Published online May 21, 2021. doi: 10.3748/wjg.v27.i19.2376
Peer-review started: January 16, 2021
First decision: February 10, 2021
Revised: February 23, 2021
Accepted: April 22, 2021
Article in press: April 22, 2021
Published online: May 21, 2021
Processing time: 116 Days and 19.3 Hours
Sepsis is a common disease in intensive care units, with high morbidity and mortality. Intestinal microecology plays a vital part in the development and progression of this disease, possibly because sepsis and its treatment cause specific changes in the composition of the intestinal flora.
To investigate the characteristics of intestinal flora disturbance in sepsis patients treated with antibiotics.
In this prospective comparative study, we enrolled ten patients with sepsis (sepsis group), hospitalized in the Department of Critical Care Medicine of the General Hospital, Ningxia Medical University, China (a class IIIa general hospital) from February 2017 to June 2017; ten patients without sepsis hospitalized in the same period (non-sepsis group) and ten healthy individuals (control group) were also enrolled. Fecal samples collected from the three groups were subjected to 16S rRNA gene sequencing and the intestinal flora diversity, structure, and composition were determined. Additionally, the dynamics of the intestinal flora diversity, structure, and composition in sepsis patients were investigated via 16S rRNA gene sequencing of samples collected 0 d, 3 d, and 7 d after admittance to the intensive care unit. Correlations between the serum levels of procalcitonin, endotoxin, diamine oxidase, and D-lactic acid and the intestinal flora composition of sepsis patients were also investigated.
Compared with the healthy control group, sepsis and non-sepsis patients showed reduced intestinal flora α-diversity and a distinct flora structure, with Firmicutes as the dominant phylum, and significantly decreased proportions of Bacte
Sepsis patients in intensive care units show dysbiosis, lasting for at least 1 wk.
Core Tip: As the largest reservoir of bacteria and endotoxins in the body, the intestinal tract is regarded as the “engine” of sepsis and multiple organ dysfunction. Through 16S rRNA gene sequencing, we observed that intestinal flora disturbance occurs in sepsis patients. Notably, here, we revealed for the first time the intestinal flora dynamic changes in sepsis patients during treatment. We found that the abundance of some intestinal bacteria in sepsis patients significantly correlated with infection- and intestinal barrier-related clinical indicators. These findings add to the understanding of the intestinal flora in sepsis, providing a basis for the reversal of dysbiosis.
- Citation: Yang XJ, Liu D, Ren HY, Zhang XY, Zhang J, Yang XJ. Effects of sepsis and its treatment measures on intestinal flora structure in critical care patients. World J Gastroenterol 2021; 27(19): 2376-2393
- URL: https://www.wjgnet.com/1007-9327/full/v27/i19/2376.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i19.2376
Sepsis refers to the life-threatening organ dysfunction caused by imbalanced responses to infection[1]. As the largest reservoir of bacteria and endotoxins in the body, the intestinal tract is regarded as the “engine” of sepsis and multiple organ dysfunction syndrome. Under normal circumstances, the intestinal flora plays beneficial roles in the context of human physiology and immunity[2-3], existing in a homeostatic state important for the maintenance of human health. However, in the context of critical illness, homeostasis is interrupted, leading to abnormal changes in the types, quantities, proportions, and locations of microorganisms in the intestinal tract[4], thus increasing susceptibility to sepsis. In fact, studies have shown that there is a dose–response relationship between the degree of intestinal microecological disturbance and the incidence of subsequent severe sepsis[5]. Therefore, an improved understanding of the status and degree of intestinal flora disturbances in sepsis patients is of great significance; for instance, the establishment of strong intestinal microecology-based biomarkers will allow for the accurate prediction of prognosis of sepsis patients and the consequent adoption of adequate treatment measures. However, there are few reports on the sepsis-related intestinal flora and its dynamic changes.
High-throughput 16S rRNA gene sequencing allows for a comprehensive understanding of the intestinal flora structure, and it is the standard technology used for human intestinal flora analysis[6]. In this study, the diversity and composition of the intestinal flora in sepsis patients, non-sepsis patients, and healthy individuals were analyzed and compared. The dynamic changes in the intestinal flora α-diversity and structure throughout a 1 wk period [1, 3, and 7 d after admittance to the intensive care unit (ICU)] were also investigated to disclose the transformation of the intestinal flora in the context of antibiotic treatment. In addition, the potential relationships between the intestinal flora imbalance and clinical indicators in sepsis were investigated via correlation analysis.
This is a prospective observational study, including ten sepsis patients (sepsis group) admitted to the ICU of the General Hospital, Ningxia Medical University, China from February 2017 to June 2017, ten patients without sepsis (non-sepsis group) admitted to the same ICU during the same time period, and ten local healthy individuals (control group). The inclusion criteria for sepsis patients were as follows: 18-75 years old; sepsis patients meeting the latest definition of sepsis in “Sepsis-3.0” issued by the Society of Critical Care Medicine (SCCM) in 2016[7]; and estimated time of more than 2 d spent in the ICU after enrollment. The inclusion criteria for the non-sepsis patients were as follows: 18-75 years old; and patients admitted to the ICU at the same period owing to diseases other than sepsis, such as multiple injuries and high-risk operations. Of note, we tried to match the age, underlying disease, and surgical site with those in patients in the sepsis group. Because there was no definite infection, the non-sepsis group was not given antibiotics before the fecal samples were collected. The exclusion criteria were as follows: Not meeting the inclusion criteria; perianal infection; patients subjected to enterostomy; and patients with chronic gastrointestinal diseases. Additionally, the requirements for the healthy control group were as follows: Matched age with the aforementioned two groups of patients; good health; no history of chronic or metabolic diseases (e.g., hypertension, coronary heart disease, diabetes, hepatitis, and hyperthyroidism); no history of digestive tract diseases or digestive tract surgery; and not using antibiotics, probiotics, enteral nutrients, or other drugs within 3 mo before enrollment. Informed consent was obtained from all patients or their immediate family members, and this study was approved by the hospital ethics committee (ethics approval number: 2016-258).
The general clinical data of patients in the sepsis and non-sepsis groups were recorded, including age, main diagnosis, operation type (abdominal cavity organ operation and non-abdominal cavity organ operation), clinical infection site, pathogenic bacterial agent (based on qualitative culture), and use of antibiotics (Tables 1-3). The acute physiology and chronic health (APACHE II) score and sequential organ failure assessment (SOFA) score of patients on the day of ICU admission were recorded (Table 1). The primary infection site, surgical site, and use of antibiotics in the sepsis group were also recorded (Table 2), as were the positive results of blood, urine, sputum, and other body fluids collected from patients with sepsis (Table 3). Additionally, venous blood samples were collected from patients with sepsis on days 1, 3, and 7 after admission to the ICU, and procalcitonin (PCT) was detected by immunochromatography [PCT-Q detection card produced by BRAHMS GmbH in Germany, provided by Thermo Fisher Scientific (China) Co., Ltd.], while the levels of serum D-lactic acid (D-Lac), bacterial endotoxin (endotoxin), and diamine oxidase (DAO) were determined using the JY-DLT intestinal barrier function biochemical index analysis system (Beijing Zhongsheng Jinyu Diagnosis Technology Co., Ltd., China.) within 0.5 h after blood collection.
| Indicator | Control group (n = 10) | Non-sepsis group (n = 10) | Sepsis group (n = 10) | H/F/χ2/Z/t value | P value |
| Age (yr, mean ± SD) | 55 (48.75, 55.25) | 59 (50.00, 74.00)a | 63.50 (44.75, 76.25)a,b | 2.05 | 0.36 |
| BMI (kg/m2, mean ± S) | 23.95 ± 2.04 | 27.19 ± 4.40a | 23.91 ± 4.09a,b | 2.64 | 0.09 |
| Underlying disease (cases) | 0.84 | 0.65 | |||
| Multiple injuries | 3 | 4 | |||
| Malignant tumor | 5 | 3 | |||
| Other diseases | 2 | 3 | |||
| Type of operation (cases) | 0.00 | 1.00 | |||
| Surgery on abdominal hollow viscera | - | 5 | 5 | ||
| Surgery on non-abdominal hollow viscera | - | 5 | 5 | ||
| APACHE II score on day 1 [points, M (P25, P75)] | - | 10.00 (7.00, 17.00) | 19.00 (15.25, 21.50) | −2.40 | 0.02 |
| SOFA score on day 1 (points, mean ± SD) | - | 3.30 ± 1.42 | 10.80 ± 2.97 | −7.20 | 0.00 |
| Patient No. | Primary infection site | Surgical site | Categories of antibiotics used within 7 d after admission to the ICU |
| S1 | Lungs | - | Broad-spectrum penicillins |
| S2 | Abdominal cavity | Hollow viscera | Carbapenems + glycopeptides |
| S3 | Lungs | Hollow viscera | Carbapenems + oxazolidinones |
| S4 | Lungs | Joint | Broad-spectrum penicillins + carbapenems |
| S5 | Abdomen | Hollow viscera | Broad-spectrum penicillins + glycopeptides |
| S6 | Lungs | - | Broad-spectrum penicillins + tetracyclines |
| S7 | Lungs | - | Carbapenems + broad-spectrum penicillins |
| S8 | Abdominal cavity | - | Carbapenems |
| S9 | Abdominal cavity | Hollow viscera | Carbapenems |
| S10 | Abdominal cavity | Hollow viscera | Carbapenems + glycopeptides |
| Pathogenic bacteria | Cultured sample | Patient No. | Corresponding sample collection time (day n after admission to the ICU) |
| Acinetobacter baumannii | Sputum | S5, S6, S8 | 4, 13, 23 |
| Stenotrophomonas maltophilia | Sputum | S6, S7 | 1, 8 |
| Enterococcus | Sputum | S7 | 23 |
| Escherichia coli | Blood | S7 | 1 |
Stool samples were collected from sepsis patients on days 1, 3, and 7 after admission to the ICU, from non-sepsis patients on day 1 after admission to the ICU, and from healthy controls. Samples from patients were taken from a deep part of the fresh stool of patients by using a sampling spoon, and the stool samples were quickly placed in a special stool sample box. The stool samples of healthy control subjects were collected in a special stool sample box after normal defecation. Then, the small stool boxes filled with samples were sealed, labeled, placed in a liquid nitrogen tank, and transferred to a -80 °C freezer for storage.
Each stool sample was added to 790 μL of lysis buffer [4 M guanidine thiocyanate, 250 μL; 10% N-lauroyl sarcosine, 40 μL; 5% N-lauroyl sarcosine-0.1 M phosphate buffer (pH 8.0), 500 μL] together with 1 g glass beads (0.1 mm, Biospec Products, Inc., United States). After sufficient vortex-based homogenization, bead beating was performed for 10 min at full speed. The subsequent extraction was carried out according to the instructions of the extraction kit manufacturer (E.Z.N.A.® Stool DNA Kit, Omega Bio-tek, Inc., GA). The V3–V4 region of the 16S rRNA gene was amplified with the primers 341F/805R (341F: 5′-CCTACGGGNGGCWGCAG-3′; 805R: 5′-GACTACHVGGGTATCTAATCC-3′), and the PCR products were sequenced on an Illumina MiSeq 2*300 bp platform. The raw sequencing data and accompanying information are available in the Sequence Read Archive database under the accession number PRJNA691455.
The clinical data were processed and analyzed using SPSS 19.0 software. All data were first tested for normality and homogeneity of variance. Data conforming to a normal distribution and homogeneity of variance are expressed as the mean ± SD, and the t-test or analysis of variance were used for statistical analysis. Data not conforming to a normal distribution are expressed as medians (P25, P75), and the rank-sum test was used for statistical analysis. The χ2 test was used to compare enumeration data, and P < 0.05 was considered statistically significant.
USEARCH 8.0 was used to process the raw sequencing data, and operational taxonomic units (OTUs) were classified according to 97% sequence similarity. The OTU representative sequences were compared against the SILVA database (SSU123; http://www.arb-silva.de) with a 70% confidence threshold, and the taxonomic status of each 16S rDNA sequence was obtained. The α-diversity of each sample was evaluated using the Shannon–Wiener and Simpson’s diversity indexes, and the significance of differences was tested using either the nonparametric Mann–Whitney U test or the Kruskal–Wallis rank-sum test. Principal coordinates analysis (PCoA) and the calculation of the linear discriminant analysis effect size (LEfSe) were performed using the R software package (http://www.R-project.org/); heatmaps were also obtained using the R software. Spearman correlation analysis was also carried out to assess the relationships between relevant parameters.
A total of ten sepsis patients, ten non-sepsis patients, and ten healthy individuals were included in this study. Table 1 shows that there were no significant differences with respect to age or body mass index among the groups, as revealed by variance analysis (P > 0.05). The underlying diseases of the patients in the sepsis and non-sepsis groups were divided into three categories: Multiple injuries, malignant tumors, and other diseases (including benign tumors and intestinal obstruction). Of note, there were no significant differences in the underlying diseases and operation types between the sepsis and non-sepsis groups as revealed by the χ2 test (P > 0.05), indicating that the groups were matched (and accurate comparisons were possible). The APACHE II and SOFA scores in the sepsis group were significantly higher than those in the non-sepsis group on day 1 of ICU admission (P < 0.05), suggesting that the patients in the sepsis group had worse pathology (with acute organ dysfunction) compared with those in the non-sepsis group.
A total of 49 fecal samples were included for analysis. Of note, one stool sample in the sepsis group (patient S3, day 3) was missing owing to a problem in specimen collection. After quality control procedures, 629665 high-quality sequences were obtained, and the qualified reads were clustered into 440 species-level OTUs using 97% as the similarity cutoff. In this study, the number of qualified reads of inpatients in the ICU was generally much lower than that of healthy controls. To understand whether these differences were due to low bacterial numbers, we randomly selected five specimens from each group to quantify the total bacteria in the feces. Regardless of whether bacteria were counted as bacterial copy number per gram fecal samples (mean bacterial copy numbers in sepsis, non-sepsis, and healthy control groups, 5.07E + 11, 1.36E + 12, and 9.27E + 12, respectively) or per nanogram bacterial DNA (9.02E + 06, 3.54E + 07, and 4.90E + 07, respectively), there was a significant overall decreasing trend in both the sepsis and non-sepsis groups relative to that in healthy controls. Therefore, altogether, these results suggest that the low number of qualified reads in some samples in the sepsis and non-sepsis group is secondary to the significantly reduced number of intestinal bacteria, probably because of the use of large amounts of antibiotics in the ICU.
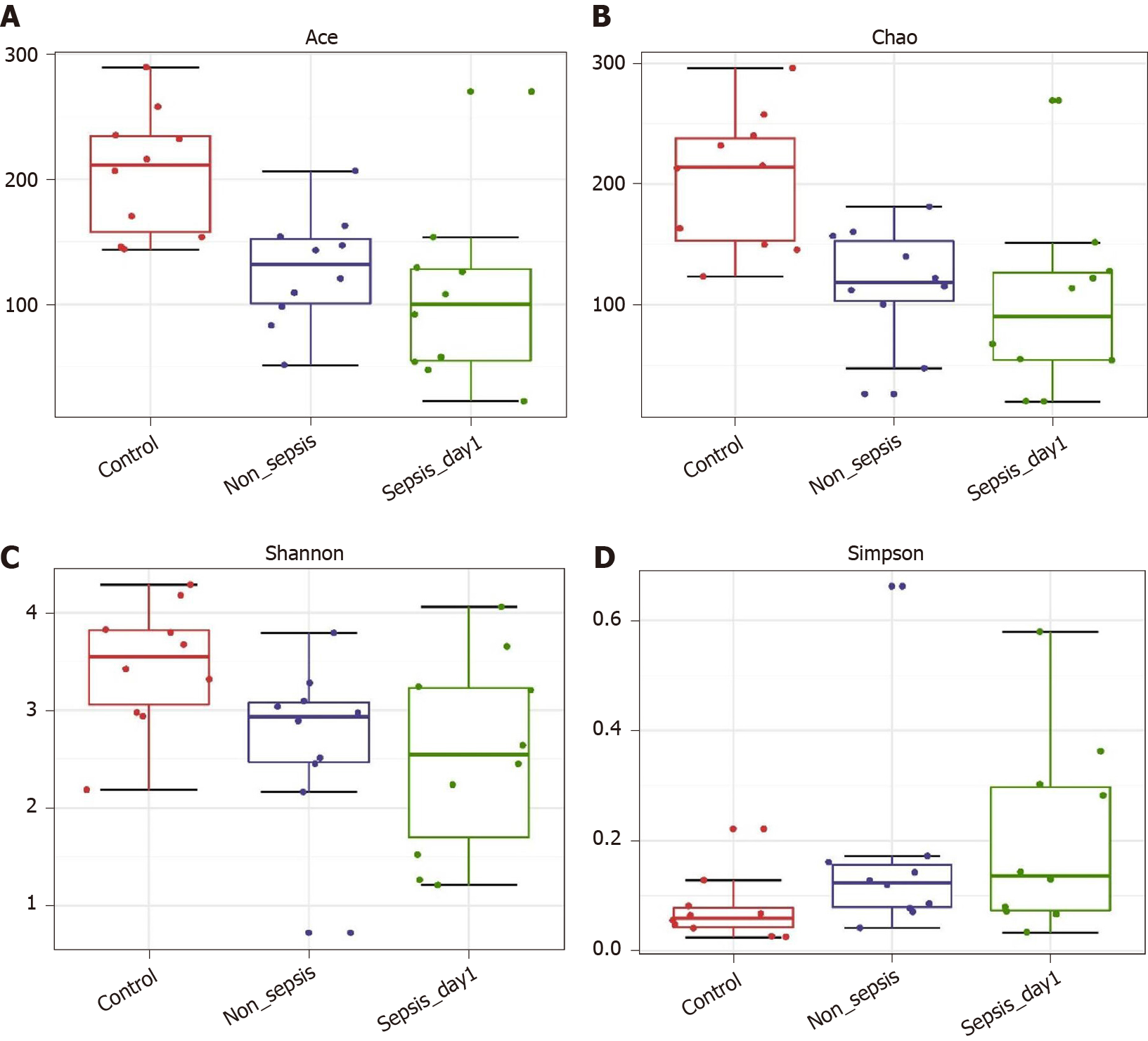
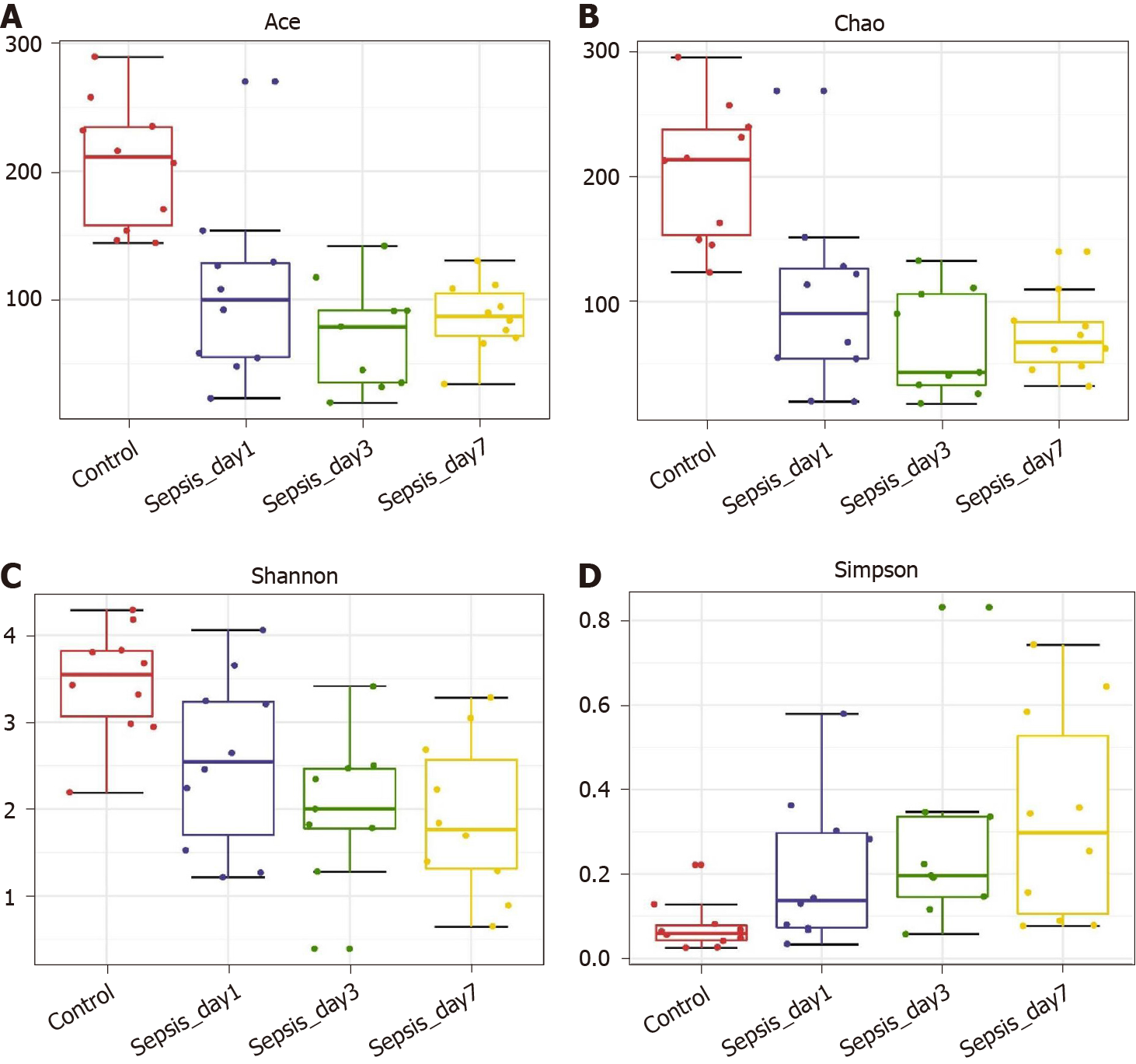
α-diversity: The Mann–Whitney test showed that the α-diversity in the non-sepsis and sepsis groups on day 1 after ICU admittance was significantly lower than that in the control group (P < 0.05). Moreover, the diversity of the intestinal flora in sepsis patients was lower than that in non-sepsis patients; however, this difference was not significant (Figure 1 and Table 4).
| α-diversity index | P value | ||
| Control-median vs non-sepsis-median | Control-median vs sepsis-median | Non-sepsis-median vs sepsis-median | |
| Ace | 0.003 | 0.002 | 0.247 |
| Chao | 0.002 | 0.003 | 0.393 |
| Shannon | 0.023 | 0.035 | 0.795 |
| Simpson | 0.035 | 0.023 | 0.739 |
| Observed OTUs | 0.001 | 0.002 | 0.623 |
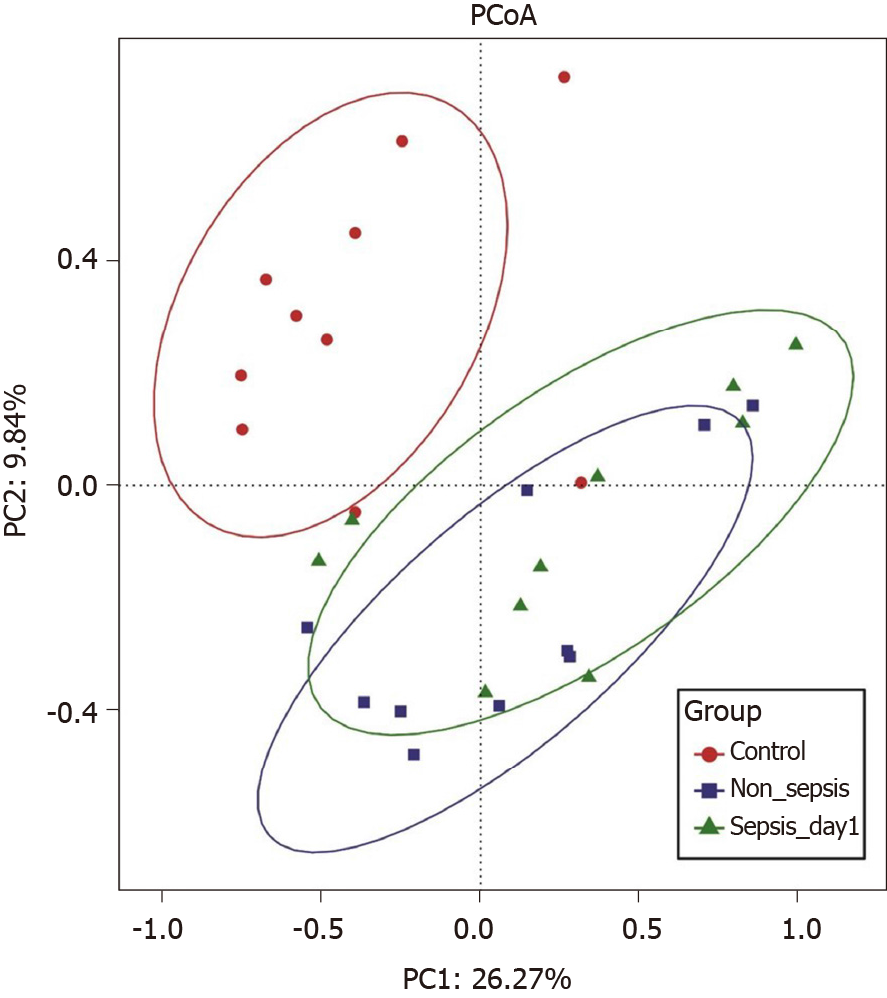
Overall structural comparison of the intestinal flora: The PCoA plot based on the Bray–Curtis dissimilarity between samples revealed that the flora composition in sepsis patients was similar to that in non-sepsis patients on day 1 after ICU admittance (Figure 2). In line with these results, Adonis analysis showed no significant difference in the composition of the intestinal flora between sepsis and non-sepsis patients on day 1 after ICU admittance (P > 0.05). However, the difference in the flora composition between these groups and normal controls was extremely significant (Adonis P < 0.05).
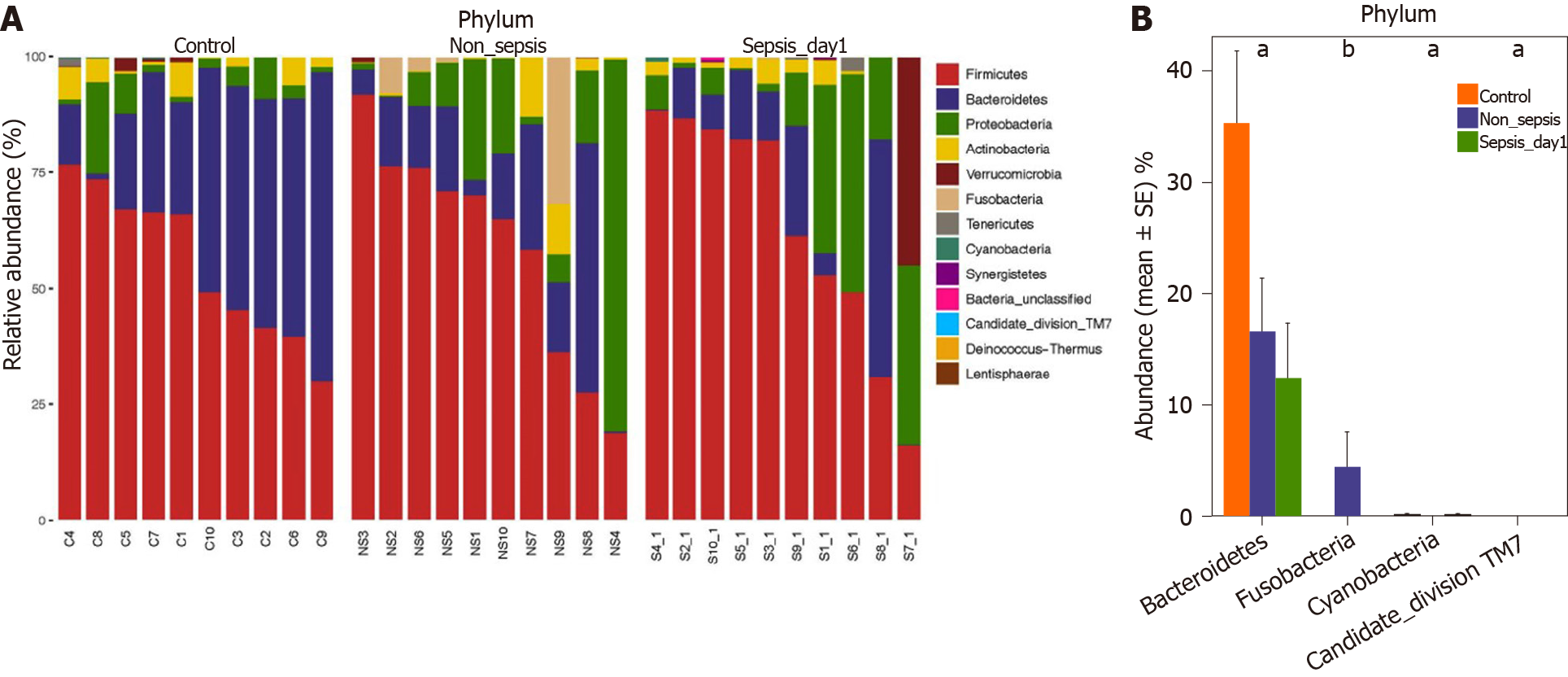
Comparison of intestinal flora compositions at the phylum level: The flora composition results revealed that Firmicutes and Bacteroidetes were the dominant phyla in healthy individuals, accounting for more than 70% of the total bacteria. Meanwhile, in sepsis and non-sepsis patients, Firmicutes was the dominant phylum in the intestinal flora (Figure 3A); the proportion of Bacteroidetes decreased significantly compared to that in healthy individuals (P < 0.05), and the relative abundance of Proteobacteria increased compared to that in normal controls, although the difference was not significant. Of note, the proportion of Fusobacteria in the intestinal tract of non-sepsis patients was significantly higher than that in both healthy controls and sepsis patients (P < 0.05; Figure 3B).
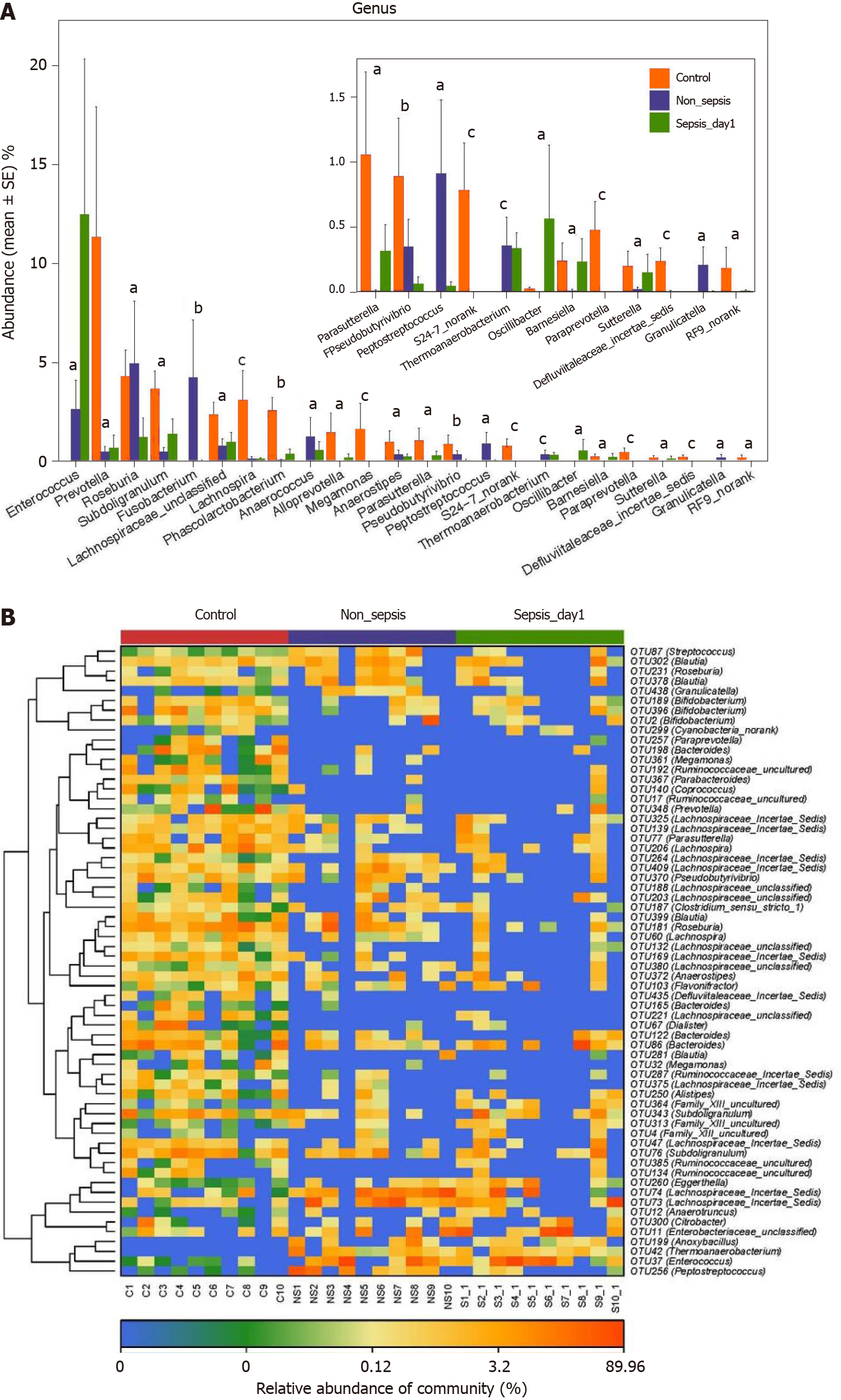
Comparison of intestinal flora compositions at the genus and OTU levels: The results of the Kruskal–Wallis test showed that the proportions of Prevotella, Subdoligranulum, Lachnospira, Phascolarctobacterium, Alloprevotella, Megamonas, and Para-sutterella in the intestinal tract of patients in the non-sepsis and sepsis groups decreased significantly compared to those in the healthy control group (P < 0.05). Furthermore, the abundance of Enterococcus was much higher in the intestinal tract of sepsis patients than in non-sepsis patients and healthy controls. In contrast, the abundance of Fusobacterium, Anaerococcus, and Peptostreptococcus was significantly higher in the intestinal tract of non-sepsis patients than in healthy controls and sepsis patients (Figure 4A).
Additionally, as per the heatmap analysis (random forest-based), 64 key OTUs were different among the three groups (Figure 4B). Among these, the relative abundance of OTUs assigned to the genera Subdoligranulum, Alistipes, Megamonas,
α-diversity: The Mann–Whitney test showed that the α-diversity in the sepsis group decreased significantly within 1 wk (from days 1 to 7 after the initiation of ICU treatment) compared to that in the control group; importantly, these differences were significant (P < 0.05). Of note, on days 1, 3, and 7 of treatment, the α-diversity of the intestinal flora decreased gradually, but pairwise comparisons did not show significant differences. This suggests that antibiotics killed many intestinal bacteria, and that the diversity of the intestinal flora not only did not recover but also showed a downward trend when antibiotics were continuously used (Figure 5 and Table 5).
| α-diversity index | P value | ||
| Day 1 vs day 3 | Day 1 vs day 7 | Day 3 vs day 7 | |
| Ace | 0.066 | 0.121 | 0.347 |
| Chao | 0.205 | 0.384 | 0.595 |
| Shannon | 0.653 | 0.307 | 0.487 |
| Simpson | 0.595 | 0.241 | 0.347 |
| Observed OTUs | 0.177 | 0.384 | 0.623 |
Overall intestinal flora composition changes in sepsis patients within 1 wk after treatment: The PCoA plot based on Bray–Curtis dissimilarities between samples showed that the intestinal flora composition in sepsis patients within 1 wk of treatment (from days 1 to 7 after ICU admittance) was different from that in normal controls (Figure 6); Adonis analysis showed that the difference between sepsis patients and normal controls was extremely significant (P < 0.05). However, the structure of the intestinal flora of sepsis patients did not differ significantly with respect to the different days after ICU admittance in the sepsis group.
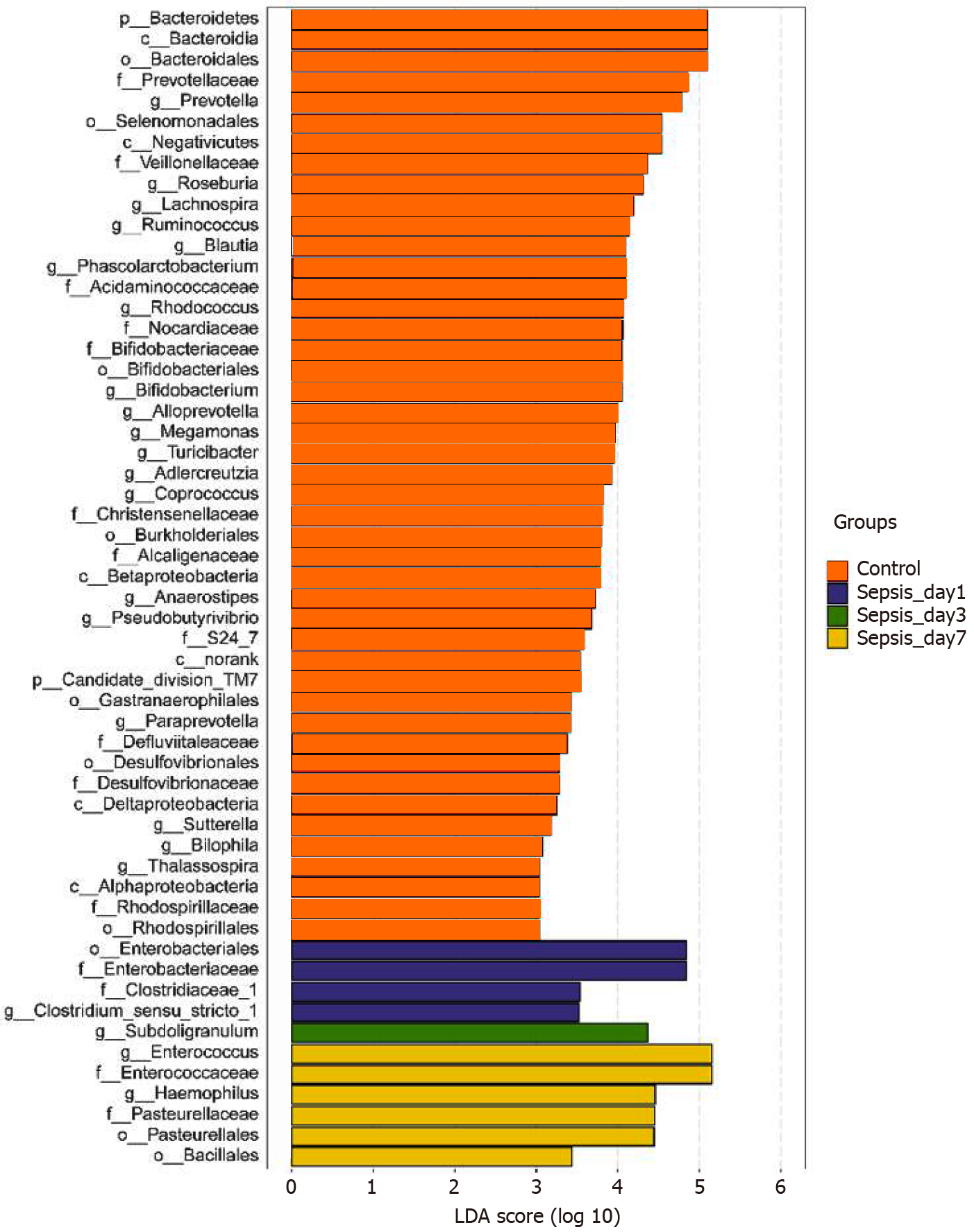
Identification of differential bacterial taxa between sepsis patients 1 wk after treatment and normal individuals: To screen for the bacterial genera associated with significant differences in the intestinal flora of sepsis patients within 1 wk of admission to the ICU, we reduced the dimensions via the calculation of LEfSe to obtain the linear discriminant analysis scores. Compared to normal controls, in sepsis patients, some harmful bacteria such as Coprococcus disappeared from the intestinal flora within 1 wk of ICU treatment, indicating the efficacy of drugs against pathogenic bacteria (Figure 7). At the same time, the abundance of most of the beneficial bacteria, such as Prevotella and Bifidobacterium, also decreased, indicating that the effect of drug treatment, especially antibiotics, was not limited to harmful bacteria. However, the abundance of some bacteria, such as Enterococcus and Hemophilus, still increased despite the action of antibiotics, indicating that these bacteria were resistant to the antibiotics used.
There were ten patients in the sepsis group (S1–S10). Venous blood samples were collected on days 1, 3, and 7 after admission to the ICU to determine the levels of permeability-related D-Lac, endotoxin, and DAO and infection-related PCT. In the blood of sepsis patients, the levels of D-Lac, endotoxin, DAO, and PCT were 20.96 ± 11.90 mg/L, 10.65 ± 7.92 U/L, 19.58 ± 17.61 U/L, and 7.23 ± 13.92 ng/mL, respectively.
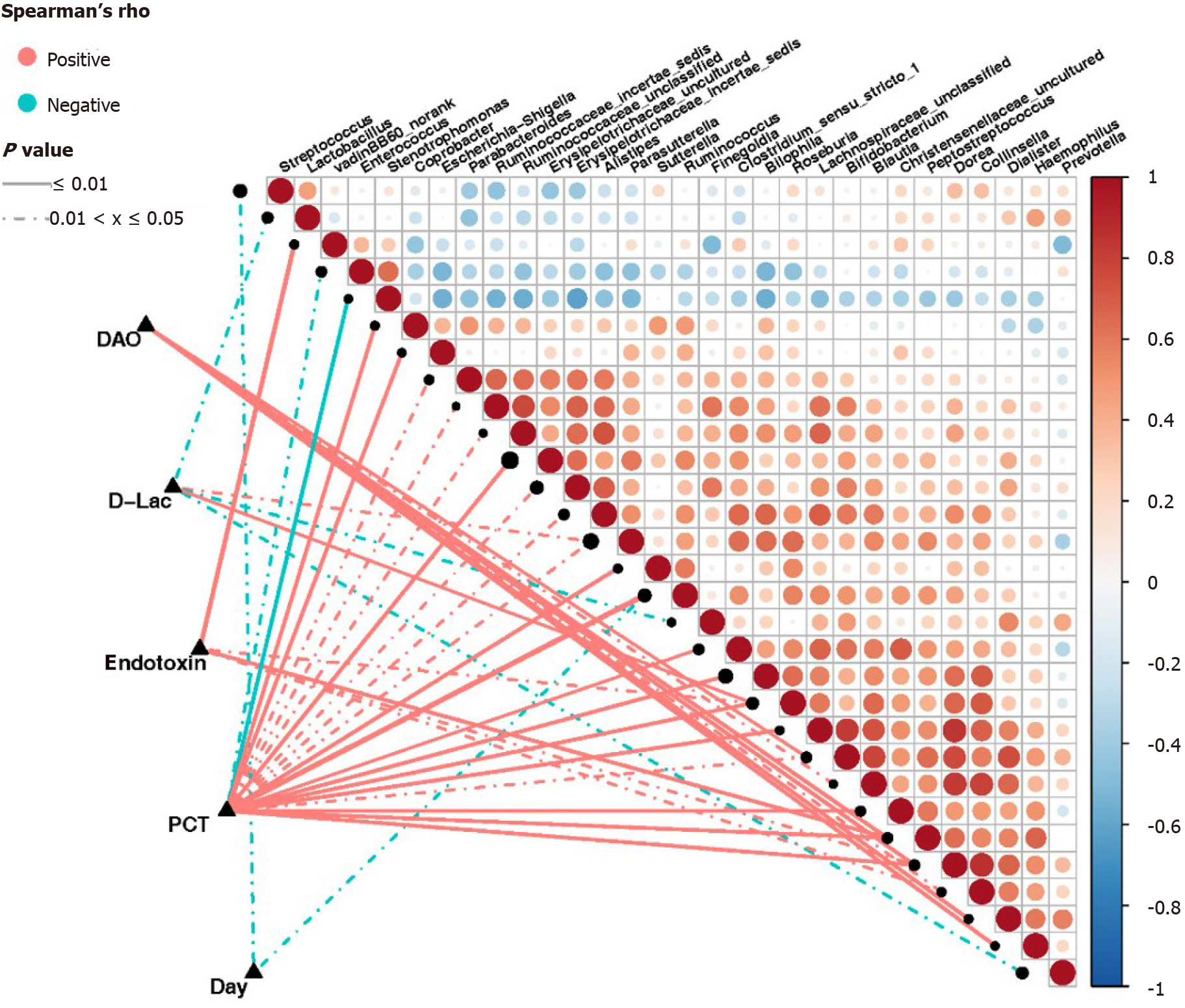
Based on the Spearman correlation analysis, significant correlations were established between clinical indicators and abundance of intestinal bacterial genera in sepsis patients (Figure 8). The number of days of admission in the ICU negatively correlated with the abundance of Streptococcus and Ruminococcus (P < 0.05). The abundance of Roseburia and Parasutterella was positively correlated (P < 0.05), while that of Prevotella, Lactobacillus, and Finegoldia was negatively correlated with the levels of D-Lac (P < 0.05). Additionally, the abundance of Ruminococcus, Roseburia, Sutterella, Peptostreptococcus, Escherichia-Shigella, Dorea, Bilophila, Coprobacter, Clostridium_sensu_ stricto_1, Parabacteroides, Bifidobacterium, Alistipes, and Parasutterella, as well as some genera belonging to Lachnospiraceae, Christensenellaceae, Ruminococcaceae, and Erysipelotrichaceae, was positively correlated with the levels of PCT (P < 0.05). Conversely, the abundance of Stenotrophomonas and Enterococcus was negatively correlated with the levels of PCT (P < 0.05). The abundance of Peptostreptococcus, Roseburia, Collinsella, Dorea, and VadinBB60 group norank was also positively correlated with the endotoxin levels (P < 0.05), while that of Dialister, Pepto streptococcus, with the endotoxin levPeptostreptococcus, Dorea,Hemophilus, Bifidobacterium, Collinsella, and Blautia was positively correlated with the levels of DAO (P < 0.05).
Sepsis is associated with high morbidity and mortality worldwide, and its timely identification and treatment are difficult. Therefore, improving the diagnosis and treatment of sepsis is essential from a global health perspective[8]. In February 2016, the SCCM and the European Society of Intensive Care Medicine jointly released the definition of sepsis in “Sepsis 3.0” as follows: “Life-threatening organ dysfunction due to a dysregulated host response to infection”[7]. This definition emphasizes the close relationship between sepsis and organ dysfunction; of note, the intestinal tract is recognized as the “engine” of organ dysfunction. The intestinal flora, as an important component of the intestinal tract, plays major physiological roles in the context of biosynthesis and metabolism; however, critical diseases lead to many changes in the diversity of the intestinal flora, causing excessive growth of pathogenic bacteria[9,10].
By comparing the intestinal flora of sepsis patients and healthy controls, we confirmed that there are marked differences in the abundance, distribution, and structure of the intestinal flora in the context of sepsis. In sepsis patients, normal bacteria decreased or disappeared, while the abundance of abnormal bacteria increased sharply. At the phylum level, the abundance of Firmicutes increased significantly, while that of Bacteroidetes decreased significantly in the intestinal flora of sepsis patients vs non-septic critically ill patients; of note, the number of bacteria in the phylum Fusobacterium also decreased significantly. At the genus level, in addition to the decrease in the abundance of beneficial symbiotic bacteria such as Prevotella and Lachnospira, as well as of other genera, including Fusobacterium and Peptostreptococcus, we found that the abundance of Enterococcus increased significantly in the intestinal flora of sepsis patients vs non-septic critically ill patients. Therefore, our results suggest that dysbiosis in sepsis shows three major features. First, as the abundance and diversity of the intestinal flora decrease, the structure of the intestinal flora changes, and the differences among individuals are greater[11]. Second, the abundance of dominant obligate anaerobes decreases and that of facultative anaerobes increases[11,12]. Third, the abundance of beneficial symbiotic bacteria decreases, while that of pathogenic bacteria increases, possibly becoming predominant[11,13].
The occurrence of intestinal microecological disorders in sepsis patients is not surprising. First, the physiological state of sepsis patients is completely different from that at homeostasis; specifically, intestinal hypoperfusion and reperfusion injury lead to changes in the intestinal environment and blood supply to the intestinal mucosa, resulting in intestinal mucosal inflammation and, consequently, a series of changes in the intestinal environment, such as increased nitrate concentrations[11] and altered mucosal oxygen gradient[12]. These changes are favorable to the growth of Proteobacteria, leading to the expansion of many clinically familiar pathogenic Gram-negative bacilli, such as Pseudomonas aeruginosa and Escherichia coli, as well as Staphylococcus aureus and Enterococcus[14,15]. Second, ICU patients are exposed to various endogenous regulators (such as increased catecholamine production and changes in glucose metabolism) and clinical interventions (such as proton pump inhibitors, opioids, nutritional support, and antibiotics), which affect the living environment of the intestinal flora to varying degrees and, thus, affect the flora structure[16]. Finally, the intestinal mucosa will be damaged and thinned in critically ill patients[17,18]; such mucosal damage leads to the loss of the normal habitat of symbiotic microorganisms, subsequently leading to intestinal microecological disorders.
Our study suggests that the abundance, distribution, and diversity of the intestinal flora in sepsis patients do not change significantly within 1 wk of ICU admittance. However, in relation to the gut microbiome of healthy subjects, the numbers of most beneficial bacteria, such as Prevotella and Bifidobacterium, were below the detection limit in sepsis patients, whereas the abundance of infection-related bacteria, such as Enterococcus and Hemophilus, increased despite the action of antibiotics (with the exception of Coprococcus, which decreased in abundance to below the detection limit). These results indicate that patients with intestinal flora disorders associated with sepsis could not recover in a short time, and while the drugs were effective against the bacteria causing infection, they also affected bacteria in the intestines. In fact, the effects of drug treatment, especially antibiotics, on bacteria were not limited to harmful bacteria; the proportion of beneficial intestinal bacteria also decreased. Other studies have also shown that the long-term use of antibiotics changes the normally healthy intestinal flora and leads to the emergence of drug resistance; worrisome enough, the long-term use of antibiotics has the potential to generate organism reservoirs with a multi-drug resistance gene pool[19]. Ma et al[20] used rat models with third-degree burns on 30% of the total surface area of the back and quantified/ identified the intestinal bacteria after treatment. The results showed that the number of cocci in the gastrointestinal contents of rats increased significantly, and the coccus/bacillus ratio was seriously inverted after treatment with Rocephin. It was considered that broad-spectrum antibiotics destroyed the intestinal microecological balance; of note, the conditional pathogenic intestinal flora showed potential to affect health, disease, and drug action. Our study also revealed that the abundance of Enterococcus increased significantly in sepsis patients, which might be related to the use of broad-spectrum antibiotics.
Our study showed a clear correlation between the abundance of intestinal flora components and clinical indicators in sepsis. The clinical indicators used in this study were D-Lac, endotoxin, DAO, and PCT. Under normal circumstances, D-Lac is produced via the methylglyoxal metabolism, and its content in the blood is very small. However, when glycolysis results in increases in a large number of gastrointestinal bacteria and the intestinal barrier function is impaired, the content of D-Lac increases sharply. Endotoxins are cell wall components of Gram-negative bacteria that are released only when bacteria are lysed and die. DAO is a highly active intracellular enzyme in the upper villi of the intestinal mucosa of mammals, including humans. Its activity is closely associated with nucleic acid and protein synthesis in mucosal cells. Therefore, the above three indicators can reflect the integrity and damage degree of the intestinal mechanical barrier. Additionally, PCT reflects the active level of the systemic inflammatory response, and the factors affecting its levels include the size and type of the infected organ, the type of pathogenic bacteria, the degree of inflammation, and the state of immune responses. Our study showed that serum PCT, endotoxin, DAO, and D-Lac levels in patients with sepsis were correlated with the abundance of various intestinal bacterial genera. Of note, some of these genera were correlated with multiple clinical indicators at the same time. For example, the abundance of Peptostreptococcus and Dorea was positively correlated with the serum levels of PCT, endotoxin, and DAO; moreover, the abundance of Roseburia was positively correlated with the serum levels of PCT, endotoxin, and D-Lac.
Among these genera, the abundance of Ruminococcus had the highest positive correlation with PCT. Ruminococcus is an important constituent of the normal intestinal microbiota. A study published in 2017[21] using samples from young Han Chinese individuals revealed changes in the intestinal flora of the Chinese population and showed that the genera Ruminococcus and Fusobacterium, among others, were relatively highly abundant in obese Chinese individuals, whereas the abundance of Bacteroides was greatly reduced. Of note, two Ruminococcus species, Ruminococcus torques and Ruminococcus gnavus, should be mentioned because they are related to inflammatory bowel disease[22] and metabolic disorder[23]. Importantly, from the significant decrease in the number and abundance of intestinal bacteria, including the decrease in abundance of the phylum Bacteroidetes, the increase in abundance of the phylum Verrucomicrobia, and the increase in abundance of the genus Ruminococcus in sepsis patients, we can establish a parallel with the results of the study of obese Chinese individuals. In clinical practice, it is known that obese individuals have more significant characteristics of insulin resistance and dyslipidemia, with a more significant inflammatory phenotype, which are risk factors for cardiovascular diseases, diabetes, osteoporosis, and some cancers among other diseases[24]. Interestingly, patients with sepsis also have clinical manifestations such as insulin resistance, dyslipidemia, and inflammatory responses, which have adverse effects on the severity of illness and prognosis[25]. Therefore, we boldly speculate that the intestinal phenotype of obese patients makes them more susceptible to the complications of sepsis, which might be of great significance for the treatment and prevention of sepsis. Of course, the sample size in this study was small, and thus, it is necessary to conduct larger, multicenter studies to support our findings; moreover, the proposed mechanisms require further validation in animal studies for clarification.
There is a correlation between intestinal flora disorders and intestinal barrier dysfunction in sepsis patients. Our research showed that the abundance of Roseburia in the intestinal tract of sepsis patients was definitely related to the levels of serum markers of intestinal barrier function. Roseburia is one of the main bacterial genera producing butyrate in the human intestinal flora[26,27]. Butyrate is the main energy source of colonic epithelial cells. Research suggests that the abundance of bacteria producing butyrate in the intestinal tract of critically ill patients decreases or disappears, which leads to a decrease in butyrate production and the apoptosis of intestinal epithelial cells due to “starvation”[26]. Importantly, one study showed that the genus Roseburia can improve the intestinal ecosystem, prevent intestinal leakage, and reduce the incidence of diabetes[27]. Conversely, other studies have shown that the abundance of Roseburia is significantly reduced in patients with Crohn’s disease[28] and inflammatory bowel disease[29]. Our study showed that the abundance of intestinal Roseburia was positively correlated with serum PCT, endotoxin, and D-Lac levels in sepsis patients, indicating that the intestinal barrier function is more severely damaged and the systemic inflammatory response is more serious with a higher content of Roseburia, which seems to contradict the results of existing research. We speculate that systemic inflammatory responses in sepsis patients cause intestinal damage and that the body has a self-regulatory effect on this damage. In addition, a recently published study[30] showed that immune cells and autoantibodies of patients with antiphospholipid syndrome (an autoimmune disease) could cross-react with the mimic epitopes of Roseburia; in line with this, Roseburia could also cause autoimmune diseases in susceptible mice. Once again, these results suggest that the positive and negative effects of intestinal bacteria are not invariable.
Bacteria can perceive the changes in the host internal environment and then change their own toxic factors to become pathogenic. Under various stimuli such as sepsis, trauma, and burns, a healthy bacterial strain might be transformed into a pathogenic bacterium within a few hours[31]. For example, Pseudomonas aeruginosa was injected into the ceca of mice in a sham operation group for culture, and then, the bacteria were collected and implanted into the undamaged abdominal cavity of mice; it was found that no death occurred. However, the same strain was injected into the ceca of mice after 30% hepatectomy for culture, and then were collected and implanted into the undamaged abdominal cavities of mice, which led to their deaths[32]. Although the mechanisms underlying such transformations remain inconclusive, these results suggest that the host environment can not only change the diversity of bacterial species but also change their virulence. Therefore, the beneficial or harmful effects of a specific bacterium are not absolute; they can be influenced by many factors.
In this study, we report that sepsis patients in the ICU showed intestinal microecological disorders, lasting for at least 1 wk. Furthermore, intestinal microecological disorder was correlated with inflammation-related and intestinal barrier-related indexes in sepsis. However, this study is not without limitations. The sample size was small, and thus, it is necessary to carry out larger and multicenter studies to support these findings. Additional animal studies might be needed to clarify the related molecular mechanisms.
Sepsis is a common disease in intensive care units, with high morbidity and mortality. Intestinal microecology plays a vital part in the development and progression of this disease, possibly because sepsis and its treatment cause specific changes to the intestinal flora. However, there are few studies on the sepsis-related intestinal flora and its dynamic changes. An improved understanding of the status and degree of intestinal flora disturbances in sepsis patients is of great significance, to allow for the accurate evaluation of the disease condition and prognosis and to optimize the treatment measures.
Studies have shown a dose–response relationship between the degree of intestinal microecological disturbance and the incidence of subsequent severe sepsis. Critical illness leading to abnormal changes in the types, quantities, proportions, and locations of microorganisms in the intestinal flora may, thus, increase susceptibility to sepsis. Therefore, an improved understanding of the status and degree of intestinal flora disturbances in sepsis patients is of great clinical significance.
The main objective of this study was to investigate the characteristics of intestinal flora disturbance in sepsis patients treated with antibiotics.
We enrolled ten patients with sepsis admitted to the intensive care unit (ICU), ten patients without sepsis admitted to the ICU in the same period, and ten healthy individuals (sepsis group, non-sepsis group, and control group, respectively). Using 16S rRNA gene sequencing technology, the fecal samples of the three groups were analyzed, and the intestinal flora diversity, structure, and composition were compared. The fecal samples of sepsis patients on days 1, 3, and 7 after ICU admittance were also analyzed, and the dynamics of the diversity, structure, and composition of the intestinal flora of sepsis patients were compared. Lastly, the serum levels of procalcitonin, endotoxin, diamine oxidase, and D-lactic acid were determined in sepsis patients on days 1, 3, and 7 after ICU admittance and correlated with the abundance of intestinal bacteria.
Sepsis patients showed a reduced intestinal flora α-diversity and a different flora structure, with Firmicutes as the dominant bacteria, and significantly decreased proportions of Bacteroidetes, as well as Prevotella, Lachnospira, and other genera. Enterococcus was significantly increased in the intestinal tract of sepsis patients. Additionally, from days 1 to 7 of treatment, the α-diversity of the intestinal flora in the sepsis group decreased gradually, although without statistical significance. Of note, some harmful bacteria such as Coprococcus disappeared, the abundance of beneficial bacteria such as Prevotella and Bifidobacterium decreased, while that of Enterococcus and other genera increased. Interestingly, the serum levels of procalcitonin, endotoxin, diamine oxidase, and D-lactic acid in sepsis patients correlated with the abundance of various intestinal bacterial genera.
In this study, we report the characteristics of sepsis intestinal flora disturbance and reveal, for the first time, the dynamic characteristics of the intestinal flora in sepsis patients under antibiotic treatment. Altogether, our results suggest that sepsis patients in the ICU show intestinal microecological disorders, lasting for at least 1 wk. Importantly, we also show that the intestinal microecological disorder in sepsis patients is correlated with inflammation-related and intestinal barrier-related indexes. Of note, the sample size of this study was small, and thus, it is necessary to conduct larger and multicenter studies to support these findings.
We plan to carry out animal studies to clarify the molecular mechanisms of intestinal flora disturbance in sepsis.
We thank all the patients who participated in this study and the physicians in the Department of Critical Care Medicine, General Hospital, Ningxia Medical University. We also thank Shanghai Mobio Biomedical Technology Co. for technical support and to Ren HY for her help in writing the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang Z S-Editor: Liu M L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381:774-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 494] [Article Influence: 41.2] [Reference Citation Analysis (1)] |
| 2. | O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1786] [Article Influence: 94.0] [Reference Citation Analysis (2)] |
| 3. | Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28:1221-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 784] [Article Influence: 71.3] [Reference Citation Analysis (1)] |
| 4. | McDonald D, Ackermann G, Khailova L, Baird C, Heyland D, Kozar R, Lemieux M, Derenski K, King J, Vis-Kampen C, Knight R, Wischmeyer PE. Extreme Dysbiosis of the Microbiome in Critical Illness. mSphere. 2016;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 286] [Article Influence: 31.8] [Reference Citation Analysis (1)] |
| 5. | Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ. Hospitalization Type and Subsequent Severe Sepsis. Am J Respir Crit Care Med. 2015;192:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 6. | Suau A, Bonnet R, Sutren M, Godon JJ, Gibson GR, Collins MD, Doré J. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65:4799-4807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 979] [Article Influence: 37.7] [Reference Citation Analysis (1)] |
| 7. | Bassetti M, Vena A, Meroi M, Cardozo C, Cuervo G, Giacobbe DR, Salavert M, Merino P, Gioia F, Fernández-Ruiz M, López-Cortés LE, Almirante B, Escolà-Vergé L, Montejo M, Aguilar-Guisado M, Puerta-Alcalde P, Tasias M, Ruiz-Gaitán A, González F, Puig-Asensio M, Marco F, Pemán J, Fortún J, Aguado JM, Soriano A, Carratalá J, Garcia-Vidal C, Valerio M, Sartor A, Bouza E, Muñoz P. Factors associated with the development of septic shock in patients with candidemia: a post hoc analysis from two prospective cohorts. Crit Care. 2020;24:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 8. | Klingensmith NJ, Coopersmith CM. The Gut as the Motor of Multiple Organ Dysfunction in Critical Illness. Crit Care Clin. 2016;32:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 271] [Article Influence: 30.1] [Reference Citation Analysis (4)] |
| 9. | Shimizu K, Ogura H, Goto M, Asahara T, Nomoto K, Morotomi M, Yoshiya K, Matsushima A, Sumi Y, Kuwagata Y, Tanaka H, Shimazu T, Sugimoto H. Altered gut flora and environment in patients with severe SIRS. J Trauma. 2006;60:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 10. | Lankelma JM, van Vught LA, Belzer C, Schultz MJ, van der Poll T, de Vos WM, Wiersinga WJ. Critically ill patients demonstrate large interpersonal variation in intestinal microbiota dysregulation: a pilot study. Intensive Care Med. 2017;43:59-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (1)] |
| 11. | Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, Bäumler AJ. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339:708-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 748] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 12. | Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H, Thom SR, Bushman FD, Vinogradov SA, Wu GD. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 2014; 147: 1055-63. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 626] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 13. | Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 761] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 14. | Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 604] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 15. | Grootjans J, Lenaerts K, Derikx JP, Matthijsen RA, de Bruïne AP, van Bijnen AA, van Dam RM, Dejong CH, Buurman WA. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol. 2010;176:2283-2291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Haak BW, Levi M, Wiersinga WJ. Microbiota-targeted therapies on the intensive care unit. Curr Opin Crit Care. 2017;23:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Lu Q, Xu DZ, Sharpe S, Doucet D, Pisarenko V, Lee M, Deitch EA. The anatomic sites of disruption of the mucus layer directly correlate with areas of trauma/hemorrhagic shock-induced gut injury. J Trauma. 2011;70:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Rupani B, Caputo FJ, Watkins AC, Vega D, Magnotti LJ, Lu Q, Xu DZ, Deitch EA. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery. 2007;141:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787-8803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1421] [Cited by in RCA: 1838] [Article Influence: 183.8] [Reference Citation Analysis (58)] |
| 20. | Ma LQ, Chen DC, Liu S. Selective action of broad-spectrum antibiotics on intestinal flora in sepsis in rats. Zhongguo Weizhongbing Jijiuyixue. 2007;19:456-459. [DOI] [Full Text] |
| 21. | Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, Shi J, Zhao S, Liu W, Wang X, Xia H, Liu Z, Cui B, Liang P, Xi L, Jin J, Ying X, Zhao X, Li W, Jia H, Lan Z, Li F, Wang R, Sun Y, Yang M, Shen Y, Jie Z, Li J, Chen X, Zhong H, Xie H, Zhang Y, Gu W, Deng X, Shen B, Yang H, Xu G, Bi Y, Lai S, Wang J, Qi L, Madsen L, Ning G, Kristiansen K, Wang W. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1032] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 22. | Peterson CT, Sharma V, Elmén L, Peterson SN. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol. 2015;179:363-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 23. | Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T; MetaHIT consortium; Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2727] [Cited by in RCA: 3215] [Article Influence: 267.9] [Reference Citation Analysis (2)] |
| 24. | Yu E, Malik VS, Hu FB. Cardiovascular Disease Prevention by Diet Modification. J Am Coll Cardiol. 2018;72:914-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 25. | Golucci APBS, Marson F, Ribeiro AF, Nogueira RJN. Lipid profile associated with the systemic inflammatory response syndrome and sepsis in critically ill patients. Nutrition. 2018;55-56:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1507] [Cited by in RCA: 1353] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 27. | Seo B, Jeon K, Moon S, Lee K, Kim WK, Jeong H, Cha KH, Lim MY, Kang W, Kweon MN, Sung J, Kim W, Park JH, Ko G. Roseburia spp. Abundance Associates with Alcohol Consumption in Humans and Its Administration Ameliorates Alcoholic Fatty Liver in Mice. Cell Host Microbe 2020; 27: 25-40. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 28. | Quan Y, Song K, Zhang Y, Zhu C, Shen Z, Wu S, Luo W, Tan B, Yang Z, Wang X. Roseburia intestinalis-derived flagellin is a negative regulator of intestinal inflammation. Biochem Biophys Res Commun. 2018;501:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Ananthakrishnan AN, Luo C, Yajnik V, Khalili H, Garber JJ, Stevens BW, Cleland T, Xavier RJ. Gut Microbiome Function Predicts Response to Anti-integrin Biologic Therapy in Inflammatory Bowel Diseases. Cell Host Microbe 2017; 21: 603-610. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 328] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 30. | Ruff WE, Dehner C, Kim WJ, Pagovich O, Aguiar CL, Yu AT, Roth AS, Vieira SM, Kriegel C, Adeniyi O, Mulla MJ, Abrahams VM, Kwok WW, Nussinov R, Erkan D, Goodman AL, Kriegel MA. Pathogenic Autoreactive T and B Cells Cross-React with Mimotopes Expressed by a Common Human Gut Commensal to Trigger Autoimmunity. Cell Host Microbe 2019; 26: 100-113. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 31. | Hayakawa M, Asahara T, Henzan N, Murakami H, Yamamoto H, Mukai N, Minami Y, Sugano M, Kubota N, Uegaki S, Kamoshida H, Sawamura A, Nomoto K, Gando S. Dramatic changes of the gut flora immediately after severe and sudden insults. Dig Dis Sci. 2011;56:2361-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Babrowski T, Romanowski K, Fink D, Kim M, Gopalakrishnan V, Zaborina O, Alverdy JC. The intestinal environment of surgical injury transforms Pseudomonas aeruginosa into a discrete hypervirulent morphotype capable of causing lethal peritonitis. Surgery. 2013;153:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |