Published online Jun 7, 2021. doi: 10.3748/wjg.v27.i21.2784
Peer-review started: January 24, 2021
First decision: February 22, 2021
Revised: February 24, 2021
Accepted: April 29, 2021
Article in press: April 29, 2021
Published online: June 7, 2021
Processing time: 122 Days and 22.3 Hours
According to Barcelona Clinic Liver Cancer recommendations, intermediate stage hepatocellular carcinomas (stage B) are excluded from liver resection and are referred to palliative treatment. Moreover, Child-Pugh B patients are not usually candidates for liver resection. However, many hepatobiliary centers in the world manage patients with intermediate stage hepatocellular carcinoma or Child-Pugh B cirrhosis with liver resection, maintaining that hepatic resection is not contraindicated in selected patients with non–early-stage hepatocellular carcinoma and without normal liver function. Several studies demonstrate that resection provides the best survival benefit for selected patients in very early/early and even in intermediate stages of Barcelona Clinic Liver Cancer classification, and this treatment gives good results in the setting of multinodular, large tumors in patients with portal hypertension and/or Child-Pugh B cirrhosis. In this review we explore this controversial topic, and we show through the literature analysis how liver resection may improve the short- and long-term survival rate of carefully selected Barcelona Clinic Liver Cancer B and Child-Pugh B hepatocellular carcinoma patients. However, other large clinical studies are needed to clarify which patients with intermediate stage hepatocellular carcinoma are most likely to benefit from liver resection.
Core Tip: According to Barcelona Clinic Liver Cancer recommendations, intermediate stage hepatocellular carcinomas are excluded from surgery. Also, Child-Pugh B patients with hepatocellular carcinoma are not usually candidates for liver resection. Nevertheless, several recent studies demonstrated that surgical resection can provide good survival benefit for patients with large and multinodular (diameter > 5 cm, number > 3) hepatocellular carcinoma or Child-Pugh B cirrhosis. In this review we discuss that liver resection may improve the short- and long-term survival of selected patients who have a stage B hepatocellular carcinoma or underlying Child-Pugh B cirrhosis.
- Citation: Romano F, Chiarelli M, Garancini M, Scotti M, Zago M, Cioffi G, De Simone M, Cioffi U. Rethinking the Barcelona clinic liver cancer guidelines: Intermediate stage and Child-Pugh B patients are suitable for surgery? World J Gastroenterol 2021; 27(21): 2784-2794
- URL: https://www.wjgnet.com/1007-9327/full/v27/i21/2784.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i21.2784
The most common primary liver tumor is hepatocellular carcinoma (HCC), which is the third leading cause of cancer mortality worldwide[1]. The incidence of HCC is increasing in Western countries due to the effects of nonalcoholic fatty liver disease[2-5]. Literature data show how surgery is the therapeutic approach for HCC associated with the most favorable results[6]. The surgical treatments available for patients with HCC are liver resection (LR) or liver transplantation (LT)[2,4,7]. LT is theoretically considered the best treatment of HCC because in addition to treating the neoplasm it also corrects the underlying liver cirrhosis, minimizing the tumor recurrence[2,4,8]. Given the limited number of liver grafts, it is applied only in a small group of patients who meet the Milan criteria[9]. LR can be applied much more widely than LT, but it is usually performed on an unhealthy liver[2,3,7]. LR is a nonprojectable therapeutic option that includes the risk of cancer progression pending a suitable organ[10]. Nevertheless, LR is associated with considerable postoperative morbidity and mortality, and the early and late recurrence rates after this surgical approach remain high[11,12].
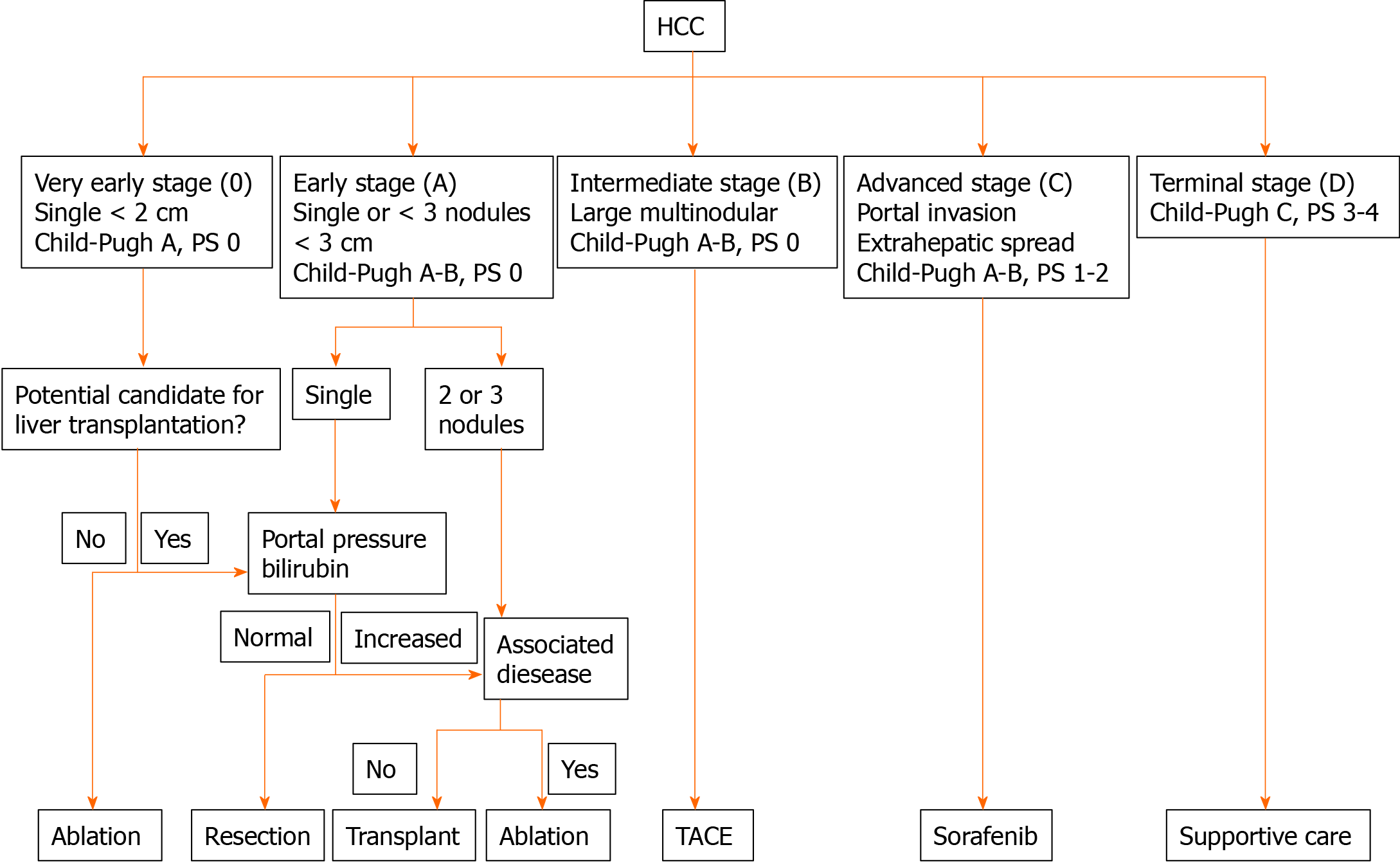
The American Association for the Study of Liver Diseases, the European Association for Study of the Liver, and the European Organization for Research and Treatment of Cancer have endorsed the Barcelona Clinic Liver Cancer (BCLC) staging and management system for treating HCC[13-16]. These Western guidelines limit LR to ideal patients, including those in BCLC stage 0 and a subset of patients in BCLC stage A, i.e. those with a single tumor without invasion of the main branches of the hepatic or portal vein, preoperative hepatic venous gradient < 10 mmHg, normal bilirubin values, Child-Pugh class A liver function, and good general condition (Figure 1). These stringent surgical indications combine low postoperative mortality rates and optimal long-term results with 5-year survival rates of up to 60%[3,5,17,18].
BCLC stage B include patients with Child-Pugh A and B scores with asymptomatic multicentric tumors (> 3) without vascular invasion or extrahepatic spread. For this group of patients, the BCLC staging system indicates transarterial chemoembolization (TACE) as the main treatment option[13]. TACE seems to provide a better 2-year survival rate than supportive treatment with an average life expectancy of 18-27 mo[19,20]. However, it is unclear whether TACE offers an effective benefit to long-term survival in these patients. BCLC stage B is a heterogeneous category that includes cases that vary widely in size of tumor, number of lesions, and liver function[19,21]. Due to this heterogeneity, TACE may not be the optimal therapy for all patients with stage B HCC[22,23]. Considering the poor prognosis associated with a nonresected HCC, several centers have moved away from these guidelines, reporting acceptable short-term survival (mortality from 0% to 5%) and long-term survival rates (ranging from 40% to 60% at 5 years) in BCLC stage B patients treated with LR[24-27]. These recent studies have verified that surgical resection can be safely performed on a subset of patients presenting BCLC stage B HCC, resulting in a long-term survival rate longer than patients that underwent TACE[28,29]. In addition, TACE can only be performed in cases of preserved liver function, and consequently it is not indicated in patients with impaired hepatic functional reserve for the high risk of postoperative liver failure[19,20,30].
In the past decades the presence of underlying cirrhosis was noted to be a negative prognostic factor for HCC recurrence in patients treated with surgery[31,32]. The 5-year survival rates after LR for HCC were different between cirrhotic and noncirrhotic patients (55% vs 80%); moreover the survival in Child-Pugh B cirrhosis was poorer than in Child-Pugh A cirrhosis (55% vs 28%)[31]. The primary reason of this marked survival difference is currently considered the multicentric carcinogenesis associated with the chronic liver disease. Consequently, in the clinical setting, surgical resection in Child-Pugh B patients tends to be avoided due to the high incidence of postoperative complications and discouraging long-term results[27,33,34]. Minimally invasive techniques have recently been applied to liver surgery with important benefits for patients. In particular, laparoscopic techniques seem to be associated with a lower rate of liver failure in the postoperative period, allowing LR to be performed with satisfactory results even in patients with Child-Pugh B or C cirrhosis[35].
The purpose of this minireview is to illustrate the possible surgical treatment of HCC in intermediate stage of BCLC classification and in Child-Pugh B patients.
About 20% of patients with HCC are classified as BCLC-B (intermediate stage). These patients present a survival of 50% at 2-year follow-up[2,5,7]. According to Western international recommendations, patients with intermediate HCC are considered unresectable, and TACE is recommended as a standard treatment[14,15]. The cumulative survival time of patients with BCLC-B HCC has been reported to vary from 24 to 60 mo (with overall 5-year survival rate ranging from 0% to 10%) for the best responders to TACE[36,37]. Advances in surgical technique and perioperative care expanded the indications for LR, and consequently surgery has recently been proposed for stage B patients[26,29]. Several studies have shown that LR for an intermediate stage HCC is associated with a promising 5-year survival rate ranging from 25% to 60% with median survival time varying from 20 to 60 mo[38-41]. Moreover, data from other groups show that LR offers satisfactory results for patients selected at intermediate and advanced stages of the BCLC classification (stage B and C), in the context of large or multinodular tumors[24,42,43].
In 2013, Torzilli et al[44] promoted a multicenter East–West observational study on LR in HCC, questioning the European Association for Study of the Liver-American Association for the Study of Liver Diseases guidelines. This study found that the patients who underwent resection for BCLC-B, defined as “single tumor with a diameter greater than 5 cm; two to three tumors with at least one greater than 3 cm in diameter; more than three tumors with any diameter” were characterized by 1-, 3-, and 5-year survival rates of 88%, 71%, and 57%, respectively. These figures were higher than those expected after TACE. Recently, a systematic review about the role of resective surgery in large (> 5 cm) and multinodular HCC found that LR was associated with a 5-year survival of 42% in Asian studies and 32% in non-Asian studies[45]. Chang et al[46] analyzed a large cohort grouped according to the tumor size and found that patients with large (between 5 and 10 cm) and huge (> 10 cm) HCCs were associated with 1-year survival of 82% and 68% respectively, and 5-year survival of 50% and 35%, respectively, after curative LR. Similar survival rates about huge (> 10 cm) HCCs were also recorded by other authors[42,47-49]. To date, patients with a single HCC larger than 5 cm are in a “grey area.” Initially, large (HCCs > 5 cm) were categorized as BCLC stage B. Currently, some Western experts and Eastern guidelines propose to classify these patients as initial stage, beyond the size[6,50,51].
Another systematic review excluded all those patients in the so-called “grey area” of intermediate BCLC stage with a single large lesion (> 5 cm). Median survival of patients resected for multinodular HCCs in BCLC stage B was 37 mo with a 5-year survival rate of 35%[22]. The 5-year survival was higher for patients with a number of carcinomas less than four vs four or more (49% vs 23%). In fact, a substantial percentage of patients with multiple HCCs in the early stage and exceeding the BCLC criteria for surgery, survived longer than expected after curative resection[52-55].
A large meta-analysis, based on high-quality studies, suggested that LR may increase the overall survival in patients with intermediate and advanced liver cancer in comparison to TACE[56]. In a recent study, Tada et al[57] concluded that LR is superior to TACE in BCLC-B patients with HCC with three nodules or less in Child-Pugh A cirrhosis. The superiority of surgery over TACE in class B BCLC patients was confirmed in four meta-analyses[58-61], one clinical trial[28], and one large observational study[62].
Not all BCLC-B HCC patients are good candidates for surgery. LR is even considered a futile procedure for patients with unfavorable short and mid-term outcomes, such as 90-d postoperative death or early tumor recurrence (within 1 year)[63]. Xu et al[64] proposed a discriminant criterion (tumor size < 11 cm or number of lesions < 4) for selecting stage B patients with the aim of receiving curative surgery. Moreover, Wada et al[65] identified three types of HCC based on the number and the diameter on imaging: type 1, up to 3 lesions < 5 cm; type 2, up to 3 lesions ≥ 5 cm or 4 tumors of any size; and type 3, > 4 tumors. These authors demonstrated that type 1 patients were associated with better survival than type 3. Intuitively the prognosis of type 2 patients was intermediate between type 1 and type 3 patients.
According to these large and high-quality studies, the Japanese Society of Hepatology, the Asian Pacific Association for the Study of the Liver, and the Chinese Liver Cancer Staging System recently proposed treatment algorithms in which patients with intermediated stage HCC are potential candidates for hepatectomy[8,50,66].
According to BCLC guidelines, the ideal candidate for LR is the early-stage cirrhotic patient with a single small size lesion[13-15]. On the contrary, it is generally accepted that early stage HCC in Child-Pugh C cirrhosis patients without significant associated diseases should be listed for LT, according to the inclusion criteria[9,67]. Moreover, the indications for TACE should be carefully evaluated in patients with impaired hepatic functional reserve for the high risk of liver failure[19,30]. Furthermore, definitive treatment indications for patients, presenting with HCC with underlying Child-Pugh B cirrhosis, are lacking, and consequently therapeutic allocation remains controversial [27,31,34,68]. In the clinical setting, the indication for LR is dictated by the severity of the hepatic dysfunction and the resection volume that should be performed on the basis of the size and number of HCCs.
In the past decades the presence of cirrhosis in HCC patients undergoing LR was associated with a higher rate of perioperative complications and reduced 5-year survival rate compared to patients treated with LT; this prognostic difference was more evident in Child-Pugh class B patients[31]. Nevertheless, as a result of recent interest in expanding eligibility for LR, many surgeons believe that well-selected Child-Pugh B patients should not be excluded a priori because good short and long-term survival rates could probably be obtained[31,33,69]. In accordance with this approach, consensus-based HCC treatment guidelines of the Japan Society of Hepatology consider LR for a wide range of HCC patients, including those with Child–Pugh B liver function, multiple tumors (regardless of size), or minimal portal invasion[66]. However, it must be highlighted that surgical resection in Child-Pugh B cirrhosis is associated with considerable postoperative morbidity and mortality, and surgical indications should be carefully weighed. In a recent study the 90-d mortality in Child-Pugh B patients was 10% in cases of major hepatectomies and 3% in segmentectomies[70]. However, it should be remembered that the frequency of adverse events in hepatic surgery still remains significant[71]. A systemic review reported a postoperative complication rate ranging from 27% to 32% and mortality rate from 2.7% to 7.3% after LR among Child-Pugh A and B patients[45].
In a cohort of 137 cases of Child-Pugh B cirrhosis with primary HCC, Harimoto et al[72] demonstrated that the overall survival rate in patients that underwent LR was not different from the rate in living donor LT. The main difference between these two types of surgical treatment consisted in the higher incidence of HCC recurrence in patients undergoing LR[31,72,73]. In this context, LR for HCC in Child B patients can be performed with acceptable postoperative morbidity and mortality with a 5-year overall survival of 50%-70%[72,73].
Laparoscopic liver surgery has recently been associated with a lower rate of hepatic failure, ascites production, and overall postoperative complications. This is mainly due to the conservation of extrahepatic collaterals and reduced liver mobilization compared to the open approach[74-76]. Therefore, the development of the minimally invasive laparoscopic approach, aiming to minimize surgical stress and improve patient recovery, allows the subsequent application of these techniques in advanced cirrhotic patients[35,77]. Laparoscopic indications for LR was recently expanded to include major hepatectomies[78]. In the English literature the laparoscopic approach to HCC is well described for Child-Pugh A cirrhosis[74,76,79]. Recently, a single institution series reported LRs in Child-Pugh B and C cirrhotic patients with acceptable results. To date, these data are scarce and come mainly from Asian studies[80-83]. Brytska et al[81] performed a subgroup analysis in advanced cirrhotic patients undergoing laparoscopic resection for HCC, including 13 Child-Pugh B and 3 Child-Pugh C patients. Laparoscopic LRs included ten tumorectomies, two segmentectomies, three left lateral sectionectomy, and one right anterior sectionectomy. The authors reported an overall complication rate of 19%, with a 12% occurrence of major postoperative complications (pleural effusion and variceal bleeding), without 90-d mortality. They reported an overall survival of about 85% and a disease-free survival of 42% at the 5-year follow-up.
Currently, TACE and sorafenib are indicated for intermediate and advanced HCCs; surgery is not recommended for HCC in BCLC stage B or associated with Child-Pugh B cirrhosis because of unproven survival benefit.
With the advancement of surgical techniques and the consequent reduction of postoperative complications, many experiences have been accumulated to support the advantages of surgery in treating intermediate HCC patients[26,28,29,62]. Recent systematic reviews show how LR is associated to better long-term survival than TACE in patients with stage B HCC. The 5-year survival rate associated with LR can be up to 35%[22,23]. Indeed, in the past decades, several studies have highlighted that LRs performed on multinodular HCC offer satisfactory long-term results, associated with acceptable perioperative morbidity and mortality[52,84,85]. Moreover, the Japanese and Chinese guidelines recommend surgery as a treatment modality for a wide spectrum of HCC patients, including those with Child–Pugh B liver function, multiple tumors, or minimal portal invasion[50,66]. Based on encouraging data, many hepatobiliary centers around the world manage patients with intermediate stage liver cancer with LR, claiming that surgical resection is not contraindicated in selected patients with non-early-stage liver cancer[17,29,38,40,41].
Hence, the restriction of surgical resection to a small subset of patients, as suggested by the Barcelona approach, can be rethought for various reasons. First, the safety of extensive hepatic surgery improved significantly, making LR a feasible option even for patients with suboptimal liver function or poor performance status[35,70]. Second, surgery does not counterpose TACE. The latter can be used as an additional procedure to decrease tumor volume and give chance for surgery in selected BCLC stage B[86-89]. Finally, the accuracy of the current diagnostic exams may be limited in particular cases of macronodular cirrhosis, leading to possible erroneous classifications with serious consequences on the therapeutic objectives (curative vs palliative). Preoperative case selection is essential to reduce surgery risks, possibly identifying the candidate who could profit rather than be harmed by LR.
In 2012, Bolondi et al[21] proposed a classification of BCLC B stage into four substages to better identify the prognosis of this heterogeneous class of patients and at the same time suggested the therapeutic options for each category. In this context, a multicenter observational study highlighted how surgical resection is associated with better results in B1/B2 stage than B3/B4[29], according to the subclassification proposed by Bolondi et al[21]. Moreover we believe that the type of resection must be balanced with the underlying liver function to direct the patient towards the safest effective oncological treatment. Indeed, the removal of the tumor and preservation of the liver parenchyma are equally important, as both contribute to determining patient survival. In this perspective the Kinki criteria simplify the Bolondi substaging system[90]. Based on Child-Pugh score (5-7 or 8-9) and “up-to-7” rule (the sum of the number of nodules plus the diameter of the largest lesion), BCLC stage B HCCs are subdivided into three subgroups (B1, B2, B3). B1 class is characterized by relatively preserved liver function (Child-Pugh A 5-6 or Child-Pugh B7) and tumor burden within “up-to-7” criterion. According to these authors only patients with B1 HCC can benefit from LR[90].
LR in Child-Pugh B cirrhosis has been reported as an alternative treatment to LT, with promising short and long-term outcomes[72,73]. Moreover laparoscopic LR can be safely performed in selected Child-Pugh B patients with acceptable perioperative outcomes, although to date long-term results are not available[80-83]. Further studies are needed to clarify which Child-Pugh B patients with HCC are suitable candidates for laparoscopic LR, hoping to optimize the chances of long-term survival[35]. To date minimally invasive surgery could be proposed in patients with a satisfactory functional reserve of the liver and less aggressive tumors[74]. Nevertheless, it should be noted that data are scanty and come mainly from single institution case series. Moreover, LR in Child-Pugh B patients is characterized by significant 90-d mortality and morbidity rates, and consequently a tailored therapeutic approach could reduce the frequency of adverse events, offering good long-term results[63,54,70].
In our opinion additional radiological and laboratory data (e.g., tumor location and distribution, serological markers), predictive of radical resection, should be incorporated into the staging system to select patients who may benefit from surgery. In this perspective, the Japanese Society of Hepatology indicated contrast-enhanced magnetic resonance imaging the pivotal exam in the preoperative work-up[66].
According to the most recent experiences, LR may improve the survival of Child-Pugh B and BCLC B carcinoma when patients are carefully selected and surgery is performed in specialized centers. Moreover, the mini-invasive approach allows pushing up the limits of LR in this stage of the disease[75,82]. Consequently, indications for resection could be expanded to include patients from therapies associated with satisfactory results. Nevertheless, randomized controlled trials must be planned to clarify which patients presenting with HCC beyond the classical indications are most likely to benefit from LR. In conclusion, these findings support the future expansion of indications for surgical resection in Western therapeutic guidelines.
We thank Dr. Gerardo Cioffi, a native speaker, for reviewing the English language.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar SKY S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55853] [Article Influence: 7979.0] [Reference Citation Analysis (132)] |
| 2. | Balogh J, Victor D 3rd, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP Jr. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 807] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 3. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3176] [Article Influence: 529.3] [Reference Citation Analysis (37)] |
| 4. | Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 524] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 6. | Surveillance group. ; Diagnosis group; Staging group; Surgery group; Local ablation group; TACE/TARE/HAI group; Target therapy/systemic therapy group; Radiotherapy group; Prevention group; Drafting group. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2018;117:381-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Llovet JM, Fuster J, Bruix J; Barcelona-Clínic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 514] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 8. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1646] [Article Influence: 205.8] [Reference Citation Analysis (0)] |
| 9. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5313] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 10. | Arii S, Sata M, Sakamoto M, Shimada M, Kumada T, Shiina S, Yamashita T, Kokudo N, Tanaka M, Takayama T, Kudo M. Management of hepatocellular carcinoma: Report of Consensus Meeting in the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol Res. 2010;40:667-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1234] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 12. | Famularo S, Donadon M, Cipriani F, Ardito F, Carissimi F, Perri P, Iaria M, Dominioni T, Zanello M, Conci S, Molfino S, LaBarba G, Ferrari C, Germani P, Patauner S, Pinotti E, Lodo E, Garatti M, Sciannamea I, Troci A, Conticchio M, Floridi A, Chiarelli M, Fumagalli L, Memeo R, Crespi M, Antonucci A, Zimmitti G, Zanus G, Zago M, Frena A, Tarchi P, Griseri G, Ercolani G, Baiocchi GL, Ruzzenente A, Jovine E, Maestri M, DallaValle R, Grazi GL, Giuliante F, Aldrighetti L, Torzilli G, Romano F; HE. RC.O.LE.S. Group. Hepatocellular carcinoma surgical and oncological trends in a national multicentric population: the HERCOLES experience. Updates Surg. 2020;72:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J; EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3244] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 14. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3031] [Article Influence: 433.0] [Reference Citation Analysis (3)] |
| 15. | European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6065] [Article Influence: 866.4] [Reference Citation Analysis (3)] |
| 16. | European Association for Study of Liver. European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 17. | Lim KC, Chow PK, Allen JC, Siddiqui FJ, Chan ES, Tan SB. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012;99:1622-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Garancini M, Nespoli S, Romano F, Uggeri F, Degrate L, Okolicsanyi S, Gianotti L. Surgical management of hepatocellular carcinoma within and beyond BCLC indications in a middle volume center. J Visc Surg. 2018;155:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 20. | Park JW, Amarapurkar D, Chao Y, Chen PJ, Geschwind JF, Goh KL, Han KH, Kudo M, Lee HC, Lee RC, Lesmana LA, Lim HY, Paik SW, Poon RT, Tan CK, Tanwandee T, Teng G, Cheng AL. Consensus recommendations and review by an International Expert Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC). Liver Int. 2013;33:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 22. | Glantzounis GK, Paliouras A, Stylianidi MC, Milionis H, Tzimas P, Roukos D, Pentheroudakis G, Felekouras E. The role of liver resection in the management of intermediate and advanced stage hepatocellular carcinoma. A systematic review. Eur J Surg Oncol. 2018;44:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Koh YX, Tan HL, Lye WK, Kam JH, Chiow AKH, Tan SS, Choo SP, Chung AYF, Goh BKP. Systematic review of the outcomes of surgical resection for intermediate and advanced Barcelona Clinic Liver Cancer stage hepatocellular carcinoma: A critical appraisal of the evidence. World J Hepatol. 2018;10:433-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Chang WT, Kao WY, Chau GY, Su CW, Lei HJ, Wu JC, Hsia CY, Lui WY, King KL, Lee SD. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection? Surgery. 2012;152:809-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Ciria R, López-Cillero P, Gallardo AB, Cabrera J, Pleguezuelo M, Ayllón MD, Luque A, Zurera L, Espejo JJ, Rodríguez-Perálvarez M, Montero JL, de la Mata M, Briceño J. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: Modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur J Surg Oncol. 2015;41:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Kim H, Ahn SW, Hong SK, Yoon KC, Kim HS, Choi YR, Lee HW, Yi NJ, Lee KW, Suh KS; Korean Liver Cancer Association. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Br J Surg. 2017;104:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Bell R, Pandanaboyana S, Lodge JPA, Prasad KR, Jones R, Hidalgo E. Primary liver resection for patients with cirrhosis and hepatocellular carcinoma: the role of surgery in BCLC early (A) and intermediate stages (B). Langenbecks Arch Surg. 2017;402:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, Wu MC, Zhou WP. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014;61:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 272] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 29. | Kariyama K, Nouso K, Wakuta A, Oonishi A, Toyoda H, Tada T, Hiraoka A, Tsuji K, Itobayashi E, Ishikawa T, Takaguchi K, Tsutsui A, Shimada N, Kumada T. Treatment of Intermediate-Stage Hepatocellular Carcinoma in Japan: Position of Curative Therapies. Liver Cancer. 2020;9:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 31. | Taura K, Ikai I, Hatano E, Yasuchika K, Nakajima A, Tada M, Seo S, Machimoto T, Uemoto S. Influence of coexisting cirrhosis on outcomes after partial hepatic resection for hepatocellular carcinoma fulfilling the Milan criteria: an analysis of 293 patients. Surgery. 2007;142:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Bilimoria MM, Lauwers GY, Doherty DA, Nagorney DM, Belghiti J, Do KA, Regimbeau JM, Ellis LM, Curley SA, Ikai I, Yamaoka Y, Vauthey JN; International Cooperative Study Group on Hepatocellular Carcinoma. Underlying liver disease, not tumor factors, predicts long-term survival after resection of hepatocellular carcinoma. Arch Surg. 2001;136:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Choi SH, Choi GH, Kim SU, Park JY, Joo DJ, Ju MK, Kim MS, Choi JS, Han KH, Kim SI. Role of surgical resection for multiple hepatocellular carcinomas. World J Gastroenterol. 2013;19:366-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Vega EA, Kutlu OC, Joechle K, De La Cruz N, Ko D, Conrad C. Preoperative Prognosticators of Safe Laparoscopic Hepatocellular Carcinoma Resection in Advanced Cirrhosis: a Propensity Score Matching Population-Based Analysis of 1799 Western Patients. J Gastrointest Surg. 2019;23:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Lin CT, Hsu KF, Chen TW, Yu JC, Chan DC, Yu CY, Hsieh TY, Fan HL, Kuo SM, Chung KP, Hsieh CB. Comparing hepatic resection and transarterial chemoembolization for Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma: change for treatment of choice? World J Surg. 2010;34:2155-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, Liu X, Li LQ. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8:e68193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Delis SG, Bakoyiannis A, Tassopoulos N, Athanassiou K, Kelekis D, Madariaga J, Dervenis C. Hepatic resection for hepatocellular carcinoma exceeding Milan criteria. Surg Oncol. 2010;19:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Kamiyama T, Orimo T, Wakayama K, Shimada S, Nagatsu A, Yokoo H, Kamachi H, Yamashita K, Shimamura T, Taketomi A. Survival outcomes of hepatectomy for stage B Hepatocellular carcinoma in the BCLC classification. World J Surg Oncol. 2017;15:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Bhandare MS, Patkar S, Shetty N, Polnaya A, Kulkarni S, Dusane RR, Shrikhande SV, Goel M. Liver resection for HCC outside the BCLC criteria. Langenbecks Arch Surg. 2018;403:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Wang H, Qian YW, Wu MC, Cong WM. Liver Resection Is Justified in Patients with BCLC Intermediate Stage Hepatocellular Carcinoma without Microvascular Invasion. J Gastrointest Surg. 2020;24:2737-2747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Furukawa K, Shiba H, Horiuchi T, Shirai Y, Haruki K, Fujiwara Y, Sakamoto T, Gocho T, Yanaga K. Survival benefit of hepatic resection for hepatocellular carcinoma beyond the Barcelona Clinic Liver Cancer classification. J Hepatobiliary Pancreat Sci. 2017;24:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, Peng T, Xie GS, Li LQ. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 376] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 44. | Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M, Morenghi E, Makuuchi M. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? Ann Surg. 2013;257:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 418] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 45. | Zhong JH, Rodríguez AC, Ke Y, Wang YY, Wang L, Li LQ. Hepatic resection as a safe and effective treatment for hepatocellular carcinoma involving a single large tumor, multiple tumors, or macrovascular invasion. Medicine (Baltimore). 2015;94:e396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 46. | Chang YJ, Chung KP, Chang YJ, Chen LJ. Long-term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg. 2016;103:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Tsoulfas G, Mekras A, Agorastou P, Kiskinis D. Surgical treatment for large hepatocellular carcinoma: does size matter? ANZ J Surg. 2012;82:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH, Ahn CS. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br J Surg. 2007;94:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Ariizumi S, Kotera Y, Takahashi Y, Katagiri S, Yamamoto M. Impact of hepatectomy for huge solitary hepatocellular carcinoma. J Surg Oncol. 2013;107:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 51. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1276] [Article Influence: 141.8] [Reference Citation Analysis (2)] |
| 52. | Ho MC, Huang GT, Tsang YM, Lee PH, Chen DS, Sheu JC, Chen CH. Liver resection improves the survival of patients with multiple hepatocellular carcinomas. Ann Surg Oncol. 2009;16:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 584] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 54. | Zhao WC, Fan LF, Yang N, Zhang HB, Chen BD, Yang GS. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. Eur J Surg Oncol. 2013;39:858-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 55. | Lee SY, Ahn CS, Yoon YI, Lee SG, Hwang S, Kim KH, Moon DB, Ha TY, Song GW, Jung DH, Park GC. Long-term outcomes of liver resection for multiple hepatocellular carcinomas: Single-institution experience with 187 patients. Ann Hepatobiliary Pancreat Surg. 2020;24:437-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, Yim HJ, Yeon JE, Byun KS. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology. 2018;68:977-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 57. | Tada T, Kumada T, Toyoda H, Tsuji K, Hiraoka A, Itobayashi E, Nouso K, Kariyama K, Ishikawa T, Hirooka M, Hiasa Y. Role of hepatic resection in patients with intermediate-stage hepatocellular carcinoma: A multicenter study from Japan. Cancer Sci. 2017;108:1414-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Liu W, Zhou JG, Sun Y, Zhang L, Xing BC. Hepatic Resection Improved the Long-Term Survival of Patients with BCLC Stage B Hepatocellular Carcinoma in Asia: a Systematic Review and Meta-Analysis. J Gastrointest Surg. 2015;19:1271-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Qi X, Wang D, Su C, Li H, Guo X. Hepatic resection vs transarterial chemoembolization for the initial treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Oncotarget. 2015;6:18715-18733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Liang L, Xing H, Zhang H, Zhong J, Li C, Lau WY, Wu M, Shen F, Yang T. Surgical resection vs transarterial chemoembolization for BCLC intermediate stage hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford). 2018;20:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Labgaa I, Taffé P, Martin D, Clerc D, Schwartz M, Kokudo N, Denys A, Halkic N, Demartines N, Melloul E. Comparison of Partial Hepatectomy and Transarterial Chemoembolization in Intermediate-Stage Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer. 2020;9:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Vitale A, Burra P, Frigo AC, Trevisani F, Farinati F, Spolverato G, Volk M, Giannini EG, Ciccarese F, Piscaglia F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Cabibbo G, Felder M, Gasbarrini A, Sacco R, Foschi FG, Missale G, Morisco F, Svegliati Baroni G, Virdone R, Cillo U; Italian Liver Cancer (ITA. LI.CA) group. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol. 2015;62:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 63. | Li C, Shen JY, Zhang XY, Peng W, Wen TF, Yang JY, Yan LN. Predictors of Futile Liver Resection for Patients with Barcelona Clinic Liver Cancer Stage B/C Hepatocellular Carcinoma. J Gastrointest Surg. 2018;22:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Xu W, Rao Q, An Y, Li M, Xu G, Sang X, Lu X, Zhang Z, Mao Y. Proposal for subclassification to select patients for hepatectomy with intermediate hepatocellular carcinoma and Child-Pugh A liver function: A double-center study from China. Medicine (Baltimore). 2018;97:e11800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 65. | Wada H, Eguchi H, Noda T, Ogawa H, Yamada D, Tomimaru Y, Tomokuni A, Asaoka T, Kawamoto K, Gotoh K, Marubashi S, Umeshita K, Nagano H, Doki Y, Mori M. Selection criteria for hepatic resection in intermediate-stage (BCLC stage B) multiple hepatocellular carcinoma. Surgery. 2016;160:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 66. | Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, Kudo M, Iijima H, Genda T, Tateishi R, Torimura T, Igaki H, Kobayashi S, Sakurai H, Murakami T, Watadani T, Matsuyama Y. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 398] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 67. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1696] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 68. | Yeh CN, Chen MF, Lee WC, Jeng LB. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol. 2002;81:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 69. | Manzini G, Henne-Bruns D, Porzsolt F, Kremer M. Is there a standard for surgical therapy of hepatocellular carcinoma in healthy and cirrhotic liver? BMJ Open Gastroenterol. 2017;4:e000129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Berardi G, Morise Z, Sposito C, Igarashi K, Panetta V, Simonelli I, Kim S, Goh BKP, Kubo S, Tanaka S, Takeda Y, Ettorre GM, Wilson GC, Cimino M, Chan CY, Torzilli G, Cheung TT, Kaneko H, Mazzaferro V, Geller DA, Han HS, Kanazawa A, Wakabayashi G, Troisi RI. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J Hepatol. 2020;72:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 71. | Giani A, Cipriani F, Famularo S, Donadon M, Bernasconi DP, Ardito F, Fazio F, Nicolini D, Perri P, Giuffrida M, Pontarolo N, Zanello M, Lai Q, Conci S, Molfino S, Germani P, Pinotti E, Romano M, La Barba G, Ferrari C, Patauner S, Manzoni A, Sciannamea I, Fumagalli L, Troci A, Ferraro V, Floridi A, Memeo R, Crespi M, Chiarelli M, Antonucci A, Zimmitti G, Frena A, Percivale A, Ercolani G, Zanus G, Zago M, Tarchi P, Baiocchi GL, Ruzzenente A, Rossi M, Jovine E, Maestri M, Dalla Valle R, Grazi G, Vivarelli M, Ferrero A, Giuliante F, Torzilli G, Aldrighetti L, Gianotti L, Romano F, Ciulli C, Braga M, Ratti F, Costa G, Razionale F, Russolillo N, Marinelli L, De Peppo V, Cremaschi E, Calabrese F, Larghi Laureiro Z, Lazzari G, Cosola D, Montuori M, Salvador L, Cucchetti A, Franceschi A, Ciola M, Sega V, Calcagno P, Pennacchi L, Tedeschi M. Performance of Comprehensive Complication Index and Clavien-Dindo Complication Scoring System in Liver Surgery for Hepatocellular Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Harimoto N, Yoshizumi T, Fujimoto Y, Motomura T, Mano Y, Toshima T, Itoh S, Harada N, Ikegami T, Uchiyama H, Soejima Y, Maehara Y. Surgery for Hepatocellular Carcinoma in Patients with Child-Pugh B Cirrhosis: Hepatic Resection Versus Living Donor Liver Transplantation. World J Surg. 2018;42:2606-2616. [PubMed] |
| 73. | Harada N, Shirabe K, Ikeda Y, Korenaga D, Takenaka K, Maehara Y. Surgical management of hepatocellular carcinoma in Child-Pugh class B cirrhotic patients: hepatic resection and/or microwave coagulation therapy vs living donor liver transplantation. Ann Transplant. 2012;17:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Ciria R, Gomez-Luque I, Ocaña S, Cipriani F, Halls M, Briceño J, Okuda Y, Troisi R, Rotellar F, Soubrane O, Abu Hilal M. A Systematic Review and Meta-Analysis Comparing the Short- and Long-Term Outcomes for Laparoscopic and Open Liver Resections for Hepatocellular Carcinoma: Updated Results from the European Guidelines Meeting on Laparoscopic Liver Surgery, Southampton, UK, 2017. Ann Surg Oncol. 2019;26:252-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 75. | Morise Z, Ciria R, Cherqui D, Chen KH, Belli G, Wakabayashi G. Can we expand the indications for laparoscopic liver resection? J Hepatobiliary Pancreat Sci. 2015;22:342-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 76. | Takahara T, Wakabayashi G, Beppu T, Aihara A, Hasegawa K, Gotohda N, Hatano E, Tanahashi Y, Mizuguchi T, Kamiyama T, Ikeda T, Tanaka S, Taniai N, Baba H, Tanabe M, Kokudo N, Konishi M, Uemoto S, Sugioka A, Hirata K, Taketomi A, Maehara Y, Kubo S, Uchida E, Miyata H, Nakamura M, Kaneko H, Yamaue H, Miyazaki M, Takada T. Long-term and perioperative outcomes of laparoscopic vs open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 77. | Di Sandro S, Bagnardi V, Najjar M, Buscemi V, Lauterio A, De Carlis R, Danieli M, Pinotti E, Benuzzi L, De Carlis L. Minor laparoscopic liver resection for Hepatocellular Carcinoma is safer than minor open resection, especially for less compensated cirrhotic patients: Propensity score analysis. Surg Oncol. 2018;27:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Lin NC, Nitta H, Wakabayashi G. Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg. 2013;257:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 79. | Yoon YI, Kim KH, Cho HD, Kwon JH, Jung DH, Park GC, Song GW, Ha TY, Lee SG. Long-term perioperative outcomes of pure laparoscopic liver resection vs open liver resection for hepatocellular carcinoma: a retrospective study. Surg Endosc. 2020;34:796-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 80. | Cai X, Liang X, Yu T, Liang Y, Jing R, Jiang W, Li J, Ying H. Liver cirrhosis grading Child-Pugh class B: a Goliath to challenge in laparoscopic liver resection? Hepatobiliary Surg Nutr. 2015;4:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 81. | Brytska N, Han HS, Shehta A, Yoon YS, Cho JY, Choi Y. Laparoscopic liver resection for hepatitis B and C virus-related hepatocellular carcinoma in patients with Child B or C cirrhosis. Hepatobiliary Surg Nutr. 2015;4:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 82. | Beard RE, Wang Y, Khan S, Marsh JW, Tsung A, Geller DA. Laparoscopic liver resection for hepatocellular carcinoma in early and advanced cirrhosis. HPB (Oxford). 2018;20:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 83. | Fuji H, Seo S, Toda R, Yoh T, Ikeno Y, Fukumitsu K, Ishii T, Taura K, Hatano E, Kaido T, Uemoto S. Optimal introduction of laparoscopic liver resection for Child-Pugh B. Asian J Endosc Surg. 2019;12:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Ruzzenente A, Capra F, Pachera S, Iacono C, Piccirillo G, Lunardi M, Pistoso S, Valdegamberi A, D'Onofrio M, Guglielmi A. Is liver resection justified in advanced hepatocellular carcinoma? J Gastrointest Surg. 2009;13:1313-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Fukami Y, Kaneoka Y, Maeda A, Kumada T, Tanaka J, Akita T, Kubo S, Izumi N, Kadoya M, Sakamoto M, Nakashima O, Matsuyama Y, Kokudo T, Hasegawa K, Yamashita T, Kashiwabara K, Takayama T, Kokudo N, Kudo M; Liver Cancer Study Group of Japan. Liver Resection for Multiple Hepatocellular Carcinomas: A Japanese Nationwide Survey. Ann Surg. 2020;272:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 86. | Ronot M, Cauchy F, Gregoli B, Breguet R, Allaham W, Paradis V, Soubrane O, Vilgrain V. Sequential transarterial chemoembolization and portal vein embolization before resection is a valid oncological strategy for unilobar hepatocellular carcinoma regardless of the tumor burden. HPB (Oxford). 2016;18:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 87. | Tang YL, Qi XS, Guo XZ. Hepatic Resection after Initial Transarterial Chemoembolization Versus Transarterial Chemoembolization Alone for the Treatment of Hepatocellular Carcinoma: A Meta-analysis of Observational Studies. Asian Pac J Cancer Prev. 2015;16:7871-7874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 88. | Tustumi F, Ernani L, Coelho FF, Bernardo WM, Junior SS, Kruger JAP, Fonseca GM, Jeismann VB, Cecconello I, Herman P. Preoperative strategies to improve resectability for hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford). 2018;20:1109-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 89. | Qi X, Liu L, Wang D, Li H, Su C, Guo X. Hepatic resection alone vs in combination with pre- and post-operative transarterial chemoembolization for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Oncotarget. 2015;6:36838-36859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 90. | Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: Proposal of Modified Bolondi's Subclassification (Kinki Criteria). Dig Dis. 2015;33:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |