Published online May 7, 2021. doi: 10.3748/wjg.v27.i17.1864
Peer-review started: January 10, 2021
First decision: February 11, 2021
Revised: March 7, 2021
Accepted: April 7, 2021
Article in press: April 7, 2021
Published online: May 7, 2021
Processing time: 108 Days and 20.8 Hours
With the growing prevalence of obesity and diabetes in the United States and across the world, a rise in the overall incidence and prevalence of non-alcoholic fatty liver disease (NAFLD) is expected. The risk factors for NAFLD are also associated with the development of chronic kidney disease (CKD). We review the epidemiology, risk factors, genetics, implications of gut dysbiosis, and specific pathogenic mechanisms linking NAFLD to CKD. Mechanisms such as ectopic lipid accumulation, cellular signaling abnormalities, and the interplay between fructose consumption and uric acid accumulation have led to the emergence of potential therapeutic implications for this patient population. Transplant evaluation in the setting of both NAFLD and CKD is also reviewed. Potential strategies for surveillance and management include the monitoring of comorbidities, the use of non-invasive fibrosis scoring systems, and the measurement of laboratory markers. Lastly, we discuss the management of patients with NAFLD and CKD, from preventative measures to experimental interventions.
Core Tip: Patients with non-alcoholic fatty liver disease (NAFLD) are at higher risk for the development of chronic kidney disease (CKD) than the general population. The prevalence of mutual comorbidities in addition to direct pathogenic mechanisms linking NAFLD to the development of CKD can explain this finding. With the breadth of data linking NAFLD to CKD, there are minimal options for treating this patient population. Regardless, we have presented strategies that can be implemented at various levels including surveillance, preventative, and management level.
- Citation: Heda R, Yazawa M, Shi M, Bhaskaran M, Aloor FZ, Thuluvath PJ, Satapathy SK. Non-alcoholic fatty liver and chronic kidney disease: Retrospect, introspect, and prospect. World J Gastroenterol 2021; 27(17): 1864-1882
- URL: https://www.wjgnet.com/1007-9327/full/v27/i17/1864.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i17.1864
Non-alcoholic fatty liver disease (NAFLD) encompasses a spectrum of chronic liver disease ranging from steatosis on one end to fibrosis and cirrhosis on the other end[1]. NAFLD and non-alcoholic steatohepatitis (NASH) are the hepatic manifestations of metabolic syndrome (MetS), which is a driving force for a multitude of comorbidities, such as insulin resistance, cardiovascular disease (CVD), chronic kidney disease (CKD), obstructive sleep apnea (OSA), as well as increased malignancy risk[2]. While NASH is the second leading indication for liver transplantation (LT), it is expected that NASH will overtake hepatitis C virus (HCV) as the leading cause, given the efficacy of direct-acting antiviral therapy[3]. A recent epidemiological study has already confirmed a downward trend for HCV-related LT in the United States[4].
NAFLD is tightly linked to underlying insulin resistance and is associated with other comorbidities related to MetS[5]. Growing evidence suggests that NAFLD is a risk factor for CKD[6] due to shared metabolic risk factors[7]. Of note, several studies have shown an association between the severity of NASH and CKD[8-11]. Interestingly, a meta-analysis of 33 studies showed that diabetes status and metabolic risk factors had no impact on the positive correlation between the severity of NASH and CKD[12], suggesting a possible unique pathogenic link between NAFLD and CKD irrespective of their shared metabolic risk factors. We review the genetic, epidemiologic, and pathogenic links between NAFLD and CKD in addition to potential preventative and management strategies.
Two meta-analyses and a retrospective cohort analysis suggest that the incidence and prevalence of CKD increase in patients with NAFLD compared to patients without NAFLD (Table 1). In all analyses, the magnitude and direction of effects remained unaffected by diabetes status, even after adjustment for other risk factors[12-14]. Moreover, the association was stronger in patients with advanced fibrosis or decompensated cirrhosis as compared to compensated cirrhosis. The studies that were included in these major meta-analyses defined advanced fibrosis was defined by histological parameters, imaging findings, and/or elevations in the NAFLD fibrosis score (NFS). Of note, among 42 studies included in these two meta-analyses, only 13 (n = 2205) utilized liver histology, which is the gold standard in diagnosing NAFLD[15]. The majority of the studies established diagnosis of NAFLD via abdominal ultrasound, liver enzyme elevation [including serum gamma-glutamyl transferase (GGT) elevation], or using international classification of disease-9 code.
| Ref. | Year | n | NAFLD diagnostic modalities | Conclusion(s) |
| Musso et al[12]. A meta-analysis of 33 studies | 2014 | 63902 | Liver biopsy, abdominal ultrasound, elevated liver enzymes | (1) 20 cross-sectional studies: Nearly two-fold increased risk of CKD in patients with NAFLD (OR 2.12, 95%CI 1.69-2.66); (2) 11 longitudinal studies: 1.8-fold increased risk of CKD in patients with NAFLD (HR 1.79, 95%CI 1.65–1.95); and (3) advanced fibrosis associated with increased prevalence (OR 5.20, 95%CI 3.14-8.61) and incidence (HR 3.29, 95%CI 2.30-4.71) of CKD in patients with NAFLD |
| Mantovani et al[13]. A meta-analysis of 9 studies | 2018 | 96595 | Abdominal ultrasound; FLI; serum GGT | Incidence of CKD: (1) 1.4-fold increased long-term risk (HR 1.37, 95%CI 1.20–1.53) in patients with NAFLD with a median follow-up period of 5.2 years; and (2) 1.5-fold increased risk (HR 1.50, 95%CI 1.25-1.74) in patients with severe NAFLD (defined as NFS ≥ -1.455 or serum GGT ≥ 109 U/L) |
| Park et al[14]. Retrospective Cohort with Propensity Score Matching (1:3) | 2019 | 262619 | ICD-9 | Incidence of CKD: 1.4-fold increased risk (aHR 1.41; 95%CI, 1.36-1.46) in patients with NAFLD after adjusting for demographics, baseline covariates, and ACEi/ARB use; Risk of incident CKD increases as the severity of NAFLD increases: (1) compensated cirrhosis (aHR, 1.47; 95%CI 1.36-1.59); and (2) decompensated cirrhosis (aHR, 2.28; 95%CI 2.12-2.46) |
Previous review articles estimated that the prevalence of CKD was 20% to 55% in patients with NAFLD, whereas the prevalence of CKD in patients without NAFLD was 5% to 30%[16,17]. However, most of these reviews evaluated the same pool of data[8,9,11,18-22], which were also included in the two meta-analyses mentioned above. Our conclusions were based on studies that were published before 2015 as several more recent studies did not use histology or imaging for NAFLD diagnosis[23-26].
Many non-hepatic and hepatic risk factors are associated with CKD in those with NAFLD.
There is minimal data on non-hepatic risk factors to predict which patients will go on to develop CKD. However, there are a few studies outlined below to identify which patients may be at higher risk (Table 2).
| Ref. | Risk factor(s) | Year | n | Comparison | Findings |
| Önnerhag et al[147] | Older age | 2019 | 120 | Biopsy-proven NAFLD vs non-NAFLD | Higher prevalence of CKD in patients ≥ 55 years old |
| Targher et al[20] | Diabetes mellitus | 2008 | 2103 | NAFLD and T2DM vs T2DM only | Patients with NAFLD and T2DM independently associated with increased risk of CKD (OR 1.87; 95%CI 1.3-4.1, P = 0.020) |
| Targher et al[33] | Diabetes mellitus | 2010 | 301 | NAFLD and T1DM vs T1DM only | Patients with NAFLD and T1DM independently associated with increased risk of CKD |
| Jang et al[29] | Elevated baseline eGFR, HTN, and current smoking | 2018 | 1525 | NAFLD vs Non-NAFLD | The decline in eGFR associated with NAFLD appeared to be stronger among patients who were current smokers, hypertensive, and lower eGFR at baseline |
Current cigarette smoking is associated with CKD or death from end-stage renal disease. Mainstream cigarette smoke includes over 4000 compounds, and nicotine is one of many biologically stable and active compounds present in tobacco. Nicotine causes kidney damage by modulating α7nAChR, NLRP6 inflammasome, ER stress, and autophagy[27,28]. Studies examining the relationship between smoking and NAFLD are lacking; however, in a cohort study of 1525 CKD patients who underwent repeated health check-up examinations over 10 years, the decline in estimated glomerular filtration rate (eGFR) associated with NAFLD was greater in current smokers, hypertensive patients, or those with lower eGFR at baseline had greater age- and sex-adjusted decline in eGFR[29].
Around one-third of patients with NAFLD have impaired renal function and its prevalence in patients with NAFLD is dependent on the severity of liver disease and presence of diabetes mellitus[30]. The development of NAFLD in patients with diabetes appears to be an important event in its natural history predisposing these patients to a higher risk for developing CKD. Type 2 diabetes mellitus (T2DM) increases the risk of serious NASH and advanced fibrosis in patients with NAFLD[31,32]. Patients with T2DM or type 1 diabetes mellitus and NAFLD are at an increased risk of developing CKD compared to diabetics without NAFLD[20,33,34]. Despite accumulating evidence for NAFLD as a driver for CKD, the shared common risk factors make it difficult to isolate diabetes as an independent risk factor for CKD in NAFLD patients.
Proper thyroid function is implicated in renal blood flow, glomerular and tubular function, electrolyte homeostasis, hepatic lipid metabolism, and fatty acid beta-oxidation[35]. Hypothyroidism can cause NAFLD through fat accumulation, while hyperthyroid can cause NAFLD through reactive oxygen species formation[36]. Additionally, the prevalence of hypothyroidism increases for each 10 mL/min/1.73 m2 decrement in eGFR[37], and patients with hypothyroidism were more than 2 times likely to have NAFLD and 4 times more likely to have NASH[38].
NAFLD-related advanced fibrosis: Patients with NAFLD-related advanced fibrosis are more likely to have CKD compared to patients with NAFLD but without advanced fibrosis[39]. The risk of albuminuria increases with the severity of NAFLD-related advanced fibrosis, according to a 2017 study of 1763 Chinese diabetic patients[40]. After adjusting for common CKD risk factors such as diabetes and other metabolic comorbidities, advanced fibrosis but not steatosis was associated with a higher risk of albuminuria (OR: 1.52; 95%CI: 1.02-2.28; P = 0.039). In a 2019 study of 594 patients with T2DM, significant liver fibrosis as detected by elastography (LSM ≥ 7.0/6.2 kPa) was independently associated with a higher risk of CKD (adjusted OR: 3.6, 95%CI: 1.3-10.1; P = 0.01) in addition to CVD and other microvascular complications[41]. Increased liver stiffness as detected by transient elastography is a predictor of CKD in patients with ultrasound-diagnosed NAFLD[42]
In a 12-year prospective cohort, patients with non-obese NAFLD had a higher risk of developing CKD than patients with obese NAFLD[43]. A recent study has noted that the risk of developing CKD is higher in metabolically unhealthy non-obese NAFLD patients than their counterparts with metabolically healthy status defined by the lack of metabolic risk factors (i.e. diabetes mellitus, low High-density lipoprotein, hypertriglyceridemia, arterial hypertension)[44].
Pathophysiology: CKD secondary to fatty liver is thought to be due to systemic low-grade inflammation[45], which may involve upregulation of the nuclear factor-κB (NF-κB) pathway[45,46]. As discussed earlier, there is circumstantial evidence to suggest that patients with NASH-related advanced fibrosis have an increased prevalence of CKD. Progression of NASH may be partly mediated by the altered renin-angiotensin-aldosterone system due to CKD has also been proposed as a mechanism for NAFLD progression[47]. Although direct pathogenic links between NAFLD and CKD seem to be confounded by common metabolic comorbidities, novel mechanisms have been described.
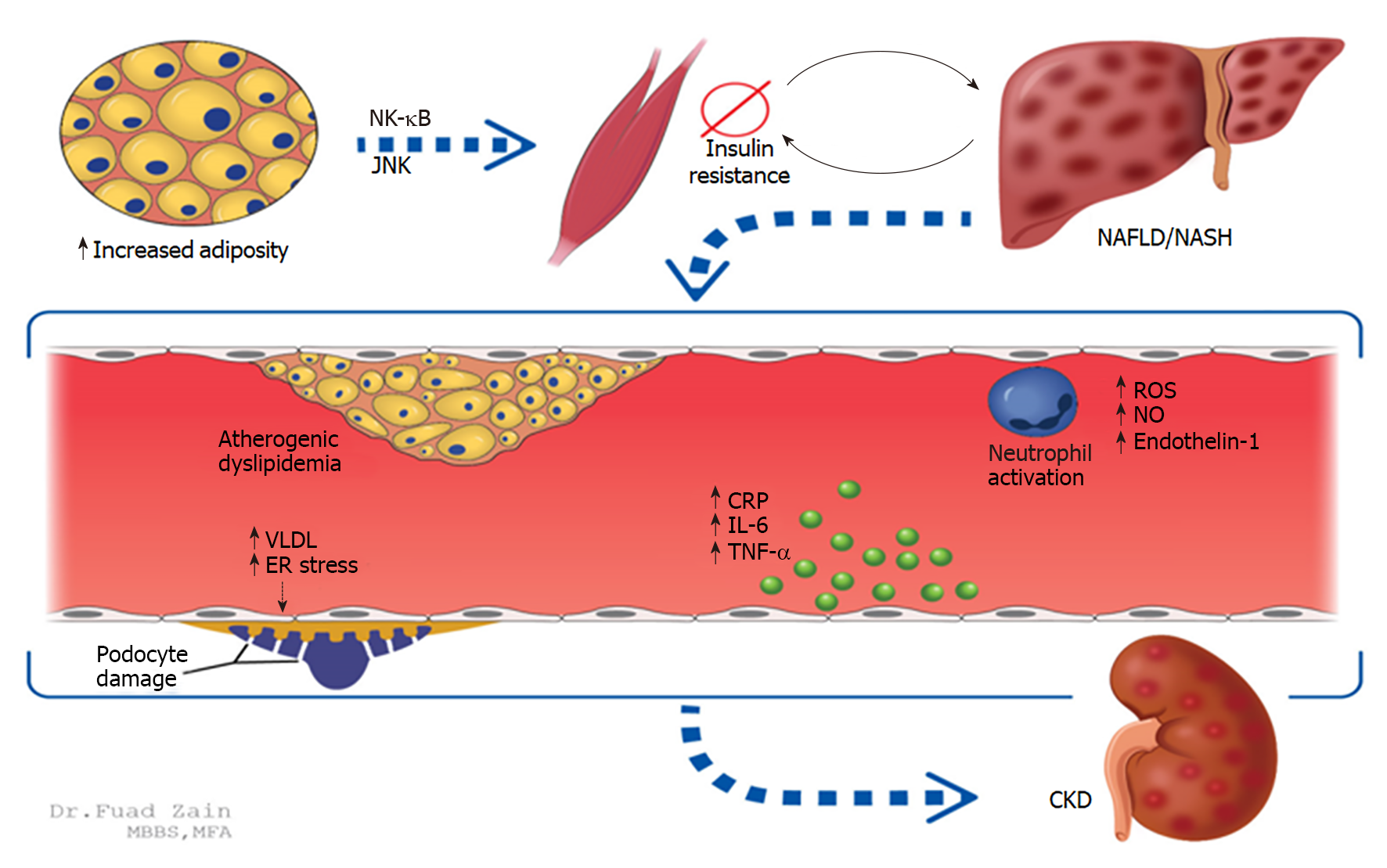
Insulin resistance: Increased adiposity leads to increased free fatty acids and pro-inflammatory cytokine release that causes systemic insulin resistance (IR), which is an established mediator of NAFLD. IR is further exacerbated by the progression of NAFLD, leading to atherogenic dyslipidemia and further release of inflammatory cytokines resulting in CKD as shown in animal models[48]. Proinflammation occurs through the NF-κB and Jun-N-terminal kinase (JNK) pathways; activation of adipose-specific JNK pathways has been shown to cause insulin resistance[48,49]. As NAFLD progresses to NASH, the inflammatory component is neutrophil-predominant and can cause systemic endothelial dysfunction (Figure 1)[50,51]. Notably, IR leads to increased production of very-low-density lipoprotein and endoplasmic reticulum stress, both of which can cause podocyte damage in glomeruli[52]. These latter two mechanisms have been linked to proteinuria and subsequent hastening of CKD[53,54].
Ectopic lipid accumulation: In animal models, a high fat/fructose diet resulted in increased urinary albumin excretion, elevated transaminases, and increased incidence of liver tumors when compared to a standard diet. Microscopically, lipid deposition leads to accelerated hepatorenal pathologies, suggesting that intracellular lipid accumulation may link NAFLD to CKD[55]. When treated with fenofibrate, slower intracellular lipid accumulation was noted in co-incidence with slower progression of renal and hepatic pathologies[55].
Wnt signaling abnormalities: Alterations in cellular pathways critical for homeostasis play an important role in the development of CKD in patients with NAFLD. Specifically, abnormalities in the Wnt (named as a fusion of the Drosophila gene wingless and its vertebrate homolog, integrated) signaling pathway have been linked to lipid accumulation, chronic inflammation, and fibrosis in the development of both NAFLD and CKD[56].
Sterol regulatory element-binding proteins: Sterol regulatory-element binding proteins are activated in a nutrient-rich (i.e., anabolic) state that leads to insulin-signaling and increased endoplasmic reticulum stress, which can cause increased lipogenesis and hepatosteatosis. These changes cause the progression of other metabolic phenomena such as CKD and MetS[57].
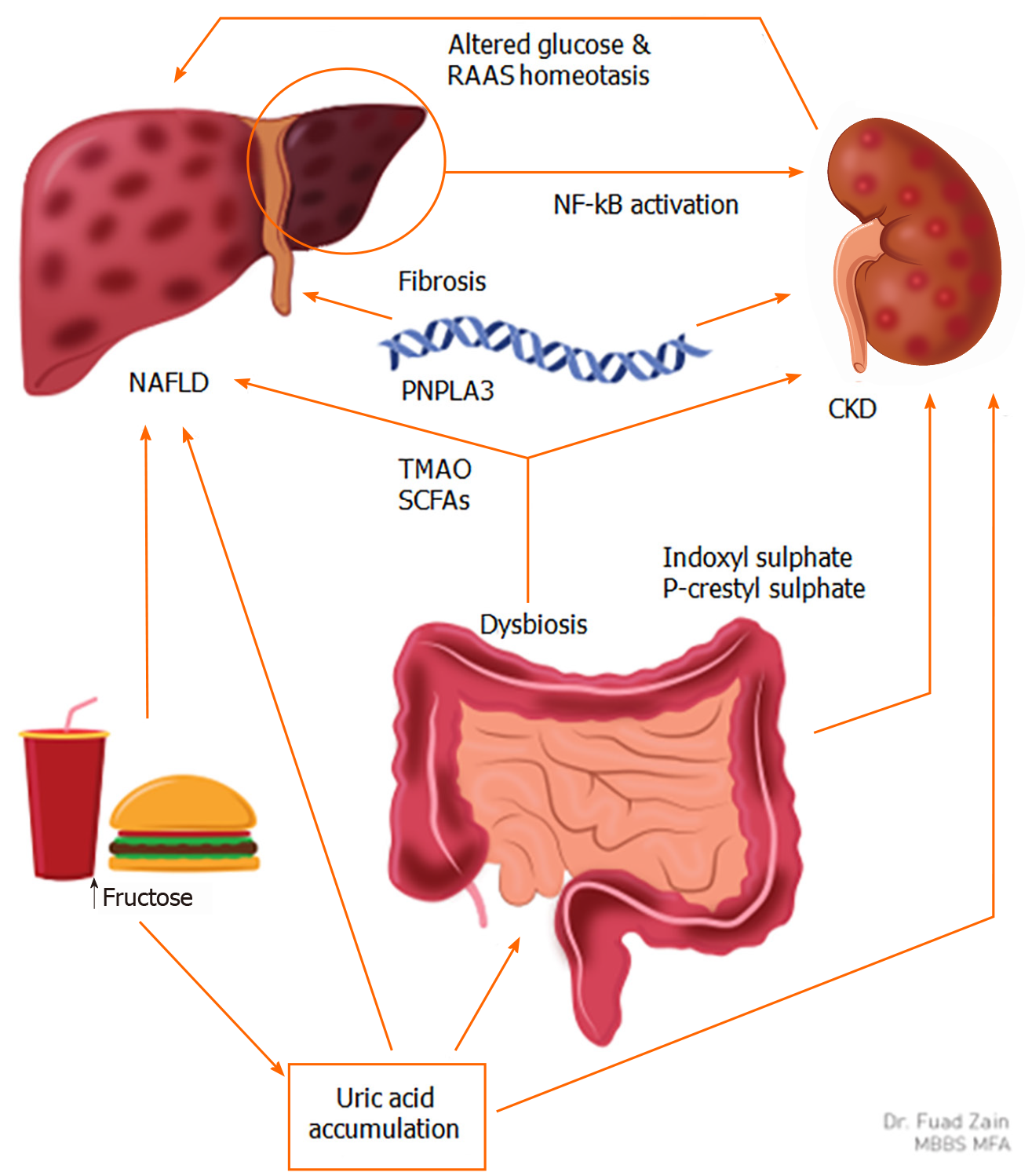
Fructose consumption and uric acid accumulation: Fructose intake has been linked to hepatorenal injury via uric acid accumulation by altering the gut microbiome (Figure 2)[45,58]. Patients with a normal body mass index (BMI) and elevated serum uric acid levels (> 10 mg/dL) have an increased prevalence of MetS when compared to patients with a serum uric acid < 6 mg/dL[59], which is corroborated by other studies[60-62]. An increase in serum uric acid levels is also associated with an increase in the incidence of NAFLD[63]. In patients with NAFLD, elevated uric acid levels are known to be pathogenic in CKD progression[42,64]. These studies suggest that MetS, NAFLD, and CKD are interconnected through elevated serum uric acid levels[65].
Uric acid stimulates fructokinase, which sensitizes hepatocytes to fructose metabolism, subsequently leading to fat deposition in the liver, thereby explaining the link between elevated uric acid and NAFLD[66]. Elevated uric acid levels in animal models lead to glomerular hypertension and tubulointerstitial fibrosis, two processes that preclude the development of CKD[64]. Decreased urate clearance in CKD patients may further exacerbate this pathology. Interestingly, xanthine oxidase inhibitors are currently being tested in patients with CKD to monitor for disease progression in the CKD-FIX[67].
Gut dysbiosis: Changes in the gut microbiome play a role in the pathogenesis of NAFLD and CKD[45]. Dietary conditions such as increased fructose intake and vitamin D deficiency are shown to cause dysbiosis, which may directly lead to low-grade inflammation responsible for the development of NAFLD and CKD[45]. Dysbiosis and subsequent microbial fermentation lead to increased production of uremic toxins indoxyl sulfate and p-cresyl sulfate, which correlate directly with the progression of CKD[68]. The liver cytochrome P450 enzymes are directly regulated by these uremic toxins derived from alterations in gut microbial metabolism, hence the gut-liver-kidney axis[69]. Animal models have also shown the gut microbiota’s ability to metabolize choline into trimethylamine N-oxide (TMAO), which is considered both nephrotoxic and hepatotoxic. In a 2015 study comparing TMAO levels in patients with CKD (n = 521) to healthy patients (n = 3166), median TMAO levels among CKD patients were significantly higher (P < 0.001)[70]. Similarly, a 2019 case-control study comparing patients with NAFLD (n = 34) to those without (n = 14) showed that TMAO has a role in aggravating liver steatosis[71]. Lastly, certain species in the gut microbiota produce short-chain fatty acids (SCFAs) such as butyrate, acetate, and propionate and diffuse through gut mucosa, which can disrupt the integrity of the intestinal barrier. In the bloodstream, SCFAs can cause systemic inflammation, the common pathogenic link between NAFLD and CKD[45].
Genetic links between NAFLD and CKD: Two gene variants associated with both CKD and NAFLD are the G allele of the patatin-like phospholipase domain-containing (PNPLA3) gene and the T allele of the transmembrane 6 superfamily member 2 (TM6SF2) gene. The G allele in the rs738409 polymorphism of the PNPLA3 gene has been shown to play a major role in the progression of NASH[72,73]. Patients with the G allele also have been shown to have lower eGFR, increased incidence of microalbuminuria, and increased prevalence of CKD, regardless of NAFLD/NASH status[74,75]. The patient population that was found to have the highest risk of CKD and NAFLD in a 2015 study were patients who carried the G allele of the PNPLA3; furthermore, these patients were not obese, which is an important risk factor for CKD[75]. Another study showed that Chinese patients with normal alanine aminotransferase levels who carried the rs738409 polymorphism in the PNPLA3 gene were at risk for early glomerular and tubular damage, which could explain why these patients develop CKD even in the absence of well-known risk factors, such as obesity or diabetes[76]. In postmenopausal women with T2DM, having the G/G allele leads to a higher prevalence of CKD, regardless of NAFLD status, further supporting the argument that this polymorphism may be an independent predictor for CKD[77]. Patients who are found to have the G/G allele in the polymorphism rs738409 should have close monitoring for the development of NAFLD as well as renal dysfunction, even in normal-weight patients[75]. On the other hand, the rs58542926 polymorphism on the TM6SF2 gene, also known as the T allele of the TMS6F2 gene, has been associated with the development of NAFLD[78] but has also been associated with a higher eGFR and lower prevalence of microalbuminuria[74]. Thus, this specific polymorphism in TM6SF2 may be nephroprotective in patients with NAFLD.
Identifying NAFLD patients at risk for progression of CKD
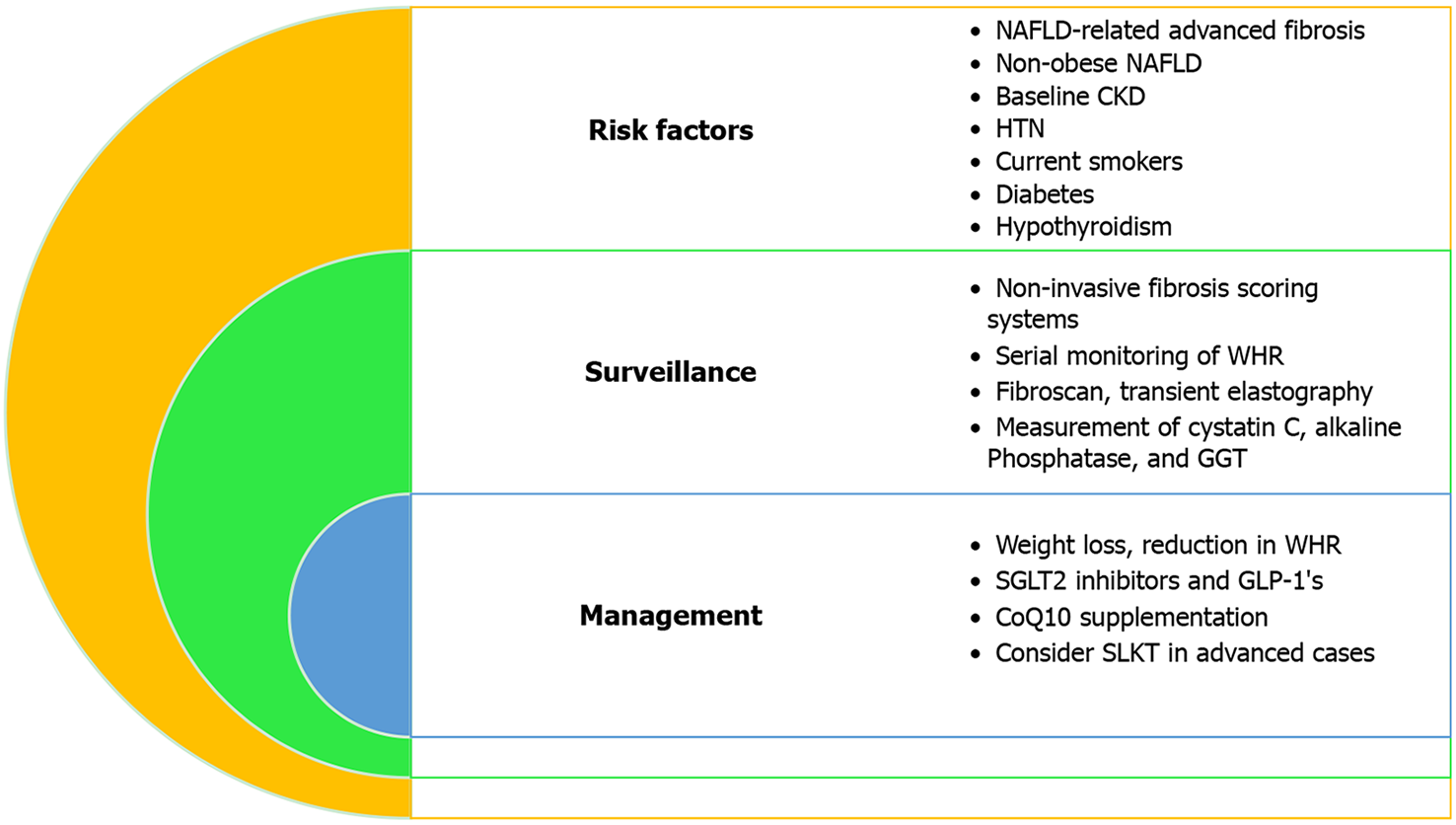
Role of non-invasive fibrosis scoring systems: Non-invasive scoring systems are utilized in assessing the severity of various chronic liver diseases. However, they have also been shown in several studies to be useful in predicting CKD in patients with NAFLD (Table 3). Incremental increases in the fatty liver index are an independent risk factor for developing CKD in a 10-year prospective analysis of 6238 adults (age 40-69 years) without CKD at baseline[23]. In another study of 11376 Taiwanese subjects, the NFS was negatively correlated with eGFR[6]. Multiple studies show that patients who have an intermediate and high-risk category of fibrosis-4 index (FIB-4)-index and NFS are at an increased risk of CKD[79,80], while a 2019 cross-sectional study of 11836 patients showed that FIB-4 is the most precise tool when estimating renal dysfunction attributable to NAFLD (area under the curve = 0.6227, 95%CI: 0.5929-0.6526, P = 0.0258) after adjusting for various demographic and clinical variables[81]. FIB-4 is the most superior predictor in other studies as well[80,82]. In summary, patients with NAFLD-related fibrosis are at increased risk for CKD, and these patients should undergo proper surveillance via non-invasive fibrosis scoring systems and/or advanced imaging techniques (i.e. Fibroscan, TE) (Figure 3).
| Ref. | Year | n | Scoring system(s) assessed | Results |
| Ciardullo et al[82] | 2020 | 2770 | APRI, FIB-4, FLI, NFS | NAFLD-related fibrosis as measured with FIB-4 associated with CKD (P < 0.01) |
| Hsieh et al[6] | 2020 | 11376 | NFS | Higher NFS associated with impaired eGFR (P < 0.0001) |
| Choi et al[81] | 2019 | 11836 | APRI, BARD, FIB-4, FLI | FIB-4 (P = 0.0258) most precise in predicting kidney dysfunction |
| Önnerhag et al[79] | 2019 | 144 | APRI, BARD, NFS, FIB-4 | High-risk NFS (P < 0.001), FIB-4 (P < 0.001), APRI (P = 0.008) predict CKD |
| Wijarnpreecha et al[80] | 2018 | 4142 | APRI, BARD, NFS, FIB-4 | High/intermediate probability of liver fibrosis on NFS (AUC = 0.75) and FIB-4 (AUC = 0.77) independently predict CKD |
| Huh et al[23] | 2017 | 6238 | FLI | NAFLD cut-off for NAFLD is an independent RF for CKD (P < 0.0001) |
Cystatin C: Serum creatinine, a widely used biomarker in assessing renal function, is inaccurate in determining GFR in patients with cirrhosis[83]. This is due to muscle wasting that occurs in cirrhosis, thus leading to diminished creatinine formation, increased tubular secretion of creatinine, and impaired assay interpretation caused by elevated bilirubin[83]. Alternatively, the measurement of cystatin C does not have the same limitations as serum creatinine due to its low molecular weight and because it does not require adjustment for gender, mass, or bilirubin level[84]. A combination of serum creatinine and cystatin C is more accurate in determining GFR than serum creatinine alone[85]. However, serum creatinine alone is superior for patients without cirrhosis[85]. Measurement of cystatin C in addition to serum creatinine may have utility for accurately assessing renal function in transplant candidates and for monitoring the development of CKD in patients with NASH cirrhosis. Although the cost of measuring eGFR using Cystatin C in addition to serum creatinine is higher, the burden of over-diagnosing CKD in patients with cirrhosis is lessened, which may lead to an overall reduction in unnecessary medical expenses for patients with cirrhosis who truly have CKD[86].
Alkaline phosphatase and GGT: In diabetic patients with NAFLD, serum alkaline phosphatase (ALP), a NAFLD-associated marker when elevate, was also significantly associated with impaired renal function[87,88]. Interestingly, ALP is associated with the release of proinflammatory cytokines from the liver that are known to disrupt the glomerular endothelial glycocalyx, leading to albuminuria, which may explain why ALP is a potential surveillance marker in patients with NAFLD who are at risk for developing CKD[89]. Furthermore, elevated serum GGT is associated with an increased risk of CKD[24,90,91]. GGT is associated with increased inflammatory markers and insulin resistance, both of which play central roles in patients with NAFLD who develop CKD[24,92]. However, elevated GGT may not be an accurate CKD parameter in Caucasian men, as GGT is confounded by BMI, lifestyle factors, and lipids, as noted in a 2017 study[25]. Therefore, elevated GGT in Caucasian men with NAFLD should be interpreted with caution when monitoring for CKD. Of importance, NAFLD was diagnosed by elevated GGT levels (in addition to ultrasound in only one study[91]; therefore, these findings may not apply to patients diagnosed by more invasive parameters (i.e. liver biopsy).
Managing the progression of CKD
Surveillance of comorbidities: In general, we recommend patients with diabetes and NAFLD undergo frequent surveillance for underlying kidney dysfunction, more so than patients with diabetes only. Monitoring thyrotropin and thyroid hormone levels may have clinical utility when evaluating the risk of developing CKD in patients with NAFLD; however, future studies are needed to specifically address the risk of CKD in patients with NAFLD and hypothyroidism (Table 4).
| Intervention | Ref. | Year | n | Findings | Recommendation |
| Decreasing WHR | Chon et al[43]. 12-yr prospective cohort | 2020 | 6137 | A decrease in the WHR of more than 5% in patients with NAFLD leads to a significantly reduced risk of CKD development, even in non-obese patients | Serial Monitoring WHR may be beneficial in identifying patients with NAFLD at risk of developing CKD and reduction can ameliorate the progression |
| Weight loss | Vilar-Gomez et al[94]. Post-hoc analysis | 2017 | 261 | Improvement in liver histology due to weight loss linked to improved renal outcomes, even after adjusting for medication profile, diabetes, and hypertension | Advocate for weight loss |
| SGLT2 Inhibitors | Shimizu et al[96]. RCT | 2019 | 57 | SGLT inhibitor (Dapagliflozin) improved liver steatosis in patients with T2DM and NAFLD and attenuates liver fibrosis in patients with NAFLD-related advanced fibrosis | Although data is not sufficient, consider using SGLT2 inhibitors in T2DM patients with NAFLD and CKD |
| Perkovic et al[95]. CREDENCE trial | 2019 | 4401 | SGLT2 inhibitor (Canagliflozin) decreased the risk of renal failure in patients with T2DM and CKD | ||
| GLP-1 | Armstrong et al[100]. LEAN trial | 2016 | 52 | Liraglutide led to weight loss, glycemic control, and histological resolution of NASH | GLP-1’s in NASH is considered effective in improving components of MetS, however, long-term studies are needed to determine NASH-related outcomes |
| Tuttle et al[101]. AWARD-7 trial | 2018 | 577 | Once-weekly dulaglutide is associated with reduced decline in eGFR, while being as effective as insulin in achieving glycemic control | GLP-1 is a safe option for patients with CKD and is associated with slower progression of CKD | |
| Coenzyme Q10 | Farhangi et al[109] and Farsi et al[110]. RCT | 2014[109] and 2016[110] | 44[109] and 41[110] | 100 mg of oral CoQ10/d improve biochemical variables of NAFLD after 4 wk[109] and 12 wk[110] of treatment | Due to lack of data in patients with both NAFLD and CKD, the benefit of CoQ10 supplementation is unknown; however, in separate trials with regards to both NAFLD and CKD, CoQ10 supplementation is beneficial |
| Yeung et al[111]. RCT | 2015 | 15 | Oral CoQ10 supplementation in patients with CKD showed significant improvement in serum creatinine when compared to placebo |
Waist-to-hip ratio: Few studies have evaluated the impact of weight loss on the progression of CKD in patients with NAFLD. Recent studies have shown that a decrease in the waist-to-hip ratio (WHR) in patients with NAFLD decreases the risk of CKD development[43]. Serial monitoring of WHR may be beneficial in identifying patients with NAFLD at risk for CKD. A drawback to this finding is that a reduction in WHR does not differentiate between a reduction in visceral fat vs subcutaneous fat. Studies have shown that visceral fat, but not subcutaneous fat, is the key driver in NAFLD pathogenesis via increased insulin resistance[93]. However, with regards to reducing the risk of CKD, the significance of reducing visceral vs subcutaneous fat is not well-studied.
Weight loss: Data from a post-hoc analysis of a clinical trial involving 261 patients with NAFLD showed a statistically significant relationship between reduction in body weight and changes in eGFR (when calculated by CKD-Epidemiology collaboration and modification of diet in renal disease equations), even after adjusting for medication profile, diabetes, and hypertension[94]. Additionally, patients with improvement in liver histology due to lifestyle modifications such as weight loss were linked with significantly improved renal outcomes[94]. Overall, patients with NAFLD who had more than 5% weight loss and/or more than a 5% reduction in WHR had improved renal outcomes.
Sodium-glucose cotransporter type-2 inhibitors: In patients with T2DM, sodium-glucose cotransporter type-2 (SGLT2) inhibitors have an established role in improving glycemic control, weight loss, cardiovascular outcomes, and lowering serum uric acid levels. In patients with type 2 diabetes, the landmark CREDENCE trial showed that patients treated with an SGLT2 inhibitor (i.e., canagliflozin) were shown to have improved outcomes related to CKD[95]. Furthermore, recent evidence has shown that SGLT2 inhibitors can also improve NAFLD progression as determined by TE[96] and biomarkers in NAFLD (i.e., liver enzymes)[96,97]. As SGLT2 inhibitors decrease serum uric acid levels, this may also contribute to this class’s positive effects on both diseases. In addition to facilitating glucosuria, SGLT2 inhibitors are thought to decrease inflammation and reactive oxygen species formation[98], which is key in the pathogenesis of NAFLD and NASH[99].
Glucagon-like peptide 1 receptor agonists: Among its multiple mechanisms of action, glucagon-like peptide 1 (GLP-1)’s aid in increasing insulin secretion, delaying gastric emptying, and decreasing appetite, all of which can lead to improved glycemic control and weight loss. Additionally, a possible anti-inflammatory mechanism makes GLP-1’s an attractive agent in NAFLD and NASH. For instance, GLP-1 Liraglutide, when compared to placebo, led to histological resolution of NASH; however, larger studies are still needed[100]. In CKD, GLP-1’s are shown to be nephroprotective, which could be due to GLP-1’s ability to lower blood pressure in addition to the aforementioned mechanisms[101,102]. GLP-1’s and SGLT2 inhibitors exhibit cardioprotective effects, and as discussed previously, patients with NAFLD and CKD are at high risk for CV events. Therefore, the use of these agents is recommended in patients with NAFLD and CKD. However, while there is landmark data to support the use of GLP-1’s and SGLT2 inhibitors to prevent CV events in patients with established CVD, data on primary prevention in patients with NAFLD and CKD is lacking[103,104]. Regardless, in patients with T2DM, CKD, and NAFLD, SGLT2 inhibitors or GLP-1’s are highly recommended not only for glycemic control but for the cardio-, hepato-, and nephroprotective effects as well.
Coenzyme Q10: Coenzyme Q10 (CoQ10) is produced endogenously and has antioxidant and anti-inflammatory effects[105]. CoQ10 also serves as an electron carrier in cellular respiration and a cofactor in pyrimidine synthesis for DNA repair and replication, among other important roles. Patients with NAFLD, CKD, and/or CVD have been reported to have CoQ10 deficiency[106]. A majority of endogenous CoQ10 is produced in the liver, and patients with NAFLD had diminished CoQ10 production[106,107]. CoQ10 deficiency will lead to oxidative stress, which plays a key pathogenic factor in NAFLD[108]. Results from separate trials assessing oral CoQ10 supplementation in patients with NAFLD and CKD are summarized in Table 4. Briefly, CoQ10 has been shown to improve NAFLD parameters and CKD parameters in separate trials[109-111]. Specific findings are summarized in Table 4. CoQ10 has positive effects on the progression of CVD as well, which is notable because patients with CKD and NAFLD are at risk for cardiovascular events[112,113]. Supplementation may be beneficial in patients with NAFLD who have CKD, but clinical data in this population is lacking.
Thiazolidinediones: Thiazolidinediones are agonists of peroxisome proliferator-activated receptors (PPARs), and they play a physiologic role in metabolism and cellular differentiation. PPARs have proven clinical utility in diseases such as hyperlipidemia (PPARα) and T2DM (PPARγ)[114]. Because CKD is a manifestation of a metabolic and/or inflammatory process, the use of PPAR agonists has been studied in patients with CKD. Specifically, pioglitazone, a PPARγ agonist, has been shown to improve cardiovascular outcomes in patients with CKD and diabetes[115]. Several RCTs have shown the beneficial effects that pioglitazone has on histopathology and metabolic function in patients with NASH[116-120]. Pioglitazone has been endorsed as a pharmacological agent in biopsy-proven NASH by the American Association for the Study of Liver Diseases[121]. Rosiglitazone has been shown to improve histological components of NASH through increasing insulin sensitivity[122] while also improving liver function[123] in a separate study, although both studies did not show improvements in liver fibrosis[122,123]. Interestingly, an extension trial showed that rosiglitazone was only beneficial in the first year of treatment, without substantial benefit noted with longer use[124]. However, Rosiglitazone is not available in most countries and its use is limited in the United States due to data concerning for increased coronary events. The most widely studied PPAR agonist, Pioglitazone has shown favorable outcomes in patients with CKD and patients with NAFLD, but data assessing the efficacy in patients with both CKD and NAFLD is lacking[114].
Vitamin D: Vitamin D deficiency is associated with increased severity of NAFLD[125] and is also associated with CKD[126]. These findings may be explained by the physiology of vitamin D activation, which requires hydroxylation by both the kidney and liver, and therefore the presence of CKD and NAFLD inevitably leads to vitamin D resistance[58]. Furthermore, experimental models have demonstrated the role hypovitaminosis D plays in the pathogenesis of both CKD and NAFLD[58]. In patients with CKD, therapeutic implications of higher vitamin D supplementation showed an ability to correct hypovitaminosis D[127], but a meta-analysis yielded a higher incidence of hypercalcemia[128]. In patients with NAFLD, vitamin D supplementation did not correct hypovitaminosis D[129], however, trials are underway for assessing the use of Vitamin D supplementation in CKD and NAFLD/NASH[130-132] (NCT00893451, NCT01623024, and NCT02098317, www.clinicaltrials.gov).
Probiotics: In rodent models, fecal microbiota transplantation[133], antibiotics in fructose-fed models[134] reduced NAFLD severity, whereas specific probiotics (Lactobacillaceae or Bifidobacteriales) alleviated proteinuria and reduced systemic inflammation in rodents with CKD. While much of this data is based on studies from animal models, human trials are needed to further evaluate the therapeutic implications of the gut-liver-kidney axis.
In recent decades, NASH has become more prevalent and will become the most common indication for LT[135]. Patients with NASH have a higher incidence of CKD compared with other etiologies, and therefore, NASH is rapidly growing as a cause for not only LT[136,137] but also simultaneous liver-kidney transplantation (SLKT) in the United States given serum creatinine and dialysis status are important components of the model for end-stage liver disease (MELD) score[138]. Considering the increased incidence of renal dysfunction at LT due to prioritization based on the MELD allocation system in the United States, SLKT rates climbed from 2.7% of all LT in 2000 to 9.3% in 2016[138-140]. NASH is currently the leading and most rapidly growing indication for SLKT in the United States[138,140] with a 200% increase for SLKT from 2002 to 2010[138]. Patients with NASH have a high probability to undergo SLKT rather than LT alone since they are highly incident for CKD for a prolonged duration, which can fulfill criteria for SLKT (patients with CKD: GFR ≤ 60 mL/min for ≥ 3 mo with recent GFR ≤ 30 mL/min or on hemodialysis, patients with AKI: Dialysis for > 6 wk GFR ≤ 25 for > 6 wk)[141].
Patients with NASH were independently associated with a higher risk of CKD or advanced kidney damage after LT compared with those without NASH[142-144]. In general, renal dysfunction after LT is affected not only by immunosuppressant medications, especially calcineurin inhibitors, pre-LT kidney dysfunction, but also persistent or de novo metabolic co-morbidities such as hypertension, diabetes, and obesity - all of which are highly likely in NASH patients undergoing LT[142,145]. Special attention to the recognition of CKD is needed for patients with NASH patients when deciding LT vs SLKT. Controlling for metabolic complications and avoiding or keeping a low dose of calcineurin inhibitors as much as possible seems to be crucially important to reduce the risk of incident CKD, and risk of progression of CKD after liver transplant in NASH patients.
Despite the breadth of research, minimal guideline-based management of patients with both NAFLD and CKD is available. However, important pathogenic links and shared risk factors between NAFLD and CKD underscore the importance of earlier surveillance and strict control of shared metabolic risk factors. Although preventative strategies for CKD in NAFLD are limited, treatment directed specifically for NASH in the future will hopefully ameliorate the progression of renal dysfunction in affected patients. There is a plethora of clinical trials underway, and if these drugs show safety and efficacy in improving NASH, they may translate into improving renal function[146]. Specific interventions for preventing CKD progression using SGLT2 inhibitors, PPAR agonists, SAM, XO inhibitors, and Vitamin D have been tried but need further confirmation. Progression from NAFLD to NAFLD-related advanced fibrosis is linked to an increased risk of CKD, and earlier intervention in those with renal dysfunction is warranted. Genetic links between NAFLD and CKD have also been proposed, specifically in the G allele of PNPLA3 and the T allele of TM6SF2, and future studies targeting patients with such genetic profiles to prevent progression to CKD is needed.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; American Association for the Study of Liver Diseases; and American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li YY, Yang SS S-Editor: Fan JR L-Editor: A P-Editor: Wu YXJ
| 1. | Satapathy SK, Sanyal AJ. Epidemiology and Natural History of Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35:221-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 2. | Kovalic AJ, Cholankeril G, Satapathy SK. Nonalcoholic fatty liver disease and alcoholic liver disease: metabolic diseases with systemic manifestations. Transl Gastroenterol Hepatol. 2019;4:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, Setiawan VW, Tran T, Ayoub WS, Lu SC, Klein AS, Sundaram V, Nissen NN. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol. 2018;113:1649-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 446] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 4. | Gadiparthi C, Spatz M, Greenberg S, Iqbal U, Kanna S, Satapathy SK, Broder A, Ahmed A. NAFLD Epidemiology, Emerging Pharmacotherapy, Liver Transplantation Implications and the Trends in the United States. J Clin Transl Hepatol. 2020;8:215-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2140] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 6. | Hsieh MH, Wu KT, Chen YY, Yang JF, Lin WY, Chang NC, Lin CY, Huang CK, Wang CL, Chuang HY, Lin SC, Hsu YK, Tsai YS, Chuang WL, Yu ML, Dai CY. Higher NAFLD fibrosis score is associated with impaired eGFR. J Formos Med Assoc. 2020;119:496-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Locatelli F, Pozzoni P, Del Vecchio L. Renal manifestations in the metabolic syndrome. J Am Soc Nephrol. 2006;17:S81-S85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Yilmaz Y, Alahdab YO, Yonal O, Kurt R, Kedrah AE, Celikel CA, Ozdogan O, Duman D, Imeryuz N, Avsar E, Kalayci C. Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: association with liver fibrosis. Metabolism. 2010;59:1327-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Yasui K, Sumida Y, Mori Y, Mitsuyoshi H, Minami M, Itoh Y, Kanemasa K, Matsubara H, Okanoue T, Yoshikawa T. Nonalcoholic steatohepatitis and increased risk of chronic kidney disease. Metabolism. 2011;60:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Park CW, Tsai NT, Wong LL. Implications of worse renal dysfunction and medical comorbidities in patients with NASH undergoing liver transplant evaluation: impact on MELD and more. Clin Transplant. 2011;25:E606-E611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Machado MV, Gonçalves S, Carepa F, Coutinho J, Costa A, Cortez-Pinto H. Impaired renal function in morbid obese patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, Hultcrantz R, Hagström H, Yoon SK, Charatcharoenwitthaya P, George J, Barrera F, Hafliðadóttir S, Björnsson ES, Armstrong MJ, Hopkins LJ, Gao X, Francque S, Verrijken A, Yilmaz Y, Lindor KD, Charlton M, Haring R, Lerch MM, Rettig R, Völzke H, Ryu S, Li G, Wong LL, Machado M, Cortez-Pinto H, Yasui K, Cassader M. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 545] [Cited by in RCA: 525] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 13. | Mantovani A, Zaza G, Byrne CD, Lonardo A, Zoppini G, Bonora E, Targher G. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: A systematic review and meta-analysis. Metabolism. 2018;79:64-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 14. | Park H, Dawwas GK, Liu X, Nguyen MH. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: a propensity-matched cohort study. J Intern Med. 2019;286:711-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Jennison E, Patel J, Scorletti E, Byrne CD. Diagnosis and management of non-alcoholic fatty liver disease. Postgrad Med J. 2019;95:314-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Corey KE, Kartoun U, Zheng H, Shaw SY. Development and Validation of an Algorithm to Identify Nonalcoholic Fatty Liver Disease in the Electronic Medical Record. Dig Dis Sci. 2016;61:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Targher G, Byrne CD. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol. 2017;13:297-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 18. | Campos GM, Bambha K, Vittinghoff E, Rabl C, Posselt AM, Ciovica R, Tiwari U, Ferrel L, Pabst M, Bass NM, Merriman RB. A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology. 2008;47:1916-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5:2166-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 20. | Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, Muggeo M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 21. | Targher G, Pichiri I, Zoppini G, Trombetta M, Bonora E. Increased prevalence of chronic kidney disease in patients with Type 1 diabetes and non-alcoholic fatty liver. Diabet Med. 2012;29:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Li G, Shi W, Hug H, Chen Y, Liu L, Yin D. Nonalcoholic fatty liver disease associated with impairment of kidney function in nondiabetes population. Biochem Med (Zagreb). 2012;22:92-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Huh JH, Kim JY, Choi E, Kim JS, Chang Y, Sung KC. The fatty liver index as a predictor of incident chronic kidney disease in a 10-year prospective cohort study. PLoS One. 2017;12:e0180951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Shen ZW, Xing J, Wang QL, Faheem A, Ji X, Li J, Bian WW, Jiang Z, Li XJ, Xue FZ, Liu J. Association between serum γ-glutamyltransferase and chronic kidney disease in urban Han Chinese: a prospective cohort study. Int Urol Nephrol. 2017;49:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Kunutsor SK, Laukkanen JA. Gamma-glutamyltransferase and risk of chronic kidney disease: A prospective cohort study. Clin Chim Acta. 2017;473:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Sinn DH, Kang D, Jang HR, Gu S, Cho SJ, Paik SW, Ryu S, Chang Y, Lazo M, Guallar E, Cho J, Gwak GY. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: A cohort study. J Hepatol. 2017;67:1274-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 27. | Zheng CM, Lee YH, Chiu IJ, Chiu YJ, Sung LC, Hsu YH, Chiu HW. Nicotine Causes Nephrotoxicity through the Induction of NLRP6 Inflammasome and Alpha7 Nicotinic Acetylcholine Receptor. Toxics. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Rezonzew G, Chumley P, Feng W, Hua P, Siegal GP, Jaimes EA. Nicotine exposure and the progression of chronic kidney disease: role of the α7-nicotinic acetylcholine receptor. Am J Physiol Renal Physiol. 2012;303:F304-F312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Jang HR, Kang D, Sinn DH, Gu S, Cho SJ, Lee JE, Huh W, Paik SW, Ryu S, Chang Y, Shafi T, Lazo M, Guallar E, Cho J, Gwak GY. Nonalcoholic fatty liver disease accelerates kidney function decline in patients with chronic kidney disease: a cohort study. Sci Rep. 2018;8:4718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Nampoothiri RV, Duseja A, Rathi M, Agrawal S, Sachdeva N, Mehta M, Dhaliwal HS, Dhiman RK, Chawla Y. Renal Dysfunction in Patients With Nonalcoholic Fatty Liver Disease is Related to the Presence of Diabetes Mellitus and Severity of Liver Disease. J Clin Exp Hepatol. 2019;9:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Bian H, Zhu X, Xia M, Yan H, Chang X, Hu X, Pan B, Guo W, Li X, Gao X. Impact of type 2 diabetes on nonalcoholic steatohepatitis and advanced fibrosis in patients with nonalcoholic fatty liver disease. Endocr Pract. 2020;26:444-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Reddy YK, Marella HK, Jiang Y, Ganguli S, Snell P, Podila PSB, Maliakkal B, Satapathy SK. Natural History of Non-Alcoholic Fatty Liver Disease: A Study With Paired Liver Biopsies. J Clin Exp Hepatol. 2020;10:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Targher G, Bertolini L, Chonchol M, Rodella S, Zoppini G, Lippi G, Zenari L, Bonora E. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and retinopathy in type 1 diabetic patients. Diabetologia. 2010;53:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Manco M, Ciampalini P, DeVito R, Vania A, Cappa M, Nobili V. Albuminuria and insulin resistance in children with biopsy proven non-alcoholic fatty liver disease. Pediatr Nephrol. 2009;24:1211-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Iglesias P, Díez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol. 2009;160:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 36. | van Tienhoven-Wind LJ, Dullaart RP. Low-normal thyroid function and the pathogenesis of common cardio-metabolic disorders. Eur J Clin Invest. 2015;45:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Rhee CM, Kalantar-Zadeh K, Streja E, Carrero JJ, Ma JZ, Lu JL, Kovesdy CP. The relationship between thyroid function and estimated glomerular filtration rate in patients with chronic kidney disease. Nephrol Dial Transplant. 2015;30:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 38. | Pagadala MR, Zein CO, Dasarathy S, Yerian LM, Lopez R, McCullough AJ. Prevalence of hypothyroidism in nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Chen PC, Kao WY, Cheng YL, Wang YJ, Hou MC, Wu JC, Su CW. The correlation between fatty liver disease and chronic kidney disease. J Formos Med Assoc. 2020;119:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Yeung MW, Wong GL, Choi KC, Luk AO, Kwok R, Shu SS, Chan AW, Lau ESH, Ma RCW, Chan HL, Chan JC, Wong VW, Kong AP. Advanced liver fibrosis but not steatosis is independently associated with albuminuria in Chinese patients with type 2 diabetes. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 41. | Lombardi R, Airaghi L, Targher G, Serviddio G, Maffi G, Mantovani A, Maffeis C, Colecchia A, Villani R, Rinaldi L, Orsi E, Pisano G, Adinolfi LE, Fargion S, Fracanzani AL. Liver fibrosis by FibroScan® independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. 2020;40:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 42. | Qin S, Wang S, Wang X, Wang J. Liver stiffness assessed by transient elastography as a potential indicator of chronic kidney disease in patients with nonalcoholic fatty liver disease. J Clin Lab Anal. 2019;33:e22657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Chon YE, Kim HJ, Choi YB, Hwang SG, Rim KS, Kim MN, Lee JH, Ha Y, Lee MJ. Decrease in waist-to-hip ratio reduced the development of chronic kidney disease in non-obese non-alcoholic fatty liver disease. Sci Rep. 2020;10:8996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Ampuero J, Aller R, Gallego-Durán R, Banales JM, Crespo J, García-Monzón C, Pareja MJ, Vilar-Gómez E, Caballería J, Escudero-García D, Gomez-Camarero J, Calleja JL, Latorre M, Albillos A, Salmeron J, Aspichueta P, Lo Iacono O, Francés R, Benlloch S, Fernández-Rodríguez C, García-Samaniego J, Estévez P, Andrade RJ, Turnes J, Romero-Gómez M; HEPAmet Registry. The effects of metabolic status on non-alcoholic fatty liver disease-related outcomes, beyond the presence of obesity. Aliment Pharmacol Ther. 2018;48:1260-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 45. | Raj D, Tomar B, Lahiri A, Mulay SR. The gut-liver-kidney axis: Novel regulator of fatty liver associated chronic kidney disease. Pharmacol Res. 2020;152:104617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 46. | Willy JA, Young SK, Stevens JL, Masuoka HC, Wek RC. CHOP links endoplasmic reticulum stress to NF-κB activation in the pathogenesis of nonalcoholic steatohepatitis. Mol Biol Cell. 2015;26:2190-2204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 47. | Marcuccilli M, Chonchol M. NAFLD and Chronic Kidney Disease. Int J Mol Sci. 2016;17:562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 48. | Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis. 2014;64:638-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 49. | Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539-1543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 477] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 50. | Khukhlina OS, Antoniv AA, Mandryk OY, Smandych VS, Matushchak MR. The role of endothelial dysfunction in the progression mechanisms of non-alcoholic steatohepatitis in patients with obesity and chronic kidney disease. Wiad Lek. 2019;72:523-526. [PubMed] |
| 51. | Hübscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006;49:450-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 52. | Sieber J, Lindenmeyer MT, Kampe K, Campbell KN, Cohen CD, Hopfer H, Mundel P, Jehle AW. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol. 2010;299:F821-F829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 53. | Joles JA, van Goor H, van der Horst ML, van Tol A, Elema JD, Koomans HA. High lipid levels in very low density lipoprotein and intermediate density lipoprotein may cause proteinuria and glomerulosclerosis in aging female analbuminemic rats. Lab Invest. 1995;73:912-921. [PubMed] |
| 54. | Lindenmeyer MT, Rastaldi MP, Ikehata M, Neusser MA, Kretzler M, Cohen CD, Schlöndorff D. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol. 2008;19:2225-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 55. | Monteillet L, Gjorgjieva M, Silva M, Verzieux V, Imikirene L, Duchampt A, Guillou H, Mithieux G, Rajas F. Intracellular lipids are an independent cause of liver injury and chronic kidney disease in non alcoholic fatty liver disease-like context. Mol Metab. 2018;16:100-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 56. | Ackers I, Malgor R. Interrelationship of canonical and non-canonical Wnt signalling pathways in chronic metabolic diseases. Diab Vasc Dis Res. 2018;15:3-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 57. | Shimano H, Sato R. SREBP-regulated lipid metabolism: convergent physiology - divergent pathophysiology. Nat Rev Endocrinol. 2017;13:710-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 791] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 58. | Musso G, Cassader M, Cohney S, De Michieli F, Pinach S, Saba F, Gambino R. Fatty Liver and Chronic Kidney Disease: Novel Mechanistic Insights and Therapeutic Opportunities. Diabetes Care. 2016;39:1830-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 59. | Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 60. | Babio N, Martínez-González MA, Estruch R, Wärnberg J, Recondo J, Ortega-Calvo M, Serra-Majem L, Corella D, Fitó M, Ros E, Becerra-Tomás N, Basora J, Salas-Salvadó J. Associations between serum uric acid concentrations and metabolic syndrome and its components in the PREDIMED study. Nutr Metab Cardiovasc Dis. 2015;25:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 61. | Zurlo A, Veronese N, Giantin V, Maselli M, Zambon S, Maggi S, Musacchio E, Toffanello ED, Sartori L, Perissinotto E, Crepaldi G, Manzato E, Sergi G. High serum uric acid levels increase the risk of metabolic syndrome in elderly women: The PRO.V.A study. Nutr Metab Cardiovasc Dis. 2016;26:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 62. | Sun HL, Pei D, Lue KH, Chen YL. Uric Acid Levels Can Predict Metabolic Syndrome and Hypertension in Adolescents: A 10-Year Longitudinal Study. PLoS One. 2015;10:e0143786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 63. | Yuan H, Yu C, Li X, Sun L, Zhu X, Zhao C, Zhang Z, Yang Z. Serum Uric Acid Levels and Risk of Metabolic Syndrome: A Dose-Response Meta-Analysis of Prospective Studies. J Clin Endocrinol Metab. 2015;100:4198-4207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 64. | Johnson RJ, Nakagawa T, Jalal D, Sánchez-Lozada LG, Kang DH, Ritz E. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant. 2013;28:2221-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 433] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 65. | Sharaf El Din UAA, Salem MM, Abdulazim DO. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J Adv Res. 2017;8:537-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 66. | Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, Li N, Roncal-Jimenez CA, Ishimoto T, Le M, Garcia GE, Thomas JB, Rivard CJ, Andres-Hernando A, Hunter B, Schreiner G, Rodriguez-Iturbe B, Sautin YY, Johnson RJ. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One. 2012;7:e47948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 67. | Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, Ito S, Yamamoto T, Tomino Y, Ohno I, Shibagaki Y, Iimuro S, Imai N, Kuwabara M, Hayakawa H, Ohtsu H, Ohashi Y; FEATHER Study Investigators. Febuxostat Therapy for Patients With Stage 3 CKD and Asymptomatic Hyperuricemia: A Randomized Trial. Am J Kidney Dis. 2018;72:798-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 253] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 68. | Wu IW, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ, Tzen CY, Wang YC, Lin CY, Wu MS. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26:938-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 372] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 69. | Liu H, Narayanan R, Hoffmann M, Surapaneni S. The Uremic Toxin Indoxyl-3-Sulfate Induces CYP1A2 In Primary Human Hepatocytes. Drug Metab Lett. 2016;10:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 918] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 71. | Tan X, Liu Y, Long J, Chen S, Liao G, Wu S, Li C, Wang L, Ling W, Zhu H. Trimethylamine N-Oxide Aggravates Liver Steatosis through Modulation of Bile Acid Metabolism and Inhibition of Farnesoid X Receptor Signaling in Nonalcoholic Fatty Liver Disease. Mol Nutr Food Res. 2019;63:e1900257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 72. | Anstee QM, Seth D, Day CP. Genetic Factors That Affect Risk of Alcoholic and Nonalcoholic Fatty Liver Disease. Gastroenterology 2016; 150: 1728-1744. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 73. | Macaluso FS, Maida M, Petta S. Genetic background in nonalcoholic fatty liver disease: A comprehensive review. World J Gastroenterol. 2015;21:11088-11111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 74. | Musso G, Cassader M, Gambino R. PNPLA3 rs738409 and TM6SF2 rs58542926 gene variants affect renal disease and function in nonalcoholic fatty liver disease. Hepatology. 2015;62:658-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 75. | Oniki K, Saruwatari J, Izuka T, Kajiwara A, Morita K, Sakata M, Otake K, Ogata Y, Nakagawa K. Influence of the PNPLA3 rs738409 Polymorphism on Non-Alcoholic Fatty Liver Disease and Renal Function among Normal Weight Subjects. PLoS One. 2015;10:e0132640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 76. | Sun DQ, Zheng KI, Xu G, Ma HL, Zhang HY, Pan XY, Zhu PW, Wang XD, Targher G, Byrne CD, Chen YP, Yuan WJ, Zheng MH. PNPLA3 rs738409 is associated with renal glomerular and tubular injury in NAFLD patients with persistently normal ALT levels. Liver Int. 2020;40:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 77. | Mantovani A, Zusi C, Sani E, Colecchia A, Lippi G, Zaza GL, Valenti L, Byrne CD, Maffeis C, Bonora E, Targher G. Association between PNPLA3rs738409 polymorphism decreased kidney function in postmenopausal type 2 diabetic women with or without non-alcoholic fatty liver disease. Diabetes Metab. 2019;45:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 78. | Sookoian S, Castaño GO, Scian R, Mallardi P, Fernández Gianotti T, Burgueño AL, San Martino J, Pirola CJ. Genetic variation in transmembrane 6 superfamily member 2 and the risk of nonalcoholic fatty liver disease and histological disease severity. Hepatology. 2015;61:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 79. | Önnerhag K, Hartman H, Nilsson PM, Lindgren S. Non-invasive fibrosis scoring systems can predict future metabolic complications and overall mortality in non-alcoholic fatty liver disease (NAFLD). Scand J Gastroenterol. 2019;54:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 80. | Wijarnpreecha K, Thongprayoon C, Scribani M, Ungprasert P, Cheungpasitporn W. Noninvasive fibrosis markers and chronic kidney disease among adults with nonalcoholic fatty liver in USA. Eur J Gastroenterol Hepatol. 2018;30:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Choi JW, Lee CH, Park JS. Comparison of laboratory indices of non-alcoholic fatty liver disease for the detection of incipient kidney dysfunction. PeerJ. 2019;7:e6524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Ciardullo S, Muraca E, Perra S, Bianconi E, Zerbini F, Oltolini A, Cannistraci R, Parmeggiani P, Manzoni G, Gastaldelli A, Lattuada G, Perseghin G. Screening for non-alcoholic fatty liver disease in type 2 diabetes using non-invasive scores and association with diabetic complications. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 83. | Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 527] [Article Influence: 52.7] [Reference Citation Analysis (1)] |
| 84. | Piano S, Romano A, Di Pascoli M, Angeli P. Why and how to measure renal function in patients with liver disease. Liver Int. 2017;37 Suppl 1:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 85. | Mindikoglu AL, Dowling TC, Weir MR, Seliger SL, Christenson RH, Magder LS. Performance of chronic kidney disease epidemiology collaboration creatinine-cystatin C equation for estimating kidney function in cirrhosis. Hepatology. 2014;59:1532-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 86. | Shardlow A, McIntyre NJ, Fraser SDS, Roderick P, Raftery J, Fluck RJ, McIntyre CW, Taal MW. The clinical utility and cost impact of cystatin C measurement in the diagnosis and management of chronic kidney disease: A primary care cohort study. PLoS Med. 2017;14:e1002400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 87. | Bulum T, Kolarić B, Duvnjak M, Duvnjak L. Alkaline phosphatase is independently associated with renal function in normoalbuminuric type 1 diabetic patients. Ren Fail. 2014;36:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Pantsari MW, Harrison SA. Nonalcoholic fatty liver disease presenting with an isolated elevated alkaline phosphatase. J Clin Gastroenterol. 2006;40:633-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Satchell SC, Tooke JE. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia. 2008;51:714-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 90. | Ryu S, Chang Y, Kim DI, Kim WS, Suh BS. gamma-Glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem. 2007;53:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 91. | Arase Y, Suzuki F, Kobayashi M, Suzuki Y, Kawamura Y, Matsumoto N, Akuta N, Sezaki H, Saito S, Hosaka T, Ikeda K, Kumada H, Ohmoto Y, Amakawa K, Tsuji H, Hsieh SD, Kato K, Tanabe M, Ogawa K, Hara S, Kobayashi T. The development of chronic kidney disease in Japanese patients with non-alcoholic fatty liver disease. Intern Med. 2011;50:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | Thamer C, Tschritter O, Haap M, Shirkavand F, Machann J, Fritsche A, Schick F, Häring H, Stumvoll M. Elevated serum GGT concentrations predict reduced insulin sensitivity and increased intrahepatic lipids. Horm Metab Res. 2005;37:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 93. | Kim D, Kim WR. Nonobese Fatty Liver Disease. Clin Gastroenterol Hepatol. 2017;15:474-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (1)] |
| 94. | Vilar-Gomez E, Calzadilla-Bertot L, Friedman SL, Gra-Oramas B, Gonzalez-Fabian L, Villa-Jimenez O, Lazo-Del Vallin S, Diago M, Adams LA, Romero-Gomez M, Chalasani N. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;45:332-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 95. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3989] [Article Influence: 664.8] [Reference Citation Analysis (0)] |
| 96. | Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, Iijima M, Takekawa H, Usui I, Hiraishi H, Aso Y. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 272] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 97. | Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016;42:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 98. | Scheen AJ. Beneficial effects of SGLT2 inhibitors on fatty liver in type 2 diabetes: A common comorbidity associated with severe complications. Diabetes Metab. 2019;45:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 99. | Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17 Suppl:S186-S190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 302] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 100. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team; Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1471] [Article Influence: 163.4] [Reference Citation Analysis (1)] |
| 101. | Tuttle KR, Lakshmanan MC, Rayner B, Busch RS, Zimmermann AG, Woodward DB, Botros FT. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 102. | Sumida Y, Yoneda M, Toyoda H, Yasuda S, Tada T, Hayashi H, Nishigaki Y, Suzuki Y, Naiki T, Morishita A, Tobita H, Sato S, Kawabe N, Fukunishi S, Ikegami T, Kessoku T, Ogawa Y, Honda Y, Nakahara T, Munekage K, Ochi T, Sawada K, Takahashi A, Arai T, Kogiso T, Kimoto S, Tomita K, Notsumata K, Nonaka M, Kawata K, Takami T, Kumada T, Tomita E, Okanoue T, Nakajima A; Japan Study Group Of Nafld Jsg-Nafld. Common Drug Pipelines for the Treatment of Diabetic Nephropathy and Hepatopathy: Can We Kill Two Birds with One Stone? Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 103. | Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 1065] [Article Influence: 177.5] [Reference Citation Analysis (0)] |
| 104. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4450] [Article Influence: 741.7] [Reference Citation Analysis (0)] |
| 105. | Hargreaves IP. Coenzyme Q10 as a therapy for mitochondrial disease. Int J Biochem Cell Biol. 2014;49:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 106. | Mantle D, Hargreaves I. Coenzyme Q10 and Degenerative Disorders Affecting Longevity: An Overview. Antioxidants (Basel). 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 107. | Yesilova Z, Yaman H, Oktenli C, Ozcan A, Uygun A, Cakir E, Sanisoglu SY, Erdil A, Ates Y, Aslan M, Musabak U, Erbil MK, Karaeren N, Dagalp K. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2005;100:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 108. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3720] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 109. | Farhangi MA, Alipour B, Jafarvand E, Khoshbaten M. Oral coenzyme Q10 supplementation in patients with nonalcoholic fatty liver disease: effects on serum vaspin, chemerin, pentraxin 3, insulin resistance and oxidative stress. Arch Med Res. 2014;45:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 110. | Farsi F, Mohammadshahi M, Alavinejad P, Rezazadeh A, Zarei M, Engali KA. Functions of Coenzyme Q10 Supplementation on Liver Enzymes, Markers of Systemic Inflammation, and Adipokines in Patients Affected by Nonalcoholic Fatty Liver Disease: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. J Am Coll Nutr. 2016;35:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 111. | Yeung CK, Billings FT 4th, Claessens AJ, Roshanravan B, Linke L, Sundell MB, Ahmad S, Shao B, Shen DD, Ikizler TA, Himmelfarb J. Coenzyme Q10 dose-escalation study in hemodialysis patients: safety, tolerability, and effect on oxidative stress. BMC Nephrol. 2015;16:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 112. | Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, Alehagen U, Steurer G, Littarru GP; Q-SYMBIO Study Investigators. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 113. | Alehagen U, Johansson P, Björnstedt M, Rosén A, Dahlström U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol. 2013;167:1860-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 114. | Cheng HS, Tan WR, Low ZS, Marvalim C, Lee JYH, Tan NS. Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 115. | Schneider CA, Ferrannini E, Defronzo R, Schernthaner G, Yates J, Erdmann E. Effect of pioglitazone on cardiovascular outcome in diabetes and chronic kidney disease. J Am Soc Nephrol. 2008;19:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 116. | Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y, Liang TJ, Yanovski JA, Kleiner DE, Hoofnagle JH. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 524] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 117. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1329] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 118. | Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 556] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 119. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2473] [Article Influence: 164.9] [Reference Citation Analysis (2)] |
| 120. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 121. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4954] [Article Influence: 707.7] [Reference Citation Analysis (9)] |
| 122. | Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 331] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 123. | Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E, Grimaldi A, Poynard T; LIDO Study Group. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 479] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 124. | Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T; LIDO Study Group. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 125. | Eliades M, Spyrou E, Agrawal N, Lazo M, Brancati FL, Potter JJ, Koteish AA, Clark JM, Guallar E, Hernaez R. Meta-analysis: vitamin D and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 126. | LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 127. | Parikh A, Chase HS, Vernocchi L, Stern L. Vitamin D resistance in chronic kidney disease (CKD). BMC Nephrol. 2014;15:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 128. | Xu L, Wan X, Huang Z, Zeng F, Wei G, Fang D, Deng W, Li Y. Impact of vitamin D on chronic kidney diseases in non-dialysis patients: a meta-analysis of randomized controlled trials. PLoS One. 2013;8:e61387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 129. | Dasarathy J, Varghese R, Feldman A, Khiyami A, McCullough AJ, Dasarathy S. Patients with Nonalcoholic Fatty Liver Disease Have a Low Response Rate to Vitamin D Supplementation. J Nutr. 2017;147:1938-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 130. | NIH. Effect on Liver Histology of Vitamin D in Patients With Non-alcoholic Steatohepatitis. [cited 7 November 2020]. Available from: https://clinicaltrials.gov/ct2/results?cond=&term=NCT01623024&cntry=&state=&city=&dist=. |
| 131. | NIH. Study of Vitamin D3 Supplementation in Patients With Chronic Kidney Disease. [cited 7 November 2020]. Available from: https://clinicaltrials.gov/ct2/results?cond=&term=NCT00893451&cntry=&state=&city=&dist=. |
| 132. | NIH. DHA and Vitamin D in Children With Biopsy-proven NAFLD. [cited 7 November 2020]. Available from: https://clinicaltrials.gov/ct2/results?cond=&term=+NCT02098317&cntry=&state=&city=&dist=. |
| 133. | García-Lezana T, Raurell I, Bravo M, Torres-Arauz M, Salcedo MT, Santiago A, Schoenenberger A, Manichanh C, Genescà J, Martell M, Augustin S. Restoration of a healthy intestinal microbiota normalizes portal hypertension in a rat model of nonalcoholic steatohepatitis. Hepatology. 2018;67:1485-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 134. | Bergheim I, Weber S, Vos M, Krämer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 402] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 135. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3800] [Article Influence: 542.9] [Reference Citation Analysis (2)] |
| 136. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1384] [Article Influence: 138.4] [Reference Citation Analysis (1)] |
| 137. | Calzadilla-Bertot L, Jeffrey GP, Jacques B, McCaughan G, Crawford M, Angus P, Jones R, Gane E, Munn S, Macdonald G, Fawcett J, Wigg A, Chen J, Fink M, Adams LA. Increasing Incidence of Nonalcoholic Steatohepatitis as an Indication for Liver Transplantation in Australia and New Zealand. Liver Transpl. 2019;25:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 138. | Singal AK, Hasanin M, Kaif M, Wiesner R, Kuo YF. Nonalcoholic Steatohepatitis is the Most Rapidly Growing Indication for Simultaneous Liver Kidney Transplantation in the United States. Transplantation. 2016;100:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 139. | Miles CD, Westphal S, Liapakis A, Formica R. Simultaneous Liver-Kidney Transplantation: Impact on Liver Transplant Patients and the Kidney Transplant Waiting List. Curr Transplant Rep. 2018;5:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 140. | Singal AK, Salameh H, Kuo YF, Wiesner RH. Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology. Transplantation. 2014;98:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 141. | Formica RN, Aeder M, Boyle G, Kucheryavaya A, Stewart D, Hirose R, Mulligan D. Simultaneous Liver-Kidney Allocation Policy: A Proposal to Optimize Appropriate Utilization of Scarce Resources. Am J Transplant. 2016;16:758-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 142. | Houlihan DD, Armstrong MJ, Davidov Y, Hodson J, Nightingale P, Rowe IA, Paris S, Gunson BK, Bramhall SB, Mutimer DJ, Neuberger JM, Newsome PN. Renal function in patients undergoing transplantation for nonalcoholic steatohepatitis cirrhosis: time to reconsider immunosuppression regimens? Liver Transpl. 2011;17:1292-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 143. | Mikolasevic I, Orlic L, Hrstic I, Milic S. Metabolic syndrome and non-alcoholic fatty liver disease after liver or kidney transplantation. Hepatol Res. 2016;46:841-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 144. | Molnar MZ, Joglekar K, Jiang Y, Cholankeril G, Abdul MKM, Kedia S, Gonzalez HC, Ahmed A, Singal A, Bhamidimarri KR, Aithal GP, Duseja A, Wong VW, Gulnare A, Puri P, Nair S, Eason JD, Satapathy SK; Global NAFLD Consortium. Association of Pretransplant Renal Function With Liver Graft and Patient Survival After Liver Transplantation in Patients With Nonalcoholic Steatohepatitis. Liver Transpl. 2019;25:399-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 145. | Mikolasevic I, Filipec-Kanizaj T, Mijic M, Jakopcic I, Milic S, Hrstic I, Sobocan N, Stimac D, Burra P. Nonalcoholic fatty liver disease and liver transplantation - Where do we stand? World J Gastroenterol. 2018;24:1491-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 146. | Ganguli S, DeLeeuw P, Satapathy SK. A Review Of Current And Upcoming Treatment Modalities In Non-Alcoholic Fatty Liver Disease And Non-Alcoholic Steatohepatitis. Hepat Med. 2019;11:159-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 147. | Önnerhag K, Dreja K, Nilsson PM, Lindgren S. Increased mortality in non-alcoholic fatty liver disease with chronic kidney disease is explained by metabolic comorbidities. Clin Res Hepatol Gastroenterol. 2019;43:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |