Published online Jan 14, 2021. doi: 10.3748/wjg.v27.i2.162
Peer-review started: September 23, 2020
First decision: November 8, 2020
Revised: November 11, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: January 14, 2021
Processing time: 108 Days and 13.2 Hours
Inflammatory bowel disease (IBD) is a chronic, relapsing inflammation of the digestive tract. Although fecal and serum biomarkers have been extremely important and supportive for monitoring of IBD, their low sensitivity and high variability characteristics limit clinical efficacy. Thus, the establishment of better biomarkers is expected. Fucosylation is one of the most important glycosylation modifications of proteins. Fucosylated haptoglobin (Fuc-Hpt) is used as a biomarker for several cancers and inflammation-related diseases. We recently established a novel glycan monoclonal antibody (mAb), designated 10-7G, which recognizes Fuc-Hpt. We developed an enzyme-linked immunosorbent assay (ELISA) to measure serum levels of Fuc-Hpt (10-7G values).
To investigate the usefulness of the serum 10-7G values as a potential biomarker for monitoring disease activity in IBD.
This was a case control study. Intestinal tissues of IBD patients (n = 10) were examined immunohistochemically using the 10-7G mAb. We determined 10-7G values using serum from patients with ulcerative colitis (UC, n = 110), Crohn’s disease (n = 45), acute enteritis (AE, n = 11), and healthy volunteers (HVs) who exhibited normal (n = 20) or high (n = 79) C-reactive protein (CRP) levels at medical check-up. We investigated the correlation between the 10-7G value and various clinical parameters of IBD patients by correlation analysis. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the usefulness of the 10-7G values as a biomarker for clinical and endoscopic remission of UC compared to conventional serum biomarkers.
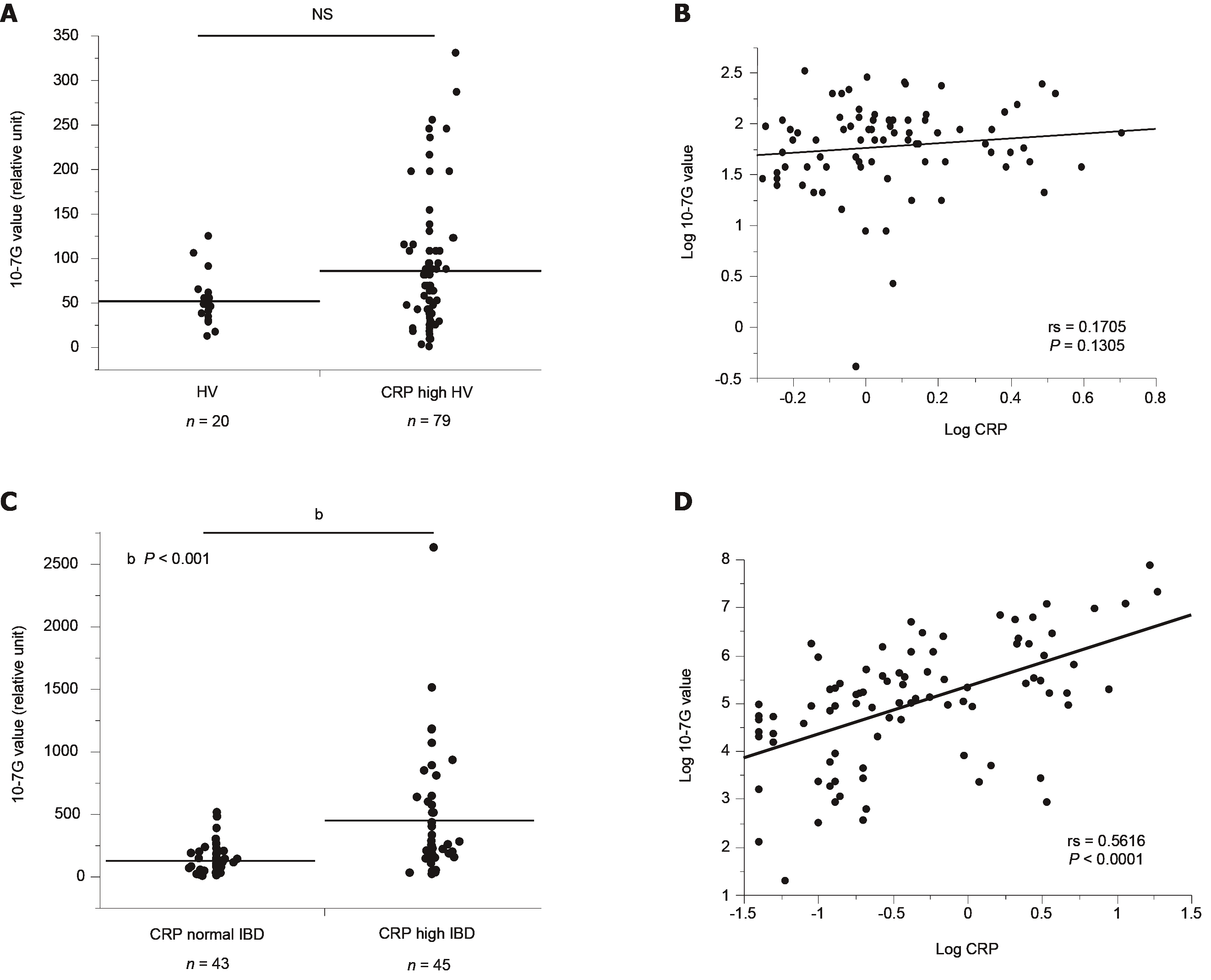
In the immunohistochemical analysis, positive 10-7G mAb staining was observed in lymphocytes infiltrating into inflammatory sites of the mucosal layer and lymphoid follicles. The 10-7G values were significantly higher in patients with IBD (P < 0.001) and AE (P < 0.05) compared with HVs. In addition, 10-7G values were correlated with clinical examination parameters related to inflammation in patients with UC, particularly the CRP level (rs = 0.525, P = 0.003) and clinical activity index score (rs = 0.435, P = 0.038). However, there was no correlation between 10-7G values and CRP in HVs with high CRP levels, suggesting that the 10-7G values is not the same as a general inflammation biomarker. ROC curve analysis showed that area under the curve (AUC) value of 10-7G values for the diagnosis of endoscopic remission was higher than other biomarkers (AUC value = 0.699).
The serum 10-7G value is a novel biomarker for evaluating intestinal inflammation and endoscopic mucosal healing in UC.
Core Tip: Fucosylation is one of the most important glycosylation involved in cancer and inflammation. Recently, we have succeeded to establish a novel glycan antibody 10-7G mAb which directly recognizes fucosylated haptoglobin (Fuc-Hpt), and developed its enzyme-linked immunosorbent assay (ELISA) system. In this study, we demonstrated the serum 10-7G values (Fuc-Hpt levels determined with 10-7G mAb ELISA) were significantly higher in patients with inflammatory bowel disease. We also found that Fuc-Hpt was produced by lymphocytes infiltrating into inflammatory sites of intestine. Moreover, statistical analyses showed that the 10-7G value could evaluate intestinal inflammation and endoscopic mucosal healing in ulcerative colitis.
- Citation: Motooka K, Morishita K, Ito N, Shinzaki S, Tashiro T, Nojima S, Shimizu K, Date M, Sakata N, Yamada M, Takamatsu S, Kamada Y, Iijima H, Mizushima T, Morii E, Takehara T, Miyoshi E. Detection of fucosylated haptoglobin using the 10-7G antibody as a biomarker for evaluating endoscopic remission in ulcerative colitis. World J Gastroenterol 2021; 27(2): 162-175
- URL: https://www.wjgnet.com/1007-9327/full/v27/i2/162.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i2.162
Inflammatory bowel disease (IBD), which comprises Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic recurrent intestinal inflammatory disease of unknown etiology, and the number of individuals affected is increasing worldwide[1]. Although the etiology of IBD remains unknown, the disease is known to involve complex interactions between genetic, environmental, and microbial factors and the immune response[2,3]. For proper management and selection of appropriate treatment strategy, it is essential to evaluate disease activity of IBD accurately. Endoscopy is the gold standard for the diagnosis and monitoring status of IBD patients, but cannot be frequently performed because of the cost and invasiveness. Although fecal and serum biomarkers, such as fecal calprotectin and serum C-reactive protein (CRP), have been important and supportive for monitoring of IBD, their low sensitivity and high variability characteristics limit clinical efficacy[4,5]. Thus, the establishment of better biomarkers is expected.
Haptoglobin, an acute-phase protein produced primarily in the liver, is rapidly upregulated following stimulation with cytokines such as tumor necrosis factor alpha, interleukin (IL)-6, and IL-1[6]. Haptoglobin modulates inflammation via its systemic anti-inflammatory activity[7]. A major function of haptoglobin is binding free plasma hemoglobin produced due to hemolysis, thereby preventing damage associated with oxidative stress[8]. Haptoglobin is an α- and β-chain heterodimer linked by disulfide bonds, with all four N-glycans located in the β chain and no potential N-glycosylation sites in the α chain[9,10].
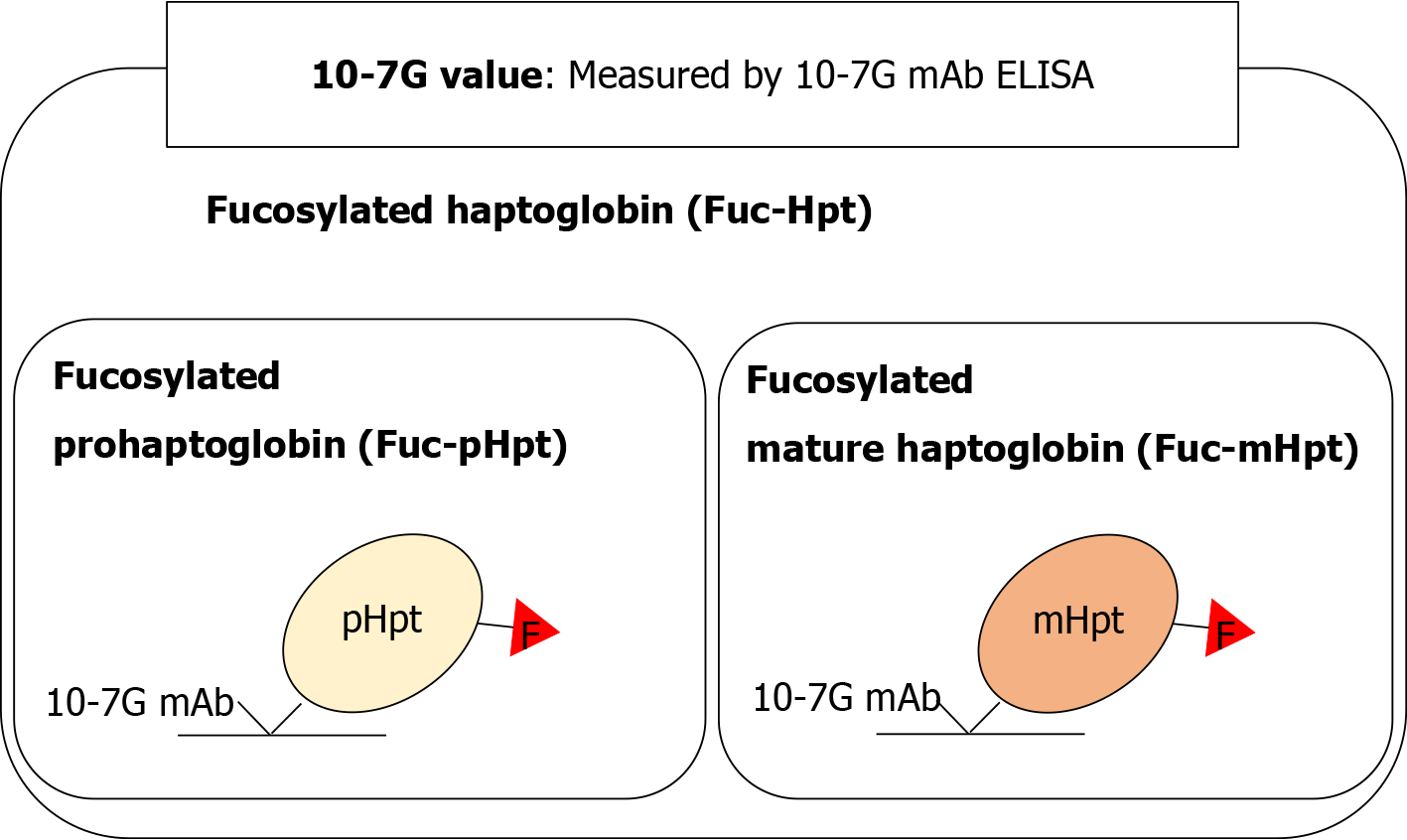
Fucosylation is one of the most important glycosylation modifications of proteins involved in inflammation and cancer[11]. We previously reported that fucosylated haptoglobin (Fuc-Hpt) is found in the serum of patients with pancreatic cancer[12] and established a lectin–antibody enzyme-linked immunosorbent assay (ELISA) to determine serum Fuc-Hpt levels[13]. Recently, we established a novel glycan antibody [10-7G monoclonal antibody (mAb)] that directly recognizes Fuc-Hpt[14]. Epitope analysis of the 10-7G mAb indicated that the peptide recognized by this antibody is in the haptoglobin α-chain, and the epitope appears as a result of aberrant glycosylation, which induces a conformational change in mature haptoglobin[15]. In human serum, the 10-7G mAb recognizes not only fucosylated mature haptoglobin (Fuc-mHpt) but also fucosylated prohaptoglobin (Fuc-pHpt), a precursor molecule. Moreover, affinity chromatography using the 10-7G mAb followed by lectin blotting and mass spectrometry analysis revealed that the 10-7G mAb predominantly recognizes both Fuc-mHpt and Fuc-pHpt[14]. The novel ELISA we developed using the 10-7G mAb enables measurement of both types of Fuc-Hpt in the serum. Here, we use Fuc-Hpt to describe both Fuc-mHpt and Fuc-pHpt, and Fuc-Hpt levels determined using the 10-7G mAb ELISA are reported as 10-7G values (Figure 1). As expected, 10-7G values are increased in pancreatic cancer patients compared with healthy volunteers (HVs)[14,16].
We recently found that the 10-7G mAb can be used for immunohistochemical staining of lymphocytes and macrophages associated with pancreatic cancer tissues. In immunoblotting analyses using the 10-7G mAb, we demonstrated that some immune cell lines produce Fuc-Hpt and that several inflammatory cytokines induce its secretion (manuscript submitted). These results suggest that the production of Fuc-Hpt by immune cells is related to local immune conditions, such as those associated with cancer and inflammation. With respect to inflammatory diseases, IBD is one of the most representative diseases in terms of the relationship between immune cells and local immunity.
In general, serum haptoglobin levels increase during acute inflammation, as do levels of CRP[6], and fucosylation of serum proteins increases dramatically in chronic inflammation[11]. As described above, the 10-7G mAb we developed recognizes characteristic types of haptoglobin, including Fuc-mHpt and Fuc-pHpt. In the present study, we investigated the usefulness of the 10-7G value as a potential serum biomarker for monitoring disease activity of IBD, comparing 10-7G values with several conventional serum biomarkers.
Serum samples were obtained from patients with CD (n = 45), UC (n = 110), and acute enteritis (AE; n = 11) at Osaka University and Osaka University Hospital. Serum samples were also obtained from HVs with normal serum CRP levels (≤ 0.3 mg/dL; n = 20) and high serum CRP levels (> 0.3 mg/dL; n = 79) who underwent medical health examinations at aMs New Otani Clinic, Osaka, Japan. Detailed characteristics of HVs and UC, CD, and AE patients are presented in Table 1. Resected intestinal tissue sections from patients with severe CD (n = 5) or UC (n = 5) were embedded in paraffin at the Department of Pathology of Osaka University Graduate School of Medicine. This study was approved by the ethics committee of Osaka University Hospital and conducted in accordance with approved guidelines (approval number 14107-10). Written informed consent was obtained from all subjects in this study at the time of enrollment or blood sampling.
| Characteristic | Patients with UC | Patients with CD | Patients with AE | HVs | CRP-high HVs |
| Number (male:female) | 110 (63:47) | 45 (36:9) | 11 (5:6) | 20 (14:6) | 79 (56:26) |
| Age, yr, mean (SD) | 48.79 (15.08) | 46.24 (11.75) | 42.89 (20.20) | 53.05 (6.58) | 56.01 (11.23) |
| Age at IBD diagnosis, yr, mean (SD) | 34.74 (16.64) | 26.89 (8.36) | |||
| UC disease location, extensive colitis/left-sided colitis/proctitis | 52/40/18 | ||||
| CD location, colic/ileal/ileo-colic | 5/17/23 | ||||
| Alb, g/dL, mean (SD) | 4.14 (0.31) | 3.71 (0.51) | 3.84 (0.41) | 4.41 (0.19) | 4.28 (0.24) |
| ALT, U/L, mean (SD) | 20.27 (17.49) | 16.00 (6.04) | 37.00 (22.19) | 16.96 (5.22) | |
| ALP, U/L, mean (SD) | 212.58 (69.81) | 244.79 (88.12) | 214.35 (54.46) | 209.84 (56.33) | |
| T-Bil, mg/dL, mean (SD) | 0.51 (0.36) | 0.45 (0.24) | 0.77 (0.22) | 0.63 (0.23) | |
| γ-GTP, U/L, mean (SD) | 30.75 (23.66) | 22.67 (15.61) | 42.78 (25.20) | 28.97 (17.30) | |
| CRP, mg/dL, mean (SD) | 0.38 (0.61) | 0.68 (1.16) | 8.15 (2.46) | 0.10 (0.08) | 1.35 (0.85) |
| Hb, g/dL, mean (SD) | 12.79 (1.87) | 12.78 (1.75) | 13.08 (1.75) | 14.53 (1.05) | 13.96 (1.32) |
| Ht, %, mean (SD) | 38.91 (7.58) | 39.65 (4.77) | 39.11 (4.59) | 42.6 (2.68) | 40.79 (3.66) |
| WBC, 10³/µL, mean (SD) | 7.35 (2.48) | 6.75 (2.17) | 12.50 (5.18) | ||
| Plt, 104/µL, mean (SD) | 27.58 (7.07) | 28.90 (6.21) | 27.34 (6.22) | 21.60 (5.62) | 23.43 (6.45) |
| Mayo, mean (SD) | 3.27 (2.83) | ||||
| CDAI, mean (SD) | 129.18 (70.48) |
Tissue sample sections (4-µm thickness) were fixed in 10% formaldehyde and embedded in paraffin. Sections were then deparaffinized using a xylene alternative, Hemo-De (Falma, Tokyo, Japan), and then rehydrated in 100% ethanol (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The sections were rinsed with phosphate-buffered saline [PBS; 137 mmol/L NaCl, 10 mmol/L Na2HPO4, 2.7 mmol/L KCl, 1.76 mmol/L KH2PO4 (pH 7.4)]. For antigen retrieval, sections were submerged in antigen-retrieval buffer [10 mmol/L Na3C6H5O7 (pH 6.0)] for 30 s at 125 °C and then submerged for 10 s at 90°C in a Pascal decloaking chamber (Dako, Glostrup, Denmark). The sections were cooled for 10 min in the chamber and 10 min at room temperature (RT) following antigen retrieval. After rinsing in Tris-buffered saline containing Tween 20 [TBST; 136 mmol/L NaCl, 2.6 mmol/L KCl, 24 mmol/L Tris, 0.005% Tween 20 (pH 7.4)], endogenous peroxidase activity was blocked by incubation in Dako REAL peroxidase-blocking solution (Dako) for 5 min at RT. The sections were incubated overnight at 4 °C with 0.22 µg/mL of the 10-7G mAb diluted in Dako REAL antibody diluent (Dako). Next, the sections were incubated with Dako ChemMate ENVISION kit/HRP (DAB) (Dako) for 30 min at RT. After each incubation step, the sections were washed with TBST three times for 5 min each. Subsequently, the sections were incubated with DAB+ chromogen solution (Dako) diluted with DAB substrate buffer (Dako) for 3 min to develop the stain. Finally, the slides were counterstained with hematoxylin (CHROMA, Nordrhein-Westfalen, Germany), dehydrated, cleared, and mounted. Images were acquired using a BZ-9000 microscope (KEYENCE, Osaka, Japan).
The 10-7G mAb ELISA was performed according to our previously reported method, with slight modifications[14]. 3,3’,5,5’-Tetramethylbiphenyl-4-4’-diamine solution was used for color development, as described previously[17]. The chromogen and the optical density were measured at 450 nm using an iMark™ microplate absorbance reader (Bio-Rad, Hercules, CA, United States).
A lectin-antibody ELISA was used to determine serum Fuc-mHpt levels, as described previously[13]. The Fab fragment of anti-haptoglobin polyclonal antibody (pAb) (Dako) was coated onto an ELISA plate to capture serum haptoglobin, and fucosylation of haptoglobin was detected using biotinylated Aleuria aurantia lectin (AAL). Serum Fuc-mHpt levels determined using this lectin-antibody ELISA are reported as AAL-mHpt.
Serum levels of leucine-rich alpha-2 glycoprotein (LRG) were determined using a human LRG assay kit (IBL, Gunma, Japan), according to the manufacturer’s instructions.
A pcDNA3.1-Hyg (+) vector (Invitrogen, Carlsbad, CA, United States) encoding the human haptoglobin transcript variant 1 (Hpt2) gene was prepared as previously described[18]. To construct the human pHpt expression vector, site-directed mutagenesis was employed to replace arginine with glutamine at residue 161, which is important for digestion by complement C1r subcomponent-like protein (C1RL). Amino acid substitution was carried out by polymerase chain reaction (PCR). In brief, the cycle parameters were as follows: initial denaturation at 95 °C for 3 min, one cycle of 98 °C for 10 s, 50 °C for 2 min, and 68 °C for 8 min, followed by 35 cycles of 10 s of denaturation at 98 °C, 15 s annealing at 57 °C, and an 8-min extension at 68 °C. The resulting DNA fragments were ligated with T4 DNA ligase (Promega, Madison, WI, United States). Primer sequences were as follows: sense, CCAAGAATCCGGCAAACCCAGTGCAGCAGATCCTGGGTGGAC; anti-sense, GGCATCCAGGTGTCCACCCAGGATCTGCTGCACTGGGTTTGCC. The mutated sequences were confirmed by sequencing analysis.
Human embryonic kidney cells (HEK293T) were obtained from the American Type Culture Collection (Manassas, VA, United States) and maintained at 37 °C in a humidified atmosphere with 5% CO2 and 95% air in DMEM high-glucose (Nacalai Tesque Inc., Kyoto, Japan) medium supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT, United States), 100 units/mL of penicillin, and 100 μg/mL of streptomycin (Nacalai Tesque Inc.).
HEK293T cells were transfected with the expression vector pcDNA3.1-Hyg(+) containing the mutated Hpt2 gene. After 24 h of incubation, transfected cells were selected using hygromycin B (Wako) for 2-3 wk to obtain the pHpt-overexpressing stable transfectants.
Fuc-pHpt products were collected from the conditioned medium of pHpt-overexpressing HEK293T cells and purified using an affinity chromatography column coupled with 10-7G mAb, as previously described[14]. The concentration of Fuc-pHpt was determined using a Nano Drop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States).
A 0.25-μL aliquot of serum in 10 μL of PBS was electrophoresed on a 10% polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. After blocking with 5% skim milk for 1 h, the membrane was incubated with 1/7500-diluted 10-7G mAb at 4 °C overnight. After three 10-min washes with TBST, the membrane was incubated with peroxidase-conjugated anti-mouse immunoglobulin G (IgG) for 1 h. After three 10-min washes with TBST, bound immunoglobulins were visualized using ImmunoStar® Zeta (Wako), according to standard protocols. Chemiluminescence signal detection and quantification were using a FUSION chemiluminescence imaging system (Vilber-Lourmat, Collegien, France). The quantity of Fuc-pHpt in each serum sample was calculated based on the ratio of the chemiluminescence signal intensity of the sample to that of the positive control. A fixed amount (5.0 ng) of purified Fuc-pHpt was used as a positive control sample for every Western blot analysis.
For haptoglobin phenotyping, serum samples were subjected to Western blotting using anti-human haptoglobin pAb, as described previously[16]. Haptoglobin phenotypes for each sample were determined after antibody signals were developed using ImmunoStar® Zeta (Wako) and detection of α-1 and/or α-2.
Statistical analyses were performed using JMP Pro 14.0 software (SAS Institute Inc., Cary, NC, United States). Data were analyzed using the Wilcoxon test, Spearman rank correlation analysis, and receiver operating characteristic (ROC) curve analysis. As the 10-7G value, AAL-mHpt, Fuc-pHpt amount, CRP levels, hemoglobin (Hb), and albumin (Alb) data did not exhibit a Gaussian distribution, these parameters were common log-transformed before correlation diagrams were drawn. In the statistical analyses, samples of the Hpt1-1 phenotype were excluded because the 10-7G values were below the level of detection. P values < 0.05 were considered indicative of a statistically significant difference.
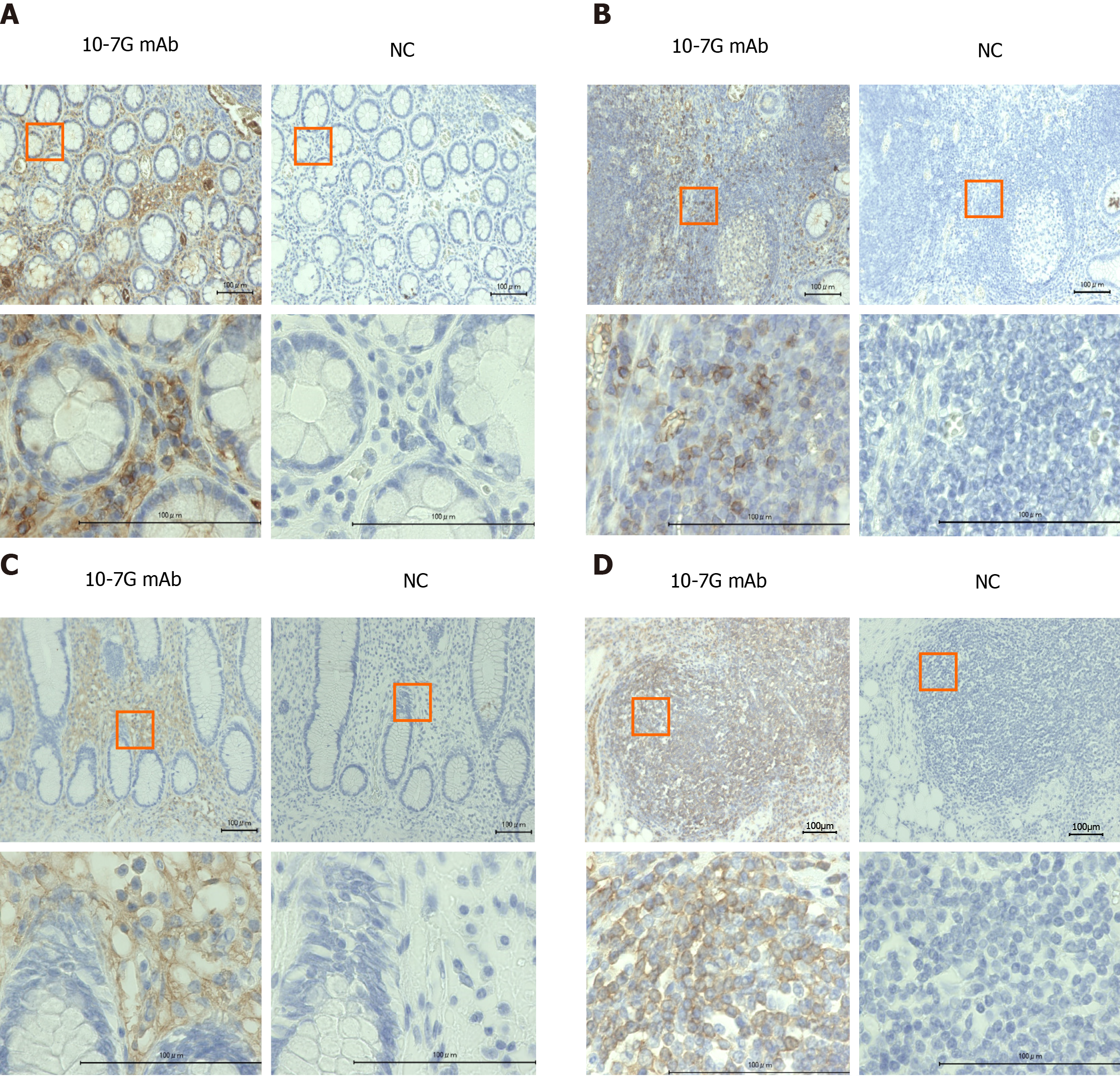
To identify Fuc-Hpt–producing cells in UC and CD intestinal tissues collected from IBD patients, we performed immunohistochemical analyses using the 10-7G mAb. As shown in Figure 2A and B, positive 10-7G mAb staining was observed in lymphocytes infiltrating into inflammatory sites of the mucosal layer and lymph nodules in the intestinal tissues of UC patients. Similar but much more diffuse 10-7G mAb staining was observed in comparable cells in CD tissues (Figure 2C and D).
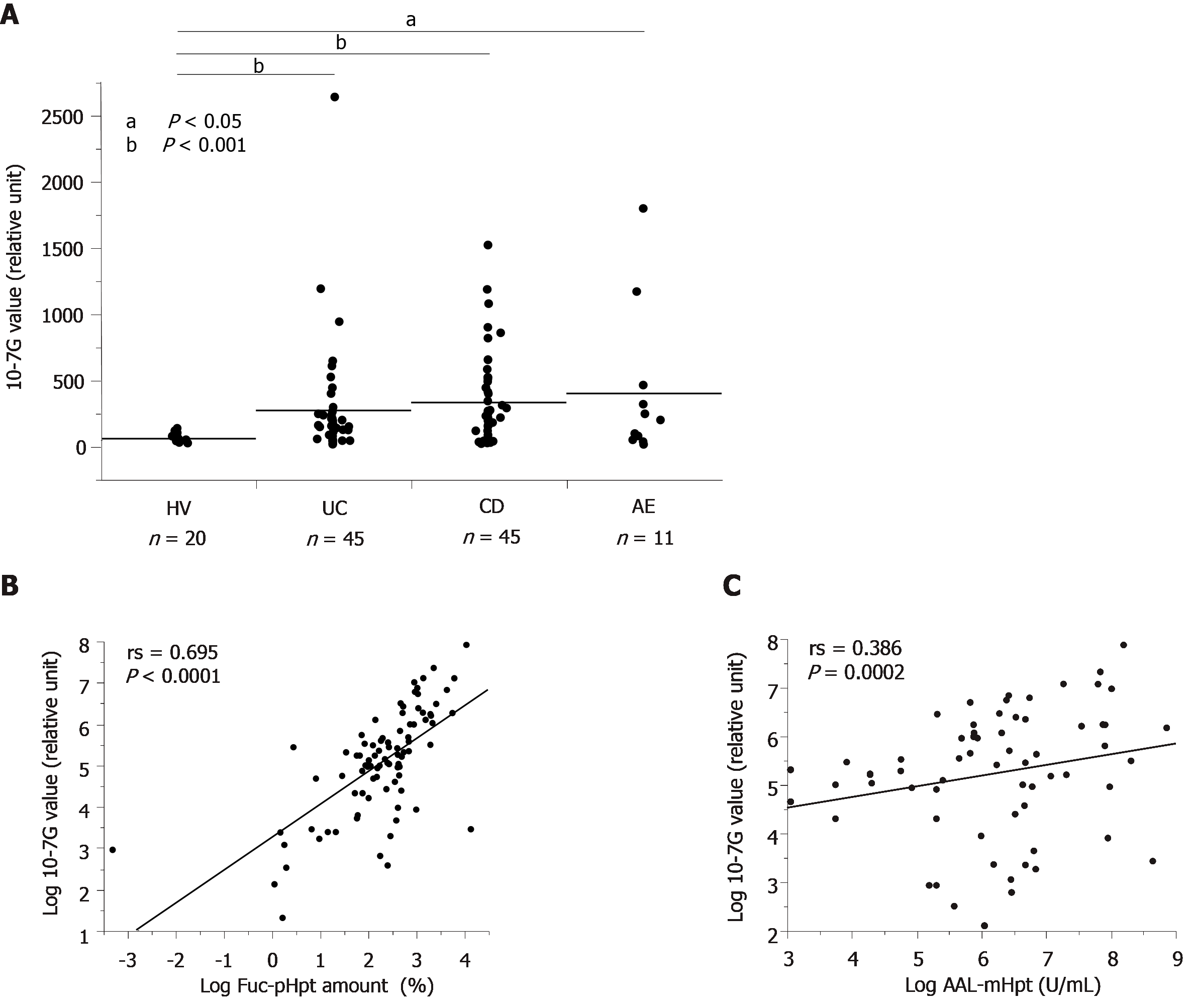
We determined serum 10-7G values for HVs and patients with UC, CD, or AE. As expected, 10-7G values were significantly higher in patients with UC, CD, and AE as compared to HVs (Figure 3A). To determine whether the 10-7G mAb primarily recognized Fuc-mHpt or Fuc-pHpt, we assessed serum Fuc-pHpt levels by immunoblot analysis using the 10-7G mAb, whereas serum Fuc-mHpt levels were determined using the lectin-antibody ELISA. Data regarding the semi-quantification of serum Fuc-pHpt levels by immunoblot analysis using the 10-7G mAb are described as Fuc-pHpt amount, whereas serum Fuc-mHpt levels determined using the lectin-antibody ELISA are described as AAL-mHpt.
Data regarding 10-7G values and Fuc-pHpt amount or AAL-mHpt were statistically analyzed using Spearman’s rank correlation coefficient (rs). As shown in Figure 3B, a marked positive correlation was observed between the 10-7G value and Fuc-pHpt amount (rs = 0.695, P < 0.0001). In contrast, a lower positive correlation was observed between serum AAL-mHpt levels and 10-7G values (rs = 0.386, P = 0.0002) (Figure 3C). These results indicate that the 10-7G value depends primarily on serum Fuc-pHpt and partly reflects serum Fuc-mHpt in patients with intestinal inflammatory diseases.
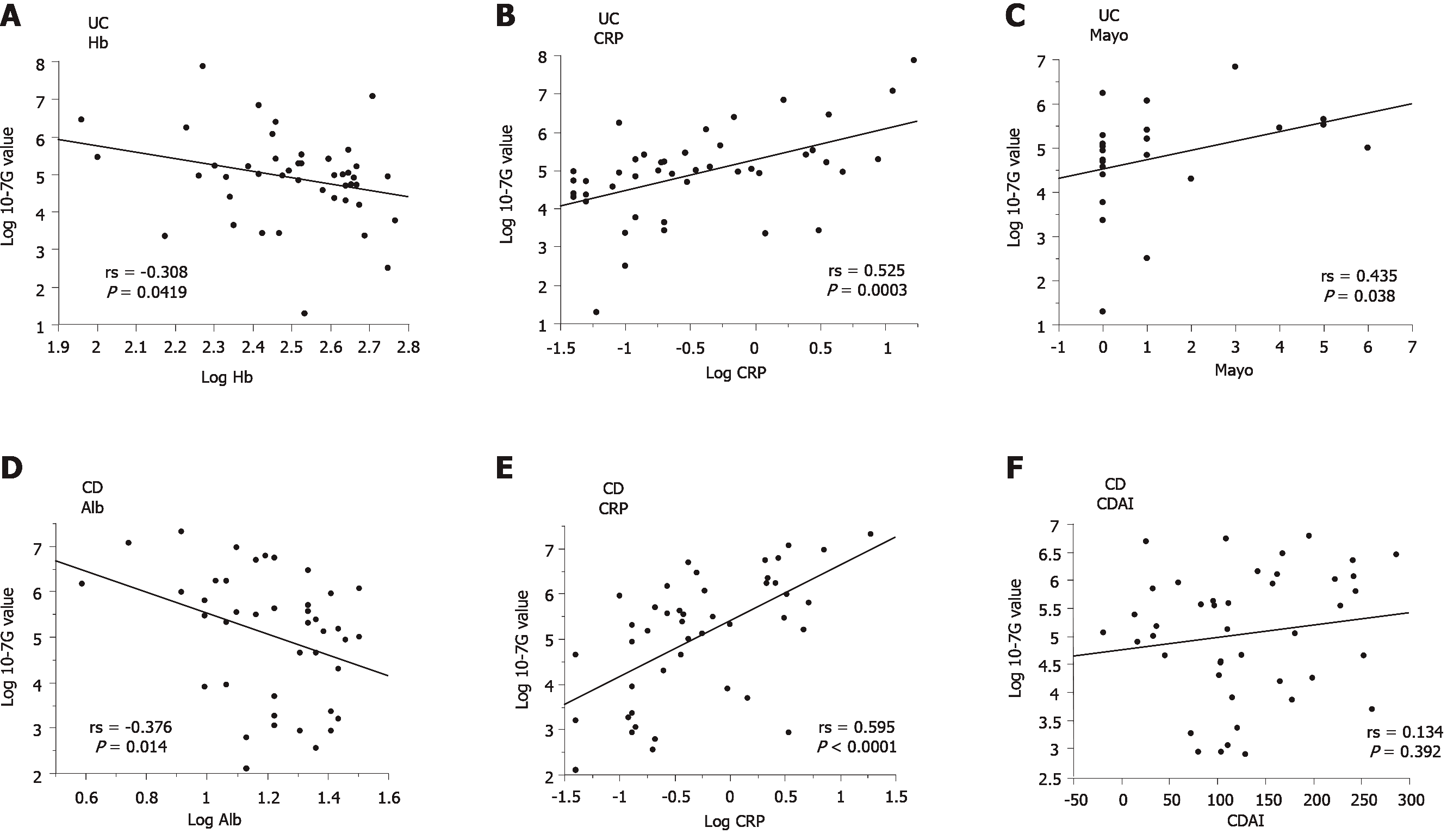
To elucidate the pathophysiologic significance of 10-7G values in IBD, we investigated the correlation between the 10-7G value and various clinical parameters of IBD patients, including Alb, alanine aminotransferase, alkaline phosphatase, total-bilirubin, γ-glutamyl transpeptidase, CRP, Hb, hematocrit, white blood cell count, platelet count, IBD activity index score, and full Mayo score (Mayo) for UC or Crohn’s disease activity index (CDAI) for CD (Table 2). As shown in Figure 4A-C the 10-7G values of UC patients were negatively correlated with Hb (rs = -0.308, P = 0.0419) and positively correlated with CRP (rs = 0.525, P = 0.0003) and Mayo (rs = 0.435, P = 0.038). In contrast, as shown in Figure 4D and E, the 10-7G values of CD patients were negatively correlated with Alb (rs = -0.376, P = 0.014) and positively correlated with CRP (rs = 0.595, P < 0.0001). However, no significant correlation was observed between 10-7G values and CDAI (Figure 4F). These results indicate that 10-7G values are correlated with some inflammation-related biomarkers, especially CRP, in both UC and CD patients and reflect an inflammatory condition.
To determine whether the 10-7G value is a suitable inflammation marker similar to CRP, we also determined 10-7G values of HVs who were found to have elevated CRP levels during routine medical examinations (CRP-high HVs, n = 79). As shown in Figure 5A, there was no significant difference in 10-7G values in CRP-high or normal HVs. In addition, there was no significant correlation between the 10-7G value and CRP level in CRP-high HVs (Figure 5B). In contrast, the 10-7G values of CRP-high IBD patients were significantly higher than those of CRP-normal IBD patients, and there was a significant correlation between 10-7G values and CRP in IBD patients (Figure 5C and D). These data suggest that the 10-7G value mainly reflects intestinal inflammation, whereas CRP reflects systemic inflammation.
| UC | CD | ||||||
| rs | P value | n | rs | P value | n | ||
| Alb | -0.182 | 0.254 | 41 | -0.376a | 0.014 | 42 | |
| ALT | -0.214 | 0.339 | 22 | -0.148 | 0.51 | 22 | |
| ALP | 0.453 | 0.052 | 19 | 0.329 | 0.169 | 19 | |
| T-Bil | -0.419 | 0.059 | 21 | -0.081 | 0.728 | 21 | |
| γ-GTP | -0.06 | 0.801 | 20 | 0.392 | 0.079 | 21 | |
| CRP | 0.525a | 0.0003 | 44 | 0.595a | < 0.0001 | 44 | |
| Hb | -0.308a | 0.0419 | 44 | -0.287 | 0.072 | 40 | |
| Ht | -0.304 | 0.064 | 38 | -0.261 | 0.091 | 43 | |
| WBC | 0.289 | 0.057 | 44 | 0.215 | 0.177 | 41 | |
| Plt | 0.06 | 0.712 | 40 | 0.145 | 0.387 | 38 | |
| Mayo | 0.435a | 0.038 | 23 | ||||
| CDAI | 0.134 | 0.392 | 43 | ||||
As the 10-7G value was found to correlate with IBD activity index score in a small number of UC patients (Figure 4C and Table 2), we investigated the usefulness of the 10-7G value as an indicator of UC disease activity using a larger number of serum samples (n = 110). We compared the 10-7G value with CRP and LRG, a novel serum biomarker for UC disease activity[19]. We first analyzed the correlation between the 10-7G value, LRG, CRP, and three clinical activity index scores: the partial Mayo score (pMayo), an evaluation of symptomatic severity; the Mayo endoscopic subscore (MES), an evaluation of endoscopic severity; and Mayo, the sum of pMayo and MES, which is an indicator of clinical severity (Supplementary Table 1). Although the 10-7G value was significantly correlated with three of the scores (pMayo: rs = 0.2433, P = 0.0224, MES: rs = 0.3540, P = 0.0064, Mayo: rs = 0.2273, P = 0.0440), the correlation coefficients were slightly lower than those for CRP (pMayo: rs = 0.2947, P = 0.0053, MES: rs = 0.4375, P = 0.0006, Mayo: rs = 0.2597, P = 0.0208) and LRG (pMayo: rs = 0.3108, P = 0.0032, MES: rs = 0.3665, P = 0.0047, Mayo: rs = 0.3010, P = 0.0070).
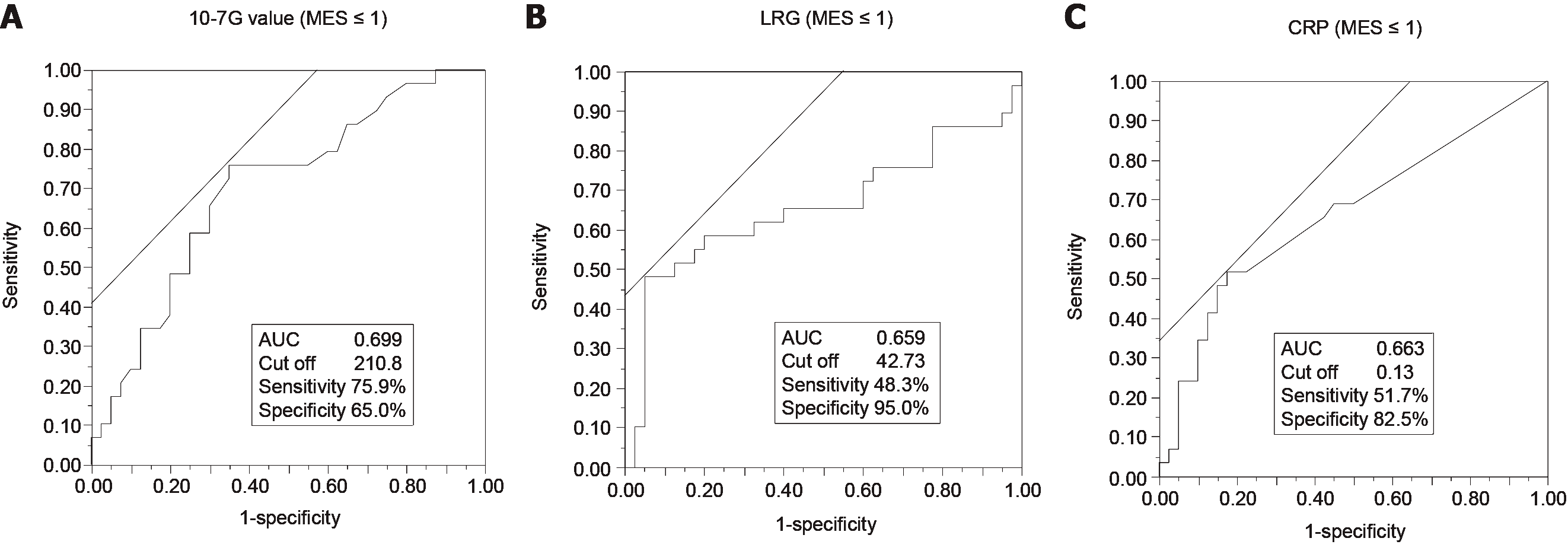
The sensitivity and specificity of the serum 10-7G value, LRG, and CRP for diagnosis of endoscopic remission and clinical remission based on MES and Mayo were determined using ROC curve analysis. Endoscopic remission (i.e., mucosal healing) was defined as MES ≤ 1[20], and clinical remission was defined as Mayo ≤ 2 (pMayo ≤ 1 and MES ≤ 1)[21]. For the diagnosis of endoscopic remission, the area under the curve (AUC) of the 10-7G value (MES ≤ 1: 0.699) (Figure 6A) was higher than that of both LRG (MES ≤ 1: 0.659) (Figure 6B) and CRP (MES ≤ 1: 0.663) (Figure 6C). In terms of clinical remission, the AUC value of the 10-7G value (Mayo ≤ 2: 0.626) (Supplementary Figure 1A) was lower than that of both LRG (Mayo ≤ 2: 0.714) (Supplementary Figure 1B) and CRP (Mayo ≤ 2: 0.678) (Supplementary Figure 1C). These results indicate that the 10-7G value is a useful biomarker for evaluating endoscopic mucosal healing in UC patients.
In this study, we demonstrated that the 10-7G value reflects intestinal inflammation. We found that the 10-7G value was not increased in the serum of HVs with elevated CRP levels, but it was significantly increased in the serum of patients with IBD and AE (Figures 3A and 5A). In addition, there was a significant correlation between 10-7G values and CRP in IBD patients, whereas no significant correlation in HVs with elevated CRP levels (Figure 5B and D). We previously reported agalactosyl IgG as a diagnostic biomarker of IBD[22]. Agalactosyl IgG is also an indicator of chronic inflammation associated with conditions such as rheumatoid arthritis[23]. The 10-7G value, on the other hand, is associated with acute and/or chronic intestinal inflammation.
We also demonstrated that the 10-7G value is a useful biomarker of disease activity in IBD patients, especially those with UC. Correlation analyses revealed that the 10-7G value is correlated with UC activity index score, non-CD Mayo score, and CDAI (Figure 4C and F). We concluded that this difference was due to the specificity of the 10-7G value for intestinal inflammation. Whereas the inflammatory lesions associated with UC are localized in the large intestine, those associated with CD can occur throughout the gastrointestinal tract, from the oral cavity to the anal canal[24]. The CDAI reflects not only intestinal inflammation but also lesions related to extraintestinal complications, such as arthritis, stomatitis, and anal fistulas[25]. Furthermore, calculation of the Mayo includes endoscopic findings that enable accurate evaluation of the intestinal condition, but the CDAI does not consider these findings. Therefore, the 10-7G value may not be correlated with the CDAI.
We also compared the 10-7G value and the LRG level, a promising tool for monitoring disease activity in IBD patients that was recently accepted for insurance coverage in Japan. As indicated in Figure 6, the AUC of the 10-7G value for a diagnosis of endoscopic remission (MES ≤ 1) was higher than that of LRG. In contrast, the AUC of LRG for the diagnosis of symptomatic remission (pMayo ≤ 1) was higher than that of the 10-7G value (Supplementary Figure 1D and E). In other words, the 10-7G value is superior to LRG for evaluating the endoscopic mucosal condition, and LRG is superior to the 10-7G value for evaluating general symptoms such as stool frequency and hematochezia. As indicated in Figure 3B, the 10-7G value of IBD patients primarily reflects Fuc-pHpt levels, and some studies have reported that pHpt increases epithelial permeability[26]. Based on our results and other reports, we attributed the usefulness of the 10-7G value for evaluating the mucosal condition of UC patients to the detection of Fuc-pHpt.
To identify Fuc-Hpt–producing cells in intestinal tissues of IBD patients, we performed immunohistochemical staining with the 10-7G mAb. As shown in Figure 2, lymphocytes infiltrating into areas of inflammation in the mucosal layer were stained with the 10-7G mAb. The staining signal was thought to depend primarily on Fuc-pHpt rather than Fuc-mHpt. Cleavage of pHpt by C1RL is required for haptoglobin maturation. C1RL is a serine protease that cleaves αβ-junctions within pHpt[27]. C1RL expression is reportedly markedly elevated in the liver[28]. Therefore, most haptoglobin produced in hepatocytes matures before it is secreted into the blood. The expression of C1RL in other tissues is typically much lower than in hepatocytes; therefore, synthesized haptoglobin does not fully mature. In addition, in an immunoblotting analysis using 10-7G mAb, we recently demonstrated that some lines of non-adherent cells produce Fuc-pHpt and that several inflammatory cytokines facilitate Fuc-pHpt secretion (manuscript submitted). These data support our hypothesis that infiltrating lymphocytes produce Fuc-pHpt in sites of intestinal inflammation and that the 10-7G value is an indicator of Fuc-pHpt production. Further studies are needed to fully elucidate the mechanism underlying the secretion of Fuc-pHpt by immune cells, however.
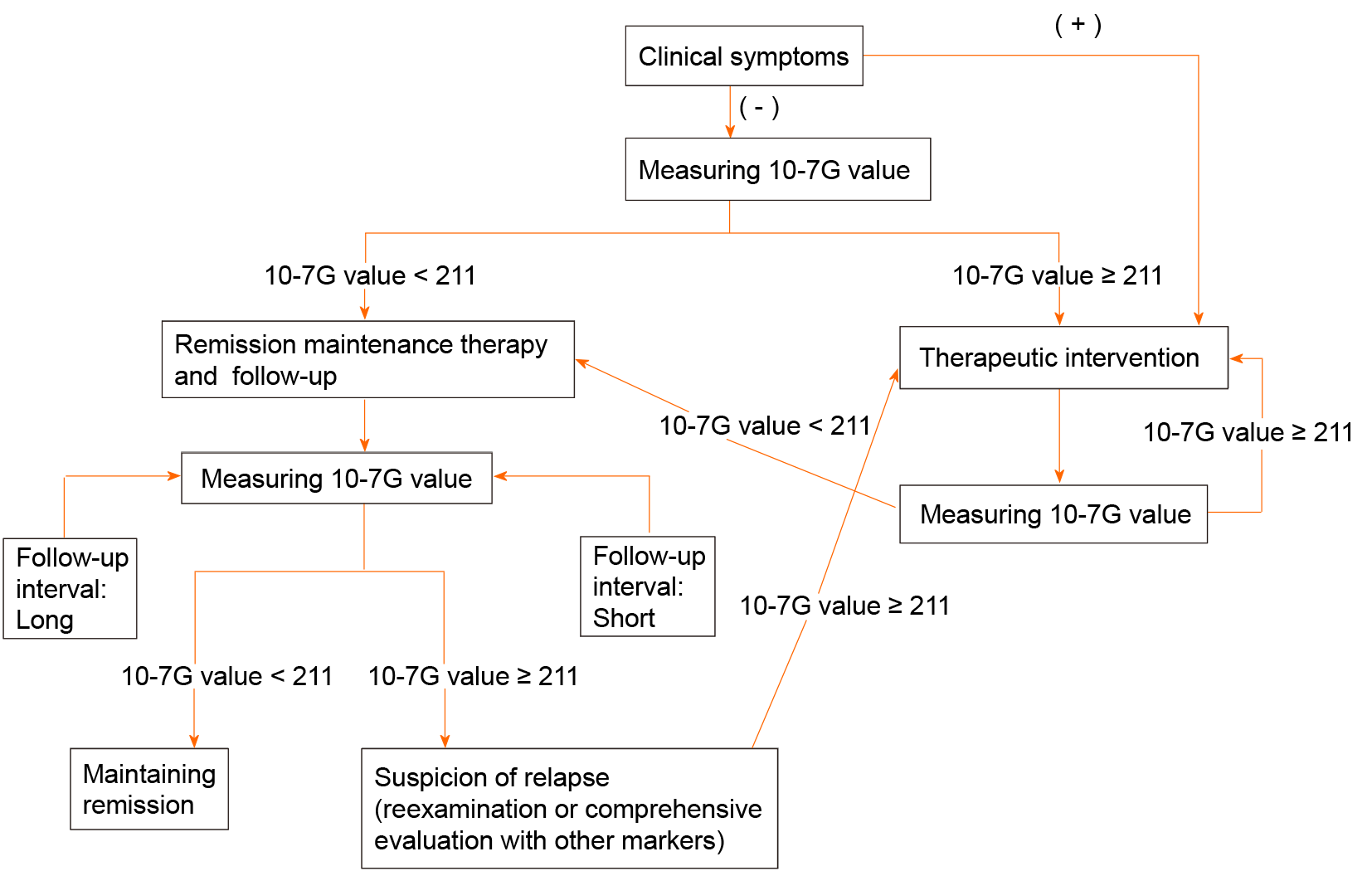
Considering our findings in this study, we proposed the flow chart of non-invasive detection methods of clinical activity of IBD, especially UC, using 10-7G value based on the cutoff value of 211 defined as ROC curve analysis (Figure 6A), the point of the best split of endoscopic remission (Figure 7). Since clinical symptoms appear after changes in mucosal inflammation status of the intestine and colonoscopy itself has a risk of exacerbating mucosal inflammation, non-invasive biomarkers such as 10-7G value are required to follow up the patients with IBD.
In conclusion, we demonstrated that the serum 10-7G value reflects intestinal inflammation and is a useful biomarker for evaluating endoscopic mucosal healing in UC, as Fuc-Hpt detected by the 10-7G mAb is produced by lymphocytes infiltrating into sites of inflammation in the intestinal mucosal layer.
Fucosylation is one of the most important glycosylation involved in cancer and inflammation. Recently, we have succeeded to establish a novel glycan antibody 10-7G mAb which directly recognizes fucosylated haptoglobin (Fuc-Hpt), and developed its enzyme-linked immunosorbent assay system.
Although fecal and serum biomarkers have been extremely important and supportive for monitoring of Inflammatory bowel disease (IBD), their low sensitivity and high variability characteristics limit clinical efficacy. Thus, the establishment of better biomarkers is expected.
This research aimed to investigate the usefulness of the serum levels of Fuc-Hpt (10-7G values) as a potential biomarker for monitoring disease activity in IBD.
Intestinal tissues of IBD patients were examined immunohistochemically using the 10-7G mAb. The 10-7G values were measured using serum from patients with IBD, acute enteritis (AE), and healthy volunteers. Correlation analysis and Receiver operating characteristic curve analysis were performed to evaluate the usefulness of the 10-7G values as a biomarker for IBD compared to conventional serum biomarkers and various clinical parameters.
Positive 10-7G mAb staining was observed in lymphocytes infiltrating into inflammatory sites of the mucosal layer. The 10-7G values were significantly higher in patients with IBD and AE. Statistical analyses showed that the 10-7G values could evaluate intestinal inflammation and endoscopic mucosal healing in ulcerative colitis (UC).
Fuc-Hpt is produced by lymphocytes infiltrating into sites of inflammation in the intestine and the serum 10-7G value is a non-invasive biomarker for evaluating endoscopic remission in UC.
Further studies are needed to fully elucidate the mechanism underlying the secretion of Fuc-Hpt by immune cells. In addition, we will measure serum 10-7G values of same patients with UC in follow-up study.
The authors would like to thank Dr. Yamada M (aMs NEW OTANI CLINIC, Osaka, Osaka 540-8570, Japan) for the excellent assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang B S-Editor: Gao CC L-Editor: A P-Editor: Wang LL
| 1. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3351] [Article Influence: 186.2] [Reference Citation Analysis (11)] |
| 2. | Uhlig HH. Monogenic diseases associated with intestinal inflammation: implications for the understanding of inflammatory bowel disease. Gut. 2013;62:1795-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 3. | Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 704] [Article Influence: 58.7] [Reference Citation Analysis (1)] |
| 4. | Carlson CS, Aldred SF, Lee PK, Tracy RP, Schwartz SM, Rieder M, Liu K, Williams OD, Iribarren C, Lewis EC, Fornage M, Boerwinkle E, Gross M, Jaquish C, Nickerson DA, Myers RM, Siscovick DS, Reiner AP. Polymorphisms within the C-reactive protein (CRP) promoter region are associated with plasma CRP levels. Am J Hum Genet. 2005;77:64-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 259] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Soubières AA, Poullis A. Emerging role of novel biomarkers in the diagnosis of inflammatory bowel disease. World J Gastrointest Pharmacol Ther. 2016;7:41-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Eckersall PD, Bell R. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet J. 2010;185:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 490] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 7. | Yang H, Wang H, Levine YA, Gunasekaran MK, Wang Y, Addorisio M, Zhu S, Li W, Li J, de Kleijn DP, Olofsson PS, Warren HS, He M, Al-Abed Y, Roth J, Antoine DJ, Chavan SS, Andersson U, Tracey KJ. Identification of CD163 as an antiinflammatory receptor for HMGB1-haptoglobin complexes. JCI Insight. 2016;1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1209] [Cited by in RCA: 1321] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 9. | Yang F, Brune JL, Baldwin WD, Barnett DR, Bowman BH. Identification and characterization of human haptoglobin cDNA. Proc Natl Acad Sci USA. 1983;80:5875-5879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Black JA, Chan GF, Hew CL, Dixon GH. Gene action in the human haptoglobins. 3. Isolation of the alpha-chains as single gene products. Isolation, molecular weight, and amino acid composition of alpha and beta chains. Can J Biochem. 1970;48:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem. 2008;143:725-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 322] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 12. | Okuyama N, Ide Y, Nakano M, Nakagawa T, Yamanaka K, Moriwaki K, Murata K, Ohigashi H, Yokoyama S, Eguchi H, Ishikawa O, Ito T, Kato M, Kasahara A, Kawano S, Gu J, Taniguchi N, Miyoshi E. Fucosylated haptoglobin is a novel marker for pancreatic cancer: a detailed analysis of the oligosaccharide structure and a possible mechanism for fucosylation. Int J Cancer. 2006;118:2803-2808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 241] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 13. | Kamada Y, Kinoshita N, Tsuchiya Y, Kobayashi K, Fujii H, Terao N, Kamihagi K, Koyama N, Yamada S, Daigo Y, Nakamura Y, Taniguchi N, Miyoshi E. Reevaluation of a lectin antibody ELISA kit for measuring fucosylated haptoglobin in various conditions. Clin Chim Acta. 2013;417:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Nishino K, Koda S, Kataoka N, Takamatsu S, Nakano M, Ikeda S, Kamamatsu Y, Morishita K, Moriwaki K, Eguchi H, Yamamoto E, Kikkawa F, Tomita Y, Kamada Y, Miyoshi E. Establishment of an antibody specific for cancer-associated haptoglobin: a possible implication of clinical investigation. Oncotarget. 2018;9:12732-12744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Morishita K, Maki Y, Takamatsu S, Ito N, Koda S, Motooka K, Kamada Y, Kajihara Y, Miyoshi E. Identification of the epitope of 10-7G glycan antibody to recognize cancer-associated haptoglobin. Anal Biochem. 2020;593:113588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Morishita K, Ito N, Koda S, Maeda M, Nakayama K, Yoshida K, Takamatsu S, Yamada M, Eguchi H, Kamada Y, Miyoshi E. Haptoglobin phenotype is a critical factor in the use of fucosylated haptoglobin for pancreatic cancer diagnosis. Clin Chim Acta. 2018;487:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Roberts IM, Jones SL, Premier RR, Cox JC. A comparison of the sensitivity and specificity of enzyme immunoassays and time-resolved fluoroimmunoassay. J Immunol Methods. 1991;143:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Narisada M, Kawamoto S, Kuwamoto K, Moriwaki K, Nakagawa T, Matsumoto H, Asahi M, Koyama N, Miyoshi E. Identification of an inducible factor secreted by pancreatic cancer cell lines that stimulates the production of fucosylated haptoglobin in hepatoma cells. Biochem Biophys Res Commun. 2008;377:792-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Serada S, Fujimoto M, Terabe F, Iijima H, Shinzaki S, Matsuzaki S, Ohkawara T, Nezu R, Nakajima S, Kobayashi T, Plevy SE, Takehara T, Naka T. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis. 2012;18:2169-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan BG, Hanauer SB, Lichtenstein GR, Present D, Sands BE, Sandborn WJ. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 728] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 21. | Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 711] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 22. | Shinzaki S, Iijima H, Nakagawa T, Egawa S, Nakajima S, Ishii S, Irie T, Kakiuchi Y, Nishida T, Yasumaru M, Kanto T, Tsujii M, Tsuji S, Mizushima T, Yoshihara H, Kondo A, Miyoshi E, Hayashi N. IgG oligosaccharide alterations are a novel diagnostic marker for disease activity and the clinical course of inflammatory bowel disease. Am J Gastroenterol. 2008;103:1173-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 931] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 24. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2200] [Article Influence: 137.5] [Reference Citation Analysis (6)] |
| 25. | Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2335] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 26. | Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S, Arrietta MC, Meddings JB, Fasano A. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA. 2009;106:16799-16804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 319] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 27. | Wicher KB, Fries E. Prohaptoglobin is proteolytically cleaved in the endoplasmic reticulum by the complement C1r-like protein. Proc Natl Acad Sci USA. 2004;101:14390-14395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Ligoudistianou C, Xu Y, Garnier G, Circolo A, Volanakis JE. A novel human complement-related protein, C1r-like protease (C1r-LP), specifically cleaves pro-C1s. Biochem J. 2005;387:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |