, Sun T, Kong F, Du Y, Li Z. Chronic Pancreatitis and Pancreatic Cancer. Gastrointest Tumors. 2014;1:123-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Munigala S, Kanwal F, Xian H, Agarwal B. New diagnosis of chronic pancreatitis: risk of missing an underlying pancreatic cancer. Am J Gastroenterol. 2014;109:1824-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 33. | Greenhalf W, Lévy P, Gress T, Rebours V, Brand RE, Pandol S, Chari S, Jørgensen MT, Mayerle J, Lerch MM, Hegyi P, Kleeff J, Castillo CF, Isaji S, Shimosegawa T, Sheel A, Halloran CM, Garg P, Takaori K, Besselink MG, Forsmark CE, Wilcox CM, Maisonneuve P, Yadav D, Whitcomb D, Neoptolemos J; Working group for the International (IAP – APA – JPS – EPC) Consensus Guidelines for Chronic Pancreatitis. International consensus guidelines on surveillance for pancreatic cancer in chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and European Pancreatic Club. Pancreatology. 2020;20:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Kennedy T, Preczewski L, Stocker SJ, Rao SM, Parsons WG, Wayne JD, Bell RH, Talamonti MS. Incidence of benign inflammatory disease in patients undergoing Whipple procedure for clinically suspected carcinoma: a single-institution experience. Am J Surg. 2006;191:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Gandhi NS, Feldman MK, Le O, Morris-Stiff G. Imaging mimics of pancreatic ductal adenocarcinoma. Abdom Radiol (NY). 2018;43:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Ichikawa T, Sou H, Araki T, Arbab AS, Yoshikawa T, Ishigame K, Haradome H, Hachiya J. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology. 2001;221:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Raman SP, Salaria SN, Hruban RH, Fishman EK. Groove pancreatitis: spectrum of imaging findings and radiology-pathology correlation. AJR Am J Roentgenol. 2013;201:W29-W39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Lekkerkerker SJ, Nio CY, Issa Y, Fockens P, Verheij J, Busch OR, van Gulik TM, Rauws EA, Boermeester MA, van Hooft JE, Besselink MG. Clinical outcomes and prevalence of cancer in patients with possible groove pancreatitis. J Gastroenterol Hepatol. 2016;31:1895-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Wolske KM, Ponnatapura J, Kolokythas O, Burke LMB, Tappouni R, Lalwani N. Chronic Pancreatitis or Pancreatic Tumor? Radiographics. 2019;39:1965-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. |
Lin E, Alexander D.
Focal Chronic Pancreatitis |
| 41. |
Guarise A, Faccioli N, Morana G, Megibow AJ.
Chronic Pancreatitis |
| 42. | Perez-Johnston R, Narin O, Mino-Kenudson M, Ingkakul T, Warshaw AL, Fernandez-Del Castillo C, Sahani VD. Frequency and significance of calcification in IPMN. Pancreatology. 2013;13:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Schima W, Böhm G, Rösch CS, Klaus A, Függer R, Kopf H. Mass-forming pancreatitis vs pancreatic ductal adenocarcinoma: CT and MR imaging for differentiation. Cancer Imaging. 2020;20:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 44. | Eloubeidi MA, Luz LP, Tamhane A, Khan M, Buxbaum JL. Ratio of pancreatic duct caliber to width of pancreatic gland by endosonography is predictive of pancreatic cancer. Pancreas. 2013;42:670-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Elmas N, Oran I, Oyar O, Ozer H. A new criterion in differentiation of pancreatitis and pancreatic carcinoma: artery-to-vein ratio using the superior mesenteric vessels. Abdom Imaging. 1996;21:331-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 47. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M, Dietrich CF, Havre R, Gheorghe C, McKay C, Gheonea DI, Ciurea T; European EUS Elastography Multicentric Study Group. Efficacy of an artificial neural network-based approach to endoscopic ultrasound elastography in diagnosis of focal pancreatic masses. Clin Gastroenterol Hepatol 2012; 10: 84-90. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | Furuya N, Kawa S, Hasebe O, Tokoo M, Mukawa K, Maejima S, Oguchi H. Comparative study of CA242 and CA19-9 in chronic pancreatitis. Br J Cancer. 1996;73:372-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Ballehaninna UK, Chamberlain RS. Biomarkers for pancreatic cancer: promising new markers and options beyond CA 19-9. Tumour Biol. 2013;34:3279-3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |