Published online May 7, 2021. doi: 10.3748/wjg.v27.i17.1883
Peer-review started: January 22, 2021
First decision: February 28, 2021
Revised: March 13, 2021
Accepted: April 7, 2021
Article in press: April 7, 2021
Published online: May 7, 2021
Processing time: 96 Days and 11.6 Hours
Diabetes mellitus type 2 and cancer share many risk factors. The pleiotropic insulin-dependent and insulin-independent effects of metformin might inhibit pathways that are frequently amplified in neoplastic tissue. Particularly, modulation of inflammation, metabolism, and cell cycle arrest are potential therapeutic cancer targets utilized by metformin to boost the anti-cancer effects of chemotherapy. Studies in vitro and in vivo models have demonstrated the potential of metformin as a chemo- and radiosensitizer, besides its chemopreven
Core Tip: Modulation of cell function into the neoplastic and around the microenvironment tissue are possible cancer targets utilized by metformin to raise chemotherapy's anti-tumor outcomes. Herein we review the studies that have demonstrated the likelihood of metformin as chemo and radiosensitizer, in addition to its chemopre
- Citation: Cunha Júnior AD, Bragagnoli AC, Costa FO, Carvalheira JBC. Repurposing metformin for the treatment of gastrointestinal cancer. World J Gastroenterol 2021; 27(17): 1883-1904
- URL: https://www.wjgnet.com/1007-9327/full/v27/i17/1883.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i17.1883
Diabetes mellitus type 2 (DM2) and cancer share several risk factors[1]. Notably, obesity and metabolic syndrome, with their inherent biological connections, such as hyperinsulinemia[2] and chronic inflammation[3]. Furthermore, some antihypergly
Metformin is a well-known oral hypoglycemic drug that belongs to the biguanide class and has been used to treat DM2 for almost a century[16]. Importantly, those patients with DM2 with long-term use of metformin have a decreased tumor incidence and lower cancer-associated mortality[17-21]. Furthermore, recent research indicates that metformin can have direct anti-cancer activity against many tumor cells, including tumor stem cells[22,23], therefore, carrying out pleiotropic effects in both the cancer cell and the neoplastic microenvironment[24]. Their potential mechanisms are insulin-dependent [via insulin growth factor (IGF) receptor, phosphatidyl inositol 3 kinase (PI3K), and Akt/mammalian target of rapamycin (mTOR)][25,26] and insulin-independent [via adenosine kinase monophosphate (AMPK), tuberous sclerosis complex (TSC), and mTOR][27,28]. Moreover, it promotes antitumor immunity-related metabolic checkpoints in T-cells, cancer cells, as well as associated with immuno
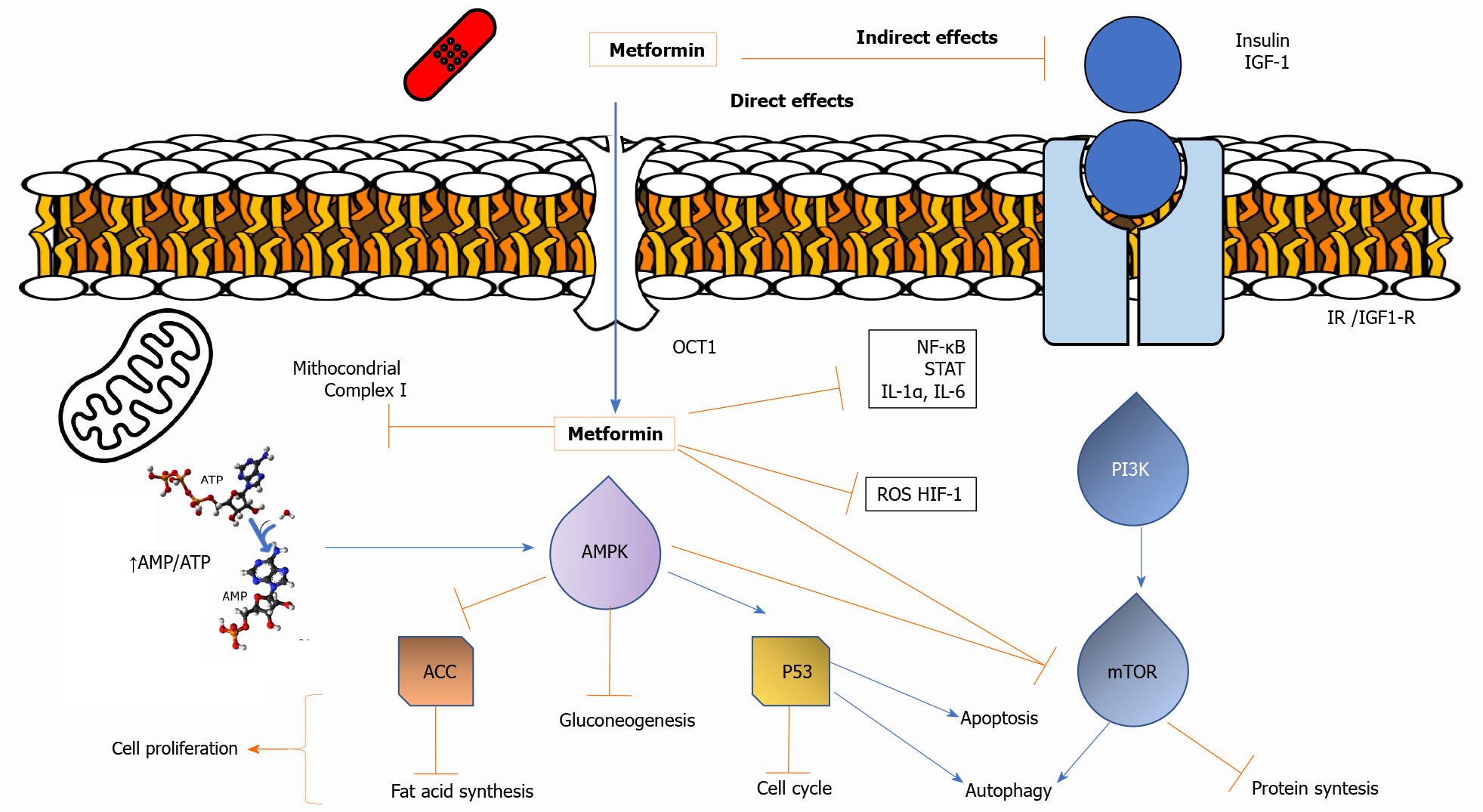
Two potential mechanisms for the antineoplastic action of metformin have been suggested (Figure 1). First, metformin can directly activate AMPK, resulting in inhibition of downstream Akt/mTOR signaling and consequent suppression of cell proliferation[32,33]. Second, metformin-induced reductions in circulating insulin and IGF concentrations may reduce activation of the IGF receptor signaling axis, resulting in decreased growth promotion and mitogenesis[2,34]. Hence, the anti-cancer effects of metformin are mediated through a systemic improvement in the metabolic milieu or directly on tumor cells[35].
The noticeable intracellular metabolic change caused by metformin is the decreased accumulation of glycolytic intermediates and a coordinated decrease in tricarboxylic acid (TCA) cycle intermediates[36,37]. Moreover, the activation of AMPK reduces fatty acid synthase (FAS) gene expression in the synthesis of fatty acids[32]. Furthermore, metformin offers other direct anti-tumor effects by (1) decreasing specific protein (Sp) transcription factors and Sp-related oncogenic proteins[38,39]; (2) decreasing AMPK-dependent c-Myc oncogene; (3) increasing other miRNAs, such as mir33a[40]; (4) increasing other miRNAs, such as miR-26a[41]; (5) reducing endogenous reactive oxygen species and associated DNA damage[42]; (6) reducing Sonic hedgehog expression[43]; (7) reducing expression of angiogenic factor CCN1, which inhibits invasion induced by the stromal cell-derived factor-1 and reducing levels of type 4 chemokine receptor[44]; and (8) inhibiting Rac1 GTPase activity[45]. Finally, metformin might interfere with the gut microbiota[30,31], as well as interfere with the balance between T-cells and associated immunosuppressive cells in the tumor milieu[29].
A central signal transduction pathway involved in cancer is the PI3K/Akt/mTOR pathway, which, when hyperactivated, leads to deregulation of survival and cell growth[28,46,47]. IGF-1 is a more potent mitogen than insulin and, like insulin, binds to its particular growth factor receptor and stimulates cell growth and anti-apoptotic activity via MAPK/ERK or Ras/Raf/MEK/ERK and PI3K/Akt/mTOR signaling[2,34]. In addition, IGF-1 inhibits PTEN, a phosphatase that deactivates PI3K/Akt/ mTOR[2]. The indirect mechanisms of metformin action include inhibition of hepatic gluconeogenesis and stimulation of peripheral glucose absorption, which ultimately lead to decreased blood glucose and insulin levels. Thus, the most apparent mechanism of insulin-dependent metformin involves decreasing insulin levels, which reduces insulin binding to the insulin receptor (IR), inhibiting tumor growth[48]. A reduction of insulin/IGF-1 levels is, at least in part, involved in the antiproliferative activity of metformin[49]. Additionally, metformin downregulates IGF-R and IR by decreasing the promoter activity of receptor genes with subsequent Akt/mTOR and MAPK/ERK signaling inhibition[50,51].
Metformin activates AMPK by inhibiting mitochondrial complex I, which leads to impaired mitochondrial function, decreased adenosine triphosphate synthesis, increased adenosine monophosphate, and subsequent phosphorylation and activation by LKB1[52]. Activated AMPK then phosphorylates TSC2, which negatively regulates mTOR activity[53]. Activation of LKB1 and AMPK, AMPK-induced stabilization of TSC1-TSC2 (inhibitor of Rheb, an mTORC1 activator), and activation of the tumor suppressor p53[54]. Moreover, independent of AMPK, metformin impedes mTORC1 by raising p53-dependent expression of REDD1 and repressing Rags[55]. Metformin also retards transformation by inhibiting mediators of the inflammatory response, including transcription factors (nuclear factor kappa, Signal transducer and activator of transcription 3, and Forkhead box O signaling), and downregulating Lin28B, most Let-7 miRNA family members, and inflammatory molecules [interleukin (IL)-1α, IL-1β, IL-6, and vascular endothelial growth factor (VEGF)][56].
Metformin has other AMPK-mediated actions that may be implicated in cancer. Through the activation of AMPK, metformin causes the suppression of FAS gene expression, which is involved in the synthesis of fatty acids, resulting in reduced lipogenesis, increased fatty acid oxidation, and decreased cell proliferation[32,57]. The activation of AMPK also modulates cyclin D1 (cell cycle protein), p21 and p27 (cyclin-dependent protein kinase), which further contribute to its anti-cancer effects[55,58]. Interestingly, metformin may act as a chemosensitizer, for example, increasing the 5-fluorouracil (5-FU) and paclitaxel sensitivities of cancer cell lines[59,60]. The ability of metformin to disconnect the electron transport chain by inhibiting complex I (NADH dehydrogenase) strongly induces cell death when glucose is limited. Metformin also reduces the hypoxic activation of hypoxia inducing factor (HIF-1), suggesting that the effects of metformin are increased in hypoglycemic and hypoxic conditions[61].
As a drug that controls metabolism, metformin promotes a coordinated decrease of TCA cycle intermediates, including succinate, fumarate, malate, citrate, and α-ketoglutarate[36,37]. The dependency of neoplastic cells on glutamine metabolism has been shown to be reprogrammed by the Kras oncogenic pathway through a single pathway involving serum glutamic-oxaloacetic transaminase, which maintains the cellular redox states essential to mitochondria and offers innovative therapeutic targets in combination with metformin[62].
Metformin can exert antitumor activity by increasing CD8+ T-cells[63,64]. It might inhibit apoptosis of CD8+ tumor infiltrating lymphocytes and prevent immune exhaustion[63,65]. Furthermore, metformin might adjust the expression profile of immune checkpoints[66], such as programmed death ligand 1, in the context of the neoplasm[37], thereby suggesting that a combination of metformin might have the potential to enhance the strength of cancer immunotherapy[63].
There is evidence that epigenomic modifications by metformin may contribute to its anti-cancer properties[67]. Metformin might regulate the activity of numerous epigenetic modifying enzymes, principally by modulating the activation of AMPK. Activated AMPK can phosphorylate several substrates, comprising epigenetic enzymes, such as histone acetyltransferases (HATs), class II histone deacetylases (HDACs) and DNA methyltransferases (DNMTs), usually resulting in their inhibition; however, HAT1 activity may be increased. Metformin has also been related to the diminished expression of various histone methyltransferases[68], enhancing the activity of the class III HDAC SIRT1 and minimizing the influence of DNMT inhibitors[69,70].
Cell lines and animal models: Metformin promotes cell cycle arrest in the G0/G1 phase in colorectal cancer (CRC) cell lines. It also decreases the expression of c-Myc and causes down-regulation of IGF-1R[71]. Consequently, up-regulation of the adenosine A1 receptor induces apoptosis[72]. Additionally, it was shown that metformin enhances the activity of the Sprouty2 gene, which suppresses colon cancer growth[73].
The combination of metformin with 5-FU was investigated on the SW620 CRC cell line and on patients with DM2. The study showed that metformin plus 5-FU treatment significantly inhibited the proliferation of SW620 cells compared with that in monotherapy. Additionally, the examination of 86 CRC tissue samples obtained from patients with DM2 revealed that treatment with metformin decreased the proportion of poorly differentiated tumors[74]. Moreover, a synergistic effect of 5-FU and metformin was observed in a 5-FU resistant cell line[74] and metformin radiosensitizer CRC cells, with reduced survival of ionization-resistant cells[75]. Consistently, the association of oxaliplatin, 5-FU and metformin also demonstrated a superior anti-tumor activity in chemoresistant HT-29, and HCT-116 cells compared to that with the drugs separately[76].
In 1977, it was firstly reported that phenformin inhibits metabolic immuno
Metformin and CRC risk: As shown in Table 1 many case-control and cohort studies and associated meta-analyses have evaluated DS cancer risk and metformin use. Specifically, a decreased risk of CRC was found in the majority of studies[82-86], but no association or an increased risk of CRC was found in some of them[87-91]. Although these different results may be related to biases, a large cohort study that used adequate methods to minimize biases also concluded that metformin use decreased the risk of CRC[92].
| Ref. | Study design and population | Inclusion criteria | Combined interventions /drugs | Main findings |
| Comparison groups | Risk estimates and 95%CI | |||
| Colorectal cancer | ||||
| Cardel et al[82], 2014 | Case-control study. Cases-controls: 2088:9060 | Cases: DM2 with CRC. Controls: DM2 without CRC | Metformin user vs nonuser | OR: 0.83 (0.68-1.00) |
| Lee et al[83], 2011 | Prospective Cohort, Taiwan. n = 480984 | DM2 and cancer free subjects | Metformin user vs nonuser | HR: 0.36 (0.13-0.98) |
| Sehdev et al[84], 2015 | Case control study. Cases-controls: 2682:5365 | Cases: DM2 with CRC. Controls: DM2 without CRC | Metformin user vs nonuser | OR: 0.85 (0.76-0.95) |
| Tseng et al[84]., 2012 | Retrospective Cohort. Men: 493704. Women: 502139 | Subjects covered by National Health Insurance without CRC | Metformin user vs nonuser | RR: 0.64 (0.49-0.84) |
| Zhang et al[86], 2011 | Meta-analysis. 108161 DM2 patients | Studies conducted in humans that evaluate metformin and CRC | Metformin user vs nonuser | RR: 0.63 (0.47-0.84) |
| Kowall et al[87], 2015 | Retrospective Cohort, United Kingdom. 80263 DM2 patients | Patients aged 30-89 years with DM2 diagnosis | Metformin user vs sulfonylurea user | HR: 1.04 (0.82-1.31) |
| Lin et al[88], 2015 | Prospective Cohort. 36270 DM2 patients. 145080 non DM2 | Patients older than 20 years old DM2 and Cancer- free | Metformin user vs nonuser | HR: 0.74 (0.53-1.03) |
| Smiechowski et al[89], 2013 | Case-control, United Kingdom. Cases-controls: 607:5837 | DM patients treated with non-insulin antidiabetic agents | Metformin user vs nonuser | RR: 0.93 (0.73-1.18) |
| Bodmer et al[90], 2012 | Case control, United Kingdom. Cases-controls: 920:5519 | Cases: DM2 with CRC. Controls: DM2 without CRC | Metformin user vs nonuser | Men: OR: 1.81 (1.25-2.62). Women: OR: 1.00 (0.63-1.58) |
| Knapen et al[91], 2013 | Retrospective Cohort, Denmark. 177281 DM2 with OHA | Oral antidiabetic drug users were matched 1:3 with population-based reference group | Biguanide user vs non-diabetic | HR: 1.19 (1.08-1.30) |
| Bradley et al[92], 2018 | Retrospective Cohort, Northern California. 47351 DM2 patients | DM2 and no history of cancer or metformin use | Long-term metformin use ( 5 years) vs nonuser | All population: HR: 0.78 (0.60-1.02). Men: HR: 0.65 (0.45-0.94) |
| Liu et al[175], 2017 | Meta-analysis. 20 case-control and cohort studies | Studies about metformin therapy and risk of adenoma/CRC in DM2 patients | Metformin user vs nonuser | Adenoma: OR: 0.75 (0.59-0.97). Carcinoma: OR: 0.781 (0.7-0.87) |
| Higurashi et al[94], 2016 | RCT, phase 3. n = 151 patients with resected adenomas or polyps | Non- diabetic adult patients who had previously had single or multiple colorectal adenomas or polyps resected by endoscopy | Metformin 250 daily or placebo (1:1) for 1 yr | Adenoma: RR 0.60 (0.39-0.92) |
| Gastric cancer | ||||
| Tseng et al[107], 2016 | Retrospective Cohort, Taiwan. 287971 DM2 with metformin. 16217 DM2 without metformin | DM2 patients newly treated with antidiabetic drugs | Metformin user vs nonuser | HR: 0.45 (0.36-0.56) |
| Dulskas et al[108], 2020 | Retrospective Cohort study. n = 99992 | DM2 patients with gastric cancer | Metformin user vs nonuser | SIR: 0.75 (0.66-0.86) |
| Ruiter et al[109], 2012 | Retrospective Cohort study. 85289 DM2 patients | DM2 with more than one prescription of antidiabetic drugs | Metformin user vs sulfonylurea user | HR: 0.90 (0.88-0.91) |
| Kim et al[110], 2014 | Retrospective cohort study. 39978 DM2 patients | DM2 receiving oral antidiabetic drugs | Metformin user and non-insulin user vs nonuser | HR: 0.73 (0.53-1.01) |
| Cheung et al[112], 2019 | Prospective Cohort study. 7266 DM2 | DM2 with prescription of therapy for H. pylori. Exclusion: history of GC | Metformin user vs nonuser | HR: 0.49 (0.24-0.98) |
| Tsilidis et al[113], 2014 | Retrospective Cohort study. 51484 metformin. 18264 sulfonylureas | DM2 receiving oral antidiabetic drugs | Metformin user vs sulfonylurea user | HR: 0.96 (0.60-1.56) |
| de Jong et al[114], 2017 | Retrospective Cohort study, Netherlands. 57621 DM2 with OHA | DM2 receiving oral antidiabetic drugs | Metformin user vs nonuser | HR: 0.97 (0.82-1.15) |
| Zheng et al[115], 2019 | Prospective Cohort study. 544130 DM2 patients | Diabetes Cohort: DM2 receiving antidiabetic drugs. Matched cohort: common-medication users. Exclusion: History of GC or gastrectomy | Metformin user vs nonuser | Non-cardia: HR: 0.93 (0.78-1.12). Cardia: HR: 1.49 (1.09-2.02) |
| Shuai et al[116], 2020 | Meta-analysis. 11 cohort studies | Studies conducted in humans that evaluate metformin and GC risk | Metformin user vs nonuser | HR: 0.79 (0.62-1.00) |
| Zhou et al[117], 2017 | Meta-analysis. 7 Cohort studies. n = 591077 | Studies conducted in humans that evaluate metformin and GC risk | Metformin user vs nonuser | HR: 0.76 (0.64-0.91) |
| Pancreatic ductal adenocarcinoma | ||||
| Currie et al[123], 2009 | Retrospective cohort study. n = 62.809 DM2. Comparison between treatment: Metformin alone; Sulfonylurea alone; metformin plus sulfonylurea; insulin | DM2 developed > 40 years of age; United Kingdom residents | Metformin vs Sulfonylurea. Metformin vs Insulin | HR: 0.20 (0.11-0.36). HR: 0.22 (0.12-0.38) |
| Li et al[124], 2009 | Hospital-based case control. Cases-controls: 973:863. Comparison between treatment: Metformin; insulin secretagogues; Other antidiabetic medications; insulin | DM subjects; cases: Newly PDAC diagnosed. Controls: Nonblood relative controls; United States residents | Metformin user vs nonuser | OR: 0.38 (0.22-0.69) |
| Soranna et al[126], 2012 | Meta-analysis of 17 case-control and cohort studies. Any cancer: 17 case-control and cohort studies; 37632 cases. PDAC: 4 case-controls and retrospective cohort studies; 1192 cases | DM2 patients exposed to metformin alone or combined to sulfonylurea | Metformin user vs nonuser | RR: 0.38 (0.14-0.91) |
| Zhang et al[125], 2013 | Meta-analysis of 37 case-control and cohort studies. n = 1535636 | DM2 patients on treatment | Metformin user vs nonuser | SRR: 0.54 (0.35-0.83) |
| Hepatocellular carcinoma | ||||
| Donadon et al[159], 2010 | Clinic-hospitalbased case control. Cases-controls: 190:359 | Cases: HCC patients. Controls: Liver cirrhosis patients and healthy controls | Metformin vs sulfonylurea. Metformin vs insulin | OR: 0.39 (0.22-0.73). OR: 0.21 (0.11-0.42) |
| Hassan et al[158], 2010 | Hospital-based case control. Cases-controls: 122:86 | Cases: HCC. Controls: Healthy controls | Metformin user vs nonuser | OR: 0.30 (0.20-0.60) |
| Ma et al[160], 2017 | Meta-analysis of 19 case-control and cohort studies and post hoc analysis of RCT of DM2 patients. n = 550.882 | DM2 exposed to metformin or biguanide | Metformin user vs nonuser | OR: 0.52 (0.40-0.68) |
| Intrahepatic cholangiocarcinoma | ||||
| Chaiteerakij et al[168], 2013 | Clinic-hospital based case-control. Cases-controls: 612:594 | Cases: ICC patients. Controls: Non-cancer patients | Metformin user vs nonuser | OR: 0.40 (0.20-0.90) |
Saliently, Cardel et al[82] demonstrated, in a case-control study, that the risk of CRC was decreased by 17% (OR: 0.83, 95%CI: 0.74-0.92) among patients treated with metformin compared to that among patients not using metformin[82], while Liu et al[93] showed a 22% risk reduction for the development of CRC[93]. Importantly, the role of metformin for CRC prophylaxis was addressed in a prospective Japanese phase III trial that demonstrated that low metformin doses for 1-year reduced polyp formation and colorectal adenomas in non-diabetic patients at high risk for new polyps[94]. However, further studies are necessary to draw a definitive conclusion.
Table 2 summarizes the clinical studies of metformin on DS cancers treatment. Specifically related to CRC, a Korean study of 595 patients with diabetes who had CRC with clinical stages I to IV showed that patients using metformin had higher overall survival (OS) and specific cancer survival compared to patients who did not use it[95]. In accordance, metformin use in 424 diabetic patients with CRC was associated with an OS of 76.9 mo vs 56.9 mo in patients not using metformin (P = 0.048)[95]. After adjusting for possible confounding factors, the study showed that patients with DM2 treated with metformin had a 30% increase in OS when compared to patients with DM2 treated with other antidiabetic drugs[95]. Recent meta-analyses demonstrated that metformin increases the OS of patients with CRC, as well as a 10% reduction in the incidence of the disease[96,97]. The ASAMET trial, an ongoing randomized, phase II, double-blind, placebo-controlled trial aims to determine the effect of low-dose aspirin and metformin in patients with stage I-III CRC in reducing CRC mortality rates and adenoma recurrence[98]. The 160 patients with CRC were divided in four arms: aspirin, metformin, aspirin plus metformin and placebo for a duration of 1 year.
| Ref. | Study design and population | Inclusion criteria | Combined interventions /drugs | Main findings |
| Colorectal cancer | ||||
| Ramjeesingh et al[99], 2016 | Retrospective cohort. 1394 all stages CRC patients | Patients with CRC | Metformin user vs nonuser | HR: 0.81 (0.60-1.08) |
| Skinner et al[100], 2013 | Retrospective cohort. 482 locally rectal cancer patients | Locally advanced rectal adenocarcinoma treated with chemoradiation and surgery | Metformin user vs nonuser | pCR: OR: 16.8 (1.6-181.1). OS at 5 and 10 years (metformin vs non): 81% and 79% vs 56% and 39% (P = 0.022) |
| Miranda et al[101], 2016 | Phase 2 Clinical trial. 50 refractory CRC patients | Refractory CRC patients | Metformin 850 mg twice a day+ 5-FU 425 mg/m2 weekly | PFS: 1.8 mo. OS: 7.9 mo. Obese vs lean: 12.4 vs 5.8 mo |
| Bragagnoli et al[102], 2021 | Phase 2 Clinical trial, 41 refractory CRC patients | Refractory CRC patients | Metformin 2500 mg a day+ Irinotecan 125 mg/m2 D1, D8, every 21 d | PFS: 2.4 mo, CI 95%, 2.0-4.5 mo. OS: 8.4 mo, CI 95%, 5.9-10.8 mo |
| El-Fatatry et al[103], 2018 | Clinical Trial, 40 Stage III CRC patients | Stage III CRC patients | FOLFOX 4 12 cycles + metformin 500 mg 3 times a day | Neuropathy grade 2-3 (metformin vs non): 60% vs 95% (P = 0.009) |
| Gastric cancer | ||||
| Lee et al[118], 2016 | Retrospective Cohort, single center in Korea. 1974 GC resected patients: – 132 DM2 with metformin; –192 DM2 without metformin; –1648 non-diabetic | GC patients who underwent curative gastrectomy | Metformin user vs nonuser | OS-HR: 0.58 (0.37-0.93). RFS-HR: 0.63 (0.41-0.98) |
| Lacroix et al[120], 2018 | Retrospective Cohort. 371 Patients | Stage I to III GC patients | Metformin user vs nonuser | OS-HR: 0.73 (0.52-1.01); cancer specific mortality-HR: 0.86 (0.56-1.33) |
| Baglia et al[121], 2019 | Prospective cohort study in Shangai. 543 GC patients | Breast, CRC, lung and GC patients | Metformin user vs nonuser | OS-HR: 1.11 (0.81-1.53). Disease-specific survival-HR: 1.03 (0.73-1.43) |
| Seo et al[119], 2019 | Retrospective cohort study. 2187 GC resected patients: – 103 DM2 with metformin; –139 DM2 without metformin; –1945 non-diabetic | GC patients who underwent curative gastrectomy | Metformin user vs nonuser | HR: 0.45 (0.30-0.66) |
| PDAC | ||||
| Sadeghi et al[128], 2012 | Retrospective cohort. n = 302 | DM2 patients. All stages. United States single center | Metformin user vs nonuser | HR: 0.64 (0.48-0.86) |
| Chaiteerakij et al[129], 2016 | Retrospective cohort. n = 980 | DM2 patients. All stages. United States single center | Metformin user vs nonuser | HR: 0.93 (0.81-1.07) |
| Lee et al[133], 2016 | Retrospective cohort. n = 237 | DM2 patients. All stages. Korean single center | Use of metformin ≥ 1-mo post-diagnosis vs nonuser | HR: 0.61 (0.46-0.81) |
| Ambe et al[130], 2016 | Prospective cohort study n = 44 | DM2 patients. Resected PDAC, stage I-II. United States single center | Metformin user vs nonuser | HR: 0.54 (0.16-1.86) |
| Cerullo et al[131], 2016 | Retrospective cohort. n = 3393 | Resected PDAC United States population based | Metformin use after surgery vs nonuser | HR: 0.79 (0.67-0.93) |
| Jang et al[132], 2017 | Prospective cohort. n = 764 | DM2, OHA user. Resected Korean population based | Metformin user vs nonuser | HR: 0.73 (0.61-0.87) |
| Hwang et al[135], 2013 | Retrospective cohort. n = 516 | DM2 patients. Locally advanced and metastatic. United Kingdom population based | Use of metformin peridiagnosis vs nonuser | HR: 1.11 (0.89-1.38) |
| Choi et al[134], 2016 | Retrospective cohort. n = 183 | DM2 patients. Locally advanced and metastatic. Korean single center | Metformin user vs nonuser | HR: 0.69 (0.49-0.97) |
| Kordes et al[137], 2015 | RCT, n = 121 | Locally advanced and metastatic. Multicentric. Netherlands | Gemcitabine-everolimus (1000 mg/m2 D1, 8, 15-every 28 d-1.000 mg/d) +/- metformin (2000 mg/d) | HR: 1.05 (0.72-1.55) |
| Reni et al[138], 2016 | RCT. n = 60 | Metastatic. Single center. Italian | PEXG (cisplatin-epirubicin-capecitabine-gemcitabine: 30 mg/m2 D1,14- 30 mg/m2 D1,14-2500 mg/m2 D1–28 – 800 mg/m2 D1–14) +/- metformin 2000 mg/d | HR: 1.56 (0.87-2.80) |
| Zhou et al[136], 2017 | Meta-analysis12 cohort studies and 2 RCT. n = 94778 | Studies that investigated metformin exposition. All stages PDAC | Metformin user vs nonuser | HR: 0.77 (0.68-0.87) |
| Li et al[139], 2017 | Meta-analysis. 9 cohort study and 2 RCT. n = 8089 | Studies that investigated metformin exposition. All stages PDAC | Metformin user vs nonuser | HR: 0.86 (0.76-0.97) |
| Wan et al[140], 2018 | Meta-analysis 15 cohort studies and 2 RCT, n = 36791 | Studies that investigated metformin exposition. All stages PDAC | Metformin user vs nonuser | HR: 0.88 (0.80-0.97). Asians only HR: 0.74 (0.58-0.94); Stage I-II HR: 0.76 (0.68-0.86); Stage III-IV HR: 1.08 (0.82-1.43) |
| Braghiroli et al[141], 2015 | Single-arm phase II. n = 20 | Locally advanced or metastatic. 2nd line treatment. Single center. Brazilian | Paclitaxel (80 mg/m2 D1, 8, 15 every 28 d) + metfomin 1750 mg/d | DCR at 8 wk 31, 6% |
| Pancreatic neuroendocrine tumor | ||||
| Pusceddu et al[153], 2018 | Retrospective cohort. n = 445 | Locally advanced or metastatic. Multicentric. Italian | No DM2 vs DM2. Metformin user vs nonuser | HR: 0.45 (0.32-0.62). HR: 0.49 (0.34-0.69) |
| Hepatocellular carcinoma | ||||
| Chen et al[163], 2011 | Retrospective cohort. n = 53 | DM2. Early-stage HCC. RFA treated. Single center. Taiwanese | Metformin user vs nonuser | HR: 0.24 (0.07-0.90) |
| Ma et al[164], 2016 | Meta-analysis. 11 cohort studies. n = 3452 | Studies that investigated metformin exposition. HCC patients | Metformin user vs nonuser | HR: 0.59 (0.42-0.83) |
| Intrahepatic cholangiocarcinoma | ||||
| Yang et al[169], 2016 | Retrospective cohort. n = 250 | DM2. Newly diagnosed ICC. United States single center | Metformin user vs nonuser | HR: 0.80 (0.60-1.20) |
The radiotherapy-induced tumor response was improved with metformin in a Korean retrospective study that evaluated patients with localized rectal cancer. The diabetic patients receiving metformin had significantly more tumor regression grade 3-4 (P = 0.029) and higher lymph node downstaging (P = 0.006) as compared to patients not receiving the medication. However, the disease-free survival (DFS) and OS was not affected[99]. Consistently, a study with 482 patients examined the effect of metformin use on pathologic complete response (pCR) rates and outcomes in patients submitted to neoadjuvant chemoradiotherapy for rectal cancer. The pCR rates were higher in patients with DM2 taking metformin (35%) compared with those in nondiabetic patients (16.6%) and patients with DM2 not using metformin (7.5%). Additionally, significantly increased DFS and OS was found in patients taking metformin[100].
A phase II clinical trial addressed the combination of metformin with 5-FU in patients with refractory CRC. It demonstrated a disease control rate in 8 wk of 22%, with a median OS of 7.9 mo and progression-free survival (PFS) of 1.8 mo[101]. A trial of our group with a similar design that analyzed the combination of irinotecan with metformin found 41% disease control rate and OS of 8.2 mo[102]. Further randomized prospective studies are needed to establish metformin as a modern drug for the treatment of refractory CRC.
Interestingly, a randomized trial that included 40 patients with stage III CRC evaluated the use of metformin in preventing oxaliplatin-induced neuropathy. After the 12th cycle of the FOLFOX-4 regimen, in the metformin group, there were fewer patients with grade 2 and 3 neuropathy as compared to the control arm (60% vs 95%, P = 0.009). Moreover, significantly higher total scores on the Ntx-12 questionnaire and pain score were found in the metformin arm. The serum levels of neurotensin and malondialdehyde were also significantly lower in the metformin arm after 6 and 12 cycles[103].
Furthermore, there are ongoing trials evaluating the role of metformin in CRC. We highlight, in adjuvant setting, a phase 3 trial (NCT02614339) with high-risk stage II and stage III CRC that aims to evaluate the impact of metformin for 48 mo on disease free survival. In refractory CRC setting, there is an interesting phase 2 trial is recruiting patients to explore the combination of the immune checkpoint inhibitors, such as nivolumab and metformin (NCT03800602).
The effect of metformin alone or in combination with cisplatin or rapamycin was studied in a tumor xenograft model[104]. It demonstrated that metformin alone decreased tumor volume. The combination of metformin with cisplatin, rapamycin or both increase the effect of each drug alone and inhibited the peritoneal dissemination of gastric cancer (GC)[104]. In accordance, Wu performed an in vitro study with AGS cell lines that analyzed how the association of metformin with cisplatin or adriamycin or paclitaxel enhanced the effects of each drug alone[105]. In striking contrast, Lesan et al[106] showed in vitro that metformin and cisplatin in combination decreased the effects of cisplatin alone[106].
In recent years, several observational studies have shown that metformin reduces the risk of GC[107-112]. The study of Tseng et al[107] demonstrated that GC risk was reduced using metformin, especially when the cumulative duration was more than 2 years[107]. In addition, metformin reduced the risk of GC, while opposite results were observed with sulfonylureas[108].
On the other hand, a study conducted in United Kingdom did not show a difference in GC incidence in patients receiving metformin compared to sulfonylureas[113]. Other reports also could not find any reduction in GC risk associated with metformin us[83,114,115]. Despite that, a meta-analysis showed a 21% reduction in the risk of GC with the use of metformin, in Asians the benefit was more prominent than in Westerners[116]. Another meta-analysis of cohort studies that included 591077 patients found a significantly lower incidence of GC with metformin therapy than other types of therapy (HR: 0.763; 95%CI: 0.642-0.905)[117].
Two retrospective studies conducted by Lee et al[118] and Seo et al[119] concluded that metformin reduced GC recurrence in patients undergoing gastrectomy[118,119]. Lacroix et al[120] showed that metformin improved OS but not cancer specific survival, in contrast, Baglia et al[121] observed that metformin use did not impact patient’s survival[120,121].
More studies are needed to confirm the effect of metformin in GC treatment and chemoprevention. Unfortunately, there are few clinical trials that are ongoing to analyze this question. An interesting phase 2 randomized trial (NCT04114136) are ongoing to evaluate the synergistic effect of metformin, rosiglitazone and anti-PD-1 on the treatment of refractory solid tumors including GC. Metformin could reduce tumor oxygen consumption creating a less hypoxic T cell environment leading to restore its anti-tumor cell function. The trial NCT04033107 analyze the combination of metformin and vitamin C in DS tumors including GC.
Pancreatic cancer is the fourth leading cause of cancer death in the United States and its prognosis remains dismal, encouraging research to discover innovative agents active in its treatment is an urgent unmet need[122]. Pancreatic ductal adenocarcinoma (PDAC) is its most common histologic type. An association between metformin use and decreased PDAC incidence in patients with DM2 was first recognized by two large clinical studies. In a large general practice retrospective cohort, Currie et al[123] reported risk reduction in metformin users related to sulfonylurea users (HR: 0.20; 95%CI: 0.11-0.36) and to insulin-based-treatment users (HR: 0.22; 95%CI: 0.12-0.38). Likewise, in a hospital-based case-control study, Li et al[124] encountered risk reduction in metformin users compared to those who did not use metformin (OR: 0.38; 95%CI: 0.21-0.67). Several meta-analyses have strongly reinforced PDAC risk reduction with metformin use in patients with DM2[18,125-127]. However, this effect should prospectively be confirmed in large prospective clinical trials.
Regarding survival, in a retrospective study, Sadeghi et al[128] reported a 36% lower risk of death (HR: 0.64; 95%CI: 0.48-0.86), OS benefit of 4 mo (15.2 mo vs 11.1 mo) and approximately 2-fold increase in 2-year survival rate (30.1% vs 15.4%) in patients who took metformin compared to those inpatients who did not take metformin. Interestingly, longer survival was only observed in non-metastatic disease, when stratified by disease stage[128]. Further evidence also encountered survival improve
Stimulated by this retrospective evidence, two European groups explored, in randomized clinical trials (RCTs), the association of metformin with gemcitabine-based chemotherapy as first-line treatment of advanced PDAC with negative results on OS improvement[137,138]. Recently, a meta-analysis, with inclusion of two RCTs, re-analyzed the improvement in OS and confirmed benefit in the whole population of diabetic patients with PDAC (HR: 0.86; 95%CI: 0.76-0.97)[139]. Analysis of subgroups in this study demonstrated improved survival in patients with resected or locally advanced tumors but not in the metastatic group. Similar results were observed in another group with a benefit in OS at various stages, which was more evident in the subgroups of less advanced stages and Asian patients[140]. Considering second-line treatment, a single arm prospective study did not reach survival gain of metformin associated to paclitaxel[141]. Results of ongoing clinical trials recently completed are expected with substantial interest. NCT01666730 explores overall survival improvement of metformin associated with modified FOLFOX6 in metastatic patients, NCT02005419 evaluates DFS at 1 year with the combination of metformin and gemcitabine in resected subjects and NCT02048384 analyses safety of metformin with or without rapamycin after disease stabilization on first line chemotherapy in metastatic individuals.
This clinical evidence is associated with the pre-clinical data that pancreatic cancer cells are sensitive to inhibition of oxidative phosphorylation, decreases in insulin-IGF signaling and inhibition of the mTOR pathway through AMPK activation, which are some of the major antineoplastic effects of metformin[39,142-145]. Identifying predictive or prognostic factors of response to metformin should be of relevance to select patients most likely to benefit from the effects of metformin[39]. Recent advances in molecular characterization might distinguish different biology and response to therapy in patients with morphologically similar PDAC and may be incorporated into clinical trials[146-148]. Moreover, the recently experienced challenge of standard of care in advanced pancreatic cancer treatment with polychemotherapy also brings new perspectives, as patients experience longer survival with the need to combine other active agents[149,150]. Future trials would include disease stage, identification of biomarkers and concentrations of metformin in neoplastic tissue to powerfully evaluate the benefit of metformin in the treatment of PDAC.
Another pancreatic neoplasm with rising incidence is pancreatic neuroendocrine tumors (panNETs)[151]. Few studies have evaluated the clinical benefit of metformin in the treatment of panNETs[152]. Pusceddu et al[153], in a multicentric retrospective cohort of patients receiving everolimus with or without somatostatin analogues, reported increased PFS in diabetic patients exposed to metformin compared to diabetic patients not exposed to metformin or non-diabetic patients [44.2 vs 20.8 mo (HR: 0.49; 95%CI: 0.34-0.69) or 15.1 mo (HR: 0.45; 95%CI: 0.32-0.62), respectively][153]. This result correlates with in vitro evidence that metformin decreases proliferation in human panNET cell lines[154,155]. A recent study demonstrated that the combination of metformin and everolimus strongly inhibited human panNET cell proliferation through mTOR suppression, compared to each agent used alone[156]. Results of the ongoing NCT02294006 prospective trial are expected to better evaluate the effects of this experimental treatment on PFS at 12 mo.
The incidence of hepatocellular carcinoma (HCC) has strongly increased in last two decades, as well as the prevalence of its metabolic risk factors[156,157]. Hassan et al[158] and Donadon et al[159], in hospital-based case-control studies, first observed the strong association of metformin use and reduced risk of HCC in subjects with DM2 (HR: 0.15; 95%CI: 0.04-0.50) (HR: 0.30; 95%CI: 0.20-0.60)[156,158,159]. This protective effect was validated by accumulated evidence of observational studies including more than 0.5 million subjects (OR: 0.52; 95%CI: 0.40-0.68), being more evident in case-control than in cohort studies and without significance in the post hoc analysis of RCTs[160-162]. These data suggest an association between metformin use and reduced HCC incidence that needs to be confirmed in prospective clinical trials.
Improvement in HCC survival was first reported by Chen et al[163] in an early-stage cohort of patients treated with radiofrequency ablation with longer OS in metformin users compared to non-users (HR: 0.24; 95%CI: 0.07-0.90)[163]. A meta-analysis of 11 cohort studies was in accordance with better prognosis related to metformin use in patients with HCC related to their counterparts (HR: 0.59; 95%CI: 0.42-0.83)[164].
Although the antineoplastic effects of metformin in liver cancer are not completely understood, pre-clinical evidence observed inhibition of proliferation and induction of cell cycle arrest and apoptosis in HCC cells through AMPK activation[165,166]. Future prospective trials should explore the potential benefit of metformin in prevention and treatment of HCC.
Intrahepatic cholangiocarcinoma (ICC) is the second most common hepatic cancer, and its incidence has markedly increased in the last decades[167]. Chaiteerakij et al[168], in a clinic-hospital-based retrospective cohort, reported 60% reduced risk of ICC in patients with DM2 who used metformin related to non-users (OR: 0.4; 95%CI: 0.2-0.9)[168]. The same group, however, did not encounter better prognosis in patients with DM2 with ICC taking metformin (HR: 0.8; 95%CI: 0.6-1.2)[169]. Although gallbladder cancer (GBC) is the most common biliary tract cancer[170], no clinical data and scarce basic evidence have explored the antineoplastic effects of metformin and its potential mechanisms of action in GBC.
Regarding comprehension of the possible mechanistic effects of metformin on ICC and GBC there are some in vitro and in vivo evidence. Overall, the studies observed the induction of apoptosis and cell cycle arrest mediated by activation of the AMPK-mTOR axis[171-173]. The association of metformin in combination with gemcitabine and cisplatin (the standard of care for advanced ICC) enhanced the antiproliferative effects of treatment in a cell model through their effects on AMPK, cyclin D1 and caspase-3[174]. Furthermore, Liu et al[175] first observed the decreased survival of GBC cells via inhibition of phosphorylated Akt (p-Akt) and Bcl-2 signaling[175]. Likewise, metformin inhibited GBC cell proliferation via downregulation of HIF-1α and VEGF and promoted cell cycle arrest by reduction of cyclin D1 expression in different animal experiments[176,177]. The association of metformin with cisplatin also promoted reduced expression of p-Akt and cyclin D1 downregulation, resulting in a synergistic antiprolife
These pre-clinical and preliminary clinical evidence highlights the need for metformin to be more deeply explored in clinical studies of ICC and GBC prevention and treatment. Considering the rationale that metformin may be active in the prevention and treatment of ICC and limited clinical data, exploratory studies should address this issue for a better understanding of its benefit in these clinical settings.
DS tumors are often associated to high morbidity and mortality and their incidence has increased over recent decades[179]. Recognition of its main risk factors and conditions of worse prognosis as well as development of strategies for prevention and treatment urges. In this context, projection of a worldwide burden of cancer attributable to diabetes and excess weight for the near future is an alarming public health concern[180]. Association of several cancers, including many DS tumors to diabetes and obesity have already been recognized (IARC, WCRF). Further strategies of prevention and treatment urge to be known. The large amount of evidence presented herein supports the idea of an important effect of metformin in decreasing risk and improving prognosis of several DS tumors.
Although most clinical studies presented here are retrospective that are often limited by immortal time and selection bias, recent discoveries of pre-clinical research on antineoplastic effects of metformin establish biological plausibility for the clinical data and reinforce the interest on its effects in carcinogenesis and cancer progression. These preclinical and clinical evidence supports running of adequately powered trials to investigate clinical use of metformin on DS tumors treatment. This should consider diabetic status, predictive biomarkers, disease stage and treatment setting. Concerning chemoprevention, safety, low cost, and widespread access are key to its feasibility. Therefore, repurposing metformin for DS cancer treatment is a scientific field of remarkable interest as it focuses on a global public health problem.
Currently, clinical research is considered a job with its inherent needed professional skills[181]. Taking in consideration that the low metformin cost does not impact the expensive process of drug repurposing, the development of this potential anti-cancer drug has been hampered. Moreover, the current stage of metformin clinical development needs testing in large, randomized, genome-guided, multicenter trials. These aspects explain, at least in part, the shortage of current studies on metformin in cancer prevention and treatment despite the large number of pre-clinical and clinical evidence indicating its potential benefit. We hope that this comprehensive review integrating the potential mechanisms, pre-clinical and clinical studies of metformin as anticancer agent alert the DS cancer community for the need of studying metformin effects in more specific clinical scenarios.
The remarkable intracellular pathway change caused by oncogenesis and the potential mechanisms of the antitumoral action of metformin have been supported. They have revealed novel target molecules and newly discovered treatment possibilities. In connection with epidemiological, pre-clinical, and clinical research, data support that metformin benefits some patients with DS tumors, requiring strict clinical trials to identify those who might obtain advantage from metformin combinations. Given that the survival outcomes are affected by a multitude of factors, such as cancer type, differentiation, staging and treatment, for adequately repurposing the use of metformin in DS cancers it is essential to take into consideration patient characteristics that may serve as predictive biomarkers of metformin antitumoral effects, such as insulin resistance, diabetes, body composition, and chronic diseases related to inflammation, as well as the specific tumor driven oncogenic pathway, which may interfere with the direct and indirect antitumoral effects of metformin.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mao Y, Ou CL, Xiao M, Yoshida S S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
| 1. | Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 685] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 2. | Pollak M. Insulin, insulin-like growth factors and neoplasia. Best Pract Res Clin Endocrinol Metab. 2008;22:625-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol. 2016;34:4270-4276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 660] [Article Influence: 73.3] [Reference Citation Analysis (1)] |
| 4. | Wu L, Zhu J, Prokop LJ, Murad MH. Pharmacologic Therapy of Diabetes and Overall Cancer Risk and Mortality: A Meta-Analysis of 265 Studies. Sci Rep. 2015;5:10147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 532] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Lega IC, Lipscombe LL. Review: Diabetes, Obesity, and Cancer-Pathophysiology and Clinical Implications. Endocr Rev. 2020;41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 7. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5283] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 8. | Singh S, Singh H, Singh PP, Murad MH, Limburg PJ. Antidiabetic medications and the risk of colorectal cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2013;22:2258-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Osório-Costa F, Rocha GZ, Dias MM, Carvalheira JB. Epidemiological and molecular mechanisms aspects linking obesity and cancer. Arq Bras Endocrinol Metabol. 2009;53:213-226. [PubMed] |
| 10. | De Bruijn KM, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CH. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg. 2013;100:1421-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Cunha Júnior AD, Pericole FV, Carvalheira JBC. Metformin and blood cancers. Clinics (Sao Paulo). 2018;73:e412s. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, Zhang H, Li Z. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 472] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 13. | Luo W, Cao Y, Liao C, Gao F. Diabetes mellitus and the incidence and mortality of colorectal cancer: a meta-analysis of 24 cohort studies. Colorectal Dis. 2012;14:1307-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Chen J, Han Y, Xu C, Xiao T, Wang B. Effect of type 2 diabetes mellitus on the risk for hepatocellular carcinoma in chronic liver diseases: a meta-analysis of cohort studies. Eur J Cancer Prev. 2015;24:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Singh PP, Shi Q, Foster NR, Grothey A, Nair SG, Chan E, Shields AF, Goldberg RM, Gill S, Kahlenberg MS, Sinicrope FA, Sargent DJ, Alberts SR. Relationship Between Metformin Use and Recurrence and Survival in Patients With Resected Stage III Colon Cancer Receiving Adjuvant Chemotherapy: Results From North Central Cancer Treatment Group N0147 (Alliance). Oncologist. 2016;21:1509-1521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Bailey CJ. Metformin: its botanical background. Pract Diab Int. 2004;21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1692] [Cited by in RCA: 1821] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 18. | Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2010;3:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 686] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 19. | Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7:e33411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 444] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 20. | Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8:e71583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 337] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 21. | Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila). 2014;7:867-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 326] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 22. | Chen G, Xu S, Renko K, Derwahl M. Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab. 2012;97:E510-E520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | Bednar F, Simeone DM. Metformin and cancer stem cells: old drug, new targets. Cancer Prev Res (Phila). 2012;5:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Gong J, Kelekar G, Shen J, Kaur S, Mita M. The expanding role of metformin in cancer: an update on antitumor mechanisms and clinical development. Target Oncol. 2016;11:447-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 404] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 26. | Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer. 2010;17:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 27. | Choi YK, Park KG. Metabolic roles of AMPK and metformin in cancer cells. Mol Cells. 2013;36:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269-10273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 842] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 29. | Verdura S, Cuyàs E, Martin-Castillo B, Menendez JA. Metformin as an archetype immuno-metabolic adjuvant for cancer immunotherapy. Oncoimmunology. 2019;8:e1633235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Rogers CJ, Prabhu KS, Vijay-Kumar M. The microbiome and obesity-an established risk for certain types of cancer. Cancer J. 2014;20:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Bryrup T, Thomsen CW, Kern T, Allin KH, Brandslund I, Jørgensen NR, Vestergaard H, Hansen T, Hansen TH, Pedersen O, Nielsen T. Metformin-induced changes of the gut microbiota in healthy young men: results of a non-blinded, one-armed intervention study. Diabetologia. 2019;62:1024-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 32. | Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3802] [Cited by in RCA: 4211] [Article Influence: 175.5] [Reference Citation Analysis (0)] |
| 33. | Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420-2425. [PubMed] |
| 34. | Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 840] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 35. | Martin M, Marais R. Metformin: a diabetes drug for cancer, or a cancer drug for diabetics? J Clin Oncol. 2012;30:2698-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Zakikhani M, Bazile M, Hashemi S, Javeshghani S, Avizonis D, St Pierre J, Pollak MN. Alterations in cellular energy metabolism associated with the antiproliferative effects of the ATM inhibitor KU-55933 and with metformin. PLoS One. 2012;7:e49513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Janzer A, German NJ, Gonzalez-Herrera KN, Asara JM, Haigis MC, Struhl K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proc Natl Acad Sci USA. 2014;111:10574-10579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 38. | Nair V, Pathi S, Jutooru I, Sreevalsan S, Basha R, Abdelrahim M, Samudio I, Safe S. Metformin inhibits pancreatic cancer cell and tumor growth and downregulates Sp transcription factors. Carcinogenesis. 2013;34:2870-2879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Nair V, Sreevalsan S, Basha R, Abdelrahim M, Abudayyeh A, Rodrigues Hoffman A, Safe S. Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factors. J Biol Chem. 2014;289:27692-27701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 40. | Blandino G, Valerio M, Cioce M, Mori F, Casadei L, Pulito C, Sacconi A, Biagioni F, Cortese G, Galanti S, Manetti C, Citro G, Muti P, Strano S. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat Commun. 2012;3:865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 41. | Li W, Yuan Y, Huang L, Qiao M, Zhang Y. Metformin alters the expression profiles of microRNAs in human pancreatic cancer cells. Diabetes Res Clin Pract. 2012;96:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Algire C, Moiseeva O, Deschênes-Simard X, Amrein L, Petruccelli L, Birman E, Viollet B, Ferbeyre G, Pollak MN. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila). 2012;5:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 269] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 43. | Nakamura M, Ogo A, Yamura M, Yamaguchi Y, Nakashima H. Metformin suppresses sonic hedgehog expression in pancreatic cancer cells. Anticancer Res. 2014;34:1765-1769. [PubMed] |
| 44. | Rios A, Hsu SH, Blanco A, Buryanek J, Day AL, McGuire MF, Brown RE. Durable response of glioblastoma to adjuvant therapy consisting of temozolomide and a weekly dose of AMD3100 (plerixafor), a CXCR4 inhibitor, together with lapatinib, metformin and niacinamide. Oncoscience. 2016;3:156-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Dirat B, Ader I, Golzio M, Massa F, Mettouchi A, Laurent K, Larbret F, Malavaud B, Cormont M, Lemichez E, Cuvillier O, Tanti JF, Bost F. Inhibition of the GTPase Rac1 mediates the antimigratory effects of metformin in prostate cancer cells. Mol Cancer Ther. 2015;14:586-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E 3rd, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci USA. 2007;104:16158-16163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 559] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 47. | Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 420] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 48. | Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. J Mol Endocrinol. 2012;48:R31-R43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 265] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 49. | Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila). 2010;3:1066-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 342] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 50. | Sarfstein R, Friedman Y, Attias-Geva Z, Fishman A, Bruchim I, Werner H. Metformin downregulates the insulin/IGF-I signaling pathway and inhibits different uterine serous carcinoma (USC) cells proliferation and migration in p53-dependent or -independent manners. PLoS One. 2013;8:e61537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 51. | Zakikhani M, Blouin MJ, Piura E, Pollak MN. Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Res Treat. 2010;123:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 52. | El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223-228. [PubMed] |
| 53. | Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577-590. [PubMed] |
| 54. | Cerezo M, Tichet M, Abbe P, Ohanna M, Lehraiki A, Rouaud F, Allegra M, Giacchero D, Bahadoran P, Bertolotto C, Tartare-Deckert S, Ballotti R, Rocchi S. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther. 2013;12:1605-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 55. | Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti JF, Giorgetti-Peraldi S, Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366-4372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 497] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 56. | Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci USA. 2013;110:972-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 348] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 57. | Rattan R, Giri S, Hartmann LC, Shridhar V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J Cell Mol Med. 2011;15:166-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 58. | Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 708] [Cited by in RCA: 690] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 59. | Kim SH, Kim SC, Ku JL. Metformin increases chemo-sensitivity via gene downregulation encoding DNA replication proteins in 5-Fu resistant colorectal cancer cells. Oncotarget. 2017;8:56546-56557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 60. | Rocha GZ, Dias MM, Ropelle ER, Osório-Costa F, Rossato FA, Vercesi AE, Saad MJ, Carvalheira JB. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin Cancer Res. 2011;17:3993-4005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 61. | Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budigner GS, Chandel NS. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 864] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 62. | Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, Kang Y, Fleming JB, Bardeesy N, Asara JM, Haigis MC, DePinho RA, Cantley LC, Kimmelman AC. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1227] [Cited by in RCA: 1524] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 63. | Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci USA. 2015;112:1809-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 434] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 64. | Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1326] [Cited by in RCA: 1280] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 65. | Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | Zhang JJ, Zhang QS, Li ZQ, Zhou JW, Du J. Metformin attenuates PD-L1 expression through activating Hippo signaling pathway in colorectal cancer cells. Am J Transl Res. 2019;11:6965-6976. [PubMed] |
| 67. | Bridgeman SC, Ellison GC, Melton PE, Newsholme P, Mamotte CDS. Epigenetic effects of metformin: From molecular mechanisms to clinical implications. Diabetes Obes Metab. 2018;20:1553-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 68. | White-Al Habeeb NM, Garcia J, Fleshner N, Bapat B. Metformin Elicits Antitumor Effects and Downregulates the Histone Methyltransferase Multiple Myeloma SET Domain (MMSET) in Prostate Cancer Cells. Prostate. 2016;76:1507-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Zhang E, Guo Q, Gao H, Xu R, Teng S, Wu Y. Metformin and Resveratrol Inhibited High Glucose-Induced Metabolic Memory of Endothelial Senescence through SIRT1/p300/p53/p21 Pathway. PLoS One. 2015;10:e0143814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 70. | DU Y, Zheng H, Wang J, Ren Y, Li M, Gong C, Xu F, Yang C. Metformin inhibits histone H2B monoubiquitination and downstream gene transcription in human breast cancer cells. Oncol Lett. 2014;8:809-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Mogavero A, Maiorana MV, Zanutto S, Varinelli L, Bozzi F, Belfiore A, Volpi CC, Gloghini A, Pierotti MA, Gariboldi M. Metformin transiently inhibits colorectal cancer cell proliferation as a result of either AMPK activation or increased ROS production. Sci Rep. 2017;7:15992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 72. | Lan B, Zhang J, Zhang P, Zhang W, Yang S, Lu D, Li W, Dai Q. Metformin suppresses CRC growth by inducing apoptosis via ADORA1. Front Biosci (Landmark Ed). 2017;22:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Feng YH, Wu CL, Shiau AL, Lee JC, Chang JG, Lu PJ, Tung CL, Feng LY, Huang WT, Tsao CJ. MicroRNA-21-mediated regulation of Sprouty2 protein expression enhances the cytotoxic effect of 5-fluorouracil and metformin in colon cancer cells. Int J Mol Med. 2012;29:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Zhang Y, Guan M, Zheng Z, Zhang Q, Gao F, Xue Y. Effects of metformin on CD133+ colorectal cancer cells in diabetic patients. PLoS One. 2013;8:e81264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 75. | Jeong YK, Kim MS, Lee JY, Kim EH, Ha H. Metformin Radiosensitizes p53-Deficient Colorectal Cancer Cells through Induction of G2/M Arrest and Inhibition of DNA Repair Proteins. PLoS One. 2015;10:e0143596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 76. | Nangia-Makker P, Yu Y, Vasudevan A, Farhana L, Rajendra SG, Levi E, Majumdar AP. Metformin: a potential therapeutic agent for recurrent colon cancer. PLoS One. 2014;9:e84369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 77. | Dilman VM. Metabolic immunodepression which increases the risk of cancer. Lancet. 1977;2:1207-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Tomimoto A, Endo H, Sugiyama M, Fujisawa T, Hosono K, Takahashi H, Nakajima N, Nagashima Y, Wada K, Nakagama H, Nakajima A. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 2008;99:2136-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 79. | Hosono K, Endo H, Takahashi H, Sugiyama M, Uchiyama T, Suzuki K, Nozaki Y, Yoneda K, Fujita K, Yoneda M, Inamori M, Tomatsu A, Chihara T, Shimpo K, Nakagama H, Nakajima A. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog. 2010;49:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 80. | Li W, Wang QL, Liu X, Dong SH, Li HX, Li CY, Guo LS, Gao JM, Berger NA, Li L, Ma L, Wu YJ. Combined use of vitamin D3 and metformin exhibits synergistic chemopreventive effects on colorectal neoplasia in rats and mice. Cancer Prev Res (Phila). 2015;8:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Mohamed Suhaimi NA, Phyo WM, Yap HY, Choy SHY, Wei X, Choudhury Y, Tan WJ, Tan LAPY, Foo RSY, Tan SHS, Tiang Z, Wong CF, Koh PK, Tan MH. Metformin Inhibits Cellular Proliferation and Bioenergetics in Colorectal Cancer Patient-Derived Xenografts. Mol Cancer Ther. 2017;16:2035-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 82. | Cardel M, Jensen SM, Pottegård A, Jørgensen TL, Hallas J. Long-term use of metformin and colorectal cancer risk in type II diabetics: a population-based case-control study. Cancer Med. 2014;3:1458-1466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 83. | Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 366] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 84. | Sehdev A, Shih YC, Vekhter B, Bissonnette MB, Olopade OI, Polite BN. Metformin for primary colorectal cancer prevention in patients with diabetes: a case-control study in a US population. Cancer. 2015;121:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 85. | Tseng CH. Diabetes, metformin use, and colon cancer: a population-based cohort study in Taiwan. Eur J Endocrinol. 2012;167:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 86. | Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, Zhao G, Kip KE. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:2323-2328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 87. | Kowall B, Stang A, Rathmann W, Kostev K. No reduced risk of overall, colorectal, lung, breast, and prostate cancer with metformin therapy in diabetic patients: database analyses from Germany and the UK. Pharmacoepidemiol Drug Saf. 2015;24:865-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 88. | Lin CM, Huang HL, Chu FY, Fan HC, Chen HA, Chu DM, Wu LW, Wang CC, Chen WL, Lin SH, Ho SY. Association between Gastroenterological Malignancy and Diabetes Mellitus and Anti-Diabetic Therapy: A Nationwide, Population-Based Cohort Study. PLoS One. 2015;10:e0125421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 89. | Smiechowski B, Azoulay L, Yin H, Pollak MN, Suissa S. The use of metformin and colorectal cancer incidence in patients with type II diabetes mellitus. Cancer Epidemiol Biomarkers Prev. 2013;22:1877-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 90. | Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin is not associated with a decreased risk of colorectal cancer: a case-control analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 91. | Knapen LM, Dittrich ST, de Vries F, Starup-Linde J, Vestergaard P, Henry RM, Stolk LM, Neef C, Bazelier MT. Use of biguanides and the risk of colorectal cancer: a register-based cohort study. Curr Drug Saf. 2013;8:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Bradley MC, Ferrara A, Achacoso N, Ehrlich SF, Quesenberry CP Jr, Habel LA. A Cohort Study of Metformin and Colorectal Cancer Risk among Patients with Diabetes Mellitus. Cancer Epidemiol Biomarkers Prev. 2018;27:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 93. | Liu F, Yan L, Wang Z, Lu Y, Chu Y, Li X, Liu Y, Rui D, Nie S, Xiang H. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Oncotarget. 2017;8:16017-16026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 94. | Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, Uchiyama T, Taniguchi L, Hata Y, Uchiyama S, Hattori A, Nagase H, Kessoku T, Arimoto J, Matsuhashi N, Inayama Y, Yamanaka S, Taguri M, Nakajima A. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 239] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 95. | Garrett CR, Hassabo HM, Bhadkamkar NA, Wen S, Baladandayuthapani V, Kee BK, Eng C, Hassan MM. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer. 2012;106:1374-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 96. | Meng F, Song L, Wang W. Metformin Improves Overall Survival of Colorectal Cancer Patients with Diabetes: A Meta-Analysis. J Diabetes Res. 2017;2017:5063239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 97. | He XK, Su TT, Si JM, Sun LM. Metformin Is Associated With Slightly Reduced Risk of Colorectal Cancer and Moderate Survival Benefits in Diabetes Mellitus: A Meta-Analysis. Medicine (Baltimore). 2016;95:e2749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 98. | Petrera M, Paleari L, Clavarezza M, Puntoni M, Caviglia S, Briata IM, Oppezzi M, Mislej EM, Stabuc B, Gnant M, Bachleitner-Hofmann T, Roth W, Scherer D, Haefeli WE, Ulrich CM, DeCensi A. The ASAMET trial: a randomized, phase II, double-blind, placebo-controlled, multicenter, 2 × 2 factorial biomarker study of tertiary prevention with low-dose aspirin and metformin in stage I-III colorectal cancer patients. BMC Cancer. 2018;18:1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 99. | Ramjeesingh R, Orr C, Bricks CS, Hopman WM, Hammad N. A retrospective study on the role of diabetes and metformin in colorectal cancer disease survival. Curr Oncol. 2016;23:e116-e122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 100. | Skinner HD, Crane CH, Garrett CR, Eng C, Chang GJ, Skibber JM, Rodriguez-Bigas MA, Kelly P, Sandulache VC, Delclos ME, Krishnan S, Das P. Metformin use and improved response to therapy in rectal cancer. Cancer Med. 2013;2:99-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 101. | Miranda VC, Braghiroli MI, Faria LD, Bariani G, Alex A, Bezerra Neto JE, Capareli FC, Sabbaga J, Lobo Dos Santos JF, Hoff PM, Riechelmann RP. Phase 2 Trial of Metformin Combined With 5-Fluorouracil in Patients With Refractory Metastatic Colorectal Cancer. Clin Colorectal Cancer 2016; 15: 321-328. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 102. | Bragagnoli AC, Araujo RLC, Ferraz MW, Dos Santos LV, Abdalla KC, Comar F, Santos FA, Oliveira MA, Carvalheira JBC, Cárcano FM, da Silveira Nogueira Lima JP. Metformin plus lrinotecan in patients with refractory colorectal cancer: a phase 2 clinical trial. Br J Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 103. | El-Fatatry BM, Ibrahim OM, Hussien FZ, Mostafa TM. Role of metformin in oxaliplatin-induced peripheral neuropathy in patients with stage III colorectal cancer: randomized, controlled study. Int J Colorectal Dis. 2018;33:1675-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 104. | Yu G, Fang W, Xia T, Chen Y, Gao Y, Jiao X, Huang S, Wang J, Li Z, Xie K. Metformin potentiates rapamycin and cisplatin in gastric cancer in mice. Oncotarget. 2015;6:12748-12762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 105. | Wu X. Effect of metformin combined with chemotherapeutic agents on gastric cancer cell line AGS. Pak J Pharm Sci. 2017;30:1833-1836. [PubMed] |
| 106. | Lesan V, Ghaffari SH, Salaramoli J, Heidari M, Rostami M, Alimoghaddam K, Ghavamzadeh A. Evaluation of antagonistic effects of metformin with Cisplatin in gastric cancer cells. Int J Hematol Oncol Stem Cell Res. 2014;8:12-19. [PubMed] |
| 107. | Tseng CH. Metformin reduces gastric cancer risk in patients with type 2 diabetes mellitus. Aging (Albany NY). 2016;8:1636-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 108. | Dulskas A, Patasius A, Kaceniene A, Linkeviciute-Ulinskiene D, Zabuliene L, Smailyte G. A Cohort Study of Antihyperglycemic Medication Exposure and Gastric Cancer Risk. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, Straus SM, Herings RM, Stricker BH. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 2012;35:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 110. | Kim YI, Kim SY, Cho SJ, Park JH, Choi IJ, Lee YJ, Lee EK, Kook MC, Kim CG, Ryu KW, Kim YW. Long-term metformin use reduces gastric cancer risk in type 2 diabetics without insulin treatment: a nationwide cohort study. Aliment Pharmacol Ther. 2014;39:854-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 111. | Valent F. Diabetes mellitus and cancer of the digestive organs: An Italian population-based cohort study. J Diabetes Complications. 2015;29:1056-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 112. | Cheung KS, Chan EW, Wong AYS, Chen L, Seto WK, Wong ICK, Leung WK. Metformin Use and Gastric Cancer Risk in Diabetic Patients After Helicobacter pylori Eradication. J Natl Cancer Inst. 2019;111:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 113. | Tsilidis KK, Capothanassi D, Allen NE, Rizos EC, Lopez DS, van Veldhoven K, Sacerdote C, Ashby D, Vineis P, Tzoulaki I, Ioannidis JP. Metformin does not affect cancer risk: a cohort study in the U.K. Clinical Practice Research Datalink analyzed like an intention-to-treat trial. Diabetes Care. 2014;37:2522-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 114. | de Jong RG, Burden AM, de Kort S, van Herk-Sukel MP, Vissers PA, Janssen PK, Haak HR, Masclee AA, de Vries F, Janssen-Heijnen ML. No Decreased Risk of Gastrointestinal Cancers in Users of Metformin in The Netherlands; A Time-Varying Analysis of Metformin Exposure. Cancer Prev Res (Phila). 2017;10:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Zheng J, Xie SH, Santoni G, Lagergren J. Metformin use and risk of gastric adenocarcinoma in a Swedish population-based cohort study. Br J Cancer. 2019;121:877-882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 116. | Shuai Y, Li C, Zhou X. The effect of metformin on gastric cancer in patients with type 2 diabetes: a systematic review and meta-analysis. Clin Transl Oncol. 2020;22:1580-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 117. | Zhou XL, Xue WH, Ding XF, Li LF, Dou MM, Zhang WJ, Lv Z, Fan ZR, Zhao J, Wang LX. Association between metformin and the risk of gastric cancer in patients with type 2 diabetes mellitus: a meta-analysis of cohort studies. Oncotarget. 2017;8:55622-55631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 118. | Lee CK, Jung M, Jung I, Heo SJ, Jeong YH, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH, Kim HS, Rha SY, Chung HC. Cumulative Metformin Use and Its Impact on Survival in Gastric Cancer Patients After Gastrectomy. Ann Surg. 2016;263:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 119. | Seo HS, Jung YJ, Kim JH, Lee HH, Park CH. The Effect of Metformin on Prognosis in Patients With Locally Advanced Gastric Cancer Associated With Type 2 Diabetes Mellitus. Am J Clin Oncol. 2019;42:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 120. | Lacroix O, Couttenier A, Vaes E, Cardwell CR, De Schutter H, Robert A. Impact of metformin on gastric adenocarcinoma survival: A Belgian population based study. Cancer Epidemiol. 2018;53:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Baglia ML, Cui Y, Zheng T, Yang G, Li H, You M, Xu L, Murff H, Gao YT, Zheng W, Xiang YB, Shu XO. Diabetes Medication Use in Association with Survival among Patients of Breast, Colorectal, Lung, or Gastric Cancer. Cancer Res Treat. 2019;51:538-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 122. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15312] [Article Influence: 3062.4] [Reference Citation Analysis (4)] |
| 123. | Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 848] [Article Influence: 53.0] [Reference Citation Analysis (1)] |
| 124. | Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 476] [Cited by in RCA: 467] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 125. | Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 126. | Soranna D, Scotti L, Zambon A, Bosetti C, Grassi G, Catapano A, La Vecchia C, Mancia G, Corrao G. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist. 2012;17:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 127. | Yu H, Zhong X, Gao P, Shi J, Wu Z, Guo Z, Wang Z, Song Y. The Potential Effect of Metformin on Cancer: An Umbrella Review. Front Endocrinol (Lausanne). 2019;10:617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 128. | Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905-2912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 129. | Chaiteerakij R, Petersen GM, Bamlet WR, Chaffee KG, Zhen DB, Burch PA, Leof ER, Roberts LR, Oberg AL. Metformin Use and Survival of Patients With Pancreatic Cancer: A Cautionary Lesson. J Clin Oncol. 2016;34:1898-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 130. | Ambe CM, Mahipal A, Fulp J, Chen L, Malafa MP. Effect of Metformin Use on Survival in Resectable Pancreatic Cancer: A Single-Institution Experience and Review of the Literature. PLoS One. 2016;11:e0151632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 131. | Cerullo M, Gani F, Chen SY, Canner J, Pawlik TM. Metformin Use Is Associated with Improved Survival in Patients Undergoing Resection for Pancreatic Cancer. J Gastrointest Surg. 2016;20:1572-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 132. | Jang WI, Kim MS, Kang SH, Jo AJ, Kim YJ, Tchoe HJ, Park CM, Kim HJ, Choi JA, Choi HJ, Paik EK, Seo YS, Yoo HJ, Kang JK, Han CJ, Kim SB, Ko MJ. Association between metformin use and mortality in patients with type 2 diabetes mellitus and localized resectable pancreatic cancer: a nationwide population-based study in korea. Oncotarget. 2017;8:9587-9596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 133. | Lee SH, Yoon SH, Lee HS, Chung MJ, Park JY, Park SW, Song SY, Chung JB, Bang S. Can metformin change the prognosis of pancreatic cancer? Dig Liver Dis. 2016;48:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 134. | Choi Y, Kim TY, Oh DY, Lee KH, Han SW, Im SA, Bang YJ. The Impact of Diabetes Mellitus and Metformin Treatment on Survival of Patients with Advanced Pancreatic Cancer Undergoing Chemotherapy. Cancer Res Treat. 2016;48:171-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 135. | Hwang AL, Haynes K, Hwang WT, Yang YX. Metformin and survival in pancreatic cancer: a retrospective cohort study. Pancreas. 2013;42:1054-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 136. | Zhou DC, Gong H, Tan CQ, Luo JQ. Prognostic significance of anti-diabetic medications in pancreatic cancer: A meta-analysis. Oncotarget. 2017;8:62349-62357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 137. | Kordes S, Pollak MN, Zwinderman AH, Mathôt RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 313] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 138. | Reni M, Dugnani E, Cereda S, Belli C, Balzano G, Nicoletti R, Liberati D, Pasquale V, Scavini M, Maggiora P, Sordi V, Lampasona V, Ceraulo D, Di Terlizzi G, Doglioni C, Falconi M, Piemonti L. (Ir)relevance of Metformin Treatment in Patients with Metastatic Pancreatic Cancer: An Open-Label, Randomized Phase II Trial. Clin Cancer Res. 2016;22:1076-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 139. | Li X, Li T, Liu Z, Gou S, Wang C. The effect of metformin on survival of patients with pancreatic cancer: a meta-analysis. Sci Rep. 2017;7:5825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 140. | Wan G, Sun X, Li F, Wang X, Li C, Li H, Yu X, Cao F. Survival Benefit of Metformin Adjuvant Treatment For Pancreatic Cancer Patients: a Systematic Review and Meta-Analysis. Cell Physiol Biochem. 2018;49:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 141. | Braghiroli MI, de Celis Ferrari AC, Pfiffer TE, Alex AK, Nebuloni D, Carneiro AS, Caparelli F, Senna L, Lobo J, Hoff PM, Riechelmann RP. Phase II trial of metformin and paclitaxel for patients with gemcitabine-refractory advanced adenocarcinoma of the pancreas. Ecancermedicalscience. 2015;9:563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 142. | Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sánchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, Kost-Alimova M, Muller F, Colla S, Nezi L, Genovese G, Deem AK, Kapoor A, Yao W, Brunetto E, Kang Y, Yuan M, Asara JM, Wang YA, Heffernan TP, Kimmelman AC, Wang H, Fleming JB, Cantley LC, DePinho RA, Draetta GF. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 982] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 143. | Ming M, Sinnett-Smith J, Wang J, Soares HP, Young SH, Eibl G, Rozengurt E. Dose-Dependent AMPK-Dependent and Independent Mechanisms of Berberine and Metformin Inhibition of mTORC1, ERK, DNA Synthesis and Proliferation in Pancreatic Cancer Cells. PLoS One. 2014;9:e114573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 144. | Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69:6539-6545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 145. | Karnevi E, Said K, Andersson R, Rosendahl AH. Metformin-mediated growth inhibition involves suppression of the IGF-I receptor signalling pathway in human pancreatic cancer cells. BMC Cancer. 2013;13:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 146. | Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung AH, Smyla JK, Anderson JM, Kim HJ, Bentrem DJ, Talamonti MS, Iacobuzio-Donahue CA, Hollingsworth MA, Yeh JJ. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47:1168-1178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1041] [Cited by in RCA: 1478] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 147. | Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2019;16:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 596] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 148. | Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM; Australian Pancreatic Cancer Genome Initiative; Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 2553] [Article Influence: 283.7] [Reference Citation Analysis (0)] |
| 149. | Vaccaro V, Sperduti I, Vari S, Bria E, Melisi D, Garufi C, Nuzzo C, Scarpa A, Tortora G, Cognetti F, Reni M, Milella M. Metastatic pancreatic cancer: Is there a light at the end of the tunnel? World J Gastroenterol. 2015;21:4788-4801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 150. | Reni M, Cereda S, Milella M, Novarino A, Passardi A, Mambrini A, Di Lucca G, Aprile G, Belli C, Danova M, Bergamo F, Franceschi E, Fugazza C, Ceraulo D, Villa E. Maintenance sunitinib or observation in metastatic pancreatic adenocarcinoma: a phase II randomised trial. Eur J Cancer. 2013;49:3609-3615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 151. | Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 612] [Article Influence: 55.6] [Reference Citation Analysis (1)] |
| 152. | Pusceddu S, Vernieri C, Prinzi N, Torchio M, Coppa J, Antista M, Niger M, Milione M, Giacomelli L, Corti F, Prisciandaro M, Monteleone M, Colombo E, Di Bartolomeo M, de Braud F. The potential role of metformin in the treatment of patients with pancreatic neuroendocrine tumors: a review of preclinical to clinical evidence. Therap Adv Gastroenterol. 2020;13:1756284820927271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 153. | Pusceddu S, Vernieri C, Di Maio M, Marconcini R, Spada F, Massironi S, Ibrahim T, Brizzi MP, Campana D, Faggiano A, Giuffrida D, Rinzivillo M, Cingarlini S, Aroldi F, Antonuzzo L, Berardi R, Catena L, De Divitiis C, Ermacora P, Perfetti V, Fontana A, Razzore P, Carnaghi C, Davì MV, Cauchi C, Duro M, Ricci S, Fazio N, Cavalcoli F, Bongiovanni A, La Salvia A, Brighi N, Colao A, Puliafito I, Panzuto F, Ortolani S, Zaniboni A, Di Costanzo F, Torniai M, Bajetta E, Tafuto S, Garattini SK, Femia D, Prinzi N, Concas L, Lo Russo G, Milione M, Giacomelli L, Buzzoni R, Delle Fave G, Mazzaferro V, de Braud F. Metformin Use Is Associated With Longer Progression-Free Survival of Patients With Diabetes and Pancreatic Neuroendocrine Tumors Receiving Everolimus and/or Somatostatin Analogues. Gastroenterology 2018; 155: 479-489. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 154. | Vlotides G, Tanyeri A, Spampatti M, Zitzmann K, Chourdakis M, Spttl C, Maurer J, Nölting S, Göke B, Auernhammer CJ. Anticancer effects of metformin on neuroendocrine tumor cells in vitro. Hormones (Athens). 2014;13:498-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 155. | Herrera-Martínez AD, Pedraza-Arevalo S, L-López F, Gahete MD, Gálvez-Moreno MA, Castaño JP, Luque RM. Type 2 Diabetes in Neuroendocrine Tumors: Are Biguanides and Statins Part of the Solution? J Clin Endocrinol Metab. 2019;104:57-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 156. | Vitali E, Boemi I, Tarantola G, Piccini S, Zerbi A, Veronesi G, Baldelli R, Mazziotti G, Smiroldo V, Lavezzi E, Spada A, Mantovani G, Lania AG. Metformin and Everolimus: A Promising Combination for Neuroendocrine Tumors Treatment. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 157. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1342] [Article Influence: 335.5] [Reference Citation Analysis (1)] |
| 158. | Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, Javle M, Moghazy DM, Lozano RD, Abbruzzese JL, Vauthey JN. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938-1946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 159. | Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 160. | Ma S, Zheng Y, Xiao Y, Zhou P, Tan H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine (Baltimore). 2017;96:e6888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 161. | Cunha V, Cotrim HP, Rocha R, Carvalho K, Lins-Kusterer L. Metformin in the prevention of hepatocellular carcinoma in diabetic patients: A systematic review. Ann Hepatol. 2020;19:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 162. | Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti G; ADOPT Study Group; RECORD Steering Committee. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia. 2010;53:1838-1845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 163. | Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol. 2011;26:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 164. | Ma SJ, Zheng YX, Zhou PC, Xiao YN, Tan HZ. Metformin use improves survival of diabetic liver cancer patients: systematic review and meta-analysis. Oncotarget. 2016;7:66202-66211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 165. | Zheng L, Yang W, Wu F, Wang C, Yu L, Tang L, Qiu B, Li Y, Guo L, Wu M, Feng G, Zou D, Wang H. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin Cancer Res. 2013;19:5372-5380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 166. | Cai X, Hu X, Cai B, Wang Q, Li Y, Tan X, Hu H, Chen X, Huang J, Cheng J, Jing X. Metformin suppresses hepatocellular carcinoma cell growth through induction of cell cycle G1/G0 phase arrest and p21CIP and p27KIP expression and downregulation of cyclin D1 in vitro and in vivo. Oncol Rep. 2013;30:2449-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 167. | Antwi SO, Mousa OY, Patel T. Racial, Ethnic, and Age Disparities in Incidence and Survival of Intrahepatic Cholangiocarcinoma in the United States; 1995-2014. Ann Hepatol. 2018;17:604-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 168. | Chaiteerakij R, Yang JD, Harmsen WS, Slettedahl SW, Mettler TA, Fredericksen ZS, Kim WR, Gores GJ, Roberts RO, Olson JE, Therneau TM, Roberts LR. Risk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer risk. Hepatology. 2013;57:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 169. | Yang Z, Zhang X, Roberts RO, Roberts LR, Chaiteerakij R. Metformin does not improve survival of cholangiocarcinoma patients with diabetes. Hepatology. 2016;63:667-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 170. | Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 490] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 171. | Ling S, Feng T, Ke Q, Fan N, Li L, Li Z, Dong C, Wang C, Xu F, Li Y, Wang L. Metformin inhibits proliferation and enhances chemosensitivity of intrahepatic cholangiocarcinoma cell lines. Oncol Rep. 2014;31:2611-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 172. | Ling S, Xie H, Yang F, Shan Q, Dai H, Zhuo J, Wei X, Song P, Zhou L, Xu X, Zheng S. Metformin potentiates the effect of arsenic trioxide suppressing intrahepatic cholangiocarcinoma: roles of p38 MAPK, ERK3, and mTORC1. J Hematol Oncol. 2017;10:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 173. | Fujimori T, Kato K, Fujihara S, Iwama H, Yamashita T, Kobayashi K, Kamada H, Morishita A, Kobara H, Mori H, Okano K, Suzuki Y, Masaki T. Antitumor effect of metformin on cholangiocarcinoma: In vitro and in vivo studies. Oncol Rep. 2015;34:2987-2996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 174. | Zhu HQ, Ma JB, Song X, Gao HJ, Ma CQ, Chang H, Li HG, Liu FF, Lu J, Zhou X. Metformin potentiates the anticancer activities of gemcitabine and cisplatin against cholangiocarcinoma cells in vitro and in vivo. Oncol Rep. 2016;36:3488-3496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 175. | Liu Y, Wang Z, Li M, Ye Y, Xu Y, Zhang Y, Yuan R, Jin Y, Hao Y, Jiang L, Hu Y, Chen S, Liu F, Wu W, Liu Y. Chloride intracellular channel 1 regulates the antineoplastic effects of metformin in gallbladder cancer cells. Cancer Sci. 2017;108:1240-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 176. | Ye J, Chen K, Qi L, Li R, Tang H, Zhou C, Zhai W. Metformin suppresses hypoxiainduced migration via the HIF1α/VEGF pathway in gallbladder cancer in vitro and in vivo. Oncol Rep. 2018;40:3501-3510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 177. | Yamashita T, Kato K, Fujihara S, Iwama H, Morishita A, Yamana H, Kobayashi K, Kamada H, Chiyo T, Kobara H, Tsutsui K, Okano K, Suzuki Y, Masaki T. Anti-diabetic drug metformin inhibits cell proliferation and tumor growth in gallbladder cancer via G0/G1 cell cycle arrest. Anticancer Drugs. 2020;31:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 178. | Bi T, Zhu A, Yang X, Qiao H, Tang J, Liu Y, Lv R. Metformin synergistically enhances antitumor activity of cisplatin in gallbladder cancer via the PI3K/AKT/ERK pathway. Cytotechnology. 2018;70:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 179. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64627] [Article Influence: 16156.8] [Reference Citation Analysis (176)] |
| 180. | Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8018] [Cited by in RCA: 7443] [Article Influence: 572.5] [Reference Citation Analysis (0)] |
| 181. | Laterre PF, François B. Strengths and limitations of industry vs. academic randomized controlled trials. Clin Microbiol Infect. 2015;21:906-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |