Published online Jun 14, 2021. doi: 10.3748/wjg.v27.i22.3130
Peer-review started: February 7, 2021
First decision: February 27, 2021
Revised: March 12, 2021
Accepted: April 21, 2021
Article in press: April 21, 2021
Published online: June 14, 2021
Processing time: 125 Days and 17.7 Hours
One third of coronavirus disease 2019 (COVID-19) patients have gastrointestinal symptoms. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA has been detected in stool samples of approximately 50% of COVID-19 individuals. Fecal calprotectin is a marker of gastrointestinal inflammation in the general population.
To investigate if fecal calprotectin correlates with SARS-CoV-2 intestinal shedding in COVID-19 patients with pneumonia.
Patients with SARS-CoV-2 pneumonia admitted to the Infectious Disease Unit (University Hospital of Trieste, Italy) from September to November 2020 were consecutively enrolled in the study. Fecal samples were collected and analyzed for quantification of fecal calprotectin (normal value < 50 mg/kg) and SARS-CoV-2 RNA presence by polymerase chain reaction (PCR). Inter-group differences were determined between patients with and without diarrhea and patients with and without detection of fecal SARS-CoV-2.
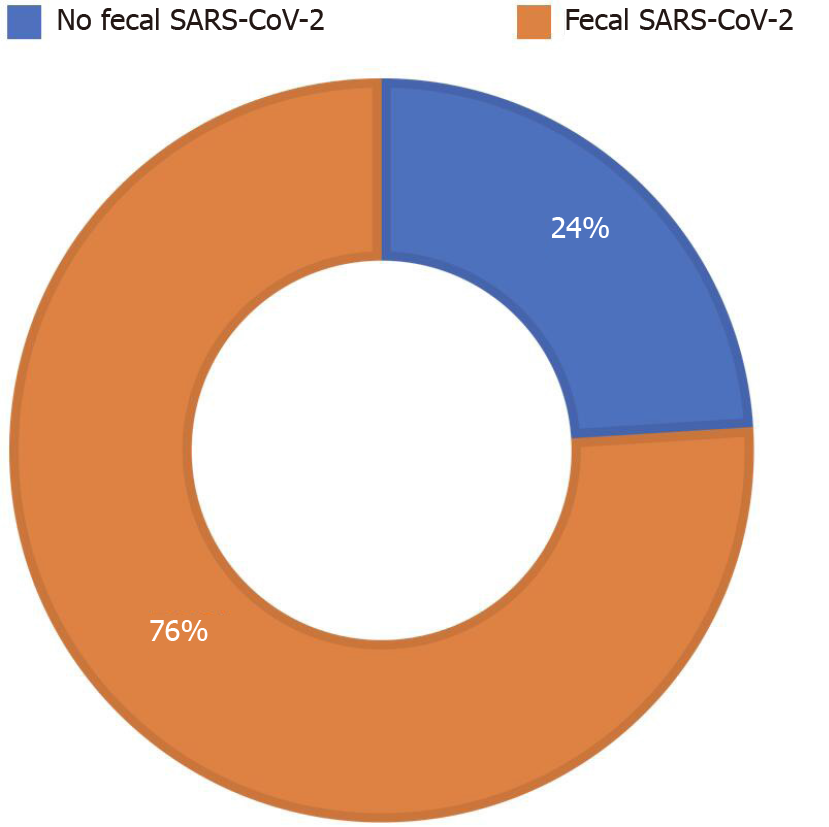
We enrolled 51 adults (40 males) with SARS-CoV-2 pneumonia. Ten patients (20%) presented with diarrhea. Real-time-PCR of SARS-CoV-2 in stools was positive in 39 patients (76%), in all patients with diarrhea (100%) and in more than two thirds (29/41, 71%) of patients without diarrhea. Obesity was one of the most common comorbidities (13 patients, 25%); all obese patients (100%) (P = 0.021) tested positive for fecal SARS-CoV-2. Median fecal calprotectin levels were 60 mg/kg [interquartile range (IQR) 21; 108]; higher fecal calprotectin levels were found in the group with SARS-CoV-2 in stools (74 mg/kg, IQR 29; 132.5) compared to the group without SARS-CoV-2 (39 mg/kg, IQR 14; 71) (P < 0.001).
High fecal calprotectin levels among COVID-19 patients correlate with SARS-CoV-2 detection in stools supporting the hypothesis that this virus can lead to bowel inflammation and potentially to the ‘leaky gut’ syndrome.
Core Tip: In this prospective study of 51 hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia, whether fecal calprotectin correlated with SARS-CoV-2 intestinal shedding was investigated. We found that high fecal calprotectin level is a common finding among hospitalized coronavirus disease 2019 (COVID-19) patients, especially those with SARS-CoV-2 fecal shedding. Obese COVID-19 patients showed high fecal viral shedding.
- Citation: Zerbato V, Di Bella S, Giuffrè M, Jaracz AW, Gobbo Y, Luppino D, Macor P, Segat L, Koncan R, D'Agaro P, Valentini M, Crocé LS, Ruscio M, Luzzati R. High fecal calprotectin levels are associated with SARS-CoV-2 intestinal shedding in COVID-19 patients: A proof-of-concept study. World J Gastroenterol 2021; 27(22): 3130-3137
- URL: https://www.wjgnet.com/1007-9327/full/v27/i22/3130.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i22.3130
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) expresses a high affinity to human angiotensin-converting enzyme 2 (ACE2) receptors. High ACE2 expression was identified within the oral cavity, type II pulmonary alveolar cells, ileal and colonic enterocytes, myocardial cells, vascular endothelium, proximal tubule, and bladder urothelial cells[1].
One-third of coronavirus disease 2019 (COVID-19) patients have gastrointestinal symptoms, with diarrhea being the most common symptom[2]. Moreover, critically ill COVID-19 patients often develop intestinal complications. Ileus, gastrointestinal bleeding, and bowel ischemia are the most common[3,4]. Spontaneous intestinal perforations among COVID-19 patients are also increasingly seen in clinical practice[4].
SARS-CoV-2 RNA has been detected in stool samples of approximately 50% COVID-19 individuals[5]. Often, viral detection in stools persists after viral clearance from respiratory samples[6], with a mean duration of fecal viral shedding of 17 d[7].
It is likely that SARS-CoV-2 can also be transmitted via the fecal–oral route and that the potential of this mode of transmission has been widely underestimated[8].
The pathogenesis of gastrointestinal symptoms caused by SARS-CoV-2 is likely multifactorial, including disruption of the intestinal mechanical barrier integrity, alteration of the gut microbiome, increased translocation of bacteria and their metabolites, and systemic inflammatory response to the virus, which in critically ill patients could be disproportionate, with uncontrolled production and release of cytokines[1].
Calprotectin is a protein derived principally from neutrophils. Upon neutrophil activation or death, calprotectin is released extracellularly, where it has a role within the innate immune response with direct antimicrobial effects. It is present in many body fluids, in proportion to the degree of inflammation. The concentration of calprotectin in feces is about six times that in plasma, and its measurement is used as a surrogate marker of gastrointestinal inflammation[1,9].
Fecal calprotectin in COVID-19 patients was found to be a marker of intestinal inflammation, both in patients with and without gastrointestinal symptoms[10,11]. Ojetti et al[12] also found a significant correlation between the development of pneumonia among COVID-19 patients and a high level of fecal calprotectin[11,12].
Serum calprotectin has been proposed as a severe COVID-19 progression marker, supporting innate immunity as a potential perpetrator of inflammation in COVID-19. It should be kept in mind that neutropenia seen in the peripheral blood of COVID-19 patients should in part reflect neutrophil migration to the tissues[12-14] and complement activation in different organs of COVID-19 patients[15] contributes to tissue damage and to the recruitment of neutrophils.
Given these premises, we aimed to investigate if fecal calprotectin correlates with SARS-CoV-2 intestinal shedding in hospitalized patients with COVID-19 pneumonia.
We performed a prospective monocentric study enrolling consecutive adults (aged >18 years) with SARS-CoV-2 pneumonia admitted to the Infectious Diseases Unit of Trieste University Hospital, Italy, from September to November 2020. Patients with inflammatory bowel diseases, gastrointestinal malignancy, and other known gastrointestinal disorders were categorically excluded.
SARS-CoV-2 detection in stool samples was determined by real-time polymerase chain reaction (RT-PCR) (LightMix® Modular SARS and Wuhan CoV E-gene and RdRp kit-TIB Molbiol, Berlin, Germany, with LightCycler Multiplex RNA Virus Master-Roche, Basel, Switzerland). According to the manufacturer’s specifications, fecal calprotectin levels were tested with the LIAISON®-Calprotectin (Diasorin, Vercelli, Italy) (normal value < 50 mg/kg). These tests were performed on hospital admission, regardless of gastrointestinal signs or symptoms.
The following data were collected on admission: Age, gender, comorbidities, gastrointestinal signs/symptoms, blood cell count, biochemical parameters, and clinical outcomes. In addition, administered drugs prior to and during hospitalization were collected. Diarrhea and obesity were defined as loose stools ≥ three times/day and a body mass index ≥ 30, respectively.
According to the size of our sample, the Shapiro-Wilk test was performed to verify the normal distribution of variables. Inter-group differences (patients with diarrhea vs patients without diarrhea and patients with fecal SARS-CoV-2 vs patients without fecal SARS-CoV-2) were determined with the Mann-Whitney U test for continuous variables and the Pearson’s Chi Square Test for discrete variables. For all analyses, two-sided statistical significance was defined as a P < 0.05. Data were analyzed using SPSS (Statistical Package for Social Science) version 25.0 (IBM SPSS Statistics for MAC OS. Armonk NY: IBM Corp.).
This study was conducted according to the declaration of Helsinki and approved by the Ethics Committee (Unique Regional Ethical Committee, Friuli Venezia-Giulia 16 April 2020), No. CEUR 2020-OS-072.
We enrolled 51 consecutive adults with SARS-CoV-2 pneumonia. Patient age ranged from 28 to 87 years [median 64 years, interquartile range (IQR) 57; 71] and 40 (78%) were males. The most common comorbidities were: Hypertension (34 patients, 67%), diabetes mellitus (5, 25%), obesity (5, 25%), heart disease (5, 25%), chronic kidney disease (5, 10%) and chronic obstructive pulmonary disease (5, 10%). Two (4%) patients were active smokers. Ten (20%) patients had diarrhea.
Median fecal calprotectin levels were 60 mg/kg (IQR 21; 108). RT-PCR of SARS-CoV-2 in stools was positive in 39 patients (76%) (Figure 1). The clinical features and biochemical parameters of enrolled patients are reported in Table 1.
| Overall, n = 51 | Patients with diarrhea, n = 10 | Patients without diarrhea, n = 41 | Significance | |
| Gender, male | 40 (78.4) | 8 (80) | 32 (78) | NS |
| Age | 64 (57; 71) | 63 (56; 70) | 65 (57; 71) | NS |
| Hypertension | 34 (66.6) | 6 (60) | 28 (68.3) | NS |
| Heart disease | 13 (25.5) | 4 (40) | 9 (21.9) | NS |
| Diabetes | 13 (25.5) | 2 (20) | 11 (26.8) | NS |
| COPD | 5 (9.8) | 1 (10) | 4 (9.75) | NS |
| CKD | 5 (9.8) | 1 (10) | 4 (9.75) | NS |
| Active smokers | 2 (3.9) | 1 (10) | 1 (2.4) | NS |
| Obesity | 13 (25.5) | 2 (20) | 11 (26.8) | NS |
| WBC (cell count/μL) | 6730 (5256; 9110) | 6595 (4672; 7420) | 6730 (5420; 9480) | NS |
| Neutrophils (cell count/μL) | 5455 (3700; 7760) | 5310 (3112; 6527) | 5310 (3112; 6725) | NS |
| CRP (mg/L) | 74 (27; 131) | 73 (25; 161) | 74 (29; 127) | NS |
| D-dimer (ng/mL FEU) | 720 (545; 1061) | 710 (560; 1511) | 720 (540; 1060) | NS |
| Fecal calprotectin(mg/kg) | 60 (21.5; 108) | 58 (31; 75) | 64 (18; 108) | NS |
| Fecal-RNA detection (n = positive) | 39 (76.5) | 10 (100) | 29 (70.8) | NS |
| Deceased | 3 (5.9) | 1 (10) | 2 (4.8) | NS |
| Hospital stay | 5 (2.5; 8) | 5 (2.75; 6.75) | 5 (3; 9.25) | NS |
No statistically significant differences in the compared variables between the two groups (patients with diarrhea vs patients without diarrhea) were found (Table 1). Both groups showed increased fecal calprotectin levels: 58 mg/kg (IQR 31; 75) in the diarrhea group and 64 mg/kg (IQR 18; 108) in those without diarrhea. Fecal SARS-CoV-2 was detected in all patients with diarrhea and in more than two thirds (29/41, 71%) of patients without diarrhea.
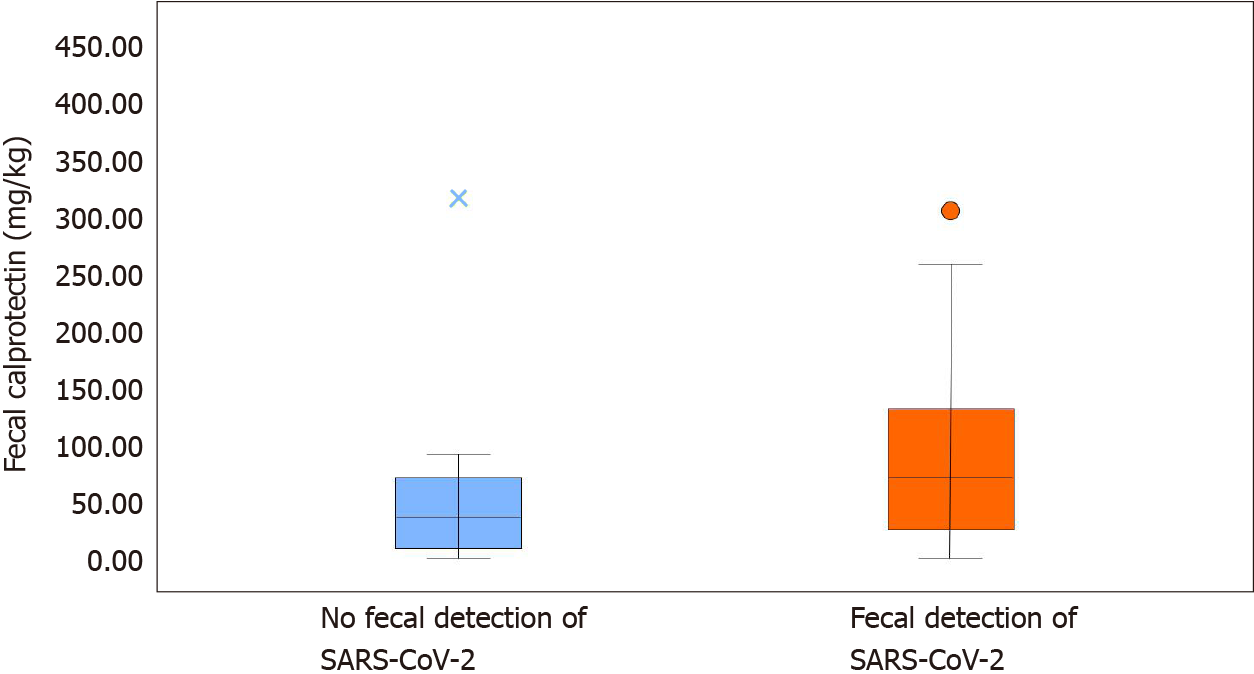
Comparing patients with and without fecal SARS-CoV-2 shedding (Table 2), we found higher fecal calprotectin levels in the former (74 mg/kg, IQR 29; 132.5) compared to the latter group (39 mg/kg, IQR 14; 71) (P < 0.001) (Figure 2). None of the patients without SARS-CoV-2 shedding had diarrhea. Neutrophil count was higher in patients with fecal SARS-CoV-2 shedding (P = 0.035), as well as D-dimer levels (P = 0.011) and white blood cell count (P = 0.038). C-reactive protein was higher in patients without fecal SARS-CoV-2 shedding (P = 0.029). Fecal SARS-CoV-2 was found in all obese patients (P = 0.021).
| Overall, n = 51 | Patients without fecal SARS-CoV-2, n = 12 | Patients with fecal SARS-CoV-2, n = 39 | Significance | |
| Gender, male | 40 (78.4) | 12 (100) | 28 (71.8) | NS |
| Age | 64 (57; 71) | 62 (57; 68) | 65 (56; 72) | NS |
| Hypertension | 34 (66.6) | 6 (50) | 28 (71.8) | NS |
| Heart disease | 13 (25.5) | 1 (8.3) | 12 (30.8) | NS |
| Diabetes | 13 (25.5) | 2 (16.6) | 11 (28.2) | NS |
| COPD | 5 (9.8) | 0 (0) | 5 (12.8) | NS |
| CKD | 5 (9.8) | 1 (8.3) | 4 (10.25) | NS |
| Active smokers | 2 (3.9) | 1 (8.3) | 1 (2.6) | NS |
| Obesity | 13 (25.5) | 0 (0) | 13 (33.3) | P = 0.021 |
| WBC (cell count/μL) | 6730 (5256; 9110) | 6105 (4870; 8400) | 7110 (5525; 9115) | P = 0.038 |
| Neutrophils (cell count/μL) | 5455 (3700; 7760) | 4390 (3485; 7417) | 5550 (3965; 8110) | P = 0.035 |
| CRP (mg/L) | 74 (27; 131) | 113 (88; 150) | 58 (24.5; 125) | P = 0.029 |
| D-dimer (ng/Ml FEU) | 720 (545; 1061) | 580 (402; 791) | 723 (562; 1157) | P = 0.011 |
| Fecal calprotectin (mg/kg) | 60 (21.5; 108) | 39 (14; 71) | 74 (29; 132.5) | P < 0.001 |
| Diarrhea | 41 (80.4) | 0 (0) | 29 (74.35) | P < 0.001 |
| Deceased | 3 (5.9) | 0 (0) | 3 (7.7) | NS |
| Hospital stay | 5 (2.5; 8) | 3 (2; 5) | 5 (3; 9.75) | NS |
SARS-CoV-2 fecal shedding is a common finding among COVID-19 patients irrespective of gastrointestinal symptoms[16,17]. Our results confirmed that fecal SARS-CoV-2 was present in approximately three quarters of our patients with COVID-19 pneumonia.
Fecal calprotectin was found to be a marker of intestinal inflammation, both in COVID-19 patients and in the general population[10,11]. Our work demonstrates that hospitalized COVID-19 patients with pneumonia have high fecal calprotectin levels, regardless of gastrointestinal symptoms.
To our knowledge this is the first study to investigate whether fecal calprotectin correlates with SARS-CoV-2 intestinal shedding in COVID-19 patients. In our study, calprotectin levels were significantly higher in those with SARS-CoV-2 fecal shedding. This finding supports the hypothesis that bowel inflammation can lead to the “leaky gut” syndrome with potential distribution of the virus to other organs[18].
While the detection of SARS-CoV-2 in feces does not necessarily lead to more gastrointestinal symptoms, the presence of SARS-CoV-2 in gastrointestinal tissue generally correlates with more severe symptoms[19].
High fecal calprotectin in COVID-19 patients is likely secondary to increased neutrophil activation in the intestinal tract. In fact, SARS-CoV-2 can activate neutrophil extracellular traps and increase levels of intracellular reactive oxygen species[20].
In our cohort, all obese patients had SARS-CoV-2 RNA detected in stools. Obesity is one of the main risk factors for severe COVID-19 and poor clinical outcomes[20,21], and is associated with a strong inflammatory response both in the general population and in COVID-19 patients[22]. This could justify a more prolonged viral shedding in this subgroup of patients.
Our work has two main limitations. First, this was a monocentric study with a small sample of patients. Second, SARS-CoV-2 was detected in stools by PCR. This technique does not discriminate between live virus and non-infectious viral particles. Only a few studies have reported live SARS-CoV-2 in the stools of COVID-19 patients[23-26]. There is also limited evidence on the duration of fecal viral shedding[7].
Our study demonstrates that higher fecal calprotectin levels correlate with SARS-CoV-2 fecal shedding in hospitalized COVID-19 patients with pneumonia. Our results support the role of neutrophils in SARS-CoV-2 pathogenesis.
In conclusion, our study provides two main results: (1) Fecal SARS-CoV-2 is present in approximately three quarters of hospitalized patients with COVID-19 pneumonia and in all patients with diarrhea; interestingly, all obese COVID-19 patients show fecal viral shedding; and (2) High fecal calprotectin levels are a common finding among hospitalized COVID-19 patients, especially those with SARS-CoV-2 fecal shedding. We believe that our results could strengthen the hypothesis that SARS-CoV-2-induced intestinal damage mediated by innate immunity (complement activation and consequent activated neutrophil migration) could contribute to COVID-19 pathogenesis.
One third of coronavirus disease 2019 (COVID-19) patients have gastrointestinal symptoms. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA has been detected in stool samples of approximately 50% of COVID-19 individuals. Fecal calprotectin is a marker of gastrointestinal inflammation.
The pathogenesis of gastrointestinal symptoms caused by SARS-CoV-2 is multifactorial and little evidence is available on this topic.
To investigate whether fecal calprotectin correlates with SARS-CoV-2 intestinal shedding in COVID-19 patients with pneumonia.
Fecal samples from patients with SARS-CoV-2 pneumonia were collected and analyzed for quantification of fecal calprotectin and SARS-CoV-2 RNA presence using polymerase chain reaction (PCR).
Real-time-PCR of SARS-CoV-2 in the stools of 51 patients with pneumonia was positive in 39 patients (76%), in all patients with diarrhea (100%) and in more than two thirds (29/41, 71%) of those without diarrhea. Higher fecal calprotectin levels were found in the group with SARS-CoV-2 in stools [74 mg/kg, interquartile range (IQR) 29; 132.5] compared to the group without SARS-CoV-2 (39 mg/kg, IQR 14; 71) (P < 0.001).
High fecal calprotectin levels in COVID-19 patients correlates with SARS-CoV-2 detection in stools.
Our results support the hypothesis that SARS-CoV-2-induced intestinal damage mediated by innate immunity could contribute to COVID-19 pathogenesis.
We thank Dr. Lisa Fusaro for her kind help.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Society for Clinical Microbiology and Infectious Diseases; Societa Italiana di Malattie Infettive e Tropicali; and SITA.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Apostolou K S-Editor: Fan JR L-Editor: Webster JR P-Editor: Liu JH
| 1. | Syed A, Khan A, Gosai F, Asif A, Dhillon S. Gastrointestinal pathophysiology of SARS-CoV2 - a literature review. J Community Hosp Intern Med Perspect. 2020;10:523-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Ye L, Yang Z, Liu J, Liao L, Wang F. Digestive system manifestations and clinical significance of coronavirus disease 2019: A systematic literature review. J Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Kaafarani HMA, El Moheb M, Hwabejire JO, Naar L, Christensen MA, Breen K, Gaitanidis A, Alser O, Mashbari H, Bankhead-Kendall B, Mokhtari A, Maurer L, Kapoen C, Langeveld K, El Hechi MW, Lee J, Mendoza AE, Saillant NN, Parks J, Fawley J, King DR, Fagenholz PJ, Velmahos GC. Gastrointestinal Complications in Critically Ill Patients With COVID-19. Ann Surg. 2020;272:e61-e62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 4. | Giuffrè M, Bozzato AM, Di Bella S, Occhipinti AA, Martingano P, Cavallaro MFM, Luzzati R, Monica F, Cova MA, Crocè LS. Spontaneous Rectal Perforation in a Patient with SARS-CoV-2 Infection. J Pers Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Gupta S, Parker J, Smits S, Underwood J, Dolwani S. Persistent viral shedding of SARS-CoV-2 in faeces - a rapid review. Colorectal Dis. 2020;22:611-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 6. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1130] [Article Influence: 226.0] [Reference Citation Analysis (1)] |
| 7. | Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13-e22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 996] [Article Influence: 249.0] [Reference Citation Analysis (0)] |
| 8. | Xiao F, Sun J, Xu Y, Li F, Huang X, Li H, Zhao J, Huang J. Infectious SARS-CoV-2 in Feces of Patient with Severe COVID-19. Emerg Infect Dis. 2020;26:1920-1922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 389] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 9. | Ayling RM, Kok K. Fecal Calprotectin. Adv Clin Chem. 2018;87:161-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 10. | Effenberger M, Grabherr F, Mayr L, Schwaerzler J, Nairz M, Seifert M, Hilbe R, Seiwald S, Scholl-Buergi S, Fritsche G, Bellmann-Weiler R, Weiss G, Müller T, Adolph TE, Tilg H. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut. 2020;69:1543-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 11. | Giuffrè M, Di Bella S, Sambataro G, Zerbato V, Cavallaro M, Occhipinti AA, Palermo A, Crescenti A, Monica F, Luzzati R, Crocè LS. COVID-19-Induced Thrombosis in Patients without Gastrointestinal Symptoms and Elevated Fecal Calprotectin: Hypothesis Regarding Mechanism of Intestinal Damage Associated with COVID-19. Trop Med Infect Dis. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Ojetti V, Saviano A, Covino M, Acampora N, Troiani E, Franceschi F; GEMELLI AGAINST COVID‐19 group. COVID-19 and intestinal inflammation: Role of fecal calprotectin. Dig Liver Dis. 2020;52:1231-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Shi H, Zuo Y, Yalavarthi S, Gockman K, Zuo M, Madison JA, Blair C, Woodward W, Lezak SP, Lugogo NL, Woods RJ, Lood C, Knight JS, Kanthi Y. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. J Leukoc Biol. 2021;109:67-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 14. | Bauer W, Diehl-Wiesenecker E, Ulke J, Galtung N, Havelka A, Hegel JK, Tauber R, Somasundaram R, Kappert K. Outcome prediction by serum calprotectin in patients with COVID-19 in the emergency department. J Infect. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Li Q, Chen Z. An update: the emerging evidence of complement involvement in COVID-19. Med Microbiol Immunol. 2021;1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | van Doorn AS, Meijer B, Frampton CMA, Barclay ML, de Boer NKH. Systematic review with meta-analysis: SARS-CoV-2 stool testing and the potential for faecal-oral transmission. Aliment Pharmacol Ther. 2020;52:1276-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 17. | Patel KP, Patel PA, Vunnam RR, Hewlett AT, Jain R, Jing R, Vunnam SR. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J Clin Virol. 2020;128:104386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 18. | Synowiec A, Szczepański A, Barreto-Duran E, Lie LK, Pyrc K. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): a Systemic Infection. Clin Microbiol Rev. 2021;34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 148] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 19. | Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 657] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 20. | Jayawardena R, Jeyakumar DT, Misra A, Hills AP, Ranasinghe P. Obesity: A potential risk factor for infection and mortality in the current COVID-19 epidemic. Diabetes Metab Syndr. 2020;14:2199-2203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Hoong CWS, Hussain I, Aravamudan VM, Phyu EE, Lin JHX, Koh H. Obesity is Associated with Poor Covid-19 Outcomes: A Systematic Review and Meta-Analysis. Horm Metab Res. 2021;53:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | McNeill JN, Lau ES, Paniagua SM, Liu EE, Wang JK, Bassett IV, Selvaggi CA, Lubitz SA, Foulkes AS, Ho JE. The role of obesity in inflammatory markers in COVID-19 patients. Obes Res Clin Pract. 2021;15:96-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Lui RN. Safety in endoscopy for patients and healthcare workers during the COVID-19 pandemic. Tech Innov Gastrointest Endosc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 2658] [Article Influence: 531.6] [Reference Citation Analysis (0)] |
| 25. | Zhang Y, Cao C, Shuangli Z. Isolation of SARs-CoV-2 from stool specimen of a confirmed case of COVID-19. [cited 16 January 2021]. Available from: https://www.cebm.net/study/isolation-of-sars-cov-2-from-a-stool-specimen-of-confirmed-case-of-covid-19/. |
| 26. | Li L, Tan C, Zeng J, Luo C, Hu S, Peng Y, Li W, Xie Z, Ling Y, Zhang X, Deng E, Xu H, Wang J, Xie Y, Zhou Y, Zhang W, Guo Y, Liu Z. Analysis of viral load in different specimen types and serum antibody levels of COVID-19 patients. J Transl Med. 2021;19:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |