Published online Jun 14, 2021. doi: 10.3748/wjg.v27.i22.3037
Peer-review started: January 27, 2021
First decision: February 25, 2021
Revised: March 8, 2021
Accepted: April 26, 2021
Article in press: April 26, 2021
Published online: June 14, 2021
Processing time: 132 Days and 7.9 Hours
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignancy. Despite the development of multimodality treatments, including surgical resection, radiotherapy, and chemotherapy, the long-term prognosis of patients with PDAC remains poor. Recently, the introduction of neoadjuvant treatment (NAT) has made more patients amenable to surgery, increasing the possibility of R0 resection, treatment of occult micro-metastasis, and prolongation of overall survival. Imaging plays a vital role in tumor response evaluation after NAT. However, conventional imaging modalities such as multidetector computed tomography have limited roles in the assessment of tumor resectability after NAT for PDAC because of the similar appearance of tissue fibrosis and tumor infiltration. Perfusion computed tomography, using blood perfusion as a biomarker, provides added value in predicting the histopathologic response of PDAC to NAT by reflecting the changes in tumor matrix and fibrosis content. Other imaging technologies, including diffusion-weighted imaging of magnetic resonance imaging and positron emission tomography, can reveal the tumor response by monitoring the structural changes in tumor cells and functional metabolic changes in tumors after NAT. In addition, with the renewed interest in data acquisition and analysis, texture analysis and radiomics have shown potential for the early evaluation of the response to NAT, thus improving patient stratification to achieve accurate and intensive treatment. In this review, we briefly introduce the application and value of NAT in resectable and unresectable PDAC. We also summarize the role of imaging in evaluating the response to NAT for PDAC, as well as the advantages, limitations, and future development directions of current imaging techniques.
Core Tip: The timely and accurate evaluation of tumor response in patients with pancreatic ductal adenocarcinoma (PDAC) after neoadjuvant therapy (NAT) is of great significance to increase the probability of tumor R0 resection and prolong survival. Ultrasound and conventional computed tomography imaging features show limited roles in the evaluation of NAT response for PDAC. Novel imaging biomarkers extracted from functional imaging technologies show promise in providing further important information for the assessment of tumor resectability and survival prediction. We reviewed the application and value of NAT in PDAC, as well as the advantages, limitations, and future development directions of current imaging techniques in tumor response assessment of PDAC after NAT in this article.
- Citation: Zhang Y, Huang ZX, Song B. Role of imaging in evaluating the response after neoadjuvant treatment for pancreatic ductal adenocarcinoma. World J Gastroenterol 2021; 27(22): 3037-3049
- URL: https://www.wjgnet.com/1007-9327/full/v27/i22/3037.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i22.3037
Pancreatic ductal adenocarcinoma (PDAC) has become a major public health issue globally[1,2]. Due to the late onset of symptoms, the high invasive potential of the disease, and the lack of accurate diagnostic markers to detect micro-metastasis, the prognosis of PDAC has not significantly improved over the past several decades[3].
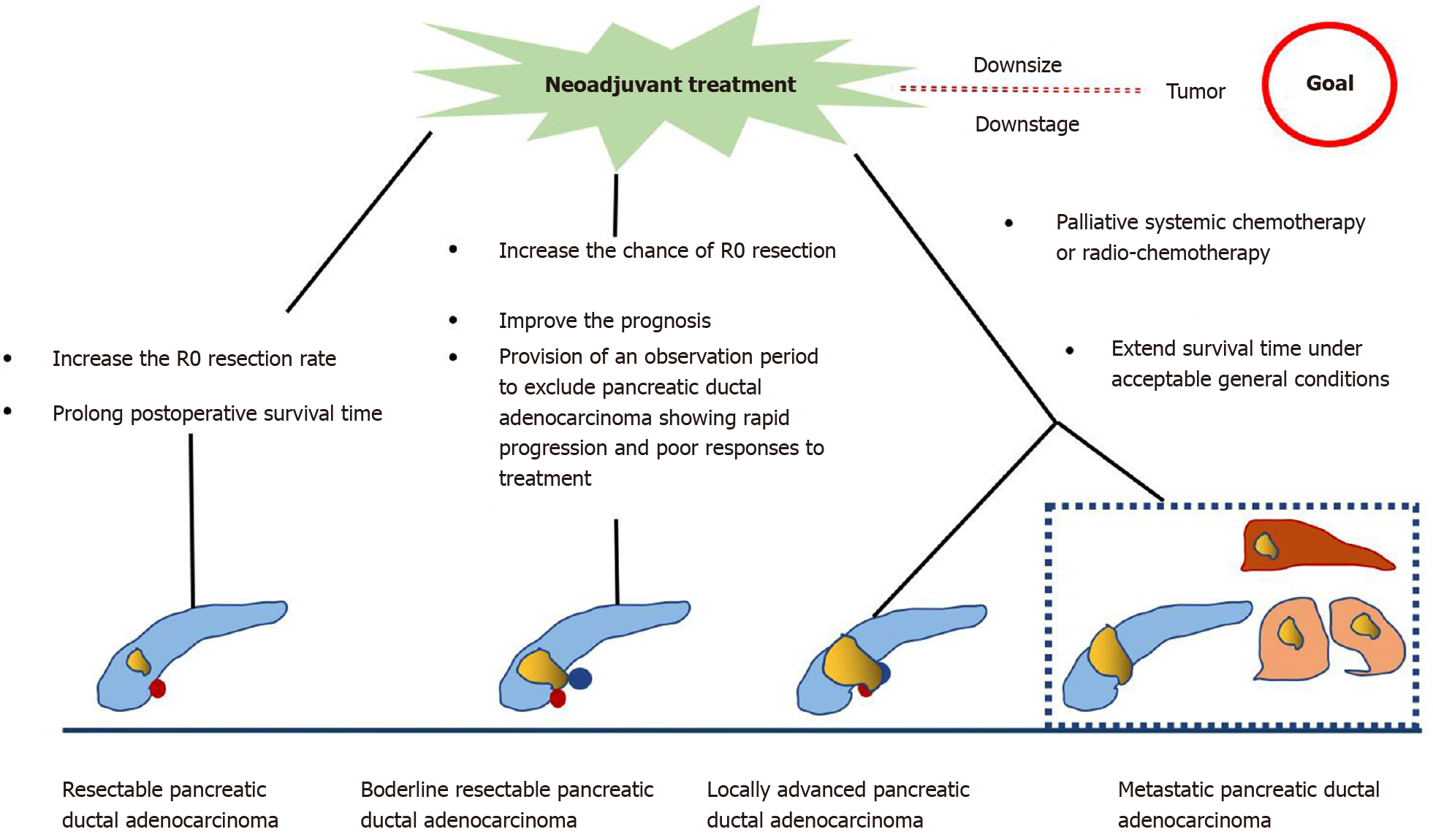
Neoadjuvant treatment (NAT) is an emerging therapy for PDAC. It provides the theoretical advantages of downstaging borderline resectable or locally advanced pancreatic tumors, allowing more patients to benefit from surgery, increasing the possibility of R0 resection, treating occult micro-metastasis, and prolonging overall survival (OS)[4,5].
With the advancements in imaging technology and the renewed interest in data acquisition and analysis, there are increasingly more studies reporting the accuracy of imaging for evaluating the response to NAT for PDAC. This review focuses on the role of imaging in the response assessment to NAT for PDAC, as well as the advantages, limitations, and future directions of current imaging techniques.
Various guidelines recommend the use of multiphase contrast-enhanced computed tomography (CT) imaging for staging PDAC and determining tumor resectability[6]. According to the National Comprehensive Cancer Network guidelines, PDAC can be divided into four categories based on the preliminary assessment of tumor location and metastasis as follows[7]: (1) Resectable. The possibility of R0 resection is very high. The tumor has no contact with the adjacent arteries [superior mesenteric artery, hepatic artery, or celiac artery (CA)] and veins [superior mesenteric vein (SMV), portal vein (PV), or their confluence (SMV/PV)], or the degree of contact is less than 180° the circumference of the vessel wall; (2) Borderline resectable. The possibility of incomplete resection of R1 or R2 is high. The degree of contact between the tumor and arteries (superior mesenteric artery or CA) is less than 180°, the tumor invades a short segment of hepatic artery that can be resected or reconstructed, but does not involve the CA, or tumor contact with the adjoining vein (SMV-PV) exceeds the circumference of the vessel wall by 180°; (3) Locally advanced. The tumor cannot be resected due to invasion of the adjacent structures; and (4) Metastasis. The tumor has distant metastasis. As seen from this classification, the tumor–vessel contact is critical to determining whether the tumor is resectable. At the same time, the realization of R0 resection (marginal negative resection) is key to prolonging the life of patients with PDAC. Surgical resection is recommended for resectable PDAC, and the 5-year survival rate of R0 resection is 18%-24%[8]. However, the 5-year survival rate of borderline and locally advanced PDAC is poor, about 8%-11%[9,10]. In addition, almost 50% of patients relapse within a short period of time after tumor resection[11]. Other therapies such as chemotherapy and radiotherapy are usually used in unresectable cases to reduce the disease burden and prolong the survival time. However, even then, the prognosis remains poor with a median survival time of 6.8-11.1 mo in patients with unresectable tumor[12,13]. Hence, to achieve better survival, downstaging of the disease by NAT followed by surgical resection if possible has been advocated for unresectable PDAC.
In the past, the standard treatment for resectable PDAC was surgical resection followed by adjuvant chemotherapy. In 2008, a large retrospective study included surgically resectable PDAC and studied the impact of NAT on the prognosis of resectable PDAC[14]. The results showed that NAT followed by surgery had a survival advantage over upfront surgery with or without adjuvant treatment, which suggested the possible role of NAT in treating resectable PDAC. A recent meta-analysis[15] showed that the median OS of patients receiving NAT was longer than that with surgery (18.8 mo vs 14.8 mo). Although the overall resection rate of NAT was lower than that of previous surgery (66% vs 81.3%), the R0 rate was higher (86.8% vs 66.9%). In addition, other studies have reported that the tumor resection rate after NAT is between 50% and 90%, with a median survival time of 23.5 mo[16-19].
Accumulating evidence indicates that patients with borderline PDAC can benefit from NAT, as it increases the chance of R0 resection, improves survival, and identifies cases of PDAC with rapid progression and poor response to treatment[20-22]. An international consensus has proposed that patients with borderline PDAC undergo surgical resection after NAT when there are no anatomical contraindications or metastatic disease[23]. A multicenter trial showed that neoadjuvant S-1 therapy in combination with radiotherapy followed by surgery achieved an R0 resection rate of 63%[24]. Inoue et al[25] reported that NAT with gemcitabine and nab-paclitaxel improved downstaging of the tumor and allowed patient selection. Anger et al[26] investigated the role of NAT in different types of borderline PDAC according to international consensus criteria. Their results showed that PDAC patients with biological (borderline resectable-B) have a relatively poor prognosis and should be considered for multimodal NAT.
In unresectable PDAC, palliative systemic chemotherapy is commonly used. In these patients, the main purpose is to extend the survival time under acceptable general conditions. FOLFIRINOX and gemcitabine in combination with other drugs are the preferred regimens for patients with good performance status, whereas capecitabine, gemcitabine, and 5-fluorouracil monotherapy are usually given to patients in poor general condition[12,27-29].
In summary, NAT for resectable PDAC performed before primary surgery increases the R0 resection rate and prolongs postoperative survival. In borderline and unresectable PDAC, NAT increases the chance of R0 resection, providing strong evidence for individualized patient treatment, eventually resulting in extending survival (Figure 1). However, there are some limitations in the current research. First, there is a lack of consensus on the best regimen for NAT. Second, some studies have not strictly separated resectable PDAC from unresectable PDAC during NAT. Finally, there is no clear consensus on the duration of NAT.
Conventional ultrasound (US) is an economical and radiation-free investigation. It helps to visualize pancreatic masses that are iso-attenuating on non-contrast CT images. However, its role in assessment of tumor response is very limited. US can be, however, a useful tool for detecting abdominal complications and drug toxicity during NAT in metastatic pancreatic cancer. The most common adverse effects of NAT regimens for metastatic pancreatic cancer are neutropenic colitis and venous thrombosis, which can be readily detected by abdominal US[30].
Endoscopic US (EUS) has developed from a strictly imaging modality to one that allows for tissue diagnosis through fine needle aspiration. It has been proved to be a valuable means for early detection and staging of PDAC, especially for lesions ≤ 3 cm, which is superior to multi-detector CT[31,32]. Recently, the role of EUS in the delivery of NAT in PDAC and its response assessment is rapidly emerging. Das et al[33] conducted a large sample study to investigate the value of EUS in preoperative tumor response prediction of PDAC after NAT. The results showed that the change in the tumor size after NAT on EUS was a sensitive marker for tumor response evaluation, and tumor size reduction ≥ 47% was an independent prognostic factor for OS in these patients. A systematic review from Barreto et al[34] compared the accuracy of imaging modalities to predict resectability and R0 resection for borderline or locally advanced PDAC after NAT. They showed that effective imaging evaluation allowed prediction of tumor resectability. Moreover, decrease in tumor stiffness of PDAC on EUS elastography may be used as a potential marker for NAT response and tumor resectability assessment. In addition, Figueiredo et al[35] reported the role of EUS-guided technology in the implementation of NAT for PDAC. The authors indicated that EUS-guided placement of fiducial markers for stereotactic body radiation therapy in PDAC helped to ensure the feasibility and security of subsequent NAT. The above studies illustrate the importance of EUS in the process of NAT for PDAC.
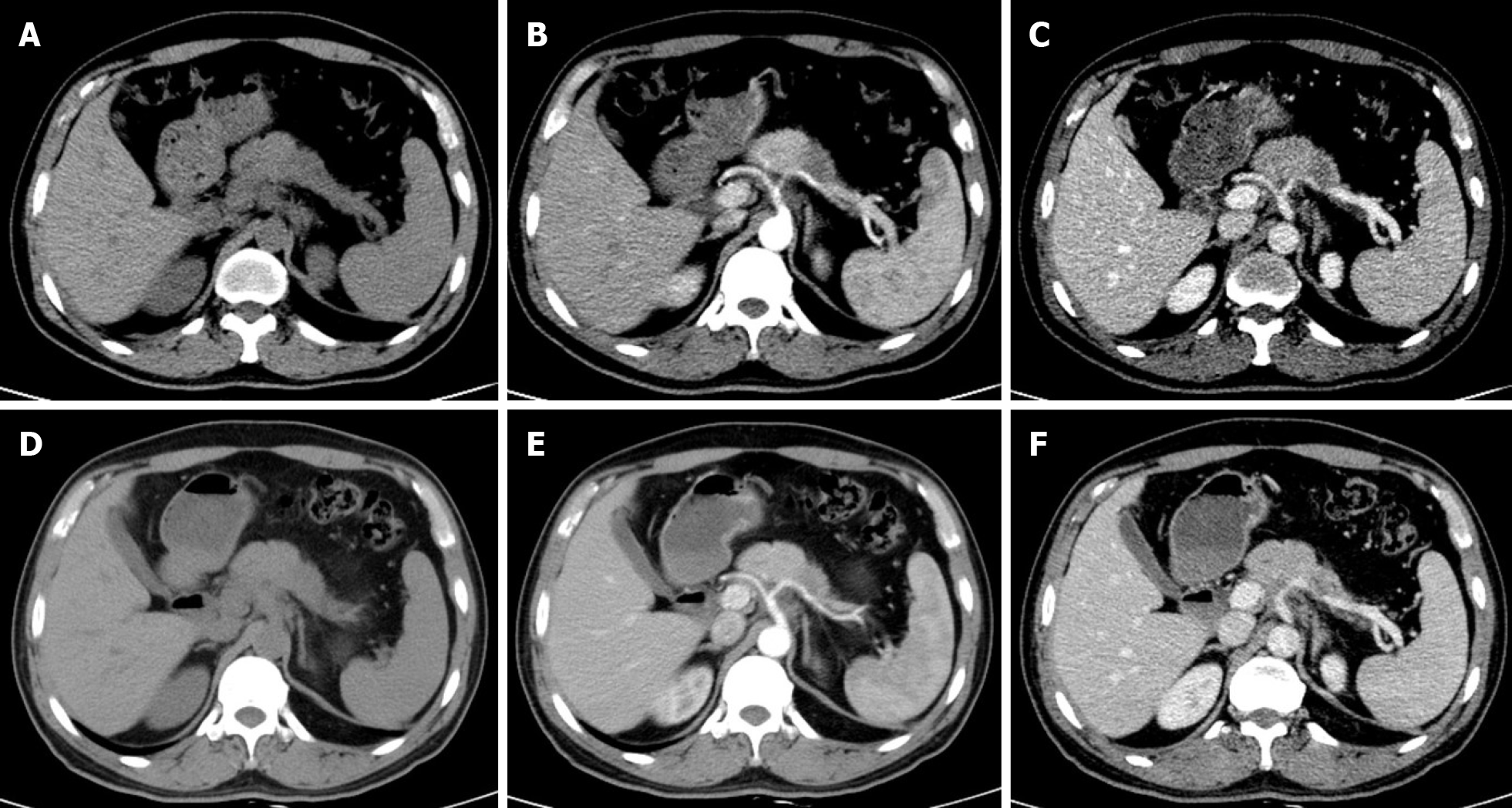
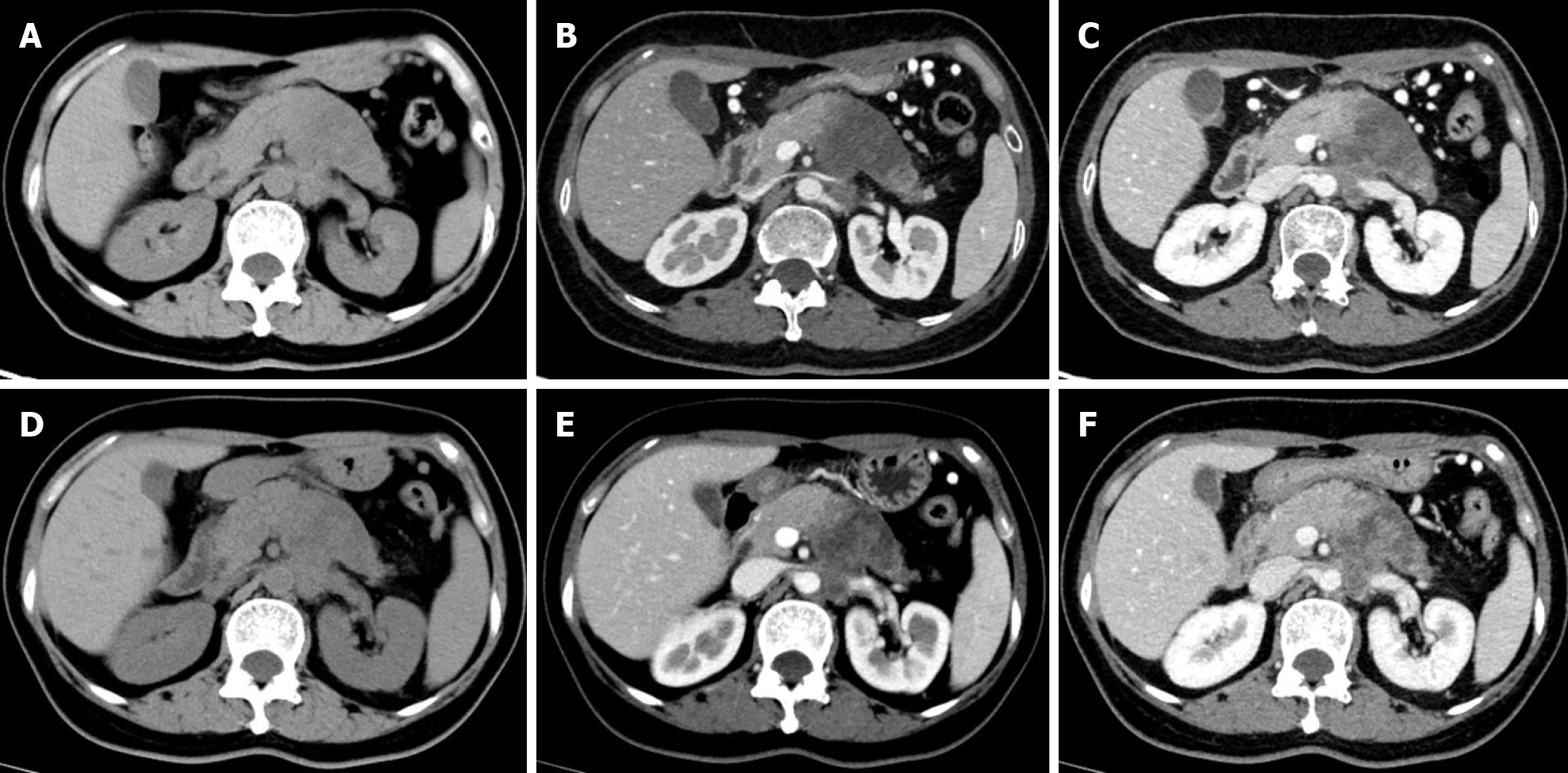
Multidetector CT (MDCT) is the most frequently used imaging method to evaluate the response of PDAC after NAT. Compared with other imaging techniques, its advantages include higher spatial resolution and multiplanar reconstruction ability[36]. However, recent studies have shown that the diagnostic performance of MDCT in evaluating tumor resectability and re-staging of borderline tumors is not very satisfactory. In a study of 129 patients with borderline PDAC, the authors found that the commonly used response evaluation criteria in solid tumors (RECIST) criteria were not suitable for evaluating tumor response after NAT, because there were few morphological changes in the imaging after treatment[37]. A systematic review reported that only a small number of patients showed tumor shrinkage after NAT (Figure 2), and most patients (53%-80%) had stable disease[34]. Similar results were reported by a recent study[38] that showed that the assessment of resectability by MDCT after NAT is relatively insensitive and non-specific to predict R0 resection, because MDCT cannot accurately distinguish between residual tumor and tissue scarring after tumor regression[39]. Moreover, local inflammatory pancreatitis also cannot be distinguished from tumor infiltration and the area of tumor infiltration is replaced by fibrotic tissue, which does not lead to apparent changes in tumor size (Figure 3). All of these factors lead to under-evaluation of tumor resectability[40-42].
Recently, some studies have started to explore whether imaging features other than tumor size and enhancement on MDCT images can be used to assess tumor response in PDAC. A study by Cassinotto et al[43] showed that the partial regression of tumor–vessel contact after NAT indicates suitability for surgical exploration, regardless of the reduction in tumor size or residual vascular involvement. Another study by Amer et al[44] suggested that changes at the PDAC/parenchyma interface may be used as an early predictor of response to NAT. A recent study from Wei et al[45] showed that the largest tumor diameter and radiological tumor volume on post-therapy MDCT were associated with the pathologic tumor staging and tumor response to NAT.
Although MDCT has a high resolution in displaying morphological characteristics of the tumor and the surrounding vascular structures, it has low specificity due to the lack of obvious tumor reduction after NAT in PDAC, as well as the presence of fibrous tissue and local pancreatitis. Hence, MDCT has low specificity and sensitivity in restaging of PDAC after NAT. However, further quantification and evaluation of imaging indicators on MDCT images can significantly improve the assessment of tumor response and prognostic value of patients with PDAC after NAT.
Magnetic resonance imaging (MRI) provides better visualization of the soft tissues and pancreatic and biliary ductal abnormalities. In PDAC, the high cellularity and potential fibrosis of the tumor hinders the free movement of water molecules. This can be quantified by diffusion-weighted imaging (DWI) on MRI, which results in a low mean apparent diffusion coefficient (ADC) on the ADC map. Several studies have investigated the utility of DWI for assessment of the NAT response in patients with PDAC[46-50]. A previous study conducted by Cuneo et al[46] reported that there was an obvious correlation between the mean ADC values before treatment and the amount of destruction of tumor cells after neoadjuvant chemoradiation. The mean pre-treatment ADC (161 × 10-5 mm2/s) of patients with a good response was significantly higher than that of non-responders (125 × 10-5 mm2/s), which may provide evidence for candidate selection of intensified therapy. Dalah et al[47] investigated the relationship between ADC values after neoadjuvant chemoradiation and the pathological treatment response in PDAC and showed that the post-mean ADC values were moderately correlated with the pathological tumor responses. The study also noted that, compared to tumors with poor pathological response, tumors with a good pathological response had a higher ratio of fibrosis to tumor cells. However, different results were reported by a recent study from Zimmermann et al[48]. The authors found that mean ADC cannot be used as a reliable marker to identify PDAC patients with a good response to NAT.
A prospective study by Okada et al[49] of 28 patients with borderline PDAC found that the post-treatment whole-tumor ADC value predicted R0 resection with 75% accuracy and histological response with 89% accuracy. Based on these findings, the authors suggested that whole-tumor ADC value can be used as the new marker for treatment response evaluation in PDAC. Bali et al[50] compared the effectiveness of DWI and RECIST criteria in evaluating tumor response to systemic chemotherapy in unresectable PDAC. This study selected three imaging biomarkers, ROI-ADC, DW volume, and diffusion parameters derived from histograms, and showed that these markers more accurately classified between responders and non-responders compared to RECIST 1.1 criteria. So, the ADC value of DWI shows better performance than morphological features in evaluating the tumor response to NAT in PDAC. However, at present, there are few related studies with inconsistent conclusions, and there are no unified standard criteria for the selection of DWI parameters. In addition, DWI has the disadvantages of large motion artifacts, long time-consuming procedures, and high cost, limiting its clinical application.
18F-fluorodeoxyglucose positron emission tomography (18-F-FDG-PET) is a diagnostic test that reflects the genetic, molecular, metabolic, and functional status of the lesions. The maximum standardized uptake value (SUVmax) obtained by PET imaging reflects the glucose metabolism of the tumors. Choi et al[51] explored the relationship between the early treatment response after neoadjuvant chemo-radiotherapy using FDG-PET and surgical outcome in locally advanced PDAC. The results suggested that FDG-PET is helpful to monitor the clinical efficacy of NAT in the treatment of locally advanced PDAC. In patients with a good tumor response (≥ 50% decrease in SUV after cycle 1), surgical resection was accomplished. Lee et al[52] investigated the role of pre-operative 18-F-FDG PET/CT in predicting survival of patients with PDAC. The results showed that metabolic tumor volume and total lesion glycolysis were independent prognostic factors for predicting recurrence-free survival and OS of patients, and PET/CT provides useful prognostic information for patients after resection of PDAC with curative intent irrespective of NAT.
Recently, some studies have further studied the value of SUVmax in PDAC to assess the tumor response after NAT[48,53,54]. The results showed that the post-NAT SUVmax can be an effective indicator to predict the prognosis and treatment response to NAT for PDAC. Moreover, the post-treatment metabolic parameters of PET/MRI also reportedly correlate with the pathological response. However, the sample size of the above studies was relatively small (< 50 cases in 2/3 articles), and the results were not convincing. Barnes et al[55] enrolled 201 consecutive patients with localized PDAC treated with NAT. The results showed that the pre-treatment SUVmax cut-off value of 7.5 on PET/CT could accurately predict the OS. The results from Yokose et al[56] showed that PET response criteria in solid tumor was more accurate in determining the NAT effects for PDAC than RECIST criteria (72.7% vs 36.4%), which puts forward a new direction for future research in this field.
The application of PET imaging indicates that the response assessment to NAT in PDAC has changed from morphological to metabolic evaluation, especially with the proposal of PET/MRI, which plays a significant role in the prediction of treatment outcome and clinical decision-making in these patients. However, due to the lack of literature, the results need to be further investigated. In addition, the PET response criteria in solid tumor criteria need to be compared with traditional evaluation criteria in future studies.
PDAC is a matrix-rich tumor, characterized by the activation of pancreatic stellate cells, which deposit a large amount of extracellular matrix[57]. The accumulation of extracellular matrix, including collagen, fibronectin, proteoglycan, and hyaluronic acid, can induce the formation of rigid extracellular matrix to compress blood vessels, leading to perfusion damage and ultimately hindering the transmission of anti-cancer drugs to the tumor cells[58] (Figure 3). Based on the above theoretical assumptions, Hamdy et al[59] investigated the value of perfusion CT in predicting the response of PDAC to neoadjuvant chemotherapy and radiation therapy. The results showed that participants who responded to NAT had higher baseline blood flow than those who did not respond (median, 44 mL/100 g/min vs 28 mL/100 g/min, respectively). In responders, the perfusion parameters increased after treatment, whereas there were no significant changes in perfusion parameters of non-responders. The authors suggested that pre-treatment perfusion CT can be helpful to predict the histopathologic response of PDAC to NAT. However, the exact role of perfusion imaging in PDAC needs to be further evaluated in a larger number of patients.
In recent years, many studies have emphasized the role of radiomics in various aspects of pancreatic tumors, such as tumor characterization, assessment of resectability, risk of recurrence, and prediction of survival. A previous study from Chen et al[42] showed changes in the CT radiomic features, such as the histograms of mean CT number, skewness, and kurtosis during the chemoradiation in patients with PDAC. The authors suggested that these changes may potentially be used for the early evaluation of the treatment response and patient stratification to achieve accurate and intensive treatment. Chakraborty et al[60] conducted a preliminary study to investigate the value of CT texture analysis in quantifying tumor heterogeneity and predicting 2-year survival in patients with PDAC. The results revealed that CT texture features can predict the heterogeneity in pancreatic tumors. Using fuzzy minimum-redundancy maximum-relevance feature selection and a naïve Bayes classifier, the area under the curve scaled up to 90%. At the same time, the accuracy of CT texture analysis in predicting the 2-year survival rate can reach 82.86%. Thus, it can be used to formulate the optimal treatment plan for PDAC patients. Ciaravino et al[61] investigated the added value of CT texture analysis in estimating tissue changes in PDAC downsized and resected after chemotherapy. The results suggested that the change of kurtosis before and after treatment showed a statistically significant difference, suggesting that CT texture analysis can assess tumor heterogeneity, tissue changes, and tumor downstaging in PDAC cases with no significant alteration in tumor size after NAT.
Recently, some studies have explored the utility of CT texture analysis in predicting resectability and prognosis in patients with PDAC[62] and the relationship between texture features and the tumor pathological response[63]. The results showed that the CT texture feature was more accurate in defining the tumor as resectable than unresectable. Moreover, higher subtracted entropy and lower subtracted gray-level co-occurrence matrix entropy indicated longer OS[62]. In the study by Borhani et al[63], texture parameters, such as pre-treatment mean positive pixel, pre-treatment kurtosis, changes in kurtosis, and pre-treatment tumor SD, were found to be statistically different between patients with poor histologic response and those with favorable histologic response. The authors concluded that pre-treatment textural features of baseline CT imaging and longitudinal changes in tumor heterogeneity can be used as biomarkers for predicting histologic response to neoadjuvant chemotherapy and disease-free survival. In addition, Nasief et al[64] reported the value of radiomics combined with carbohydrate antigen 19-9 (CA19-9) in the evaluation of NAT for PDAC. The results showed that reduction of CA19-9 levels and delta radiomics features were predictors of survival in these patients. The delta radiomics features-CA19-9 combination has the potential to increase the possibility for response-based treatment adaptation. There is no denying that radiomics or texture analysis has wide prospects in the management of PDAC after NAT. The current limitations of radiomics include the time-consuming segmentation and non-robustness conclusion. Further large-scale studies are required to determine its real potential.
In all stages of NAT for PDAC, imaging plays an essential role in the diagnosis, assessment of resectability, tumor re-staging, and response evaluation after NAT (Table 1). The change in tumor size as assessed by EUS after NAT can provide valuable information on tumor response and survival prediction. Moreover, change in tumor stiffness of PDAC on EUS elastography may be used as a potential marker for NAT response and tumor resectability assessment. MDCT, which relies on its high-density resolution and the speed of data acquisition, is often used to evaluate the resectability and tumor response of PDAC after NAT. However, because of the fibrotic and infiltrative nature of PDAC, changes in the size of the tumor after NAT are not apparent, and it is difficult to distinguish accurately between residual tumor and scarring from tumor regression. Hence, the value of conventional CT imaging features in evaluating NAT for PDAC is limited. ADC value quantified by DWI on MRI can reflect the cellularity and potential fibrotic changes of PDAC after NAT. Moreover, pre-treatment ADC can be used as an imaging biomarker to distinguish responders from non-responders after NAT. However, due to the shortcomings of large motion artifacts, the time-consuming process and high cost, as well as the inconsistency of existing results, the application of DWI in NAT for PDAC needs further confirmation. PET has great prospects for the prediction and evaluation of NAT for PDAC, which represents the transformation of imaging markers from morphological to metabolic. However, due to the lack of substantial evidence and the costly nature of the PET technique, the role of PET in PDAC is limited and needs further investigation. Perfusion CT, as a new imaging method to assess the response to NAT in PDAC, has proved to be beneficial. It can reveal the changes of extracellular matrix by monitoring the changes in the tumor blood perfusion, so as to predict and evaluate the efficacy of NAT in PDAC. However, more research is needed to confirm the findings of the preliminary studies. Radiomics and texture analysis have gradually become research hotspots in the field of NAT for PDAC, providing additional values for evaluating tumor heterogeneity. However, the time-consuming segmentation and non-robustness of current data cannot be denied.
| Imaging technologies | Imaging parameters | Pathological basis | Roles and advantages | Limitations | Improvement |
| US | Conventional US imaging features | Iso-/low-attenuating pancreatic masses | Non-radiation and high economy; a tool for PDAC early detection and staging; detection of systemic therapies associated adverse events in metastatic PDAC | Play the limited role for tumor response of PDAC after NAT | Further exploration |
| EUS | Tumor size | Change in tumor size after NAT | Provides additional information for response assessment and survival prediction | Invasive procedure, preliminary research, lack of credible results | Further large-scale research is needed in the future |
| Tumor stiffness (EUS elastography) | Decrease of change in tumor stiffness after NAT | Potential marker for NAT response and tumor resectability assessment | Poor objectivity and low reproducibility; the pressure of the probe on the lesion area is not easily controlled | Technology Optimization; further investigation is needed | |
| CT | MDCT conventional imaging features Tumor size; Enhancement; Tumor-vessel contact | Size or enhancement reduction; changes in tumor-vessel contact | High-density resolution; speed of data acquisition; multiplanar reconstruction ability | Low specificity and sensitivity (53%-80% of patients are in a stable state on MDCT after NAT) | Further quantification, exploration and evaluation of imaging indicators on MDCT conventional imaging features |
| Perfusion CT; Blood flow; Blood volume Permeability–surface area product | Changes in extracellular matrix causing changes in the tumor blood flow | Pre-treatment perfusion CT can be helpful to predict the histopathologic response of PDAC to NAT | Preliminary research, lack of credible results | Further studies are needed to evaluate the value of perfusion imaging in a large number of patients | |
| Texture analysis; Texture features | CT texture features after NAT can reveal the heterogeneity in PDAC | Providing additional value for judging tumor heterogeneity; judging tissue changes and tumor downstaging in those cases with no significant alteration in tumor size after NAT | Time consuming segmentation and non-robustness conclusion | Further large-scale research is needed in the future | |
| Radiomics; Radiomics features and delta radiomics features combined with common clinical index (e.g., CA-199) | Radiomics features after NAT can reveal the heterogeneity change of PDAC | Increasing the possibility for response-based treatment adaptation; has a broad prospect in the management of PDAC after NAT | The lack of enough research; time consuming segmentation and non-robustness conclusion | ||
| MRI | ADC value of DWI | Cellularity and potential fibrotic changes of PDAC after NAT | Improve the prediction possibility of R0 resection rate (75% accuracy) in resectable PDAC; pre-treatment ADC can be used to distinguish responding patients from non-responding patients after NAT | Few related studies and inconsistent conclusions; no unified standard for the selection of DWI scanning technology and parameters; large motion artifacts, time-consuming and high cost | Large-scale studies to validate the role of DWI in PDAC to NAT; establish a unified scanning standard; reduce motion artifacts and scan time; reduce costs by optimizing sequences |
| PET imaging | SUVmax | Changes in glucose metabolism of tumors before and after NAT | Aid in monitoring the clinical outcome of patients with locally advanced PDAC treated with NAT; distinguish responding patients from non-responding patients after NAT; plays a significant role in the clinical decision-making of patients | Lack of relevant research and high cost of inspection | Application of PERCIST criteria and comparison with the accuracy of traditional evaluation criteria will be the future research direction |
The role of conventional CT imaging features in the evaluation of NAT response for PDAC is limited. Other imaging techniques, including EUS, DWI, PET, and perfusion CT, have enormous potential to become powerful tools for the assessment of tumor resectability and survival prediction of PDAC after NAT. In addition, the derivate techniques based on artificial intelligence, such as texture analysis and radiomics, have gradually begun to show their prominence in the field of NAT for PDAC. Although current research is limited and the conclusions are inconsistent, additional research conducted in this field will address the shortcomings of the existing evaluation system for PDAC and promote the implementation of precision medicine.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hu R S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Liu JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55827] [Article Influence: 7975.3] [Reference Citation Analysis (132)] |
| 2. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1673] [Article Influence: 334.6] [Reference Citation Analysis (1)] |
| 3. | Deplanque G, Demartines N. Pancreatic cancer: are more chemotherapy and surgery needed? Lancet. 2017;389:985-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA, Charnsangavej C, Lano EA, Ho L, Lenzi R, Abbruzzese JL, Wolff RA. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496-3502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 547] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 5. | Roland CL, Yang AD, Katz MH, Chatterjee D, Wang H, Lin H, Vauthey JN, Pisters PW, Varadhachary GR, Wolff RA, Crane CH, Lee JE, Fleming JB. Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol. 2015;22:1168-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Abrams RA, Lowy AM, O'Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, Benson AB 3rd, Binder E, Cardin DB, Cha C, Chiorean EG, Chung V, Czito B, Dillhoff M, Dotan E, Ferrone CR, Hardacre J, Hawkins WG, Herman J, Ko AH, Komanduri S, Koong A, LoConte N, Lowy AM, Moravek C, Nakakura EK, O'Reilly EM, Obando J, Reddy S, Scaife C, Thayer S, Weekes CD, Wolff RA, Wolpin BM, Burns J, Darlow S. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1028-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 735] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 8. | Gilbert JW, Wolpin B, Clancy T, Wang J, Mamon H, Shinagare AB, Jagannathan J, Rosenthal M. Borderline resectable pancreatic cancer: conceptual evolution and current approach to image-based classification. Ann Oncol. 2017;28:2067-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, Hong TS, Kwak EL, Lauwers GY, Ryan DP, Wargo JA, Lillemoe KD, Ferrone CR. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? Ann Surg. 2013;257:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 10. | Bilimoria KY, Talamonti MS, Sener SF, Bilimoria MM, Stewart AK, Winchester DP, Ko CY, Bentrem DJ. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg. 2008;207:510-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, Zheng H, Szymonifka J, Wargo JA, Thayer SP, Lauwers GY, Deshpande V, Mino-Kenudson M, Fernández-del Castillo C, Lillemoe KD, Warshaw AL. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery. 2012;152:S43-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1945] [Article Influence: 277.9] [Reference Citation Analysis (0)] |
| 13. | Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J, Staugaard C, Indukala D, Boustani AM, Patel V, Cha CH, Salem RR, Chang B, Hochster HS, Lacy J. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114:737-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | Stessin AM, Meyer JE, Sherr DL. Neoadjuvant radiation is associated with improved survival in patients with resectable pancreatic cancer: an analysis of data from the surveillance, epidemiology, and end results (SEER) registry. Int J Radiat Oncol Biol Phys. 2008;72:1128-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, van Eijck CHJ, Groot Koerkamp B, Rasch CRN, van Tienhoven G; Dutch Pancreatic Cancer Group. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105:946-958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 389] [Article Influence: 55.6] [Reference Citation Analysis (1)] |
| 16. | Kim SS, Nakakura EK, Wang ZJ, Kim GE, Corvera CU, Harris HW, Kirkwood KS, Hirose R, Tempero MA, Ko AH. Preoperative FOLFIRINOX for borderline resectable pancreatic cancer: Is radiation necessary in the modern era of chemotherapy? J Surg Oncol. 2016;114:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, Honda G, Matsumoto I, Wada K, Furuse J, Matsuyama Y, Unno M; Study Group of Preoperative Therapy for Pancreatic Cancer (Prep) and Japanese Study Group of Adjuvant Therapy for Pancreatic cancer (JSAP). Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019;49:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 330] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 18. | Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh Rde W, Collisson E, Schwartz L, Frankel W, Martin R, Conway W, Truty M, Kindler H, Lowy AM, Bekaii-Saab T, Philip P, Talamonti M, Cardin D, LoConte N, Shen P, Hoffman JP, Venook AP. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 2016;151:e161137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 360] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 19. | Versteijne E, Suker M, Groen JV, Besselink MG, Bonsing BA, Bosscha K, Busch OR, de Hingh IHJT, De Jong KP, Molenaar IQ, van Santvoort HC, Verkooijen HM, Van Eijck CH, van Tienhoven G; Dutch Pancreatic Cancer Group. External Validity of the Multicenter Randomized PREOPANC Trial on Neoadjuvant Chemoradiotherapy in Pancreatic Cancer: Outcome of Eligible But Non-Randomized Patients. Ann Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | di Sebastiano P, Grottola T, di Mola FF. Borderline resectable pancreatic cancer and the role of neoadjuvant chemoradiotherapy. Updates Surg. 2016;68:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Barnes CA, Chavez MI, Tsai S, Aldakkak M, George B, Ritch PS, Dua K, Clarke CN, Tolat P, Hagen C, Hall WA, Erickson BA, Evans DB, Christians KK. Survival of patients with borderline resectable pancreatic cancer who received neoadjuvant therapy and surgery. Surgery. 2019;166:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Rose JB, Rocha FG, Alseidi A, Biehl T, Moonka R, Ryan JA, Lin B, Picozzi V, Helton S. Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann Surg Oncol. 2014;21:1530-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 23. | Kulkarni NM, Mannelli L, Zins M, Bhosale PR, Arif-Tiwari H, Brook OR, Hecht EM, Kastrinos F, Wang ZJ, Soloff EV, Tolat PP, Sangster G, Fleming J, Tamm EP, Kambadakone AR. White paper on pancreatic ductal adenocarcinoma from society of abdominal radiology's disease-focused panel for pancreatic ductal adenocarcinoma: Part II, update on imaging techniques and screening of pancreatic cancer in high-risk individuals. Abdom Radiol (NY). 2020;45:729-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Takahashi S, Ohno I, Ikeda M, Konishi M, Kobayashi T, Akimoto T, Kojima M, Morinaga S, Toyama H, Shimizu Y, Miyamoto A, Tomikawa M, Takakura N, Takayama W, Hirano S, Otsubo T, Nagino M, Kimura W, Sugimachi K, Uesaka K. Neoadjuvant S-1 With Concurrent Radiotherapy Followed by Surgery for Borderline Resectable Pancreatic Cancer: A Phase II Open-Label Multicenter Prospective Trial (JASPAC05). Ann Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 25. | Inoue Y, Saiura A, Oba A, Ono Y, Mise Y, Ito H, Sasaki T, Ozaka M, Sasahira N, Takahashi Y. Neoadjuvant gemcitabine and nab-paclitaxel for borderline resectable pancreatic cancers: Intention-to-treat analysis compared with upfront surgery. J Hepatobiliary Pancreat Sci. 2021;28:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Anger F, Döring A, van Dam J, Lock JF, Klein I, Bittrich M, Germer CT, Wiegering A, Kunzmann V, van Eijck C, Löb S. Impact of Borderline Resectability in Pancreatic Head Cancer on Patient Survival: Biology Matters According to the New International Consensus Criteria. Ann Surg Oncol. 2021;28:2325-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5639] [Article Influence: 402.8] [Reference Citation Analysis (1)] |
| 28. | Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA, Cunningham D, Wadsley J, Darby S, Meyer T, Gillmore R, Anthoney A, Lind P, Glimelius B, Falk S, Izbicki JR, Middleton GW, Cummins S, Ross PJ, Wasan H, McDonald A, Crosby T, Ma YT, Patel K, Sherriff D, Soomal R, Borg D, Sothi S, Hammel P, Hackert T, Jackson R, Büchler MW; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1327] [Cited by in RCA: 1394] [Article Influence: 174.3] [Reference Citation Analysis (0)] |
| 29. | Mornex F, Girard N, Delpero JR, Partensky C. Radiochemotherapy in the management of pancreatic cancer--part I: neoadjuvant treatment. Semin Radiat Oncol. 2005;15:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Smith DA, Somarouthu B, Ramaiya NH. An imaging-based review of systemic therapies and associated toxicities in metastatic pancreatic cancer as per the 2018 ASCO guidelines: what every radiologist should know. Abdom Radiol (NY). 2019;44:2182-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2115] [Article Influence: 151.1] [Reference Citation Analysis (3)] |
| 32. | Dewitt J, Devereaux BM, Lehman GA, Sherman S, Imperiale TF. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol. 2006;4:717-25; quiz 664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Das R, McGrath K, Seiser N, Smith K, Uttam S, Brand RE, Fasanella KE, Khalid A, Chennat JS, Sarkaria S, Singh H, Slivka A, Zeh HJ, Zureikat AH, Hogg ME, Lee KK, Paniccia A, Ongchin MC, Pingpank JF, Boone BA, Dasyam AK, Bahary N, Gorantla VC, Rhee JC, Thomas R, Ellsworth S, Landau MS, Ohori NP, Henn P, Shyu S, Theisen BK, Singhi AD. Tumor Size Differences Between Preoperative Endoscopic Ultrasound and Postoperative Pathology for Neoadjuvant-Treated Pancreatic Ductal Adenocarcinoma Predict Patient Outcome. Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 34. | Barreto SG, Loveday B, Windsor JA, Pandanaboyana S. Detecting tumour response and predicting resectability after neoadjuvant therapy for borderline resectable and locally advanced pancreatic cancer. ANZ J Surg. 2019;89:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 35. | Figueiredo M, Bouchart C, Moretti L, Mans L, Engelholm JL, Bali MA, Van Laethem JL, Eisendrath P. EUS-guided placement of fiducial markers for stereotactic body radiation therapy in pancreatic cancer: feasibility, security and a new quality score. Endosc Int Open. 2021;9:E253-E257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Ginès MA, Real MI, Gilabert R, Quintó L, Trilla A, Feu F, Montanyà X, Fernández-Cruz L, Navarro S. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 37. | Katz MH, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, Wang H, Abbruzzese J, Pisters PW, Vauthey JN, Charnsangavej C, Tamm E, Crane CH, Balachandran A. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749-5756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 38. | Jang JK, Byun JH, Kang JH, Son JH, Kim JH, Lee SS, Kim HJ, Yoo C, Kim KP, Hong SM, Seo DW, Kim SC, Lee MG. CT-determined resectability of borderline resectable and unresectable pancreatic adenocarcinoma following FOLFIRINOX therapy. Eur Radiol. 2021;31:813-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Windsor JA, Barreto SG. The concept of 'borderline resectable' pancreatic cancer: limited foundations and limited future? J Gastrointest Oncol. 2017;8:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Balthazar EJ. Pancreatitis associated with pancreatic carcinoma. Preoperative diagnosis: role of CT imaging in detection and evaluation. Pancreatology. 2005;5:330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, Sabbatino F, Santos DD, Allen JN, Blaszkowsky LS, Clark JW, Faris JE, Goyal L, Kwak EL, Murphy JE, Ting DT, Wo JY, Zhu AX, Warshaw AL, Lillemoe KD, Fernández-del Castillo C. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 652] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 42. | Chen X, Oshima K, Schott D, Wu H, Hall W, Song Y, Tao Y, Li D, Zheng C, Knechtges P, Erickson B, Li XA. Assessment of treatment response during chemoradiation therapy for pancreatic cancer based on quantitative radiomic analysis of daily CTs: An exploratory study. PLoS One. 2017;12:e0178961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Cassinotto C, Mouries A, Lafourcade JP, Terrebonne E, Belleannée G, Blanc JF, Lapuyade B, Vendrely V, Laurent C, Chiche L, Wagner T, Sa-Cunha A, Gaye D, Trillaud H, Laurent F, Montaudon M. Locally advanced pancreatic adenocarcinoma: reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology. 2014;273:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 44. | Amer AM, Zaid M, Chaudhury B, Elganainy D, Lee Y, Wilke CT, Cloyd J, Wang H, Maitra A, Wolff RA, Varadhachary G, Overman MJ, Lee JE, Fleming JB, Tzeng CW, Katz MH, Holliday EB, Krishnan S, Minsky BD, Herman JM, Taniguchi CM, Das P, Crane CH, Le O, Bhosale P, Tamm EP, Koay EJ. Imaging-based biomarkers: Changes in the tumor interface of pancreatic ductal adenocarcinoma on computed tomography scans indicate response to cytotoxic therapy. Cancer. 2018;124:1701-1709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Wei D, Zaid MM, Katz MH, Prakash LR, Kim M, Tzeng CD, Lee JE, Agrawal A, Rashid A, Wang H, Varadhachary G, Wolff RA, Tamm EP, Bhosale PR, Maitra A, Koay EJ. Clinicopathological correlation of radiologic measurement of post-therapy tumor size and tumor volume for pancreatic ductal adenocarcinoma. Pancreatology. 2021;21:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Cuneo KC, Chenevert TL, Ben-Josef E, Feng MU, Greenson JK, Hussain HK, Simeone DM, Schipper MJ, Anderson MA, Zalupski MM, Al-Hawary M, Galban CJ, Rehemtulla A, Feng FY, Lawrence TS, Ross BD. A pilot study of diffusion-weighted MRI in patients undergoing neoadjuvant chemoradiation for pancreatic cancer. Transl Oncol. 2014;7:644-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | Dalah E, Erickson B, Oshima K, Schott D, Hall WA, Paulson E, Tai A, Knechtges P, Li XA. Correlation of ADC With Pathological Treatment Response for Radiation Therapy of Pancreatic Cancer. Transl Oncol. 2018;11:391-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Zimmermann C, Distler M, Jentsch C, Blum S, Folprecht G, Zöphel K, Polster H, Troost EGC, Abolmaali N, Weitz J, Baumann M, Saeger HD, Grützmann R. Evaluation of response using FDG-PET/CT and diffusion weighted MRI after radiochemotherapy of pancreatic cancer: a non-randomized, monocentric phase II clinical trial-PaCa-DD-041 (Eudra-CT 2009-011968-11). Strahlenther Onkol. 2021;197:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Okada KI, Kawai M, Hirono S, Kojima F, Tanioka K, Terada M, Miyazawa M, Kitahata Y, Iwahashi Y, Ueno M, Hayami S, Murata SI, Shimokawa T, Yamaue H. Diffusion-weighted MRI predicts the histologic response for neoadjuvant therapy in patients with pancreatic cancer: a prospective study (DIFFERENT trial). Langenbecks Arch Surg. 2020;405:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Bali MA, Pullini S, Metens T, Absil J, Chao SL, Marechal R, Matos C, Peerboccus BM, Van Laethem JL. Assessment of response to chemotherapy in pancreatic ductal adenocarcinoma: Comparison between diffusion-weighted MR quantitative parameters and RECIST. Eur J Radiol. 2018;104:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Choi M, Heilbrun LK, Venkatramanamoorthy R, Lawhorn-Crews JM, Zalupski MM, Shields AF. Using 18F-fluorodeoxyglucose positron emission tomography to monitor clinical outcomes in patients treated with neoadjuvant chemo-radiotherapy for locally advanced pancreatic cancer. Am J Clin Oncol. 2010;33:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, Lee JH, Lee JD. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis on Preoperative ¹⁸F-FDG PET/CT in Patients with Pancreatic Cancer. J Nucl Med. 2014;55:898-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 53. | Panda A, Garg I, Truty MJ, Kline TL, Johnson MP, Ehman EC, Suman G, Anaam DA, Kemp BJ, Johnson GB, Halfdanarson TR, Venkatesh SK, Fidler JL, Goenka AH. Borderline Resectable and Locally Advanced Pancreas Cancer: FDG PET/MRI and CT Tumor Metrics for Assessment of Neoadjuvant Therapy Pathologic Response and Prediction of Survival. AJR Am J Roentgenol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 54. | Itchins M, Chua TC, Arena J, Jamieson NB, Nahm CB, O'Connell RL, Bailey EA, Schembri GP, Gill AJ, Kneebone A, Hruby G, Mittal A, Pavlakis N, Clarke SJ, Samra JS. Evaluation of Fluorodeoxyglucose Positron Emission Tomography Scanning in the Neoadjuvant Therapy Paradigm in Pancreatic Ductal Adenocarcinoma. Pancreas. 2020;49:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Barnes CA, Aldakkak M, Clarke CN, Christians KK, Bucklan D, Holt M, Tolat P, Ritch PS, George B, Hall WA, Erickson BA, Evans DB, Tsai S. Value of Pretreatment 18F-fluorodeoxyglucose Positron Emission Tomography in Patients With Localized Pancreatic Cancer Treated With Neoadjuvant Therapy. Front Oncol. 2020;10:500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Yokose T, Kitago M, Matsusaka Y, Masugi Y, Shinoda M, Yagi H, Abe Y, Oshima G, Hori S, Endo Y, Toyama K, Iwabuchi Y, Takemura R, Ishii R, Nakahara T, Okuda S, Jinzaki M, Kitagawa Y. Usefulness of 18 F-fluorodeoxyglucose positron emission tomography/computed tomography for predicting the prognosis and treatment response of neoadjuvant therapy for pancreatic ductal adenocarcinoma. Cancer Med. 2020;9:4059-4068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 57. | Dunér S, Lopatko Lindman J, Ansari D, Gundewar C, Andersson R. Pancreatic cancer: the role of pancreatic stellate cells in tumor progression. Pancreatology. 2010;10:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 58. | Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, Tuveson DA. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 628] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 59. | Hamdy A, Ichikawa Y, Toyomasu Y, Nagata M, Nagasawa N, Nomoto Y, Sami H, Sakuma H. Perfusion CT to Assess Response to Neoadjuvant Chemotherapy and Radiation Therapy in Pancreatic Ductal Adenocarcinoma: Initial Experience. Radiology. 2019;292:628-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 60. | Chakraborty J, Langdon-Embry L, Cunanan KM, Escalon JG, Allen PJ, Lowery MA, O'Reilly EM, Gönen M, Do RG, Simpson AL. Preliminary study of tumor heterogeneity in imaging predicts two year survival in pancreatic cancer patients. PLoS One. 2017;12:e0188022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 61. | Ciaravino V, Cardobi N, DE Robertis R, Capelli P, Melisi D, Simionato F, Marchegiani G, Salvia R, D'Onofrio M. CT Texture Analysis of Ductal Adenocarcinoma Downstaged After Chemotherapy. Anticancer Res. 2018;38:4889-4895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Kim BR, Kim JH, Ahn SJ, Joo I, Choi SY, Park SJ, Han JK. CT prediction of resectability and prognosis in patients with pancreatic ductal adenocarcinoma after neoadjuvant treatment using image findings and texture analysis. Eur Radiol. 2019;29:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 63. | Borhani AA, Dewan R, Furlan A, Seiser N, Zureikat AH, Singhi AD, Boone B, Bahary N, Hogg ME, Lotze M, Zeh HJ III, Tublin ME. Assessment of Response to Neoadjuvant Therapy Using CT Texture Analysis in Patients With Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma. AJR Am J Roentgenol. 2020;214:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Nasief H, Hall W, Zheng C, Tsai S, Wang L, Erickson B, Li XA. Improving Treatment Response Prediction for Chemoradiation Therapy of Pancreatic Cancer Using a Combination of Delta-Radiomics and the Clinical Biomarker CA19-9. Front Oncol. 2019;9:1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |