Published online May 14, 2021. doi: 10.3748/wjg.v27.i18.2090
Peer-review started: January 3, 2021
First decision: January 23, 2021
Revised: February 27, 2021
Accepted: April 5, 2021
Article in press: April 5, 2021
Published online: May 14, 2021
Processing time: 126 Days and 23.5 Hours
Hepatitis E virus (HEV) is an important cause of repeated waterborne outbreaks of acute hepatitis. Recently, several extrahepatic manifestations (EHMs) have been described in patients with HEV infection. Of these, neurological disorders are the most common EHM associated with HEV. The involvement of both the peripheral nervous system and central nervous system can occur together or in isolation. Patients can present with normal liver function tests, which can often be misleading for physicians. There is a paucity of data on HEV-related neurological manifestations; and these data are mostly described as case reports and case series. In this review, we analyzed data of 163 reported cases of HEV-related neurological disorders. The mechanisms of pathogenesis, clinico-demographic profile, and outcomes of the HEV-related neurological disorders are described in this article. Nerve root and plexus disorder were found to be the most commonly reported disease, followed by meningoencephalitis.
Core Tip: Neurological involvement in patients with hepatitis E virus (HEV) infection is rare. There is a paucity of data on HEV-related neurological manifestations. This review comprehensively describes the mechanisms of pathogenesis, clinico-demographic profile, and outcomes of HEV-related neurological disorders. Nerve root and plexus disorder were the most commonly reported diseases followed by meningoencephalitis. These patients can present with normal liver function tests, which can often be misleading for physicians.
- Citation: Jha AK, Kumar G, Dayal VM, Ranjan A, Suchismita A. Neurological manifestations of hepatitis E virus infection: An overview. World J Gastroenterol 2021; 27(18): 2090-2104
- URL: https://www.wjgnet.com/1007-9327/full/v27/i18/2090.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i18.2090
The hepatitis E virus (HEV) is a non-enveloped, positive-sense, single-stranded ribonucleic acid (RNA) virus. It belongs to the Hepeviridae family and is one of the most common causes of acute hepatitis globally. Currently, HEV is represented by one serotype and eight genotypes (GTs)[1]; of these, four GTs (GT1-4) are mainly responsible for human infection. Epidemiological characteristics of GT1-4 of HEV have been summarized in Table 1.
| Genotype | Hosts/reservoir | Mode of transmission | Geographic distribution | Remarks | Reported neurological manifestations1 |
| GT1 | Only humans | Fecal-oral transmission | Mostly Asia and Africa | Large outbreaks; Self-limiting; High mortality in pregnancy | Yes (2 cases) |
| GT2 | Only humans | Fecal-oral transmission | Africa and Mexico | Large outbreaks; Self-limiting; High mortality in pregnancy | None |
| GT3 | Human, swine, wild boar, goat, cattle, deer, camel and yak | Zoonotic infections in humans; Blood transfusion | Worldwide including Europe and America | Can cause chronic infection in organ transplant patients | Yes (56 cases) |
| GT4 | Human, swine, wild boar, goat, cattle, deer, camel and yak | Zoonotic infections in humans; Blood transfusion | China, Japan, South-east Asia | Can cause chronic infection in organ transplant patients | Yes (4 cases) |
HEV is an important cause of repeated waterborne outbreaks of acute hepatitis. While HEV outbreaks have mainly been reported in Asia and Africa, it has recently been identified as an emerging issue in public health in Western countries. However, the global burden of HEV infection remains unclear. The recent meta-analysis estimated that approximately 939 million of the global population have experienced HEV infection once, and 15-110 million individuals have had a recent or have an ongoing infection. The seroprevalence rates of HEV infection in Africa, Asia, Europe, and America are 21.76%, 15.80%, 9.31%, and 7%-8% respectively[2]. The World Health Organization (WHO) estimated 14 million symptomatic cases of HEV infection, with 0.3 million deaths and 5.2 thousand stillbirths each year worldwide[3]. More than 50% of global HEV deaths were recorded in the WHO South-East Asia Region (SEAR), which includes India, Bangladesh, Thailand, the Democratic People’s Republic of Korea, Myanmar, Nepal, Bhutan, Sri Lanka, Indonesia, Maldives, and Timor-Leste. In the WHO SEAR countries, annual symptomatic cases of HEV are estimated to be 6.5 million, with 0.16 million deaths and 2.7 thousand stillbirths[3].
Symptomatic HEV infection can present with anicteric or icteric acute hepatitis. It can also rarely lead to fulminant liver failure, and HEV infection further carries high mortality (25%) in pregnancy. While most cases of HEV-related acute hepatitis spontaneously resolve within 4-8 wk, HEV sometimes causes chronic infection, especially in immunosuppressed patients[4]. Chronic HEV infection has been defined as the persistence of HEV replication for more than 6 mo[5]. GT3 and GT4 (mainly GT3) can cause chronic infection, resulting in liver fibrosis and cirrhosis. HEV infection is also an important cause of acute-on-chronic liver failure (ACLF), especially in South-East Asian countries. In our prospective study, we observed that the hepatitis virus infection is the most common acute insult identified in patients with ACLF. Overall, the most common hepatitis virus superinfection was HEV (23% cases)[6]. In another study, Kumar et al[7] demonstrated a 44% HEV superinfection rate in a sample of patients with chronic liver disease.
Recently, several extrahepatic manifestations (EHMs) were described in patients with HEV infection (Table 2). These EHMs were reported as case–control studies and case series or reports during the last decades. In a recent systemic review, Rawla et al[8] analyzed data of 324 reported cases of EHM patients with HEV infection. The most common EHMs were neurological (55%), cardiovascular or hematological (35%), and gastrointestinal manifestations (7%). Rare manifestations included renal (1.24%), endocrine (0.31%), and skin disease (0.31%), as well as manifestations in the respiratory (0.31%), muscular (0.31%), and immune system (0.31%).
| Extrahepatic manifestations | Likelihood of causal relationship, Hill criteria[9,97] | Evidence to support a casual role |
| Neurological disorders | ||
| Neuralgic amyotrophy | Very probable | Good |
| Guillain-Barré syndrome | Very probable | Good |
| Meningoencephaltis | Very probable | Good |
| Others1 | Possible/under debate | Remains to be established |
| Kidney disorders | ||
| Membranoproliferative glomerulonephritis | Very probable | Good |
| Membranous glomerulonephritis | Very probable | Good |
| IgA nephropathy | Very probable | Good |
| Gastrointestinal disorder | ||
| Acute pancreatitis | Very probable | Remains to be established |
| Hematological diseases | ||
| Thrombocytopenia, monoclonal gammopathy of uncertain significance, Cryoglobulinemia, hemolytic anemia, aplastic anemia | Possible | Remains to be established |
| Miscellaneous | ||
| Autoimmune hepatitis, myocarditis, thyroiditis | Doubtful/under debate | Remains to be established |
This review is summarizes the available evidence regarding neurological manifestations in patients with HEV infection. To this end, we analyzed the literature related to the neurological manifestations associated with HEV. These reported manifestations were classified as central nervous system (CNS) disorders, nerve root and plexus disorders, neuropathy, cranial neuropathy, neuromuscular junction disorders, muscle disorders, and other miscellaneous manifestations.
Various EHMs have a temporal relationship with HEV infections; however, whether it is a coincidence or an actual causal relationship remains unclear. A causal relationship between HEV and neurological has been proposed on the basis of a few well-described case-control studies, which showed a significantly higher seroprevalence of HEV-associated neuralgic amyotrophy (NA) and Guillain-Barré syndrome (GBS) compared to non-HEV-associated cases. Moreover, several case studies have documented the presence of HEV RNA in the cerebrospinal fluid (CSF). Although the authors explained this association on the basis of existing evidence, the pathophysiological basis of a causal relationship remains unknown. There is good evidence to support a causal role of HEV in associated conditions such as NA, GBS, meningoencephalitis, membranoproliferative glomerulonephritis, membranous glomerulonephritis, and immunoglobulin (Ig) A nephropathy[5,9]. Relationships between other EHMs and HEV are based on case reports only; therefore, causality has yet to be established (Table 2).
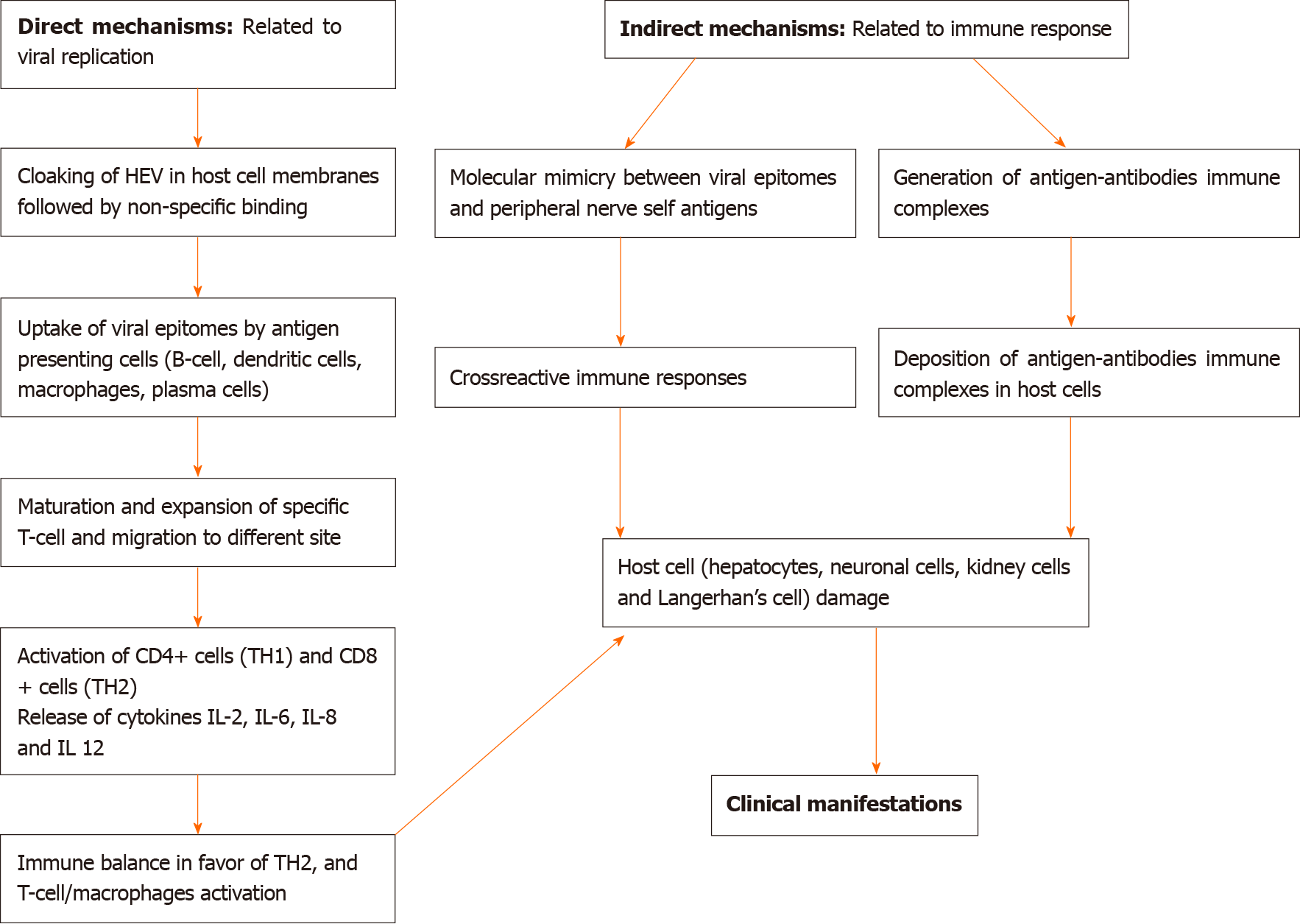
The pathogenesis of EHMs in HEV infection is unclear. The mechanisms by which HEV can induce EHM may be caused by either direct or indirect mechanisms. Direct mechanisms involve HEV replication in affected tissues, resulting in cellular damage, whereas indirect mechanisms are caused by cross-reactive immune triggers, the formation of immune complexes, or by secondary infection[10]. Direct mechanisms are supported by in vitro studies. HEV can infect neuronal cells in vitro and human neuronal-derived cells support the full-length replication of viral RNA and translation of viral capsid protein[11,12]. Indirect immune trigger-mediated mechanisms appear to be more relevant in pathogenesis. Both humoral and cellular immune responses are likely to be involved in the pathogenesis of hepatic and EHMs. Furthermore, the prevalence of EHM is more common in immunocompetent patients than immunocompromised patients. In a study, the neurological manifestations were found to be significantly more common (22.6% as against 3.2%) in immunocompetent patients (n = 137) compared to immunocompromised patients (n = 63). The higher frequency of neurological disorders in immunocompetent patients suggests immune-mediated mechanisms causing EHMs[13]. In another study, all patients with NA were immunocompetent[14]. A schematic diagram of the potential mechanisms of EHM is shown in Figure 1.
The neurologic manifestations of HEV infection are being increasingly recognized. Retrospective studies from Europe revealed 5.5%-7.5% of neurological manifestations in HEV-infected patients[15,16]. Furthermore, two recent European prospective studies showed an even higher prevalence of neurological symptoms (16.5%-31%) in HEV-infected patients. Ripellino et al[14] indicated 31% prevalence of neurological symptoms in HEV-infected patients. However, two-third of patients had myalgia only, and none of these patients underwent brachial plexus MRI or muscle biopsy. In another recent prospective case-control study, out of 200 HEV-infected patients, 33 (16.5%) showed neurological symptoms. The most frequent manifestation was neuropathic pain (42%), which suggests small fiber neuropathy. However, the results of their neurological examination were normal, and extensive investigations were not performed to detect small fiber neuropathy[13]. The prevalence of HEV infection in patients with non-traumatic neurological injury ranges from 2.4% to 6.9%. In a multi-centric European study, 2.4% of 464 patients with non-traumatic neurological injury showed evidence of recent HEV infection. Symptoms of hepatitis were mild or absent and all patients were anicteric[17]. Another study from France demonstrated a high seroprevalence (6.9%) of positive anti-HEV IgM in a cohort (n = 159) of patients with acute non-traumatic, non-vascular neurological injuries as compared to seroprevalence in blood donors (0.4%) at the same period of time[18,19]. However, a recent study from China showed a similar HEV seroprevalence in patients with acute non-traumatic neuropathy (n = 1117) and healthy controls (n = 1415) (0.54% vs 0.68%; P = 0.65)[20].
Involvement of both the peripheral nervous system and CNS can occur together or in isolation. The various neurologic syndromes that have been reported in patients with HEV infection are shown in Table 3. Approximately 163 cases have been described to date, wherein the mean age of patients was 53.9 years with a male to female ratio (M:F) of 2.84:1. Neurological disorders are most commonly reported in Europe (74%) and SEAR nations (15%), mainly France, India, and Bangladesh. Nerve root and plexus disorders [NA (39%) and GBS (37%)] were the most commonly reported HEV-related neurological disorders, followed by CNS disorder [meningoencephalitis (4%)]. The majority of patients (88%) had normal bilirubin levels. Alanine aminotransferase (ALT) levels were widely variable [median (range): 345.5 (16-4502) IU/L]. HEV RNA was detected in serum in 94 (58%) cases. Genotyping was performed in 62 (38%) cases, which revealed that GT3 is the main GT (90%) associated with neurological disorders. HEV RNA was identified in CSF in 12 (7%) cases. Patients were mainly treated with supportive measures, intravenous Ig (IVIG), plasmapheresis, steroids, and physiotherapy. Antiviral treatment (ribavirin) was given to 16 (10%) patients only. Of the 163 patients, the follow-up details of 130 patients were available. Complete recovery was observed in 60 (46%) patients, while partial recovery or long-term disability and death were noted in 68 (53%) and 2 patients respectively.
| Diseases | n (%) | Geographic distribution | Mean age in yr | M:F ratio | Serological diagnosis | Serum HEV-RNA | Genotype | CSF HEV-RNA | Elevated bilirubin, n | Median (range) ALT in IU/L | Use of anti-viral | Recovery |
| Nerve root and plexus disorders | ||||||||||||
| Neuralgic amyotrophy[13,15-17,28-56] | 64 (39) | Europe: 62; SEAR: 1; United States: 1 | 45.7 | 6.5:1 | 51 | 44 | GT3: 28 | 0 | 4 | 1007 (22-2579) | 9 | CR: 9; PR: 47; NM: 8 |
| Guillain-Barré syndrome[13,15,16,20,36,58-85] | 61 (37) | SEAR: 21; Europe: 30; China/Hong Kong: 5; Japan: 4; Iraq: 1 | 51.16 | 2.42:1 | 46 | 20 | GT3: 10; GT4: 1; GT1: 2 | 3 | 11 | 1950 (57-4502) | 1 | CR: 23; PR: 14; Died: 1; NM: 23 |
| Central nervous system disorder | ||||||||||||
| Meningoencephalitis[17,20,35,86,87] | 6 (4) | Europe: 2; China: 2; SEAR: 1; United States: 1 | 44 | 1:1 | 6 | 5 | GT 3: 2 | 2 | 0 | 142 (20-479) | 1 | CR: 5; NM: 1 |
| Cerebral ischemia[17,20] | 5 (3) | Europe: 3; China: 2 | 66 | 4:1 | 5 | 4 | GT3: 2 | 0 | 0 | 18 (8-28) | 0 | CR: 3; PR: 2 |
| Seizures[17] | 2 (1) | Europe | 71 | 1:1 | 2 | 1 | GT3: 1 | 0 | 0 | 19 (10-28) | 0 | CR: 2 |
| Transverse myelitis[88] | 1 (0.6) | Europe | 62 | 0:1 | 1 | 1 | GT3: 1 | 1 | 0 | 1152 | 0 | PR: 1 |
| Neuropathy | ||||||||||||
| Mononeuritis multiplex[36] | 6 (4) | Europe | 53.16 | 1:1 | 6 | 4 | GT3: 3 | 0 | 1 | 188 (118-3641) | 1 | CR: 5; PR: 1 |
| Peripheral neuropathy[16,20,89] | 5 (3) | Europe: 4; China: 1 | 57.4 | 3:2 | 5 | 5 | GT3: 2 | 2 | 1 | 285 (30-1606) | 1 | CR: 2; PR: 3 |
| Cranial neuropathy | ||||||||||||
| Cranial neuropathy[16,17,90-92] | 5 (3) | SEAR: 2; Europe: 2; Japan: 1 | 53 | 4:1 | 2 | 2 | GT3: 1; GT4: 3; ND | 0 | 0 | 1200 (60-3866) | 0 | CR: 5 |
| Neuromuscular junction and muscle disorders | ||||||||||||
| Myositis[93] | 1 (0.6) | Europe | 57 | 1:0 | 1 | 1 | ND | 0 | 1 | 1030 | 1 | CR:1 |
| Myasthenia gravis[18] | 1 (0.6) | Europe | 33 | 0:1 | 1 | 1 | GT3: 1 | 0 | 0 | 190 | 1 | NM: 1 |
| Others neurological disorders | ||||||||||||
| Meningoradiculitis[36,94,95] | 6 (4) | Europe | 53.33 | 1:1 | 6 | 6 | GT3: 5 | 4 | 1 | 406 (40-822) | 1 | CR: 5; Died: 1 |
| Total cases, n (%) | ||||||||||||
| Total | 163 | Europe: 120 (74); WHO SEAR: 25 (15); China/Hong Kong: 10 (6), Japan: 4; United States: 2; Iraq: 1 | 53.9 | 2.84:1 | 132 (81) | 94 (58) | GT3: 56 (90); GT4: 4; GT1: 2 | 12 (7) | 19 (12) | 345.5 (16-4502) | Ribavirin: 16 (10) | CR: 60 (46); PR: 68 (53) Died:2; NM 33 (20) |
Neurological disorders have also been described in cases of viral hepatitis A, B, and C[21-26]. Hepatitis C virus infection is often associated with neuropsychiatric disorders including peripheral neuropathy (mainly sensory), cognitive impairment, and cerebrovascular accidents. Encephalitis, myelitis, encephalomyelitis, and GBS are rarely described in patients with hepatitis C virus infection[21,22]. Hepatitis A virus infection is rarely associated with neurological disorders, especially GBS and encephalitis. Other hepatitis A-related neurological disorders include meningoencephalitis, meningitis, transverse myelitis, peripheral neuropathy, optic neuritis, and neuromuscular junction and muscular disorders[23-25]. Hepatitis B virus infection is rarely associated with neurological disorders, especially GBS and peripheral neuropathy[26]. GBS and peripheral neuropathy have been reported with viral hepatitis A, B, C, and E. It is interesting to note that NA is described in HEV infection alone.
The causal association of HEV infection with GBS, NA, and meningoencephalitis is supported by good evidence, including well-described case cohorts and a large number of case reports. However, the association of HEV infection with other neurological diseases is primarily based on a small number of case reports without sufficient evidence to establish a causal relationship.
NA — also known as Parsonage-Turner syndrome or brachial plexus neuritis—is a distinct disorder, with core features that include episodes of severe shoulder and arm pain at symptom onset, rapid multifocal paresis, and atrophy of the upper extremity muscles, and a slow recovery, requiring a few months to several years. The disease is characterized by the patchy distribution of motor, sensory, and autonomic symptoms. Any part of the brachial plexus and the lumbosacral plexus can be involved, with any combination of motor and sensory impairment. Involvement of nerves from regions other than the brachial plexus is noted in about one-fourth of patients. The pathogenesis of NA is unknown, but it may share its pathogenesis mechanism with post-infectious GBS. No currently available tests can unequivocally confirm or exclude NA, although needle electromyography (EMG) can help in estimating axonal damage and reinnervation. Exclusion of other disorders such as cervical disc herniation and neoplasms of the superior sulcus region are usually required[27].
NA is likely to be causally associated with HEV infection. In a study, acute HEV infection was identified in 10.6% of NA patients (n = 47)[28]. Another study demonstrated a high seroprevalence (6.9%) of acute HEV infection in a cohort of patients with acute non-traumatic, non-vascular neurological injuries (n = 159). Notably, more than half of the NA patients (57%) showed positive anti-HEV IgM assays[18]. Recently, a multi-center retrospective data analysis was performed for the comparison of HEV-associated NA (n = 57) and NA without HEV infection (n = 61). HEV-NA patients were found to have significantly more bilateral involvement (80% vs 9%), damage outside the brachial plexus (59% vs 11%), including phrenic nerve and lumbosacral plexus injury (25% vs 4% and 26% vs 7.0% respectively), sensory symptoms, and reduced reflexes. Despite more extensive damage to the brachial plexus in HEV-associated NA, the outcome was similar in the two groups. About 90% of patients with HEV-associated NA were anicteric[29].
Approximately 64 cases of HEV-associated NA have been reported to date[13,15-17,28-56]. The salient clinico-demographic features of NA are summarized in Tables 3 and 4. HEV-associated NA is predominantly seen in middle-aged males (mean age: 45.7 years; M:F = 6.5:1). Genotyping was conducted in 28 cases; all were found to be GT3. All patients were immunocompetent. All patients, except two, were from European countries. About 90% of the cases had bilateral involvement (89% vs 11% unilateral). Predominantly right-sided involvement was observed in 60% of the patients with bilateral involvement, whereas right-sided involvement was seen in 83% of the patients with unilateral involvement. An EMG was performed in 34 of the 64 cases and revealed denervation and/or damage of brachial plexus in 31 cases. Almost all patients (94%) had normal bilirubin levels and ALT levels were variable [median (range): 1007 (22-2579) IU/L]. HEV RNA was detected in serum in 44 cases but not detected in CSF. Patients were primarily treated with supportive measures, including physiotherapy. A total of nine patients were treated with ribavirin and IVIG. Complete recovery was seen in six patients only. Partial recovery and long-term disability was noted in 40 and 7 patients respectively.
| Nerve root and plexus disorders | Source of infection | Hepatitis-neurological involvement interval | Type of involvement, n (%) | Specific remarks, n (%) |
| Neuralgic amyotrophy (n = 64) | Described in 9. Sausage figatelli: 3; Vegetables: 2; Uncooked pork: 1; Manipulation of horse manure: 1; Travel history: 2 | Described in 29. Mean delay: 8.4 d | Described in 54. Brachial plexus involvement: Bilateral 48 (89) [right > left: 15 (60); left > right: 10 (40); not described: 23]; Unilateral 6 (11) [right: 5 (83); left: 1] | 94% had normal bilirubin levels. EMG findings mentioned: 34; Denervation and/or damage of brachial plexus: 31 (91) |
| Guillain-Barré syndrome (n = 61) | Described in 5. Sausage figatelli: 2; Uncooked pork: 1; Dear meat: 1; Contact with farm animals: 1 | Described in 51. Mean delay:15 d; Concomitant: 6 patients | Described in 38. Acute inflammatory demyelinating polyneuropathy: 28 (74); Acute motor-sensory axonal neuropathy: 5 (13); Acute motor axonal neuropathy: 4 (11); Miller Fisher syndrome: 1 (3) | 82% had normal bilirubin levels; Anti-ganglioside GM1 antibodies: 4; Anti-ganglioside GM 2 antibodies: 4; Anti-GQ1b ganglioside antibody: 1 |
GBS is an inflammatory disease of the nerve root and plexus and is the most common cause of acute flaccid paralysis. It should be considered a diagnosis in patients who have experienced rapidly progressive bilateral weakness of the legs and/or arms, in the absence of CNS involvement or other obvious causes. Patients with the classic sensorimotor form of GBS present with distal paresthesia or sensory loss, accompanied or followed by weakness that starts in the legs and progresses to the arms and cranial muscles. Reflexes are decreased or absent in most patients at presentation and in almost all patients at nadir. GBS is a clinical diagnosis, but additional investigations are mostly performed for confirmation. The diagnosis of GBS can be supported by a CSF examination finding of classical cytoalbuminologic dissociation—the combination of a normal cell count and increased protein level. Nerve conduction studies can be helpful but are generally not required to diagnose GBS[57].
Prior respiratory or gastrointestinal tract infection has been reported in approximately two-thirds of GBS patients. A specific pathogen can be identified in about 50% of cases of GBS with a suspected infectious precipitant. Epidemiological studies have shown that Campylobacter jejuni, Mycoplasma pneumoniae, Haemophilus influenzae, Cytomegalovirus, and Epstein-Barr virus infections are strongly associated with GBS. These pathogens appear to act as potential triggers of a postinfectious immune-mediated process leading to GBS. Molecular mimicry and cross-reactive immune triggers play an important role in the immunopathogenesis of GBS. Antibodies to gangliosides following infection with C. jejuni have been demonstrated in patients with GBS.
Recently, case-control studies and several case reports reported an association between acute HEV infection and GBS. The seroprevalence of acute HEV infection in patients with GBS has been found to range from 5% to 11%[57-61]. In a retrospective cohort study, 8% of patients (n = 73) with GBS showed positive IgM assays for HEV[58]. The association of HEV infection with GBS has been described in at least three well-described case-control studies. A study conducted in the Netherlands indicated a significantly higher frequency of acute HEV infection in GBS patients (5%) compared to healthy controls (0.5%)[59]. Another study from Japan demonstrated that 4.8% of the patients (n = 63) with GBS showed an association with acute HEV infection[60]. A study in Bangladesh documented that 11% of GBS patients (n = 100) were associated with acute HEV infection and seroprevalence was significantly higher for this group compared to patients with other neurological disorders as well as healthy controls[61]. In all studies, no clinical differences were observed between HEV-associated GBS and other GBS cases[58-61].
We found 61 cases of HEV-associated GBS reported in the medical literature[13,15,16,20,36,58-85]. Salient clinico-demographic features of GBS are summarized in Tables 3 and 4. The mean age of the patients was 51.16 years with an M:F of 2.42:1. Genotyping was performed in 13 cases. All patients, except three, were GT3. HEV-associated GBS is most commonly reported in Europe and Southeast Asia, followed by East Asia and China. The patients most commonly presented with acute inflammatory demyelinating polyneuropathy. The majority of patients (82%) had normal bilirubin levels, and ALT levels widely varied (median [range]: 1950 [57-4502] IU/L). HEV RNA was detected in serum in 20 cases, while HEV RNA was detected in CSF in 3 cases. Further, anti-ganglioside antibodies were documented in nine patients. Patients were mainly treated with supportive measures, including IVIG and physiotherapy. The follow-up details of 38 of 61 patients were available. Complete recovery was seen in only 23 patients. Partial recovery was noted in 14 patients and 1 patient died.
The association between HEV infection and CNS disorders, including meningoencephalitis, cerebral ischemia, seizure, and transverse myelitis was reported in a few case reports. Meningoencephalitis was reported in six cases with acute HEV infection (mean age: 44 years; M:F = 1:1)[17,20,35,86,87]. Genotyping done in two cases revealed GT3, and all patients were anicteric. The median (range) ALT was 142 (20-479) IU/L. HEV RNA was detected in serum in all patients, except one. CSF examination for HEV RNA performed in four cases revealed the presence of HEV RNA in two cases. Of the six cases, complete recovery was seen in five.
Cerebral ischemia was reported in six cases[17,20], and all patients were anicteric. Serum HEV RNA was detected in four patients with cerebral ischemia. However, HEV RNA was not detected in CSF. Seizures were reported in two cases with HEV infection[17]. Transverse myelitis was reported in an anicteric patient with acute HEV (GT3) infection, and HEV RNA was detected in both serum and CSF[88].
The association between HEV infection and neuropathy was reported in case series and reports. Mononeuritis multiplex, defined by asymmetric, asynchronous involvement of the noncontiguous nerve trunks has been documented in six patients with acute HEV infection (GT3) from France (mean age: 53.16 years; M:F = 1:1)[36]. All patients presented with neuropathic pain and paresthesia in multiple sites with hyporeflexia or areflexia, and all patients, except one, were anicteric. The median (range) ALT was 188 (118-3641) IU/L. HEV RNA was detected in serum in four cases. All patients, except one, had a complete recovery.
Peripheral neuropathy and small fiber neuropathy were reported in five cases, mostly from Europe (4/5; mean age: 57.4 years; M:F = 3:2)[16,20,89]. Genotyping done in two cases revealed GT3. Patients presented with neuropathic pain, paresthesia and weakness of limbs. All patients, except one, were anicteric. Median (range) ALT was 285 (30-1606) IU/L. HEV RNA was detected in serum in all five cases. HEV RNA was detected in CSF in two cases. Out of five, complete recovery was seen in two-cases only.
Cranial nerve involvement was seen in five patients with acute HEV infection (mean age: 53 years; M:F = 4:1)[16,17,90-92]. Cases were reported from Europe (n = 2), India (n = 2), and Japan (n = 1) respectively. Isolated facial nerve palsy (Bell’s palsy) and vestibular nerve involvement were seen in three and one patient respectively. Combined facial nerve and vestibular nerve involvement was noted in one patient. All patients were anicteric. Median (range) ALT was 1200 (60-3866) IU/L. HEV RNA was detected in serum in two cases. Genotyping was done in two cases and revealed GT3 and GT4 (Europe: GT1; Japan: GT4). CSF examination for HEV RNA was performed in none of the patients, and complete recovery was observed in all.
Cases of myasthenia gravis (n = 1) and myositis (n = 1) were also reported in serum HEV RNA positive patients[18,93]. However, HEV RNA was not detected in CSF.
Meningoradiculitis refers to the combined involvement of meninges and nerve roots, and the lumbosacral region is the most common site of involvement. Etiology includes inflammatory, infectious, and neoplastic disorders. Recently, meningoradiculitis was reported in six patients with acute HEV infection from Europe[36,94,95]. The mean age of patients was 53.33 years with M:F of 1:1. Genotyping was done in five cases and showed GT3. Patients mainly presented with arthromyalgia and asthenia. All patients, except one, were anicteric. Median (range) ALT was 406 (40-822) IU/L. HEV RNA was detected in serum in all six cases. HEV RNA was detected in CSF in four cases. Of the six, complete recovery was noted in five cases.
Acute HEV infection is diagnosed through the detection of serum and/or stool HEV RNA by polymerase chain reaction (PCR), serum anti-HEV immunoglobulin M, HEV antigen, and rising anti-HEV immunoglobulin G titer. A negative PCR does not exclude acute infection, and serology sometimes gives false negatives in the case of immunocompromised patients. Therefore, serology and PCR testing are best used in combination. Testing for HEV should be conducted in all patients with suspicion of acute hepatitis, immunosuppressed patients with unexplained deranged liver function tests (LFTs), and in patients with NA and GBS. Testing is also suggested in patients with unexplained ACLF, and encephalitis or myelitis. Moreover, testing for proteinuria is suggested in HEV-infected patients[5].
The HEV is spontaneously cleared in almost all patients with acute infection. Therefore, acute hepatitis does not require antiviral therapy. In a few case studies, ribavirin treatment of severe acute HEV infection showed rapid normalization of liver enzymes and viremia. Therefore, ribavirin treatment may be considered in cases of severe acute hepatitis E or ACLF[5,96]. Antiviral treatment is also suggested for patients with chronic HEV infection, and HEV-associated glomerular disease. There is no sufficient evidence of ribavirin use in HEV-associated neurological disorders.
Several epidemiological factors associated with HEV infection are described in Table 1. HEV is principally transmitted via the fecal-oral route due to fecal contamination of drinking water. As such, it has been detected in sewage water, rivers, pork liver sausages, shellfish, cattle milk, unpeeled fruit, strawberries, berries, salads, and leafy green vegetables. Poor hygiene, exposure to contaminated environments, consumption of raw meat, exposure to soil, travel to endemic areas, contact with farm animals and pets, living in rural areas, and receiving lower levels of education were identified as risk factors for HEV infection. Transmission from infected blood products and vertical transmission are other modes of transmission[13]. The spread of HEV can be reduced by maintaining quality standards for public water supplies, ensuring proper disposal of human faces, maintaining individual hygienic practices, and avoiding consumption of foods of unknown purity. A recombinant subunit vaccine demonstrated efficacy against HEV infection. However, no vaccine is commercially available yet for this infection, except in China.
The neurologic manifestations of HEV infection are increasingly recognized worldwide, especially in Europe and Southeast Asia. Nerve root and plexus disorder (NA and GBS) are the most commonly reported diseases followed by meningoencephalitis. NA, GBS, and meningoencephalitis appear to be causally associated with HEV-infection. Further, GT3 is the most common GT identified in these patients. The majority of patients present without jaundice. However, the absence of jaundice and normal LFTs can mislead the physician. Thus, detailed neurological evaluation is warranted in patients with HEV infection and neurological symptoms. Patients with neurological disorders and deranged LFTs require further investigation to diagnose HEV infection. Testing for HEV in patients with neurological disorders and unexplained etiology can also be rewarding. Finally, there is no sufficient evidence of routine use of an anti-viral agent in HEV-related neurological disorders.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology, No. 56353; Indian Society of Gastroenterology, No. LM002279.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chawla S, Granito A, Shimizu Y S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | Purdy MA, Harrison TJ, Jameel S, Meng XJ, Okamoto H, Van der Poel WHM, Smith DB; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Hepeviridae. J Gen Virol. 2017;98:2645-2646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 2. | Li P, Liu J, Li Y, Su J, Ma Z, Bramer WM, Cao W, de Man RA, Peppelenbosch MP, Pan Q. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. 2020;40:1516-1528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Viral hepatitis in the WHO South-East Asia Region. [cited 15 November 2020]. In: World Health Organization [Internet]. Available from: https://apps.who.int/iris/handle/10665/206521. |
| 4. | Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 999] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 5. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 442] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 6. | Jha AK, Nijhawan S, Rai RR, Nepalia S, Jain P, Suchismita A. Etiology, clinical profile, and inhospital mortality of acute-on-chronic liver failure: a prospective study. Indian J Gastroenterol. 2013;32:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Kumar A, Aggarwal R, Naik SR, Saraswat V, Ghoshal UC, Naik S. Hepatitis E virus is responsible for decompensation of chronic liver disease in an endemic region. Indian J Gastroenterol. 2004;23:59-62. [PubMed] |
| 8. | Rawla P, Raj JP, Kannemkuzhiyil AJ, Aluru JS, Thandra KC, Gajendran M. A Systematic Review of the Extra-Hepatic Manifestations of Hepatitis E Virus Infection. Med Sci (Basel). 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Pischke S, Hartl J, Pas SD, Lohse AW, Jacobs BC, Van der Eijk AA. Hepatitis E virus: Infection beyond the liver? J Hepatol. 2017;66:1082-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (1)] |
| 10. | Feng Z. Causation by HEV of extrahepatic manifestations remains unproven. Liver Int. 2016;36:477-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Zhou X, Huang F, Xu L, Lin Z, de Vrij FMS, Ayo-Martin AC, van der Kroeg M, Zhao M, Yin Y, Wang W, Cao W, Wang Y, Kushner SA, Marie Peron J, Alric L, de Man RA, Jacobs BC, van Eijk JJ, Aronica EMA, Sprengers D, Metselaar HJ, de Zeeuw CI, Dalton HR, Kamar N, Peppelenbosch MP, Pan Q. Hepatitis E Virus Infects Neurons and Brains. J Infect Dis. 2017;215:1197-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Drave SA, Debing Y, Walter S, Todt D, Engelmann M, Friesland M, Wedemeyer H, Neyts J, Behrendt P, Steinmann E. Extra-hepatic replication and infection of hepatitis E virus in neuronal-derived cells. J Viral Hepat. 2016;23:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Abravanel F, Pique J, Couturier E, Nicot F, Dimeglio C, Lhomme S, Chiabrando J, Saune K, Péron JM, Kamar N, Evrard S, de Valk H, Cintas P, Izopet J; HEV study group. Acute hepatitis E in French patients and neurological manifestations. J Infect. 2018;77:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Ripellino P, Pasi E, Melli G, Staedler C, Fraga M, Moradpour D, Sahli R, Aubert V, Martinetti G, Bihl F, Bernasconi E, Terziroli Beretta-Piccoli B, Cerny A, Dalton HR, Zehnder C, Mathis B, Zecca C, Disanto G, Kaelin-Lang A, Gobbi C. Neurologic complications of acute hepatitis E virus infection. Neurol Neuroimmunol Neuroinflamm. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Kamar N, Bendall RP, Peron JM, Cintas P, Prudhomme L, Mansuy JM, Rostaing L, Keane F, Ijaz S, Izopet J, Dalton HR. Hepatitis E virus and neurologic disorders. Emerg Infect Dis. 2011;17:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 16. | Woolson KL, Forbes A, Vine L, Beynon L, McElhinney L, Panayi V, Hunter JG, Madden RG, Glasgow T, Kotecha A, Dalton HC, Mihailescu L, Warshow U, Hussaini HS, Palmer J, Mclean BN, Haywood B, Bendall RP, Dalton HR. Extra-hepatic manifestations of autochthonous hepatitis E infection. Aliment Pharmacol Ther. 2014;40:1282-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Dalton HR, van Eijk JJJ, Cintas P, Madden RG, Jones C, Webb GW, Norton B, Pique J, Lutgens S, Devooght-Johnson N, Woolson K, Baker J, Saunders M, Househam L, Griffiths J, Abravanel F, Izopet J, Kamar N, van Alfen N, van Engelen BGM, Hunter JG, van der Eijk AA, Bendall RP, Mclean BN, Jacobs BC. Hepatitis E virus infection and acute non-traumatic neurological injury: A prospective multicentre study. J Hepatol. 2017;67:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Belbézier A, Deroux A, Sarrot-Reynauld F, Colombe B, Bosseray A, Wintenberger C, Dumanoir P, Lugosi M, Boccon-Gibod I, Leroy V, Maignan M, Collomb-Muret R, Viglino D, Vaillant M, Minotti L, Lagrange E, Epaulard O, Dumestre-Perard C, Lhomme S, Lupo J, Larrat S, Morand P, Schwebel C, Vilotitch A, Bosson JL, Bouillet L. Screening of hepatitis E in patients presenting for acute neurological disorders. J Infect Public Health. 2020;13:1047-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Mansuy JM, Gallian P, Dimeglio C, Saune K, Arnaud C, Pelletier B, Morel P, Legrand D, Tiberghien P, Izopet J. A nationwide survey of hepatitis E viral infection in French blood donors. Hepatology. 2016;63:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Wang S, Wu J, Jiang Y, Zhang H, Li S, Liu H, Yang C, Tang H, Guo N, Peppelenbosch MP, Wei L, Pan Q, Zhao J. Hepatitis E virus infection in acute non-traumatic neuropathy: A large prospective case-control study in China. EBioMedicine. 2018;36:122-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Adinolfi LE, Nevola R, Lus G, Restivo L, Guerrera B, Romano C, Zampino R, Rinaldi L, Sellitto A, Giordano M, Marrone A. Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World J Gastroenterol. 2015;21:2269-2280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 22. | Mapoure NY, Budzi MN, Eloumou SAFB, Malongue A, Okalla C, Luma HN. Neurological manifestations in chronic hepatitis C patients receiving care in a reference hospital in sub-Saharan Africa: A cross-sectional study. PLoS One. 2018;13:e0192406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Menon D, Jagtap SA, Nair MD. Guillain-Barré syndrome following acute viral hepatitis A. J Neurosci Rural Pract. 2014;5:204-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Lee JJ, Kang K, Park JM, Kwon O, Kim BK. Encephalitis associated with acute hepatitis a. J Epilepsy Res. 2011;1:27-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Chonmaitree P, Methawasin K. Transverse Myelitis in Acute Hepatitis A Infection: The Rare Co-Occurrence of Hepatology and Neurology. Case Rep Gastroenterol. 2016;10:44-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Kappus MR, Sterling RK. Extrahepatic manifestations of acute hepatitis B virus infection. Gastroenterol Hepatol (N Y). 2013;9:123-126. [PubMed] |
| 27. | van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129:438-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 392] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 28. | van Eijk JJ, Madden RG, van der Eijk AA, Hunter JG, Reimerink JH, Bendall RP, Pas SD, Ellis V, van Alfen N, Beynon L, Southwell L, McLean B, Jacobs BC, van Engelen BG, Dalton HR. Neuralgic amyotrophy and hepatitis E virus infection. Neurology. 2014;82:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | van Eijk JJJ, Dalton HR, Ripellino P, Madden RG, Jones C, Fritz M, Gobbi C, Melli G, Pasi E, Herrod J, Lissmann RF, Ashraf HH, Abdelrahim M, Masri OABAL, Fraga M, Benninger D, Kuntzer T, Aubert V, Sahli R, Moradpour D, Blasco-Perrin H, Attarian S, Gérolami R, Colson P, Giordani MT, Hartl J, Pischke S, Lin NX, Mclean BN, Bendall RP, Panning M, Peron JM, Kamar N, Izopet J, Jacobs BC, van Alfen N, van Engelen BGM. Clinical phenotype and outcome of hepatitis E virus-associated neuralgic amyotrophy. Neurology. 2017;89:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Fong F, Illahi M. Neuralgic amyotrophy associated with hepatitis E virus. Clin Neurol Neurosurg. 2009;111:193-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Rianthavorn P, Thongmee C, Limpaphayom N, Komolmit P, Theamboonlers A, Poovorawan Y. The entire genome sequence of hepatitis E virus genotype 3 isolated from a patient with neuralgic amyotrophy. Scand J Infect Dis. 2010;42:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Carli P, Landais C, Poisnel E, Cournac JM, Aletti M, Paris JF, Martinez V. [Shoulder pain in a 30-year-old man]. Rev Med Interne. 2012;33:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Moisset X, Vitello N, Bicilli E, Courtin R, Ferrier A, Taithe F, Lahaye C, Hssain AA, Garrouste C, Pierre C. Severe bilateral amyotrophic neuralgia associated with major dysphagia secondary to acute hepatitis E. F1000Res. 2013;2:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Motte A, Franques J, Weitten T, Colson P. Hepatitis E-associated Parsonage-Turner syndrome, France. Clin Res Hepatol Gastroenterol. 2014;38:e11-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Deroux A, Brion JP, Hyerle L, Belbezier A, Vaillant M, Mosnier E, Larrat S, Morand P, Pavese P. Association between hepatitis E and neurological disorders: two case studies and literature review. J Clin Virol. 2014;60:60-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Perrin HB, Cintas P, Abravanel F, Gérolami R, d'Alteroche L, Raynal JN, Alric L, Dupuis E, Prudhomme L, Vaucher E, Couzigou P, Liversain JM, Bureau C, Vinel JP, Kamar N, Izopet J, Peron JM. Neurologic Disorders in Immunocompetent Patients with Autochthonous Acute Hepatitis E. Emerg Infect Dis. 2015;21:1928-1934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Décard BF, Grimm A, Andelova M, Deman A, Banderet B, Garcia M, Fuhr P. Hepatitis-E virus associated neuralgic amyotrophy with sustained plexus brachialis swelling visualized by high-resolution ultrasound. J Neurol Sci. 2015;351:208-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Theochari E, Vincent-Smith L, Ellis C. Neuralgic amyotrophy complicating acute hepatitis E infection: a rare association. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Martínez Rodríguez L, Carvajal P, Morís G. [Neuralgic amyotrophy associated to hepatitis E virus infection]. Med Clin (Barc). 2015;145:462-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Dartevel A, Colombe B, Bosseray A, Larrat S, Sarrot-Reynauld F, Belbezier A, Lagrange E, Bouillet L. Hepatitis E and neuralgic amyotrophy: Five cases and review of literature. J Clin Virol. 2015;69:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Avila JD, Lacomis D, Lam EM. Neuralgic Amyotrophy Associated With Hepatitis E Virus Infection: First Case in the United States. J Clin Neuromuscul Dis. 2016;18:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Silva M, Wicki B, Tsouni P, Cunningham S, Doerig C, Zanetti G, Aubert V, Sahli R, Moradpour D, Kuntzer T. Hepatitis E virus infection as a direct cause of neuralgic amyotrophy. Muscle Nerve. 2016;54:325-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Altuna-Azkargorta M, Torne-Hernandez L, Aznar-Gomez P, Ibiricu-Yanguas MA, Ducouret A. [Infection by the hepatitis E virus as a precipitating factor of Parsonage-Turner syndrome]. Rev Neurol. 2016;62:572-574. [PubMed] |
| 44. | Pischke S, Ryll U, De Weerth A, Ufer F, Gelderblom M. [Neuralgic amyotrophy: an extrahepatic manifestation of hepatitis E]. Dtsch Med Wochenschr. 2016;141:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Bisciglia M, Van den Bergh P, Duprez T, Kabamba BM, Ivanoiu A. Neuralgic amyotrophy associated with hepatitis E virus (HEV) infection: a case report. Acta Neurol Belg. 2017;117:555-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Velay A, Kack-Kack W, Abravanel F, Lhomme S, Leyendecker P, Kremer L, Chamouard P, Izopet J, Fafi-Kremer S, Barth H. Parsonage-Turner syndrome due to autochthonous acute genotype 3f hepatitis E virus infection in a nonimmunocompromised 55-year-old patient. J Neurovirol. 2017;23:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Scanvion Q, Perez T, Cassim F, Outteryck O, Lanteri A, Hatron PY, Lambert M, Morell-Dubois S. Neuralgic amyotrophy triggered by hepatitis E virus: a particular phenotype. J Neurol. 2017;264:770-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Fraga M, Doerig C, Moulin H, Bihl F, Brunner F, Müllhaupt B, Ripellino P, Semela D, Stickel F, Terziroli Beretta-Piccoli B, Aubert V, Telenti A, Greub G, Sahli R, Moradpour D. Hepatitis E virus as a cause of acute hepatitis acquired in Switzerland. Liver Int. 2018;38:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Fritz M, Berger B, Schemmerer M, Endres D, Wenzel JJ, Stich O, Panning M. Pathological Cerebrospinal Fluid Findings in Patients With Neuralgic Amyotrophy and Acute Hepatitis E Virus Infection. J Infect Dis. 2018;217:1897-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Sánchez Azofra M, Romero Portales M, Tortajada Laureiro L, García-Samaniego J, Mora Sanz P. Hepatitis E virus in neurological disorders: a case of Parsonage-Turner syndrome. Rev Esp Enferm Dig. 2018;110:402-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Njabom CN, Gilbert A, Brasseur E, Zandona R, Ghuysen A, D'Orio V. Parsonage-Turner Syndrome as a Rare Extrahepatic Complication of Hepatitis E Infection. Eur J Case Rep Intern Med. 2019;6:001208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Arányi Z, Szpisjak L, Szőke K. Multiphasic presentation of neuralgic amyotrophy associated with hepatitis E virus infection. Muscle Nerve. 2020;61:108-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Mendoza-Lopez C, Lopez-Lopez P, Atienza-Ayala S, Rivero-Juarez A, Benito R. Parsonage-Turner syndrome associated with hepatitis E infection in immunocompetent patients. Virus Res. 2020;290:198165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Inghilleri ML, Grini Mazouzi M, Juntas Morales R. [Neuralgic amyotrophy as a manifestation of hepatitis E infection]. Rev Neurol (Paris). 2012;168:383-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Diebold M, Fischer-Barnicol B, Tsagkas C, Kuhle J, Kappos L, Derfuss T, Décard BF. Hepatitis E virus infections in patients with MS on oral disease-modifying treatment. Neurol Neuroimmunol Neuroinflamm. 2019;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Cheung MC, Maguire J, Carey I, Wendon J, Agarwal K. Review of the neurological manifestations of hepatitis E infection. Ann Hepatol. 2012;11:618-622. [PubMed] |
| 57. | van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10:469-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 682] [Article Influence: 62.0] [Reference Citation Analysis (2)] |
| 58. | Stevens O, Claeys KG, Poesen K, Saegeman V, Van Damme P. Diagnostic Challenges and Clinical Characteristics of Hepatitis E Virus-Associated Guillain-Barré Syndrome. JAMA Neurol. 2017;74:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 59. | van den Berg B, van der Eijk AA, Pas SD, Hunter JG, Madden RG, Tio-Gillen AP, Dalton HR, Jacobs BC. Guillain-Barré syndrome associated with preceding hepatitis E virus infection. Neurology. 2014;82:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 60. | Fukae J, Tsugawa J, Ouma S, Umezu T, Kusunoki S, Tsuboi Y. Guillain-Barré and Miller Fisher syndromes in patients with anti-hepatitis E virus antibody: a hospital-based survey in Japan. Neurol Sci. 2016;37:1849-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 61. | Geurtsvankessel CH, Islam Z, Mohammad QD, Jacobs BC, Endtz HP, Osterhaus AD. Hepatitis E and Guillain-Barre syndrome. Clin Infect Dis. 2013;57:1369-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 62. | Sood A, Midha V, Sood N. Guillain-Barré syndrome with acute hepatitis E. Am J Gastroenterol. 2000;95:3667-3668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Kamani P, Baijal R, Amarapurkar D, Gupte P, Patel N, Kumar P, Agal S. Guillain-Barre syndrome associated with acute hepatitis E. Indian J Gastroenterol. 2005;24:216. [PubMed] |
| 64. | Khanam R, Faruq M, Basunia R, Ahsan A. Guillain-Barré Syndrome Associated with Acute HEV Hepatitis. IMCJ. 2008;2:32-34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 65. | Loly JP, Rikir E, Seivert M, Legros E, Defrance P, Belaiche J, Moonen G, Delwaide J. Guillain-Barré syndrome following hepatitis E. World J Gastroenterol. 2009;15:1645-1647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Cronin S, McNicholas R, Kavanagh E, Reid V, O'Rourke K. Anti-glycolipid GM2-positive Guillain-Barre syndrome due to hepatitis E infection. Ir J Med Sci. 2011;180:255-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Maurissen I, Jeurissen A, Strauven T, Sprengers D, De Schepper B. First case of anti-ganglioside GM1-positive Guillain-Barré syndrome due to hepatitis E virus infection. Infection. 2012;40:323-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 68. | Del Bello A, Arné-Bes MC, Lavayssière L, Kamar N. Hepatitis E virus-induced severe myositis. J Hepatol. 2012;57:1152-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Tse AC, Cheung RT, Ho SL, Chan KH. Guillain-Barré syndrome associated with acute hepatitis E infection. J Clin Neurosci. 2012;19:607-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Santos L, Mesquita JR, Rocha Pereira N, Lima-Alves C, Serrão R, Figueiredo P, Reis J, Simões J, Nascimento M, Sarmento A. Acute hepatitis E complicated by Guillain-Barre syndrome in Portugal, December 2012--a case report. Euro Surveill. 2013;18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Sharma B, Nagpal K, Bakki Sannegowda R, Prakash S. Hepatitis E with Gullain-Barré syndrome: still a rare association. J Neurovirol. 2013;19:186-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Chen XD, Zhou YT, Zhou JJ, Wang YW, Tong DM. Guillain-Barré syndrome and encephalitis/encephalopathy of a rare case of Northern China acute severe hepatitis E infection. Neurol Sci. 2014;35:1461-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 73. | Scharn N, Ganzenmueller T, Wenzel JJ, Dengler R, Heim A, Wegner F. Guillain-Barré syndrome associated with autochthonous infection by hepatitis E virus subgenotype 3c. Infection. 2014;42:171-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 74. | Comont T, Bonnet D, Sigur N, Gerdelat A, Legrand-Abravanel F, Kamar N, Alric L. [Acute hepatitis E infection associated with Guillain-Barré syndrome in an immunocompetent patient]. Rev Med Interne. 2014;35:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Bandyopadhyay D, Ganesan V, Choudhury C, Kar SS, Karmakar P, Choudhary V, Banerjee P, Bhar D, Hajra A, Layek M, Mukhopadhyay S. Two Uncommon Causes of Guillain-Barré Syndrome: Hepatitis E and Japanese Encephalitis. Case Rep Neurol Med. 2015;2015:759495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Higuchi MA, Fukae J, Tsugawa J, Ouma S, Takahashi K, Mishiro S, Tsuboi Y. Dysgeusia in a Patient with Guillain-Barré Syndrome Associated with Acute Hepatitis E: A Case Report and Literature Review. Intern Med. 2015;54:1543-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Ji SB, Lee SS, Jung HC, Kim HJ, Kim TH, Jung WT, Lee OJ, Song DH. A Korean patient with Guillain-Barré syndrome following acute hepatitis E whose cholestasis resolved with steroid therapy. Clin Mol Hepatol. 2016;22:396-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Lei JH, Tian Y, Luo HY, Chen Z, Peng F. Guillain-Barré syndrome following acute co-super-infection of hepatitis E virus and cytomegalovirus in a chronic hepatitis B virus carrier. J Med Virol. 2017;89:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Salim OJ, Davidson A, Li K, Leach JP, Heath C. Brainstem encephalitis and acute polyneuropathy associated with hepatitis E infection. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Troussière AC, Sudaveschi V, Collardelle P, Marque Julliet S, Servan J, Pico F. Guillain-Barré syndrome due to hepatitis E. Rev Neurol (Paris). 2018;174:72-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 81. | Zheng X, Yu L, Xu Q, Gu S, Tang L. Guillain-Barre syndrome caused by hepatitis E infection: case report and literature review. BMC Infect Dis. 2018;18:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Choudhary MC, Bajpai V, Anand L, Gupta E. Guillain-Barré syndrome in a patient of acute Hepatitis E virus infection associated with genotype 1: Case report and literature review. Intractable Rare Dis Res. 2019;8:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Kumar R, Bhoi S, Kumar M, Sharma B, Singh BM, Gupta BB. Guillain-Barré syndrome and acute hepatitis E: A rare association. JIACM. 2002;4:389-391. |
| 84. | Oh HW, Cha RR, Lee SS, Lee CM, Kim WS, Jo YW, Kim JJ, Lee JM, Kim HJ, Ha CY, Kim TH, Jung WT, Lee OJ. Comparing the Clinical Features and Outcomes of Acute Hepatitis E Viral Infections with Those of Acute Hepatitis A, B, and C Infections in Korea. Intervirology. 2017;60:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Al-Saffar A, Al-Fatly B. Acute Motor Axonal Neuropathy in Association with Hepatitis E. Front Neurol. 2018;9:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 86. | Pasha SA, Pasha SA, Suhasini T, Rao DA. Hepatitis E Virus-Associated Acute Encephalitic Parkinsonism. J Assoc Physicians India. 2018;66:92-93. [PubMed] |
| 87. | Murkey JA, Chew KW, Carlson M, Shannon CL, Sirohi D, Sample HA, Wilson MR, Vespa P, Humphries RM, Miller S, Klausner JD, Chiu CY. Hepatitis E Virus-Associated Meningoencephalitis in a Lung Transplant Recipient Diagnosed by Clinical Metagenomic Sequencing. Open Forum Infect Dis. 2017;4:ofx121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 88. | Sarkar P, Morgan C, Ijaz S. Transverse myelitis caused by hepatitis E: previously undescribed in adults. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 89. | Bennett S, Li K, Gunson RN. Hepatitis E virus infection presenting with paraesthesia. Scott Med J. 2015;60:e27-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Dixit VK, Abhilash VB, Kate MP, Jain AK. Hepatitis E infection with Bell's palsy. J Assoc Physicians India. 2006;54:418. [PubMed] |
| 91. | Jha AK, Nijhawan S, Nepalia S, Suchismita A. Association of Bell's Palsy with Hepatitis E Virus Infection: A Rare Entity. J Clin Exp Hepatol. 2012;2:88-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Yazaki Y, Sugawara K, Honda M, Ohnishi H, Nagashima S, Takahashi M, Okamoto H. Characteristics of 20 Patients with Autochthonous Acute Hepatitis E in Hokkaido, Japan: First Report of Bilateral Facial Palsy Following the Infection with Genotype 4 Hepatitis E Virus. Tohoku J Exp Med. 2015;236:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Mengel AM, Stenzel W, Meisel A, Büning C. Hepatitis E-induced severe myositis. Muscle Nerve. 2016;53:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 94. | Despierres LA, Kaphan E, Attarian S, Cohen-Bacrie S, Pelletier J, Pouget J, Motte A, Charrel R, Gerolami R, Colson P. Neurologic disorders and hepatitis E, France, 2010. Emerg Infect Dis. 2011;17:1510-1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 95. | Belliere J, Abravanel F, Nogier MB, Martinez S, Cintas P, Lhomme S, Lavayssière L, Cointault O, Faguer S, Izopet J, Kamar N. Transfusion-acquired hepatitis E infection misdiagnosed as severe critical illness polyneuromyopathy in a heart transplant patient. Transpl Infect Dis. 2017;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Péron JM, Dalton H, Izopet J, Kamar N. Acute autochthonous hepatitis E in western patients with underlying chronic liver disease: a role for ribavirin? J Hepatol. 2011;54:1323-4; author reply 1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (2)] |
| 97. | Hill AB. The environment and disease: Association or causation? Proc R Soc Med. 1965;58:295-300. [PubMed] |